Abstract
The Hsp90 chaperone is required for the maturation of signal transduction clients, including many kinases and nuclear steroid hormone receptors. The binding and hydrolysis of ATP by Hsp90 drive conformational rearrangements in three structure domains. Two intrinsically disordered regions of Hsp90 located between these domains and at the C terminus have traditionally been considered to impart flexibility. We discovered that the charged nature of these acid-rich disordered regions imparts a solubility-promoting function to Hsp90 that is important for its cellular activity in yeast. Both the solubility-promoting function and ATPase activity must occur in the same Hsp90 molecule in order to support robust growth, suggesting that the solubility-promoting function is required during the ATP-driven client maturation process. Expression of model clients together with Hsp90 variants indicated interdependent solubilities mediated by the aggregation propensities of both the client and Hsp90. We propose a model whereby the charge-rich disordered regions of Hsp90 serve a solubility-promoting function important for complexes with aggregation-prone clients. These findings demonstrate a novel biological function of the intrinsically disordered regions in Hsp90 and provide a compelling rationale for why their charged properties are conserved throughout eukaryotic evolution.
INTRODUCTION
Chaperones are essential in all organisms, where they perform a number of functions, including promoting the solubility of aggregation-prone proteins. The expression of misfolded proteins capable of aggregating has been shown to decrease fitness in yeast and is correlated with neurodegenerative diseases, including Huntington's disease, amyotrophic lateral sclerosis (ALS), and Alzheimer's disease (8, 15, 16). For these reasons, understanding the role of chaperones in promoting solubility is of central importance to biology and medicine.
The Hsp90 chaperone is required for the maturation of many signal transduction clients, including numerous kinases and steroid hormone receptors (43). Hsp90 clients form signaling pathways that are important in many cancers, and cancer cells can require greater Hsp90 activity than healthy cells (44, 51). These observations have stimulated many efforts to understand the chaperone mechanism of Hsp90 and to develop inhibitors as anticancer therapeutics.
Hsp90 is composed of three domains: the N-terminal (N) domain, which contains an ATPase site; the middle (M) domain; and the C-terminal (C) domain, which forms a thermodynamically stable dimer (1, 33, 35). In addition, Hsp90 has two large regions that are intrinsically disordered: the charged linker (CL) is a region of about 30 amino acids that connects the N and M domains, and the charged extension (CX) is a region of about 25 amino acids at the C terminus. While the structured domains are rigid, large hinge motions between the domains lead to dramatically distinct conformations (1, 40, 41). The two N domains in the Hsp90 homodimer contact one another in the structure of yeast Hsp90 cocrystallized with an ATP analogue and the cochaperone Sba1 (1). In contrast, the N domains are separated by 30 Å in the structure of bacterial Hsp90 cocrystallized with ADP (40). Electron microscopy and fluorescence resonance energy transfer (FRET) experiments have further demonstrated that ATPase-driven conformational changes result in large changes to N-domain distances and that these conformational changes are conserved from bacteria to humans (6, 13, 17, 22, 41). How these ATPase-driven conformational changes mediate binding with cochaperones and maturation of clients remains an active area of investigation.
The role of the two intrinsically disordered regions of Hsp90 (CL and CX) have been investigated, but their role in antiaggregation function in vivo was unclear. The amino acid sequences in these regions are poorly conserved in phylogenetic alignments, yet throughout eukaryotes these regions are consistently rich in negatively charged amino acids (48). These observations suggest that physical properties associated with negative charge, but not the specific sequence of negatively charged amino acids, provides a fitness benefit in eukaryotes. Conformational models of Hsp90 have postulated that the CL and CX may be important for mediating structural rearrangements of the N, M, and C domains (10, 37, 46). In addition, Tsutsumi et al. recently observed that the sequence of the CL can impact in vivo function, including the growth rescue of an otherwise lethal N-domain mutation (47). The deletion of unstructured regions of the CL supports yeast growth indistinguishable from that with wild-type Hsp90 across a broad temperature range (10, 21). Deletions that extend into regions ordered within the Hsp90 crystal structure resulted in reduced growth at temperatures of above 37°C (10). While flexibility is clearly inherent to Hsp90 (17, 41), it can be dramatically reduced (10, 34, 46) without impairing yeast growth. In addition, flexibility alone is insufficient to explain the evolutionary conservation of negative charge in the CL.
Disordered regions of proteins populate multiple conformations. Depending on the amino acid composition and sequence, this conformational heterogeneity can lead to protein association or aggregation (36). For this reason, intrinsically disordered regions are frequently deleted to enable efficient protein purification for in vitro biochemical and structural analyses (19). However, the contribution of intrinsically disordered regions to solubility depends on additional biophysical properties, including amino acid composition (23). The high concentration of negative charge in the CL and CX of Hsp90 should interact favorably with aqueous solvent and promote solubility.
Our previous work demonstrated that the CL and CX of Hsp90 promote the solubility of model substrates in vitro (48). Both citrate synthase and the nucleotide binding domain of cystic fibrosis transmembrane regulator aggregate at elevated temperature in vitro. This aggregation can be dramatically reduced by Hsp90 (14, 48, 54). Individual deletions of the CL or CX resulted in modest decreases in this Hsp90 solubility-promoting function. The double deletion (ΔCLΔCX) was severely defective for promoting the solubility of these substrates. Of note, these CL and CX deletions were made to remove the bulk of the acid-rich regions without impacting the structured domains of Hsp90. All of the deletion variants were appropriately folded and retained ATPase activity, indicating that the structured domains were not severely perturbed. Importantly, the in vitro solubility-promoting function of ΔCLΔCX was regained in a charge rescue (CR) variant where an exogenous charge-rich region was appended to the C terminus. These results demonstrated that the intrinsically disordered regions of Hsp90 contribute to solubility promotion with model substrates in vitro.
Here we investigated the solubility-promoting role of these intrinsically disordered regions in vivo. There are a number of important distinctions between in vitro and in vivo experiments with Hsp90. Hsp90 is essential in yeast and higher eukaryotes (2, 3). In addition, it is an ATPase, and mutations that abrogate either binding of ATP or hydrolysis do not support viability (29, 33). Thus, the essential function of Hsp90 in vivo requires ATP binding and hydrolysis. In contrast, the solubility-promoting activity of Hsp90 observed in vitro is not influenced by ATP and is not perturbed by mutations that prevent ATP binding or hydrolysis (48). Experiments with Hsp90 in vitro, in the absence of ATP, and with model substrates may not accurately reflect in vivo function. To analyze the role of the intrinsically disordered CL and CX in vivo, we studied the function of a panel of Hsp90 constructs (wild type, ΔCLΔCX, and CR) in yeast. Importantly, the CL deletion in these constructs (Δ221-252) is within a region (positions 217 to 261) that appears to be unstructured in the Hsp90 crystal structure (1). In addition, a larger deletion (Δ211-259) was shown to support growth similar to that of the wild type across all temperatures (10). Utilizing our unstructured deletion constructs, we observed that the deletion of both the CL and CX impairs growth as well as client solubility and activity.
