Abstract
Legume biological nitrogen (N) fixation is the most important N source in agroecosystems, but it is also a process requiring a considerable amount of phosphorus (P). Therefore, developing legume varieties with effective N2 fixation under P-limited conditions could have profound significance for improving agricultural sustainability. We show here that inoculation with effective rhizobial strains enhanced soybean (Glycine max) N2 fixation and P nutrition in the field as well as in hydroponics. Furthermore, we identified and characterized a nodule high-affinity phosphate (Pi) transporter gene, GmPT5, whose expression was elevated in response to low P. Yeast heterologous expression verified that GmPT5 was indeed a high-affinity Pi transporter. Localization of GmPT5 expression based on β-glucuronidase staining in soybean composite plants with transgenic roots and nodules showed that GmPT5 expression occurred principally in the junction area between roots and young nodules and in the nodule vascular bundles for juvenile and mature nodules, implying that GmPT5 might function in transporting Pi from the root vascular system into nodules. Overexpression or knockdown of GmPT5 in transgenic composite soybean plants altered nodulation and plant growth performance, which was partially dependent on P supply. Through both in situ and in vitro 33P uptake assays using transgenic soybean roots and nodules, we demonstrated that GmPT5 mainly functions in transporting Pi from roots to nodules, especially under P-limited conditions. We conclude that the high-affinity Pi transporter, GmPT5, controls Pi entry from roots to nodules, is critical for maintaining Pi homeostasis in nodules, and subsequently regulates soybean nodulation and growth performance.
Legume crops are important food, nutrition, and energy sources, accounting for over 30% of crop yields in the world (Graham and Vance, 2003). Most legumes form symbioses with soil nitrogen (N)-fixing bacteria (Lindström et al., 2010). The amount of organic N produced in legume-rhizobia symbiosis totals 20 to 22 million tons each year (Herridge et al., 2008). According to the FAO (http://faostat.fao.org/), the average proportion of crop N derived from atmospheric N2 is nearly 70% worldwide; therefore, legume N2 fixation is considered the most important N source in the agroecosystems.
Besides N, phosphorus (P) is also an essential macronutrient for plants, which is particularly critical for legumes due to the huge demands for P in protein and fat synthesis and energy consumption for N2 fixation (Vance et al., 2003). However, low P availability is a global problem limiting agriculture, because of high P fixation rates by soil particles even under conditions where total soil P levels are reasonably high (Tiessen, 2008; Sánchez-Calderón et al., 2010). To reduce N and P deficiencies and maintain crop productivity, nearly 100 million tons of N and 40 million tons of P fertilizers are estimated to be applied every year. Yet, up to 60% and 80% of the applied N and P fertilizer, respectively, might not be absorbed by crops due to the low use efficiencies (http://faostat.fao.org/). Unlike N, mineral P sources are nonrenewable, and high-grade rock phosphate (Pi) minerals are expected to be depleted in the near future (Cordell et al., 2009). With the growing global population, the demand for food and energy is increasing, and intensive large-scale fertilization and excessive consumption of natural resources has led to many environmental problems (Domagalski et al., 2007). Developing legume varieties with effective N2 fixation under P-limited conditions will be important to reduce N and P fertilization and enhance agricultural sustainability.
P deficiency severely inhibits both nodule growth and N2-fixing capacity (Chaudhary et al., 2008; Hernández et al., 2009). In order to maintain growth and high N2 fixation rate, P levels need to be maintained in the nodules. In the literature, there is only one report investigating the sources of P for nodules in legumes (Al-Niemi et al., 1998). Using the 32P labeling approach, two pathways of Pi entry into nodules have been demonstrated. One is the direct uptake pathway, where the Pi is directly absorbed by nodules from the growth medium. The second pathway is indirect and involves Pi translocation from the roots of host plants to nodules. However, no further physiological and/or molecular studies on how legumes facilitate and maintain Pi homeostasis in nodules have been reported.
P is transported into and within plants mainly via Pi transporters coupled to the proton gradient generated by plasma membrane H+-ATPase (Ullrich-Eberius et al., 1981). Induction of gene expression encoding high-affinity Pi transporters is one strategy plants use to adapt to low-P soils (Raghothama, 1999). Among the Pi transporter families, the Pht1 family has been most widely studied due to its key roles in Pi acquisition from the soil and Pi translocation within the plants. Most Pht1 family members are root-specific Pi transporters expressed in root epidermal cells (Mudge et al., 2002; Rae et al., 2003) or cortical cells after arbuscular mycorrhizal colonization (Nagy et al., 2005). To the best of our knowledge, none of the Pi transporters have been reported to be involved in the P nutrition of the legume-rhizobia symbiosis system.
Soybean (Glycine max) is one of the most widely grown leguminous crops, comprising approximately 68% of global crop legume production and 57% of world oilseed production (Herridge et al., 2008). Its global production has doubled over the past 20 years (http://faostat.fao.org/). Soybean also has a superior capacity to fix atmospheric N2 via soil rhizobia. In Brazil, over 70% of the N required for soybean growth is derived primarily from symbiotic N2 fixation (Peoples et al., 2009). Furthermore, a large proportion of the N2 fixed by nodules in soybean is available for the growth of subsequent crops in rotation systems. Therefore, the soybean-rhizobia symbiosis is an efficient way to sustain agricultural development due to its superior N2 fixation, reducing the dependence on N fertilizers and thereby avoiding the overexploitation of natural resources. However, several environmental factors limit soybean nodulation and production, especially low P availability in soils (Kantar et al., 2010). Therefore, understanding the detailed mechanisms of Pi homeostasis and the possible roles Pi transporter genes play in nodule P nutrition could assist in the development of new approaches to enhance biological N2 fixation under P-limited conditions in soybean as well as in other legumes.
Here, we report that effective nodulation is important not only for N2 fixation but also for P nutrition in soybean in both field and hydroponic studies. Furthermore, we identify a nodule-expressed and low-P-enhanced soybean gene, GmPT5, which encodes a high-affinity Pi transporter. Overexpression or knockdown of GmPT5 in transgenic composite soybean plants altered nodulation and Pi transport from roots to nodules when [33P]Pi was supplied to the roots and subsequently affected N2 fixation and crop growth performance.
RESULTS
Enhancement of Soybean Yield, Nodulation, and Contents of N and P in the Field
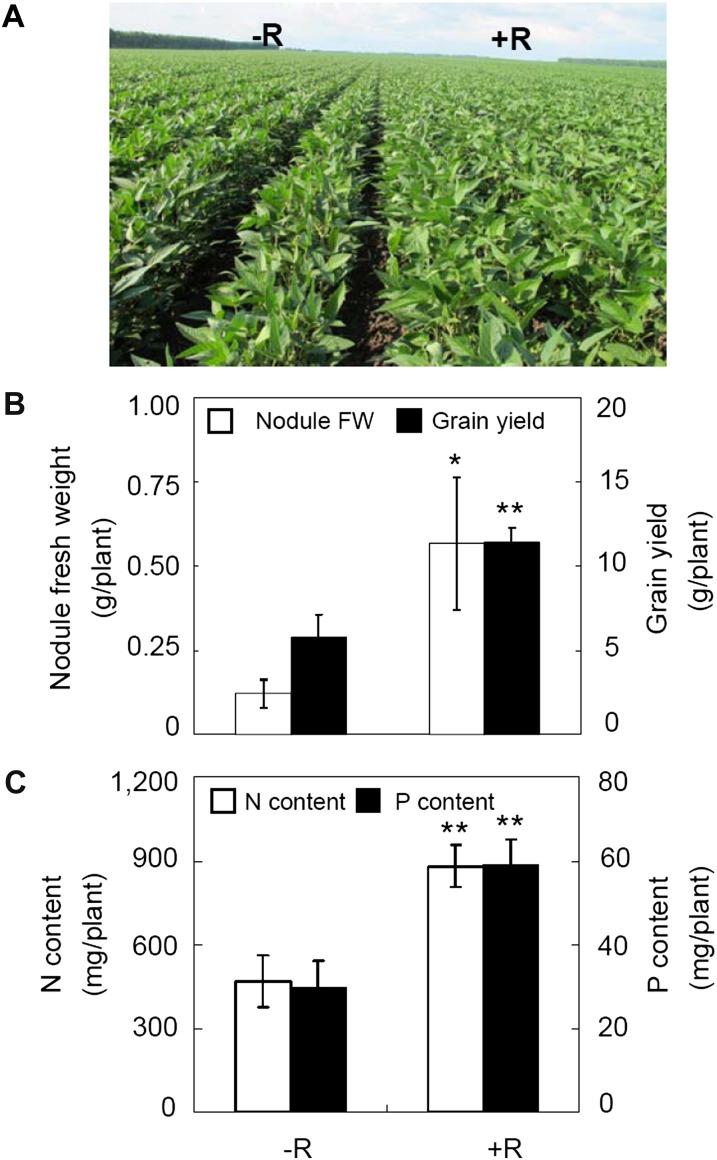
Significant enhancement of soybean growth was observed after inoculation with effective rhizobium strains in the field (Fig. 1A). Soybean yields were increased 92% after inoculation, which coincided with a 4.5-fold increase in nodule fresh weight per plant at 50 d after emergence (Fig. 1B). Meanwhile, the total N and P contents of plants at final harvest were increased after inoculation by 85% and 95%, respectively (Fig. 1C), indicating that effective nodulation not only increases N efficiency through symbiotic N2 fixation but also enhances P uptake, which could subsequently contribute to improve soybean yield.
