Abstract
Studies from diverse systems have shown that distinct interchromosomal interactions are a central component of nuclear organization. In some cases, these interactions allow an enhancer to act in trans, modulating the expression of a gene encoded on a separate chromosome held in close proximity. Despite recent advances in uncovering such phenomena, our understanding of how a regulatory element acts on another chromosome remains incomplete. Here, we describe a transgenic approach to better understand enhancer action in trans in Drosophila melanogaster. Using phiC31-based recombinase-mediated cassette exchange (RMCE), we placed transgenes carrying combinations of the simple enhancer GMR, a minimal promoter, and different fluorescent reporters at equivalent positions on homologous chromosomes so that they would pair via the endogenous somatic pairing machinery of Drosophila. Our data demonstrate that the enhancer GMR is capable of activating a promoter in trans and does so in a variegated pattern, suggesting stochastic interactions between the enhancer and the promoter when they are carried on separate chromosomes. Furthermore, we quantitatively assessed the impact of two concurrent promoter targets in cis and in trans to GMR, demonstrating that each promoter is capable of competing for the enhancer’s activity, with the presence of one negatively affecting expression from the other. Finally, the single-cell resolution afforded by our approach allowed us to show that promoters in cis and in trans to GMR can both be activated in the same nucleus, implying that a single enhancer can share its activity between multiple promoter targets carried on separate chromosomes.
Keywords: transvection; interchromosomal interactions; long-range enhancer; RMCE, phiC31
IN an oversimplified view of the genome, each chromosome could be considered a linear arrangement of genic units, with each gene controlled solely by nearby cis-acting regulatory sequences. However, extensive analyses based on DNA fluorescence in situ hybridization (DNA FISH) and chromatin conformation capture (3C) have shown that the three-dimensional organization of the nucleus involves physical interactions between distant genomic regions, suggesting that gene regulation is more complex than this simplified model (reviewed by Gondor and Ohlsson 2009; Naumova and Dekker 2010). Further complicating our view, long-distance interactions are not limited to genomic regions on the same chromosomes (in cis), but are also observed between specific loci on separate chromosomes (in trans) (reviewed by Williams et al. 2010). Through genome-wide 3C-based approaches, extensive interchromosomal interactions have been identified in yeast (Duan et al. 2010), Drosophila (Sexton et al. 2012), mice (Zhang et al. 2012), and humans (Lieberman-Aiden et al. 2009) and are thus a component of nuclear organization in diverse organisms.
Long-distance intra- and interchromosomal interactions can result from colocalization of unlinked genomic regions to a common nuclear compartment, including loci undergoing active transcription or that bind insulator or Polycomb Group proteins (reviewed by Bantignies and Cavalli 2011; Bulger and Groudine 2011; Dean 2011). In some cases, long-distance interactions could reflect communication between regulatory sequences that will directly affect gene expression, as is the case when a distant enhancer loops toward a promoter to activate transcription (reviewed by Bartkuhn and Renkawitz 2008; Bulger and Groudine 2011). Interchromosomal interactions that directly affect gene expression have been demonstrated in several developmental contexts, including X-inactivation (Bacher et al. 2006; Xu et al. 2006), Ifng expression in naive T helper cells (Spilianakis et al. 2005), and the establishment of replication timing of imprinted loci (Sandhu et al. 2009) in mice, and IFN-β expression in response to virus infection in humans (Apostolou and Thanos 2008). In addition, physical interactions between chromosomes have been postulated to explain epigenetic phenomena such as repeat-induced point mutation in Neurospora (reviewed by Galagan and Selker 2004) and paramutation in maize (Chandler and Stam 2004). However, our ability to identify and catalog interchromosomal interactions has grown more quickly than our ability to determine their potential impact on gene expression. Thus, a central question remains: Is it common for sequences that regulate gene expression to communicate between chromosomes when they are physically juxtaposed?
Drosophila provides an excellent model system to better understand the potential impact of interchromosomal interactions on gene regulation. In Drosophila, homologous chromosomes are stably paired from end to end in virtually all cells of the organism (reviewed by McKee 2004). The potential for communication between Drosophila homologs was first postulated >100 years ago by Nettie Stevens (Stevens 1908) and was demonstrated genetically in 1954 by Ed Lewis, who coined the term transvection to describe the phenomenon of pairing-dependent gene regulation (Lewis 1954). Transvection has since been shown to influence many genes in Drosophila and can lead to either upregulation or silencing of gene expression by several mechanisms (reviewed by Duncan 2002; Kennison and Southworth 2002).
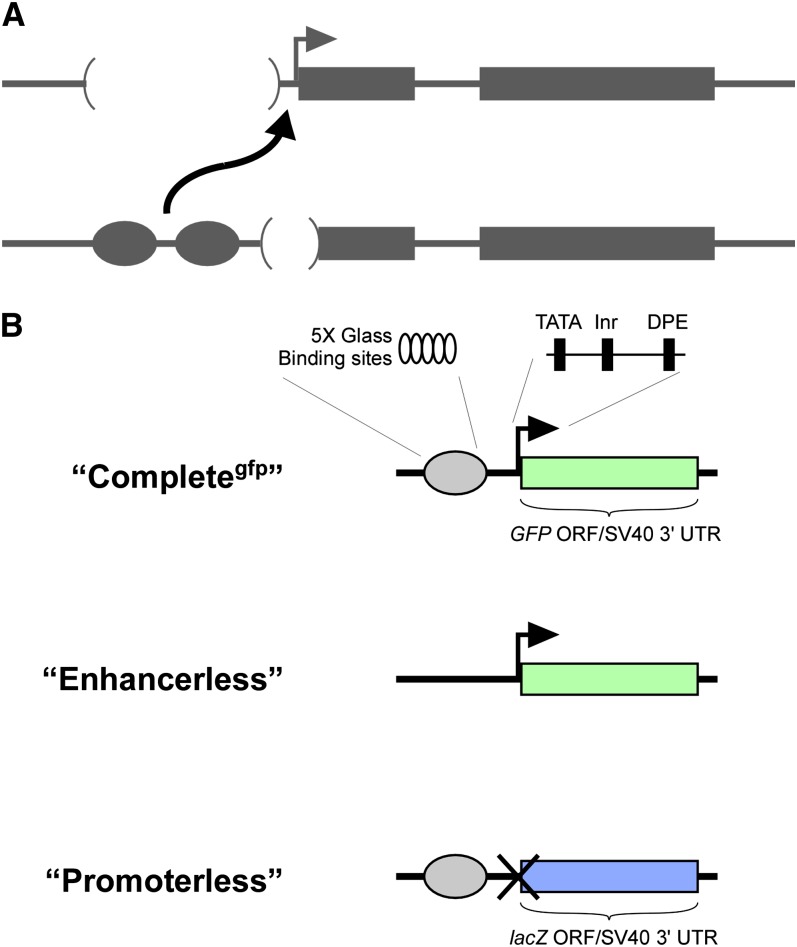
In one form of transvection, an enhancer on one homolog acts in trans to activate a promoter on a paired homolog, leading to upregulation of a linked gene. Enhancer action in trans is often uncovered as an unexpected case of intragenic complementation involving specific types of mutant alleles. For example, one mutant allele may carry a nonfunctional enhancer while another carries a compromised promoter; acting alone, neither allele can support gene expression, but when paired, the remaining functional enhancer and promoter interact in trans to activate transcription (Figure 1A). For many genes, enhancer action in trans is thought to produce reduced levels of transcription relative to activation in cis, as evidenced by the partial complementation of mutant phenotypes that result from trans-action (e.g., Lewis 1954; Geyer et al. 1990; Leiserson et al. 1994). However, precise quantification of specific enhancer–promoter interactions in trans has been difficult due to the complexity of regulatory sequences surrounding most genes.
Figure 1 .
Schematic view of constructs used to establish a transgenic system for transvection. (A) A schematic of typical alleles that show transvection at endogenous genes. In the top allele, the enhancers (circles) are deleted, while in the bottom allele, the promoter (bent arrow) is deleted. When paired, the remaining enhancers of the bottom allele act in trans (curved arrow) on the intact promoter of the top allele. Boxes represent exons, and straight lines represent chromosomal DNA. (B) Schematic view of transgenic constructs inspired by the alleles in A. Completegfp carries the GMR enhancer (gray oval), consisting of five tandem Glass binding sites, the hsp70 promoter (black bent arrow), which has TATA, Inr, and DPE core promoter elements, and a transcriptional region consisting of a GFP ORF fused to the SV40 3′-UTR (green box). The Enhancerless construct is identical to Completegfp with the omission of GMR sequences. The Promoterless construct carries GMR, with no promoter (indicated by an uppercase X), and replaces the GFP ORF with that of lacZ (blue box). All three constructs are flanked by phiC31 attB sites to allow for site-specific transformation via RMCE.
