Abstract
Background:
The prevalence of obstructive sleep apnea syndrome (OSAS) is higher in children with sickle cell disease (SCD) as compared with the general pediatric population. It has been speculated that overgrowth of the adenoid and tonsils is an important contributor.
Methods:
The current study used MRI to evaluate such an association. We studied 36 subjects with SCD (aged 6.9 ± 4.3 years) and 36 control subjects (aged 6.6 ± 3.4 years).
Results:
Compared with control subjects, children with SCD had a significantly smaller upper airway (2.8 ± 1.2 cm3 vs 3.7 ± 1.6 cm3, P < .01), and significantly larger adenoid (8.4 ± 4.1 cm3 vs 6.0 ± 2.2 cm3, P < .01), tonsils (7.0 ± 4.3 cm3 vs 5.1 ± 1.9 cm3, P < .01), retropharyngeal nodes (3.0 ± 1.9 cm3 vs 2.2 ± 0.9 cm3, P < .05), and deep cervical nodes (15.7 ± 5.7 cm3 vs 12.7 ± 4.0 cm3, P < .05). Polysomnography showed that 19.4% (seven of 36) of children with SCD had OSAS compared with 0% (zero of 20) of control subjects (P < .05) and that in children with SCD the apnea-hypopnea index correlated positively with upper airway lymphoid tissues size (r = 0.57, P < 001). In addition, children with SCD had lower arterial oxygen saturation nadir (84.3% ± 12.3% vs 91.2% ± 4.2%, P < .05), increased peak end-tidal CO2 (53.4 ± 8.5 mm Hg vs 42.3 ± 5.3 mm Hg, P < .001), and increased arousals (13.7 ± 4.7 events/h vs 10.8 ± 3.8 events/h, P < .05).
Conclusions:
Children with SCD have reduced upper airway size due to overgrowth of the surrounding lymphoid tissues, which may explain their predisposition to OSAS.
Sleep-disordered breathing (SDB), a condition associated with abnormalities in respiratory gas exchange during sleep (particularly intermittent oxyhemoglobin desaturation), has been implicated as a possible risk factor for deleterious cerebrovascular complications in children with sickle cell disease (SCD).1‐3 Although the prevalence of SDB in these children has not been well established, estimates range from 5% to 79%4‐10 and are far greater than the prevalence of 1% to 4% in children without SCD.11 In addition, the pathophysiology of SDB in these children is not well understood. Possible mechanisms include hypoventilation due to chronic lung disease,12,13 obstructive sleep apnea syndrome (OSAS)6 (a disorder characterized by recurrent events of partial or complete upper airway obstruction during sleep14 that is associated with distinct neurocognitive deficits and cardiovascular morbidities15), left ventricular diastolic dysfunction,16 pulmonary hypertension,17 and the presence of dyshemoglobins.18
This study was part of a large investigation at the Children’s Hospital of Philadelphia (CHOP) on the prevalence, contributory mechanisms, and pulmonary and vascular consequences of oxyhemoglobin desaturations in children with SCD-hemoglobin. In this particular study, we aimed to determine the possible anatomic changes in the upper airway that may predispose to SDB and particularly OSAS.
In children without SCD, adenotonsillar hypertrophy is the most common cause of OSAS.19‐21 Children with SCD may be predisposed to the development of adenoidal and tonsillar hypertrophy due to a compensatory response for their commonly described functional asplenia.4,6,9 Thus, we hypothesized that children with SCD may have excess upper airway lymphoid tissue proliferation leading to a decrease in their upper airway size. Such alterations may in turn result in abnormal polysomnographic findings and increase the prevalence of SDB and OSAS in this population. If such an association exists, greater attention to diagnosis and management of compromised airway size in this population would be warranted to reduce oxyhemoglobin desaturation during sleep. To this end, we used MRI to delineate the size of the upper airway and surrounding lymphoid tissues and overnight polysomnography to evaluate for SDB and/or OSAS in unselected children with SCD as compared with control subjects.
Materials and Methods
Subjects
The study was approved by the institutional review boards of CHOP (IRB#07-005188) and the Children’s Hospital at Montefiore (CCI#2007-941). Informed assent was obtained from each child > 7 years old, and informed consent was obtained from a parent/guardian of each child.
