Abstract
The activity of voltage-gated sodium channels has long been linked to disorders of neuronal excitability such as epilepsy and chronic pain. Recent genetic studies have now expanded the role of sodium channels in health and disease, to include autism, migraine, multiple sclerosis, cancer as well as muscle and immune system disorders. Transgenic mouse models have proved useful in understanding the physiological role of individual sodium channels, and there has been significant progress in the development of subtype selective inhibitors of sodium channels. This review will outline the functions and roles of specific sodium channels in electrical signalling and disease, focusing on neurological aspects. We also discuss recent advances in the development of selective sodium channel inhibitors.
Keywords: ion channel, genetics, pain, epilepsy, SCN1A
Sodium channels
Structure and activity
The voltage-gated sodium channel (VGSC) gene family comprises nine homologous members SCN1A to SCN11A, which encode the sodium selective ion channels NaV1.1 to NaV1.9. Nax (SCN6A/SCN7A), though structurally related to VGSCs, is not activated by membrane depolarization, but rather by altered sodium concentrations (Goldin et al., 2000). Each large α-subunit (∼260 kDa) contains four homologous domains DI–DIV, with each domain containing six transmembrane segments. One α-subunit alone is necessary and sufficient to form a functional channel; however α-subunits also associate with β-subunits (SCN1B to SCN4B), which modulate channel biophysics and trafficking. At resting membrane potentials, VGSCs are closed, requiring depolarization to be activated. Upon activation they contribute to the upstroke of the action potential in excitable cells (Fig. 1A and B). Channel opening results in a rapid influx of sodium ions into the cell and further depolarization of the membrane potential towards the equilibrium potential for sodium (∼+60 mV in most neurons). VGSCs close within milliseconds of opening, a process called fast inactivation that contributes to the downstroke of the action potential (Fig. 1C). In many neurons, inactivation of VGSCs is incomplete, resulting in a small persistent Na+ current, which inactivates over a time period of tens of seconds. Functionally, the structure of the VGSCs can be divided into two parts with the transmembrane domains S1–S4 contributing to the voltage sensor and S5–S6 arranging to form the sodium selective pore (Stuhmer et al., 1989; Catterall et al., 2005). The molecular mechanism by which changes in membrane voltage confer a conformational change on voltage-gated ion channel proteins is through the movement of modular voltage sensors contained within the S4 segment of domains I–IV (Fig. 1C) (Alabi et al., 2007). The voltage sensors contain repeated motifs of positively charged amino acids followed by hydrophobic residues arranged in an α-helix with a linear array of positively charged residues. Depolarization of the cell alters the electric field across the cell membrane resulting in the rapid movement of the DI–III S4 voltage sensors and a conformational change in the protein which opens the ion channel pore. Inactivation follows activation due to the intrinsically slower movement of the DIV voltage sensor (Bosmans et al., 2008). The VGSC inactivation gate contains a trio of amino acids (IFM) located in a highly conserved intracellular loop connecting domains III and IV (West et al., 1992). Upon inactivation, the inactivation gate moves into the channel pore as shown by the altered accessibility of antibodies targeted to this domain (Vassilev et al., 1988, 1989), resulting in occlusion of the pore often depicted as a ball and chain type block (Fig. 1C). The channels remain in a refractory inactivated state until the cell membrane potential repolarizes, normally facilitated by the delayed activation of voltage-gated potassium channels.
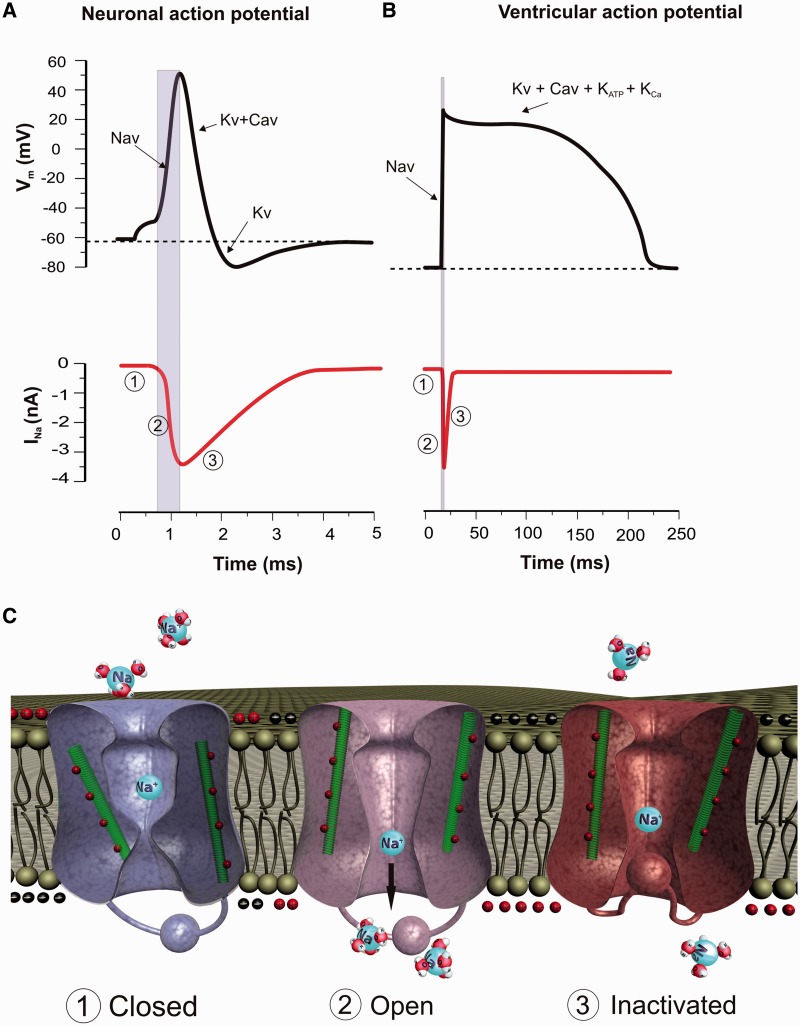
Figure 1.
Gating model and contribution of voltage-gated sodium channels to neuronal and cardiac action potential firing. Upper traces depict a cartoon representation of a whole-cell current clamp recording from a typical neuron (A) or cardiac myocyte (B). Dotted line indicates the resting membrane potential (Vm). Lower trace is temporally aligned to the upper trace and shows the change in sodium current (INa) during an action potential. Note a downward deflection of the trace reflects an inward movement of sodium ions into the cell. (1) At the resting membrane potential VGSCs are closed. A small depolarization of the neuronal membrane potential in response to sensory input or receptor input depolarizes the neuronal membrane potential to the threshold for VGSC activation (∼−50 mV). (2) VGSCs activate rapidly (∼1 ms to peak) allowing the influx of sodium and depolarizing the membrane potential further, forming the upstroke of the action potential. Note that the peak sodium current correlates with the peak of the action potential. (3) Following activation the sodium channels inactivate resulting in a decrease in sodium current and repolarization of the neuronal membrane potential, contributing to the downstroke of the action potential. Recovery from inactivation allows the channels to participate in the next action potential. (C) Mechanism of voltage sensitive gating of VGSCs. The left channel represents a VGSC in a deactivated (closed) state. A small depolarization of the membrane potential causes a movement of the positively charged S4 voltage-sensor domain (green) leading to a conformational change in the protein and opening of the pore (middle channel). Following activation, the pore is rapidly occluded by the inactivation gate, resulting in inactivation of the sodium channel (right channel).
Much of what is known about the molecular mechanism of voltage sensing derives from studies on voltage-gated potassium channels for which high-resolution crystal structures have been obtained. Using X-ray crystallography the structure of the S4 voltage sensor in the Archaea voltage-dependent potassium channel KvAP was modelled as a paddle (Jiang et al., 2003a, b). However, recent modelling of the bacterial sodium channel NaChBac reveals that the S4 voltage sensor segment is arranged in a 3(10) alpha helical conformation that slides in a linear fashion through a narrow groove formed by the S1, S2 and S3 segments (Yarov-Yarovoy et al., 2012). Recently, the crystal structure of a VGSC has been reported (Payandeh et al., 2011). Comparison with the previous open potassium channel structures showed that the voltage-sensor domains and the S4–S5 linkers dilate the central pore by pivoting together around a hinge at the base of the pore module (Payandeh et al., 2011). This newly described crystal structure of a prokaryotic VGSC shows that the basis of ion channel selectivity for Na+ is different from potassium channels (Corry and Thomas, 2012).
Persistent sodium currents
All the kinetically fast transient channels (NaV1.1–1.7) appear quite similar in functional properties, but sodium channels sometimes generate much longer openings as a result of incomplete or defective fast inactivation. NaV1.9 gives rise to a low-threshold, persistent tetrodotoxin (TTX)-resistant Na+ current in sensory neurons (Baker et al., 2003). Persistent sodium currents have also been recorded in cells that do not express NaV1.9, including cardiac and skeletal muscle (Patlak and Ortiz, 1986; Bohle and Benndorf, 1995), large diameter dorsal root ganglion neurons (Baker and Bostock, 1997) and cortical pyramidal neurons (Alzheimer et al., 1993; Schwindt and Crill, 1995). In some voltage-clamp protocols the amplitude of persistent current is just a few per cent of that of the transient current at the same potentials but is still functionally important. For mammalian primary sensory neurons, persistent currents activate at more negative potentials than the associated transient currents. The hyperpolarized voltage dependence of activation of persistent sodium currents allows these channels to operate as amplifiers of subthreshold depolarization, because their activation kinetics are fast and they operate over a strategic subthreshold membrane potential range with low potassium channel activation. Evidence from muscle fibre recordings indicates that NaV1.4 can generate persistent currents, and NaV1.6 generates such currents in cerebellar Purkinje neurons (Raman et al., 1997; Raman and Bean, 1997). The functional importance of persistent currents can be seen from the effects of SCN8A mutations that change neuron firing patterns and lead to ataxia in mice (Meisler et al., 2002, 2004). In addition, many epileptogenic mutations of SNC1A, SCN2A and SCN3A exhibit increased persistent currents (Meisler and Kearney, 2005). The implication of these findings is that specific blocking of channels with inactivation-defective gating might be a useful way of controlling membrane excitability within the nervous system (Lampl et al., 1998). In primary sensory neurons, persistent currents are preferentially targeted by local anaesthetics (Baker, 2000). Local anaesthetics can suppress ectopic firing in damaged sensory neurons without altering action potential properties (Devor et al., 1992), probably because the block of persistent currents takes the membrane potential further away from firing threshold.
Resurgent sodium currents
Resurgent currents, first observed in cerebellar Purkinje neurons (Raman and Bean, 1997) and present in dorsal root ganglion neurons (Cummins et al., 2005), may arise following relief of ultra-fast open-channel block (faster than pore block by the inactivation gate) mediated by yet undetermined proteins. In some neurons, sodium channels transiently open upon recovery from inactivation when the membrane potential is repolarized. This transient opening gives rise to a large inward tail current termed ‘resurgent current’ (Cannon and Bean, 2010). One possible mechanism for resurgent current involves the blockade of the channel pore by the C-terminus of β4 subunit of sodium channels (Grieco et al., 2005; Bant and Raman, 2010). Resurgent currents have recently become an important topic in pain and myopathy research as sodium channel mutations involved in these pathologies were found to increase resurgent currents (Jarecki et al., 2010). Mutations in NaV1.4, NaV1.5 and NaV1.7 that lead to paramyotonia congenita, cardiac long QT syndrome/sudden infant death syndrome and paroxysmal extreme pain disorder, respectively, all enhance resurgent currents, thereby affecting the firing properties of the cells. Although resurgent current can be observed with NaV1.4-, NaV1.5-, NaV1.6- and NaV1.7-based sodium channels in expression studies, in vivo resurgent currents have only been recorded from neurons and never from cardiac or skeletal muscle.
Many toxins affect sodium channel function by altering the gating of these channels. The wasp venom β-pompilidotoxin (β-PMTX) is able to produce resurgent currents through a molecular mechanism involving the slowing of sodium channel inactivation (Grieco and Raman, 2004). Another toxin, the Cn2 β-scorpion peptide, shifts the activation of NaV1.2 and NaV1.6 towards more hyperpolarized potentials by trapping the DII S4 voltage sensor in the inactivated rather than the closed state. A β-scorpion toxin also has the capability of producing resurgent currents by trapping the voltage sensor of human NaV1.6 channels and VGSC in mouse Purkinje cells (Schiavon et al., 2006). The data from these two studies strongly suggest that an increased open probability of sodium channels is a key requirement for the generation of resurgent currents. Very recently it has been suggested that the transient, resurgent and persistent phases of the sodium current in cerebellar granule cells are all interlinked through the β4 subunit (Bant and Raman, 2010). These results highlight the critical role that toxins play in unravelling gating mechanisms of sodium channels.
Voltage-gated sodium channel function and disease association
The nine different VGSC α-subunits are differentially expressed (Table 1), and disruption of VGSC function can lead to a broad range of pathologies. The role of VGSCs in epilepsy and pain has been well established; however, there is increasing evidence of a role for VGSCs in other disorders including cancer, multiple sclerosis, muscle and immune disorders, autism, neurodegeneration and cardiovascular complications (Table 1). Human heritable disorders can now be mapped with relative ease. A number of disorders have been ascribed to mutations in genes encoding sodium channels and further genetic insights have been provided by analysis of targeted sodium channel mutations and gene deletion in mice (Table 1). The correlation between the clinical phenotype of patients with channel mutations and channel biophysical properties and observations in mouse models will be discussed later.
Table 1.
Expression patterns in relation to known effects of human and mouse mutants of VGSCs
| Channel | Gene | Major expression | Channel disease association | Phenotype of mouse mutants |
|---|---|---|---|---|
| Nav1.1 | SCN1A | CNS (Westenbroek et al., 1989), PNS (Black et al., 1996) | Epilepsy (Escayg et al., 2000), migraine (Dichgans et al., 2005), autism (Weiss et al., 2003) | (−/−) Ataxia and death at P15 (Yu et al., 2006); (+/−) spontaneous seizures and sporadic deaths after P21 (Yu et al., 2006) |
| Nav1.2 | SCN2A | CNS (Westenbroek et al., 1992), PNS (Black et al., 1996) | Epilepsy (Sugawara et al., 2001; Heron et al., 2002; Liao et al., 2010) (GOF), autism (Weiss et al., 2003), episodic ataxia (GOF) (Liao et al., 2010) | (−/−) Perinatal lethal (Planells-Cases et al., 2000) |
| Nav1.3 | SCN3A | CNS (Whitaker et al., 2001; Hains et al., 2003), PNS (elevated following nerve injury) (Waxman et al., 1994b) | Epilepsy (GOF) (Holland et al., 2008b) | (−/−) and (−/− nociceptor specific) normal acute inflammatory and neuropathic pain (Nassar et al., 2006) |
| Nav1.4 | SCN4A | Skeletal muscle (Haimovich et al., 1987) | Hyperkalaemic periodic paralysis (GOF) (Ptacek et al., 1991); paramyotonia congenita (GOF); hypokalaemic periodic paralysis (altered gating pore current) (Sokolov et al., 2007) | Unknown |
| Nav1.5 | SCN5A | Cardiac muscle (Rogart et al., 1989) | Brugada syndrome (Chen et al., 1998) (LOF); long QT syndrome 3 (Zimmer and Surber, 2008) (GOF); atrial fibrillation | (−/−) Intrauterine lethality with severe defects in ventricular morphogenesis; (+/−) decreased atrial and atrio-ventricular conduction progressed with age (Papadatos et al., 2002). (Δ/+) knockin model of LQT3 resulted in spontaneous ventricular arrhythmias (Nuyens et al., 2001) |
| Nav1.6 | SCN8A | CNS, PNS (Black et al., 2002), smooth muscle (Saleh et al., 2005) | Mental retardation, pancerebellar atrophy, ataxia (Trudeau et al., 2006); infantile experimental encephalopathy (Veeramah et al., 2012) | med mutant (−/−): severe muscle atrophy, progressive paralysis and juvenile death; ataxia, tremor, and impaired coordination (Meisler et al., 2004); sleep deficits (Papale et al., 2010); Purkinje and granule cells specific (−/−): ataxia (Levin et al., 2006) D981V produces paroxysmal tremors and mild deafness (Mackenzie et al., 2009) |
| Nav1.7 | SCN9A | PNS (Toledo-Aral et al., 1997) | Pain free (LOF) (Cox et al., 2006, 2010); erythromalalgia (GOF) (Yang et al., 2004), paroxysmal extreme pain disorder (reduced fast inactivation) (Fertleman et al., 2006), anosmia (Goldberg et al., 2007) | (−/−) Perinatal lethal (Nassar et al., 2004); (−/− Nociceptor specific deletion) insensitivity to blunt mechanical pressure and lack of inflammatory pain (Nassar et al., 2004); (−/− sensory and sympathetic): pain free (Minett et al., 2012) |
| Nav1.8 | SCN10A | PNS (Akopian et al., 1996), heart (Chambers et al., 2010) | SNP rs6795970 (G>A) results in prolonged cardiac conduction (Chambers et al., 2010) | (−/−) Insensitivity to blunt mechanical pressure; some deficits in inflammatory pain(Akopian et al., 1999; Papadatos et al., 2002; Abrahamsen et al., 2008); prolonged cardiac PR interval (Chambers et al., 2010) |
| Nav1.9 | SCN11A | PNS (Dib-Hajj et al., 1998) | Unknown | (−/−) Reduced cold allodynia in a neuropathic pain model; increased visceral inflammatory pain (Leo et al., 2010); reduced thermal but not mechanical hyperalgesia in inflammatory model (Priest et al., 2005) |
| NaX | SCN7A | Circumventricular organs (Hiyama et al., 2004) | Unknown | Abnormal salt intake under dehydrated conditions (Watanabe et al., 2000; Hiyama et al., 2004) |
| β1 | SCN1B | CNS, cardiac muscle (Isom et al., 1992) | Epilepsy (GEFS+) (Scheffer et al., 2007), Dravet’s syndrome (Patino et al., 2009) Brugada syndrome (Watanabe et al., 2008) (defective trafficking); atrial fibrillation (LOF) (Watanabe et al., 2009) | (−/−) Spontaneous seizures (Patino et al., 2009); (+/−) normal seizure susceptibility (Patino et al., 2009); (−/−) reduced resurgent sodium current in cebellar neurons (Brackenbury et al., 2010); (−/−) hypoglycaemia (Ernst et al., 2009); (−/−) long QT interval (Lopez-Santiago et al., 2007) |
| β2 | SCN2B | PNS (Lopez-Santiago et al., 2006) | Atrial fibrillation (LOF) (Watanabe et al., 2009) | (−/−) Neuroprotective in model of multiple sclerosis (O'Malley et al., 2009); (−/−) increased sensitivity to noxious heat and reduced inflammatory pain (Lopez-Santiago et al., 2006) |
| β3 | SCN3B | Cardiac muscle, CNS, skeletal muscle (Stevens et al., 2001) | Idiopathic ventricular fibrillation (Valdivia et al., 2010), atrial fibrillation (DN mutant) (Wang et al., 2010a), Brugada syndrome (defective trafficking) (Hu et al., 2009), sudden infant death syndrome (Tan et al., 2010) | Atrial and ventricular conduction abnormalities (Hakim et al., 2008, 2010) |
| β4 | SCN4B | CNS, PNS (Yu et al., 2003) | Long QT syndrome (Medeiros-Domingo et al., 2007), Sudden infant death syndrome (Tan et al., 2010) | Unknown |
DN = dominant negative; GOF = gain-of-function; LOF = loss-of-function; PNS = peripheral nervous system; GEF+ = generalized epilepsy with febrile seizures plus; −/− = homozygous knockout; +/− = heterozygous knockout; med = motor endplate disease.