MATERIALS AND METHODS
Plasmid construction.
Wild-type, ΔCLΔCX (48), and CR (48) Hsp90 constructs were generated from the yeast HSP82 gene and cloned into the Trp-marked p414GPD plasmid (25) for expression in yeast. To increase steady-state levels, ΔCLΔCX Hsp90 was cloned into the high-copy p424GPD (25) plasmid to generate ΔCLΔCXHC. A GCN4-based coiled coil computationally optimized for stability (11) was appended to the C termini of wild-type and D79N Hsp90 to generate constructs that do not heterodimerize with subunits lacking the coil (50). WTcoil and D79Ncoil Hsp90 were cloned into the His-marked p413GPD plasmid (25). All Hsp90 constructs contained a His6 sequence (GGHHHHHHGGH) at the N terminus to facilitate detection by Western blotting.
Sole Hsp90 in yeast.
The haploid Saccharomyces cerevisiae strain ECU82a (26) is a derivative of W303 in which both endogenous Hsp90 genes, HSP82 and HSC82, are knocked out and wild-type HSC82 is constitutively expressed from pKAT6, a URA3-marked plasmid that is amenable to negative selection. Lithium acetate was used to introduce Trp-marked Hsp90 constructs into ECU82a, and plating in the absence of tryptophan was used to select for transformants. Transformants were grown in liquid medium lacking tryptophan to cell density of 1 × 107 cells/ml, serially diluted, and plated on synthetic dextrose (SD) medium lacking tryptophan (SD-W medium) at 25°C in the presence or absence of 5-fluoroorotic acid (5-FOA), which selects for loss of the pKAT6 plasmid.
Yeast growth.
For monitoring yeast growth at elevated temperature, single colonies of ECU82a strains grown on restreaked plates in the presence of 5-FOA were selected and grown in liquid cultures in SD-W medium to a cell density of 1 × 107 cells/ml. Cultures were serially diluted, plated on SD-W, and grown at the indicated temperatures for 3 to 6 days. To monitor growth in liquid culture, strains were grown for 32 h in SD-W medium at the indicated temperature with dilution to sustain log-phase growth. Cell growth was monitored from 8 to 32 h at the indicated temperature using a Victor X5 multilabel plate reader (Perkin Elmer). The log of relative optical density at 600 nm (OD600) versus time was fit to a linear equation to determine the growth rate.
To measure steady-state levels of the Hsp90 constructs, cultures were grown to a cell density of 1 × 107 cells/ml in SD-W. Cell pellets were lysed by vortexing with glass beads in yeast lysis buffer (50 mM Tris [pH 7.5], 5 mM EDTA, 10 mM phenylmethylsulfonyl fluoride [PMSF]) followed by the addition of sodium dodecyl sulfate (SDS) to 2%. After pelleting of the cellular debris, the protein concentration in the lysates was assessed using the bicinchoninic acid (BCA) assay (Pierce) and the expression level monitored by Western blotting with anti-HisG antibody (Invitrogen).
v-src assay.
Lithium acetate was used to introduce p316Galv-srcv5 (49) into ECU82a cells containing wild-type, ΔCLΔCXHC, or CR Hsp90 constructs as the sole copy of Hsp90. On plates, cells were grown with dextrose as the sugar source to repress v-src expression. Liquid cultures were then grown overnight in synthetic raffinose medium lacking uracil and tryptophan (SR-U-W) at 25°C to a cell density of 1 × 107 cells/ml. In order to monitor v-src activity in cells completely equilibrated to growth at 36°C, cells were then diluted and grown for 6 h in a shaking incubator at 36°C in SR-U-W to a cell density of 7 × 106 cells/ml. Cell pellets were resuspended and inoculated into medium containing either 2% raffinose (SR-U-W) or 2% galactose (SGal-U-W) as the sugar source. Cells were then grown for an additional 6 h at 36°C. Cell pellets were collected by centrifugation, washed once with water, and frozen at −80°C. Cells were lysed as described previously (49) except that Src lysis buffer contained 10 mM PMSF to inhibit v-src degradation. Phosphotyrosine levels were analyzed by Western blotting (3 μg protein/lane) with antibody 4G10 (Upstate). The levels of v-src and Hsp90 were analyzed by Western blotting (20 μg protein/lane) with anti-v5 antibody (Invitrogen) and anti-HisG antibody, respectively.
To monitor the degradation of v-src, a protein synthesis inhibitor, cycloheximide (CHX) (A.G. Scientific, Inc.) was applied to cells and the time-dependent decrease in v-src monitored by Western blotting. Cells were grown as described above except that l-proline was substituted for ammonium sulfate as the sole nitrogen source in the growth medium and 0.003% SDS was added to the SGal-U-W medium to increase the permeability of the yeast cell wall to CHX (20). Cells were grown for 3 h in SGal-U-W, and then CHX was added to 100 μg/ml and samples were collected after various time in CHX. Cell pellets were collected by centrifugation, washed once with water, and frozen at −80°C. Cells were lysed as described above and v-src levels analyzed by Western blotting.
To determine whether galactose-induced expression was impaired in ΔCLΔCXHC Hsp90 cells, plasmid p316GalSspBv5 (50) was introduced with lithium acetate into ECU82a cells containing wild-type, ΔCLΔCXHC, or CR Hsp90 constructs as the sole copy of Hsp90. Cells were grown as for v-src growth described above with ammonium sulfate as the sole nitrogen source in the growth medium. After allowing cells to equilibrate to growth at 36°C for 6 h in SR-U-W medium to a cell density of 7 × 106 cells/ml, cell pellets were resuspended and inoculated into SGal-U-W medium. Cell pellets were collected by centrifugation at the indicated time points, washed once with water, and frozen at −80°C. Cells were lysed with Src lysis buffer and 10 mM PMSF as described above and the levels of SspB and Hsp90 analyzed by Western blotting.
GR assay.
P2A/GRGZ (26) containing a FLAG epitope tag (50) was introduced into ECU82a cells with wild-type, ΔCLΔCXHC, or CR Hsp90 constructs as the sole copy of Hsp90. Cells were grown overnight in synthetic dextrose medium without adenine and tryptophan (SD-Ade-W) at 25°C to a cell density of 1 × 107 cells/ml. In order to monitor glucocorticoid receptor (GR) activity in cells completely equilibrated to growth at 36°C, cells were then diluted and grown for 8 h in a shaking incubator at 36°C to a cell density of 5 × 106 cells/ml. After 8 h at 36°C, cultures were split in half, and deoxycorticosterone (DOCS) dissolved in ethanol was added to 0 or 10 μM with a final concentration of 0.1% ethanol in all samples. Cells were grown for an additional hour at 36°C and collected by centrifugation. After being washed once with water, cell pellets were frozen at −80°C. Cells were lysed as described previously (49) except that βGal lysis buffer contained 10 mM PMSF to inhibit GR degradation. Galactosidase levels were measured as described previously (49). GR and Hsp90 levels were monitored by Western blotting (20 μg protein/lane) with anti-FLAG antibody (Sigma) for GR and anti-HisG antibody for Hsp90.