Despite decades of genetic studies of transvection, several questions regarding enhancer action in trans remain. First, is the capacity to act in trans a common property of enhancers? Only a handful of the thousands of enhancers encoded in the Drosophila genome have been demonstrated to act in trans, which could reflect that this is a rare property, but could also be a consequence of the specific types of alleles required to easily observe trans activity. Second, are specific sequences required to promote enhancer action in trans? Some examples of transvection at the Abdominal-B locus require a 10-kb sequence dubbed the transvection-mediating region (tmr) (Hopmann et al. 1995), and transgenic studies have shown that the tmr can positively affect pairing (Ronshaugen and Levine 2004). Similarly, long-distance interactions between enhancers and promoters in cis can be facilitated by promoter tethering sequences (Zhou and Levine 1999; Calhoun et al. 2002; Calhoun and Levine 2003; Lin 2003; Akbari et al. 2008; Fujioka et al. 2009; Kwon et al. 2009). It is as yet unclear whether similar sequences are required for any enhancer to act in trans or, alternatively, whether transvection between regulatory elements is a natural consequence once chromosomes are physically paired. Finally, in light of the extensive pairing in the Drosophila genome, how does an enhancer choose a target promoter when simultaneously presented with promoters in cis and in trans? For several genes, the presence of a promoter in cis reduces the strength of enhancer action in trans (Martinez-Laborda et al. 1992; Casares et al. 1997; Gohl et al. 2008), suggesting that promoters in cis and in trans compete for an enhancer’s activity. Because these observations have been largely based on mutant phenotypes in whole animals, the cellular basis for this competition is not yet clear. Does an enhancer from each cell choose either the promoter in cis or the promoter in trans? Or can a single enhancer in one cell activate both targets?
Here we describe a transgenic approach to the study of enhancer action in trans. Notably, the use of transgenic reporters greatly simplifies the manipulation of regulatory sequences via the use of straightforward in vitro cloning techniques, permitting the construction of various “alleles” with which to better understand transvection. Our approach was informed by a prior experiment that used transposons carrying yellow alleles, including flanking regulatory sequences, that were designed to mimic mutations known to support enhancer action in trans at the natural yellow locus. That study showed the Drosophila genome to be generally permissive to transvection, demonstrating the feasibility of a transgenic approach to the analysis of enhancer action in trans (Chen et al. 2002). In the present study, our goal was to construct transgenes based on fluorescent reporters such as GFP and mCherry to facilitate quantification of gene expression and to provide cellular resolution of transgene activation. Using the eye-specific enhancer GMR, we first established that simple constructs containing different combinations of enhancer, promoter, and transcriptional unit can support enhancer action in trans, suggesting that special sequences are not required to mediate this form of transvection. Furthermore, we showed that GMR action in trans occurs in a variegated pattern in cells of the eye disc. Variegation was not seen when GMR acts in cis, even when the distance between the enhancer and the promoter was increased, suggesting that stochastic interactions between the enhancer and the promoter are specific to enhancer action in trans. Finally, we quantitatively assessed the impact of promoter competition on GMR function, supporting a model wherein promoters in cis and in trans will both compete for an enhancer’s activity.
Materials and Methods
Fly stocks and husbandry
Flies carrying the recombinase-mediated cassette exchange (RMCE) target cassette P[attP.w+.attP] at positions 37B and 53F were previously described (Bateman and Wu 2008); the target line 38F is an insertion of the same target cassette at polytene band 38F. All insertions are intergenic. Flies were maintained at 25° on standard Drosophila cornmeal, yeast, sugar, and agar medium with p-hydroxybenzoic acid methyl ester as a mold inhibitor (Morris et al. 1998).
Plasmid construction
The RMCE donor vector piB-GFP was previously described (Bateman et al. 2006). To create GMR-GFP carrying the Completegfp construct, the GMR enhancer was amplified by PCR from the plasmid pGMR (a gift from J. Settleman), using primers 5xGMR1 and 5xGMR2 carrying additional ClaI recognition sequences at their 5′ ends (note that two additional cloning steps below make use of the same primers that instead carry BamHI or AscI sites), and subcloned into pcr2.1 using a TOPO-TA cloning kit (Invitrogen, Carlsbad, CA). The enhancer was then digested from pcr2.1 and inserted upstream of the minimal hsp70 promoter in piB-GFP using ClaI.
To create GMR-lacZ carrying the CompletelacZ construct, we first digested piB-GFP with XbaI and HindIII to remove all promoter, GFP, and UTR sequences. We then isolated a 4.6-kb SpeI-HindIII fragment carrying the minimal hsp70 promoter, the β-galactosidase open reading frame, and the SV40 3′-UTR from the plasmid pCaSpeR-hs43-lacZ and cloned it into the remaining piB-GFP backbone to create piB-lacZ (consisting of the lacZ transgene flanked by phiC31 attB sequences to allow for insertion into RMCE sites). The GMR enhancer was then cloned into a BamHI site upstream of the hsp70 minimal promoter.
To create the Promoterless construct, a 4.5-kb SalI fragment consisting of the β-galactosidease open reading frame and the SV40 3′-UTR but omitting the minimal hsp70 promoter was isolated from pCaSpeR-hs43-lacZ. This fragment was cloned into the SalI-digested backbone of piB-GFP, producing piB-lacZ(P-), carrying a promoterless lacZ transgene flanked by attB sites. Next, we introduced a minimal hsp70 promoter flanked by loxP sites that could be removed in vivo by treatment with the Cre recombinase. We amplified the hsp70 promoter from piB-GFP using loxTATA1 and loxTATA2, subcloned this fragment into pcr2.1 using a TOPO-TA kit, and then liberated the loxP-promoter-loxP fragment from pcr2.1 using HindIII and inserted it into a unique HindIII site upstream of the lacZ gene in piB-lacZ(P-) to create piB-LTL-lacZ. Finally, the GMR enhancer was inserted into a BamHI site upstream of the loxP-promoter-loxP cassette to create GMR-LTL-lacZ. Once integrated into flies via RMCE (see below), the final Promoterless transgene was created by removing the promoter between the two loxP sites via crosses to flies carrying the Cre recombinase on the balancer CyO (Siegal and Hartl 1996). Promoter deletions were verified by PCR using primers Pry1 and RNXG2.
To create GMR-mCherry carrying the CompletemC construct, we first used gene splicing by overlap extension (SOEing) (Horton et al. 1990) to combine an mCherry ORF from pmCherry (Clonetech) with the minimal hsp70 promoter. Briefly, the hsp70 promoter was amplified from piB-GFP using cherrySOE1_for and cherrySOE2_3RC_rev primers, and the mCherry ORF was amplified using cherrySOE3_2RC_for and cherrySOE4_rev. The two products were combined in a PCR reaction containing cherrySOE1_for and cherrySOE4_rev, and the resulting product was subcloned into pcr2.1 via TOPO-TA cloning. The hsp70-mCherry fragment was then digested from pcr2.1 using BamHI and XbaI and cloned into BamHI/XbaI-digest piB-GFP(-S) (a piB-GFP derivative generated by religation after XbaI/SpeI digestion, removing BamHI, SpeI, and XbaI sites from downstream of the 3′-UTR) to create piB-mCherry, carrying the hsp70 promoter, an mCherry ORF, and the SV40 3′-UTR flanked by attB sites. Finally, the GMR enhancer was inserted into the BamHI site upstream of the promoter.
To create GMR-λ3-GFP carrying the Complete+3.0 construct, a 3.0-kb fragment that has previously been demonstrated to lack insulator activity (Chung et al. 1993; Singer et al. 2009) was amplified from phage lambda DNA using primers lambda3kb_Bam_3 and lambda3kb_BamAsc_5, and the resulting fragment was subcloned into pcr2.1 via TOPO-TA cloning. Next, the GMR enhancer flanked with AscI sites was cloned into the AscI site at one end of the lambda fragment. Finally, the GMR-lambda fragment was liberated with BamHI and cloned into the BamHI site upstream of the promoter in piB-GFP(-S).
Site-specific transformation via RMCE
All constructs were integrated into RMCE target sites as previously described (Bateman and Wu 2008), using a genomic source of the phiC31 integrase (Groth et al. 2004; Bischof et al. 2007). Following integration, the orientation of insertion was assessed using primers lac4 or 3′Pend1, which are complementary to the 5′ and 3′ P-element ends flanking the RMCE cassette, respectively, and RNXG9, which is complementary to the SV40 3′-UTR. All constructs were analyzed in the same orientation with the enhancer or promoter near the 3′ P-element end, with the exception of the Complete+3.0 construct, which generated only one insertion in the opposite orientation.