NaV1.1 (SCN1A)
Broadly expressed in the CNS, NaV1.1 expression may be preferentially expressed in inhibitory gamma-aminobutyric acidergic (GABAergic) neurons (Yu et al., 2006). The majority of the >700 associated SCN1A mutations are nonsense causing the autosomal dominant disorder Dravet’s syndrome. In addition, approximately two dozen SCN1A mutations have been identified in families with the milder disorder, generalized epilepsy with febrile seizures plus, which is characterized by short-lasting tonic–clonic seizures accompanied by fever (Meisler et al., 2010). Generalized epilepsy with febrile seizures plus mutations change expression and function of NaV1.1 channels due to both gain- and loss-of-function mutations. For example, the D188V mutation leads to impaired slow inactivation (Cossette et al., 2003), while the T875M mutation enhances slow inactivation (Spampanato et al., 2001). Both mutations lead to the same phenotype. Thus, the relationship between the clinical phenotypes and the functional defects is complex (reviewed in Ragsdale, 2008). Some linkage between specific genetic abnormalities and phenotype has been shown; SCN1A mutations are associated with early onset of febrile seizures/febrile seizures plus while SCN1B mutations are associated with later onset (Sijben et al., 2009).
The more detrimental severe myoclonic epilepsy of infancy (or Dravet’s syndrome) is associated with haploinsufficiency for SCN1A in 50–80% of severe myoclonic epilepsy of infancy patients caused by more deleterious nonsense and frameshift mutations in NaV1.1 (De Jonghe, 2011). In contrast to generalized epilepsy with febrile seizures plus, these mutations prevent channel expression or severely impair channel function. While loss-of-function mutations are common in Dravet’s syndrome, a gain-of-function mutation in SCN1A (R865G) has also been found (Volkers et al., 2011). SCN1A duplications and deletions are also found in patients with Dravet’s syndrome (Marini et al., 2009). More recently, de novo SCN1A mutations have been found in patients with the severe early infantile onset syndrome of malignant migrating partial seizures, also a severe epileptic encephalopathy (Carranza et al., 2011).
The severity of channel impairment has been suggested to underlie the different efficacies of some anti-epileptic drugs in treating either generalized epilepsy with febrile seizures plus or severe myoclonic epilepsy of infancy, of which many act through inhibiting VGSCs. For example, the sodium channel blocker lamotrigine is very effective for treating generalized epilepsy with febrile seizures plus, while it aggravates symptoms in patients with severe myoclonic epilepsy of infancy (Guerrini et al., 1998). The efficacy of some frontline anti-epileptic drugs, which often work through stabilizing channels in the inactive state, has been suggested to be influenced by a polymorphism that modifies splicing of NaV1.1 leading to altered inactivation (Fletcher et al., 2011). Studies on Scn1a+/− mice have shown that deletion of NaV1.1 leads to impaired firing of GABAergic inhibitory hippocampal interneurons (Catterall and Kalume, 2010) and cerebellar GABAergic Purkinje neurons (Kalume et al., 2007). The impaired functioning of inhibitory GABAergic neurons may contribute to seizures, ataxia, spasticity and failure of motor coordination observed in these mice.
It is interesting to note that targeting NaV1.1 to treat epilepsy is not the only possible therapeutic strategy. Mice with haploinsufficiency for both NaV1.1 and NaV1.6 showed reduced susceptibility to drug-induced seizures compared with NaV1.1 heterozygotes (Martin et al., 2007). In contrast with NaV1.1, NaV1.6 is highly expressed in excitatory neurons (Caldwell et al., 2000). Thus it seems that the excitatory and inhibitory balance in the brain is restored by NaV1.6 mutations that reduce firing of excitatory neurons. Finally, NaV1.1 mutations are also associated with familial hemiplegic migraine type 3 (Dichgans et al., 2005), an autosomal dominant severe subtype of migraine with aura characterized by hemiparesis during the attacks. Genome-wide linkage analysis revealed three families with the same missense mutation in SCN1A (Q1489K). This mutation resulted in complex changes in channel gating including a depolarizing shift in the voltage dependence of inactivation, accelerated recovery from inactivation and increased persistent current (Cestele et al., 2008). These biophysical changes could cause either hyper- or hypoexcitability depending on the firing frequency and resting membrane potential of the neuron. More recently, whole exome sequencing has identified candidate genes with de novo mutations, including SCN1A, in sporadic autism spectrum disorders (O'Roak et al., 2011, 2012).
NaV1.2 (SCN2A)
NaV1.2 is abundantly expressed in the adult CNS, particularly in the cortex and hippocampus (Westenbroek et al., 1989), where it is primarily expressed in unmyelinated axons and dendrites (Boiko et al., 2001). Early in development, NaV1.2 is highly expressed in regions destined to become nodes of Ranvier and is replaced during development by NaV1.6 (Boiko et al., 2001; Kaplan et al., 2001). NaV1.2 knockout mice die perinatally from neuronal apoptosis and hypoxia (Planells-Cases et al., 2000). In humans, NaV1.2 mutations are associated with inherited epilepsy, mainly benign familial neonatal-infantile seizures (Heron et al., 2002) and less frequently with other forms of epilepsy such as generalized epilepsy with febrile seizures plus (Sugawara et al., 2001). Benign familial neonatal-infantile seizure is an autosomal dominant disorder characterized by afebrile seizures with onset within 4 months after birth and spontaneous remission within the first year of life, without residual neurological deficits. Three separate benign familial neonatal-infantile seizures causing mutations in NaV1.2 resulted in reduced plasma membrane expression while having varied effects on channel activation and inactivation (Misra et al., 2008). Although there is little consensus over the pathological mechanisms, studies have demonstrated that either gain- or loss-of-function mutations of NaV1.2 are associated with disease. A link between NaV1.2 and autism has been reported at low frequency (1/229 autism families studied), resulting in mutation of the calmodulin binding domain of NaV1.2 and reduced calmodulin-binding affinity (Weiss et al., 2003). De novo mutations revealed by whole-exome sequencing including two independent nonsense mutations in SCN2A, are strongly associated with autism (Sanders et al., 2012).
NaV1.3 (SCN3A)
NaV1.3 is widely expressed in the human brain and has a predominantly somatodendritic expression pattern (Whitaker et al., 2001). In contrast to many of the other VGSC genes, there are as yet no clear monogenic diseases associated with SCN3A mutation. However, a small study of patients with cryptogenic paediatric partial epilepsy revealed a mutation in SCN3A (K354Q) that led to an increase in persistent current and induced epileptiform hyperexcitability in hippocampal neurons (Holland et al., 2008a; Estacion et al., 2010). Animal studies have focused on a possible role of NaV1.3 in neuropathic pain. Following axotomy and inflammation in mice, NaV1.3 transcript levels increase in sensory neurons (Waxman et al., 1994b; Dib-Hajj et al., 1999). Antisense knockdown of NaV1.3 expression attenuates pain-related behaviour associated with spinal cord injury and chronic constriction injury (Hains et al., 2004) but not allodynia in the spared nerve injury model (Lindia et al., 2005). However, NaV1.3 knockout mice, where the gene was deleted globally or selectively in nociceptive neurons, showed normal pain behaviour and normal development of neuropathic pain in the Chung model of neuropathic pain (Nassar et al., 2006). Although several lines of investigation have implicated NaV1.3 as a candidate drug target to treat neuropathic pain, this study does not support an essential role for NaV1.3 in neuropathic pain.
NaV1.4 (SCN4A)
NaV1.4 is responsible for the generation and propagation of action potentials that initiate muscle contraction. Currently, five hereditary sodium channelopathies of skeletal muscle involving NaV1.4 mutations have been identified, such as hyperkalaemic periodic paralysis, hypokalaemic periodic paralysis, paramyotonia congenita, potassium-aggravated myotonia and congenital myasthenic syndrome (Jurkat-Rott et al., 2010). Hypokalaemic periodic paralysis and normokalaemic peridodic paralysis causing mutations map to the NaV1.4 voltage sensor, resulting in ionic leak through the gating pore allowing sustained inward sodium flux at negative membrane potentials (Sokolov et al., 2010). In contrast, mutations associated with paramyotonia congenita and hyperkalaemic periodic paralysis are widespread in the NaV1.4 protein and either enhance activation or impair inactivation resulting in hyperexcitability. Mutations in KCNA1 and SCN4A have been found in a patient with episodic ataxia and paramyotonia congenita. Coexistence of these two ion channelopathies in this patient raises the possibility of a role of sodium channels in episodic ataxias (Rajakulendran et al., 2009).
NaV1.5 (SCN5A)
Several syndromes leading to sudden cardiac death have been linked to NaV1.5. For example, over 80 SCN5A mutations have been identified in patients with long QT syndrome type 3 (Zimmer and Surber, 2008). These mutations mostly disrupt fast inactivation, thereby causing persistent sodium currents (Bennett et al., 1995). Similarly, Brugada syndrome also leads to sudden cardiac death that may account for up to 50% of all sudden cardiac deaths in young individuals without structural heart disease. SCN5A mutations were found in ∼20% of patients with Brugada syndrome (Kapplinger et al., 2010) resulting in channel loss-of-function through a number of different mechanisms including expression of non-functional NaV1.5 (Valdivia et al., 2004; Hsueh et al., 2009), decreased protein expression (Kyndt et al., 2001), impaired membrane trafficking (Baroudi et al., 2001, 2002) or defective channel inactivation (Hsueh et al., 2009). Although NaV1.5 has been mainly linked to cardiac disease, a more recent report shows a novel SCN5A mutation in a patient with idiopathic epilepsy who died in sudden unexpected death in epilepsy (SUDEP) (Aurlien et al., 2009)
Interestingly, VGSC upregulation has been associated with several strongly metastatic carcinomas, leading to the hypothesis that VGSC upregulation may ‘switch’ the cancerous cell to a highly invasive state (Onkal and Djamgoz, 2009). Some cancers express embryonic/neonatal VGSC splice variants, for example, a neonatal isoform of NaV1.5 (seven amino acid differences) is the predominant (>80%) VGSC in human metastatic breast cancer (Fraser et al., 2005) as well as neuroblastoma (Ou et al., 2005).
NaV1.6 (SCN8A)
NaV1.6 is broadly expressed in the nervous system in a variety of cells including Purkinje cells, motor neurons, pyramidal and granule neurons, glial cells and Schwann cells and is enriched at the nodes of Ranvier (Caldwell et al., 2000; Kearney et al., 2002). Mutation in SCN8A is not a common cause of human disease although a patient with a heterozygous mutation in SCN8A has been described with mental retardation, pancerebellar atrophy and ataxia (Trudeau et al., 2006). This mutation caused a C-terminal truncation of NaV1.6 resulting in predicted loss of channel function. Naturally occurring med mutant NaV1.6 knockout mice show a range of movement disorders including tremor, ataxia, dystonia and paralysis (Meisler et al., 2002, 2004). Mutant mice are also reported to have disordered sleep patterns with a chronic impairment of REM sleep and enhanced spatial memory (Papale et al., 2010). In Purkinje cells of NaV1.6 knockout mice, resurgent currents are reduced and spontaneous and evoked firing was attenuated (Raman et al., 1997). Recently a de novo pathogenic SCN8A mutation with greatly increased persistent current was identified in a case of SUDEP with infantile epileptic encephalopathy (Veeramah et al., 2012). Persistent NaV1.6 activity can trigger axonal injury within white matter axons during experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis (Craner et al., 2004). In non-excitable cells such as macrophages, NaV1.6 is expressed at an intracellular location on podosomes (Carrithers et al., 2009a). In this study, inhibition of NaV1.6 with TTX or by genetic ablation was effective at reducing invasion of macrophages into melanoma. Similarly, a non-neuronal role for NaV1.6 contributing to the invasiveness of cervical carcinomas has been suggested (Hernandez-Plata et al., 2012). This suggests that targeting NaV1.6 in non-neuronal tissue might have therapeutic potential to treat cancer or autoimmune disorders such as multiple sclerosis (Carrithers et al., 2009b). However, the essential role of NaV1.6 in many neurological functions may make this a difficult task.
NaV1.7 (SCN9A)
NaV1.7 is expressed in peripheral sensory neurons innervating the skin, viscera and orofacial region (dorsal root and trigeminal ganglia) as well as sympathetic neurons and olfactory epithelia (Toledo-Aral et al., 1997; Weiss et al., 2011). A number of human heritable pain disorders map to mutations in SCN9A, the gene encoding NaV1.7 (Dib-Hajj et al., 2010). Dominant gain-of-function mutations lead to inherited primary erythromelalgia, which is characterized by bilateral burning pain of the feet/lower legs and hands, elevated skin temperature of affected areas and reddened extremities (Yang et al., 2004). Additionally, dominant gain-of-function mutations can cause paroxysmal extreme pain disorder which is characterized by episodic burning pain of the rectum, ocular and mandibular regions (Fertleman et al., 2006). Rare recessive loss-of-function conditions can cause an inability to experience pain (Cox et al., 2006; Ahmad et al., 2007) and anosmia (Weiss et al., 2011).
Biophysical characterization of the NaV1.7 mutations present in patients with erythromelalgia shows a significant hyperpolarizing shift in voltage dependence of activation (Cummins et al., 2007), resulting in gain-of-function. The NaV1.7 mutations underlying paroxysmal extreme pain disorder, where mechanical stimulation evokes excruciating pain (Fertleman et al., 2006), attenuate the fast inactivation of NaV1.7 resulting in persistent sodium currents. Such a deficit in inactivation is predicted to produce prolonged bursts of action potentials leading to increased nociceptive signalling. In mouse studies, selective knockout of NaV1.7 expression in NaV1.8-positive nociceptors lead to a loss of acute noxious mechanosensation and inflammatory pain (Nassar et al., 2004), while deletion of NaV1.7 in all sensory neurons leads to additional loss of noxious thermosensation (Minett et al., 2012). These data suggest that NaV1.7 expressed within NaV1.8-positive sensory neurons are important for acute noxious mechanosensation, whilst NaV1.7 expressed within NaV1.8 negative dorsal root ganglion neurons are essential for acute noxious thermosensation. Furthermore, no effect on neuropathic pain behaviour was observed in mice that lack expression of NaV1.7 in NaV1.8-positive sensory neurons (Nassar et al., 2005). This is also true for mice in which NaV1.7 has been deleted from all dorsal root ganglion neurons. In contrast, mice in which NaV1.7 is deleted from all sensory neurons as well as sympathetic neurons show a dramatic reduction in mechanical hypersensitivity following a surgical model of neuropathic pain, demonstrating an important role for NaV1.7 in sympathetic neurons in the development of neuropathic pain (Minett et al., 2012). Overall, the role of NaV1.7 in human as well as animal pain perception highlights NaV1.7 as an important analgesic drug target.