The solubilities of Hsp90 and GR were measured in cells grown at 36°C with the addition of 10 μM DOCS as described above. After 9 h at 36°C, cultures were split in half, and cell pellets were collected by centrifugation, washed once with water, and frozen at −80°C. The frozen cell pellets were lysed by vortexing with glass beads in solubility buffer (50 mM Tris [pH 7.5], 5 mM EDTA, 150 mM sodium chloride, 10 mM PMSF, and 1:20 dilution protease inhibitor cocktail [Sigma], with 5% [final concentration] dimethyl sulfoxide [DMSO] used to solubilize the protease inhibitors). For whole-cell lysate samples, SDS was added to 2% immediately after vortexing with glass beads. To isolate soluble fractions, samples were lysed by vortexing with glass beads and insoluble material pelleted by centrifugation. The supernatant representing the soluble protein content of the cell was removed to a fresh tube and SDS added to 2%. The protein concentration in these lysates was assessed using the BCA assay (Pierce). All samples were analyzed by SDS-PAGE with Coomassie blue staining to ensure that loading was appropriately normalized and that lysis was efficient such that endogenous high-molecular-weight proteins were well represented in all soluble fraction samples. The amounts of GR and Hsp90 were measured by Western blotting as described above. The solubility of Hsp90 in the absence of GR was tested under the same conditions in cells that lacked the GR expression plasmid.
RESULTS
The solubility-promoting function of Hsp90 is required for robust yeast growth at elevated temperature.
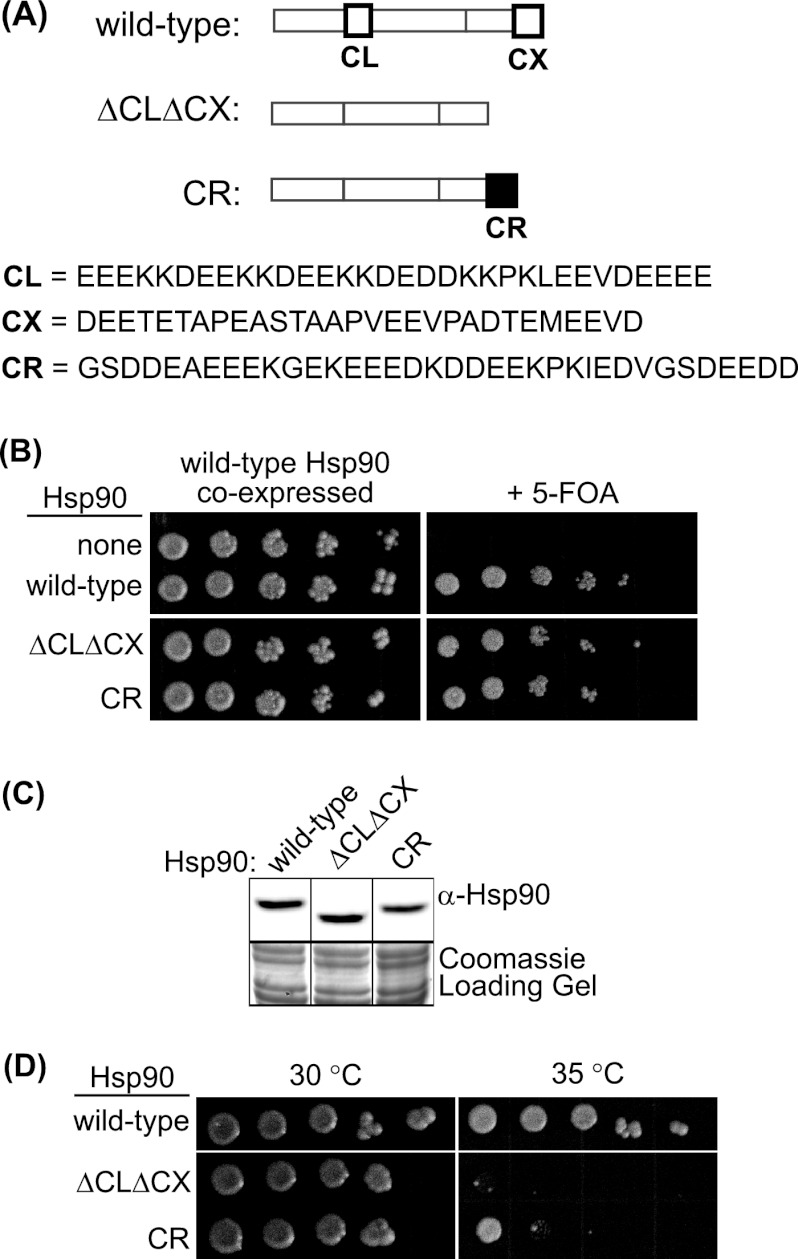
To investigate the solubility-promoting function of Hsp90 activity in unstressed cells, we compared the abilities of wild-type, ΔCLΔCX, and CR Hsp90 (Fig. 1A) to support yeast viability as the sole copy of Hsp90 at modest temperature. Each construct was introduced into a yeast strain whose only other source of Hsp90 was encoded on a URA3 plasmid that could be swapped out using 5-FOA (26, 49). ΔCLΔCX and CR Hsp90 both supported yeast growth as the sole Hsp90 in yeast (Fig. 1B), and both constructs accumulated to wild-type levels in yeast grown at 25°C (Fig. 1C).
Fig 1.
The solubility-promoting function of Hsp90 is required for yeast growth at elevated temperatures. (A) Hsp90 constructs used in these studies. Primary structures of wild-type ΔCLDCX, and CR Hsp90 are shown. Deletions in ΔCLΔCX were limited to unstructured regions. The CR sequence is from the CL region of human Hsp90. (B) ΔCLΔCX and CR Hsp90 supported yeast viability as the sole copy of Hsp90 at 25°C. (C) ΔCLΔCX and CR Hsp90 accumulated to wild-type levels at 25°C based on Western blotting of yeast lysates. Intervening lanes were removed for presentation purposes. (D) Growth of yeast strains expressing wild-type, ΔCLΔCX, or CR Hsp90 as the sole copy of Hsp90 at 30 and 35°C.
We conjectured that yeast harboring ΔCLΔCX Hsp90 may exhibit growth defects at elevated temperature, where robust growth shows an increased dependence on Hsp90 levels (3) that has been linked to cell wall integrity (CWI) pathways (12, 39, 45). We examined growth at elevated temperatures (Fig. 1D) and observed that ΔCLΔCX Hsp90 was deficient for growth at 35°C compared to wild-type Hsp90. CR Hsp90, which displays efficient solubility-promoting activity in vitro (48), was less severely impaired for growth at elevated temperature than ΔCLΔCX Hsp90 (Fig. 1D).