Figure 1.
Enhancement of yield, nodule fresh weight, and N and P contents in the field by inoculation with effective rhizobium strains in soybean. −R, Not inoculated, +R, inoculated with rhizobia. A, Photograph showing soybean growth performance at 50 d after emergence. B, Nodule fresh weight (FW) and grain yield. C, Plant N and P contents. There were five replicates for each treatment and three plants for each replicate. Bars show means ± se. Asterisks indicate significant differences of the same trait between −R and +R in t tests: * P < 0.05, ** 0.001 < P < 0.01.
Plant Growth, Nodulation, and Soluble Pi Concentration in Hydroponics
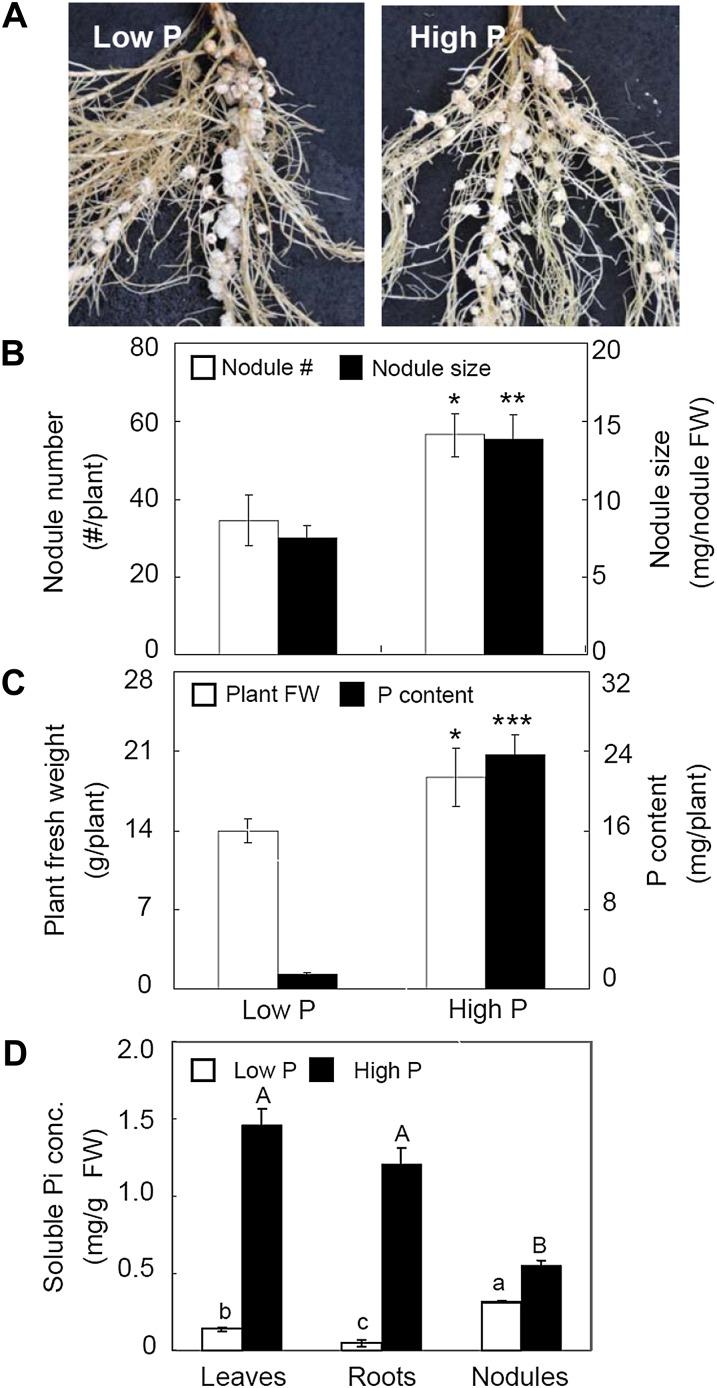
In hydroponic studies, P supply significantly affected soybean growth, nodulation, and Pi concentrations in different tissues (Fig. 2). Sufficient P supply promoted soybean growth. Compared with the total P content, soybean fresh weight was much less affected by the changes of P supply (Fig. 2C). Soybean nodulation was significantly enhanced by high P supply, as indicated by 63% and 85% increases in nodule number and nodule size compared with plants in low P, respectively (Fig. 2, A and B). Regardless of the large fluctuations in Pi status of leaves and roots associated with changes in P supply, soybean nodules maintained relatively stable Pi concentrations. Leaf and root soluble Pi concentrations in high P were 10 and 26 times higher than in low P, respectively, while the Pi concentration in nodules was only increased 1.75 times in high P versus low P (Fig. 2D). This suggests that stabilizing Pi homeostasis in nodules under P-deficient conditions might be important for legume growth and symbiotic N2 fixation.
Figure 2.
Plant growth, nodulation, and soluble Pi concentration as affected by P supply in hydroponics. A, Photographs showing nodule growth performance. B, Nodule number and size. C, Plant fresh weight (FW) and P content. D, Soluble Pi concentrations of leaves, roots, and nodules. Seedlings inoculated with rhizobia were grown at two P levels (low P and high P were 5 and 250 μm P, respectively) for 50 d after planting. There were four replicates for each treatment, and bars show means ± se. Asterisks in B and C indicate significant difference of the same trait between two P levels in t tests: * P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001. Different letters in D indicate that Pi concentrations in different tissues were significantly different (P < 0.05).
Identification of a Nodule-Expressed and Low-P-Enhanced Pi Transporter Gene, GmPT5
In order to understand the underlying mechanisms for the maintenance of soybean Pi homeostasis in nodules under varying P supply, we cloned the members of the soybean Pht1 family and characterized the expression of each in response to varied P supply. A total of 14 putative Pht1 family members (GmPTs) were identified in soybean (Supplemental Fig. S1). Among them, only one member, GmPT5, exhibited high transcript abundance that was enhanced up to 22-fold in nodules in response to P deficiency (Supplemental Fig. S2). GmPT5 was predicted to be localized to the plasma membrane through WoLF PSORT analysis. Results from onion (Allium cepa) epidermal cells with a GFP reporter gene fused to the GmPT5 coding region showed that GmPT5 was localized to the plasma membrane (Supplemental Fig. S3), suggesting that GmPT5 mediates Pi transport across the plasma membrane.
