Abstract
Background: Polonium-210 (210Po) concentrations that exceed 1 Bq/L in drinking-water supplies have been reported from four widely separated U.S. states where exposure to it went unnoticed for decades. The radionuclide grandparents of 210Po are common in sediments, and segments of the public may be chronically exposed to low levels of 210Po in drinking water or in food products from animals raised in contaminated areas.
Objectives: We summarized information on the environmental behavior, biokinetics, and toxicology of 210Po and identified the need for future research.
Methods: Potential linkages between environmental exposure to 210Po and human health effects were identified in a literature review.
Discussion: 210Po accumulates in the ovaries where it kills primary oocytes at low doses. Because of its radiosensitivity and tendency to concentrate 210Po, the ovary may be the critical organ in determining the lowest injurious dose for 210Po. 210Po also accumulates in the yolk sac of the embryo and in the fetal and placental tissues. Low-level exposure to 210Po may have subtle, long-term biological effects because of its tropism towards reproductive and embryonic and fetal tissues where exposure to a single alpha particle may kill or damage critical cells. 210Po is present in cigarettes and maternal smoking has several effects that appear consistent with the toxicology of 210Po.
Conclusions: Much of the important biological and toxicological research on 210Po is more than four decades old. New research is needed to evaluate environmental exposure to 210Po and the biological effects of low-dose exposure to it so that public health officials can develop appropriate mitigation measures where necessary.
Keywords: cigarettes, drinking water, Fallon, human health, lead-210, leukemia, ovary, polonium-210
In 2007, very high levels of the naturally occurring radioisotope polonium-210 (210Po) were discovered in drinking water from numerous private wells in Lahontan Valley, Churchill County, in northern Nevada. Of the 60 private wells and 3 public supply wells sampled in the valley (Seiler 2011), more than one-third of the private wells had 210Po levels exceeding 0.55 Bq/L, and 10% had levels that exceeded 2 Bq/L. This level is extremely unusual—fewer than 100 wells in the United States have reported 210Po activities that exceed 0.55 Bq/L, and the maximum value measured in Lahontan Valley, 6.59 Bq/L, is the fourth highest value reported in the United States (Seiler 2012).
210Po is one of the most toxic substances known because of its intense radioactivity, with 1 µg of 210Po having an activity of 1.66 × 108 Bq. After the death of Alexander Litvinenko from 210Po poisoning in 2006, several papers were published that addressed the diagnosis, treatment, and toxicity of acute 210Po poisoning (Harrison et al. 2007; Jefferson et al. 2009; Scott 2007) and the need for medical toxicology expertise about 210Po because of its potential use in radiation terrorism events (Nemhauser 2010). The estimated acute lethal dose from oral ingestion for an adult if untreated is 10–30 µg (Jefferson et al. 2009), and as little as 1 µg might be lethal to the most radiosensitive members of the population (Scott 2007).
As an alpha-particle–emitting decay product of radium-226 (226Ra), 210Po is classified as a Group 1 human carcinogen [International Agency for Research on Cancer (IARC) 2001]. Numerous epidemiological studies, which were reviewed by Canu et al. (2011), have investigated associations between exposure to members of the uranium-238 (238U) decay series in drinking water and cancers such as leukemia (e.g., Hoffmann et al. 1993; Lyman et al. 1985; Raaschou-Nielsen et al. 2008). Because 210Po is the last radioactive member of the 238U decay series, a common feature of all these studies is the presence of 210Po in the environment, even if it was not measured. Because 210Po is a human carcinogen and because information on environmental exposure to it is poorly known, we reviewed the literature on 210Po.
In this paper, we summarize relatively unknown and disparate information on the environmental behavior, biokinetics, and toxicology of 210Po and suggest supportive research that could help guide future investigations.
Environmental Behavior of 210Po
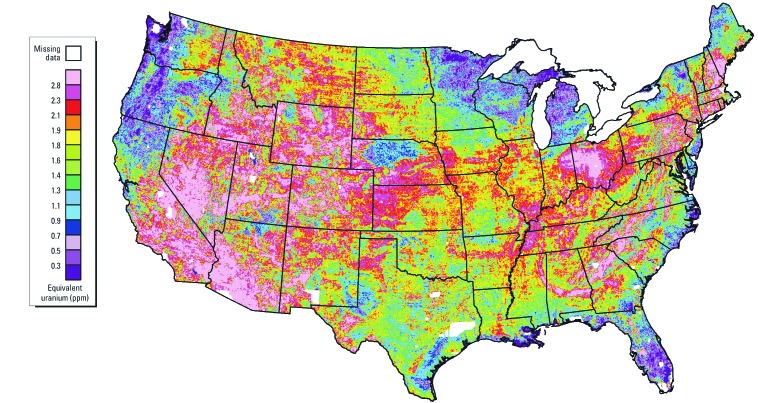
210Po has a relatively short half-life of 138.4 days and decays to lead-206 (206Pb) by emitting an alpha particle. It is the last unstable isotope in the 238U decay series and is present in the environment wherever 238U or any other members of the 238U decay series, such as 226Ra, radon, or 210Pb, are present. The average crustal U concentration is approximately 2.7 mg/kg (Siegel and Bryan 2003), and > 99% of natural U is 238U (Bordujenko 2002). Large areas in the United States have surficial uranium concentrations exceeding 2.7 mg/kg (Figure 1). The specific activity of 238U is 12.4 Bq/mg, and in old sediments where radon is not lost to the atmosphere, the activity of 210Po in secular equilibrium with 2.7 mg/kg of 238U would be about 33.5 Bq/kg.
Figure 1.
Equivalent uranium concentrations in surface soils and rocks of the conterminous United States. Adapted from Duval et al. (2005).
The main source of 210Po in the atmosphere and oceans is from the emanation of radon gas from surficial sediments to the atmosphere where it is spread by diffusion and winds (Parfenov 1974; Persson and Holm 2011). In addition, high 210Po can be introduced into the atmosphere in volcanic emissions because of the high volatility of 210Po compared with 210Pb (Kim et al. 2011). Radon’s decay products, 210Pb and 210Po, have strong affinities for particles and become widely distributed on the earth’s surface as the 210Pb and 210Po that are formed from decay of radon in the atmosphere adsorb to aerosol particles and fall onto the land or sea.
210Po activities exceeding 1 Bq/L have been found in private wells in Florida and Nevada and public supply wells in Louisiana and Maryland (Seiler et al. 2011). 210Po in groundwater is normally < 30 mBq/L because it strongly adsorbs to aquifer materials (Harada et al. 1989). In typical aquifer sediments, however, 210Po concentrations are high enough that geochemical and microbial processes that mobilize only a small percentage of the 210Po that is present can yield groundwater with 210Po concentrations that greatly exceed safe levels in drinking water. Wells with high 210Po in Florida are typically shallow wells located in the central part of the state (Harada et al. 1989) where the ultimate source of the 210Po is uranium-rich phosphate rock in the aquifers and phosphogypsum waste products from mining operations (Cherrier et al. 1995). U concentrations in aquifer sediments near contaminated wells in Nevada range from 1.4 to 4.5 mg/kg (Seiler et al. 2011) and are typical of the western United States (Figure 1). U concentrations in sediments near contaminated wells in Maryland and Louisiana have not been described.
A comparison of naturally contaminated wells in the United States and Finland (Seiler et al. 2011) suggested that, in areas where 238U and radon are present in the environment, elevated 210Po activities in groundwater may be found in two distinct groundwater environments, one with oxic or anoxic groundwater with extremely high radon levels, and the other with anoxic, sulfate-reducing groundwater with low hydrogen sulfide concentrations and low-to-high radon levels. In wells in Florida, Maryland, and Nevada, aqueous 210Pb concentrations were much less than 210Po concentrations (Harada et al. 1989; Outola et al. 2008; Seiler 2011), indicating that the 210Po was being mobilized from the sediments. Aqueous 210Po concentrations were the same as 210Pb concentrations in the Finnish wells (Seiler et al. 2011)—a finding that indicates secular equilibrium between the two radioisotopes and that the 210Po was derived from the decay of 210Pb in the water. Elevated 210Po levels in Finnish wells were associated with radon activities up to 43,000 Bq/L (Seiler et al. 2011). A Maryland well that had a 210Po activity of 1.7 Bq/L did not contain high radon (only 8.5 Bq/L) (Outola et al. 2008), an indication that elevated 210Po does not require the presence of elevated radon.