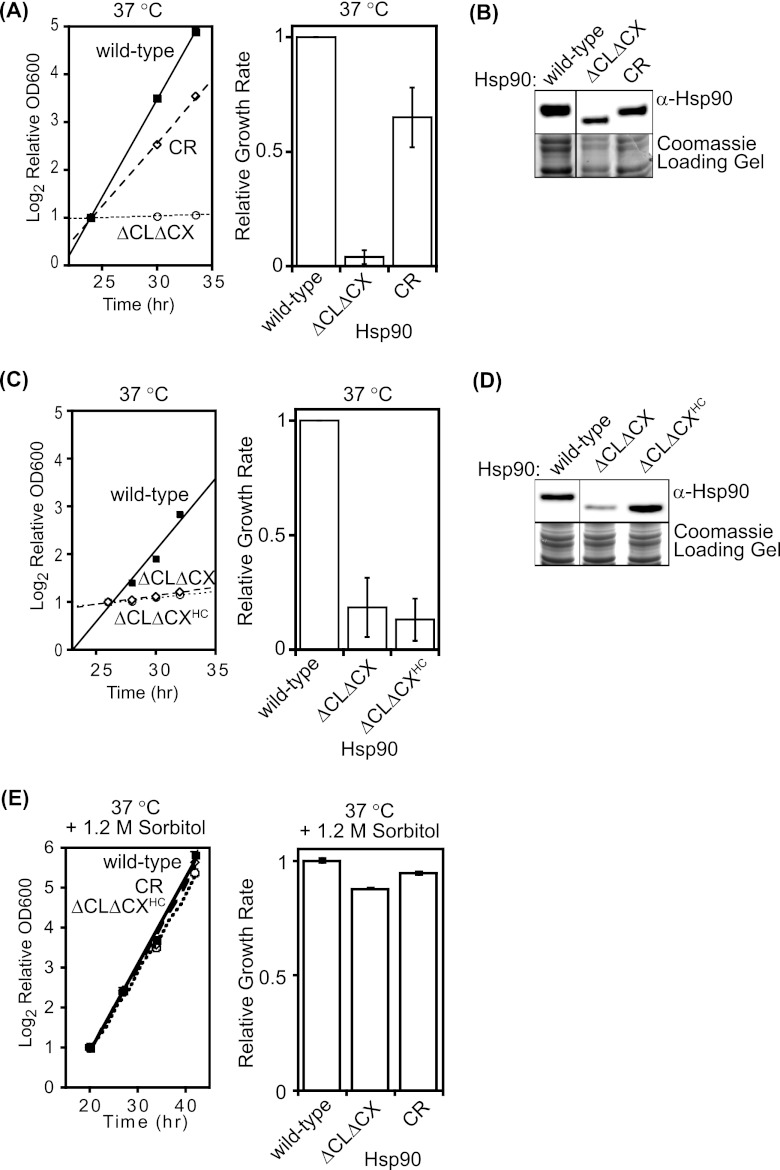
To accurately quantify growth defects, we examined the growth properties of wild-type, ΔCLΔCX, and CR Hsp90 in liquid culture at elevated temperature (Fig. 2A). At 37°C we observed that ΔCLΔCX Hsp90 had essentially stopped growing compared to cells with wild-type Hsp90 and that CR Hsp90 recovered about 60% of the growth rate of the wild type. Culture temperatures were monitored using a thermocoupler submerged in a mock culture and were found to vary by ±0.2°C around the set temperature. Of note, similar analyses of temperatures on plates indicated a wider temperature variation. At 37°C in liquid culture, the expression level of ΔCLΔCX Hsp90 is clearly reduced relative to those of both wild-type and CR Hsp90 (Fig. 2B). In order to distinguish expression-level effects (3) from other biochemical functions such as client solubility, we increased expression of ΔCLΔCX Hsp90 by generating a high-copy expression plasmid (ΔCLΔCXHC). ΔCLΔCXHC Hsp90 was expressed to similar levels as wild-type Hsp90 but did not improve the growth rate of yeast at 37°C (Fig. 2C and D). Of note, the C-terminal peptide (MEEVD) of wild-type Hsp90 binds to cochaperones, and this region is deleted in both ΔCLΔCX and CR Hsp90. Individual deletion of the MEEVD region from Hsp90 has been reported and was not observed to compromise yeast viability (21). The absence of the MEEVD motif from CR Hsp90 together with our observation that CR strongly rescues the growth defect of ΔCLΔCXHC (Fig. 2A) indicates that the dominant functional effect of deleting the CX is not due to the MEEVD sequence. Instead, our results indicate that the charged property of Hsp90 is essential for growth at elevated temperature independent of expression level. The improved growth of the CR construct compared to ΔCLΔCXHC indicates that the charged nature of the unstructured regions is a primary contributor to growth rate under these conditions.
Fig 2.
Quantification of growth defects of ΔCLΔCX Hsp90. (A) Growth of ΔCLΔCX Hsp90 in liquid culture was significantly impaired at 37°C. Experiments were performed in triplicate (on the left are results from a representative experiment) and analyzed to determine the average growth effect and the variation between replicates. (B) The steady-state level of ΔCLΔCX Hsp90 was reduced compared to that of wild-type Hsp90 at 37°C based on Western blotting of yeast lysates. Intervening lanes were removed for presentation purposes. (C) In order to increase ΔCLΔCX Hsp90 expression, we generated a high-copy expression plasmid (ΔCLΔCXHC). ΔCLΔCXHC Hsp90 was defective for growth in liquid culture at 37°C. Experiments were performed in triplicate (on the left are results from a representative experiment) and analyzed to determine the average growth effect and the variation between replicates. (D) ΔCLΔCXHC Hsp90 accumulated to wild-type levels at 37°C as determined by Western blotting of yeast lysates. Intervening lanes were removed for presentation purposes. (E) Growth rates at 37°C with 1.2 M sorbitol. For growth measurements, error bars represent standard deviations (n = 3).
Robust growth of yeast at elevated temperature requires Hsp90-mediated activation of the cell wall integrity (CWI) signaling pathway (18, 45). To examine potential CWI defects in yeast harboring our Hsp90 constructs, we examined growth in the presence of the osmotic stabilizer sorbitol, which was previously shown to enable robust yeast growth in the presence of CWI signaling defects (45). We observed that 1.2 M sorbitol almost fully rescued the growth defects of ΔCLΔCXHC Hsp90 at 37°C (Fig. 2E). These results suggest that CWI signaling is compromised by ΔCLΔCXHC Hsp90.
Coexpression of ΔCLΔCX with wild-type Hsp90.
