Abstract
Delivery of proteins to the lytic vacuole in plants is a complex cascade of selective interactions that specifically excludes residents of the endoplasmic reticulum and secreted proteins. Vacuolar transport must be highly efficient to avoid mistargeting of hydrolytic enzymes to locations where they could be harmful. While plant vacuolar sorting signals have been well described for two decades, it is only during the last 5 years that a critical mass of data was gathered that begins to reveal how vacuolar sorting receptors (VSRs) may complete a full transport cycle. Yet, the field is far from reaching a consensus regarding the organelles that could be involved in vacuolar sorting, their potential biogenesis, and the ultimate recycling of membranes and protein machinery that maintain this pathway. This review will highlight the important landmarks in our understanding of VSR function and compare recent transport models that have been proposed so that an emerging picture of plant vacuolar sorting mechanisms can be drawn.
INTRODUCTION
Unlike prokaryotes, which can transport proteins only across or into the plasma membrane, eukaryotes possess a complex secretory pathway consisting of several compartments with distinct morphologies and lipid/protein compositions. The protein components of this pathway can be classified into cargo and transport machinery. Biosynthetic cargo transport occurs via multiple transport steps in a vectorial manner (Palade, 1975), starting with synthesis in the endoplasmic reticulum (ER). Further transport via the Golgi apparatus and/or other intermediate organelles can lead to two end locations, the plasma membrane or the vacuole. Endocytic transport also occurs in a vectorial manner but starts from the plasma membrane and leads to intracellular organelles.
Cargo trafficking can occur via two fundamental mechanisms. Transport between existing organelles is regulated by protein–protein and protein–lipid interactions to shape vesicular or tubular transport carriers that leave one organelle and fuse with another (Bonifacino and Glick, 2004). This is complemented by organelle maturation, a process by which selective removal of specific constituents leads to a gradual change in the biochemical composition until the organelle itself assumes another identity (Luini, 2011). However, not all transport events fall strictly into either one of these two mechanisms, and intermediate scenarios, such as organelle stratification followed by asymmetrical fission, will be discussed later. All the organelles of the secretory pathway are seemingly involved in both biosynthetic and endocytic transport (Pelham et al., 1992). It is the inevitable crosstalk between the numerous branches and recycling routes of the pathway that have made the study of the machinery so challenging.
In contrast with linear cargo transport, membranes and machinery need to be continuously recycled to mediate multiple transport reactions and to maintain organelle function. As a consequence, a number of secretory pathway components move in a bidirectional manner between two adjacent organelles. Besides the in vivo complexity, technical limitations include the low abundance of certain key regulators, obstructing biochemical identification, as well as pleiotropic effects of some loss-of function mutations, causing inaccurate assignment of gene function. In addition, the resolution of current microscopy techniques still does not permit direct observation of most biomolecular interactions.
The reconstitution of membrane traffic in cell-free systems (Haselbeck and Schekman, 1986; Beckers et al., 1987) has helped to isolate specific transport events within a less complex biochemical remit. Likewise, genetic screens with the model organism Saccharomyces cerevisiae for conditional mutants defective in secretion or vacuolar transport (Novick and Schekman, 1979; Schekman, 1985) have identified numerous key regulators that would not have been amenable to biochemical purification. The current challenge is to integrate the identification of gene products involved in the secretory pathway with experiments that dissect the complete cycle of events in a transport reaction.
This review will summarize the established and emerging pieces of information required to understand the full process of vacuolar sorting in plants. We will approach the problem from a biosynthetic perspective, and for an appraisal of endocytic trafficking, the reader is referred to the recent review by Reyes et al. (2011). When appropriate, key machinery to the corresponding transport steps in yeast and mammals will be compared with show similarities and differences between the workings of the secretory pathway among eukaryotes.
HISTORIC BACKGROUND ON TRANSPORT TO LYTIC COMPARTMENTS
The combination of electron microscopy (EM) with analytical biochemistry led to the establishment of the fundamental principles in secretory protein trafficking and vesicular transport (de Duve, 1975; Palade, 1975). This set the stage for our current understanding of lysosomal transport. Both the principle of coated vesicles as transport carriers (Roth and Porter, 1964) and the description of basket-shaped structures around coated vesicles (Kanaseki and Kadota, 1969) were based on morphological analyses of structures visualized by EM. It was not until this was combined with analytical biochemistry to include purification and characterization of their protein composition that clathrin-coated vesicles (CCVS) could be established as transport carriers (Pearse, 1975, 1976).