210Po Biokinetics and Biodistribution after Ingestion
Absorption and excretion. The fraction of 210Po absorbed from the digestive tract into the blood (f1) ranges from 3% to 80% (Harrison et al. 2007). Experimental data indicate greater absorption of biologically incorporated 210Po than of inorganic 210Po (Haines et al. 1993). In some areas, caribou and shellfish are important sources of 210Po in the diet of humans. Thomas et al. (2001) found that the average f1 was 56% among 13 volunteers who consumed caribou meat, and Hunt and Rumney (2007) reported an average f1 of approximately 51% among five volunteers who consumed shellfish. In a review article, Harrison et al. (2007) cited three different studies that measured f1 in rats that had orally ingested different Po compounds. The results showed that the f1 was 6% for Po chloride, 5% for Po nitrate, and 7–9% for Po citrate. The f1 of 210Po is unknown for ingestion of naturally contaminated groundwater, where the 210Po may be present in volatile organic forms such as dimethyl polonium (Hussain et al. 1995), in hydrophilic complexes with fulvic acids (Oural et al. 1988), or as an anionic colloid (Seiler et al. 2011). With prolonged chronic intake, absorption of 210Po through the digestive tract may increase sharply (Moroz and Parfenov 1972), possibly because of damage to the intestinal wall through persistent bombardment by alpha particles.
Following exposure to 210Po, much more 210Po is excreted in feces than in urine (Moroz and Parfenov 1972). Renal excretion of 210Po is slow compared with other elements because it binds strongly to hemoglobin and plasma proteins and is not filtered by the kidneys (Thomas et al. 2001). 210Po is also excreted through the cutaneous glands and the hair (Moroz and Parfenov 1972) and in milk (Parfenov 1974). In an experiment that used a lactating goat, Schreckhise and Watters (1969) estimated the transfer coefficient (percent intake by the goat per liter of milk produced) for 210Po was 0.18%.
Hunt and Rumney (2007) examined the retention of 210Po from consuming shellfish and concluded its biological half-life was about 40 days, which is consistent with the International Commission on Radiological Protection (ICRP 1993) value of 50 days. On the other hand, Thomas et al. (2001) concluded that the biological half-life of 210Po in humans following consumption of caribou meat is > 100 days and that once absorbed 210Po is excreted very slowly if at all. Assuming that the biological half-life is 50 days, then the effective half-life in humans would be about 37 days.
Biodistribution. The tissue distribution of 210Po across mammalian species is broadly similar (Thomas et al. 2001). 210Po concentrations in the bones of farm animals are 2–3 orders of magnitude greater than in the muscles (Parfenov 1974). Average relative 210Po activities in the liver, kidney, and muscle were 19, 13, and 1, respectively, in caribou and 24, 19, and 1, respectively, in wolves (Thomas et al. 1994). In adult female baboons, the highest concentrations of injected 210Po were found in the liver and kidney, and the lowest were in the muscle, skeleton, and brain (Fellman et al. 1994).
Ingested 210Po in humans is initially concentrated in red blood cells, followed by the liver, kidneys, bone marrow, gastrointestinal tract, and gonads (Jefferson et al. 2009). Once absorbed into the blood, about 30% goes to the liver, 10% to red bone marrow, 10% to the kidney, 5% to the spleen, and the remainder to the body in general (Thomas et al. 2001). Average wet weight 210Po concentrations for nonsmokers were 0.055, 0.53, and 0.48 Bq/kg in skeletal muscle, liver, and kidney, respectively (Parfenov 1974). Based on reported measurements of 210Po in the various organs and the weights of the organs in the standard reference person, the total 210Po content of the human body was estimated to be about 20 Bq (Parfenov 1974).
Median wet weight 210Po concentrations from cattle in an uncontaminated agricultural area in Germany were 1.2 Bq/kg in the liver and 6.6 Bq/kg in the kidney (Bunzl et al. 1979). In northern Poland with background levels of radioactivity, Skwarzec and Prucnal (2007) found that the average wet weight 210Po concentrations in deer from these areas were 0.16 Bq/kg in muscle, 1.08 Bq/kg in liver, and 1.58 Bq/kg in kidney. Levels for animals from contaminated areas can be significantly higher. In the Arctic, lichens accumulate large amounts of 210Po from the atmosphere and are the main winter forage for caribou (Thomas et al. 2001). In Canadian caribou that consume the lichens, 210Po concentrations ranged from 9 to 40 Bq/kg in meat and from 90 to 620 Bq/kg in liver and kidney (Thomas et al. 2001). In cattle raised in an area in New Mexico that was frequently flooded by untreated effluent from a nearby uranium mine, Lapham et al. (1989) found that the average wet weight 210Po concentrations were 3.4, 56, and 65 Bq/kg in muscle, liver, and kidney, respectively.
In general, testes and ovaries contain elevated 210Po concentrations compared with most other tissues. An assessment of 210Po biokinetics suggested that transfer coefficients to the testes and ovaries are 0.1% and 0.05% per day, respectively, for 210Po in blood plasma that originated from the gastrointestinal tract or from systemic tissues returning to the blood (Leggett and Eckerman 2001). 210Po in the ovaries accumulates preferentially in the cells of the follicular epithelium and in the connective tissue cells of the corpora lutea (Moroz and Parfenov 1972). Ten days after injecting 925,000 Bq/kg into mice, Finkel et al. (1953) found that the 210Po concentrations in the ovaries were very high and caused severe damage to the ovaries. At 10 days, concentrations in the ovary were exceeded only by concentrations in the spleen; however, within 120 days after the injection, 210Po concentrations in the ovaries had decreased and had become undetectable.
Published values for 210Po concentration in cow’s milk ranged from 3.3 to 18.5 mBq/L and were > 1 order of magnitude lower than in meat (Parfenov 1974). Concentrations can be much higher in milk from areas known to be contaminated. For example, the geometric mean 210Po concentration in milk samples from cattle raised in a uranium-mining district in India was 1.08 Bq/L (Giri et al. 2010), with a maximum of 2.94 Bq/L.
After injecting 210Po into pregnant rats and guinea pigs, Haines et al. (1995) reported that fetal and placental tissues contained about 3–4% of the injected 210Po in rats and about 10% in guinea pigs. The authors also observed that at birth each rat pup had about 0.1% of the injected 210Po and that on day 57 each guinea pig fetus contained 0.6%. The highest concentrations of 210Po, about 4% of the injected 210Po per gram, were measured in the yolk sacs of the embryonic rats during their hematopoietic stage (Haines et al. 1995). The data suggest that the placenta acts as a barrier against movement of 210Po from mother to fetus. In one population of caribou in Canada, fetal 210Po averaged 62% of the 210Pb and 41% in a second population of caribou (Thomas et al. 1994). We calculated that within the caribou gestation period of 230 days, it would take 204 and 117 days, respectively, in these two populations, for the decay of 210Pb to generate this much 210Po. Thus, decay of fetal 210Pb could have provided all or most of the of the measured fetal 210Po in the caribou. Paquet et al. (1998) found that approximately 0.7–1% of 210Po injected into pregnant baboons at 5 months was present in the fetus 7 days later, which indicates that small amounts of 210Po do pass through the placenta. Söremark and Hunt (1966) found that the autoradiographs of mice 4 days after being injected with 210Po showed 210Po was present in the placenta but not in the fetus; however, by the fifth day, 210Po was detected in the fetus. By this time, the placenta was showing damage, which suggests that 210Po transport across the placenta may change with dose and time.