Subjects With SCD:
Children were recruited from the Comprehensive Sickle Cell Center at CHOP. Inclusion criteria were as follows: (1) SCD-hemoglobin; (2) age 2 to 21 years; (3) intact adenoid and tonsils; (4) steady state, defined as a period of at least 3 months since the last RBC transfusion and at least 4 weeks since the last acute chest syndrome or painful episode. Exclusion criteria were as follows: (1) treatment with hydroxyurea within the past 3 months; (2) chronic lung disease unrelated to SCD, other than asthma; (3) chronic transfusion protocol.
Control Subjects:
Control subjects were selected from patients who underwent a head or neck MRI at CHOP for medical indications such as concussion, headache, or seizures. Control subjects were matched to subjects with SCD by age, sex, ethnicity, weight, and height. Inclusion criteria were (1) normal growth and development, (2) intact adenoid and tonsils. Exclusion criteria included (1) evidence of a brain tumor or a seizure disorder requiring therapy, (2) genetic disorders associated with any craniofacial anomaly, (3) chronic respiratory disease other than asthma, (4) history of OSAS.
MRI
Studies were performed in the Department of Radiology at CHOP. For children < 7 years of age, studies were performed under sedation with IV pentobarbital 2 to 6 mg/kg until sleep was achieved; a maximum of 200 mg was administered. All subjects were monitored continuously by pulse oximetry and observed by an anesthesiologist throughout the study until recovery (~1 h).
MRI was performed with a 1.5T Siemens Vision system. Images were acquired using a commercially available head coil. Axial and sagittal sequential T1-weighted (TR650/TE14) and T2-weighted (TR6000/TE90) images with 3-mm slice thickness and 1 NEX were obtained from the orbital cavity to the larynx and from the midline bilaterally, respectively, as previously described.21
Image Processing and Upper Airway Measurements
The acquired MRI studies were anonymized and converted to a multidimensional version of the digital imaging and communications in medicine (DICOM) format. These were transferred via the CHOP’s picture archiving system to a workstation at the Division of Respiratory and Sleep Medicine at the Children’s Hospital at Montefiore. Image analysis was performed by a blinded scorer using AMIRA, version 4.1.1, software (Visage Imaging, GmbH), and using intensity threshold after normalization.
Volumetric Measurements
The volumes of the following structures were determined as follows:
Airway: The upper airway including the nasopharynx, defined as the region located superior to the level of the soft palate and continuous anteriorly, through the choanae, with the nasal cavities; oropharynx, defined as the region located between the level of the soft palate and the larynx, communicating anteriorly with the oral cavity, and having the posterior one-third of the tongue as its anterior border; and the hypopharynx, defined as the region posterolateral to the larynx, and communicating with the cavity of the larynx through the auditus and included the pyriform recesses and the valleculae.
Lymphoid tissues: Adenoid, combined palatine tonsils, combined retropharyngeal nodes (defined as lymph nodes located between the internal carotid arteries from the base of the skull to the hyoid bone), and the combined deep cervical lymph nodes (defined as level 2 nodes, located along the internal jugular vein from base of the skull to the level of the hyoid bone).
Polysomnography
Overnight polysomnography was performed in the sleep laboratory at CHOP. The following parameters were recorded (using Embla): EEGs (C4/A1, C3/A2, O1/A2, O2/A1), right and left electrooculogram, submental and tibial electromyograms, chest and abdominal wall movement (Respitrace Systems; Ambulatory Monitoring Inc), ECG, end-tidal CO2 (etco2) by capnography (Novametrix 7000; Novametrix), airflow by nasal pressure (Pro-Tech) and three-pronged thermistor (Nihon Kohden), arterial oxygen saturation (Spo2) and pulse waveform (Masimo), and infrared video. Sleep staging and respiratory events were consistent with the American Academy of Sleep Medicine pediatric scoring rules.22 Accordingly, central apneas, obstructive apneas, and obstructive hypopneas were scored. An obstructive apnea-hypopnea index (AHI) was calculated as the number of obstructive apneas and hypopneas per hour. The reported Spo2 nadir was the lowest oxygen saturation measured during polysomnography and not limited to an apnea or hypopnea event. We considered the diagnosis of OSAS when the AHI was ≥ 1.5/h.23‐25
Sleep Questionnaire
Since not all control subjects underwent polysomnography, we used a validated questionnaire developed by Brouilette et al26 to assess the likelihood of OSAS in these subjects. Accordingly, no subject with score < −1 would be expected to have OSAS; a score between −1 and 3.5 is considered indeterminate, and a score > 3.5 is considered highly predictive of OSAS.