NaV1.7 is not only implicated in pain perception. Weiss et al. (2011) demonstrated that NaV1.7 is an essential requirement for odour perception in both mice and humans. Surprisingly, NaV1.7 is required for synaptic signalling from the primary olfactory neurons to mitral cells, and the release of substance P from nociceptive neurons has also been shown to be NaV1.7-dependent (Weiss et al., 2011; Minett et al., 2012).
NaV1.8 (SCN10A)
NaV1.8 is a TTX-resistant sodium channel subtype that is expressed in nociceptive sensory neurons (Akopian et al., 1999) and acts as a major contributor to the upstroke of action potentials (Renganathan et al., 2001). NaV1.8 is essential in maintaining the excitability of nociceptors at low temperatures (Zimmermann et al., 2007), becoming the sole electrical impulse generator at temperatures <10°C. This is caused by enhanced slow inactivation of TTX-sensitive channels in response to cooling, whereas inactivation of NaV1.8 is cold resistant. Behavioural studies of mice in which NaV1.8 expressing sensory neurons are ablated show loss of response to noxious cold and noxious mechanical stimuli (Abrahamsen et al., 2008). Antisense studies have shown an important role for NaV1.8 channels in inflammatory pain (Khasar et al., 1998). Antisense oligonucleotides attenuate the development and maintenance of neuropathic pain in rats (Lai et al., 2002; Joshi et al., 2006) while small interfering RNA selective knockdown of NaV1.8 reverses mechanical allodynia (Dong et al., 2007). However, NaV1.8 knockout mice as well as NaV1.7/1.8 double knockout mice show normal neuropathic pain behaviour (Kerr et al., 2001; Nassar et al., 2005). However, selective blockers of NaV1.8 such as A-803467 (Jarvis et al., 2007) and ambroxol (Gaida et al., 2005) successfully suppress various pain symptoms and neuropathic pain in rats. A recent genome wide association study has identified a single nucleotide polymorphism in NaV1.8 which was associated with prolonged cardiac conduction (Chambers et al., 2010) (longer P-wave duration, PR interval and QRS duration), thereby providing evidence that NaV1.8 has a functional role in the heart, probably through effects on innervation rather than cardiac muscle.
NaV1.9 (SCN11A)
NaV1.9 is the most recently discovered VGSC subtype (Dib-Hajj et al., 1998). It is a marker of primary nociceptors (Fang et al., 2002) and is also expressed in the enteric nervous system (Rugiero et al., 2003). NaV1.9 is a biophysically unique sodium channel which generates TTX-resistant currents that have very slow gating kinetics (Dib-Hajj et al., 2002). The current generated by NaV1.9 is ‘persistent’ and can be activated at potentials close to resting membrane potential (∼−60 mV). Although the activation kinetics are too slow to contribute to the up-stroke of an action potential, the channel acts as a modulator of membrane excitability by contributing regenerative inward currents over a strategic membrane potential range both negative to, and overlapping with the voltage-threshold for other transient sodium channels.
While a selective blocker of NaV1.9 does not exist at present, SCN11A knockout mice exhibit a clear analgesic phenotype (Priest et al., 2005; Amaya et al., 2006), confirming NaV1.9 is an important player in generating hyperalgesia in inflammatory pain states. This appears to be explicable by changes in the properties of distal primary afferents. The response to inflammatory mediators is suppressed in NaV1.9 knockout mice consistent with the immunocytochemical localization of the channel at unmyelinated nerve endings (Black and Waxman, 2002; Padilla et al., 2007), and the remarkable functional plasticity of the current, known to be under G-protein pathway control via protein kinase C (Baker et al., 2003; Baker, 2005). Overall, therapeutically targeting NaV1.9 may help regulate pain thresholds following inflammation or injury.
β-Subunits of voltage-gated sodium channels
β-Subunits of VGSCs belong to the immunoglobulin superfamily of cell adhesion molecules and associate with α-subunits in two ways: covalently in the case of β2 and β4 subunits and non-covalently for β1 and β3 subunits (Patino and Isom, 2010). VGSC β-subunit expression is widespread both in excitable and non-excitable tissues (Patino and Isom, 2010). Although reported effect sizes vary, β-subunits shift the voltage-dependent gating of VGSC in heterologous expression systems (Zhao et al., 2011). In humans, mutations in β-subunits have been linked to numerous cardiac and epilepsy related diseases (Table 1). Heterozygous β1 mutations have been identified in seven families with generalized epilepsy with febrile seizures plus (Scheffer et al., 2007), (4–6% of generalized epilepsy with febrile seizures plus patients) with the most common mutation being C121W, which leads to impaired trafficking of VGSC to the axon initial segment (Wimmer et al., 2010). A human SCN1B epilepsy-related mutation (G257R) unique to a splice variant of β1BA has been proposed to contribute to epilepsy through a mechanism that includes intracellular retention β1 resulting in aberrant neuronal path-finding (Patino et al., 2011). Mice heterozygous for β1 (C121W) displayed behavioural arrest at elevated core temperatures and enhanced axon initial segment excitability, which is proposed to be due to a hyperpolarized shift in the voltage dependence of activation of VGSC expressed at the axon initial segment (Wimmer et al., 2010). Mutations in all four β-subunits have been linked to cardiac pathologies including Brugada syndrome (β1 and β3), atrial fibrillation (β1, β2 and β3), ventricular fibrillation (β3) and long QT syndrome (β4) (Table 1). Mutations in β3 and β4 have also been linked to sudden infant death syndrome (found in 1% of cases) due to reduced peak sodium current through NaV1.5 and enhanced ‘late sodium current’ (Tan et al., 2010).
Expression levels of VGSC β-subunits vary in different pathological conditions (nerve injury, pain, Huntington’s disease) and knockout models of VGSC β-subunits display pain, epilepsy and ataxia phenotypes (Patino and Isom, 2010), suggesting that the range of VGSC β-subunit roles in pathological conditions may be wider than known. Interestingly, recent reports also show that the affinity and efficacy of VGSC inhibitors can be dramatically altered by changing β-subunit expression levels (Uebachs et al., 2010; Wilson et al., 2011) and that β-subunit expression levels change during diseases such as Huntington’s disease (mouse model) (Oyama et al., 2006) and after nerve injury (Pertin et al., 2005). It remains to be seen whether this altered pharmacology of α-β complexes can be utilized to produce VGSC blockers with higher selectivity and efficacy in vivo.
VGSC β-subunits also interact with the extracellular matrix as well as the cytoskeleton and intracellular signalling molecules (Isom, 2002; Brackenbury and Isom, 2011). Enzymatic cleavage leads to production of a soluble ectodomain and membrane bound C-terminal fragment, which have been implicated in the regulation of cell–cell contact and neurite outgrowth (Wong et al., 2005). The β4-subunit was recently identified as a novel substrate of the β-secretase, BACE1, an enzyme implicated in the pathogenesis of Alzheimer’s disease (Huth et al., 2011). In BACE1 knockout mice, the decay of the resurgent sodium current recorded from Purkinje cells was found to be slowed and could be modelled as a decrease in open pore block consistent with proteolytic modification of β4.
Sodium channel trafficking and disease
The pivotal role of sodium channels in electrical signalling requires targeting of VGSCs to the correct cellular location. High channel densities of VGSCs can be found at the axon initial segment and nodes of Ranvier as part of complex protein aggregates (Hedstrom and Rasband, 2006). Cytoplasmatic proteins regulate expression and function of VGSCs through binding to the intracellular domain of VGSCs, that are, in contrast to the extracellular domain, relatively divergent (Wood et al., 2004). To date, several studies have focused on identifying VGSC-associated proteins of which some are involved in trafficking (Diss et al., 2004; Shao et al., 2009; Leterrier et al., 2010). For example NaV1.8 requires the expression of the annexin p11 subunit, which binds to the N-terminal region of the channel to facilitate cell-surface expression of the channel (Okuse et al., 2002). Nerve growth factor upregulation of functional NaV1.8 expression, important in inflammatory pain appears to be indirectly mediated through enhanced p11 expression and trafficking (Okuse et al., 2002; Poon et al., 2004). In addition, the interaction of the N-terminus of NaV1.6 with microtubule-associated protein Map1b facilitates trafficking of NaV1.6 to neuronal surfaces (O'Brien et al., 2012).
A variety of protein kinases have been shown to regulate the trafficking of VGSCs to the cell membrane or to specialized membrane domains, such as lipid rafts (reviewed in Shao et al., 2009). Stimulation of the β2-adrenergic receptor leads to localization of cardiac NaV1.5 to caveolin-enriched membrane domains resulting in increased function and thereby possibly promoting cardiac arrhythmias (Yarbrough et al., 2002). Moreover, trafficking of intracellular pools of the sensory neuron-specific VGSCs NaV1.8 and NaV1.9 has been implicated in enhanced pain sensitivity (Dib-Hajj et al., 2010).
The co-factors required for NaV1.9 expression have not been defined, but this channel can only be functionally expressed in dorsal root ganglion neurons where it rescues the expression of persistent current in NaV1.9 knockout neurons (Ostman et al., 2008).
A number of VGSC mutants found in several human diseases have been found to be trafficking-deficient and may give insights into key protein regions/domains important for the regulation of VGSC trafficking (Table 1). Trafficking defects may arise due to improper protein folding or altered binding to essential chaperones within the endoplasmic reticulum, ultimately leading to endoplasmic reticulum retention and/or protein degradation. Alternatively, VGSC domains that are crucial for binding to associated proteins regulating VGSCs localization/functioning may be affected. An important family of scaffolding proteins, ankyrins, is responsible for the localization of structurally diverse membrane-associated and cytosolic protein, including VGSCs. Ankyrin-G is important in clustering NaV1.2 and NaV1.6 into nodes of Ranvier and axon initial segments (Jenkins and Bennett, 2001; Garrido et al., 2003). A nine-residue motif has been characterized in the DII–III loop that is critical for ankyrin-G binding. This sequence is highly conserved within all VGSC isoforms and is almost identical between NaV1.2, NaV1.5 and NaV1.6 (Lemaillet et al., 2003). Mutation of the ankyrin-G binding site of NaV1.6 prevents clustering at the axon initial segments (Gasser et al., 2012). A mutation associated with Brugada syndrome has been found within this ankyrin-G-binding motif of NaV1.5. This mutation (E1053K) abolished ankyrin-G binding resulting in a loss of membrane expression in cardiac myocytes (Mohler et al., 2004). Other Brugada syndrome mutations in NaV1.5 have been shown to be associated with defective trafficking/surface localization emphasizing the importance of correct targeting of this protein for cardiac function (Baroudi et al., 2001, 2002; Kyndt et al., 2001; Valdivia et al., 2002; Bezzina et al., 2003; Herfst et al., 2003; Ruan et al., 2010).
In patients with long QT syndrome, mutations in NaV1.5-associated genes have been found, such as ankyrin-B and SCN4B (Saenen and Vrints, 2008). β-Subunits regulate the surface density and the biophysical properties of the channel complex (Shao et al., 2009) and knockout mice lacking β2 subunit show reduced VGSC surface expression (Chen et al., 2002). Moreover, a recent report showed that a loss-of-function mutation of the SCN3B-encoded channel β3 subunit (Navb3–V54G) is associated with a case of idiopathic ventricular fibrillation. This mutation caused a trafficking defect of NaV1.5 to the plasma membrane (Valdivia et al., 2010). Conversely, β-subunits have also been shown to rescue a trafficking-defective NaV1.1 mutant (Rusconi et al., 2007).
Four mutations in the SCN1B gene have been described that lead to an inherited generalized epilepsy with febrile seizures plus. Both mutations occur in a domain of the β1 subunit that is important for the regulation of the subcellular localization of VGSCs within neurons (Wimmer et al., 2010). An epilepsy causing SCN1A loss-of-function mutation within the region of the C-terminal cytoplasmatic domain (M1841T) that is involved in interactions with accessory subunits has been identified as trafficking defective. Importantly, trafficking of this mutant could be rescued by modulatory proteins, such as β-subunits, calmodulin or G protein β2γ3 and the anti-epileptic drug phenytoin (Rusconi et al., 2007). However, phenytoin cannot be used therapeutically as it also blocks channel function.
The broadly expressed 20 amino acid IQ motif also found in VGSCs binds to the ubiquitously expressed Ca2+-sensing protein calmodulin. Mutation of the NaV1.4 calmodulin-binding IQ motif showed that this domain is indispensable for normal channel expression and functioning (Biswas et al., 2008). A mutation of SCN2A that reduced affinity for calcium-bound calmodulin was observed in a patient with autism (Weiss et al., 2003). Finally, a recent study has identified two novel non-truncating missense mutations in families with congenital insensitivity to pain that were mapped within the pore domain of SCN9A. These mutations cause complete loss-of-function as well as membrane expression of the channel (Cox et al., 2010).
There are no effective drugs in use that target trafficking of VGSC. However, some reports have shown that mexiletine, a drug used to inhibit persistent sodium current and to shorten QT interval, rescues trafficking in defective SCN5A mutants (Valdivia et al., 2002; Ruan et al., 2010). The rescue of trafficking of these mutants, however, counteracts the effectiveness of the drug as the increased trafficking may exacerbate the QT prolongation due to increased expression of the mutant protein. In contrast, phenytoin not only rescues a trafficking-defective SCN1A mutant but also blocks the channel (Rusconi et al., 2007). Thus, drugs that can act as folding chaperones to rescue mutant protein, but do not block channel function, are required.
Although effective drugs have yet to be designed to modulate VGSC expression by interfering with the trafficking pathway, some promising results have been obtained with the anti-epileptic and anti-nociceptive drug gabapentin and its derivatives. These drugs exert their effect primarily via inhibition of trafficking of the voltage-gated α2δ2 calcium channel subunit (Heblich et al., 2008; Hendrich et al., 2008) indicating that drugs targeting trafficking may be useful in VGSC-related pathologies.
Toxins as useful tools to understand voltage-gated sodium channel function and pharmacology
Toxins have been useful in understanding the structural and molecular determinants of VGSC gating through their modifying actions on the gating of VGSCs (Catterall et al., 2007). At least six toxin-binding sites (sites 1–6) for toxins have been localized to specific regions of sodium channels. The site of interaction of a number of more recently characterized toxins, including the inhibitory µO-conotoxins and spider toxins, remains to be fully characterized. Binding to these sites affects channel ion conduction or gating, and sequence differences in the residues involved contribute to subtype specificity (Catterall, 2000). Site 1 toxins such as TTX and the µO-conotoxins inhibit current, while most site 2–6 toxins enhance sodium current through effects on channel gating. While channel enhancers have helped to characterize gating and inactivation mechanisms of sodium channels, and the allosteric interactions between toxin binding sites, these classes of toxins invariably produce toxic effects at all doses.
The usefulness of toxins as clinically relevant drugs is limited in part by their high molecular weights and lack of subtype specificity. However, a peptide derived from tarantula venom, peptide ProTx-II is two orders of magnitude more selective for NaV1.7 compared with other heterologously expressed VGSCs and blocked action potential propagation in nociceptors (Schmalhofer et al., 2008a). Moreover, a µO-conotoxin selectively blocks NaV1.8 currents and chronic pain behaviour in animal models (Ekberg et al., 2006).
Mechanisms of drug binding to voltage-gated sodium channels
VGSCs, like other voltage-gated ion channels, can be differentially modulated by compounds that bind selectively to distinct conformational states of the channels. Upon changes in membrane potential, VGSCs undergo voltage-dependent gating that consists of a succession of conformational transitions: non-conducting closed states upon depolarization adopt an activated conducting open state followed by open-state inactivation in which the flow of ions through the channel pore is blocked by the movement of a molecular ‘ball’ into the cytoplasmic side of the newly opened pore (Armstrong and Hille, 1998) (Fig. 1). Repolarization of the membrane leads to another conformational transition, the deactivation gate (Kuo and Bean, 1994), which consists in a brief opening of the channel before reaching the closed states again. Other conformational states of VGSCs include closed-state inactivation (Armstrong, 2006), slow inactivation and for some VGSCs the ‘resurgent current’ gate. Closed-state inactivation is engaged upon small depolarizations (these allow the movement of the S4 voltage sensors in domains III and IV only, which is enough for the inactivation particle to move into the pore before the channel opens). Slow inactivation is reached during prolonged depolarizations.