Effects of 210Pb on 210Po biokinetics and biodistribution. 210Pb biokinetics affect 210Po biokinetics and biodistribution because 210Pb decays to 210Po. Lead accumulates in skeletal bone: 67% of the lead burden in children is in bone and 95% in adults (Barry and Mossman 1970). 210Pb readily passes the placenta (Goyer 1990) and 210Pb-supported 210Po (210Po originating from in situ decay of 210Pb) can irradiate fetal tissues. 210Po produced by decay of skeletal 210Pb remains in the bone (James et al. 2004); however, 210Po from the decay of soft-tissue 210Pb likely would follow typical 210Po biokinetics. 210Po activity begins to increase after 210Pb ingestion and reaches 25% of the 210Pb activity in 65 days, 50% in 145 days, and approximately 99% of the 210Pb activity in 2 years. Because 210Po concentrations increase as 210Pb in the body decays, exposure of the fetus and children to 210Pb-supported 210Po would be least during early pregnancy and would increase with age.
Toxicology of 210Po
Unlike some alpha emitters such as 238U, 210Po is toxic because of its radioactivity rather than its chemical properties (Jefferson et al. 2009). Acute lethal doses cause fatal damage to the bone marrow and, in addition, cause severe damage to the kidneys, spleen and gastrointestinal tract (Scott 2007). 210Po presents a radiation hazard only if taken into the body because its alpha particles have a range of only 40–50 µm in biological tissue and are easily stopped by surface layers of the skin (Jefferson et al. 2009).
210Po is a known human carcinogen (IARC 2001), and its carcinogenicity to animals has been demonstrated by numerous animal studies (reviewed by Moroz and Parfenov 1972). Exposure of the lungs to 210Po alone is capable of causing lung cancer in rats and hamsters (Little and O’Toole 1974; Yuile et al. 1967). Injecting 210Po into CF-1 female mice caused an increased incidence of lymphomas and soft-tissue and malignant-bone tumors (Finkel and Hirsch 1950). At a dose of 33.3 Bq/g body weight, 42% of autopsied mice had developed lymphomas within 250 days of injection compared with 21% of the controls.
Injected 210Po was taken up by the ovaries in mice and localized primarily in the follicle cells, whereas in the uterus it was distributed diffusely through the myometrium (Samuels 1966). Significant killing of primary oocytes occurred after exposure to as little as 0.037 Bq/g body weight 210Po, with higher doses killing almost all the oocytes and causing gross distortion of the ovarian architecture. Radioautographs led Samuels (1966) to conclude that 210Po appears to concentrate in the ovary in such a manner that it selectively irradiated the oocytes. Finkel et al. (1953) concluded that the ovary may be the critical organ in determining the lowest injurious dose for 210Po because of its radiosensitivity and tendency to concentrate 210Po.
For alpha radiation, substantial doses of radiation (0.5–1.3 Gy) are delivered to individual cells that are traversed by a single alpha particle no matter how low the dose to the whole body (Lorimore et al. 1998; Purnell et al. 1999). Alpha particles traversing the nucleus of HeLa cells produced a track averaging 10 µm, with an average of 22 double-strand chromosomal breaks (DSBs) (Aten et al. 2004). Breaks found at distant locations can subsequently be brought together and form chromosomal translocations (Aten et al. 2004). Alpha emitters can induce DNA lesions in stem cells that result in the transmission of chromosomal instability to their progeny (Kadhim et al. 1992), and even a single alpha particle can induce long-term chromosomal instability in primary human T lymphocytes (Kadhim et al. 2001). Chromosomal translocation and aneuploidy are a hallmark of the childhood leukemias regardless of specific histology (Szczepański et al. 2010). Because 210Po is an alpha emitter, toxicological investigations on the cellular and molecular effects of alpha radiation using other alpha emitters likely will also apply to 210Po. Irrespective of their source, alpha particles produce the same patterns of secondary ionizations and localized damage to biological molecules (IARC 2001), thus the important difference between different alpha emitters would be in what tissues they preferentially accumulate in, and thus what tissues are preferentially irradiated.
Radiation-induced bystander effects result when ionizing radiation, such as that from an alpha particle traversing a cell, causes targeted cells to produce signals that damage unirradiated neighboring cells (Hall 2003). Biological consequences to bystander cells include apoptosis, micronucleus induction, sister-chromatid exchange, chromosomal rearrangements, gene mutations, cell transformations, and delayed effects on their progeny such as genomic instability (Morgan and Sowa 2007). Bystander signals initiated by very low doses of alpha particles in irradiated normal human diploid fibroblasts induced DSB damage in unirradiated cells, and the percentage of DSBs in bystander cells was not dependent on the dose delivered (Hu et al. 2006). Exposure of only four human fibroblast cells to five alpha particles each was sufficient to induce a significant increase in micronucleated and apoptotic cells in a population of > 3,000 neighboring cells (Prise et al. 1998). In experiments where < 1% of the Chinese Hamster ovary cells in a culture were actually traversed by an alpha particle, Nagasawa and Little (1992) observed an increase in sister-chromatid exchange in > 30% of the cells. In a normal human three-dimensional skin-tissue system, Belyakov et al. (2005) saw significant increases in micronuclei formation and apoptosis in unirradiated cells up to 0.6 mm and 1.0 mm distant, respectively, from irradiated cells. The significance of the bystander effect for alpha particles is that linear extrapolation of the risk of carcinogenesis from high doses to low doses would underestimate the risks at low doses (Hall 2003).
Direct and Indirect Ingestion of 210Po
In uncontaminated settings, only about 14% of the human body burden is due to direct ingestion of 210Po, principally in food, and the remainder is indirectly due to ingestion of 210Pb, which decays to 210Po (Parfenov 1974). In soft tissues, about half of the 210Po originates from 210Pb, whereas in bone almost all of the 210Po originates from 210Pb (Parfenov 1974). Atmospheric inhalation, diet, and domestic radon are estimated to provide 12%, 86%, and 2%, respectively, of total 210Pb uptake; however, in some locations, inhalation can be the largest source of 210Pb (Salmon et al. 1998). Smoking and consumption of alcoholic beverages can add an extra 75% to the total 210Pb uptake (Salmon et al. 1998). In the absence of 210Po ingestion or inhalation, the 210Po activity will be approximately 99% of the 210Pb activity within 2 years.
Direct ingestion of 210Po-contaminated water. It is unusual to find 210Po activities greater than approximately 30 mBq/L in groundwater because it is rapidly adsorbed onto particles (Harada et al. 1989); however, in some geochemical settings, much higher levels of 210Po are found (Seiler et al. 2011). Exposure to contaminated drinking water is particularly insidious because it may be chronic and unrecognized, especially in rural areas where the population relies on private wells that are unregulated and not tested for radioactivity. Analyses for the presence of 210Po in drinking water are rarely made, and 210Po is not measured directly to determine compliance with drinking-water standards for public supply wells in the United States. In the United States, compliance is assessed indirectly by analyzing the U activity and the gross alpha radioactivity (GAR), a measure of emitted alpha radiation that does not identify the specific isotopes involved. The water samples are evaporated to dryness, which drives off radon gas, and the standard is violated if the GAR of the sample exceeds the U activity by 0.55 Bq [U.S. Environmental Protection Agency (EPA) 2000].
210Po activities in private wells in Lahontan Valley, Nevada, were measured at levels as high as 6.59 Bq/L (Seiler 2011), and these private wells serve hundreds to thousands of people in areas where 210Po commonly exceeds 0.5 Bq/L. In one well, the 210Po activity was stable in measurements made six half-lives (830 days) apart (Seiler 2012), which suggests users of contaminated wells in Lahontan Valley have been exposed to 210Po since the wells were drilled. Several wells with high 210Po in West Central Florida were used as household drinking-water sources or for lowering groundwater levels near phosphate mines (Burnett et al. 1988). One intensively studied well in Hillsborough County, Florida, with 210Po activities ranging between 9.6 Bq/L and 95 Bq/L (Burnett et al. 1988) was a private well used by several families as their main source of drinking water until the 210Po contamination was discovered. Contaminated municipal wells have served large numbers of people for more than a decade before the contamination was discovered. For example, a municipal well in service since 1992 provided water for 95 houses in a subdivision in Charles County, Maryland, and was discovered to contain 1.7 Bq/L 210Po in 2004 (Outola et al. 2008). The presence of 210Po was not discovered earlier because before 2002 it took about 1 year before samples were analyzed (Outola et al. 2008), during which time 210Po was being lost through radioactive decay.