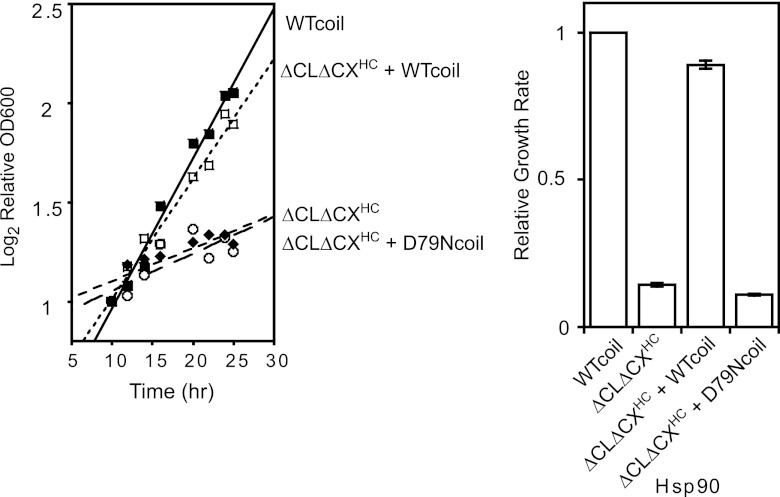
To gain mechanistic insights into the deficiency of ΔCLΔCXHC, we performed coexpression experiments. Because Hsp90 is a dimer, coexpression experiments can be complicated to analyze due to the formation of heterodimer Hsp90 species. To prevent cross dimerization in coexpression experiments, we utilize a coiled coil on one construct. The coiled coil results in superstabilization of one homodimer, which shifts the dimer exchange reaction to disfavor any heterodimer formation (50). Using this strategy, we observed that coexpression of WTcoil Hsp90 with ΔCLΔCXHC largely rescued the growth defect of ΔCLΔCXHC (Fig. 3). Of note, the coiled coil caused a slow-growth phenotype at 37°C compared to that of wild-type Hsp90, suggesting that transient C-domain dissociation may be important for robust yeast growth at elevated temperatures. For this reason, it was important in these experiments to compare rescue (WTcoil Hsp90 with ΔCLΔCXHC) to yeast with WTcoil as the sole Hsp90. This comparison indicates that the primary effect of ΔCLΔCXHC at elevated temperatures is a loss of function.
Fig 3.
Solubility-promoting and ATPase activities were required in the same Hsp90 molecule for robust growth. The growth deficiency of ΔCLΔCXHC was not dominant and could be rescued by coexpression of a functional WTcoil Hsp90. In contrast, coexpression of ΔCLΔCXHC with an ATP-deficient D79Ncoil failed to support robust growth. Experiments were performed in triplicate (on the left are results from a representative experiment) and analyzed to determine the average growth effect and the variation between replicates. Experiments were performed at 37,°C and error bars represent standard deviations (n = 3). Coiled-coil constructs were used to prevent cross-dimerization.
Both the ATPase function and the solubility-promoting function are important for robust yeast growth. We examined whether these functions were required in the same Hsp90 molecule or if a solubility-promoting mutant (ΔCLΔCXHC) coexpressed with an ATPase mutant (D79Ncoil) would support robust growth. D79Ncoil Hsp90 contains a point mutation that abolishes ATP binding and does not support viability on its own (27, 28, 50) but retains the ability to function as a solubility-promoting chaperone in vitro (48). D79Ncoil also includes an engineered coiled coil to prevent cross dimerization (50) with ΔCLΔCXHC. D79Ncoil coexpression failed to rescue the growth defect of ΔCLΔCXHC Hsp90 at 37°C (Fig. 3). This result indicates that both the solubility-promoting function and ATPase function of Hsp90 must occur in the same molecule, likely because the solubility-promoting function is required during the ATP-driven client maturation process.
In order to facilitate examination of the ATP-dependent activation of clients, we examined the sensitivity of our Hsp90 panel to small changes in temperature by monitoring growth at 36°C. At this temperature, the rank order of growth rates for wild-type, ΔCLΔCXHC, and CR Hsp90 is the same as at 37°C, but the magnitude of growth defects is reduced about 2-fold (see Fig. S1 in the supplemental material). Of note, large changes in growth rates are frequently observed for small temperature changes close to the critical temperature required to stall growth (26). The ability of all Hsp90 variants to grow at this temperature enabled further biochemical analysis of client proteins under these conditions. Because the growth was sensitive to small changes in temperature, care was taken to perform experiments on all strains in parallel and the culture temperature was monitored periodically using a sterilized thermocoupler.
ΔCLΔCX is deficient for client expression and activation at elevated temperature.
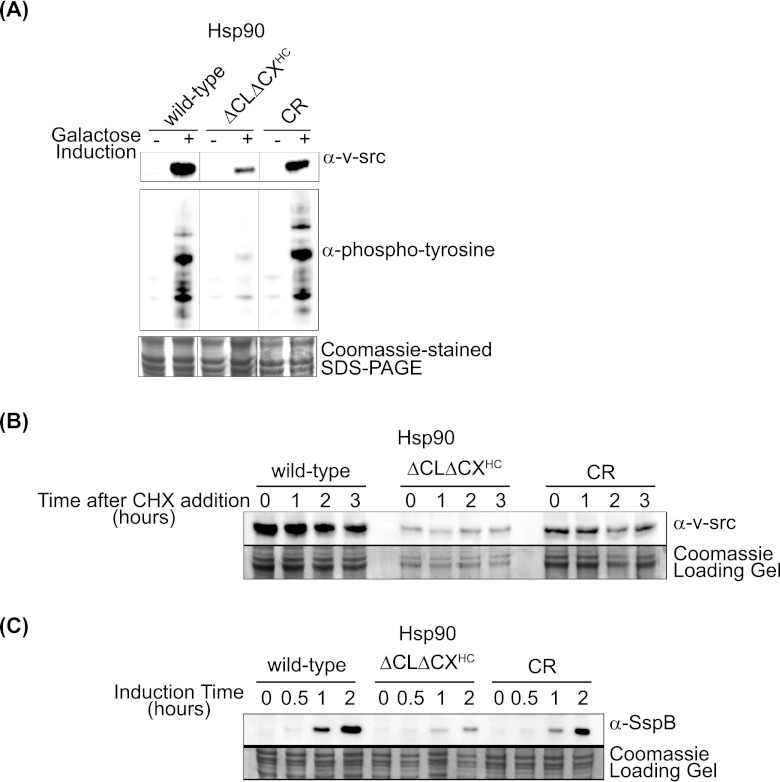
We investigated the ability of ΔCLΔCXHC to mature the v-src tyrosine kinase, which has a well-established dependence on Hsp90 for activity in yeast (5, 26, 53). Yeasts have very low levels of phosphotyrosine, and v-src activity can be readily observed by following tyrosine phosphorylation of endogenous yeast proteins by active v-src. We observed that in ΔCLΔCXHC yeast, v-src accumulated at low levels and did not exhibit src kinase activity (Fig. 4A). In contrast, CR Hsp90 supported v-src expression and kinase activity indistinguishably from wild-type Hsp90.
Fig 4.
Yeast with ΔCLΔCXHC Hsp90 was deficient for galactose-induced expression and activity of v-src kinase. (A) Yeast with ΔCLΔCXHC Hsp90 had reduced v-src activity a judged by phosphotyrosine buildup and had reduced v-src levels. CR Hsp90 activated v-src to wild-type levels. Intervening lanes were removed for presentation purposes. (B) Degradation of v-src over time was monitored following the addition of the protein synthesis inhibitor CHX by Western blotting for v-src in yeast lysates. (C) Galactose-induced expression was impaired in yeast with ΔCLΔCXHC Hsp90 as monitored with the non-Hsp90 client SspB.