Tissue preparation, immunostaining, and microscopy
Eye-antennal discs were dissected from wandering third-instar larvae in 1× phosphate-buffered saline (PBS) and fixed in 4% formaldehyde (Electron Microscopy Sciences) in PBS for 20 min. Discs were then rinsed three times in PBS, 0.1% Triton X-100 (PBST). For direct visualization of fluorescent reporters, discs were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA) or Fluoromount G with DAPI (Southern Biotech). For immunostaining, discs were blocked in 4% normal goat serum (NGS) in PBST for 1 hr and then incubated in primary antibodies overnight at 4°. GFP was visualized with a polyclonal rabbit anti-GFP (Invitrogen) diluted 1:2000, and Elav was visualized using a concentrated supernatent of mouse monoclonal antibody elav-9F8A9 (Developmental Studies Hybridoma Bank) at 1:400. After primary antibody incubation, discs were rinsed and then washed 2 × 20 min in PBST, blocked in 4% NGS/PBST for 1 hr, and then incubated for 1 hr in secondary antibodies, goat anti-mouse Cy3 (Jackson ImmunoResearch) at 1:250 and goat anti-rabbit AlexaFluor-488 (Invitrogen) at 1:2000. Discs were then washed 4 × 20 min in PBST and mounted in Vectashield with DAPI or Fluoromount G with DAPI.
For low-magnification imaging, discs were visualized on an Olympus BX51, using a Media Cybernetics Evolution VF color camera. For higher-magnification imaging, samples were visualized using a Zeiss Axioplan 2 microscope with a 510 Meta confocal laser scanning system, with optical sections collected at 0.7-µm increments. At least 10 discs of each genotype were analyzed; all discs with the same genotype showed similar patterns of expression.
Quantification of variegation
To quantify the degree of variegation in GFP cis- and trans-activation, confocal z-stacks of immunostained discs were first max-projected into a single-plane image. Using ImageJ imaging software, a small elliptical region of interest (ROI) was placed over cell R4 of each ommatidial cluster of the five posterior-most rows and the mean GFP fluorescence was measured for each R4 cell. Next, the mean background fluorescence, determined by averaging the mean fluorescence of 10 ROIs in nonfluorescent regions of the disc, was subtracted from each measurement. Finally, each background-corrected measurement was converted to a relative intensity by expressing it as a percentage of the highest-intensity cell in the field of view.
Reverse transcription and quantitative PCR
For each sample, eye-antennal discs were dissected from 10 wandering third-instar larvae (20 discs total) and frozen at −80°. Tissue homogenization, genomic DNA elimination, and RNA purification were carried out using a QIAGEN RNeasy plus kit according to the manufacturer’s protocol. Briefly, discs were suspended in RLTplus buffer, passed through a 25-gauge needle eight times, and then spun through a qiashredder spin column. The lysate was then spun through a gDNA eliminator spin column, and the flow-through was transferred to an RNeasy spin column for RNA purification. Generation of cDNA was performed using a QIAGEN QuantiTect Reverse Transcription Kit according to the manufacturer’s protocol, including pretreatment with gDNA Wipeout Buffer to eliminate any remaining traces of genomic DNA.
Quantitative PCR was carried out on a StepOne Real-Time PCR system (Applied Biosystems, Foster City, CA). For each reaction, cDNA was diluted 1:5 in water and combined with SYBR green PCR mastermix (Applied Biosystems) and primers. GFP cDNA was amplified using primers GFPRT_1 and GFPRT_2, and mCherry cDNA was assayed using primers cherryRT_1F and cherryRT_1R. Primers RP49-58F and RP49-175R targeting the housekeeping rp49 cDNA (Moon et al. 2008) were used as an internal reference. Each reaction was performed in triplicate, and each genotype was assayed using at least three independent cDNA samples from separate crosses. Relative levels of transcript were calculated via the ΔΔCt method, using StepOne software. Unpaired t-tests were performed using GraphPad Software’s online t-test calculator (www.graphpad.com).
Primer sequences
Primers used in this study are indicated below in 5′ to 3′ orientation:
5XGMR1: GATCCCCCTAGAATCCCAAA
5XGMR2: TATACTCCGGCGCTCTTTTC
loxTATA1: AAGCTTATAACTTCGTATAGCATACATTATACGAAGTTATAAGAGCGCCGGAGTATAAATAG
loxTATA2: AAGCTTATAACTTCGTATAATGTATGCTATACGAAGTTATCTGCAGATTGTTTAGCTTGTTCAG
RNXG2: CAGCTGCGCTTGTTTATTTG
Pry1: CCTTAGCATGTCCGTGGGGTTTGAAT
cherrySOE1_for: ATGTCGAGGTCGACGGTATC
cherrySOE2_3RC_rev: ATGGTGGCGACCGGTACTCTTCTTTCTCGGTAACTTGTTGA
cherrySOE3_2RC_for: TCAACAAGTTACCGAGAAAGAAGAGTACCGGTCGCCACCAT
cherrySOE4_rev: CGGCGCTCAGTTGGAAT
lac4: ACTGTGCGTTAGGTCCTGTTCATTGT
3′Pend1: GTCGGCAAGAGACATCCACT
RNXG9: GTGGTTTGTCCAAACTCATCAA
GFPRT_1: ATTCTCGTGGAACTGGATGG
GFPRT_2: AGCTTTCCAGTGGTGCAGAT
cherryRT_1F: CCCGCCGACATCCCCGACTA
cherryRT_1R: CTGGGTCACGGTCACCACGC
RP49-58F: TACAGGCCCAAGATCGTGAAG
RP49-175R: GACGCACTCTGTTGTCGATACC.
Results
Our goal was to better understand enhancer action in trans and its relationship to expression in cis by comparing these modes of gene activation under controlled conditions. We reasoned that a transgenic approach would be advantageous, permitting us to easily manipulate the identities of the transgene’s components, to vary simple parameters such as deletions and relative distances between regulatory regions, and to accurately quantify levels of gene expression. We chose to focus our analysis on the simple enhancer GMR, which consists of five tandem binding sites for the transcription factor Glass and drives tissue-specific expression of reporter constructs in the photoreceptors, or R cells, posterior to the morphogenetic furrow of the developing eye (Moses and Rubin 1991).
To assess cis expression, we created a “Completegfp” reporter carrying GMR, the hsp70 minimal promoter, and an open reading frame encoding GFP fused to the SV40 3′-UTR. To address trans expression, we designed “Enhancerless” and “Promoterless” transgenes that were modeled after alleles demonstrating transvection at other Drosophila genes, with the former construct lacking an enhancer and the latter lacking a promoter (Figure 1). Additionally, we designed the Enhancerless construct to encode GFP and the Promoterless construct to encode β-galactosidase to differentiate potential expression of one transgene from the other. Importantly, neither the Enhancerless nor the Promoterless transgene is capable of reporter gene expression in isolation. We anticipated that pairing of the two nonfunctional transgenes would permit GMR to activate the hsp70 promoter in trans, producing GFP fluorescence specifically in developing R cells.
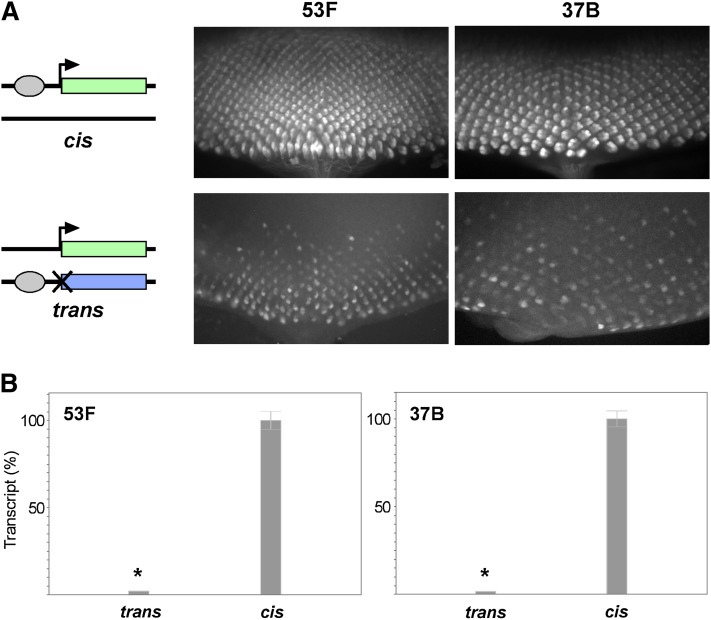
We began by integrating our transgenes into an RMCE target site at polytene band 53F (Bateman and Wu 2008). As expected, the Completegfp reporter produced robust GFP expression in mature retinal cells posterior to the morphogenetic furrow of third-instar eye discs, whereas neither the Enhancerless nor the Promoterless transgene alone produced detectable GFP (Figure 2A, data not shown). Next, we established crosses to produce progeny carrying paired Enhancerless and Promoterless constructs in trans to one another at 53F. Excitingly, we observed GFP fluorescence specifically in the photoreceptors of the developing retina, consistent with our prediction if GMR were to function in trans. To quantify relative levels of expression, we isolated RNA from whole eye-antennal discs expressing GFP under the control of GMR in cis or in trans and prepared cDNA for quantitative RT-PCR. We found that total levels of GFP transcript in the eye-antennal disc were strongly diminished when GMR acted in trans, with 2.2% [95% confidence interval (C.I.), 2.0–2.3%] of transcript levels relative to discs where GMR acted in cis (Figure 2B).