Extensive mutagenesis studies of VGSCs have identified the local anaesthetic binding site as the intracellular surface of the S6 helices (Ragsdale et al., 1994, 1996), binding to which causes occlusion of the pore. The modulated receptor hypothesis (Hille, 1977) first predicted that the local anaesthetic binding site could be accessed via two distinct mechanisms: a hydrophilic pathway requiring binding of the drug from the intracellular side during channel opening and a hydrophobic pathway whereby local anaesthetics can access the water filled pore directly. Indeed, the recent crystal structure of the Arcobacter butzleri VGSC NavAb confirmed the existence of hydrophobic fenestrations within the protein lipid interface composed of fatty acyl chains (Payandeh et al., 2011, 2012). These observations suggest a molecular mechanism for closed state and use/frequency-dependent inhibition of VGSCs. Highly hydrophilic local anaesthetics have limited access to the hydrophobic pathway and instead require opening of VGSCs, allowing access of local anaesthetics to the pore and promoting binding of local anaesthetics in the inactivated state. In this case cumulative block occurs with high-frequency opening when dissociation from the local anaesthetic binding site occurs with a time constant slower than the association rate. This results in accumulation of inhibition and makes potency dependent on opening frequency. By contrast, neutral or hydrophobic local anaesthetics can access the local anaesthetic binding site through both the hydrophobic pathway when the channel is in the closed state and the hydrophilic pathway during channel opening, resulting in a combination of tonic and use-dependent blocking properties. In addition, quaternary amines such as QX-222 or QX-314 may have restricted access to the local anaesthetic binding site in accordance with the guarded receptor hypothesis (Starmer and Hollett, 1985), which may also contribute to use-dependent block. Many neurological pathological conditions result from neurons firing action potentials at higher frequencies than normal, which lead to these cells displaying a tonically depolarized membrane potential. Therefore, voltage-dependent compounds that exhibit frequency-dependent inhibition of VGSCs are desirable as they will tend to only target VGSCs in affected areas, leaving healthy tissues safe. The voltage dependence of VGSC ligands along with their pharmacokinetic properties (on and off rates) is critical in determining the mode of action of these compounds. Chemical entities with affinity for the resting state of VGSCs, like TTX, simply bind to the extracellular regions of VGSCs, block the passage of ions and cannot be removed by either changing the membrane voltage or the gating of the channel. On the contrary, compounds with affinity for the open-inactivated state need channel opening, and therefore membrane depolarization, to bind to the inner pore of VGSCs. The pharmacokinetic properties of these ligands determine the optimal frequency at which blockade is strongest: slow dissociation rates promote use-dependent block at low frequencies, whereas fast off rates favour block at high frequencies. In other words, voltage-dependent drugs that dissociate quickly from VGSCs when the membrane potential is returned to resting values upon action potential repolarization, such as anti-epileptic VGSC blockers, are best at affecting high-frequency firing as observed in epileptic conditions. On the contrary, voltage-dependent compounds that dissociate more slowly, such as anti-arrhythmic and local anaesthetic VGSC blockers, tend to be more effective in blocking low-frequency firing.
Sodium channel targeted drugs
Voltage-gated sodium channel blockers as local anaesthetics
Cocaine was one of the first topical anaesthetics used by humans. Although cocaine is well known as a serotonin–norepinephrine–dopamine reuptake inhibitor, it also has VGSC blocking properties (Ruetsch et al., 2001). Over the years, novel VGSC blockers that can be used as local anaesthetics have been synthesized that target VGSC more specifically, have higher efficacy and have fewer side-effects. Currently, many different VGSC blockers are used as local anaesthetics such as lidocaine, bupivacaine and ropivacaine (Ruetsch et al., 2001). Local anaesthetics are weak bases that require to be injected as hydrochloride salts in acid solution to be dissolved. At the site of injection, where the pH is higher, local anaesthetics dissociate according to their pKa and release a free base. The free base is able to cross the nerve cell membrane and once inside the nerve, it becomes re-ionized due to the lower cytoplasmic pH and is unable to diffuse out of the cell (ion trapping).
The most common systemically applied local anaesthetics are lidocaine and mexiletine, which have been demonstrated to be effective drugs in treating neuropathic pain in controlled clinical studies (Challapalli et al., 2005). These anti-nociceptive effects of local anaesthetics can be observed even at plasma concentrations that would be too low to physiologically block nerve conduction. However, these low concentrations of local anaesthetics are still sufficient to block/attenuate impulse generation/ectopic discharges that cause pain while nerve conduction is unaffected (Mao and Chen, 2000). Importantly, the anti-inflammatory as well as anti-nociceptive effects of local anaesthetics cannot be explained solely by their action on VGSCs (Hollmann and Durieux, 2000; Mao and Chen, 2000). For example, systemic lidocaine enhances spinal inhibitory glycinergic neurotransmission independent of VGSC inhibition (Muth-Selbach et al., 2009).
Subtype selective blockers to treat pain
NaV1.7 and NaV1.8 have expression patterns restricted predominantly to the PNS and are both essential for normal pain transmission. Selective antagonists (Table 2) of these channels therefore make attractive targets for the treatment of pain due to the reduced chance of CNS or cardiac side-effects (although NaV1.8 may play a role in cardiac conduction). A-803467 is a NaV1.8 selective small molecule showing selective block of both recombinant and native NaV1.8 currents (Jarvis et al., 2007). In vitro studies performed on isolated small-diameter dorsal root ganglion neurons have demonstrated that A-803467 blocks NaV1.8 currents in a voltage-dependent manner and inhibits action potential firing. A-803467 shows efficacy in alleviating acute mechanical pain, inflammatory thermal hyperalgesia and neuropathic pain in rodents (Jarvis et al., 2007). These behavioural data are consistent with data showing systemic injection of A-803467 decreases both mechanically evoked and spontaneous firing of spinal neurons in nerve-injured rats (McGaraughty et al., 2008). The identification of this compound provides an important proof of concept that it is possible to develop isoform-specific blockers of sodium channels that are analgesic. Following the development of the NaV1.8 selective blocker A-830467, Abbott Labs have succeeded in developing an orally active preparations based on a modified structure of A-830467 that is effective in rodent models of neuropathic pain (Drizin et al., 2008). These compounds generally inhibited NaV1.8 with IC50s in the sub-micromolar range and had some selectivity for NaV1.8 over other Nav isoforms with the best compound displaying a 5-fold and 20-fold greater potency for NaV1.8 over NaV1.2 and NaV1.5, respectively. Importantly, this class of drugs shows improved effects after oral application and better safety profiles than currently clinically used sodium channel blockers such as mexiletine and lamotrigine (Drizin et al., 2008).
Table 3.
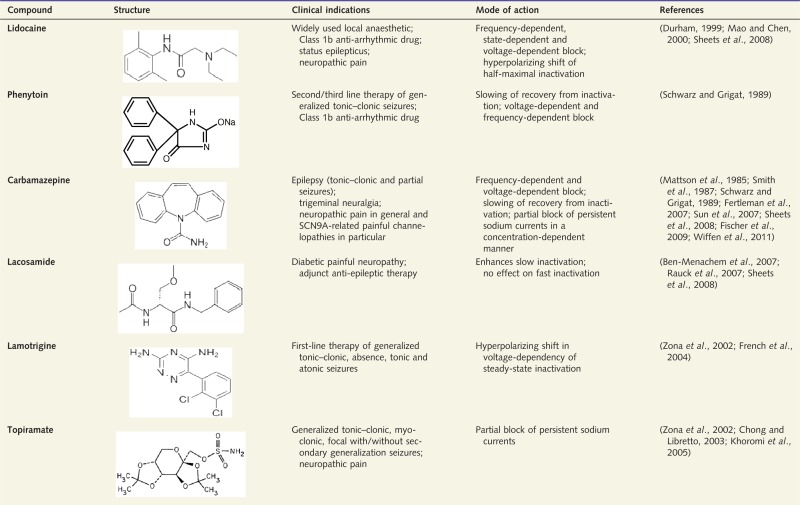
Most commonly used classic, non-selective VGSC blockers
 |
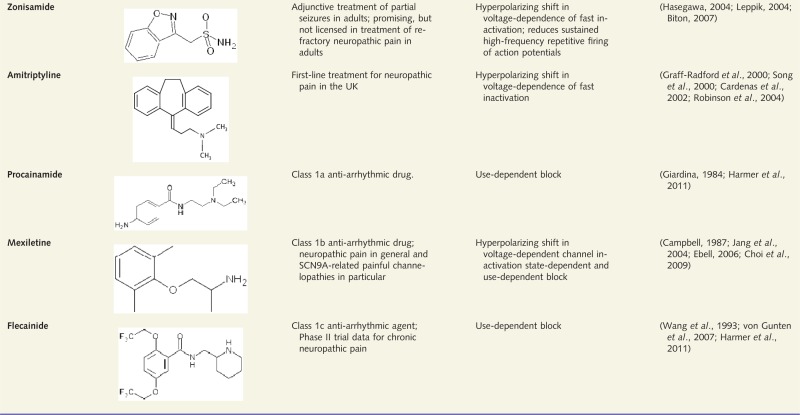 |
Although many of these blockers also act through mechanisms other than blocking VGSCs, only VGSC-related indications and mechanisms are reported.
Table 2.
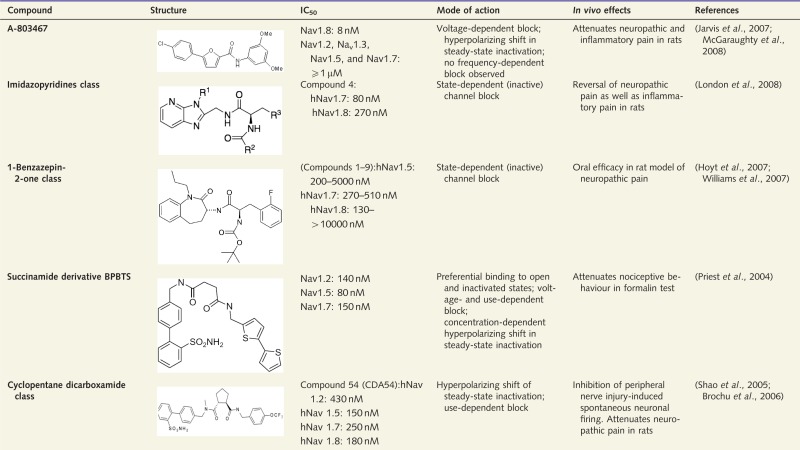
Isoform-selective compounds
 |
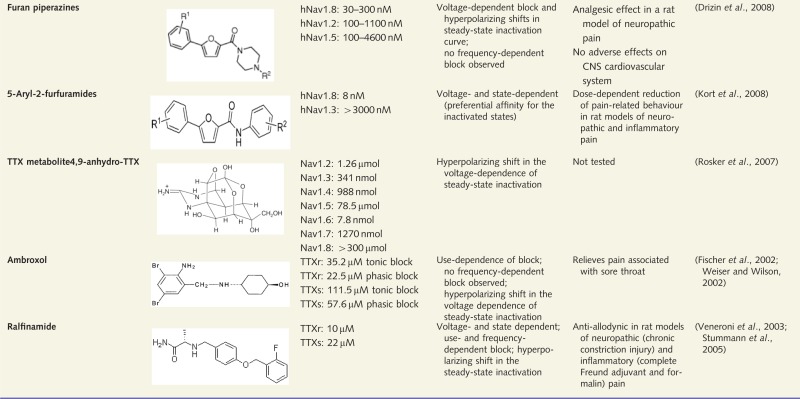 |
This table summarizes examples of VGSC isoform-selective compounds that have been investigated in an approach to identify potentially therapeutically useful drugs. TTXr = TTX-resistant; TTXs = TTX-sensitive.
NaV1.7 blockers have also been developed, but the benzazepinone structures have equipotent actions on Nav 1.2 and 1.5, suggesting that side-effects may be an issue (Williams et al., 2007).
Currently, specific NaV1.7 blockers are being tested by a number of companies in human trials. For example, Convergence Pharmaceuticals are evaluating a NaV1.7 inhibitor in phase II trials of trigeminal neuralgia.
A selective small molecule NaV1.7 blocker (BZP) is an example of an approach to facilitate inhibition of peripherally expressed VGSCs by designing compounds that poorly penetrate the CNS (McGowan et al., 2009). BZP was demonstrated to have anti-nociceptive effects in animal models of inflammatory and neuropathic pain after oral administration, while inducing fewer CNS-related side-effects compared to mexiletine.
State-dependent acting agents to treat pain
Biophysical characterization of rare gain-of-function mutations affecting pain signalling has provided us with invaluable insight into the way various sodium channel blocking drugs differentially modulate the transition between the states of VGSCs. NaV1.7 mutations in primary erythromelalgia and paroxysmal extreme pain disorder exhibit gain-of-function. Interestingly, patients with paroxysmal extreme pain disorder respond favourably to carbamazepine treatment, while carbamazepine is generally ineffective in patients with inherited primary erythromelalgia (Dib-Hajj et al., 2007; Fertleman et al., 2007). Paroxysmal extreme pain disorder mutations enhance recovery from inactivation and mutant channels can give rise to persistent and enhanced resurgent currents (Dib-Hajj et al., 2008; Jarecki et al., 2008; Theile et al., 2011). Carbamazepine specifically targets these deficits by shifting the voltage dependence of fast inactivation towards more hyperpolarized potentials and targets persistent currents while leaving normal currents relatively unaffected. In contrast, in most patients with inherited primary erythromelalgia, negative shifts in the voltage dependence of activation are observed. These altered properties of the channel are not affected by carbamazepine. In addition, sodium channel inhibitors such as riluzole that effectively target persistent currents and accelerate the rate of inactivation display enhanced efficacy towards inhibiting Navβ4-peptide-mediated resurgent currents and also paroxysmal extreme pain disorder mutant currents (Theile et al., 2011). In agreement with this view, it was recently demonstrated that patients with primary erythromelalgia with a (V400M) mutation in SCN9A also display a modified VGSC fast inactivation and can be successfully treated with carbamazepine (Fischer et al., 2009).
The local anaesthetics mexiletine and lidocaine are effective in some cases of primary erythromelalgia (Iqbal et al., 2009; Kuhnert et al., 1999). Importantly, the effectiveness of these drugs can be affected by the causative mutation. For example, a specific primary erythromelalgia causing mutation (V872G) can lead to increased use-dependent block of this mutant channel, indicating some patients might have a favourable response to mexilitine (Choi et al., 2009). On the contrary, another primary erythromelalgia mutation (N395K) has been found to cause a loss in lidocaine sensitivity and this was associated with ineffectiveness of treatment with Las (Sheets et al., 2007).
Lacosamide is a novel amino acid derivative with anti-convulsant activity that is also effective as an analgesic (Stohr et al., 2006) in several animal models of neuropathic pain (Beyreuther et al., 2006, 2007a) and as a therapy for painful diabetic neuropathy (Doty et al., 2007). Lacosamide selectively enhances sodium channel slow inactivation with no effects on fast inactivation (Errington et al., 2008) as demonstrated on recombinant NaV1.3, NaV1.7 and neuronal NaV1.8 currents (Sheets et al., 2008). Slow inactivation is induced under conditions of sustained depolarization and repeated firing, conditions relevant for the pathophysiology of chronic pain. The difference in affinity of lacosamide for binding inactivated channels rather than channels in the resting state was much higher than that for carbamazepine or lidocaine. Recently, it has been shown that lacosamide mediates some actions on VGSC through binding to collapsin response mediator protein 2 (now known as DPYSL2) and this interaction with collapsin response mediator protein 2 results in lacosamide-induced slowing of inactivation (Wang et al., 2010b). This novel class of VGSC blockers, which targets VGSC channels in specific conformations associated with certain pathologies, opens a new avenue of drug development that may lead to blockers of ‘pathological’ VGSCs. Recently, it has been shown that uncoupling of collapsin response mediator protein 2 from N-type voltage-gated calcium channels also suppresses inflammatory and neuropathic pain (Brittain et al., 2011). However, the relative importance of lacosamide effects on VGSC and voltage-gated calcium channels is uncertain (Wang and Khanna, 2011).
Alternative modes of action to treat pain
To limit the side-effects of VGSC blockers, one possibility is to develop compounds that target VGSCs to the desired tissue only (Clare, 2010). One example of such a drug is cyclopentane dicarboxamide CDA54, a non-selective VGSC blocker developed by Merck (Shao et al., 2005). Oral administration of CDA54 is effective at reducing pain responses in models of inflammatory and neuropathic pain (Shao et al., 2005; Brochu et al., 2006). Importantly, after oral administration of CDA54 into rats, brain homogenate concentrations were found to be 33-fold lower than plasma concentrations, thus reducing the likelihood of side effects caused by actions within the CNS. In addition, compound 54 showed less cardiotoxicity than mexiletine (Brochu et al., 2006).