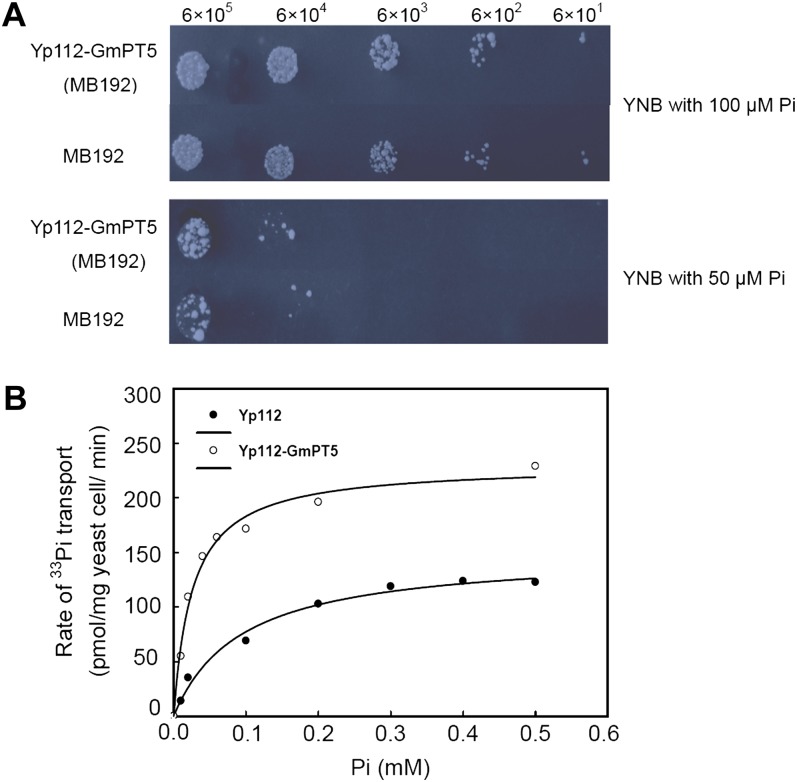
In order to study the Pi transport properties of GmPT5, the coding region was cloned into a yeast expression vector and transferred into a yeast mutant, MB192, which is defective in high-affinity Pi uptake. The mutant cells expressing GmPT5 (Yp112-GmPT5) grew much better than the MB192 mutant cells, especially in low P (50 μm; Fig. 3A), suggesting that GmPT5 functions as a high-affinity Pi transporter. This was directly investigated by using a 33P influx technique in yeast. As seen in Figure 3B, the concentration-dependent kinetics for yeast Pi uptake by GmPT5 followed Michaelis-Menten kinetics, with a Km of 25 μm and a Vmax of 231 pmol Pi mg−1 yeast cell min−1, which demonstrates that, indeed, GmPT5 is a high-affinity Pi transporter.
Figure 3.
Functional characterization of GmPT5 in yeast. MB192 is a yeast mutant defective in high-affinity Pi uptake. Yp112-GmPT5 contained GmPT5 in the expression vector p112A1NE (abbreviated Yp112) and transformed into MB192. A, Growth of yeast cells. Equal volumes of 10-fold serial dilution with the original cell number of 6 × 105 were applied to YNB medium supplied with 100 or 50 μm Pi and then incubated at 30°C for 3 d. B, Rate of [33P]Pi transport by Yp112-GmPT5 and Yp112 at different Pi concentrations. Nonlinear regression of Pi uptake of strain Yp112-GmPT5 and Yp112 versus external Pi concentration at pH 6 was used to estimate the Km value.
GmPT5 Expression Pattern in Soybean Nodules
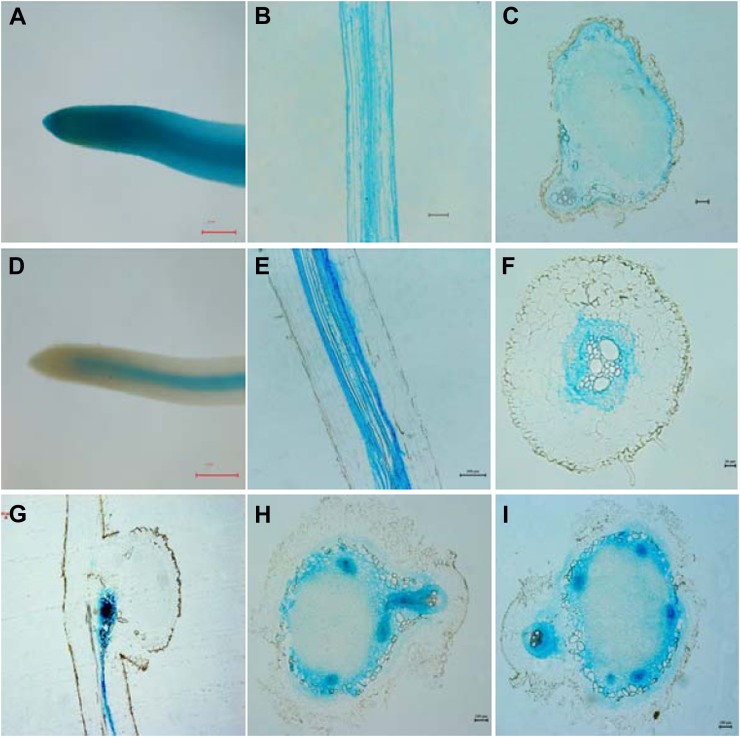
Without rhizobial inoculation, clear GUS staining was detected in the root vascular tissues of transgenic plants with hairy roots expressing proGmPT5::GUS (Fig. 4, D–F). Fifteen days after inoculation, strong GmPT5 expression based on GUS staining was observed within the conjunction region between nodule initials and roots and root vascular tissues (Fig. 4G). Thirty days after inoculation, strong GmPT5 expression was mainly observed in the vascular bundles within the nodule peripheral zone of juvenile and mature nodules (Fig. 4, H and I). This suggests that GmPT5 functions in Pi translocation between host roots and nodules in vascular tissues.
Figure 4.
Observation of GUS staining in transgenic soybean roots and nodules harboring the GmPT5 promoter::GUS fusion. A to C, Expression of empty vector with 35S promoter in roots (A and B) and nodules (C). d to F, Expression of proGmPT5::GUS in roots without rhizobium inoculation. D, Root tips. E and F, Longitudinal section (E) and cross-section (F) of mature roots. G to I, Expression of proGmPT5::GUS in nodules. G, Conjunction region between nodule initial and root at an early stage of nodulation. H and I, Intermediate (H) and mature (I) nodules. Soybean transgenic plants were grown in low-N and low-P nutrient solution for 15 d (G) and 30 d (A–F, H, and I). Bars = 500 μm for A and D, 20 μm for F and G, and 100 μm for all other images.
Overexpression and Suppression of GmPT5 Significantly Affected Soybean Growth and Nodulation
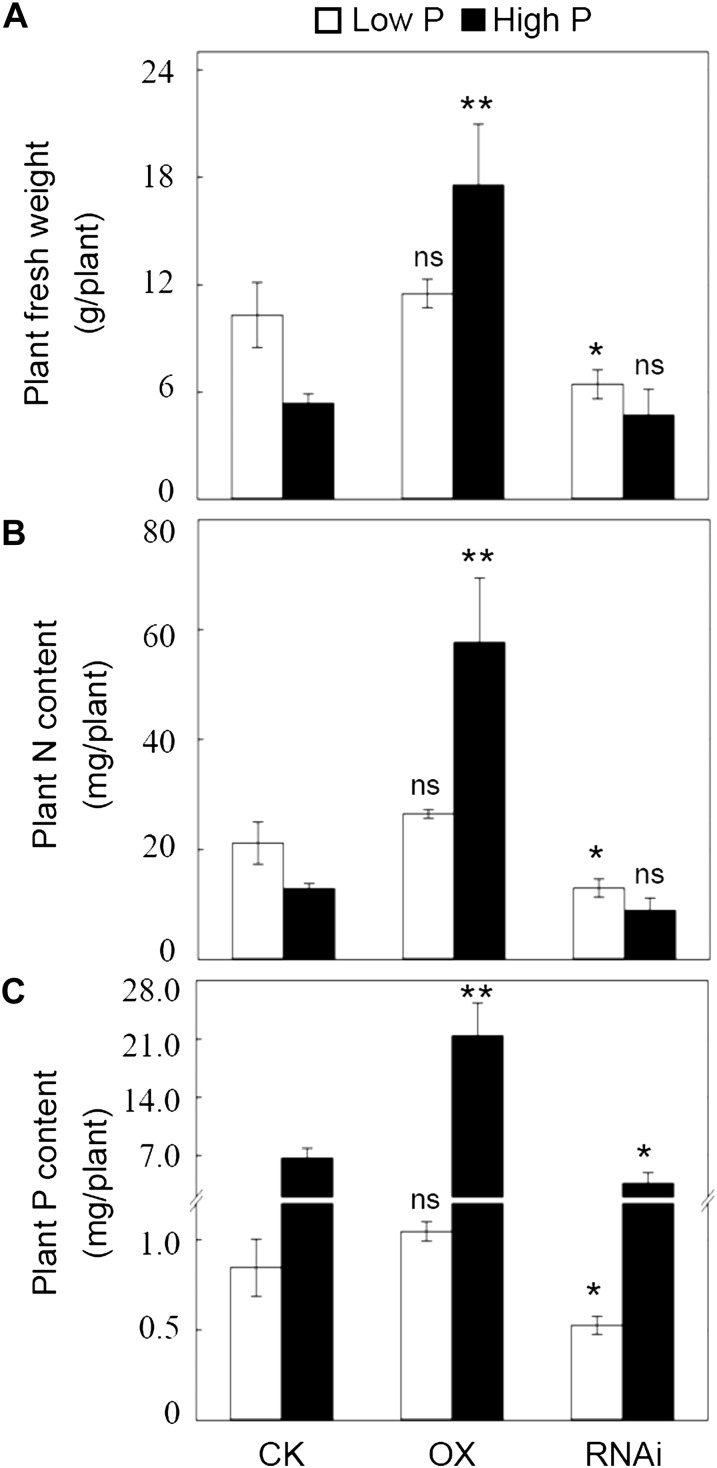
To further evaluate the effects of GmPT5 on soybean growth and nodulation, transgenic plants either overexpressing (OX) or suppressing (RNA interference [RNAi]) GmPT5 were generated, and the corresponding transcripts of GmPT5 in OX and RNAi lines at low P and high P levels were verified by quantitative real-time (qRT)-PCR. The OX lines had 65% and 178% higher, while the RNAi lines had 92% and 62% lower, expression of GmPT5 than that of empty vector (CK) lines in low P and high P, respectively (Supplemental Fig. S4).