The residents of parts of Lahontan Valley, where 210Po in private wells exceeds 0.5 Bq/L, can potentially be exposed to substantial doses of radioactivity. Using the dose conversion factor of 1.2 × 10–3 mSv/Bq for 210Po 1 year after ingestion (ICRP 2010), the annual effective dose would be > 0.4 mSv/year for adults who consumed 2 L/day of water from wells with > 0.5 Bq/L of 210Po. For adults using the well with 6.59 Bq/L of 210Po, the annual effective dose would be almost 6 mSv/year. For comparison, the World Health Organization (WHO 2006) concluded that no deleterious radiological health effects are expected from consumption of drinking water if the committed effective dose (i.e., the total effective dose received over a lifetime), does not exceed 0.1 mSv/year.
Effective dose coefficients are higher for infants than for adults, in part because the fraction of 210Po absorbed from the alimentary tract into the blood, f1, is assumed to be 100% for a child < 1 year of age, and 50% for older ages (ICRP 2010). For a 3-month-old child, the effective dose coefficient is 2.6 × 10–2 mSv/Bq for 210Po 1 year after ingestion, and for a 1-year-old child, it is 8.8 × 10–3 mSv/Bq (ICRP 2010). For a 3-month-old child who consumed 1 L/day of well water used to prepare infant formula, the annual effective dose would be almost 5 mSv/year for well water that contained 0.5 Bq/L of 210Po and about 60 mSv/year for well water that contained 6.59 Bq/L of 210Po. For a 3-month-old child, the committed equivalent dose coefficient is 5.7 × 10–3 mSv/Bq for the ovary, 8.1 × 10–2 mSv/Bq for red bone marrow, and 2.2 × 10–1 mSv/Bq for the spleen (ICRP 2010). Therefore, a 3-month-old child who consumed 1 L/day of well water that contained 0.5 Bq/L of 210Po would receive doses to the ovary, red bone marrow, and spleen of about about 1, 15, and 40 mSv/year, respectively.
Direct ingestion of 210Po-contaminated food. A review of food-chain transport of 210Po concluded that the greatest sources of ingested 210Po were found in populations with large dietary complements of seafood (Japan) or caribou and reindeer muscle and organs (Arctic) (Watson 1985). In shellfish and crustaceans, Lee et al. (2009) reported 210Po concentrations ranging from 19.1 to 33 Bq/kg in Korea, and Carvalho (1995) reported levels from 49 to 152 Bq/kg in Portugal. Because all marine organisms accumulate 210Po in high concentrations due to its solubility in seawater and its affinity to organic matter, seafood is a notable source of 210Po in the human diet (Alam and Mohamed 2011).
Caribou, which are a main dietary staple for many northern Canadians, bioaccumulate 210Po from lichens—their main winter forage (Thomas and Gates 1999). The surfaces of lichens are highly efficient at capturing 210Pb and 210Po from atmospheric fallout, with concentrations of about 250 Bq/kg dry weight (Persson and Holm 2011). The average 210Po concentration in lichen samples from 20 sites in northern Saskatchewan, Canada, was 232 Bq/kg. Thomas and Gates (1999) collected samples from 18 caribou that had consumed the lichen and found that the average 210Po concentration in the liver, kidney, and muscles was 332 Bq/kg (wet weight), 156 Bq/kg, and 14 Bq/kg, respectively.
Tobacco Toxicology and 210Po
Talhout et al. (2011) listed 98 hazardous compounds in mainstream cigarette smoke, including 210Po, and 51 other components that are known, probable, and possible human carcinogens. The 210Po concentration in tobacco has a mean ± SD of 13 ± 2 Bq/kg (Persson and Holm 2011), and the estimated daily inhalation of 210Po in smokers was between 13 and 590 mBq/day (Watson 1985). Zagà et al. (2011) concluded that 210Po is one of the most powerful carcinogens in tobacco smoke and, as previously noted, exposure of the lungs to 210Po alone is capable of causing lung cancer in rats and hamsters (Little and O’Toole 1974; Yuile et al. 1967).
Cigarette smoking has several effects that appear consistent with exposure to 210Po. A 60% increase in the risk of infertility among smokers may be related to ovarian toxicity (Augood et al. 1998). Cigarette smoking appears to significantly reduce ovarian reserves in women (El-Nemr et al. 1998), and Tuttle et al. (2009) found that the ovaries of mice that had been exposed to cigarette smoke were on average 20% smaller and had significantly fewer follicles. These outcomes appear similar to those described by Samuels (1966) for mice exposed to 210Po. Cigarette smoking also significantly increases a woman’s risk of developing mucinous ovarian cancer (Gram et al. 2011; Jordan et al. 2006). This may be linked to exposure to 210Po, which is known to accumulate in the ovary (Finkel et al. 1953).
Cigarette smoking has been linked to hematopoietic cancers, and a possible, although unstudied, contributing factor could be 210Po. For example, a case–control study on childhood cancer (Stjernfeldt et al. 1986) concluded that the risk of non-Hodgkin lymphoma and acute lymphoblastic leukemia (ALL) were doubled in the offspring of women who smoked > 10 cigarettes per day. Benzene is clearly a leukemogen in adults (Schnatter et al. 2005) and is typically indicated as the primary leukemogen in cigarettes. The contribution of tobacco carcinogens to leukemia, however, is predicated by an ability to target the appropriate organs, and 210Po shares with benzene a tropism to the bone marrow as well as fetal hematopoietic organs. To be sure, 210Po is not likely to be the major constituent in cigarette smoke’s contributions to leukemogenesis, but constitutes a dose- and mechanism-relevant constituent that deserves further scrutiny.
210Po and Leukemia
Leukemogenesis. The best substantiated cause for acute leukemia is via ionizing radiation (Greaves 1997), and recently published risk models suggest that about 15% of childhood leukemia in Great Britain is associated with natural background radiation (Kendall et al. 2011). Although experimental data are limited, the disposition and biological activities of 210Po in human and animal tissues are consistent with a role as a myelotoxicant and leukemogen. Those adult leukemias epidemiologically associated with chronic exposure to cigarettes or contaminated drinking water have cytogenetic characteristics that are typical of an alpha emitter such as 210Po. These leukemias commonly harbor broken chromosomes as well as aneuploidy, including loss of the q arm of chromosomes 5 and 7 and complete loss of chromosome 7 (Pedersen-Bjergaard and Rowley 1994).
210Po is equally plausible as a leukemogen in children. Epidemiologic studies on atomic bomb survivors indicated that young children were much more sensitive to radiation-induced leukemia than were adults (Little 2003). 210Po accumulates in several tissues with the capacity for hematopoiesis, including the fetal yolk sac and bone marrow and liver in mature animals as explained above. Both ALL and acute myelocytic leukemia (AML) in children are known to originate in the fetus, as demonstrated by the presence of cells with leukemia clone-specific mutations present at birth in children who later contract the disease (Wiemels 2008). Indirect evidence for the fetal origin of childhood ALL stems from the immature nature of immunoglobulin rearrangements as well as lack of evidence for recombinase activating gene-induced alterations at chromosomal translocations (Steenbergen et al. 1994; Wasserman et al. 1992). These translocations as well as chromosomal deletions and aneuploidy are the characteristic hallmark of childhood ALL (Greaves and Wiemels 2003; Mullighan et al. 2007). Translocations are the primary method of dosimetry of ionizing radiation exposure (Tucker 2008). Ionizing radiation from sources such as alpha particles has the capacity to both break DNA, a precursor to translocations and deletions (Cohen-Jonathan et al. 1999), and to elicit mitotic catastrophe, a type of cell death that occurs during mitosis (Castedo et al. 2004). Cells that fail to execute apoptosis in response to mitotic failure are likely to divide asymmetrically, with the consequent generation of aneuploid cells (Castedo et al. 2004). It is notable that up to 35% of childhood leukemias exhibit “high hyperdiploidy,” which is defined as having five or more extra chromosomes; this cytogenetic feature is known to occur after a single mitotic failure in utero (Gruhn et al. 2008; Paulsson et al. 2005).