Figure 2 .
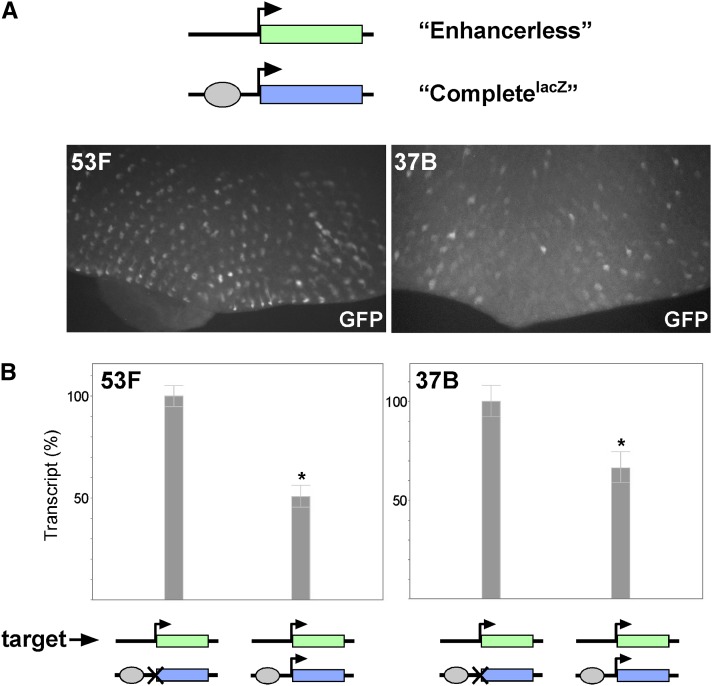
GMR activates the hsp70 promoter in trans. (A) Schematics of genotypes and images of GFP expression in third-instar eye discs. Top row, GFP fluorescence resulting from cis-activation by an insertion of the Completegfp on one homolog. Bottom row, GFP fluorescence from trans-expression of GFP in flies with the Enhancerless construct on one homolog and the Promoterless construct on the other. Transgenes were placed at an RMCE target at polytene position 53F (left) or 37B (right). The posterior of the eye disc is oriented to the bottom of each image. (B) Relative quantification of GMR activation of GFP in cis and in trans by quantitative RT-PCR. Expression in cis was defined as 100% for both 53F and 37B. Error bars indicate 95% confidence intervals. *P < 0.05 (t-test).
To ensure that our observations were representative of other genomic regions, we integrated the same constructs into RMCE targets located at polytene bands 37B and 38F and observed nearly identical patterns of expression in both cis and trans (Figure 2A and Supporting Information, Figure S1). Additionally, quantitative RT-PCR showed that relative levels of GFP transcripts from constructs inserted at 37B were similar to those from transgenes at 53F, with 1.7% [95% C.I., 1.6–1.8%] of total GFP transcript in eye discs where GMR acts in trans relative to those where activation is in cis (Figure 2B). Notably, expression in trans was dependent on pairing of Enhancerless and Promoterless transgenes, as flies carrying an Enhancerless transgene at 53F and a Promoterless transgene at 37B produced no detectable fluorescence (data not shown). We conclude that the enhancer GMR, and therefore the transcription factor Glass that is responsible for its expression, is capable of activating the hsp70 promoter in trans. Furthermore, as our transgenes carry only minimal enhancer and promoter elements in addition to exogenous reporter sequences, we infer that, once homologous chromosomes are paired, GMR and the hsp70 promoter are capable of communicating in trans without the need for additional sequences (see Discussion).
GMR action in trans is variegated
In eye discs where GMR acts in trans, we noted that the pattern of GFP expression was uneven, or variegated, in cells across the eye disc (Figure 2A). The variegated expression was unlikely to result from a position effect as has been observed for some transgenic insertions near heterochromatic regions, because we noted a similar pattern for GMR expression in trans at all three genomic locations tested, whereas GMR action in cis produced no variegation. Furthermore, we found no evidence that the variegated expression produced when GMR acts in trans was altered by reduction of mRNA encoding the heterochromatin protein HP1, nor did we observe any evidence of variegated eye pigmentation for insertions of mini-white or a white transgene under the control of the hsp70 promoter in the same location (Figure S2, Figure S3, and data not shown). Thus, the variegated pattern appears to be specific to GMR action in trans.
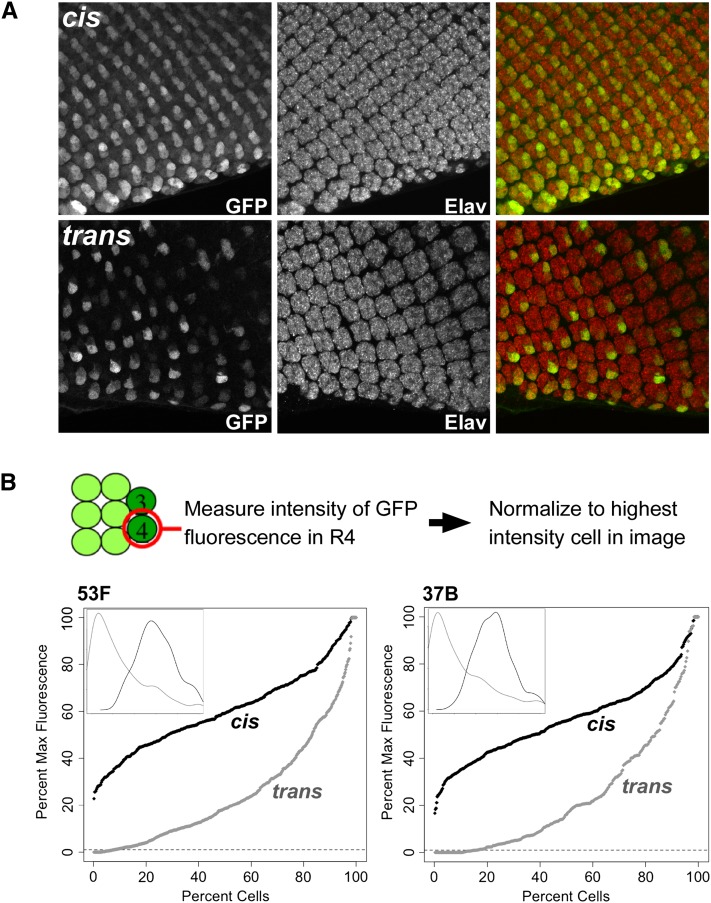
To better understand the variegated phenotype, we used confocal microscopy to produce high-magnification three-dimensional z-stacks of developing retinal cells expressing GFP under the control of GMR in cis and in trans (Figure 3). The retinal photoreceptors undergo a stereotyped pattern of development as the morphogenetic furrow passes from posterior to anterior across the eye disc, resulting in a repeating pattern of ommatidial clusters, each possessing eight photoreceptor subtypes, R1–R8. All subtypes are highlighted using antibodies targeting Elav, a neuronal-specific cell marker (Robinow and White 1988) (Figure 3). In discs where GMR acts in cis, GFP fluorescence was evident in all of the R cells of each ommatidial cluster, with the highest levels of expression in R3 and R4. When GMR acts in trans, expression in R3 and R4 once again predominated, but some of these cells expressed GFP strongly, some weakly, and some undetectably.
Figure 3 .
GMR action in trans is variegated. (A) Max-projected confocal z-stacks showing GMR-driven expression of GFP in cis (top) or in trans (bottom). Constructs are identical to those in Figure 2 and are integrated at 53F. GFP is visualized by immunostaining, and ommatidial clusters are shown by Elav immunostaining. GFP expression is highest in cells R3 and R4 in both genotypes, with trans-activation showing variegated expression. Posterior is oriented to the bottom of each image. (B) Quantification of relative fluorescent intensities. Top, rough schematic of cell positions in each ommatidial cluster; intensities of GFP fluorescence were measured for each R4 cell in the posterior-most five rows and normalized to the brightest cell of the image. Percentile plots show data for expression in cis (black) and in trans (gray) at 53F (n = 427 cells from four discs for cis and 532 cells from five discs for trans) and 37B (n = 467 cells from five discs for cis and 321 cells from four discs for trans). Dashed line represents a threshold of 1% of the maximum intensity, below which expression is not detectable. Insets show density plots of the same data, demonstrating the approximately normal distribution from expression in cis and strong leftward shift from expression in trans.