Data Analysis
Statistical analysis was conducted using SPSS, version 18 (SPSS Inc). Means and SDs were used to summarize continuous variables. For comparisons between the groups for MRI data demographics, anthropometrics, and polysomnography data, we used a two-tailed unpaired t test and χ2 test as appropriate. Pearson correlations were derived between AHI and upper airway lymphoid tissues within the SCD group. A P value < .05 was considered significant.
Results
We studied 36 children with SCD with a mean age of 6.9 ± 4.3 years (2.0-16.8 years) and 36 control subjects with a mean age of 6.6 ± 3.4 years (2.2-15.8 years). Subjects with SCD were similar to control subjects in age, ethnicity, sex, height, and weight (Table 1). However, their mean BMI z score was significantly lower (Table 1).
Table 1.
—Demographics and Anthropometric Measures
| Measure | SCD (n = 36) | Control Subjects (n = 36) | P Value |
| Age, y | 6.9 ± 4.3 | 6.6 ± 3.4 | NS |
| Range, y | 2.0-16.8 | 2.2-15.8 | |
| Ethnicity: black, No. | 36 | 36 | NS |
| Sex, male, % | 55.6 | 55.6 | NS |
| Height, cm | 117.6 ± 24.8 | 117.4 ± 21.8 | NS |
| Weight, kg | 23.5 ± 13.3 | 25.2 ± 11.7 | NS |
| BMI z score | −0.4 ± 1.2 | 0.6 ± 1.0 | < .05 |
Data are displayed as mean ± SD unless otherwise noted. NS = not significant; SCD = sickle cell disease.
Upper Airway Volumetric Measurements
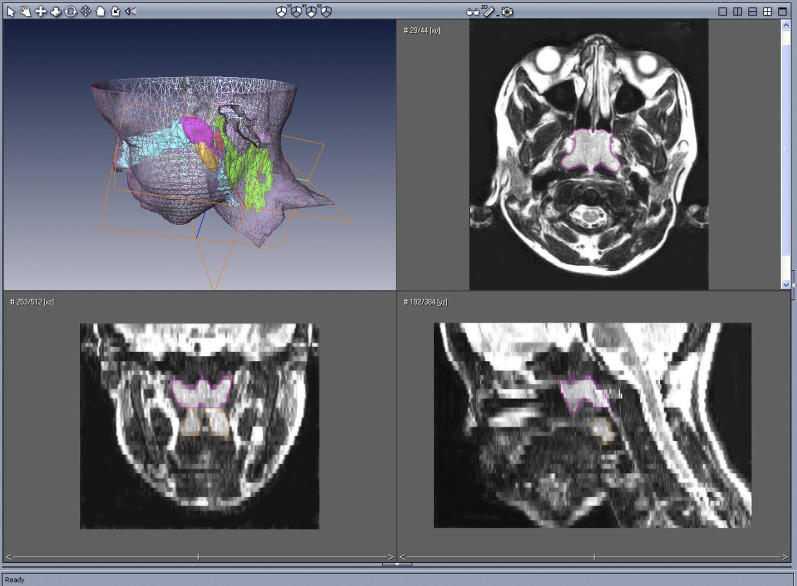
Volumetric analysis based on 3-mm axial images of the upper airway is shown in Table 2. Upper airway lymphoid tissue analysis of an 11.8-year-old male subject with SCD with OSAS, using AMIRA, is presented graphically in Figure 1. Figure 2 depicts a three-dimensional rendering of the face, upper airway, and lymphoid tissues of the same subject.