As seen in Figure 5A, overexpression of GmPT5 significantly enhanced soybean growth in high P, with over 3.2 times increase of fresh weight compared with CK, while suppression of GmPT5 only inhibited soybean growth in low P, as indicated by 37% decrease in fresh weight compared with CK (Fig. 5, B and C). Similar effects were found on plant N and P contents. Overexpression of GmPT5 significantly increased plant N and P contents in high P, but not in low P, while knockdown of GmPT5 reduced plant P content at both P levels but only reduced plant N content in low P (Fig. 5, B and C).
Figure 5.
Effects of OX and RNAi lines of GmPT5 on soybean growth and N and P contents. A, Plant fresh weight. B, Plant N content. C, Plant P content. Soybean transgenic plants inoculated with rhizobia were grown in the low-N nutrient solution with low P (5 μm P) and high P (250 μm P) for 50 d. CK, Soybean transgenic plants carrying empty vector. There were four biological replicates for each treatment, and bars show means ± se. Asterisks indicate significant differences of the same trait between OX or RNAi and CK at the same P level in t tests: * P < 0.05, ** 0.001 < P < 0.01; ns, not significant at 0.05.
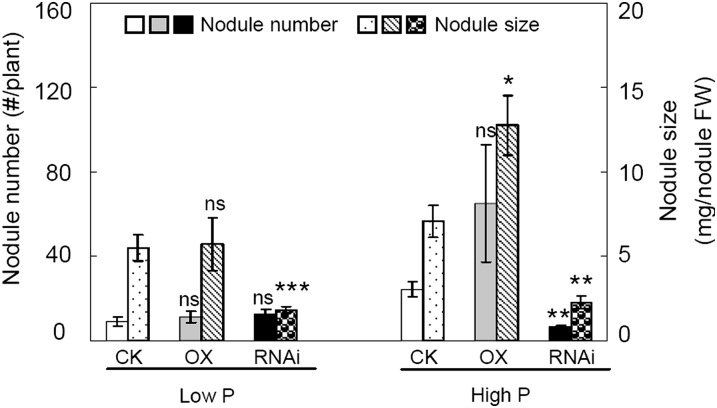
The results in Figure 6 revealed that GmPT5 transcript levels significantly affected nodulation, and this effect was partially dependent on P supply. In low P, knockdown of GmPT5 suppressed nodule growth, as indicated by 67% and 66% reductions in nodule size compared with CK and OX lines, respectively. In high P, both nodule number and size were significantly reduced in the RNAi lines, while only nodule size was enhanced in the OX lines.
Figure 6.
Effects of overexpression (OX) and knockdown (RNAi) of GmPT5 on the nodulation of soybean transgenic composite plants. Soybean transgenic composite plants inoculated with rhizobia were grown in the low-N nutrient solution with low P (5 μm P) or high P (250 μm P) for 50 d. CK, Soybean transgenic composite plants carrying empty vector. There were four biological replicates for each treatment, and bars show means ± se. Asterisks indicate significant differences of the same trait between OX or RNAi and CK at the same P level in t tests: * P < 0.05, ** 0.001 < P < 0.01, *** P < 0.001; ns, not significant at 0.05. FW, Fresh weight.
Altered Expression of GmPT5 Changes Pi Translocation from Roots to Nodules
To determine the roles of GmPT5 on nodule Pi translocation and uptake, GmPT5 transgenic plants were grown in low P and high P for 50 d for in situ [33P]Pi transport assays. Transgenic plants were first monitored during an 8-h uptake period when supplied with 10 μCi of H333PO4 solution. Neither the [33P]Pi activity in the upper root regions not exposed to 33P-labeled solution (Supplemental Fig. S6A) nor the bottom 6 cm of the root regions that were directly exposed to 33P-labeled solution significantly varied in transgenic soybean lines pretreated at two P levels (Supplemental Fig. S6B). This indicates that GmPT5 expression does not significantly affect direct Pi uptake by roots or internal Pi translocation within roots.
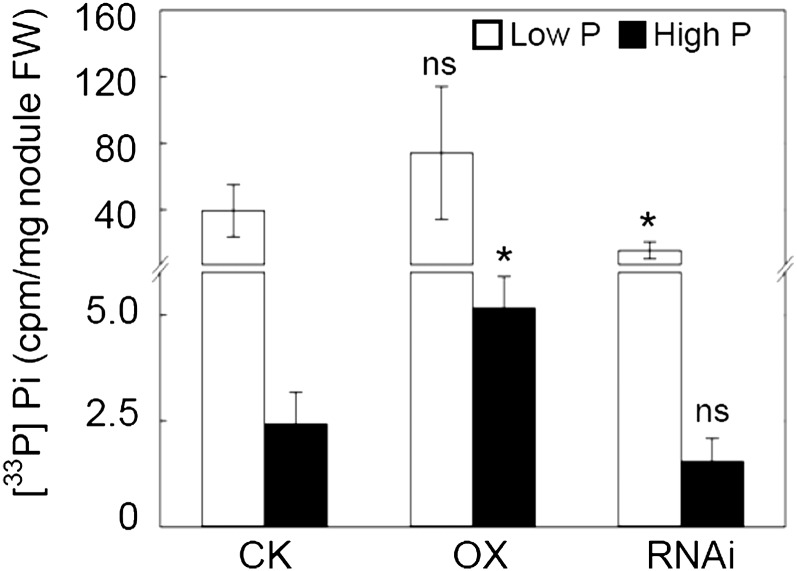
In low P, the [33P]Pi activity in nodules not exposed to the 33P-labeled solution was more than 20-fold higher than similar nodules in high P (Fig. 7), suggesting that pretreatment with low P increases the capacity for Pi transport from roots to nodules. Furthermore, GmPT5-OX transgenic lines in high P with 112% higher [33P]Pi activity and GmPT5-RNAi lines in low P with 60% lower [33P]Pi activity displayed significant differences in nodule [33P]Pi activities compared with CK lines (Fig. 7). This suggests that altered expression of GmPT5 regulates at least in part Pi translocation from roots to nodules, and this activity is partially dependent on P supply.
Figure 7.