To quantify the variegation, we assessed the relative GFP fluorescence intensity specifically in R4 cells of the posterior-most five rows of ommatidial clusters in eye discs expressing GFP in cis and in trans (see Materials and Methods) and plotted the data in percentile plots. For transgenes at position 53F, expression in cis showed a near-normal distribution, with 28.3% of R4 cells having less than half the fluorescent intensity of the brightest cells in the image and no cells having <20% of the intensity of the brightest cells (Figure 4B). In contrast, expression in trans showed a strong shift in the distribution of relative intensities, with 83.1% of R4 cells showing less than half the fluorescent intensity of the brightest cells; among these, 7.7% of cells showed no detectable fluorescence (defined as <1% of highest-intensity cells), which could reflect either weak expression that was below our limit of detection or a complete lack of enhancer activity. Analysis of transgenes inserted at 37B produced nearly identical results, with 83.4% of R4 cells that expressed GFP in trans showing less than half the fluorescent intensity of the brightest cells and 15.9% of cells showing no detectable fluorescence. Thus, GMR action in trans results in weak expression in the majority of R4 cells, with sporadic “jackpot” events resulting in bright fluorescence in a small proportion of the cell population.
Figure 4 .
Increasing enhancer–promoter distance in cis delays GFP expression, but does not lead to variegation. (A) Discs where GMR acts in trans (left) or is separated from the hsp70 promoter by 3 kb in cis (right). GFP is visualized by immunostaining, and ommatidial clusters are shown by Elav immunostaining. The distant enhancer in cis does not produce the variegated pattern of GFP fluorescence; rather, GFP expression is highest in the posterior-most cells that were the earliest to commit to the R cell fate and lowest or undetectable in the newly committing cells. (B) Quantification of relative R4 cell fluorescent intensities for GMR action in trans and action from a distance in cis. Schematic shows the positions of row 1 (posterior-most, longest committed) through row 5 that label the x-axis in the box plots below. Left, GMR action in trans (n = 984 cells from nine discs); right, GMR action in cis with 3 kb separation from the promoter (n = 458 cells from six discs). Outliers are indicated by white circles; note the presence of cells at 0% and at 100% of the maximum fluorescence in all rows for trans-action, but not for cis-action. A significant difference in relative intensities is observed between row 1 cells expressing GFP in cis vs. in trans by either parametric (t-test) or nonparametric (Mann–Whitney) statistical tests.
Variegation is not reproduced by a distant enhancer in cis
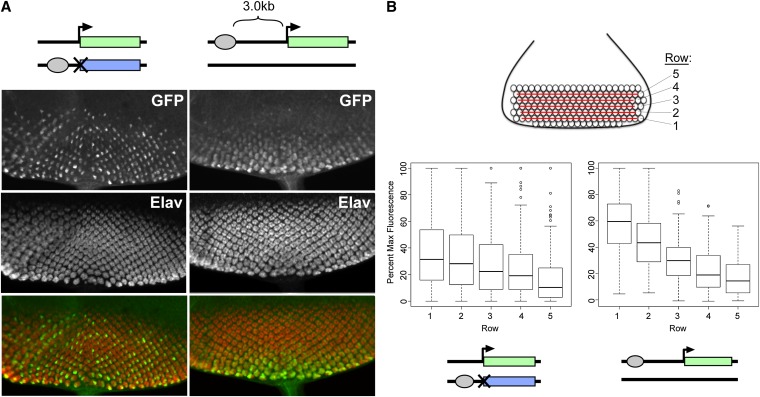
We reasoned that the weak and variegated expression produced by GMR action in trans might be related to changes in the distance between the enhancer and the promoter when the two communicate in trans relative to their interactions in cis. Indeed, in our Completegfp transgene, the enhancer and promoter are separated by only 50 bp, which likely facilitates efficient enhancer–promoter communication. In contrast, we presumed that when GMR was carried on a separate chromosome from the promoter, the two would be less likely to lie in close physical proximity; thus, one possible explanation for the variegated pattern of GFP activation is that the increased distance between enhancer and promoter leads to stochastic activation of the promoter as the probability of interaction is decreased.
As a test of whether a simple increase in distance between GMR and the hsp70 promoter can account for the variegated expression in trans, we created a variant of our Complete transgene wherein the GMR enhancer is separated from the hsp70 promoter by 3.0 kb of DNA derived from the lambda phage genome (“Complete+3.0” transgene), thereby increasing the distance between enhancer and promoter in cis relative to our initial Completegfp construct. After integrating the Complete+3.0 construct into position 53F, we assessed total transcript levels by quantitative RT-PCR. Similar to the case when GMR acts in trans, we observed a reduction in transcript levels to 15.2% (95% C.I., 12.8–18.1%) relative to the original Completegfp construct (data not shown), consistent with the notion that increasing the distance between enhancer and promoter decreases the efficiency of transcriptional activation. However, when we examined discs carrying Complete+3.0 by microscopy, the pattern of GFP did not appear variegated; instead, we observed a “delayed” pattern of expression where GFP fluorescence was primarily observed in the posterior-most ommatidial clusters that were the earliest to commit to photoreceptor cell fates (Figure 4A).
To quantify this effect, we assessed the relative fluorescent intensities of R4 cells in the five posterior-most ommatidial rows as above; in this case, we isolated data from each row in separate box plots to better dissect changing levels of expression between early- and late-committing cells (Figure 4B). In discs where GMR acts in trans, we saw great variation in all rows; notably, some cells in the newly committed rows are among the brightest cells, whereas cells with undetectable levels of fluorescence persist in the longest-expressing rows. In contrast, discs where GMR acts at a distance in cis show a strong trend for increased expression over time, with all nonexpressing cells limited to the newly committed rows and all of the brightest cells found in the early-committed cells. Importantly, the distributions of relative intensities in the posterior-most row are significantly different between the two genotypes (P = 3.7 × 10−9, Welch’s t-test). In sum, our data indicate that increasing the distance between GMR and the hsp70 promoter in cis has a negative impact on transcriptional activation, but does not lead to variegation.
Promoter targets in cis and in trans compete for GMR enhancer activity
In the experiments above, the GMR enhancer was provided with just one target hsp70 promoter provided either in cis or in trans. We next assessed how GMR would respond when given a choice between promoters in cis and in trans concurrently. First, we created a new Complete construct encoding β-galactosidase instead of GFP (CompletelacZ), which is identical to the Promoterless construct except for the addition of a functional hsp70 promoter. After targeting CompletelacZ to RMCE sites at 53F and 37B, we verified that it was capable of robust expression of β-galactosidase in cis (Figure S4). We then placed the CompletelacZ construct in trans to the Enhancerless GFP construct and once again observed variegated GFP expression in the eye disc, indicating that GMR is capable of activating a promoter in trans in the presence of a promoter in cis (Figure 5A). Next, we prepared cDNA from discs carrying the Enhancerless construct in trans to either the Promoterless or the CompletelacZ construct and quantified relative levels of GFP transcript between the two genotypes via quantitative RT-PCR (Figure 5B). At both 53F and 37B, we observed a near twofold reduction in GFP transcript levels in flies carrying the Enhancerless transgene paired with CompletelacZ relative to those with the Promoterless construct [50.1% (95% C.I., 45.8–56.4%) and 66.4% (95% C.I., 59.1–74.7%) at 53F and 37B, respectively], consistent with the model that the promoter in cis competes for the enhancer’s activity and thereby interferes with GMR’s ability to act in trans.
Figure 5 .
A promoter in cis decreases GMR action in trans. (A) Top, schematic showing the Enhancerless construct paired with CompletelacZ. This combination of paired alleles produces variegated GFP fluorescence when integrated at 53F or 37B. (B) Relative quantification of GMR activation of GFP in trans with and without a promoter in cis by quantitative RT-PCR. For both 53F and 37B, total levels of GFP transcript generated from the Enhancerless construct are decreased in discs with an intact promoter in cis to GMR (right column) relative to those without a promoter in cis (defined as 100%, left column). Error bars indicate 95% confidence intervals. *P < 0.05 (t-test).