Table 2.
—Airway and Lymphoid Tissues Volumes
| Area Measured | SCD (n = 36) | Control Subjects (n = 36) | % Difference | P Value |
| Airway | 2.8 ± 1.2 | 3.7 ± 1.6 | −24.3 | < .01 |
| Lymphoid tissues | ||||
| Adenoid | 8.4 ± 4.1 | 6.0 ± 2.2 | 40.0 | < .01 |
| Tonsils | 7.0 ± 4.3 | 5.1 ± 1.9 | 37.3 | < .01 |
| Retropharyngeal nodes | 3.0 ± 1.9 | 2.2 ± 0.9 | 36.4 | < .05 |
| Deep cervical nodes | 15.7 ± 5.7 | 12.7 ± 4.0 | 23.6 | < .05 |
Data are displayed a mean ± SD. Units are cm3. % Difference = percent mean volume difference. See Table 1 legend for expansion of abbreviation.
Figure 1.
Upper left, Surface rendering of the head and neck with three-dimensional reconstruction of the adenoid (magenta), tonsils (orange), retropharyngeal nodes (red), and deep cervical lymph nodes (green), of an 11.8-year-old male subject with sickle cell disease with obstructive sleep apnea syndrome using AMIRA software. Upper right, Axial T2-weighted image at the nasopharyngeal level outlining the adenoid (magenta). Lower left, Coronal reconstructed image outlining the adenoid (magenta) and tonsils (orange). Lower right, Midsagittal reconstructed image outlining the adenoid (magenta) and tonsils (orange).
Figure 2.
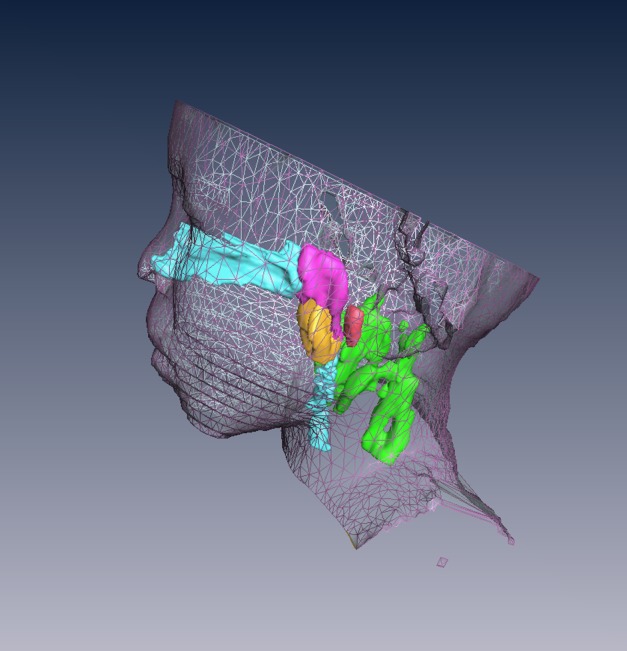
Surface rendering of the head and neck and three-dimensional reconstruction of the upper airway and lymphoid tissues of the subject shown in Figure 1: airway (light blue), adenoid (magenta), tonsils (orange), retropharyngeal nodes (red), and deep cervical lymph nodes (green).
Airway:
We noted a significantly smaller upper airway in the SCD group. In comparison with control subjects, subjects with SCD had an upper airway volume of 2.8 ± 1.2 cm3 compared to 3.7 ± 1.6 cm3 in control subjects (P < .01).