Transdermal drug application may be an advantageous way of targeting the PNS. The effectiveness of this administration route has been demonstrated by the success of lidocaine patches. Lidocaine patches are approved for the relief of pain associated with post-herpetic neuralgia and have proved efficacious in the treatment of peripheral neuropathies, lower back pain, myofascial pain, osteoarthritis, leg ulceration, erythromelalgia and carpal tunnel syndrome (Nalamachu et al., 2006).
One often undesired effect of classic VGSC blockers such as lidocaine and its derivatives is that they block action potential firing not only in nociceptive neurons after perineural injection but also in other neurons, thereby inhibiting tactile and mechanical sensation as well as motor function. Thus, targeting derivates of lidocaine specifically to nociceptive neurons, while leaving tactile and mechanical sensation unaffected, is an attractive strategy to treat pain. One strategy for selectively inhibiting nociceptors is cell-specific targeting of the quaternary lidocaine-derivative QX-314 to nociceptive neurons. QX-314 produces a long-lasting non-selective neuronal block with a slow onset (Lim et al., 2007). The slow onset of neuronal block is most probably linked to the low membrane permeability of QX-314 reducing the capacity of QX-314 to reach the intracellular blocking site of VGSCs. However, the transient receptor potential cation channel (TRPV1) agonist capsaicin facilitates the selective cellular entry of QX-314 into nociceptive sensory neurons through TRPV1, which is selectively expressed in nociceptors (Binshtok et al., 2007). By combining QX-314 with other TRPV1 agonists such as lidocaine itself or protons (lowering pH), similar effects can be achieved (Binshtok et al., 2009; Liu et al., 2011; Roberson et al., 2011). One report also showed that QX-314 itself acts as a TRPV1 agonist (Rivera-Acevedo et al., 2011). Unfortunately, intrathecal application of QX-314 causes serious irritation and death (Schwarz et al., 2010) and is twice as toxic as lidocaine when applied systemically in mice (Cheung et al., 2011). Activation of TRPV1 results in intense pain and does therefore not appear to be the most appealing route to affect analgesia through the use of sodium channel blockers.
Biologicals as the next generation of analgesics
Over recent years, biological compounds such as peptides and antibodies have begun to feed into drug discovery programmes for many disease indications including pain. These include analgesic peptides based on venom toxins, which interact with VGSC. The perceived advantage of venom peptides over conventional small molecule inhibitors is that the toxins are often highly potent and efficacious (low nanomolar IC50s) and have a greater potential for selectivity due to their larger drug target interface. Subsequent mutagenesis of the wild-type toxin can then further improve potency and strive to improve selectivity for a given VGSC isoform. ProTx-II is a venom toxin from the tarantula Thrixopelma prurient and has been reported to block NaV1.7 channels (IC50 = 0.3 nM) with >100-fold selectivity over other Nav isoforms (Middleton et al., 2002; Priest et al., 2007; Schmalhofer et al., 2008a). ProTx-II was effective at reducing C-fibre action potential firing frequency in an isolated skin nerve preparation in which the nerve had been de-sheathed (Schmalhofer et al., 2008b); however, no effect of ProTx-II was observed with an intact nerve sheath indicating that this peptide cannot access sodium channels in intact tissues. Intravenously applied ProTx-II was also ineffective at reducing complete Freund’s adjuvant-induced mechanical hyperalgesia. These data confirm that targeting NaV1.7 with a potent selective inhibitor is sufficient to dampen peripheral nociceptive drive (reduce firing of C fibres); however, due to the low permeability of ProTx-II, it is ineffective as an analgesic. Future toxin-derived analgesics targeted to peripherally expressed proteins must therefore overcome this limitation. Importantly, it is possible to use a herpes viral vector to specifically deliver biologicals to sensory neurons of the dorsal root ganglion (Fink et al., 2011).
Monoclonal antibodies targeted to essential pain pathway proteins also have the potential to revolutionize analgesic drug discovery due to their potential for high selectivity, high affinity (femtomolar range), low cardiotoxicity and long half-life (monthly subcutaneous injections are achievable). Monoclonal antibodies targeted against nerve growth factor (e.g. tanezumab, Pfizer) have successfully been used to treat chronic joint pain in osteoarthritic patients (Cattaneo, 2010). Polyclonal antibodies targeted against the second or third extracellular loop of ion channels have been successfully used as isoform selective channel blockers of TRP channels (Klionsky et al., 2006; Naylor et al., 2008), VGSCs (Chioni et al., 2005) and voltage-gated calcium channels (Liao et al., 2008). However, to date there are no published records of a therapeutically useful monoclonal antibody with ion channel blocking function, although a patent has been filed (US2011/0135662 A1) which describes a NaV1.7 (E3 loop) targeted rabbit antibody which inhibits NaV1.7 currents in a frequency-dependent manner, indicating that this approach may be valid.
Voltage-gated sodium channel blockers in neurological diseases
Voltage-gated sodium channel blockers as anti-epileptic drugs
Phenytoin and carbamazepine are the most widely used compounds to treat epilepsy. Both these drugs act in a state-dependent manner and slow the recovery from inactivation, thereby reducing the availability of channels for subsequent opening (Rogawski and Loscher, 2004). Phenytoin and carbamazepine are both effective in combating partial and generalized tonic–clonic seizures in humans and in animal models of these conditions (Perucca and Tomson, 2011). However, phenytoin and carbamazepine do not show efficacy against absence seizures (very brief generalized epileptic seizures of sudden onset and termination) both in humans and in the animal models of this condition (Dreifuss, 1983; Mantegazza et al., 2010). Phenytoin is most effective at depolarized membrane potentials and high-frequency action potential firing. The state dependence of phenytoin causes minimal effects on cognitive functions (low-frequency firing). Carbamazepine displays the same pharmacological properties as phenytoin (VGSC specificity, voltage and state dependence). However, carbamazepine binds VGSCs less effectively, but with a much faster rate than phenytoin, rendering carbamazepine more effective in blocking high-frequency firing (Mantegazza et al., 2010). These differences in properties might explain why some epileptic patients respond better to one or the other drug if they carry different VGSC mutations.
Lamotrigine, like phenytoin and carbamazepine, is effective against partial and generalized tonic–clonic seizures and also shows efficacy for the treatment of absence seizures and Lennox–Gastaut syndrome, a rare and intractable form of childhood epilepsy associated with learning difficulties. The mode of action of lamotrigine on VGSCs is similar to that of phenytoin and carbamazepine (voltage and use dependence). However, lamotrigine also acts on other molecular targets, such as the hyperpolarization-gated cationic current Ih in dendrites of pyramidal neurons (Poolos et al., 2002), N- and P-type voltage-gated Ca2+ channels in cortical neurons (Stefani et al., 1996) and neocortical potassium currents (Zona et al., 2002). Therefore, the anti-epileptic action of lamotrigine may have a different biophysical basis to carbamazepine and phenytoin.
Topiramate, a sulphamate derivative of the naturally occurring sugar d-fructose, is another broad-spectrum anti-epileptic drug prescribed in cases of partial and generalized tonic–clonic seizures. It blocks both VGSCs and voltage-gated Ca2+ channels and enhances potassium channel activity (Shank and Maryanoff, 2008). Interestingly, the action of topiramate on VGSCs consists of slowing down the opening of the channels and protein kinase C activation limits the effect of topiramate of blocking persistent sodium currents (Curia et al., 2004).
Introduced as an anti-epileptic drug in 1962, valproate has an even more extensive range of pharmacological actions, being effective against partial and generalized tonic–clonic seizures, absence seizures, and myoclonic seizures. Valproate is a widely prescribed anti-epileptic drug, in both adults and children, and its success is likely to be due to modes of actions different from blocking VGSCs. However, valproate selectively inhibits persistent sodium currents over transient ones in neocortical and sympathetic neurons (Taverna et al., 1998; Lamas et al., 2009), although the exact mechanism remains unknown. In part, its therapeutic effects are caused by increases in the turnover of GABA, inhibition of NMDA (N-methyl-d-aspartic acid) receptors and reduction in gamma-hydroxybutyrate release (Maitre, 1997).
Riluzole was first developed as an anti-epileptic drug but is now used as the first-in-line drug for treatment of amyotrophic lateral sclerosis. It has neuroprotective effects due to blockade of VGSCs on presynaptic terminals and enhancing glutamate uptake by astrocytes thereby inhibiting glutamatergic transmission. Additionally, riluzole has been demonstrated recently to protect against cardiac ischaemia and reperfusion injury by inhibiting persistent sodium currents and is now being tested as a possible treatment for psychiatric disorders (Pittenger et al., 2008; Weiss et al., 2010).
Voltage-gated sodium channel blockers for the treatment of migraine
Migraine is thought to originate from the activation of meningeal and blood vessel nociceptive fibres, in conjunction with neurogenic inflammation and a change in central pain modulation (Kalra and Elliott, 2007). In common with epilepsy, migraine is characterized by recurrent episodes of nervous system dysfunction with a return to baseline between attacks. Rare forms of familial migraine are caused by mutation of SCN1A (Dichgans et al., 2005; Vahedi et al., 2009). Migraine is treated with a wide range of drugs, including tryptans that, through their agonist effects on serotonin receptors, block the release of vasoactive neuropeptides such as CGRP. Other treatments include the use of non-steroidal anti-inflammatory drugs, anti-depressants and calcium channel blockers (Bolcskei et al., 2009). Epilepsy is a co-morbid condition of migraine. Several anti-epileptic drugs targeting VGSCs have been tested for efficacy in migraine conditions. Anti-epileptic compounds, such as valproate, topiramate and lamotrigine, which act through VGSC, have been shown to be effective at reducing the frequency of migraine attacks. However, these drugs all act on other targets as well and therefore their therapeutic effect may be unrelated to their effect on VGSCs (Calabresi et al., 2007). It is important to note that use-dependent selective VGSC blockers, such as phenytoin and carbamazepine, have not been documented to be efficacious against migraine attacks (Rogawski and Loscher, 2004). Finally, intranasal application of the local anaesthetic and anti-arrhythmic compound lidocaine was reported to be an effective treatment for some refractory migraines (Kudrow et al., 1995; Bolcskei et al., 2009).
Voltage-gated sodium channel blockers in neurodegenerative disorders and neuroinflammation
Multiple sclerosis is a condition that may be linked to an autoimmune reaction. However, drug treatment to suppress immune responses is of limited effectiveness. Neurodegeneration as a consequence of progression of multiple sclerosis also involves the activation of VGSCs (Smith, 2007). In particular, demyelination of axons that occurs in patients with multiple sclerosis leads to ectopic action potential firing that is caused by slow sodium-dependent membrane potential oscillations (Kapoor et al., 1997).
VGSCs have also been implicated in anoxia/injury-induced neurodegeneration. Energy loss leads in part to persistent sodium currents that cause an increase in axonal intracellular sodium leading to membrane depolarization and further activation of VGSCs. These events promote the reversal of Na+/Ca2+ exchanger and overload of axonal calcium (Stys, 2004).
VGSC blockers such as TTX, lidocaine, procaine, mexiletine, phenytoin and carbamazepine protect against white matter axonal damage in multiple sclerosis models (Waxman et al., 1994a; Carter, 1998; Hewitt et al., 2001; Kapoor et al., 2003; Black and Waxman, 2008). These protective effects can be observed at concentrations that do not compromise the conduction of action potentials. Lidocaine and flecainide can also protect axons from nitric oxide-triggered degeneration (Kapoor et al., 2003). However, withdrawal of phenytoin or carbamazepine in experimental autoimmune encephalomyelitis, a mouse model for multiple sclerosis, resulted in increased inflammatory infiltrate, worsening of symptoms and high incidence of mortality, leading to the suspension of clinical trials (Black and Waxman, 2008). In other trials, lamotrigine did not positively affect clinical outcome measures of patients with secondary progressive multiple sclerosis (Kapoor et al., 2010).
Other clinical trials involving a combined therapy with interferon β1a and topiramate, riluzole and lamotrigine, respectively, are still ongoing (Conway and Cohen, 2010). Thus, whether VGSC blockers will ultimately provide an effective new strategy for the treatment of multiple sclerosis is unclear.
Effects of VGSC blockers might also occur through inhibition of phagocytic functions of microglia. Expression of NaV1.6 is upregulated in activated microglia and inhibition of VGSC reduces their phagocytic capacity and reduces inflammatory cells infiltration in brain tissue of mice with experimental autoimmune encephalomyelitis (Craner et al., 2005)
NaV1.5 is present in late endosomes of human macrophages, which play an important role in phagocytosis. NaV1.6 is also expressed in macrophages where it associates with the cytoskeleton, possibly aiding macrophage motility (Carrithers et al., 2007). VGSCs were also shown to have a role in T-lymphocyte motility (Fraser et al., 2004). However, given the possible side-effects, the value of VGSC blockers to modulate immune responses is unclear (Roselli et al., 2006).
Voltage-gated sodium channel blockers in neuromuscular disorders
Currently, 12 channelopathies affecting skeletal muscle have been described. All five VGSC channelopathies affecting skeletal muscle are found in SCN4A/NaV1.4 (Jurkat-Rott et al., 2010). These channelopathies, as described earlier, are classified as potassium-aggravated myotonia, paramyotonia congenita, hyperkalaemic periodic paralysis, hypokalaemic periodic paralysis and a form of congenital myasthenic syndrome. Depending on the functional consequence of the mutation (gain- or loss-of-function), treatment options are to either use VSGC blockers to directly block the channel or to reduce the fraction of inactivated channels by restoring the skeletal muscle membrane potential (Jurkat-Rott et al., 2010). Pharmacological treatment in myotonia is aimed at decreasing muscle stiffness by mitigating the involuntary action potential bursts without blocking voluntary high-frequency muscle stimulation (Jurkat-Rott et al., 2010). VGSC blockers reduce muscle stiffness in potassium-aggravated myotonia and paramyotonia congenita by promoting the inactivated state of NaV1.4 by inducing a hyperpolarized shift in steady-state inactivation and by prolonging recovery time from inactivation. VSGC blockers such as mexiletine, flecainide and other lidocaine analogues can reduce repetitive firing of action potential because of their use-depended properties, a mechanism that leads to a preferential action on channels with pathogenic gain-of-function mutations (Mohammadi et al., 2005). Symptoms of muscle weakness are often caused by other pathogenic factors and cannot be treated sufficiently with VGSC blockers. However, influencing potassium concentration or blocking potassium channels have been proven to be beneficial (Jurkat-Rott et al., 2010). Burge and Hanna (2012) performed a more detailed analysis of mechanisms and therapeutic options in neuromuscular disorders.
Voltage-gated sodium channel blockers in non-neurological diseases
Voltage-gated sodium channel blockers as anti-arrhythmic drugs
VGSCs are important therapeutic targets in the management of cardiac arrhythmias. According to the Singh Vaughan Williams classification (Walker, 2006), the group of anti-arrhythmic drugs is subdivided into four categories depending on whether they block VGSCs, β-adrenergic receptors, potassium channels or Ca2+ channels. Class I anti-arrhythmics are primarily VGSC blockers that are further subdivided into three subclasses (Nattel, 1993), based upon their effect on the length of the action potential. VGSCs blockers as anti-arrhythmic drugs have been discussed extensively previously (Ganjehei et al., 2011)
Potential dangers of voltage-gated sodium channel blockers on human development
A missense mutation in the SCN9A gene encoding NaV1.7 has been linked to abnormal limb development (Hoeijmakers et al., 2012). Some VGSC blockers may have teratogenic effects. Anti-epileptic medication during pregnancy might elevate the risk for congenital malformations, particularly when the treatment involves multiple compounds and/or valproate (Morrow et al., 2006). Anti-epileptic treatment with valproate during pregnancy was also linked to significantly lower intelligence in children (Bromley et al., 2009). Lacosamide is not used in young children because it was demonstrated to interact with the collapsin response mediator protein 2, which is involved in neuronal differentiation and control of axonal growth (Beyreuther et al., 2007b; Wang et al., 2010b).
Conclusions and future directions
Sodium channel blockers originally derived from cocaine, such as lidocaine, have been in clinical use for more than a century. Progress in understanding the molecular basis of channel activity and the mechanisms of action of some analgesic drugs that have been found to act on sodium channels have provided a clear framework in which to pursue medicinal chemistry approaches. Toxins also provide important models for the development of novel analgesic families, based on natural peptides or on organic peptidomimetics. Recent advances in the development of orally active small molecule-specific NaV1.8 blockers will eventually be followed by antagonists of NaV1.7 and NaV1.9. In the meantime the state-dependent blocker lacosamide looks set to join the broad-spectrum sodium channel blockers already in clinical use in Europe. While pain control and epilepsy are still the major focus of interest in terms of sodium channel drug development, it remains possible that indications ranging from autism to immune disorders may be modulated by compounds targeted at sodium channel activity. Manipulation of VGSCs in particular cell types is desirable for many of these indications, as global channel blocking is likely to have deleterious consequences. Topical application, targeted delivery or drugs that lower functional channel expression are all potential future approaches to these problems.