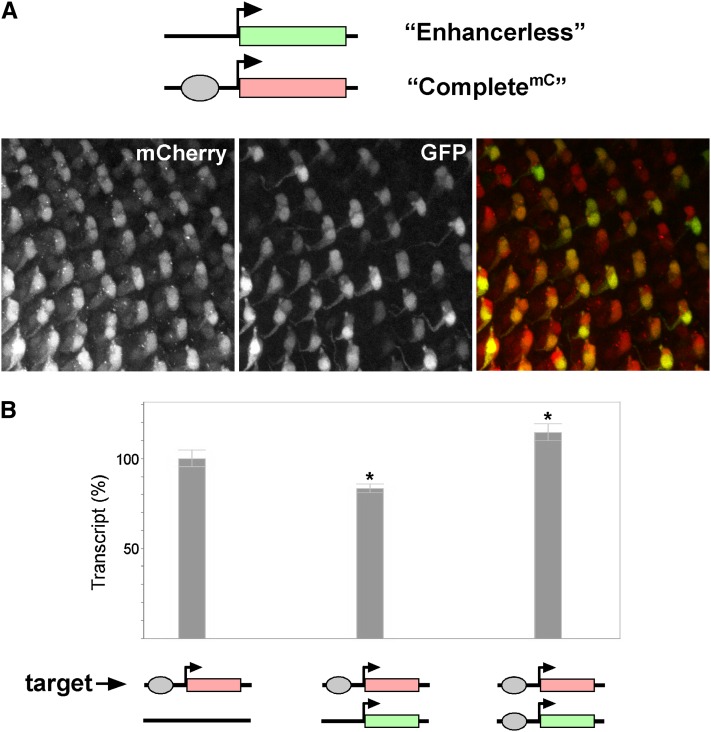
Our quantitative data suggest that GMR acts on both promoters in cis and in trans in flies carrying paired Enhancerless and CompletelacZ constructs. We reasoned that this could happen in one of two ways: first, that the single copy of GMR in each photoreceptor would exclusively act on one promoter, with different cells choosing the promoter either in cis or in trans. Alternatively, the enhancer in each cell could have the capacity to activate both promoters, resulting in expression from cis- and trans-promoters in the same nucleus. To distinguish between these possibilities, we created a variant of our complete construct encoding the red fluorescent reporter mCherry (CompletemC) so that when CompletemC was paired with the Enhancerless construct, we could easily observe expression in cis via red fluorescence and expression in trans via green fluorescence. In flies carrying CompletemC alone at 53F, we observed prominent red fluorescence in all R3 and R4 cells with lower levels of fluorescence in other cells of each ommatidial cluster, similar to the expression of GFP observed from Completegfp alone (data not shown). We therefore focused our analysis on R3 and R4 where expression could be confidently scored. When CompletemC was paired with the Enhancerless construct at 53F, we never observed R3 or R4 cells exhibiting green fluorescence in the absence of red fluorescence (0/∼400 cells), indicating that the promoter in cis was not ignored in favor of the promoter in trans (Figure 6A). Rather, many R3 and R4 cells expressed both mCherry and GFP, indicating that a GMR enhancer in a single cell can activate promoters in cis and in trans. The lack of “green-only” cells was true even for the newly committed photoreceptors where the fluorescent reporters were first detectable (n = 56), implying either that promoters in cis and in trans are activated simultaneously by one enhancer or that potential switching of the enhancer between promoters occurs rapidly (see Discussion).
Figure 6 .
Promoters in cis and in trans compete for enhancer activity. (A) Top, schematic showing the Enhancerless construct paired with CompletemC. GMR activates mCherry expression in cis in all R3 and R4 cells, including those expressing GFP, indicating that GMR can act in cis and in trans within the same cell. (B) Relative quantification of GMR activation of mCherry in cis via quantitative RT-PCR. Expression is reduced in the presence of a promoter in trans to 83.3% of transcript levels generated from the CompletemC construct alone. In contrast, pairing with a Completegfp construct increases mCherry transcript levels by 14.5% relative to CompletemC alone. Transcript levels from CompletemC alone are defined as 100%. Error bars indicate 95% confidence intervals. *P < 0.05 (t-test) in comparison to the CompletemC construct alone.
The data above support a model where promoters in cis and in trans will compete for the activity of a single GMR enhancer when they are paired. As a final test of this model, we assessed whether the presence of a promoter in trans could affect GMR activation of a promoter in cis. To do so, we quantified levels of mCherry transcript generated from the CompletemC construct either alone or when paired with the Enhancerless construct. Consistent with a competitive role for the promoter in trans, we observed a significant reduction in mCherry transcript levels generated in cis when the Enhancerless construct was paired with CompletemC (83.3%; 95% C.I., 80.7–85.9%) relative to levels from the CompletemC construct alone (Figure 6B). To ensure that the change in mCherry expression in cis was not due to possible topological changes when CompletemC is or is not paired to another transgene, we also assessed mCherry transcript levels in flies carrying paired CompletemC and Completegfp transgenes, where the latter differs from the Enhancerless construct only by the addition of GMR. Notably, pairing with Completegfp did not decrease transcript levels from CompletemC; in fact, we observed a significant increase in mCherry transcripts by 14.5% (95% C.I., 10.0–19.4%) (Figure 6B), suggesting that the paired enhancers could augment expression from the CompletemC promoter, perhaps via the concurrent action of one enhancer in cis and the other in trans (see Discussion). In sum, our data support a model wherein, in each cell, an enhancer has the capacity to share its activity between promoters in cis and in trans, with an overall bias toward the promoter in cis.
Discussion
Using a transgenic approach, we have demonstrated several key aspects of enhancer action in trans. First, we showed that the enhancer GMR, which had never before been tested for the ability to act in trans, can activate the hsp70 promoter on a paired homolog, thereby adding a new enhancer–promoter pair to a growing list of those that can communicate in trans. Second, because our transgenes carried only a simple enhancer, minimal promoter, and reporter sequences derived from other organisms, it is unlikely that putative transvection-mediating sequences are required for an enhancer to act in trans once homologs are paired. Third, because our approach provided single-cell resolution, we were able to show that GMR action in trans results in a variegated pattern of expression across the cells of the eye disc. Fourth, by simultaneously presenting GMR with multiple promoter targets, we showed that the enhancer can activate promoters in cis and in trans within the same cell. Finally, the use of fluorescent reporters permitted us to precisely quantify relative levels of gene expression in the presence of varying regulatory components and to demonstrate that promoters in cis and in trans will each compete for the activity of an enhancer.
Although we expected GMR action in trans to produce lower levels of transcript than those resulting from expression in cis, we were surprised at the large divergence between the two modes of activation, with an ∼50-fold difference detected at two different loci. Importantly, due to the variegated pattern of expression in discs where GMR acts in trans, the levels of GFP transcript assessed by quantitative RT-PCR represent an average of mRNA from cells with little to no detectable expression and those with robust expression. It is possible that aspects of our transgenes could be optimized for higher expression in trans; for example, we designed our transgenes to encode different proteins to differentiate expression in cis and in trans, and the lack of homology over the coding regions may adversely affect the overall strength of trans-activation. However, our results may be consistent with existing data from the yellow locus. Here, promoter mutations show that full phenotypic complementation can be achieved with 11% of wild-type levels of yellow transcript generated in cis, implying that enhancer action in trans, which shows only partial complementation, contributes an even lower amount of transcript (Morris et al. 2004). In contrast, quantitative analyses of transvection at the genes encoding Gpdh and Men suggest that their enhancers can contribute up to 100% of normal cis-activity via enhancer action in trans, but that strength of trans-activity varies greatly with allele structure (Gibson et al. 1999; Lum and Merritt 2011). Thus, it is likely that local chromosomal environment and enhancer identity are important factors in determining the strength of an enhancer’s trans-activity.
GMR action in trans is variegated
When GMR acts in trans, the majority of R4 cells express GFP at low or undetectable levels, with occasional jackpot cells producing robust fluorescence. The variegated pattern is specific to trans action; we saw no evidence of variegation for any transgenic construct where a reporter was controlled by an enhancer in cis, including a construct where GMR was separated from the hsp70 promoter by 3 kb. The latter construct, Complete+3.0, resulted in diminished levels of target gene expression relative to Completegfp, suggesting reduced transcriptional activation with increasing distance between the enhancer and the promoter as previously described (Dobi and Winston 2007). Although Complete+3.0 did not show evidence of variegation, in other cases, long-distance cis-interactions between enhancers and promoters may produce variegated expression, as previously reported for a transgenic insertion into the engrailed locus (Kwon et al. 2009). It should be noted that some topological aspects of expression in cis may be distinct from those required for enhancer action in trans, complicating straightforward comparisons of expression from the Complete+3.0 transgene to that from paired Enhancerless and Promoterless constructs; for example, the intervening sequence between an enhancer and a promoter in cis must bend to permit enhancer–promoter contact (reviewed by Bulger and Groudine 2011), but no such intervening sequence exists between enhancers and promoters carried on different chromosomes. Thus, aspects of enhancer action in trans, including local topology and patterns of gene expression, are distinct from long-distance interactions in cis.
Variegated and/or stochastic gene expression has been observed in other cases of interchromosomal gene regulation. For example, introduction of an ectopic human β-globin locus control region (LCR) into transgenic mice leads to interactions with and upregulation of the endogenous β-globin genes, which are encoded on a different chromosome (Noordermeer et al. 2011). In this system, single-cell analysis showed that only a small number of cells have elevated levels of endogenous β-globin mRNA, suggesting stochastic activation of the native β-globin promoter. Similarly, expression of IFN-β in response to viral infection in humans is stochastic, with only a subset of the cells of a population showing detectable transcript (Zawatzky et al. 1985). A recent analysis has led to a model wherein IFN-β expression depends on interchromosomal associations between multiple unlinked loci, which occur only in a subset of cells (Apostolou and Thanos 2008). In the case of GMR action in trans, cell–cell variability in the proximity of GMR and the hsp70 promoter may account for the variegated pattern of expression.