Lymphoid Tissues:
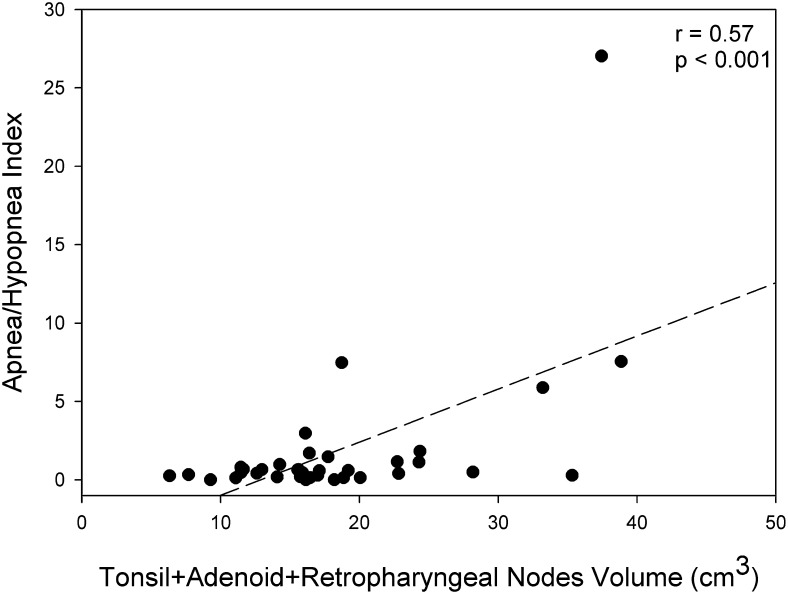
All lymphoid tissues surrounding the upper airway, including adenoid, tonsils, and retropharyngeal nodes, as well as the deep cervical nodes, were significantly larger in children with SCD as compared with control subjects (Table 2). For the SCD groups, we noted the following correlations between AHI and the various lymphoid tissues: adenoid, r = 0.71 (P < .001); retropharyngeal nodes, r = 0.61 (P < .001); tonsils, r = 0.1 (P = not significant [NS]), and deep cervical nodes, r = 0.1 (P = NS). A positive correlation was noted between AHI and the combined volume of the lymphoid tissues surrounding the upper airway (tonsils, adenoid, and retropharyngeal nodes; r = 0.57, P < .001) (Fig 3).
Figure 3.
Correlation between apnea-hypopnea index and the combined upper airway lymphoid tissue volume (cm3) in the group with sickle cell disease.
Polysomnography
Polysomnography data were available for all 36 subjects with SCD and for 20 control subjects and are shown in Table 3. Abnormalities in gas exchange in SCD, including lower baseline Spo2, lower Spo2 nadir, increased baseline etco2, and increased peak etco2, are suggestive of SDB in this group. In addition, subjects with SCD exhibited differences in sleep quality, including decreased sleep efficiency and increased arousals, as compared with control subjects.
Table 3.
—Polysomnography
| Measure | SCD (n = 36) | Control Subjects (n = 20) | P Value |
| Total sleep time, h | 7.3 ± 1.2 | 7.7 ± 0.8 | NS |
| Sleep efficiency, % | 83.7 ± 12.4 | 90.4 ± 5.3 | < .05 |
| Arousal index, events/h | 13.7 ± 4.7 | 10.8 ± 3.8 | < .05 |
| Baseline Spo2, % | 95.3 ± 2.9 | 97.1 ± 0.9 | < .05 |
| Spo2 nadir, % | 84.3 ± 12.3 | 91.1 ± 4.2 | < .05 |
| Baseline etco2, mm Hg | 43.0 ± 3.1 | 37.5 ± 4.6 | < .001 |
| Peak etco2, mm Hg | 53.4 ± 8.5 | 42.3 ± 5.3 | < .001 |
| Obstructive apnea index, events/h | 0.7 ± 2.0 | 0.2 ± 0.3 | NS |
| AHI | 1.9 ± 4.7 | 0.4 ± 0.3 | NS |
| OSAS (AHI ≥ 1.5) | 7 of 36 | 0 of 20 | < .05 |
Data are displayed as mean ± SD. AHI = apnea-hypopnea index; etco2 = end-tidal CO2; OSAS = obstructive sleep apnea syndrome; Spo2 = arterial oxygen saturation. See Table 1 legend for expansion of other abbreviations.