We examined the possibility that the poor expression of v-src with ΔCLΔCXHC was due to rapid v-src degradation by utilizing cycloheximide to block new protein synthesis and following v-src levels as a function of time (Fig. 4B). We found that v-src levels declined slowly and similarly over a 3-h time course for ΔCLΔCXHC, wild-type, and CR Hsp90. These results indicate that variations in the v-src degradation rate between the strains were modest and suggested that reduced v-src levels may be caused instead by reduced induction from the galactose-inducible promoter used to drive v-src expression.
We probed whether ΔCLΔCXHC Hsp90 was deficient for expression from the galactose-inducible promoter by using the same promoter to drive the expression of SspB, a non-Hsp90-dependent protein that is highly soluble (50). We observed that galactose-induced expression of SspB is reduced and/or delayed in cells expressing ΔCLΔCXHC Hsp90 compared to wild-type Hsp90 (Fig. 4C). Previous studies have shown that Hsp90 activity is required for efficient transcriptional activation from the galactose promoter (7). Our results implicate the charge-rich regions of Hsp90 in this process, though this hindered our ability to investigate activation of v-src.
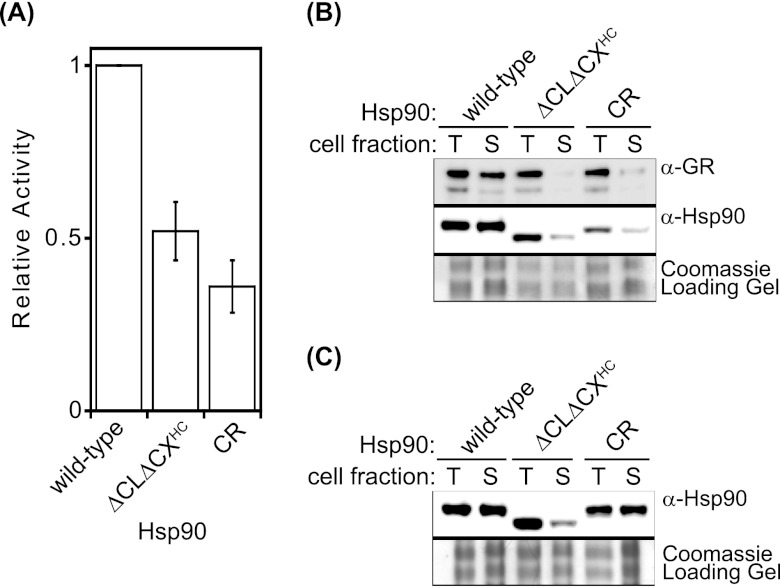
In order to analyze an Hsp90 client that does not require induced client expression, we turned to the glucocorticoid receptor (GR). Like v-src, GR is a well established Hsp90-dependent client in yeast (31), but unlike v-src, it can be constitutively expressed. Upon stimulation with agonist, GR transcriptional activity in yeast was monitored with a β-galactosidase reporter. At elevated temperature, we observed that cells with either ΔCLΔCXHC or CR Hsp90 were deficient for GR activity compared to cells with wild-type Hsp90 (Fig. 5A). Consistent with our observations, previous reports have demonstrated that the sequence of the CL can affect GR maturation in yeast (47). In the absence of agonist, all samples had baseline β-galactosidase levels that were less than 10% of those in wild-type Hsp90 samples with agonist (data not shown). The total levels of GR in cells with ΔCLΔCXHC, CR, and wild-type Hsp90 were similar (Fig. 5B), indicating that ΔCLΔCXHC and CR Hsp90 are deficient at maturing GR to an active state and not in maintaining the expression level.
Fig 5.
ΔCLΔCXHC Hsp90 was unable to mature the hormone receptor GR in yeast. (A) GR reporter activity levels were lower than those of the wild type for both ΔCLΔCXHC and CR Hsp90. Error bars represent standard deviations (n = 3). (B) Western blotting for GR in whole-cell lysates (T) and soluble fractions (S) indicated that the ratio of soluble to total GR is drastically reduced in ΔCLΔCXHC and CR Hsp90 cells. The solubilities of ΔCLΔCXHC and CR Hsp90 were also reduced compared to that of wild-type Hsp90. (C) In the absence of exogenous client proteins, the solubility of ΔCLΔCXHC Hsp90 was drastically reduced, while CR Hsp90 maintained wild-type solubility.
Motivated by previous observations that the GR protein is poorly soluble in its purified form (38), we investigated the solubility of GR in cells. In cells with wild-type Hsp90, GR was abundant in the soluble fraction, while in cells with either ΔCLΔCXHC or CR Hsp90, the soluble fraction contained very low levels of GR (Fig. 5B). Thus, low levels of GR in the soluble fraction correspond to reduced GR-driven reporter activity. These results suggest that Hsp90 assists GR maturation by affecting the solubility of GR and that the CL and CX regions of Hsp90 are required for this solubility.
While all of the Hsp90 constructs in this study are readily soluble in purified form (48), we considered the possibility that they may be partly insoluble under cellular conditions, where Hsp90 is one of the most concentrated proteins in the cytosol (3) and binds to hundreds of partner proteins (55). We had previously observed that ΔCLΔCX is indistinguishable from wild-type Hsp90 for aggregation at 45°C but that in the presence of a purified aggregation-prone client ΔCLΔCX was dramatically less stable than wild-type Hsp90 (48). Motivated by these in vitro observations, we examined the impact of exogenous expression of the aggregation-prone client, GR, in yeast. When GR was expressed, we observed that both ΔCLΔCXHC and CR Hsp90 accumulated at reduced levels in soluble fractions compared to wild-type Hsp90 (Fig. 5B). We further examined Hsp90 solubility in cells without GR and found that ΔCLΔCXHC Hsp90 accumulated at reduced levels in soluble fractions but that CR Hsp90 had solubility properties similar to those of wild-type Hsp90 (Fig. 5C). These results indicate that the charge-rich CL and CX regions of Hsp90 can have a large impact on the solubility and activity of Hsp90 in cells with endogenous clients and that this can be magnified by exogenous expression of the aggregation-prone GR client.