Because the majority of prior studies of transvection have focused on developmental phenotypes affecting adult structures, it is not yet clear whether cell–cell variability in expression will be common to other Drosophila genes that undergo transvection. One prior study used immunostaining to compare levels of Ubx expression directed by the Contrabithorax1 (Cbx1) regulatory insertion in cis and in trans (Castelli-Gair et al. 1990). While this study demonstrated that expression in trans was limited to a reduced population of wing disc cells relative to expression in cis, it is not clear whether this change reflects variegation or some other patterning change. It is likely that variables including local pairing dynamics, efficiencies of promoter activation and transcriptional elongation, and mRNA or protein stability will strongly influence whether variegation will be a consequence of enhancer action in trans.
Cis- and trans-promoters compete for enhancer activity
Our data show that GMR is biased toward a promoter in cis relative to a promoter in trans, as evidenced by its relative strength of action in cis vs. trans as well as the strong reduction in trans-activation when a cis-promoter is present. Given that the sequences of the promoters in our experiments were identical, this bias likely reflects the closer proximity of the promoter in cis, consistent with other studies relating distance to promoter competition (Dillon et al. 1997; Kmita et al. 2002). We also found that a promoter in trans negatively affects expression from a promoter in cis, supporting a competitive role for the trans-promoter to the detriment of the cis-promoter. To our knowledge, this represents the first demonstration of a negative impact on expression in cis by a promoter in trans. We speculate that other transgenic insertions of strong promoters may attract the activity of compatible enhancers located nearby on a paired homolog, analogous to their recognized ability to trap enhancer activity in cis (O’Kane and Gehring 1987).
Prior genetic analyses have shown that, for many genes, enhancer action in trans is reduced in the presence of a promoter in cis (Martinez-Laborda et al. 1992; Casares et al. 1997; Gohl et al. 2008). Our data are consistent with the model that this reduction results from an enhancer sharing its activity between the two promoters. An apparent exception occurs at the well-studied yellow gene, where the presence of a functional promoter in cis abolishes all evidence of enhancer action in trans, a phenomenon termed “cis-preference” (Geyer et al. 1990; Morris et al. 1998, 1999). While this may reflect a novel mechanism, it is possible that cis-preference results from a highly skewed competition between promoters in cis and in trans such that trans-interactions, while permitted, are so reduced that the few transcripts resulting from enhancer action in trans have no impact on phenotype. According to this reasoning, the ratio of enhancer activity between promoter targets in cis and in trans, and thus the overall availability of an enhancer to act in trans, may vary among different enhancers and/or loci. Excitingly, these concepts can be easily tested using a transgenic system similar to that used here.
Our study demonstrates that a single enhancer can activate promoters in cis and in trans in the same cell. Due to the time delay required for detection of fluorescence and the perdurance of GFP and mCherry following translation, we were unable to determine whether GMR periodically switches from one target to the other over the time that we assayed or, alternatively, whether transcription is actively promoted simultaneously from both promoters in those cells expressing reporters in cis and in trans to the enhancer. We assayed relative fluorescent intensities of newly committed R4 cells when fluorescence was first detectable and found no evidence that the intensity of one channel was negatively correlated with that of the other channel (data not shown), suggesting that, if switching takes place, it must do so on a relatively short timescale. An analysis using reagents and strategies better suited to short-term dynamics, including observations of nascent transcripts (Kosman et al. 2004) or the use of destabilized fluorescent reporters (Li et al. 1998), may help to differentiate between these possibilities.
Finally, we note that our quantitative data show a significant elevation of expression from our CompletemC reporter when paired with the Completegfp transgene that carries its own enhancer and promoter, suggesting a possible synergistic effect when “Complete” genes are paired. This synergism could result from the activities of both enhancers upon the promoter of the CompletemC construct, as would be predicted if both enhancers acted on promoters in cis and in trans simultaneously. It is also possible that the nearby Completegfp construct increases the likelihood of both transgenes residing in a transcription factory, which may lead to elevated expression levels (reviewed by Bulger and Groudine 2011; Dean 2011). If the phenomenon of increased expression from paired genes is common to other enhancer–promoter interactions in Drosophila, it could represent a means to augment transcriptional activation across the genome when all chromosomes are paired, ensuring robust responses to environmental cues. Notably, a similar phenomenon has been reported in cells from a human renal oncocytoma, where extensive pairing between the q arms of chromosome 19 is correlated with elevated expression from genes along the length of that arm (Koeman et al. 2008), suggesting that increased gene expression resulting from pairing also occurs in other systems.
Transvection as a model for interchromosomal interactions
The study of transvection in Drosophila enjoys a rich history of genetic and cytological studies from some of the pioneers of genetics. Our recognition of the prevalence of interchromosomal interactions has continued to grow in other model systems, expanding the possibilities of potential effects on gene regulation resulting from interactions in trans. While the mechanisms that identify and pair homologs in Drosophila are not yet fully understood, they are likely to differ from many of the forces that bring together nonhomologous loci in other systems such as vertebrates. However, irrespective of the pairing mechanism, once chromosomes are brought in close proximity, the potential for trans-interactions between regulatory sequences is likely similar in different organisms, particularly given the strong evolutionary conservation of mechanisms for gene regulation in cis. Transgenic analyses of transvection in Drosophila have the potential to address the capacity of diverse enhancers and promoters to interact in trans and allow precise quantification of subtle effects on gene expression. Furthermore, the varying dynamics of pairing at different genomic locations in the Drosophila genome (Fung et al. 1998) afford the ability to contrast effects on transvection when chromosomes are stably or poorly paired. Thus, detailed analyses of transvection have the potential to inform our understanding of interchromosomal interactions in diverse systems.
Supplementary Material
Acknowledgments
We thank Jeff Settleman for the gift of pGMR, Chris Smith at the Mount Desert Island Biological Laboratory DNA Sequencing Core, and the Bloomington Drosophila Stock Center. We specially thank Ting Wu, Pam Geyer, Judy Kassis, Lori Wallrath, and Gio Bosco for stimulating conversations and ideas. This work was supported by grants from the National Center for Research Resources (5P20RR016463-12) and the National Institute of General Medical Sciences (8 P20 GM103423-12) from the National Institutes of Health.
Note added in proof: In this issue of GENETICS, Mellert and Truman (pp. 1129–1141) describe an independent study of enhancer action in trans between transgenes inserted using site-specific transformation methods. In agreement with our data, they demonstrate that promoters in cis and in trans to an enhancer will compete for the enhancer’s activity, with a bias toward the promoter in cis. Furthermore, they report that enhancer action in trans is limited to a smaller group of cells relative to activation in cis, with evidence of stochastic activation in trans for several enhancers.