Lahontan Valley childhood leukemia cluster. Between 1997 and 2002, 15 cases of ALL and 1 case of AML were diagnosed in children who lived in Lahontan Valley and in the city of Fallon in Churchill County, Nevada, before their diagnosis (Rubin et al. 2007). In the year 2000 alone, 9 cases of ALL were diagnosed in this county of only 26,000 people. Because > 12 cases were diagnosed during a 4-year period in which fewer than 2 cases would be expected, the U.S. Centers for Disease Control and Prevention led a multiagency effort to conduct a comprehensive cross-sectional exposure assessment that began in March 2001 (Rubin et al. 2007). Most research on the leukemia cluster focused on chemical exposures such as pesticides, jet fuel, volatile organic compounds, radon, arsenic, and tungsten (Rubin et al. 2007; Seiler 2004); however, no plausible exposure was identified. Instead, the temporal and spatial patterning of the leukemia cluster suggested involvement of an infectious disease (Francis et al. 2012). The temporal and spatial patterning was inconsistent with exposure to 210Po in drinking water (Seiler 2012); however, other possible sources of 210Po exposure were not investigated.
Discussion and Research Needs
Animal research studies indicate that 210Po accumulates in the reproductive and blood-forming organs and that some 210Po is probably transferred from the mother to the embryo and fetus where its high-energy alpha particles can potentially kill or damage critical cells. These effects are consistent with exposure to 210Po in cigarettes because maternal smoking is known to affect maternal fertility and possibly the development of cancer in offspring. Although 210Po is not the only carcinogen in cigarettes, daily inhalation of 210Po by smokers exposes them to between 0.013 and 0.59 Bq/day (Watson 1985). Relatively small amounts of 210Po in drinking water near the current U.S. standard (U.S. EPA 2000), or in food, could result in greater exposure to women to 210Po than from smoking, and subtle unrecognized health effects may be occurring in women who are exposed to environmental 210Po.
Little is specifically known about the effects of 210Po exposure to the embryo and fetus, however, fetal exposure could cause failure of implantation or miscarriage or major malfunctions (Jefferson et al. 2009), as well an increased incidence of chromosomal breaks and translocations resulting in childhood cancer. At particular stages of development a future tissue, or stem cells for many cell lineages, can be represented by one or a few cells, and alpha irradiation may cause mutagenic effects in these cells that is later manifested as cancer (Goodhead 1991). The distribution of 210Po to the ovaries and the embryo (Finkel et al. 1953; Haines et al. 1995; Samuels 1966) and the potential for a single alpha particle traversing a stem cell to induce chromosomal instability (Kadhim et al. 2001) suggests that even low-level alpha-particle irradiation to reproductive or embryonic cells may have significant downstream effects. New, basic, biological, and toxicological research is needed on 210Po because of its potential to kill or damage critical reproductive, embryonic, or hematopoietic cells at low doses. New research is needed on transplacental transfer of 210Po and the effects of low-level exposure to the embryo, fetus, and infant because the high degree of cell kinetics needed for normal growth of embryonic and hematopoietic cells and other tissues in the fetus and child imparts a high sensitivity to children from environmental insults such as ionizing radiation from 210Po.
210Po may be involved in leukemogenesis by exposing the hematopoietic stem cells in the embryonic yolk sac and fetal spleen, liver, and bone marrow to alpha-particle radiation. The placenta provides at least a partial barrier to transfer of 210Po; however, 210Pb readily passes the placenta (Goyer 1990). Fetal 210Pb is incorporated into bone and 210Pb-supported 210Po could irradiate hematopoietic stem cells in fetal marrow. 210Po remains in place following decay of calcified 210Pb (James et al. 2004), and up to 80% of the marrow in lumbar vertebrae would be within range of alpha particles from 210Po on bone surfaces because bone-marrow spaces are small in the fetus (Purnell et al. 1999). Maternal 210Po that passes the placenta, or 210Pb-supported 210Po in soft tissues, could migrate in the blood to the fetal liver or spleen and potentially expose hematopoietic stem cells. Fat is present in adult but not fetal bone marrow and, being fat soluble, inhaled or ingested radon could accumulate in fat in adult marrow and expose it to alpha-particle irradiation from the radon itself or its daughters 218Po and 214Po (Richardson et al. 1991). New research is needed on toxicological and health effects of adult and embryonic and fetal exposure to Po isotopes, regardless of whether the exposure originates from ingestion of 210Po itself or its radionuclide grandparents radon and 210Pb. The emergence of exquisitely sensitive modern leukemia mouse models should be used to explore mechanisms of carcinogenesis by 210Po and 210Pb administered by appropriate routes and doses. Such models are currently used primarily to explore genetic pathways but increasingly are being made available for environmental research.
The linkage between smoking and female infertility (Augood et al. 1998) and the smaller size and reduced number of follicles in the ovaries of mice exposed to cigarette smoke (Tuttle et al. 2009) appear similar to outcomes described by Samuels (1966) for mice where primary oocytes were killed at low 210Po doses and the structure of the ovaries affected at higher doses. An unrecognized outcome for women exposed to environmental 210Po may be subfertility. Dose calculations together with a cell-survival function for primary oocytes could be used to estimate doses to the ovary and provide information on the expected level of killing of reproductive cells by environmental levels of 210Po. A single alpha particle can induce bystander effects in a population of cells, thus the sensitivity of primary oocytes to low doses of 210Po noted by Samuels (1966) may be the result of bystander effects. There may be tissue-specific differences in bystander responses, and research is needed on the susceptibility of reproductive and embryonic cells to bystander signals.
Numerous epidemiological studies reviewed by Canu et al. (2011) have investigated associations between cancers, including leukemia, and ingestion of naturally radioactive water. Even if exposure to 210Po was not occurring, 210Po must have been present in the environment at these studies because 238U, 226Ra, and radon ultimately decay to 210Po. Future investigations of natural radioactivity should consider the possibility that exposure to 210Po is occurring.
Residents of the subdivision in Charles County, Maryland (Outola et al. 2008), and rural areas of Lahontan Valley, Nevada (Seiler 2012), were exposed to levels of 210Po exceeding 1.5 Bq/L in their drinking water for decades before it was discovered. If the 210Po activity exceeds 0.55 Bq/L, then the GAR standard for 210Po would be exceeded (U.S. EPA 2000). Hence, even levels that meet the current drinking-water standard could expose the public to more 210Po than they would receive if they were smokers. The occurrence of 210Po concentrations in groundwater > 0.55 Bq/L is currently assumed to be extremely rare, but this rarity may be an artifact of groundwater seldom being directly tested for 210Po. Furthermore, the standard test for GAR (U.S. EPA 2000) may not adequately identify samples with 210Po concentrations that exceed safety thresholds. The recommended procedure allows quarterly samples to be composited as long as they are analyzed within 1 year of the first sample being collected (U.S. EPA 2000). However, during this 1-year period, > 60% of the 210Po in the composited samples can be lost by radioactive decay before analysis. Furthermore, sample heating during the analytical procedure for gross alpha radioactivity can drive off volatile radionuclides such as 210Po (Arndt and West 2008). Research on the environmental occurrence of 210Po and 210Pb is needed, particularly in drinking water, because of potential health effects at low levels. Development of new, less expensive, analyses for 210Po and 210Pb is needed because of the potential that current screening tests for them do not adequately protect public health.