In situ assay for radioactive [33P]Pi translocation from roots to nodules in transgenic soybean plants. Transgenic soybean plants inoculated with rhizobia were grown in the low-N nutrient solution with low P (5 μm P) or high P (250 μm P) for 50 d before being used in in situ assays. The bottom 6 cm of root regions were directly immersed in 33P-labeled nutrient solution for an 8-h uptake period. [33P]Pi accumulation in the nodules that were not exposed to the 33P-labeled nutrient solution was quantified at both low P and high P levels. cpm represents radioactive cpm measured by a liquid scintillation analyzer. CK, Transgenic soybean plants carrying empty vector; FW, fresh weight. There were four biological replicates for each treatment, and bars show means ± se. Asterisks indicate significant differences between OX or RNAi and CK at the same P level in t tests: * P < 0.05; ns, not significant at 0.05.
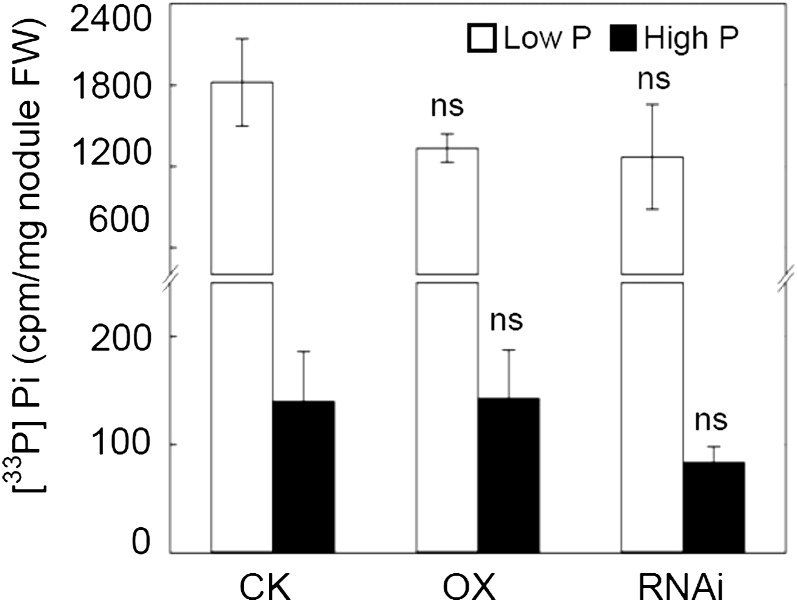
Direct [33P]Pi absorption by nodules was further evaluated using in vitro assays. The results showed that nodules could directly take up Pi from the growth medium, with the direct [33P]Pi uptake by nodules when supplied with 0.25 μCi of H333PO4 after plants were grown in low P for 50 d being more than 10-fold higher than when grown in high P. This indicates that soybean nodules have great capacities to take up Pi from the environment, especially after long-term growth in Pi starvation conditions. However, direct [33P]Pi uptake by nodules was not affected in either OX or RNAi lines (Fig. 8), which suggest that GmPT5 expression does not significantly affect direct Pi uptake by nodules.
Figure 8.
In vitro assays for radioactive [33P]Pi directly taken up by nodules in GmPT5 transgenic soybean plants. Transgenic soybean plants inoculated with rhizobia were grown in low-N nutrient solution with low P (5 μm P) or high P (250 μm P) for 50 d before being used in in vitro assays. Three nodules from each transgenic line pretreated by low P or high P for 50 d were immersed in the same 33P-labeled nutrient solution for a 2-h uptake assay. [33P]Pi accumulation in nodules was quantified at both low P and high P levels. cpm represent radioactive cpm measured by a liquid scintillation analyzer. CK, Transgenic soybean plants carrying empty vector; FW, fresh weight. There were four biological replicates for each treatment, and bars show means ± se. ns, Not significant at 0.05.
DISCUSSION
N and P are the two most important macronutrients for plant growth. Since most soils cannot supply sufficient N and P to meet the demands of crop production, N and P chemical fertilizers account for at least 90% of total fertilizer consumption in the world (http://faostat.fao.org/). As an environment-friendly N source, biologically fixed N2 by legumes plays vital roles in sustainable agricultural systems. However, P is particularly critical for legumes due to their high demands for this nutrient (Sánchez-Calderón et al., 2010). Therefore, the ability to improve N2 fixation with limited P supply in legumes should have a great impact on agricultural sustainability.
Legumes are high-P-demanding species, not only due to their growth requirements and synthesis of high levels of protein and oil in seeds but also because of the strong demand for P by nodules (Schulze et al., 2006). A primary reason for the high P requirement of nodules is the high energy consumption of nodule N2 fixation (Sa and Israel, 1991), thereby supporting elevated synthesis and metabolic activity (Gaude et al., 2004). It is well known that low P limits nodulation and nodule growth (Kouas et al., 2005; Le Roux et al., 2009). Nodules are strong P sinks and maintain higher Pi concentrations than other organs, which has been suggested to play a role in legume adaptation to low-P soils (Al-Niemi et al., 1997; Schulze et al., 2006). Our studies in hydroponics also demonstrate that Pi homeostasis in nodules is critical for legume growth and symbiotic N2 fixation, especially under P-deficient conditions (Fig. 2). Even though low P severely inhibits nodule biomass and number, the Pi concentration in nodules is still much higher than in roots and leaves, and the relative Pi concentration in nodules without P supply was more stable than in leaves and roots (Fig. 2D), suggesting that stabilization of Pi homeostasis in soybean nodules supports the stronger P demand of nodule growth and N2 fixation (Jakobsen, 1985) and further improves the N2 fixation rate and growth performance of nodulated plants, as we found in the field (Fig. 1).
The maintenance of Pi homeostasis in plants under P-deficient conditions is regulated by enhanced acquisition of external Pi and by the remobilization of internal Pi resources (Raghothama, 1999). Plant Pi transporters involved in Pi homeostasis regulation have been documented in previous reports, and these Pi transporters are primarily responsible for translocation of Pi from roots to shoots or from the elder parts to sink tissues (Daram et al., 1999; Jia et al., 2011; Nagarajan et al., 2011; Sun et al., 2012). But there is no report on legume Pi transporters involved in maintaining Pi homeostasis in nodules. Recently, most studies on soybean nodules have analyzed transcriptome and protein profiles of low-P-stressed nodules or bacteroids within nodules, identifying some nodule-specific genes and proteins involved in carbon and N metabolism or genes related to transcription and signaling (Hernández et al., 2009; Chen et al., 2011), but no nodule-specific Pi transporter has been found in soybean. As a strong Pi sink, especially under P-limiting conditions, the import of Pi from other organs has been speculated to be important to nodule P nutrition. In this study, we identified a nodule-expressed and low-P-enhanced Pi transporter gene, GmPT5, in soybean. Bioinformatic analysis together with heterologous expression in yeast and onion epidermal cells verifies that GmPT5 is indeed a high-affinity plasma membrane-localized Pi transporter (Fig. 3; Supplemental Figs. S1 and S3).
Further functional analysis using transgenic soybean composite plants demonstrates that GmPT5 plays vital roles in Pi entry from roots to nodules in soybean. First, GmPT5 expression was shown here to be primarily in the root vascular system, not in root tips and epidermal cells (Fig. 4, D–F), suggesting that GmPT5 might function more in Pi translocation within plants. At the early stage of nodule initiation, strong GmPT5 expression via GUS staining was detected in the junction area between roots and nodules. For juvenile and mature nodules, GmPT5 expression occurred principally in the nodule vascular bundles (Fig. 4, G–I), implying that GmPT5 might help transport Pi from roots into nodules for nodule growth and N2 fixation. This was further proven by in situ [33P]Pi uptake using transgenic soybean plants. Overexpression of GmPT5 in high P or knockdown of GmPT5 in low P increased or decreased [33P]Pi activity in nodules, which appears to be the result of Pi transported from the root regions exposed to [33P]Pi solution (Fig. 7). The alteration of nodule [33P]Pi transported from roots was well consistent with the GmPT5 expression in the transgenic soybean plants, in which GmPT5 expression was greatly enhanced in high P in the OX lines and suppressed in low P in the RNAi lines (Supplemental Fig. S4). Moreover, the [33P]Pi activity in both the lower root regions responsible for direct [33P]Pi uptake and the upper root regions not in contact with the [33P]Pi uptake solution showed no differences among the different transgenic lines (Supplemental Fig. S6). This indicates that GmPT5 does not function in direct Pi absorption by roots and Pi translocation within roots. The in vitro nodule Pi uptake assay also demonstrates that GmPT5 does not directly function in direct Pi absorption into nodules from the rhizosphere, as there was no significant difference of [33P]Pi absorption in nodules from overexpression or knockdown and empty vector lines (Fig. 8). All of these findings together demonstrate that GmPT5 mainly functions in transporting Pi from the root vascular system to nodules.