Footnotes
Communicating editor: P. K. Geyer
Literature Cited
- Akbari O. S., Bae E., Johnsen H., Villaluz A., Wong D., et al. , 2008. A novel promoter-tethering element regulates enhancer-driven gene expression at the bithorax complex in the Drosophila embryo. Development 135: 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou E., Thanos D., 2008. Virus infection induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell 134: 85–96 [DOI] [PubMed] [Google Scholar]
- Bacher C. P., Guggiari M., Brors B., Augui S., Clerc P., et al. , 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell Biol. 8: 293–299 [DOI] [PubMed] [Google Scholar]
- Bantignies F., Cavalli G., 2011. Polycomb group proteins: repression in 3D. Trends Genet. 27: 454–464 [DOI] [PubMed] [Google Scholar]
- Bartkuhn M., Renkawitz R., 2008. Long range chromatin interactions involved in gene regulation. Biochim. Biophys. Acta 1783: 2161–2166 [DOI] [PubMed] [Google Scholar]
- Bateman J. R., Wu C. T., 2008. A simple polymerase chain reaction-based method for the construction of recombinase-mediated cassette exchange donor vectors. Genetics 180: 1763–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. R., Lee A. M., Wu C. T., 2006. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics 173: 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M., Groudine M., 2011. Functional and mechanistic diversity of distal transcription enhancers. Cell 144: 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V. C., Levine M., 2003. Long-range enhancer-promoter interactions in the Scr-Antp interval of the Drosophila Antennapedia complex. Proc. Natl. Acad. Sci. USA 100: 9878–9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V. C., Stathopoulos A., Levine M., 2002. Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex. Proc. Natl. Acad. Sci. USA 99: 9243–9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares F., Bender W., Merriam J., Sanchez-Herrero E., 1997. Interactions of Drosophila Ultrabithorax regulatory regions with native and foreign promoters. Genetics 145: 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli-Gair J. E., Micol J. L., Garcia-Bellido A., 1990. Transvection in the Drosophila Ultrabithorax gene: a Cbx1 mutant allele induces ectopic expression of a normal allele in trans. Genetics 126: 177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Stam M., 2004. Chromatin conversations: mechanisms and implications of paramutation. Nat. Rev. Genet. 5: 532–544 [DOI] [PubMed] [Google Scholar]
- Chen J. L., Huisinga K. L., Viering M. M., Ou S. A., Wu C. T., et al. , 2002. Enhancer action in trans is permitted throughout the Drosophila genome. Proc. Natl. Acad. Sci. USA 99: 3723–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. H., Whiteley M., Felsenfeld G., 1993. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74: 505–514 [DOI] [PubMed] [Google Scholar]
- Dean A., 2011. In the loop: long range chromatin interactions and gene regulation. Brief. Funct. Genomics 10: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon N., Trimborn T., Strouboulis J., Fraser P., Grosveld F., 1997. The effect of distance on long-range chromatin interactions. Mol. Cell 1: 131–139 [DOI] [PubMed] [Google Scholar]
- Dobi K. C., Winston F., 2007. Analysis of transcriptional activation at a distance in Saccharomyces cerevisiae. Mol. Cell. Biol. 27: 5575–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z., Andronescu M., Schutz K., McIlwain S., Kim Y. J., et al. , 2010. A three-dimensional model of the yeast genome. Nature 465: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. W., 2002. Transvection effects in Drosophila. Annu. Rev. Genet. 36: 521–556 [DOI] [PubMed] [Google Scholar]
- Fujioka M., Wu X., Jaynes J. B., 2009. A chromatin insulator mediates transgene homing and very long-range enhancer-promoter communication. Development 136: 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. C., Marshall W. F., Dernburg A. F., Agard D. A., Sedat J. W., 1998. Homologous chromosome pairing in Drosophila melanogaster proceeds through multiple independent initiations. J. Cell Biol. 141: 5–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan J. E., Selker E. U., 2004. RIP: the evolutionary cost of genome defense. Trends Genet. 20: 417–423 [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G., 1990. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 9: 2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. B., Reed D. S., Bartoszewski S., Wilks A. V., 1999. Structural changes in the promoter region mediate transvection at the sn-glycerol-3-phosphate dehydrogenase gene of Drosophila melanogaster. Biochem. Genet. 37: 301–315 [DOI] [PubMed] [Google Scholar]
- Gohl D., Muller M., Pirrotta V., Affolter M., Schedl P., 2008. Enhancer blocking and transvection at the Drosophila apterous locus. Genetics 178: 127–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondor A., Ohlsson R., 2009. Chromosome crosstalk in three dimensions. Nature 461: 212–217 [DOI] [PubMed] [Google Scholar]
- Groth A. C., Fish M., Nusse R., Calos M. P., 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166: 1775–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann R., Duncan D., Duncan I., 1995. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics 139: 815–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Cai Z. L., Ho S. N., Pease L. R., 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques 8: 528–535 [PubMed] [Google Scholar]
- Kennison J. A., Southworth J. W., 2002. Transvection in Drosophila. Adv. Genet. 46: 399–420 [DOI] [PubMed] [Google Scholar]
- Kmita M., Fraudeau N., Herault Y., Duboule D., 2002. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature 420: 145–150 [DOI] [PubMed] [Google Scholar]
- Koeman J. M., Russell R. C., Tan M. H., Petillo D., Westphal M., et al. , 2008. Somatic pairing of chromosome 19 in renal oncocytoma is associated with deregulated EGLN2-mediated oxygen-sensing response. PLoS Genet. 4: e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosman D., Mizutani C. M., Lemons D., Cox W. G., McGinnis W., et al. , 2004. Multiplex detection of RNA expression in Drosophila embryos. Science 305: 846. [DOI] [PubMed] [Google Scholar]
- Kwon D., Mucci D., Langlais K. K., Americo J. L., DeVido S. K., et al. , 2009. Enhancer-promoter communication at the Drosophila engrailed locus. Development 136: 3067–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiserson W. M., Bonini N. M., Benzer S., 1994. Transvection at the eyes absent gene of Drosophila. Genetics 138: 1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B., 1954. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 88: 225 [Google Scholar]
- Li X., Zhao X., Fang Y., Jiang X., Duong T., et al. , 1998. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 273: 34970–34975 [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E., van Berkum N. L., Williams L., Imakaev M., Ragoczy T., et al. , 2009. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., 2003. The promoter targeting sequence facilitates and restricts a distant enhancer to a single promoter in the Drosophila embryo. Development 130: 519–526 [DOI] [PubMed] [Google Scholar]
- Lum T. E., Merritt T. J., 2011. Nonclassical regulation of transcription: interchromosomal interactions at the malic enzyme locus of Drosophila melanogaster. Genetics 189: 837–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Laborda A., Gonzalez-Reyes A., Morata G., 1992. Trans regulation in the Ultrabithorax gene of Drosophila: alterations in the promoter enhance transvection. EMBO J. 11: 3645–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee B. D., 2004. Homologous pairing and chromosome dynamics in meiosis and mitosis. Biochim. Biophys. Acta 1677: 165–180 [DOI] [PubMed] [Google Scholar]
- Moon N.-S., Di Stefano L., Morris E. J., Patel R., White K., et al. , 2008. E2F and p53 induce apoptosis independently during Drosophila development but intersect in the context of DNA damage. PLoS Genet. 4: e1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Chen J. L., Geyer P. K., Wu C. T., 1998. Two modes of transvection: enhancer action in trans and bypass of a chromatin insulator in cis. Proc. Natl. Acad. Sci. USA 95: 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Geyer P. K., Wu C. T., 1999. Core promoter elements can regulate transcription on a separate chromosome in trans. Genes Dev. 13: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. R., Petrov D. A., Lee A. M., Wu C. T., 2004. Enhancer choice in cis and in trans in Drosophila melanogaster: role of the promoter. Genetics 167: 1739–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K., Rubin G. M., 1991. Glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 5: 583–593 [DOI] [PubMed] [Google Scholar]
- Naumova N., Dekker J., 2010. Integrating one-dimensional and three-dimensional maps of genomes. J. Cell Sci. 123: 1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer D., de Wit E., Klous P., van de Werken H., Simonis M., et al. , 2011. Variegated gene expression caused by cell-specific long-range DNA interactions. Nat. Cell Biol. 13: 944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane C. J., Gehring W. J., 1987. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA 84: 9123–9127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S., White K., 1988. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev. Biol. 126: 294–303 [DOI] [PubMed] [Google Scholar]
- Ronshaugen M., Levine M., 2004. Visualization of trans-homolog enhancer-promoter interactions at the Abd-B Hox locus in the Drosophila embryo. Dev. Cell 7: 925–932 [DOI] [PubMed] [Google Scholar]
- Sandhu K. S., Shi C., Sjolinder M., Zhao Z., Gondor A., et al. , 2009. Nonallelic transvection of multiple imprinted loci is organized by the H19 imprinting control region during germline development. Genes Dev. 23: 2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T., Yaffe E., Kenigsberg E., Bantignies F., Leblanc B., et al. , 2012. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148: 458–472 [DOI] [PubMed] [Google Scholar]
- Siegal M. L., Hartl D. L., 1996. Transgene coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics 144: 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. D., Hily J.-M., Liu Z., 2009. A 1-kb bacteriophage lambda fragment functions as an insulator to effectively block enhancer–promoter interactions in Arabidopsis thaliana. Plant Mol. Biol. Rep. 28: 69–76 [Google Scholar]
- Spilianakis C. G., Lalioti M. D., Town T., Lee G. R., Flavell R. A., 2005. Interchromosomal associations between alternatively expressed loci. Nature 435: 637–645 [DOI] [PubMed] [Google Scholar]
- Stevens N. M., 1908. A study of the germ cells of certain Diptera with reference to the heterochromosomes and the phenomena of synapsis. J. Exp. Zool. 5: 359–374 [Google Scholar]
- Williams A., Spilianakis C. G., Flavell R. A., 2010. Interchromosomal association and gene regulation in trans. Trends Genet. 26: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Tsai C. L., Lee J. T., 2006. Transient homologous chromosome pairing marks the onset of X inactivation. Science 311: 1149–1152 [DOI] [PubMed] [Google Scholar]
- Zawatzky R., De Maeyer E., De Maeyer-Guignard J., 1985. Identification of individual interferon-producing cells by in situ hybridization. Proc. Natl. Acad. Sci. USA 82: 1136–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., McCord R. P., Ho Y. J., Lajoie B. R., Hildebrand D. G., et al. , 2012. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148: 908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Levine M., 1999. A novel cis-regulatory element, the PTS, mediates an anti-insulator activity in the Drosophila embryo. Cell 99: 567–575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.