Additional research on the occurrence of 210Po in groundwater and the f1 of naturally occurring 210Po is also needed because of the potential for contaminated food chains resulting from contaminated wells being used as water supplies for food animals. Chemical or radiological testing of water used for stock animals is not required by state or federal governments, and testing of meat and dairy products for contaminants found in animal’s stockwater is not required by the U.S. Food and Drug and Administration (FDA). When the discovery of 210Po in Lahontan Valley was first reported, two dairies that used wells with > 2 Bq/L 210Po were required to dump thousands of gallons of milk by the local dairy cooperative until the FDA could test whether the milk was safe. Samples of raw milk, beef, liver, and kidney from the dairies were analyzed by the FDA (Lin and Wu 2009) and the FDA concluded that the milk was safe (Associated Press 2007). However, the analytical results and risk analyses were never released to the public.
Summary
The extent of environmental exposure to 210Po is poorly known, and environmentally relevant exposure to it may have subtle unrecognized health effects that merit further investigation because 210Po accumulates in reproductive and embryonic and fetal tissues where a single alpha particle can kill or damage critical cells. 210Po is present in cigarettes, and maternal smoking is known to affect ovarian reserve and possibly the development of cancer in the offspring. Daily exposure from 210Po in cigarettes is less than a person could receive from drinking water that meets U.S. drinking-water standards (U.S. EPA 2000), which raises the possibility that subtle health effects similar to those observed in women who smoke might be observed in exposed members of the public.
In uncontaminated settings, only about 14% of the human body burden is due to direct ingestion of 210Po; the remainder is indirectly due to ingestion of 210Pb. Nonetheless, exposure to 210Po through drinking water may be more widespread than currently assumed and a comprehensive national reconnaissance is needed to determine what levels of 210Po and 210Pb the U.S. population is being exposed to in its drinking water. 210Po concentrations in typical aquifer sediments are high enough that mobilization of only a small percentage of the 210Po present in the sediments could yield water with sufficient 210Po to affect the health of people drinking the water. For adults and infants using wells with 0.5 Bq/L of 210Po, which would meet the current U.S. drinking-water standard, the annual effective dose would exceed the limit of 0.1 mSv/year recommended by the WHO (2006). Private wells used in rural areas and wells used for stockwater are unregulated, and even gross 210Po contamination of one of these wells would almost never be detected.
Much of the important biological and toxicological research on 210Po is more than four decades old. Modern tools are required to assess environmentally relevant exposure to 210Po and its capacity to kill or damage critical reproductive, embryonic, or hematopoietic cells in humans. We call attention to these research needs to assess whether current regulatory standards to protect public health are appropriate so effective mitigation measures can be developed if necessary.
Acknowledgments
The authors thank the U.S. Environmental Protection Agency for funding their work on the Fallon Leukemia Cluster (grant EM-96963201), and J.L.W. thanks the Children with Cancer UK (formerly Children with Leukaemia) for providing additional support (grant 10-14-19). R.L.S. thanks T. Rees for encouraging him to get involved in the Fallon Leukemia Cluster Investigation. An earlier version of this article was greatly improved by the valuable comments of three anonymous reviewers, and R.L.S. appreciates the helpful comments by former colleagues at U.S. Geological Survey (USGS).
Footnotes
R.L.S. is retired from the USGS, and the views expressed in this paper are those of the authors and do not reflect the position of the U.S. government or the USGS.
The authors declare they have no actual or potential competing financial interests.
References
- Alam L, Mohamed CAR. A mini-review on bioaccumulation of 210Po by marine organisms. Int Food Res J. 2011;18:1–10. [Google Scholar]
- Arndt MF, West L. An experimental analysis of the contribution of 210Po and of 210Po produced by 210Pb decay to the gross alpha-particle activity of water samples. Health Phys. 2008;94:310–316. doi: 10.1097/01.HP.0000315807.81067.04. [DOI] [PubMed] [Google Scholar]
- Associated Press. FDA clears Fallon dairies. Las Vegas Review-Journal (Las Vegas, NV) 7 August. 2007. Available: http://www.lvrj.com/news/8961877.html [accessed 30 November 2010]
- Aten JA, Stap J, Krawcyzyk PM, van Oven CH, Hoebe RA, et al. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science. 2004;303:92–95. doi: 10.1126/science.1088845. [DOI] [PubMed] [Google Scholar]
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–1539. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- Barry PSI, Mossman DB. Lead concentrations in human tissues. Br J Ind Med. 1970;27:339–351. doi: 10.1136/oem.27.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov OV, Michell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, et al. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci USA. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordujenko A. Military medical aspects of depleted uranium munitions. ADF Health. 2002;3:50–57. [Google Scholar]
- Bunzl K, Kracke W, Kreuzer W. 210Pb and 210Po in liver and kidneys of cattle—I. Animals from an area with little traffic or industry. Health Phys. 1979;37:323–330. doi: 10.1097/00004032-197909000-00007. [DOI] [PubMed] [Google Scholar]
- Burnett WC, Chin P, Deetae S, Panik P. Release of Radium and Other Decay-Series Isotopes from Florida Phosphate Rock. Florida Institute of Phosphate Research Publication No. 05-016-059. 1988. Available: http://www1.fipr.state.fl.us/fipr/fipr1.nsf/129fc2ac92d337ca85256c5b00481502/31573005bc536bd885256b2f005ad314!OpenDocument [accessed 25 July 2012]
- Canu IG, Laurent O, Pires N, Laurier D, Dublineau I. Health effects of naturally radioactive water ingestion: the need for enhanced studies. Environ Health Perspect. 2011;119:1676–1680. doi: 10.1289/ehp.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FP. 210Po and 210Pb intake by the Portuguese population: the contribution of seafood in the dietary intake of 210Po and 210Pb. Health Phys. 1995;69:469–480. doi: 10.1097/00004032-199510000-00004. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Cherrier J, Burnett WC, LaRock PA. Uptake of polonium and sulfur by bacteria. Geomicrobiol J. 1995;13:103–115. [Google Scholar]
- Cohen-Jonathan E, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Duval JS, Carson JM, Holman PB, Darnley AG. Reston, VA: U.S. Geological Survey; 2005. Terrestrial Radioactivity and Gamma-ray Exposure in the United States and Canada. Open-file Report 2005-1413. [Google Scholar]
- El-Nemr A, Al-Shawaf T, Sabatini L, Wilson C, Wower AM, Grudzinskas GG. Effect of smoking on ovarian reserve and ovarian stimulation in in vitro fertilization and embryo transfer. Hum Reprod. 1998;13:2192–2198. doi: 10.1093/humrep/13.8.2192. [DOI] [PubMed] [Google Scholar]
- Fellman A, Ralston L, Hickman D, Ayres L, Cohen N. Polonium metabolism in adult female baboons. Radiat Res. 1994;137:238–250. [PubMed] [Google Scholar]
- Finkel MP, Hirsch GM. In: Argonne National Laboratory. ANL-4531 Health and Biology. Division of Biological and Medical Research. Quarterly Report, August, September, October 1950 (Brues AM, ed). Chicago:Argonne National Laboratory, 80–92; 1950. Progress of the polonium mouse experiment. II. Analysis at 500 days. [Google Scholar]
- Finkel MP, Norris WP, Kisioloski WE, Hirsch GM. The toxicity of polonium 210 in mice. I. The 30-day LD50, retention, and distribution. Am J Roentgenol Radium Ther Nucl Med. 1953;10:477–485. [PubMed] [Google Scholar]