The identification of mannose-6-phosphate (M6P) and its receptor as causal agent for lysosomal sorting (Kaplan et al., 1977) followed almost immediately after the first isolation of CCVs. Although they were first identified as derived from the plasma membrane to mediate endocytosis, a potential role of CCVs in biosynthetic transport from intracellular organelles also emerged rapidly (Pearse and Bretscher, 1981). This was substantiated by the observation that the M6P receptor and lysosomal cargo were shown to exit from the Golgi in CCVs (Campbell et al., 1983; Campbell and Rome, 1983; Brown and Farquhar, 1984; Geuze et al., 1985; Lemansky et al., 1987). However, when the concept of a trans-Golgi network (TGN) was first established (Griffiths and Simons, 1986), it was still controversial if M6P receptors leave the Golgi from the cis- or the trans-cisternae. Clathrin mainly labeled the trans-Golgi (Orci et al., 1985), but M6P receptors were reported to exit from the cis-Golgi (Brown and Farquhar, 1984; Farquhar, 1985). The fact that two types of M6P receptors operate in the same pathway but follow different transport routes did not simplify the dissection of individual CCV budding sites by immunocytochemistry (Hoflack and Kornfeld, 1985; Klumperman et al., 1993).
A role of CCVs in transport to the plasma membrane was convincingly ruled out by the observation that constitutively secreted invertase was unaffected by deletion of the clathrin heavy chain in the yeast S. cerevisiae (Payne and Schekman, 1985). The logical consequence was to propose a role of CCVs in transport to the yeast vacuole. Around this time, plant research entered the field of protein trafficking in the secretory pathway with the distinction between signal-mediated vacuolar sorting and secretion by bulk flow being one of the first breakthroughs (Shinshi et al., 1988; Dorel et al., 1989; Denecke et al., 1990).
DISCOVERY OF THE PLANT VACUOLAR SORTING RECEPTOR
Plant lytic vacuoles are considered equivalent to mammalian lysosomes and accumulate a number of lytic hydrolases, but plants also contain specialized vacuoles for storage of proteins and other components (Wink, 1993; Marty, 1999). The identification of plant vacuolar sorting signals was facilitated via deletion and transplantation experiments by genetic engineering of artificial cargo molecules. The use of plant protoplasts for transient expression and quantitative secretion experiments was then developed, enabling reproducible protein transport assays in plants.
Similar to vacuolar proteases in yeast and unlike lysosomal hydrolases in mammals, soluble plant vacuolar proteins do not use modified glycans as sorting signals (Voelker et al., 1989) but instead possess short peptide sequences as vacuolar sorting signals (Shinshi et al., 1988; Bednarek et al., 1990; Matsuoka and Nakamura, 1991; Neuhaus et al., 1991; Holwerda et al., 1992). These sorting signals mediate segregation from secreted proteins destined to the plasma membrane, a process that was suggested to occur by passive bulk flow in mammals (Wieland et al., 1987) and that was later shown for plants using cytoplasmic proteins introduced to the ER lumen with N-terminal signal peptides (Denecke et al., 1990).
To study the vacuolar transport machinery in yeast, smart mutant screens scoring vacuolar morphology (vam), vacuolar protein sorting (vps), and vacuolar protease activity (pep) led to the identification of numerous key genes by shotgun complementation cloning. Among these was a gene that encoded the yeast vacuolar sorting receptor (VSR) VPS10 (Marcusson et al., 1994). The search for the plant VSR was based on an elegant biochemical approach (Kirsch et al., 1994) inspired by the observation that vacuolar proteins, such as pea (Pisum sativum) lectin precursor and a number of hydrolases, were found to be enriched in CCVs (Harley and Beevers, 1989). Detergent-solubilized proteins extracted from purified CCVs from developing pea cotyledons were passed over an affinity matrix displaying a peptide harboring the vacuolar sorting motif from proaleurain (Holwerda et al., 1992), a vacuolar thiol protease. Binding of pea BP80 (for binding protein of 80 kD) to this affinity column was strong at pH 6.5 to 7, and elution from the column was efficient at pH 4, consistent with the concept of receptor-ligand release in an acidic compartment en route to the vacuole (Kirsch et al., 1994). These pioneering experiments set the stage for all subsequent research into plant VSRs.