Stabilizing Pi homeostasis in soybean nodules through GmPT5 is critical for soybean nodulation as well as growth performance. At low P level, [33P]Pi activity in nodules measured both through in situ and in vitro assays is much higher than in high P (Figs. 7 and 8). This suggests that under P-deficient conditions, Pi homeostasis in nodules becomes more important and soybean plants might evolutionarily have developed mechanisms to maintain Pi homeostasis in nodules either through enhancing Pi translocation from host roots or by directly taking up Pi into nodules. From the greatly enhanced expression of GmPT5 in nodules in low P (Supplemental Fig. S2), together with the decreased [33P]Pi activity in the nodules of GmPT5-RNAi lines pretreated by low P supply from in situ assays (Fig. 7), we suggest that GmPT5 is the key Pi transporter involved in Pi translocation from host roots to nodules in soybean, especially under P-limited conditions. The great capacity of Pi directly taken up by nodules pregrown in low P (Fig. 8) also indicates that one or more other Pi transporters act in nodules, mainly in direct Pi uptake from growth medium, especially when resupplying Pi under P-deficient conditions. The increased fresh weight, N and P contents, as well as nodule number and nodule size in GmPT5-OX lines in high P, together with the decreased growth and nodulation in GmPT5-RNAi lines at in low P, further suggest that GmPT5 expression plays a vital role in soybean nodulation (Fig. 6) and subsequently in soybean growth performance (Fig. 5).
Since biochemical reactions associated with symbiotic N2 fixation produce Pi (Schuize et al., 1999), too much Pi should inhibit N2 fixation, probably due to substrate feedback effects. Therefore, the maintenance of Pi homeostasis may also be important when supplied with excess Pi. This is supported by our results in hydroponics under high-P conditions, where Pi concentrations in nodules are much lower than in the host roots and leaves (Fig. 2). Suppression of GmPT5 by high P (Supplemental Fig. S2) and the lower [33P]Pi directly taken up by nodules pregrown in high P (Fig. 8) may be the responses of plants to maintain Pi homeostasis under high-P conditions. Therefore, there may be some other important pathways involved in Pi acquisition and translocation in nodules under Pi-sufficient conditions, and the underlying mechanisms need to be further elucidated.
Altogether, we conclude that the high-affinity Pi transporter, GmPT5, regulates Pi entry from roots to the region of plant tissues in nodules and maintains Pi homeostasis in nodules, particularly under P-limiting conditions. This activity subsequently affects soybean nodulation and growth performance.
MATERIALS AND METHODS
Plant Growth Conditions
A field experiment was carried out on the black soil at Friendship Farm (E131.82°, N46.76°) in the Heilongjiang province of China with a local soybean (Glycine max) variety, KF50, in 2011. Basic soil chemical characteristics of the top 30-cm layer in the field were as follows: pH, 5.6; organic matter, 35.0 g kg−1; available P (Olsen-P), 22.0 mg kg−1; available N, 192 mg kg−1; available potassium, 188 mg kg−1. There were two inoculation treatments, with (+R) and without (−R) rhizobium inoculation. The inoculant was a mixture of three highly effective rhizobium strains, BXYD3, BXBL9, and BDYD1, that were previously identified belonging to Bradyrhizobium elkanii based on morphological and 16s ribosomal DNA sequence analysis (Cheng et al., 2009). Before sowing by machine, soybean seeds were uniformly mixed with rhizobium inoculants. Seventy kilograms P2O5 ha−1 as diammonium Pi and 45 kg K2O ha−1 as KCl were applied following the local practice. For the −R treatment, 55 kg N ha−1 as urea was added to supplement N. The field plot was 1.66 km long and 1.2 km wide. There were five blocks, and three plants were harvested from each block at 50 d after emergence for measuring nodule fresh weight and at the R7 stage for determination of biomass, grain yield, and total N and P contents.
For the hydroponic experiment, soybean genotype HN89 and rhizobial strain BXYD3 were employed. The sterilized seeds were germinated and grown in sterile sand supplied with one-half-strength nutrient solution for 5 d, and then uniform seedlings were inoculated with BXYD3 before transplanting. The seedlings were grown in the low-N nutrient solution containing 50 µm NH4NO3, 1,200 µm CaCl2, 1,000 µm K2SO4, 500 µm MgSO4, 25 µm MgCl2, 2.5 µm NaB4O7·10H2O, 1.5 µm MnSO4, 1.5 µm ZnSO4, 0.5 µm CuSO4, 0.15 µm (NH4)6Mo7O24, and 40 µm Fe-Na-EDTA and treated with 5 μm (low P) or 250 μm (high P) P added as KH2PO4. The pH value of the nutrient solution was adjusted to 5.8, and nutrient solution was changed every week. After 50 d, soybean leaves, roots, and nodules were separately harvested for determining fresh weight, soluble Pi concentration, total P content, nodule number, and nodule fresh weight. Nodule size was calculated as the average fresh weight of a single nodule.
For spatial expression analysis of GmPTs in response to P supply and rhizobium inoculation, 1 week after germination, soybean seedlings of uniform height and development were inoculated with rhizobial strain BXYD3 and then transplanted into low-N nutrient solution containing 5 µm (low P) or 250 µm (high P) P as described above. Thirty days after inoculation, leaves, roots, and nodules were separately harvested. All samples were stored at –80°C for RNA extraction and qRT-PCR analysis.
Identification and Bioinformatic Analysis of Pht1 Family Genes in Soybean
All of the Pht1 family members from Arabidopsis (Arabidopsis thaliana; Mudge et al., 2002) and rice (Oryza sativa; Paszkowski et al., 2002) were used as query sequences in a BLAST search at the Phytozome Web site http://www.phytozome.net/search.php?show=blast&method=Org_Gmax. Predicted amino acid sequences of soybean Pi transporters (GmPTs) with conserved transmembrane domains belonging to the Pht1 family were identified in the soybean genome. The soybean Pi transporters were named according to their positions on the chromosomes. The nucleic acid sequences, protein sequences, and promoter regions of the candidate genes were available in the Phytozome Web site (http://www.phytozome.net/soybean). A phylogenetic tree was constructed based on protein sequence alignment of the candidate genes and Pht1 family members of other legumes, such as Medicago sativa, Lupinus albus, Lotus japonicus, and Arabidopsis using the ClustalW and MEGA 4.1 programs. The subcellular location of the candidate protein was predicted by WoLF PSORT analysis (http://wolfpsort.org/).
RNA Extraction, Complementary DNA Synthesis, and qRT-PCR Analysis
The total RNA was separately extracted from leaves, roots, and nodules using TRIzol reagent (Takara). The extracted RNA was treated with DNaseI to remove contaminating genomic DNA and synthesized to the first-strand complementary DNA using the Moloney murine leukemia virus reverse transcription kit (Promega) according to the manufacturer’s instructions. Gene-specific primers for GmPTs in the Pht1 family and a soybean housekeeping gene, TefS1 (encoding the elongation factor EF-1a; accession no. X56856), are listed in Supplemental Table S1. The complementary DNA reverse transcription products were used as templates for qRT-PCR. The reactions were carried out in a 20-µL volume containing 2 µL of 1:100 diluted complementary DNA product, 0.2 µm primers, and 10 µL of SYBR Premix Ex Taq (Takara). All of the reactions were carried on a Rotor-Gene 3000 (Corbett Research). The PCR conditions for thermal cycling were as follows: 95°C for 1 min, 40 cycles of 95°C for 15 s, 54°C to 62°C (adjusted according to the annealing temperatures of individual candidate genes) for 15 s, and 72°C for 30 s. Fluorescence data were collected during the cycle at 72°C. The relative two standard curve method was used to analyze the quantity of target genes with Rotor-Gene 6.1 software. The relative expression value was calculated by the ratio of the expression value of the target gene to that of the soybean housekeeping gene TefS1 (accession no. X56856).