In regard to OSAS, on average the SCD group did not have more obstructive apneas or obstructive hypopneas. However, seven of 36 (19.4%) of the SCD group had an AHI elevated above a suggestive threshold for OSAS as compared with zero of 20 (0%) in the control group (P < .05). These seven subjects with OSAS had a mean obstructive apnea index of 3.0 ± 3.9 events/h (median, 2.5 events/h), a mean AHI of 7.8 ± 8.9 events/h (median, 5.9 events/h), a mean Spo2 nadir of 80.4% ± 8.1% (median, 83%), a mean peak etco2 of 52.7 ± 4.3 mm Hg (median, 54.6 mm Hg), and a mean arousal-awakening index of 14.9 ± 5.1 events/h (median, 15.2 events/h).
Sleep Questionnaire
All control subjects had an OSAS questionnaire score < −1, suggesting that none had evidence of the disorder. In addition, scores in control subjects who had polysomnography were similar to those who did not (−3.0 ± 0.8 vs −3.1 ± 0.9, respectively; P = NS).
Discussion
The current study is the first, to our knowledge, to quantify the upper airway and surrounding lymphoid tissues in children with SCD. Our findings suggest that SCD is associated with a generalized increase in size of upper airway lymphoid tissues and concomitant decrease in upper airway size. We believe the current analysis can explain the high occurrence of OSAS in the subjects with SCD in our study and complements previous studies demonstrating a much higher prevalence of OSAS in subjects with SCD compared with the general population.4‐10
A few methodologic issues deserve comment. First, MRI is considered a reliable and accurate tool to evaluate the upper airway and surrounding soft tissues. However, to minimize movement artifact during imaging, light sedation was provided to all children < 7 years of age. It is possible that sedation altered upper airway dimensions in these children. However, this effect was indeed controlled by studying a similar number of sedated children in each group. Sedation should not have affected the volumetric measurements of the lymphoid tissues. Second, in spite of our attempt to match control subjects to subjects with SCD by demographics and anthropometrics, BMI z score was significantly lower in subjects with SCD. This is probably due to suboptimal growth described in children with SCD.27,28 We believe, however, that this finding of larger lymphoid tissues in the SCD group despite having a lower BMI z score further enhances our findings. Third, we studied subjects at steady state and excluded those requiring chronic blood transfusion or hydroxyurea or those with episodes of acute chest syndrome or pain episodes within 4 weeks of the study.
Our hypothesis considered existence of general lymphoid hypertrophy in proximity to the upper airway in children with SCD. Therefore, our analysis was not limited to the adenoid and tonsils but also included the retropharyngeal nodes. The retropharyngeal nodes are located posterior to the pharyngeal constrictor muscles and between the internal carotid arteries from the base of the skull to the hyoid bone. These nodes may contribute to upper airway restriction and risk for OSAS when they are enlarged, in a similar way to the adenoid and tonsils.29 This point is not well documented in the literature since these nodes are not visible on examination and are not routinely evaluated by radiographic measures. Our findings of larger adenoid, tonsils, and retropharyngeal nodes restricting the upper airway could explain the propensity of children with SCD to have OSAS. This is also supported by the demonstration of a positive correlation between the AHI and the size of lymphoid tissues surrounding the upper airway.
The large deep cervical nodes noted in these subjects should not directly impact upper airway size because of their more distant location from the airway. However, the finding that the size of deep cervical nodes is also increased in SCD lends support to the observation of overall lymphoid hypertrophy in these children.
The main polysomnographic findings in our study included abnormalities in gas exchange in subjects with SCD; these findings are not considered specific and may represent abnormalities in ventilation/perfusion ratio, hypoventilation, or abnormalities in gas diffusion. However, the more specific finding is of OSAS in 19% (seven of 36), which supports other studies evaluating the increased incidence of SDB and OSAS in this population.4‐10
Three important weaknesses related to our study should be mentioned. First, control subjects were excluded if any had a history of OSAS and/or previously underwent adenotonsillectomy. Thus, a possible selection bias may have been introduced by such criteria. However, we do not think this should significantly affect our results, assuming a background prevalence of 2% of children in this age group with OSAS.
Second, not all control subjects who had an upper airway MRI agreed to have polysomnography. For this reason, we introduced a standardized sleep questionnaire to exclude the possibility of OSAS in any of the 16 additional control subjects. Interestingly, a subgroup analysis comparing the 36 subjects with SCD to the 20 control subjects who agreed to have polysomnography demonstrated similar findings to our primary analysis. The SCD group had a smaller upper airway (P = .0005) and larger tonsils (P = .03). However, despite the fact that the adenoid and deep cervical nodes were identical in size to the primary analysis, the P values only approached significance (P = .08 and P = .06, respectively). The latter finding may reflect the smaller sample size within this subanalysis.