VSR-LIGAND BINDING SPECIFICITY IN VITRO
The affinity-based receptor isolation instantly offered a method to interrogate the receptor binding affinity of a number of different peptide sequences from vacuolar proteins via binding and competition assays in vitro (Kirsch et al., 1994, 1996). Vacuolar sorting signals were initially classified into N-terminal, C-terminal, and internal signals, many of which were shown to be transplantable but others appeared to be context dependent. In addition, there was also a trend to allocate specific signals for sorting to either lytic or storage vacuoles, but due to an increasing number of exceptions, it became apparent that a general classification of vacuolar sorting signals was difficult, if not impossible (Matsuoka and Neuhaus, 1999; Matsuoka, 2000). Work with pea BP80, the first established plant VSR, contributed significantly to our understanding of receptor binding specificity. Kirsch and coworkers established that both the N-terminal targeting signals from barley (Hordeum vulgare) aleurain and sweet potato (Ipomoea batatas) sporamin as well as the C-terminal sorting signal from the Brazil nut (Bertholletia excelsa) 2S storage albumin show strong BP80 binding, while the C-terminal signal of barley lectin exhibited only weak, albeit detectable, BP80 binding. Weak in vitro receptor binding of the barley lectin C terminus (VFAEAIAANSTLVAE) was abolished by shortening the sequence to VFAEAI, even though this shorter sequence was earlier reported to convey vacuolar sorting in vivo (Dombrowski et al., 1993). It is likely that binding affinities are higher in vivo because the sorting signal may be better displayed within the context of the entire cargo molecule compared with in vitro assays with short peptides.
The notion that a wide range of vacuolar sorting signals could interact with plant VSRs was supported by several important follow-up studies. First, the stigma of Nicotiana alata was used as a source for an organelle fraction enriched in prevacuolar compartments (PVCs), leading to successful cross-linking of a vacuolar proteinase inhibitor precursor with a VSR-like protein that cross-reacted strongly with antibodies to pea BP80 (Miller et al., 1999). The work provided the first evidence supporting a role of VSR–ligand interactions in situ and confirmed that C-terminal vacuolar sorting signals may be recognized by plant VSRs. This finding corresponded well with an earlier study showing that plant VSRs and the plant homolog of the yeast prevacuolar t-SNARE Pep12p accumulate in PVCs where they colocalize (Sanderfoot et al., 1998). Another study showed that storage proteins containing either C-terminal or internal sorting signals can act as VSR ligands (Jolliffe et al., 2004). Vacuolar sorting signals of castor bean (Ricinus communis) proricin and pro2S albumin were shown to bind to VSR-like proteins with strong cross-reactivity to a variety of antibodies generated against pea BP80. Work with recombinant PV72, a VSR-like protein from pumpkin (Cucurbita sp cv Kurokawa Amakuri Nankin), revealed binding to pro2S albumin via affinity chromatography and surface plasmon resonance (Watanabe et al., 2002), providing further evidence for a role of VSRs in transport to storage vacuoles. The most recent in vitro binding study with the largest portfolio of peptide-borne vacuolar sorting motifs (Suen et al., 2010) confirms that VSR–ligand interactions are certainly not restricted to lytic vacuolar cargo.
Several of the in vitro affinity studies were strongly supported by control experiments involving point mutagenesis to replace the hydrophobic Ile in the conserved NPIR motif of the barley aleurain and sweet potato sporamin. This specific modification in the sorting signals completely abolished BP80 binding in vitro (Kirsch et al., 1996), which corresponded well with the findings in vivo showing that the corresponding I-to-G substitution caused induced secretion of sweet potato sporamin (Nakamura and Matsuoka, 1993). The Brazil nut 2S albumin C terminus does not show the conserved NPIR motif; instead, the C-terminal peptide IAGF was reported to be crucial for BP80 binding, possibly dependent on the context of the preceeding five amino acids that may help display the tetrapeptide in a biologically meaningful context (Kirsch et al., 1996). Later VSR binding studies with the sorting signals of Castor bean proricin and pro2S albumin were subject to the same experimental rigor, and point mutations that abolish functionality of the vacuolar sorting signals in vivo (Frigerio et al., 2001; Brown et al., 2003) also effectively prevented VSR binding in vitro (Jolliffe et al., 2004; Suen et al., 2010). Importantly, the cofractionation of proricin and pro2S albumin with VSRs and clathrin on gradients strongly suggests that CCVs mediate selective anterograde transport of VSRs with their ligands (Jolliffe et al., 2004).