Yeast Manipulations
The yeast Pi uptake-defective mutant MB192 (Bun-Ya et al., 1991) and the expression vector in MB192, p112A1NE (abbreviated as Yp112; kindly provided by Dr. M. Feng, Fudan University [http://www.fudan.edu.cn/englishnew]) were used in yeast heterologous expression experiments. The coding sequence of GmPT5 was amplified and cloned into Yp112 (Yp112-GmPT5). Yeast strains Yp112-GmPT5 and MB192 were grown to the logarithmic phase in YNB medium (yeast N base, 6.7 g; amino acid mix, 1.98 g; Glc monohydrate, 20 g; adenine genisulfate, 20 mg; double-distilled water, to 1 L) and were harvested and washed in Pi-free YNB medium. Then, equal volumes of 10-fold serial dilution with the original cell number as 6 × 105 were applied to solid YNB medium supplied with 100 or 50 μm Pi and incubated at 30°C for 3 d to observe the growth of different yeast strains.
In order to elucidate the Pi uptake kinetics of GmPT5, 33P uptake experiments in yeast were performed according to previously described methods (Ai et al., 2009). The Km and Vmax values of GmPT5 for Pi uptake were calculated using the software Sigmaplot (version 10.0).
Subcellular Localization of GmPT5 in Onion Epidermal Cells
For subcellular localization analysis, the coding region of GmPT5 was amplified using specific primers (Supplemental Table S2). The PCR product was digested with XbaI and BamHI (underlined in primer sequences) and ligated into the pBEGFP vector with a cauliflower mosaic virus (CaMV) 35S promoter.
After checking by sequencing, the constructs were transiently transformed into onion (Allium cepa) epidermal cells (Scott et al., 1999) on agar plates by a helium-driven accelerator (PDS/1000; Bio-Rad). In order to eliminate the possibility of cell wall localization, the bombarded epidermal cells were plasmolyzed by adding 30% Suc solution for 20 min before confocal scanning, with red propidium iodide fluorescence being used as an indicator of the cell wall. One day after culturing, GFP expression of the target protein in the bombarded epidermal cells was viewed using a confocal scanning microscope (TCS SP2; Leica) with 488-nm laser light for fluorescence excitation of GFP and detection using a 515- to 545-nm filter (green; GFP fluorescence) and a 610-nm filter (red; propidium iodide fluorescence).
Vector Construction and Generation of Transgenic Composite Soybean Plants
For the promoter analysis, the putative promoter region of GmPT5 was amplified using the primers shown in Supplemental Table S2. After digestion with XbaI and NcoI (underlined in primer sequences), a 2,496-bp promoter and a 5′ untranslated region of GmPT5 (proGmPT5) were fused to the pCAMBIA 3301 vector (CAMBIA) with a GUS reporter gene, and expression driven by a CaMV 35S promoter in empty vector was used as the control.
For the overexpression construct, the open reading frame region of GmPT5 was amplified and inserted into the pYLRNAi vector with a CaMV 35S promoter. For the RNAi construct, 400 bp of the GmPT5 coding region was amplified and inserted into the pYLRNAi vector in the sense and antisense orientations.
All of the above constructs were transformed into Agrobacterium rhizogenes strain K599 (kindly provided by Dr. Peter M. Gresshoff, University of Queensland) by the heat shock method and then transformed into soybean as described previously (Guo et al., 2011) to obtain composite plants with transgenic hairy roots.
Histochemical Localization of GUS Expression
For histochemical analysis of GUS expression, the transgenic hairy roots without rhizobium inoculation and nodules (after 15- and 30-d inoculations) were incubated in the GUS staining solution (0.2 m Na2HPO4-NaH2PO4 buffer, pH 7.0, and 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) at 37°C for 12 h, followed by washing in 70% ethanol according to a published method (Jefferson et al., 1987). After GUS staining, root segments (about 5 mm long) and nodules were sampled, then fixed in fixative solution (5% [v/v] formaldehyde, 5% [v/v] glacial acetic acid, and 63% [v/v] ethanol), vacuumed overnight, dehydrated gradually in a graded ethanol series (50%, 65%, 75%, 85%, and 95%), and infiltrated with graded ethanol/xylene (50:50, 0:100). After achieving transparency, paraffin fragments were gradually added overnight into xylene until saturation with paraffin and then replaced with pure paraffin twice. Finally, root and nodule samples were embedded in the paraffin, and then sectioned transversely to a thickness of 7 μm with a microtome (CUT 5062) for observation with a light microscope (LEICA DM5000B).
Characterization of Nodule Growth Using Transgenic Soybean Plants
After removing the main roots, uniform transgenic soybean plants with the empty vector (CK), overexpression GmPT5 (OX), and knockdown GmPT5 (RNAi) lines were inoculated with rhizobium BXYD3 as described above for 0.5 h and then cultured in nutrient solution with two P supplies (low P, 5 μm P; high P, 250 μm P) and 100 μm N. For each transgenic plant, only one positive transgenic hairy root checked by qRT-PCR was kept and represented an independent transgenic line. One independent transgenic line carrying the same construct was considered as one biological replication. Nodules were partially sampled from the same transgenic root to verify the GmPT5 expression before being used in other analyses. Fifty days after planting, plants and nodules were harvested separately to determine plant fresh weight, N and P contents of plants, as well as nodule number and nodule fresh weight. Nodule size was calculated as described above.
Radioactive [33P]Pi Uptake Assay
Transgenic composite plants of CK, OX, and RNAi lines were used in two radioactive [33P]Pi uptake assays: in situ Pi translocation (Supplemental Fig. S5A) and in vitro nodule Pi uptake assays. Before being used in the [33P]Pi uptake assays, the transgenic plants were gown in low P (5 μm P) or high P (250 μm P) for 50 d after inoculation with rhizobia as described above. For the in situ assay, the 6-cm bottom root regions (without nodules) of different transgenic composite plants were directly exposed to 100 mL of the nutrient solution containing 10 μCi of H333PO4. After 8 h of Pi uptake, the roots exposed to 33P were washed with nutrient solution until no radioactive 33P was detected in the liquid. After that, the seedlings were fixed on paper and autoradiographed with photographic film plates (X-omat, Kodak; Supplemental Fig. S5B). The radioactivity of the 6-cm bottom root regions, the upper roots, and the nodules from the upper roots that did not directly contact the 33P solution were separately weighed and analyzed following previously described methods (Jia et al., 2011) using scintillation counting (LS6500 Multipurpose Scintillation Counter; Beckman Coulter).
In order to determine the functions of GmPT5 on the direct Pi uptake pathway in soybean nodules, the nodules from GmPT5-OX and GmPT5-RNAi lines were pretreated with low P or high P for 50 d and assayed for in vitro Pi uptake. The fresh weight of uniform nodules from different plants was measured, and then nodules were transferred to 1 mL of 33P-labeled nutrient solution containing 0.25 μCi of H333PO4 for 2 h. They were then washed with nutrient solution until no radioactive [33P]Pi was detected in the liquid. Four milliliters of scintillation cocktail was added to each sample, and the radioactivity was measured by scintillation counting (LS6500 Multipurpose Scintillation Counter; Beckman Coulter).
Measurement of Soluble Pi Concentration and Plant N and P Contents
For the measurement of soluble Pi concentration in plant tissues, about 0.1 g of fresh samples was ground and extracted with distilled, deionized water. After centrifugation at 12,000g for 30 min, soluble Pi concentration in the supernatant was measured using the P-molybdate blue color reaction (Murphy and Riley, 1962). After the dry weight of each sample was determined, total N content in transgenic plants was measured using the semimicro Kjedahl procedure with a N analyzer (Kjedahl 2300; FOSS), and total P content was measured using the P-molybdate blue color reaction (Murphy and Riley, 1962).
Statistical Analysis
All means and se values were calculated using Microsoft Excel 2003. Comparisons between groups were performed using Student’s t test in Microsoft Excel 2003 or one-way ANOVA in the SAS system (SAS Institute) as appropriate.