Funding
J.N.W. thanks the Medical Research Council, The Wellcome Trust and the Biochemistry and Biotechnology Research Council for generous financial support. N.E. is supported by a Rubicon fellowship of The Netherlands Organisation for Scientific Research (NWO). R.W. is supported by a research fellowship (We 4860/1-1) from Deutsche Forschungsgemeinschaft, Bonn, Germany.
Acknowledgements
We thank members of the Molecular Nociception Group and London Pain Consortium for helpful comments.
Glossary
Abbreviations
- TTX
tetrodotoxin
- VGSC
voltage-gated sodium channel
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–5. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Dahllund L, Eriksson AB, Hellgren D, Karlsson U, Lund PE, et al. A stop codon mutation in SCN9A causes lack of pain sensation. Hum Mol Genet. 2007;16:2114–21. doi: 10.1093/hmg/ddm160. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–62. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–8. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Alabi AA, Bahamonde MI, Jung HJ, Kim JI, Swartz KJ. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–5. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–73. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–60. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM. Na channel inactivation from open and closed states. Proc Natl Acad Sci USA. 2006;103:17991–6. doi: 10.1073/pnas.0607603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–80. doi: 10.1016/s0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- Aurlien D, Leren TP, Tauboll E, Gjerstad L. New SCN5A mutation in a SUDEP victim with idiopathic epilepsy. Seizure. 2009;18:158–60. doi: 10.1016/j.seizure.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Baker MD. Selective block of late Na+ current by local anaesthetics in rat large sensory neurones. Br J Pharmacol. 2000;129:1617–26. doi: 10.1038/sj.bjp.0703261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–67. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophysiol. 1997;77:1503–13. doi: 10.1152/jn.1997.77.3.1503. [DOI] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–82. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc Natl Acad Sci USA. 2010;107:12357–62. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudi G, Acharfi S, Larouche C, Chahine M. Expression and intracellular localization of an SCN5A double mutant R1232W/T1620M implicated in Brugada syndrome. Circ Res. 2002;90:E11–6. [PubMed] [Google Scholar]
- Baroudi G, Pouliot V, Denjoy I, Guicheney P, Shrier A, Chahine M. Novel mechanism for Brugada syndrome: defective surface localization of an SCN5A mutant (R1432G) Circ Res. 2001;88:E78–83. doi: 10.1161/hh1201.093270. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007:48. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in a rat model for painful diabetic neuropathy. Eur J Pharmacol. 2006;539:64–70. doi: 10.1016/j.ejphar.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Beyreuther BK, Callizot N, Brot MD, Feldman R, Bain SC, Stohr T. Antinociceptive efficacy of lacosamide in rat models for tumor- and chemotherapy-induced cancer pain. Eur J Pharmacol. 2007a;565:98–104. doi: 10.1016/j.ejphar.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Beyreuther BK, Freitag J, Heers C, Krebsfanger N, Scharfenecker U, Stohr T. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007b;13:21–42. doi: 10.1111/j.1527-3458.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina CR, Rook MB, Groenewegen WA, Herfst LJ, van der Wal AC, Lam J, et al. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ Res. 2003;92:159–68. doi: 10.1161/01.res.0000052672.97759.36. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–10. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, et al. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–37. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Deschenes I, Disilvestre D, Tian Y, Halperin VL, Tomaselli GF. Calmodulin regulation of Nav1.4 current: role of binding to the carboxyl terminus. J Gen Physiol. 2008;131:197–209. doi: 10.1085/jgp.200709863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin Neuropharmacol. 2007;30:230–40. doi: 10.1097/wnf.0b013e3180413d7d. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, et al. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–31. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Black JA, Renganathan M, Waxman SG. Sodium channel Na(v)1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Mol Brain Res. 2002;105:19–28. doi: 10.1016/s0169-328x(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp Eye Res. 2002;75:193–9. doi: 10.1006/exer.2002.2014. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Phenytoin protects central axons in experimental autoimmune encephalomyelitis. J Neurol Sci. 2008;274:57–63. doi: 10.1016/j.jns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Bohle T, Benndorf K. Multimodal action of single Na+ channels in myocardial mouse cells. Biophys J. 1995;68:121–30. doi: 10.1016/S0006-3495(95)80166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, et al. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Bolcskei H, Farkas B, Kocsis P, Tarnawa I. Recent advancements in anti-migraine drug research: focus on attempts to decrease neuronal hyperexcitability. Recent Pat CNS Drug Discov. 2009;4:14–36. doi: 10.2174/157488909787002537. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclaire MF, Swartz KJ. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–8. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, Oyama F, et al. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci USA. 2010;107:2283–8. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. Na channel beta subunits: overachievers of the ion channel family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2) channel complex. Nat Med. 2011;17:822–9. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochu RM, Dick IE, Tarpley JW, McGowan E, Gunner D, Herrington J, et al. Block of peripheral nerve sodium channels selectively inhibits features of neuropathic pain in rats. Mol Pharmacol. 2006;69:823–32. doi: 10.1124/mol.105.018127. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Baker GA, Meador KJ. Cognitive abilities and behaviour of children exposed to antiepileptic drugs in utero. Curr Opin Neurol. 2009;22:162–6. doi: 10.1097/WCO.0b013e3283292401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge JA, Hanna MG. Novel insights into the pathomechanisms of skeletal muscle channelopathies. Curr Neurol Neurosci Rep. 2012;12:62–9. doi: 10.1007/s11910-011-0238-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Galletti F, Rossi C, Sarchielli P, Cupini LM. Antiepileptic drugs in migraine: from clinical aspects to cellular mechanisms. Trends Pharmacol Sci. 2007;28:188–95. doi: 10.1016/j.tips.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci USA. 2000;97:5616–20. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RW. Mexiletine. N Engl J Med. 1987;316:29–34. doi: 10.1056/NEJM198701013160106. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Bean BP. Sodium channels gone wild: resurgent current from neuronal and muscle channelopathies. J Clin Invest. 2010;120:80–3. doi: 10.1172/JCI41340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–73. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- Carranza RD, Hamiwka L, McMahon JM, Dibbens LM, Arsov T, Suls A, et al. De novo SCN1A mutations in migrating partial seizures of infancy. Neurology. 2011;77:380–3. doi: 10.1212/WNL.0b013e318227046d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, et al. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. 2009a;284:8114–26. doi: 10.1074/jbc.M801892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, et al. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. 2009b;284:8114–26. doi: 10.1074/jbc.M801892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Dib-Hajj S, Carrithers LM, Tokmoulina G, Pypaert M, Jonas EA, et al. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–32. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- Carter AJ. The importance of voltage-dependent sodium channels in cerebral ischaemia. Amino Acids. 1998;14:159–69. doi: 10.1007/BF01345257. [DOI] [PubMed] [Google Scholar]
- Cattaneo A. Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain. Curr Opin Mol Ther. 2010;12:94–106. [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Cestele S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–41. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Catterall W, Kalume F. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849–59. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestele S, Scalmani P, Rusconi R, Terragni B, Franceschetti S, Mantegazza M. Self-limited hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci. 2008;28:7273–83. doi: 10.1523/JNEUROSCI.4453-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005:CD003345. doi: 10.1002/14651858.CD003345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, et al. Genetic variation in SCN10A influences cardiac conduction. Nat Genet. 2010;42:149–52. doi: 10.1038/ng.516. [DOI] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, et al. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci USA. 2002;99:17072–7. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–6. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- Cheung HM, Lee SM, Macleod BA, Ries CR, Schwarz SK. A comparison of the systemic toxicity of lidocaine versus its quaternary derivative QX-314 in mice. Can J Anaesth. 2011;58:443–50. doi: 10.1007/s12630-011-9479-5. [DOI] [PubMed] [Google Scholar]
- Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, et al. A novel polyclonal antibody specific for the Na(v)1.5 voltage-gated Na(+) channel 'neonatal' splice form. J Neurosci Methods. 2005;147:88–98. doi: 10.1016/j.jneumeth.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Choi JS, Zhang L, Dib-Hajj SD, Han C, Tyrrell L, Lin Z, et al. Mexiletine-responsive erythromelalgia due to a new Na(v)1.7 mutation showing use-dependent current fall-off. Exp Neurol. 2009;216:383–89. doi: 10.1016/j.expneurol.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clin J Pain. 2003;19:59–68. doi: 10.1097/00002508-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Clare JJ. Targeting voltage-gated sodium channels for pain therapy. Expert Opin Investig Drugs. 2010;19:45–62. doi: 10.1517/13543780903435340. [DOI] [PubMed] [Google Scholar]
- Conway D, Cohen JA. Emerging oral therapies in multiple sclerosis. Curr Neurol Neurosci Rep. 2010;10:381–8. doi: 10.1007/s11910-010-0125-3. [DOI] [PubMed] [Google Scholar]
- Corry B, Thomas M. Mechanism of ion permeation and selectivity in a voltage gated sodium channel. J Am Chem Soc. 2012;134:1840–6. doi: 10.1021/ja210020h. [DOI] [PubMed] [Google Scholar]
- Cossette P, Loukas A, Lafreniere RG, Rochefort D, Harvey-Girard E, Ragsdale DS, et al. Functional characterization of the D188V mutation in neuronal voltage-gated sodium channel causing generalized epilepsy with febrile seizures plus (GEFS) Epilepsy Res. 2003;53:107–17. doi: 10.1016/s0920-1211(02)00259-0. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–8. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JJ, Sheynin J, Shorer Z, Reimann F, Nicholas AK, Zubovic L, et al. Congenital insensitivity to pain: novel SCN9A missense and in-frame deletion mutations. Hum Mutat. 2010;31:E1670–86. doi: 10.1002/humu.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Damarjian TG, Liu S, Hains BC, Lo AC, Black JA, et al. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia. 2005;49:220–9. doi: 10.1002/glia.20112. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127:294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579:2166–70. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131:243–57. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Aracri P, Sancini G, Mantegazza M, Avanzini G, Franceschetti S. Protein-kinase C-dependent phosphorylation inhibits the effect of the antiepileptic drug topiramate on the persistent fraction of sodium currents. Neuroscience. 2004;127:63–8. doi: 10.1016/j.neuroscience.2004.04.040. [DOI] [PubMed] [Google Scholar]
- De Jonghe P. Molecular genetics of Dravet syndrome. Dev Med Child Neurol. 2011;53(Suppl 2):7–10. doi: 10.1111/j.1469-8749.2011.03965.x. [DOI] [PubMed] [Google Scholar]
- Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–8. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 2002;25:253–9. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007;30:555–63. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–47. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Estacion M, Jarecki BW, Tyrrell L, Fischer TZ, Lawden M, et al. Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol Pain. 2008;4:37. doi: 10.1186/1744-8069-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, et al. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83:591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998;95:8963–8. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–7. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- Diss JK, Fraser SP, Djamgoz MB. Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J. 2004;33:180–93. doi: 10.1007/s00249-004-0389-0. [DOI] [PubMed] [Google Scholar]
- Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, et al. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–21. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Doty P, Rudd GD, Stoehr T, Thomas D. Lacosamide. Neurotherapeutics. 2007;4:145–8. doi: 10.1016/j.nurt.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss FE. Treatment of the nonconvulsive epilepsies. Epilepsia. 1983;24(Suppl 1):S45–54. doi: 10.1111/j.1528-1157.1983.tb04642.x. [DOI] [PubMed] [Google Scholar]
- Drizin I, Gregg RJ, Scanio MJ, Shi L, Gross MF, Atkinson RN, et al. Discovery of potent furan piperazine sodium channel blockers for treatment of neuropathic pain. Bioorg Med Chem. 2008;16:6379–86. doi: 10.1016/j.bmc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Durham D. Management of status epilepticus. Crit Care Resusc. 1999;1:344–53. [PubMed] [Google Scholar]
- Ebell MH. Systemic lidocaine or mexiletine for neuropathic pain. Am Fam Physician. 2006;74:79. [PubMed] [Google Scholar]
- Ekberg J, Jayamanne A, Vaughan CW, Aslan S, Thomas L, Mould J, et al. muO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci USA. 2006;103:17030–5. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst SJ, Aguilar-Bryan L, Noebels JL. Sodium channel beta1 regulatory subunit deficiency reduces pancreatic islet glucose-stimulated insulin and glucagon secretion. Endocrinology. 2009;150:1132–9. doi: 10.1210/en.2008-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington AC, Stohr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73:157–69. doi: 10.1124/mol.107.039867. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–5. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Estacion M, Gasser A, Dib-Hajj SD, Waxman SG. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp Neurol. 2010;224:362–8. doi: 10.1016/j.expneurol.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN. The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22:7425–33. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–74. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Fertleman CR, Ferrie CD, Aicardi J, Bednarek NA, Eeg-Olofsson O, Elmslie FV, et al. Paroxysmal extreme pain disorder (previously familial rectal pain syndrome) Neurology. 2007;69:586–95. doi: 10.1212/01.wnl.0000268065.16865.5f. [DOI] [PubMed] [Google Scholar]
- Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, et al. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol. 2011;70:207–12. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Pschorn U, Vix JM, Peil H, Aicher B, Muller A, et al. Efficacy and tolerability of ambroxol hydrochloride lozenges in sore throat. Randomised, double-blind, placebo-controlled trials regarding the local anaesthetic properties. Arzneimittelforschung. 2002;52:256–63. doi: 10.1055/s-0031-1299889. [DOI] [PubMed] [Google Scholar]
- Fischer TZ, Gilmore ES, Estacion M, Eastman E, Taylor S, Melanson M, et al. A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia. Ann Neurol. 2009;65:733–41. doi: 10.1002/ana.21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EV, Kullmann DM, Schorge S. Alternative splicing modulates inactivation of type 1 voltage-gated sodium channels by toggling an amino acid in the first S3-S4 linker. J Biol Chem. 2011;286:36700–8. doi: 10.1074/jbc.M111.250225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–9. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Lloyd LJ, Pani F, Chioni AM, George AJ, et al. T-lymphocyte invasiveness: control by voltage-gated Na+ channel activity. FEBS Lett. 2004;569:191–4. doi: 10.1016/j.febslet.2004.05.063. [DOI] [PubMed] [Google Scholar]
- French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1261–73. doi: 10.1212/01.wnl.0000123695.22623.32. [DOI] [PubMed] [Google Scholar]
- Gaida W, Klinder K, Arndt K, Weiser T. Ambroxol, a Nav1.8-preferring Na+ channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology. 2005;49:1220–7. doi: 10.1016/j.neuropharm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Ganjehei L, Massumi A, Nazeri A, Razavi M. Pharmacologic management of arrhythmias. Tex Heart Inst J. 2011;38:344–9. [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Fernandes F, Moussif A, Fache MP, Giraud P, Dargent B. Dynamic compartmentalization of the voltage-gated sodium channels in axons. Biol Cell. 2003;95:437–45. doi: 10.1016/s0248-4900(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Gasser A, Ho TS, Cheng X, Chang KJ, Waxman SG, Rasband MN, et al. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of ranvier. J Neurosci. 2012;32:7232–43. doi: 10.1523/JNEUROSCI.5434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina EG. Procainamide: clinical pharmacology and efficacy against ventricular arrhythmias. Ann N Y Acad Sci. 1984;432:177–88. doi: 10.1111/j.1749-6632.1984.tb14519.x. [DOI] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71:311–9. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–8. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16:188–92. doi: 10.1097/00002508-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–44. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Raman IM. Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin. J Neurosci. 2004;24:35–42. doi: 10.1523/JNEUROSCI.3807-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39:508–12. doi: 10.1111/j.1528-1157.1998.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Haimovich B, Schotland DL, Fieles WE, Barchi RL. Localization of sodium channel subtypes in adult rat skeletal muscle using channel-specific monoclonal antibodies. J Neurosci. 1987;7:2957–66. doi: 10.1523/JNEUROSCI.07-09-02957.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–92. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci. 2004;24:4832–9. doi: 10.1523/JNEUROSCI.0300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, et al. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol. 2010;198:47–59. doi: 10.1111/j.1748-1716.2009.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, Lawrence J, et al. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog Biophys Mol Biol. 2008;98:251–66. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer AR, Valentin JP, Pollard CE. On the relationship between block of the cardiac Na channel and drug-induced prolongation of the QRS complex. Br J Pharmacol. 2011;164:260–73. doi: 10.1111/j.1476-5381.2011.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H. Utilization of zonisamide in patients with chronic pain or epilepsy refractory to other treatments: a retrospective, open label, uncontrolled study in a VA hospital. Curr Med Res Opin. 2004;20:577–80. doi: 10.1185/030079904125003313. [DOI] [PubMed] [Google Scholar]