Third, in this study we focused on upper airway lymphoid tissues as the main cause for OSAS in SCD. However, it is possible that children with SCD have other anatomic abnormalities, such as those affecting their craniofacial structure particularly due to extramedullary hematopoiesis,30 and/or functional factors that may increase upper airway collapsibility,31 thereby increasing their risk for SDB or OSAS. Thus, additional studies are warranted to elucidate such potential contributors in this population.
Although the reason for upper airway lymphoid hypertrophy in SCD remains unknown, it has been speculated that it may result from hematopoietic lymphoid compensation secondary to functional asplenia.4,6,9 In this regard, we would like to point out that in SCD, chronic hemolysis and vasoocclusive events are associated with local and systemic inflammatory responses to oxidative stress, ischemia-reperfusion injury, and release of NO.32‐34 Similar pathways of oxidative stress reaction have been noted in children without SCD who have SDB and/or OSAS with adenotonsillar hypertrophy.35‐39 Thus, we propose that similar mechanisms of inflammatory response that occur in children with SCD may at least partly explain their upper airway lymphoid hypertrophy.
In conclusion, we suggest that children with SCD should be screened for OSAS by an appropriate questionnaire and physical examination because of their risk of developing adenotonsillar hypertrophy. Adenotonsillectomy should be considered once diagnosis of OSAS is confirmed to reduce the deleterious effects of the disorder in this particular group.
Acknowledgments
Author contributions: Dr Arens had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ms Strauss: contributed to performing research, contributed new analytical tools, analyzed data, and wrote the manuscript.
Mr Sin: contributed to performing research, contributed new analytical tools, analyzed data, and wrote the manuscript.
Dr Marcus: contributed to designing research, performed research, analyzed data, and wrote the manuscript.
Dr Mason: contributed to designing research, performed research, analyzed data, and wrote the manuscript.
Mr McDonough: contributed to designing research, analyzed data, and wrote the manuscript.
Dr Allen: contributed to designing research, performed research, and wrote the manuscript.
Dr Caboot: contributed to designing research, performed research, and analyzed data.
Dr Bowdre: contributed to performing research and wrote the manuscript.
Dr Jawad: contributed to designing research, analyzed data, and wrote the manuscript.
Dr Smith-Whitley: contributed to designing research, performed research, and wrote the manuscript.
Dr Ohene-Frempong: contributed to designing research, performed research, and wrote the manuscript.
Dr Pack: contributed to designing research, contributed new analytical tools, and wrote the manuscript.
Dr Arens: contributed to designing research, performed research, contributed new analytical tools, analyzed data, and wrote the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Marcus has funding from Phillips Respironics (not related to the current project) and Dr Pack received an endowed chair from the Respironics Foundation (not related to the current project). Ms Strauss, Messrs Sin and McDonough, and Drs Mason, Allen, Caboot, Bowdre, Jawad, Smith-Whitley, Ohene-Frempong, and Arens have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at the Children’s Hospital of Philadelphia and Children’s Hospital at Montefiore.