- Francis SS, Selvin S, Yang W, Buffler PA, Wiemels JL. Unusual space-time patterning of the Fallon, Nevada, leukemia cluster: evidence of an infectious etiology. Chem Biol Interact. 2012;196(3):102–109. doi: 10.1016/j.cbi.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Singh G, Jha VN, Tripathi RM. Risk assessment due to ingestion of natural radionuclides and heavy metals in the milk samples: a case study from a proposed uranium mining area, Jharkhand. Environ Monit Assess. 2010;175:157–166. doi: 10.1007/s10661-010-1502-8. [DOI] [PubMed] [Google Scholar]
- Goodhead DT. Microscopic features of dose from radionuclides, particularly emitters of α-particles and Auger electrons. Int J Radiat Biol. 1991;60:550–553. [PubMed] [Google Scholar]
- Goyer RA. Transplacental transfer of lead. Environ Health Perspect. 1990;89:101–105. doi: 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram IT, Lukanova A, Brill I, Braaten T, Lund E, Lundin E, et al. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer. 2011;130:2204–2210. doi: 10.1002/ijc.26235. [DOI] [PubMed] [Google Scholar]
- Greaves MF. Aetiology of acute leukaemia. Lancet. 1997;349:344–349. doi: 10.1016/s0140-6736(96)09412-3. [DOI] [PubMed] [Google Scholar]
- Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Gruhn B, Taub JW, Ge Y, Beck JF, Zell R, Häfer R, et al. Prenatal origin of childhood acute lymphoblastic leukemia, association with birth weight and hyperdiploidy. Leukemia. 2008;22:1692–1697. doi: 10.1038/leu.2008.152. [DOI] [PubMed] [Google Scholar]
- Haines JW, Harrison JW, Pottinger HE, Phipps AW. Transfer of polonium to the embryo and foetus of rat and guinea pig. Int J Radiat Biol. 1995;67:381–390. doi: 10.1080/09553009514550431. [DOI] [PubMed] [Google Scholar]
- Haines JW, Naylor GPI, Pottinger H, Harrison JD. Gastrointestinal absorption and retention of polonium in adult and newborn rats and guinea pigs. Int J Radiat Biol. 1993;64:127–132. doi: 10.1080/09553009314551181. [DOI] [PubMed] [Google Scholar]
- Hall EJ. The bystander effect. Health Phys. 2003;85:31–35. doi: 10.1097/00004032-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Harada K, Burnett WC, LaRock PA, Cowart JB. Polonium in Florida groundwater and its possible relationship to the sulfur cycle and bacteria. Geochim Cosmochim Acta. 1989;53:143–150. [Google Scholar]
- Harrison J, Leggett R, Lloyd D, Phipps A, Scott B. Polonium-210 as a poison. J Radiol Prot. 2007;27:17–40. doi: 10.1088/0952-4746/27/1/001. [DOI] [PubMed] [Google Scholar]
- Hoffmann W, Kranefeld A, Schmitz-Feuerhake I. Radium-226-contaminated drinking water: hypothesis on an exposure pathway in a population with elevated childhood leukemia. Environ Health Perspect. 1993;101(supp 3):113–115. doi: 10.1289/ehp.93101s3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wu L, Han W, Zhang L, Chen S, Xu A, et al. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- Hunt GJ, Rumney HS. The human alimentary tract transfer and body retention of environmental polonium-210. J Radiol Prot. 2007;27:405–426. doi: 10.1088/0952-4746/27/4/002. [DOI] [PubMed] [Google Scholar]
- Hussain N, Ferdelman TG, Church TM, Luther GW., III Bio-volatilization of polonium: results from laboratory analyses. Aquat Geochem. 1995;1:175–188. [Google Scholar]
- IARC (International Agency for Research on Cancer) Ionizing Radiation, Part 2: Some Internally Deposited Radionuclides. IARC Monogr Eval Carcinog Risks Hum. 2001;78(Pt 2):1–559. [PMC free article] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Age-Dependent Doses to Members of the Public from the Intake of Radionuclides: Part 2. Ingestion Dose Coefficients. A Report of a Task Group of Committee 2. ICRP Publication No. 67. Ann ICRP. 1993;23(3–4):1–167. [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Free Educational CD Downloads. ICRP Database of Dose Coefficients: Workers and Members of the Public. Version 3. 2010. Available: http://www.icrp.org/page.asp?id=145 [accessed 15 December 2011]
- James PR, Close JJ, Keitch PA, Allen JE, Fews AP, Henshaw DL. Morphological features of the microdistribution of naturally occurring 210Pb/210Po and 226Ra in the teeth of children and juveniles. Int J Radiat Biol. 2004;80:185–198. doi: 10.1080/09553000410001665681. [DOI] [PubMed] [Google Scholar]
- Jefferson RD, Goans RE, Blain PG, Thomas SHL. Diagnosis and treatment of polonium poisoning. Clin Toxicol (Phila) 2009;47:379–392. doi: 10.1080/15563650902956431. [DOI] [PubMed] [Google Scholar]
- Jordan SJ, Whiteman DC, Purdie DM, Green AC, Webb PM. Does smoking increase risk of ovarian cancer? A systematic review. Gynecol Oncol. 2006;103(3):1122–1129. doi: 10.1016/j.ygyno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG.1992Transmission of chromosomal instability after plutonium α-particle irradiation, Nature 255738–740. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Marsden SJ, Goodhead DT, Malcolmson AM, Folkard M, Prise KM, et al. Long-term genomic instability in human lymphocytes induced by single-particle irradiation. Radiat Res. 2001;155:122–126. doi: 10.1667/0033-7587(2001)155[0122:ltgiih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kendall G, Little MP, Wakeford R. Numbers and proportions of leukemias in young people and adults induced by radiation of natural origin. Leuk Res. 2011;35:1039–1043. doi: 10.1016/j.leukres.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kim T, Church TM. In: Handbook of Environmental Isotope Geochemistry, Part 2 (Baskaran M, ed). Berlin:Springer-Verlag, 271–284; 2011. Po-210 in the environment: Biogeochemical cycling and bioavailability. [Google Scholar]
- Lapham SC, Millard JB, Samet JM. Health implications of radionuclide levels in cattle raised near U mining and milling facilities in Ambrosia Lake, New Mexico. Health Phys. 1989;56:327–340. doi: 10.1097/00004032-198903000-00008. [DOI] [PubMed] [Google Scholar]
- Lee CW, Kang MJ, Lee W, Choi GS, Cho YH, Kim HR, et al. Assessment of 210Po in foodstuffs consumed in Korea. J Radioanal Nucl Ch. 2009;279:519–522. [Google Scholar]
- Leggett RW, Eckerman KF. A systemic biokinetic model for polonium. Sci Total Environ. 2001;275:109–125. doi: 10.1016/s0048-9697(00)00858-5. [DOI] [PubMed] [Google Scholar]
- Lin Z, Wu Z. Analysis of polonium-210 in food products and bioassay samples by isotope-dilution alpha spectrometry. Appl Radiat Isot. 2009;67:907–912. doi: 10.1016/j.apradiso.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Little JB, O’Toole WF. Respiratory tract tumors in hamsters induced by benzo(a)pyrene and 210Po α-radiation. Cancer Res. 1974;34:3026–3029. [PubMed] [Google Scholar]
- Little MP. Risks associated with ionizing radiation. Br Med Bull. 2003;68:259–275. doi: 10.1093/bmb/ldg031. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Kadhim MA, Pocock DA, Papworth D, Stevens DL, Goodhead DT, et al. Chromosomal instability in the descendants of unirradiated surviving cells after α-particle irradiation. P Natl Acad Sci USA. 1998;95:5730–5733. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GH, Lyman CG, Johnson W. Association of leukemia with radium groundwater contamination. JAMA. 1985;254:621–626. [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Nontargeted bystander effects induced by ionizing radiation. Mutat Res. 2007;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Moroz BB, Parfenov YD. Metabolism and biological effects of polonium-210. At Energy Rev. 1972;(10):175–232. [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Nemhauser JB. The polonium-210 public health assessment: The need for medical toxicology expertise in radiation terrorism events. J Med Toxicol. 2010;6:355–359. doi: 10.1007/s13181-010-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oural CR, Upchurch SB, Brooker HR. Radon progeny as souces of gross-α radioactivity anomalies in ground water. Health Phys. 1988;55:880–894. doi: 10.1097/00004032-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Outola I, Nour S, Kurosaki H, Inn K, LaRosa J, Lucas L, et al. Investigation of radioactivity in selected drinking water samples from Maryland. J Radioanal Nucl Chem. 2008;277:155–159. [Google Scholar]