Sequence data from this article can be found in the GenBank data libraries under accession numbers FJ814697 to JQ518271 (GmPT1–GmPT14).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phylogenetic tree analysis among soybean Pht1 family members and Pi transporters in other plant species.
Supplemental Figure S2. Fold change of the expression of GmPT genes between low-P and high-P treatments in soybean nodules.
Supplemental Figure S3. Subcellular localization of GmPT5 fused to GFP in epidermal onion cells.
Supplemental Figure S4. Relative expression of GmPT5 in CK, OX, and RNAi transgenic soybean plants grown with low P (5 μm P) or high P (250 μm P) levels.
Supplemental Figure S5. In situ assays for [33P]Pi uptake using transgenic soybean plants.
Supplemental Figure S6. In situ assays for [33P]Pi uptake by GmPT5 transgenic soybean roots.
Supplemental Table S1. Soybean Pht1 family genes and gene-specific primers used for qRT-PCR analysis.
Supplemental Table S2. Gene-specific primers used in this work.
Supplementary Material
Acknowledgments
We are grateful to Drs. Leon Kochian, Thomas Walk, and Xiurong Wang for critical reading of the manuscript and to Drs. Xinping Chen, Yaoguang Liu, Feng Ming, Hongfang Jia, Hong Wu, and Xiping Ning for technical assistance.
Glossary
- N
nitrogen
- P
phosphorus
- Pi
phosphate
- OX
overexpressing
- RNAi
RNA interference
- YNB medium
yeast N base, 6.7 g; amino acid mix, 1.98 g; Glc monohydrate, 20 g; adenine genisulfate, 20 mg; distilled, deionized water, to 1 L
- CaMV
cauliflower mosaic virus
- qRT
quantitative real-time
References
- Ai PH, Sun SB, Zhao JN, Fan XR, Xin WJ, Guo Q, Yu L, Shen QR, Wu P, Miller AJ, Xu GH. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809 [DOI] [PubMed] [Google Scholar]
- Al-Niemi TS, Kahn ML, McDermott TR. (1997) P metabolism in the bean-Rhizobium tropici symbiosis. Plant Physiol 113: 1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Niemi TS, Kahn ML, McDermott TR. (1998) Phosphorus uptake by bean nodules. Plant Soil 198: 71–78 [Google Scholar]
- Bun-Ya M, Nishimura M, Harashima S, Oshima Y. (1991) The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11: 3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary MI, Adu-Gyamfi JJ, Saneoka H, Nguyen NT, Suwa R, Kanai S, El-Shemy HA, Lightfoot DA, Fujita K. (2008) The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in mashbean, mungbean and soybean. Acta Physiol Plant 30: 537–544 [Google Scholar]
- Chen ZJ, Cui QQ, Liang CY, Sun LL, Tian J, Liao H. (2011) Identification of differentially expressed proteins in soybean nodules under phosphorus deficiency through proteomic analysis. Proteomics 11: 4648–4659 [DOI] [PubMed] [Google Scholar]
- Cheng FX, Cao GQ, Wang XR, Zhao J, Yan XL, Liao H. (2009) Isolation and application of effective nitrogen fixation rhizobial strains on low-phosphorus acid soils in South China. Chin Sci Bull 54: 412–420 [Google Scholar]
- Cordell D, Drangert JO, White S. (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19: 292–305 [Google Scholar]
- Daram P, Brunner S, Rausch C, Steiner C, Amrhein N, Bucher M. (1999) Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 11: 2153–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalski J, Lin C, Luo Y, Kang J, Wang S, Brown LR, Munn MD. (2007) Eutrophication study at the Panjiakou-Daheiting Reservoir system, northern Hebei Province, People’s Republic of China: chlorophyll-a model and sources of phosphorus and nitrogen. Agric Water Manage 94: 43–53 [Google Scholar]
- Gaude N, Tippmann H, Flemetakis E, Katinakis P, Udvardi M, Dörmann P. (2004) The galactolipid digalactosyldiacylglycerol accumulates in the peribacteroid membrane of nitrogen-fixing nodules of soybean and Lotus. J Biol Chem 279: 34624–34630 [DOI] [PubMed] [Google Scholar]
- Graham PH, Vance CP. (2003) Legumes: importance and constraints to greater use. Plant Physiol 131: 872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WB, Zhao J, Li XX, Qin L, Yan XL, Liao H. (2011) A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66: 541–552 [DOI] [PubMed] [Google Scholar]
- Hernández G, Valdés-López O, Ramírez M, Goffard N, Weiller G, Aparicio-Fabre R, Fuentes SI, Erban A, Kopka J, Udvardi MK, et al. (2009) Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol 151: 1221–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge DF, Peoples MB, Boddey RM. (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311: 1–18 [Google Scholar]
- Jakobsen I. (1985) The role of phosphorus in nitrogen fixation by young pea plants (Pisum sativum). Physiol Plant 64: 190–196 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G. (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156: 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantar F, Shivakumar B, Arrese-Igor C, Hafeez F, González E, Imran A, Larrainzar E. (2010) Efficient biological nitrogen fixation under warming climates. In SS Yadav, DL McNeil, R Redden, SA Patil, eds, Climate Change and Management of Cool Season Grain Legume Crops. Springer, Dordrecht, The Netherlands, pp 283–306.
- Kouas S, Labidi N, Debez A, Abdelly C. (2005) Effect of P on nodule formation and N fixation in bean. Agron Sustain Dev 25: 389–393 [Google Scholar]
- Le Roux M, Khan S, Valentine A. (2009) Nitrogen and carbon costs of soybean and lupin root systems during phosphate starvation. Symbiosis 48: 102–109 [Google Scholar]
- Lindström K, Murwira M, Willems A, Altier N. (2010) The biodiversity of beneficial microbe-host mutualism: the case of rhizobia. Res Microbiol 161: 453–463 [DOI] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW. (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353 [DOI] [PubMed] [Google Scholar]
- Murphy J, Riley JP. (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27: 31–36 [Google Scholar]
- Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. (2011) Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol 156: 1149–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht MB, Xu G, Jakobsen I, Levy AA, Amrhein N, Bucher M. (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J 42: 236–250 [DOI] [PubMed] [Google Scholar]
- Paszkowski U, Kroken S, Roux C, Briggs SP. (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99: 13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples M, Brockwell J, Herridge D, Rochester I, Alves BJR, Urquiaga S, Boddey R, Dakora F, Bhattarai S, Maskey S. (2009) The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48: 1–17 [Google Scholar]
- Rae AL, Cybinski DH, Jarmey JM, Smith FW. (2003) Characterization of two phosphate transporters from barley: evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol Biol 53: 27–36 [DOI] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Sa TM, Israel DW. (1991) Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol 97: 928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, Chacon-López A, Pérez-Torres CA, Herrera-Estrella L. (2010) Phosphorus: plant strategies to cope with its scarcity. In R Hell, RR Mendel, eds, Cell Biology of Metals and Nutrients, Vol 17. Springer, Heidelberg, pp 173–198.
- Schuize J, Adgo E, Merbach W. (1999) Carbon costs associated with N2 fixation in Vicia faba L. and Pisum sativum L. over a 14-day period. Plant Biol 1: 625–631 [Google Scholar]
- Schulze J, Temple G, Temple SJ, Beschow H, Vance CP. (2006) Nitrogen fixation by white lupin under phosphorus deficiency. Ann Bot (Lond) 98: 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Wyatt S, Tsou PL, Robertson D, Allen NS. (1999) Model system for plant cell biology: GFP imaging in living onion epidermal cells. Biotechniques 26: 1125–, 1128–1132. [DOI] [PubMed] [Google Scholar]
- Sun SB, Gu M, Cao Y, Huang XP, Zhang X, Ai PH, Zhao JN, Fan XR, Xu GH. (2012). A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in Pi-replete rice. Plant Physiol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen H. (2008) Phosphorus in the global environment. In PJ White, JP Hammond, eds, The Ecophysiology of Plant-Phosphorus Interactions. Springer, Netherlands, pp 1–7.
- Ullrich-Eberius CI, Novacky A, Fischer E, Lüttge U. (1981) Relationship between energy-dependent phosphate uptake and the electrical membrane potential in Lemna gibba G1. Plant Physiol 67: 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.