- Heblich F, Tran Van MA, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels. 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Rasband MN. Intrinsic and extrinsic determinants of ion channel localization in neurons. J Neurochem. 2006;98:1345–52. doi: 10.1111/j.1471-4159.2006.04001.x. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci USA. 2008;105:3628–33. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfst LJ, Potet F, Bezzina CR, Groenewegen WA, Le MH, Hoorntje TM, et al. Na+ channel mutation leading to loss of function and non-progressive cardiac conduction defects. J Mol Cell Cardiol. 2003;35:549–57. doi: 10.1016/s0022-2828(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Plata E, Ortiz CS, Marquina-Castillo B, Medina-Martinez I, Alfaro A, Berumen J, et al. Over expression of Na(V) 1.6 channels is associated with the invasion capacity of human cervical cancer. Int J Cancer. 2012;130:2013–23. doi: 10.1002/ijc.26210. [DOI] [PubMed] [Google Scholar]
- Heron SE, Crossland KM, Andermann E, Phillips HA, Hall AJ, Bleasel A, et al. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet. 2002;360:851–2. doi: 10.1016/S0140-6736(02)09968-3. [DOI] [PubMed] [Google Scholar]
- Hewitt KE, Stys PK, Lesiuk HJ. The use-dependent sodium channel blocker mexiletine is neuroprotective against global ischemic injury. Brain Res. 2001;898:281–7. doi: 10.1016/s0006-8993(01)02195-3. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama TY, Watanabe E, Okado H, Noda M. The subfornical organ is the primary locus of sodium-level sensing by Na(x) sodium channels for the control of salt-intake behavior. J Neurosci. 2004;24:9276–81. doi: 10.1523/JNEUROSCI.2795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JG, Han C, Merkies IS, Macala LJ, Lauria G, Gerrits MM, et al. Small nerve fibres, small hands and small feet: a new syndrome of pain, dysautonomia and acromesomelia in a kindred with a novel NaV1.7 mutation. Brain. 2012;135:345–58. doi: 10.1093/brain/awr349. [DOI] [PubMed] [Google Scholar]
- Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, et al. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett. 2008a;433:65–70. doi: 10.1016/j.neulet.2007.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland KD, Kearney JA, Glauser TA, Buck G, Keddache M, Blankston JR, et al. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci Lett. 2008b;433:65–70. doi: 10.1016/j.neulet.2007.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–75. doi: 10.1097/00000542-200009000-00038. [DOI] [PubMed] [Google Scholar]
- Hoyt SB, London C, Gorin D, Wyvratt MJ, Fisher MH, Abbadie C, et al. Discovery of a novel class of benzazepinone Na(v)1.7 blockers: potential treatments for neuropathic pain. Bioorg Med Chem Lett. 2007;17:4630–4. doi: 10.1016/j.bmcl.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Hsueh CH, Chen WP, Lin JL, Tsai CT, Liu YB, Juang JM, et al. Distinct functional defect of three novel Brugada syndrome related cardiac sodium channel mutations. J Biomed Sci. 2009;16:23. doi: 10.1186/1423-0127-16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, et al. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–8. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth T, Rittger A, Saftig P, Alzheimer C. beta-Site APP-cleaving enzyme 1(BACE1)cleavescerebellar Na+ channel beta4-subunit and promotes Purkinje cell firing by slowing the decay of resurgent Na+ current. Pflugers Arch. 2011;461:355–71. doi: 10.1007/s00424-010-0913-2. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Bhat MI, Charoo BA, Syed WA, Sheikh MA, Bhat IN. Experience with oral mexiletine in primary erythromelalgia in children. Ann Saudi Med. 2009;29:316–8. doi: 10.4103/0256-4947.55316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL. The role of sodium channels in cell adhesion. Front Biosci. 2002;7:12–23. doi: 10.2741/isom. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, et al. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992;256:839–42. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Jang HS, Jung D, Kim S, Jo J, Lee J, Kim M, et al. A case of primary erythromelalgia improved by mexiletine. Br J Dermatol. 2004;151:708–10. doi: 10.1111/j.1365-2133.2004.06167.x. [DOI] [PubMed] [Google Scholar]
- Jarecki BW, Piekarz AD, Jackson JO, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. J Clin Invest. 2010;120:369–78. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarecki BW, Sheets PL, Jackson JO, Cummins TR. Paroxysmal extreme pain disorder mutations within the D3/S4-S5 linker of Nav1.7 cause moderate destabilization of fast inactivation. J Physiol. 2008;586:4137–53. doi: 10.1113/jphysiol.2008.154906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA. 2007;104:8520–5. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–46. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003a;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003b;423:42–8. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh CC, Neelands T, et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123:75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F. Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch Eur J Physiol. 2010;460:239–48. doi: 10.1007/s00424-010-0814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra AA, Elliott D. Acute migraine: current treatment and emerging therapies. Ther Clin Risk Manag. 2007;3:449–59. [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–74. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–19. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Davies M, Blaker PA, Hall SM, Smith KJ. Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann Neurol. 2003;53:174–80. doi: 10.1002/ana.10443. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681–8. doi: 10.1016/S1474-4422(10)70131-9. [DOI] [PubMed] [Google Scholar]
- Kapoor R, Li YG, Smith KJ. Slow sodium-dependent potential oscillations contribute to ectopic firing in mammalian demyelinated axons. Brain. 1997;120 (Pt 4):647–52. doi: 10.1093/brain/120.4.647. [DOI] [PubMed] [Google Scholar]
- Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JA, Buchner DA, De Haan G, Adamska M, Levin SI, Furay AR, et al. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6) Hum Mol Genet. 2002;11:2765–75. doi: 10.1093/hmg/11.22.2765. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–80. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829–36. doi: 10.1016/j.jpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Klionsky L, Tamir R, Holzinger B, Bi X, Talvenheimo J, Kim H, et al. A polyclonal antibody to the prepore loop of transient receptor potential vanilloid type 1 blocks channel activation. J Pharmacol Exp Ther. 2006;319:192–8. doi: 10.1124/jpet.106.108092. [DOI] [PubMed] [Google Scholar]
- Kort ME, Drizin I, Gregg RJ, Scanio MJ, Shi L, Gross MF, et al. Discovery and biological evaluation of 5-aryl-2-furfuramides, potent and selective blockers of the Nav1.8 sodium channel with efficacy in models of neuropathic and inflammatory pain. J Med Chem. 2008;51:407–16. doi: 10.1021/jm070637u. [DOI] [PubMed] [Google Scholar]
- Kudrow L, Kudrow DB, Sandweiss JH. Rapid and sustained relief of migraine attacks with intranasal lidocaine: preliminary findings. Headache. 1995;35:79–82. doi: 10.1111/j.1526-4610.1995.hed3502079.x. [DOI] [PubMed] [Google Scholar]
- Kuhnert SM, Phillips WJ, Davis MD. Lidocaine and mexiletine therapy for erythromelalgia. Arch Dermatol. 1999;135:1447–9. doi: 10.1001/archderm.135.12.1447. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Bean BP. Na+ channels must deactivate to recover from inactivation. Neuron. 1994;12:819–29. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Kyndt F, Probst V, Potet F, Demolombe S, Chevallier JC, Baro I, et al. Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation. 2001;104:3081–6. doi: 10.1161/hc5001.100834. [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–52. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Romero M, Reboreda A, Sanchez E, Ribeiro SJ. A riluzole- and valproate-sensitive persistent sodium current contributes to the resting membrane potential and increases the excitability of sympathetic neurones. Pflugers Arch. 2009;458:589–99. doi: 10.1007/s00424-009-0648-0. [DOI] [PubMed] [Google Scholar]
- Lampl I, Schwindt P, Crill W. Reduction of cortical pyramidal neuron excitability by the action of phenytoin on persistent Na+ current. J Pharmacol Exp Ther. 1998;284:228–37. [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–9. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Leo S, D'Hooge R, Meert T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav Brain Res. 2010;208:149–57. doi: 10.1016/j.bbr.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Leppik IE. Zonisamide: chemistry, mechanism of action, and pharmacokinetics. Seizure. 2004;13:S5–9. doi: 10.1016/j.seizure.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Brachet A, Fache MP, Dargent B. Voltage-gated sodium channel organization in neurons: protein interactions and trafficking pathways. Neurosci Lett. 2010;486:92–100. doi: 10.1016/j.neulet.2010.08.079. [DOI] [PubMed] [Google Scholar]
- Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, et al. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J Neurophysiol. 2006;96:785–93. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- Liao Y, Anttonen AK, Liukkonen E, Gaily E, Maljevic S, Schubert S, et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010;75:1454–8. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc Natl Acad Sci USA. 2008;105:2705–10. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TK, Macleod BA, Ries CR, Schwarz SK. The quaternary lidocaine derivative, QX-314, produces long-lasting local anesthesia in animal models in vivo. Anesthesiology. 2007;107:305–11. doi: 10.1097/01.anes.0000270758.77314.b4. [DOI] [PubMed] [Google Scholar]
- Lindia JA, Kohler MG, Martin WJ, Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117:145–53. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang HX, Hou HY, Lu XF, Wei JQ, Wang CG, et al. Acid solution is a suitable medium for introducing QX-314 into nociceptors through TRPV1 channels to produce sensory-specific analgesic effects. PLoS One. 2011;6:e29395. doi: 10.1371/journal.pone.0029395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London C, Hoyt SB, Parsons WH, Williams BS, Warren VA, Tschirret-Guth R, et al. Imidazopyridines: a novel class of hNav1.7 channel blockers. Bioorg Med Chem Lett. 2008;18:1696–701. doi: 10.1016/j.bmcl.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–47. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, et al. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci. 2006;26:7984–94. doi: 10.1523/JNEUROSCI.2211-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie FE, Parker A, Parkinson NJ, Oliver PL, Brooker D, Underhill P, et al. Analysis of the mouse mutant Cloth-ears shows a role for the voltage-gated sodium channel Scn8a in peripheral neural hearing loss. Genes Brain Behav. 2009;8:699–713. doi: 10.1111/j.1601-183X.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–61. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–24. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87:7–17. doi: 10.1016/S0304-3959(00)00229-3. [DOI] [PubMed] [Google Scholar]
- Marini C, Scheffer IE, Nabbout R, Mei D, Cox K, Dibbens LM, et al. SCN1A duplications and deletions detected in Dravet syndrome: implications for molecular diagnosis. Epilepsia. 2009;50:1670–8. doi: 10.1111/j.1528-1167.2009.02013.x. [DOI] [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–9. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado-Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med. 1985;313 doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Scanio MJ, Kort ME, Faltynek CR, Jarvis MF. A selective Nav1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther. 2008;324:1204–11. doi: 10.1124/jpet.107.134148. [DOI] [PubMed] [Google Scholar]
- McGowan E, Hoyt SB, Li X, Lyons KA, Abbadie C. A peripherally acting Na(v)1.7 sodium channel blocker reverses hyperalgesia and allodynia on rat models of inflammatory and neuropathic pain. Anesth Analg. 2009;109:951–8. doi: 10.1213/ane.0b013e3181b01b02. [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, et al. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–42. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115:2010–7. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA, Sprunger LK, MacDonald BT, Buchner DA, Escayg A. Mutations of voltage-gated sodium channels in movement disorders and epilepsy. Novartis Found Symp. 2002;241:72–81. [PubMed] [Google Scholar]
- Meisler MH, O'Brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588:1841–8. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Plummer NW, Burgess DL, Buchner DA, Sprunger LK. Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica. 2004;122:37–45. doi: 10.1007/s10709-004-1441-9. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Warren VA, Kraus RL, Hwang JC, Liu CJ, Dai G, et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry. 2002;41:14734–47. doi: 10.1021/bi026546a. [DOI] [PubMed] [Google Scholar]
- Minett MS, Nassar MA, Clark AK, Passmore G, Dickenson AH, Wang F, et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat Commun. 2012;3:791. doi: 10.1038/ncomms1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra SN, Kahlig KM, George AL., Jr Impaired NaV1.2 function and reduced cell surface expression in benign familial neonatal-infantile seizures. Epilepsia. 2008;49:1535–45. doi: 10.1111/j.1528-1167.2008.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B, Jurkat-Rott K, Alekov A, Dengler R, Bufler J, Lehmann-Horn F. Preferred mexiletine block of human sodium channels with IVS4 mutations and its pH-dependence. Pharmacogenet Genomics. 2005;15:235–44. doi: 10.1097/01213011-200504000-00007. [DOI] [PubMed] [Google Scholar]
- Mohler PJ, Rivolta I, Napolitano C, Lemaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA. 2004;101:17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth-Selbach U, Hermanns H, Stegmann JU, Kollosche K, Freynhagen R, Bauer I, et al. Antinociceptive effects of systemic lidocaine: involvement of the spinal glycinergic system. Eur J Pharmacol. 2009;613:68–73. doi: 10.1016/j.ejphar.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Nalamachu S, Crockett RS, Mathur D. Lidocaine patch 5 for carpal tunnel syndrome: how it compares with injections: a pilot study. J Fam Pract. 2006;55:209–14. [PubMed] [Google Scholar]
- Nassar MA, Baker MD, Levato A, Ingram R, Mallucci G, McMahon SB, et al. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol Pain. 2006;2:33. doi: 10.1186/1744-8069-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MA, Levato A, Stirling LC, Wood JN. Neuropathic pain develops normally in mice lacking both Nav1.7 and Nav1.8. Mol Pain. 2005;1:24. doi: 10.1186/1744-8069-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA. 2004;101:12706–11. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattel S. Comparative mechanisms of action of antiarrhythmic drugs. Am J Cardiol. 1993;72:13F–7F. doi: 10.1016/0002-9149(93)90959-g. [DOI] [PubMed] [Google Scholar]
- Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol. 2008;155:567–73. doi: 10.1038/bjp.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–7. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- O'Brien JE, Sharkey LM, Vallianatos CN, Han C, Blossom JC, Yu T, et al. Interaction of voltage-gated sodium channel Nav1.6 (SCN8A) with microtubule associated protein Map1b. J Biol Chem. 2012;287:18459–66. doi: 10.1074/jbc.M111.336024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley HA, Shreiner AB, Chen GH, Huffnagle GB, Isom LL. Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci. 2009;40:143–55. doi: 10.1016/j.mcn.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, et al. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–6. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- Onkal R, Djamgoz MBA. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–19. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Ostman JA, Nassar MA, Wood JN, Baker MD. GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9. J Physiol. 2008;586:1077–87. doi: 10.1113/jphysiol.2007.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S-W, Kameyama A, Hao L-Y, Horiuchi M, Minobe E, Wang W-Y, et al. Tetrodotoxin-resistant Na+ channels in human neuroblastoma cells are encoded by new variants of Nav1.5/SCN5A. Eur J Neurosci. 2005;22:793–801. doi: 10.1111/j.1460-9568.2005.04280.x. [DOI] [PubMed] [Google Scholar]
- Oyama F, Miyazaki H, Sakamoto N, Becquet C, Machida Y, Kaneko K, et al. Sodium channel beta4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington's disease transgenic mice. J Neurochem. 2006;98:518–29. doi: 10.1111/j.1471-4159.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- Padilla F, Couble ML, Coste B, Maingret F, Clerc N, Crest M, et al. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci. 2007;35:138–52. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA. 2002;99:6210–5. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papale LA, Paul KN, Sawyer NT, Manns JR, Tufik S, Escayg A. Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory. J Biol Chem. 2010;285:16553–61. doi: 10.1074/jbc.M109.090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O'Malley HA, Chen C, et al. Voltage-gated Na+ channel beta1B: a secreted cell adhesion molecule involved in human epilepsy. J Neurosci. 2011;31:14577–91. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–78. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel beta subunits in development and disease. Neurosci Lett. 2010;486:53–9. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB, Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986;87:305–26. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–9. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–8. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertin M, Ji RR, Berta T, Powell AJ, Karchewski L, Tate SN, et al. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–80. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10:446–56. doi: 10.1016/S1474-4422(11)70047-3. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS drugs. 2008;22:761–86. doi: 10.2165/00023210-200822090-00004. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Caprini M, Zhang J, Rockenstein EM, Rivera RR, Murre C, et al. Neuronal death and perinatal lethality in voltage-gated sodium channel alpha(II)-deficient mice. Biophys J. 2000;78:2878–91. doi: 10.1016/S0006-3495(00)76829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–74. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Poon WY, Malik-Hall M, Wood JN, Okuse K. Identification of binding domains in the sodium channel Na(V)1.8 intracellular N-terminal region and annexin II light chain p11. FEBS Lett. 2004;558:114–8. doi: 10.1016/S0014-5793(03)01512-6. [DOI] [PubMed] [Google Scholar]