DISCUSSION
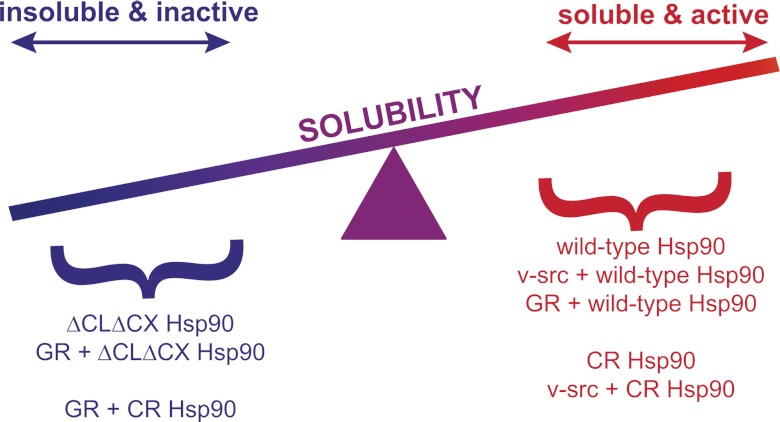
The results presented here indicate that the charge-rich regions of Hsp90 (CL and CX) contribute solubility-promoting properties to Hsp90 and Hsp90-client complexes in cells. We propose that the solubility-promoting properties of these charge-rich regions serves to improve the solubility of Hsp90 itself and complexes of Hsp90 with aggregation-prone clients (Fig. 6). Many Hsp90 client proteins are aggregation prone, particularly steroid hormone receptors such as GR (30, 32). Our results suggest that Hsp90 is required for maintaining the balance between soluble, active clients and insoluble, inactive proteins (Fig. 6). Our results indicate that the net charge of Hsp90 contributed by its two charge-rich regions (CL and CX) is important for maintaining this balance.
Fig 6.
Hsp90 plays an important role in maintaining the balance between soluble, active clients and insoluble, inactive proteins. The solubilities and/or activities of Hsp90 variants and clients monitored in this study are outlined. The high charge of Hsp90 concentrated in two charge-rich regions modulates this solubility-promoting function. Deletion of both charge-rich regions of Hsp90 impairs the solubility of Hsp90 and in turn reduces the solubility of complexes with clients.
Based on our results and those of others (47), the solubility-promoting contribution of charge-rich regions likely depends on their structural location, net charge, and sequence. For example, we observed that Hsp90 lacking both charge-rich regions (ΔCLΔCXHC) is aggregation prone on its own in yeast but that both wild-type Hsp90 and CR Hsp90, where one of these regions is replaced with an exogenous charge-rich sequence, are highly soluble. These results indicate that having a single charge-rich sequence is sufficient to imbue Hsp90 in yeast cells with high solubility. In contrast, when an aggregation-prone client (GR) was exogenously expressed, we observed that only wild-type Hsp90 maintained it in a soluble form and that both CR and ΔCLΔCXHC Hsp90 became aggregation prone. In our model (Fig. 6), the aggregation properties of GR require the solubility-promoting function of both CL and CX in order to confer net solubility on Hsp90-GR complexes.
The solubility-promoting effects of the CL and CX regions may be particularly important during the process of client maturation. Studies using chemical modification of residues in the hormone binding domain of GR suggest that Hsp90 is required for opening the steroid binding site of GR, making it accessible for hormone binding (42). Opening of the hormone binding domain results in exposure of the hydrophobic binding pocket (9, 24, 42, 52), which likely causes a transient state that is highly prone to aggregate. The solubility-promoting function of Hsp90 may play a role in enhancing the solubility of GR in this hormone binding-competent conformation.
The negatively charged properties of the Hsp90 CL and CX regions are maintained throughout the eukaryotic evolutionary lineage (21, 48), implying that they contribute to the function of Hsp90 in these organisms. The CX is at the C terminus of Hsp90 and contains a peptide motif that binds to tetratricopeptide repeat domain (TPR)-containing cochaperones (4). However, binding to these cochaperones does not appear to be critical to function in yeast, as deletion of the CX is compatible with yeast viability and efficient maturation of nuclear steroid receptors in yeast (21). The CL connects the N and M domains, which both contribute catalytic amino acids for ATP hydrolysis. The CL has been implicated in the ATP- and cochaperone-driven conformational cycle of Hsp90 (10, 22, 34, 41). Our results indicate an additional critical function of CL and CX: imparting high solubility on Hsp90 in complex with aggregation-prone clients.
Multiple lines of evidence and reasoning indicate that the charge-mediated Hsp90 solubility imparted by the disordered CL and CX regions should be advantageous. The expression of aggregation-prone proteins results in an inefficient use of cellular resources in terms of both wasted biosynthetic energy and additional energy resources required to rescue or degrade proteins in aggregates. The fitness impact due to expression of aggregation prone proteins can be magnified because initial aggregates can seed the aggregation of other proteins. The fitness cost caused by the expression of a misfolded protein has been directly observed in yeast (8) and indicates that solubility is under selective pressure. Because the fitness cost of an aggregation-prone protein should be related to its expression level, the solubility of Hsp90, which is one of the most highly expressed proteins, should be under strong selective pressure. This conceptual framework combined with our observations of the solubility-promoting properties of the charge-rich regions provides a compelling explanation for conservation of the charged properties of Hsp90 CL and CX throughout eukaryotic phylogeny.
Future directions.
Chaperones are critical to all cells because of the inherent challenges of protein folding in the complex and crowded cellular environment. However, mechanistic investigations of chaperones in cells have been hindered because of overlapping/redundant chaperone function. The unique specificity of Hsp90 clients combined with the ability to modulate the solubility-promoting function of Hsp90 through deletions of the charge-rich regions provides a promising system to study aggregation events in cells. Our results indicate that Hsp90 is involved in maintaining the balance between soluble, active clients and insoluble, inactive proteins. Solubility-deficient ΔCLΔCXHC Hsp90 provides a novel tool for identifying endogenous aggregation-prone clients of Hsp90 in cells. This should be a promising route to enhance our understanding of the role of chaperones such as Hsp90 in the solubility of cellular proteins.
Supplementary Material
ACKNOWLEDGMENTS
We express appreciation to M. Munson and R. Gilmore for useful discussions.
This work was supported in part by grants from the National Institutes of Health (R01-GM083038) and the American Cancer Society (RSG-08-17301-GMC) to D.N.A.B.