VSRs ARE ENCODED BY LARGE GENE FAMILIES IN PLANTS
After the initial purification of pea BP80 in 1994, experimental evidence presented itself suggesting that two routes existed to deliver vacuolar proteins to the vacuole. One argument was derived from experiments in which vacuolar sorting mediated by C-terminal but not N-terminal (sequence-specific) vacuolar sorting signals was inhibited by the drug Wortmannin (Matsuoka et al., 1995). Multiple reports suggest that a subset of soluble vacuolar proteins and different types of aquaporins (Di Sansebastiano et al., 1998; Isayenkov et al., 2011) potentially segregate to lytic and storage vacuoles fueled this idea even further (Paris et al., 1996). Although Wortmannin was later shown strongly to inhibit vacuolar sorting of cargo with sequence-specific signals (Pimpl et al., 2003; daSilva et al., 2005) and the alleged segregating aquaporins were found in the same vacuole of Arabidopsis thaliana (Hunter et al., 2007), this appealing concept of plant-unique multiple pathways for different vacuoles was one of the major driving forces to investigate plant vacuolar sorting in more detail.
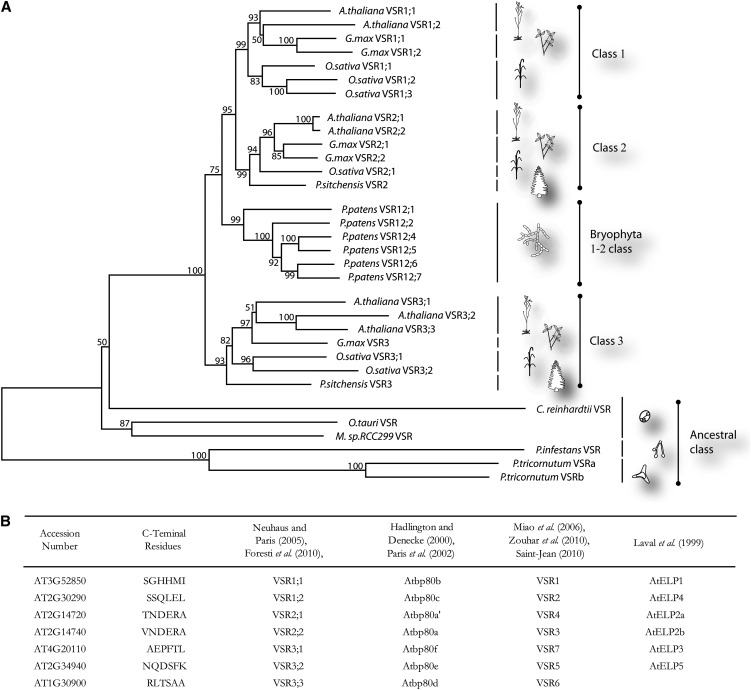
With these issues in mind, the full-length pea BP80 gene was cloned together with two highly homologous full-length VSR genes from Arabidopsis (Paris et al., 1997). Simultaneously, other research groups identified closely related sequences in pumpkin, named PV72 and PV78 (Shimada et al., 1997), and one of the two Arabidopsis genes (Ahmed et al., 1997), alternatively termed At-ELP. The sudden explosion of the number of available ESTs and genomic sequences in the late 1990s would soon reveal that VSRs are encoded by large gene families in all plants investigated, and it was popular to ask if different isoforms could operate in divergent vacuolar trafficking routes. Paris and Neuhaus (2002) and Neuhaus and Paris (2005) proposed the VSR nomenclature, but an alternative VSR1-7 numbering system was later introduced in which the seven members were numbered differently (Shimada et al., 2003; Miao et al., 2006; Zouhar et al., 2010). Figure 1B summarizes the diverse names used for the seven Arabidopsis VSR paralogs in the past.
Figure 1.
VSR Phylogenetic Tree and Nomenclature.