- Paquet F, Poncy J-L, Ham GJ, Prosser SL, Harrison JD. Transfer of polonium, neptunium, plutonium and americium to the primate fetus. Radiat Prot Dosimetry. 1998;79:303–306. [Google Scholar]
- Parfenov YD. Polonium-210 in the environment and in the human organism. At Energy Rev. 1974. pp. 75–143. [PubMed]
- Paulsson K, Mörse H, Fioretos T, Behrendtz M, Strömbeck B, Johansson B. Evidence for a single-step mechanism in the origin of hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2005;44:113–122. doi: 10.1002/gcc.20222. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J, Rowley JD. The balanced and unbalanced chromosome aberrations of acute myeloid leukemia may develop in different ways and may contribute differently to malignant transformation. Blood. 1994;83:2780–2786. [PubMed] [Google Scholar]
- Persson BRR, Holm E. Polonium-210 and lead-210 in the terrestrial environment: a historical review. J Environ Radioact. 2011;102:420–429. doi: 10.1016/j.jenvrad.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Prise KM, Folkard M, Michael BD. Radiation-induced bystander and adaptive responses in cell and tissue models. Dose Response. 1998;4:263–276. doi: 10.2203/dose-response.06-113.Prise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell SJ, Allen JE, Oyedepo AC, Henshaw DL. Fetal dosimetry from natural alpha-particle emitters. Radiat Res. 1999;152:S133–S136. [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Andersen CE, Andersen HP, Gravesen P, Lind M, Schüz J, et al. Domestic radon and childhood cancer in Denmark. Epidemiology. 2008;19:536–543. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Richardson RB, Eatough JP, Henshaw DL. Dose to red bone marrow from natural radon and thoron exposure. Br J Radiol. 1991;64:608–624. doi: 10.1259/0007-1285-64-763-608. [DOI] [PubMed] [Google Scholar]
- Rubin CS, Holmes AK, Belson MG, Jones RL, Flanders WD, Kieszak SM, et al. Investigating childhood leukemia in Churchill County, Nevada. Environ Health Perspect. 2007;115:151–157. doi: 10.1289/ehp.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon PL, Berkovsky VI, Henshaw DL. Relative importance of inhalation and ingestion as sources of uptake of 210Pb from the environment. Radiat Prot Dosimetry. 1998;78:279–293. [Google Scholar]
- Samuels LD. Effects of polonium-210 on mouse ovaries. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;11:117–130. doi: 10.1080/09553006614550881. [DOI] [PubMed] [Google Scholar]
- Schnatter AR, Rosamilia K, Wojcik NC. Review of the literature on benzene exposure and leukemia subtypes. Chem Biol Interact. 2005;153–154:9–21. doi: 10.1016/j.cbi.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Schreckhise RG, Watters RL. Internal distribution and milk secretion of polonium-210 after oral administration to a lactating goat. J Dairy Sci. 1969;52:1867–1869. doi: 10.3168/jds.s0022-0302(69)86860-8. [DOI] [PubMed] [Google Scholar]
- Scott BR. Health risk evaluations for ingestion exposure of humans to polonium-210. Dose Response. 2007;5:94–122. doi: 10.2203/dose-response.06-013.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler RL. Temporal changes in water quality at a childhood leukemia cluster. Ground Water. 2004;42:446–455. doi: 10.1111/j.1745-6584.2004.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Seiler RL. 210Po in Nevada groundwater and its relation to gross alpha radioactivity. Ground Water. 2011;49:160–171. doi: 10.1111/j.1745-6584.2010.00688.x. [DOI] [PubMed] [Google Scholar]
- Seiler RL. Physical setting and natural sources of exposure to carcinogenic trace elements and radionuclides in Lahontan Valley, Nevada. Chem Biol Interact. 2012;196:79–86. doi: 10.1016/j.cbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Seiler RL, Stillings LL, Cutler N, Salonen L, Outola I. Biogeochemical factors affecting the presence of 210Po in groundwater. Appl Geochem. 2011;26:526–539. [Google Scholar]
- Siegel MD, Bryan CR. Environmental geochemistry of radioactive contamination. Treatise Geochem. 2003;9:205–262. [Google Scholar]
- Skwarzec B, Prucnal M. Accumulation of polonium 210Po in tissues and organs of deer carvidae from Northern Poland. J Environ Sci Health B. 2007;42:335–341. doi: 10.1080/03601230701229346. [DOI] [PubMed] [Google Scholar]
- Söremark R, Hunt VR. Autoradiographic studies of the distribution of polonium-210 in mice after a single intravenous injection. Int J Radiat Biol Relat Stud Phys Chem Med. 1966;11:43–50. doi: 10.1080/09553006614550781. [DOI] [PubMed] [Google Scholar]
- Steenbergen EJ, Verhagen OJ, van Leeuwen EF, Behrendt H, Merle PA, Wester MR, et al. B precursor acute lymphoblastic leukemia third complementarity-determining regions predominantly represent an unbiased recombination repertoire: leukemic transformation frequently occurs in fetal life. Eur J Immunol. 1994;24:900–908. doi: 10.1002/eji.1830240418. [DOI] [PubMed] [Google Scholar]
- Stjernfeldt M, Lindsten J, Berglund K, Ludvigsson J. Maternal smoking during pregnancy and risk of childhood cancer. Lancet. 1986;327(8494):1350–1352. doi: 10.1016/s0140-6736(86)91664-8. [DOI] [PubMed] [Google Scholar]
- Szczepański T, Harrison CJ, van Dongen JJ. Genetic aberrations in paediatric acute leukaemias and implications for management of patients. Lancet Oncol. 2010;11:880–889. doi: 10.1016/S1470-2045(09)70369-9. [DOI] [PubMed] [Google Scholar]
- Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PA, Fisenne I, Chorney D, Baweja AS, Tracy BL. Human absorption and retention of polonium-210 from caribou meat. Radiat Prot Dosimetry. 2001;97:241–250. doi: 10.1093/oxfordjournals.rpd.a006669. [DOI] [PubMed] [Google Scholar]
- Thomas PA, Gates TE. Radionuclides in the lichen-caribou-human food chain near uranium mining operations in northern Saskatchewan, Canada. Environ Health Perspect. 1999;107:527–537. doi: 10.1289/ehp.99107527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PA, Sheard JW, Swanson S. Transfer of 210Po and 210Pb through the lichen-caribou-wolf food chain of northern Canada. Health Phys. 1994;66:666–677. doi: 10.1097/00004032-199406000-00006. [DOI] [PubMed] [Google Scholar]
- Tucker JD. Low-dose ionizing radiation and chromosome translocations: a review of the major considerations for human biological dosimetry. Mutat Res. 2008;659:211–220. doi: 10.1016/j.mrrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Tuttle AM, Stampfli M, Foster WG. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum Reprod. 2009;24:1452–1459. doi: 10.1093/humrep/dep023. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Enivronmental Protection Agency) National primary drinking water regulations; radionuclides; final rule. Fed Reg. 2000;65(236):76708–76753. [Google Scholar]
- Wasserman R, Galili N, Ito Y, Reichard BA, Shane S, Rovera G. Predominance of fetal type DJH joining in young children with B precursor lymphoblastic leukemia as evidence for an in utero transforming event. J Exp Med. 1992;176:1577–1581. doi: 10.1084/jem.176.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AP. Polonium-210 and lead-210 in food and tobacco products: transfer parameters and normal exposure and dose. Nuclear Safety. 1985;26:179–191. [Google Scholar]
- Wiemels J. Chromosomal translocations in childhood leukemia: natural history, mechanisms, and epidemiology. J Natl Cancer Inst Monogr. 2008;39:87–90. doi: 10.1093/jncimonographs/lgn006. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Guideline for Drinking-water Quality. First Addendum to Third Edition. Volume 1: Recommendations. 2006. Available: www.who.int/water_sanitation_health/dwq/gdwq0506.pdf [accessed 30 January 2011]
- Yuile CL, Berke HL, Hull T. Lung cancer following polonium-210 inhalation in rats. Radiat Res. 1967;31:760–774. [Google Scholar]
- Zagà V, Lygidakis C, Chaouachi K, Gattavecchia E.2011Polonium and lung cancer. J Oncol doi: 10.1155/2011/860103[Online 23 June 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]