Footnotes
Published ahead of print 1 June 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Ali MM, et al. 2006. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440: 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birnby DA, et al. 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in caenorhabditis elegans. Genetics 155: 85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. 1989. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 9: 3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinker A, et al. 2002. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 × Hop × Hsp90 complexes. J. Biol. Chem. 277: 19265–19275 [DOI] [PubMed] [Google Scholar]
- 5. Chang HC, Lindquist S. 1994. Conservation of Hsp90 macromolecular complexes in Saccharomyces cerevisiae. J. Biol. Chem. 269: 24983–24988 [PubMed] [Google Scholar]
- 6. Cunningham CN, Krukenberg KA, Agard DA. 2008. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J. Biol. Chem. 283: 21170–21178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Floer M, Bryant GO, Ptashne M. 2008. HSP90/70 chaperones are required for rapid nucleosome removal upon induction of the GAL genes of yeast. Proc. Natl. Acad. Sci. U. S. A. 105: 2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geiler-Samerotte KA, et al. 2011. Misfolded proteins impose a dosage-dependent fitness cost and trigger a cytosolic unfolded protein response in yeast. Proc. Natl. Acad. Sci. U. S. A. 108: 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giannoukos G, Silverstein AM, Pratt WB, Simons SS., Jr 1999. The seven amino acids (547-553) of rat glucocorticoid receptor required for steroid and hsp90 binding contain a functionally independent LXXLL motif that is critical for steroid binding. J. Biol. Chem. 274: 36527–36536 [DOI] [PubMed] [Google Scholar]
- 10. Hainzl O, Lapina MC, Buchner J, Richter K. 2009. The charged linker region is an important regulator of Hsp90 function. J. Biol. Chem. 284: 22559–22567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Havranek JJ, Harbury PB. 2003. Automated design of specificity in molecular recognition. Nat. Struct. Biol. 10: 45–52 [DOI] [PubMed] [Google Scholar]
- 12. Hawle P, et al. 2007. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p). Eukaryot. Cell 6: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hessling M, Richter K, Buchner J. 2009. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 16: 287–293 [DOI] [PubMed] [Google Scholar]
- 14. Jakob U, Lilie H, Meyer I, Buchner J. 1995. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J. Biol. Chem. 270: 7288–7294 [DOI] [PubMed] [Google Scholar]
- 15. Johnson BS, McCaffery JM, Lindquist S, Gitler AD. 2008. A yeast TDP-43 proteinopathy model: exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc. Natl. Acad. Sci. U. S. A. 105: 6439–6444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kopito RR. 2000. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10: 524–530 [DOI] [PubMed] [Google Scholar]
- 17. Krukenberg KA, Forster F, Rice LM, Sali A, Agard DA. 2008. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure 16: 755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levin DE. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linding R, et al. 2003. Protein disorder prediction: implications for structural proteomics. Structure 11: 1453–1459 [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Apodaca J, Davis LE, Rao H. 2007. Proteasome inhibition in wild-type yeast Saccharomyces cerevisiae cells. Biotechniques 42: 158–162 [DOI] [PubMed] [Google Scholar]
- 21. Louvion JF, Warth R, Picard D. 1996. Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc. Natl. Acad. Sci. U. S. A. 93: 13937–13942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T. 2009. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat. Struct. Mol. Biol. 16: 281–286 [DOI] [PubMed] [Google Scholar]
- 23. Monsellier E, Chiti F. 2007. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep. 8: 737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morishima Y, Murphy PJ, Li DP, Sanchez ER, Pratt WB. 2000. Stepwise assembly of a glucocorticoid receptor.hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 275: 18054–18060 [DOI] [PubMed] [Google Scholar]
- 25. Mumberg D, Muller R, Funk M. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156: 119–122 [DOI] [PubMed] [Google Scholar]
- 26. Nathan DF, Lindquist S. 1995. Mutation analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 15: 3917–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. 1998. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 143: 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panaretou B, et al. 1998. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17: 4829–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panaretou B, et al. 2002. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell 10: 1307–1318 [DOI] [PubMed] [Google Scholar]
- 30. Picard D. 2006. Chaperoning steroid hormone action. Trends Endocrinol. Metab. 17: 229–235 [DOI] [PubMed] [Google Scholar]
- 31. Picard D, et al. 1990. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348: 166–168 [DOI] [PubMed] [Google Scholar]
- 32. Prima V, Depoix C, Masselot B, Formstecher P, Lefebvre P. 2000. Alteration of the glucocorticoid receptor subcellular localization by non steroidal compounds. J. Steroid Biochem. Mol. Biol. 72: 1–12 [DOI] [PubMed] [Google Scholar]
- 33. Prodromou C, et al. 1997. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90: 65–75 [DOI] [PubMed] [Google Scholar]
- 34. Pullen L, Bolon DN. 2011. Enforced N-domain proximity stimulates Hsp90 ATPase activity and is compatible with function in vivo. J. Biol. Chem. 286: 11091–11098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richter K, Muschler P, Hainzl O, Buchner J. 2001. Coordinated ATP hydrolysis by the Hsp90 dimer. J. Biol. Chem. 276: 33689–33696 [DOI] [PubMed] [Google Scholar]
- 36. Ross CA, Poirier MA. 2004. Protein aggregation and neurodegenerative disease. Nat. Med. 10(Suppl.): S10–S17 [DOI] [PubMed] [Google Scholar]
- 37. Scheibel T, et al. 1999. The charged region of Hsp90 modulates the function of the N-terminal domain. Proc. Natl. Acad. Sci. U. S. A. 96: 1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seitz T, et al. 2010. Enhancing the stability and solubility of the glucocorticoid receptor ligand-binding domain by high-throughput library screening. J. Mol. Biol. 403: 562–577 [DOI] [PubMed] [Google Scholar]
- 39. Shaner L, Gibney PA, Morano KA. 2008. The Hsp110 protein chaperone Sse1 is required for yeast cell wall integrity and morphogenesis. Curr. Genet. 54: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shiau AK, Harris SF, Southworth DR, Agard DA. 2006. Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127: 329–340 [DOI] [PubMed] [Google Scholar]
- 41. Southworth DR, Agard DA. 2008. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol. Cell 32: 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stancato LF, Silverstein AM, Gitler C, Groner B, Pratt WB. 1996. Use of the thiol-specific derivatizing agent N-iodoacetyl-3-[125I]iodotyrosine to demonstrate conformational differences between the unbound and hsp90-bound glucocorticoid receptor hormone binding domain. J. Biol. Chem. 271: 8831–8836 [DOI] [PubMed] [Google Scholar]
- 43. Taipale M, Jarosz DF, Lindquist S. 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11: 515–528 [DOI] [PubMed] [Google Scholar]
- 44. Trepel J, Mollapour M, Giaccone G, Neckers L. 2010. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 10: 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Truman AW, et al. 2007. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot. Cell 6: 744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsutsumi S, et al. 2009. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat. Struct. Mol. Biol. 16: 1141–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsutsumi S, et al. 2012. Charged linker sequence modulates eukaryotic heat shock protein 90 (Hsp90) chaperone activity. Proc. Natl. Acad. Sci. U. S. A. 109: 2937–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wayne N, Bolon DN. 2010. Charge-rich regions modulate the anti-aggregation activity of Hsp90. J. Mol. Biol. 401: 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wayne N, Bolon DN. 2007. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J. Biol. Chem. 282: 35386–35395 [DOI] [PubMed] [Google Scholar]
- 50. Wayne N, Lai Y, Pullen L, Bolon DN. 2010. Modular control of cross-oligomerization: analysis of superstabilized Hsp90 homodimers in vivo. J. Biol. Chem. 285: 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U. S. A. 91: 8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu M, Dittmar KD, Giannoukos G, Pratt WB, Simons SS., Jr 1998. Binding of hsp90 to the glucocorticoid receptor requires a specific 7-amino acid sequence at the amino terminus of the hormone-binding domain. J. Biol. Chem. 273: 13918–13924 [DOI] [PubMed] [Google Scholar]
- 53. Xu Y, Lindquist S. 1993. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc. Natl. Acad. Sci. U. S. A. 90: 7074–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Youker RT, Walsh P, Beilharz T, Lithgow T, Brodsky JL. 2004. Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol. Biol. Cell 15: 4787–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhao R, et al. 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120: 715–727 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.