Abbreviations
- AHI
apnea-hypopnea index
- CHOP
Children’s Hospital of Philadelphia
- etco2
end-tidal CO2
- NS
not significant
- OSAS
obstructive sleep apnea syndrome
- SCD
sickle cell disease
- SDB
sleep-disordered breathing
- Spo2
arterial oxygen saturation
Footnotes
Funding/Support: Funded by National Institutes of Health [Grants HL-62408 and HL-79911].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Davies SC, Stebbens VA, Samuels MP, Southall DP. Upper airways obstruction and cerebrovascular accident in children with sickle cell anaemia. Lancet. 1989;2(8657):283-284 [DOI] [PubMed] [Google Scholar]
- 2.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357(9269):1656-1659 [DOI] [PubMed] [Google Scholar]
- 3.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362(9394):1450-1455 [DOI] [PubMed] [Google Scholar]
- 4.Brooks LJ, Koziol SM, Chiarucci KM, Berman BW. Does sleep-disordered breathing contribute to the clinical severity of sickle cell anemia? J Pediatr Hematol Oncol. 1996;18(2):135-139 [DOI] [PubMed] [Google Scholar]
- 5.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659-665 [DOI] [PubMed] [Google Scholar]
- 6.Maddern BR, Reed HT, Ohene-Frempong K, Beckerman RC. Obstructive sleep apnea syndrome in sickle cell disease. Ann Otol Rhinol Laryngol. 1989;98(3):174-178 [DOI] [PubMed] [Google Scholar]
- 7.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6(4):374-381 [PMC free article] [PubMed] [Google Scholar]
- 8.Salles C, Ramos RT, Daltro C, Barral A, Marinho JM, Matos MA. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35(11):1075-1083 [DOI] [PubMed] [Google Scholar]
- 9.Samuels MP, Stebbens VA, Davies SC, Picton-Jones E, Southall DP. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67(7):925-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spivey JF, Uong EC, Strunk R, Boslaugh SE, DeBaun MR. Low daytime pulse oximetry reading is associated with nocturnal desaturation and obstructive sleep apnea in children with sickle cell anemia. Pediatr Blood Cancer. 2008;50(2):359-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minter KR, Gladwin MT. Pulmonary complications of sickle cell anemia. A need for increased recognition, treatment, and research. Am J Respir Crit Care Med. 2001;164(11):2016-2019 [DOI] [PubMed] [Google Scholar]
- 13.Vichinsky EP, Neumayr LD, Earles AN, et al. National Acute Chest Syndrome Study Group Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342(25):1855-1865 [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866-878 [DOI] [PubMed] [Google Scholar]
- 15.Schechter MS.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):e69. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MC, Kirkham FJ, Redline S, et al. Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood. 2010;116(1):16-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onyekwere OC, Campbell A, Teshome M, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29(2):309-312 [DOI] [PubMed] [Google Scholar]
- 18.Needleman JP, Setty BN, Varlotta L, Dampier C, Allen JL. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28(6):423-428 [DOI] [PubMed] [Google Scholar]
- 19.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704-712 [DOI] [PubMed] [Google Scholar]
- 20.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27(5):997-1019 [DOI] [PubMed] [Google Scholar]
- 21.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164(4):698-703 [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 23.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22-30 [DOI] [PubMed] [Google Scholar]
- 24.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125(3):872-878 [DOI] [PubMed] [Google Scholar]
- 25.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168(12):1540. [DOI] [PubMed] [Google Scholar]
- 26.Brouilette R, Hanson D, David R, et al. A diagnostic approach to suspected obstructive sleep apnea in children. J Pediatr. 1984;105(1):10-14 [DOI] [PubMed] [Google Scholar]
- 27.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61(5 pt 1):607-613 [DOI] [PubMed] [Google Scholar]
- 28.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311(1):7-12 [DOI] [PubMed] [Google Scholar]
- 29.Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2011;183(6):782-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins WO, Younis RT, Garcia MT. Extramedullary hematopoiesis of the paranasal sinuses in sickle cell disease. Otolaryngol Head Neck Surg. 2005;132(6):954-956 [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Pinto SJ, Allen JL, et al. Upper airway genioglossal activity in children with sickle cell disease. Sleep. 2011;34(6):773-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagar W, Vichinsky E. Advances in clinical research in sickle cell disease. Br J Haematol. 2008;141(3):346-356 [DOI] [PubMed] [Google Scholar]
- 33.Krishnan S, Setty Y, Betal SG, et al. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. Br J Haematol. 2010;148(5):797-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu X, Nouraie M, Campbell A, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PLoS ONE. 2009;4(11):e7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172(3):364-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25(1):59-65 [DOI] [PubMed] [Google Scholar]
- 37.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35-51 [DOI] [PubMed] [Google Scholar]
- 39.Tauman R, Ivanenko A, O’Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113(6):e564-e569 [DOI] [PubMed] [Google Scholar]