- Priest BT, Blumenthal KM, Smith JJ, Warren VA, Smith MM. ProTx-I and ProTx-II: gating modifiers of voltage-gated sodium channels. Toxicon. 2007;49:194–201. doi: 10.1016/j.toxicon.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Priest BT, Garcia ML, Middleton RE, Brochu RM, Clark S, Dai G, et al. A disubstituted succinamide is a potent sodium channel blocker with efficacy in a rat pain model. Biochemistry. 2004;43:9866–76. doi: 10.1021/bi0493259. [DOI] [PubMed] [Google Scholar]
- Priest BT, Murphy BA, Lindia JA, Diaz C, Abbadie C, Ritter AM, et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc Natl Acad Sci USA. 2005;102:9382–7. doi: 10.1073/pnas.0501549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptacek LJ, George AL, Jr, Griggs RC, Tawil R, Kallen RG, Barchi RL, et al. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991;67:1021–7. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS. How do mutant Nav1.1 sodium channels cause epilepsy? Brain Res Rev. 2008;58:149–59. doi: 10.1016/j.brainresrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S, Tan SV, Matthews E, Tomlinson SE, Labrum R, Sud R, et al. A patient with episodic ataxia and paramyotonia congenita due to mutations in KCNA1 and SCN4A. Neurology. 2009;73:993–5. doi: 10.1212/WNL.0b013e3181b87959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–26. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–91. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rauck RL, Shaibani A, Biton V, Simpson J, Koch B. Lacosamide in painful diabetic peripheral neuropathy: a phase 2 double-blind placebo-controlled study. Clin J Pain. 2007;23:150–58. doi: 10.1097/01.ajp.0000210957.39621.b2. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–40. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Rivera-Acevedo RE, Pless SA, Ahern CA, Schwarz SK. The quaternary lidocaine derivative, QX-314, exerts biphasic effects on transient receptor potential vanilloid subtype 1 channels in vitro. Anesthesiology. 2011;114:1425–34. doi: 10.1097/ALN.0b013e318216ea0c. [DOI] [PubMed] [Google Scholar]
- Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol. 2011;164:48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LR, Czerniecki JM, Ehde DM, Edwards WT, Judish DA, Goldberg ML, et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Arch Phys Med Rehabil. 2004;85:1–6. doi: 10.1016/s0003-9993(03)00476-3. [DOI] [PubMed] [Google Scholar]
- Rogart RB, Cribbs LL, Muglia LK, Kephart DD, Kaiser MW. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci USA. 1989;86:8170–4. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–92. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- Roselli F, Livrea P, Jirillo E. Voltage-gated sodium channel blockers as immunomodulators. Recent Pat CNS Drug Discov. 2006;1:83–91. doi: 10.2174/157488906775245255. [DOI] [PubMed] [Google Scholar]
- Rosker C, Lohberger B, Hofer D, Steinecker B, Quasthoff S, Schreibmayer W. The TTX metabolite 4,9-anhydro-TTX is a highly specific blocker of the Na(v1.6) voltage-dependent sodium channel. Am J Physiol Cell Physiol. 2007;293:C783–9. doi: 10.1152/ajpcell.00070.2007. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Denegri M, Liu N, Bachetti T, Seregni M, Morotti S, et al. Trafficking defects and gating abnormalities of a novel SCN5A mutation question gene-specific therapy in long QT syndrome type 3. Circ Res. 2010;106:1374–83. doi: 10.1161/CIRCRESAHA.110.218891. [DOI] [PubMed] [Google Scholar]
- Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1:175–82. doi: 10.2174/1568026013395335. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Mistry M, Sage D, Black JA, Waxman SG, Crest M, et al. Selective expression of a persistent tetrodotoxin-resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J Neurosci. 2003;23:2715–25. doi: 10.1523/JNEUROSCI.23-07-02715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi R, Scalmani P, Cassulini RR, Giunti G, Gambardella A, Franceschetti S, et al. Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J Neurosci. 2007;27:11037–46. doi: 10.1523/JNEUROSCI.3515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen JB, Vrints CJ. Molecular aspects of the congenital and acquired Long QT Syndrome: clinical implications. J Mol Cell Cardiol. 2008;44:633–46. doi: 10.1016/j.yjmcc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Saleh S, Yeung SY, Prestwich S, Pucovsky V, Greenwood I. Electrophysiological and molecular identification of voltage-gated sodium channels in murine vascular myocytes. J Physiol. 2005;568:155–69. doi: 10.1113/jphysiol.2005.090951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–9. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- Schiavon E, Sacco T, Cassulini RR, Gurrola G, Tempia F, Possani LD, et al. Resurgent current and voltage sensor trapping enhanced activation by a beta-scorpion toxin solely in Nav1.6 channel. Significance in mice Purkinje neurons. J Biol Chem. 2006;281:20326–37. doi: 10.1074/jbc.M600565200. [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, et al. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008a;74:1476–84. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- Schmalhofer WA, Calhoun J, Burrows R, Bailey T, Kohler MG, Weinglass AB, et al. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008b;74:1476–84. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Grigat G. Phenytoin and carbamazepine: potential- and frequency-dependent block of Na currents in mammalian myelinated nerve fibers. Epilepsia. 1989;30:286–94. doi: 10.1111/j.1528-1157.1989.tb05300.x. [DOI] [PubMed] [Google Scholar]
- Schwarz SK, Cheung HM, Ries CR, Lee SM, Wang JT, Macleod BA. Lumbar intrathecal administration of the quaternary lidocaine derivative, QX-314, produces irritation and death in mice. Anesthesiology. 2010;113:438–44. doi: 10.1097/ALN.0b013e3181dfd31b. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995;74:2220–4. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–42. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Okuse K, Djamgoz MB. Protein-protein interactions involving voltage-gated sodium channels: Post-translational regulation, intracellular trafficking and functional expression. Int J Biochem Cell Biol. 2009;41:1471–81. doi: 10.1016/j.biocel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Shao PP, Ok D, Fisher MH, Garcia ML, Kaczorowski GJ, Li C, et al. Novel cyclopentane dicarboxamide sodium channel blockers as a potential treatment for chronic pain. Bioorg Med Chem Lett. 2005;15:1901–7. doi: 10.1016/j.bmcl.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sheets PL, Heers C, Stoehr T, Cummins TR. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther. 2008;326:89–99. doi: 10.1124/jpet.107.133413. [DOI] [PubMed] [Google Scholar]
- Sheets PL, Jackson JO, Waxman SG, Dib-Hajj SD, Cummins TR. A Nav1.7 channel mutation associated with hereditary erythromelalgia contributes to neuronal hyperexcitability and displays reduced lidocaine sensitivity. J Physiol. 2007;581:1019–31. doi: 10.1113/jphysiol.2006.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben AE, Sithinamsuwan P, Radhakrishnan A, Badawy RA, Dibbens L, Mazarib A, et al. Does a SCN1A gene mutation confer earlier age of onset of febrile seizures in GEFS+? Epilepsia. 2009;50:953–6. doi: 10.1111/j.1528-1167.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- Smith DB, Mattson RH, Cramer JA, Collins JF, Novelly RA, Craft B. Results of a nationwide Veterans Administration Cooperative Study comparing the efficacy and toxicity of carbamazepine, phenobarbital, phenytoin, and primidone. Epilepsia. 1987;28:50–8. doi: 10.1111/j.1528-1157.1987.tb05778.x. [DOI] [PubMed] [Google Scholar]
- Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol. 2007;17:230–42. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446:76–8. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- Sokolov S, Scheuer T, Catterall WA. Ion permeation and block of the gating pore in the voltage sensor of NaV1.4 channels with hypokalemic periodic paralysis mutations. J Gen Physiol. 2010;136:225–36. doi: 10.1085/jgp.201010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Ham SS, Shin YK, Lee CS. Amitriptyline modulation of Na(+) channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2000;401:297–305. doi: 10.1016/s0014-2999(00)00460-x. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci. 2001;21:7481–90. doi: 10.1523/JNEUROSCI.21-19-07481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer CF, Hollett MD. Mechanisms of apparent affinity variation of guarded receptors. J Theor Biol. 1985;115:337–49. doi: 10.1016/s0022-5193(85)80196-x. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Siniscalchi A, Bernardi G. Lamotrigine inhibits Ca2+ currents in cortical neurons: functional implications. Eur J Pharmacol. 1996;307:113–6. doi: 10.1016/0014-2999(96)00265-8. [DOI] [PubMed] [Google Scholar]
- Stevens EB, Cox PJ, Shah BS, Dixon AK, Richardson PJ, Pinnock RD, et al. Tissue distribution and functional expression of the human voltage-gated sodium channel beta3 subunit. Pflugers Arch. 2001;441:481–8. doi: 10.1007/s004240000449. [DOI] [PubMed] [Google Scholar]
- Stohr T, Krause E, Selve N. Lacosamide displays potent antinociceptive effects in animal models for inflammatory pain. Eur J Pain. 2006;10:241–9. doi: 10.1016/j.ejpain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, et al. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Stummann TC, Salvati P, Fariello RG, Faravelli L. The anti-nociceptive agent ralfinamide inhibits tetrodotoxin-resistant and tetrodotoxin-sensitive Na+ currents in dorsal root ganglion neurons. Eur J Pharmacol. 2005;510:197–208. doi: 10.1016/j.ejphar.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr Mol Med. 2004;4:113–30. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, et al. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci USA. 2001;98:6384–9. doi: 10.1073/pnas.111065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamazepine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia. 2007;48:774–82. doi: 10.1111/j.1528-1167.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, et al. Sudden infant death syndrome-associated mutations in the sodium channel beta subunits. Heart Rhythm. 2010;7:771–8. doi: 10.1016/j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Mantegazza M, Franceschetti S, Avanzini G. Valproate selectively reduces the persistent fraction of Na+ current in neocortical neurons. Epilepsy Res. 1998;32:304–8. doi: 10.1016/s0920-1211(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Theile JW, Jarecki BW, Piekarz AD, Cummins TR. Nav1.7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navbeta4 peptide-mediated resurgent sodium currents. J Physiol. 2011;589:597–608. doi: 10.1113/jphysiol.2010.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, Koszowski AG, Whisenand T, Levinson SR, et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA. 1997;94:1527–32. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MM, Dalton JC, Day JW, Ranum LP, Meisler MH. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J Med Genet. 2006;43:527–30. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann MT, Isom LL, et al. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents. J Neurosci. 2010;30:8489–501. doi: 10.1523/JNEUROSCI.1534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K, Depienne C, Le FD, Riant F, Chaine P, Trouillard O, et al. Elicited repetitive daily blindness: a new phenotype associated with hemiplegic migraine and SCN1A mutations. Neurology. 2009;72:1178–83. doi: 10.1212/01.wnl.0000345393.53132.8c. [DOI] [PubMed] [Google Scholar]
- Valdivia CR, Ackerman MJ, Tester DJ, Wada T, McCormack J, Ye B, et al. A novel SCN5A arrhythmia mutation, M1766L, with expression defect rescued by mexiletine. Cardiovasc Res. 2002;55:279–89. doi: 10.1016/s0008-6363(02)00445-5. [DOI] [PubMed] [Google Scholar]
- Valdivia CR, Medeiros-Domingo A, Ye B, Shen WK, Algiers TJ, Ackerman MJ, et al. Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. 2010;86:392–400. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia CR, Tester DJ, Rok BA, Porter C-BJ, Munger TM, Jahangir A, et al. A trafficking defective, Brugada syndrome-causing SCN5A mutation rescued by drugs. Cardiovasc Res. 2004;62:53–62. doi: 10.1016/j.cardiores.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Vassilev P, Scheuer T, Catterall WA. Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc Natl Acad Sci USA. 1989;86:8147–51. doi: 10.1073/pnas.86.20.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Scheuer T, Catterall WA. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–61. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- Veeramah KR, O'Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012;90:502–10. doi: 10.1016/j.ajhg.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneroni O, Maj R, Calabresi M, Faravelli L, Fariello RG, Salvati P. Anti-allodynic effect of NW-1029, a novel Na(+) channel blocker, in experimental animal models of inflammatory and neuropathic pain. Pain. 2003;102:17–25. doi: 10.1016/s0304-3959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- Volkers L, Kahlig KM, Verbeek NE, Das JH, van Kempen MJ, Stroink H, et al. Nav 1.1 dysfunction in genetic epilepsy with febrile seizures-plus or Dravet syndrome. Eur J Neurosci. 2011;34:1268–75. doi: 10.1111/j.1460-9568.2011.07826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten CF, Eappen S, Cleary JF, Taylor SGt, Moots P, Regevik N, et al. Flecainide for the treatment of chronic neuropathic pain: a Phase II trial. Palliat Med. 2007;21:667–72. doi: 10.1177/0269216307083031. [DOI] [PubMed] [Google Scholar]
- Walker MJ. Antiarrhythmic drug research. Br J Pharmacol. 2006;147:222–31. doi: 10.1038/sj.bjp.0706500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yang Q, Wu X, Yang Y, Shi L, Wang C, et al. Functional dominant-negative mutation of sodium channel subunit gene SCN3B associated with atrial fibrillation in a Chinese GeneID population. Biochem Biophys Res Commun. 2010a;398:98–104. doi: 10.1016/j.bbrc.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brittain JM, Jarecki BW, Park KD, Wilson SM, Wang B, et al. In silico docking and electrophysiological characterization of lacosamide binding sites on collapsin response mediator protein-2 identifies a pocket important in modulating sodium channel slow inactivation. J Biol Chem. 2010b;285:25296–307. doi: 10.1074/jbc.M110.128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Khanna R. Voltage-gated calcium channels are not affected by the novel anti-epileptic drug lacosamide. Transl Neurosci. 2011;2:13–22. doi: 10.2478/s13380-011-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue. J Pharmacol Exp Ther. 1993;267:575–81. [PubMed] [Google Scholar]
- Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, et al. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci. 2000;20:7743–51. doi: 10.1523/JNEUROSCI.20-20-07743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, et al. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:268–75. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, et al. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–8. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Black JA, Ransom BR, Stys PK. Anoxic injury of rat optic nerve: ultrastructural evidence for coupling between Na+ influx and Ca(2+)-mediated injury in myelinated CNS axons. Brain Res. 1994a;644:197–204. doi: 10.1016/0006-8993(94)91680-2. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994b;72:466–70. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser T, Wilson N. Inhibition of tetrodotoxin (TTX)-resistant and TTX-sensitive neuronal Na(+) channels by the secretolytic ambroxol. Mol Pharmacol. 2002;62:433–8. doi: 10.1124/mol.62.3.433. [DOI] [PubMed] [Google Scholar]
- Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature. 2011;472:186–90. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, et al. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol Psychiatry. 2003;8:186–94. doi: 10.1038/sj.mp.4001241. [DOI] [PubMed] [Google Scholar]
- Weiss S, Benoist D, White E, Teng W, Saint DA. Riluzole protects against cardiac ischaemia and reperfusion damage via block of the persistent sodium current. Br J Pharmacol. 2010;160:1072–82. doi: 10.1111/j.1476-5381.2010.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci USA. 1992;89:10910–4. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Noebels JL, Catterall WA. Elevated expression of type II Na+ channels in hypomyelinated axons of shiverer mouse brain. J Neurosci. 1992;12:2259–67. doi: 10.1523/JNEUROSCI.12-06-02259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD005451.pub2. : CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BS, Felix JP, Priest BT, Brochu RM, Dai K, Hoyt SB, et al. Characterization of a new class of potent inhibitors of the voltage-gated sodium channel Nav1.7. Biochemistry. 2007;46:4693–703. doi: 10.1021/bi7018207. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Zhang MM, Azam L, Olivera BM, Bulaj G, Yoshikami D. NaVbeta subunits modulate the inhibition of NaV1.8 by the analgesic gating modifier muO-conotoxin MrVIB. J Pharmacol Exp Ther. 2011;338:687–93. doi: 10.1124/jpet.110.178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, Leaw BT, et al. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J Clin Invest. 2010;120:2661–71. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, et al. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–17. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. J Neurobiol. 2004;61:55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–4. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res. 2002;90:443–9. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, et al. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci USA. 2012;109:E93–102. doi: 10.1073/pnas.1118434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, et al. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–85. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, O'Leary ME, Chahine M. Regulation of Na(v)1.6 and Na(v)1.8 peripheral nerve Na(+) channels by auxiliary beta-subunits. J Neurophysiol. 2011;106:608–19. doi: 10.1152/jn.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T, Surber R. SCN5A channelopathies–an update on mutations and mechanisms. Prog Biophys Mol Biol. 2008;98:120–36. doi: 10.1016/j.pbiomolbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, et al. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–8. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- Zona C, Tancredi V, Longone P, D'Arcangelo G, D'Antuono M, Manfredi M, et al. Neocortical potassium currents are enhanced by the antiepileptic drug lamotrigine. Epilepsia. 2002;43:685–90. doi: 10.1046/j.1528-1157.2002.51401.x. [DOI] [PubMed] [Google Scholar]