(A) Protein sequences were obtained using publicly available data (http://www.ncbi.nlm.nih.gov/), aligned using the BLOSUM62 algorithm with the ClustalW alignment tool (http://www.ebi.ac.uk/Tools/msa/clustalw2/), and subsequently manually adjusted the position of the TM domain of the C. reinhardtii VSR (the complete alignment can be found in Supplemental Data Set 1 online). The aligned sequences were assembled into a phylogenetic tree using the boot-strapped neighbor-joining algorithm (Saitou and Nei, 1987) and the Jones, Taylor, and Thornton amino acid substitution model (Jones et al., 1992) in MEGA 5.05 with 1000 trials (http://www.megasoftware.net/). Bootstrap values are indicated as percentages of the 1000 trials at their respective node. Included in this analysis were VSR sequences of Arabidopsis, G. max, O. sativa, P. patens, O. tauri, P. sitchensis, C. reinhardtii, Micromonas strain RCC299, P. infestans, and P. tricornutum. Due to the recent genome duplication in soybean (Shoemaker et al., 1996) leading to five pairs of almost identical VSR orthologs, only one representative of each was used to avoid unnecessary complexity. P. patens XP_001759820 (VSR12;2) has a very close homolog, XP_001759928 (VSR2;3), which has been omitted in our analysis due to a small gap in the sequence contig preventing accurate gene modeling and sequence alignment. In parallel, the phylogenetic tree was also resolved using a boot-strapped maximum likelihood algorithm, resulting in a comparable topology and the same three angiosperm VSR classes (data not shown).
(B) A table comparing the various VSR nomenclatures used in the last 15 years. To avoid future confusion, the second column refers to the last six terminal residues that act as a fingerprint helping to differentiate quickly between the different isoforms.
From all attempts, the latest Neuhaus classification (Neuhaus and Paris, 2005) appears to reflect best the evolution of this gene family and was confirmed by an independent study of the Raikhel group (Avila et al., 2008) as well as this analysis. Figure 1A shows a phylogenetic tree including the seven VSR sequences from Arabidopsis, in comparison with representatives of other flowering plants, including the dicot soybean (Glycine max), the monocot rice (Oryza sativa), and the gymnosperm spruce (Picea sitchensis). In addition, we included VSR sequences of a Bryophyte moss (Physcomitrella patens), green unicellular algae (Ostreococcus tauri, Micromonas sp RCC299, and Chlamydomonas reinhardtii), a diatom (Phaeodactylum tricornutum), and a fungus representative of the oomycete family (Phytophthora infestans).
All VSR sequences strictly show a predicted type 1 single membrane spanning topology with an N-terminal signal peptide, a large lumenal domain of remarkably similar size between the different organisms, and a transmembrane (TM) domain followed by a highly conserved sequence of 23 amino acids (see Supplemental Data Set 1 online). VSR sequences from angiosperms consistently fall into the same three categories as described earlier (Neuhaus and Paris, 2005; Avila et al., 2008). Spruce appears to contain only class 2 and 3 VSRs, while the moss Physcomitrella appears to form a separate clade of VSRs seemingly ancestral to classes 1 and 2 of flowering plants. Green algae, diatoms, and the oomycete appear to represent a VSR ancestral clade. Class 3 of flowering plants appears to be due to an early segregation, prior to the class 1 and 2 divide, and may indicate a specialized role unique to flowering plants yet to be uncovered by experimental analysis.
We encourage the scientific community to adopt the three-class system proposed by the Neuhaus group in 2005 to avoid the various independent nomenclatures for Arabidopsis VSRs used in the last decade (Figure 1B). The original pea BP80 shares the highest homology with the class 2 group, whereas pumpkin PV72 is more closely related to the class 1 group, including Arabidopsis ELP1/VSR1;1.
VSRs ARE RESPONSIBLE FOR VACUOLAR SORTING OF SOLUBLE PROTEINS
Attempts to specify biological functions in vivo by genetic approaches have been much less straightforward compared with the initial biochemical binding studies (Kirsch et al., 1996). Overexpression of VSR isoforms does not yield any obvious morphological phenotype in Arabidopsis or tobacco (Nicotiana tabacum; Laval et al., 2003; daSilva et al., 2006). VSR1 antisense inhibition in Arabidopsis leads to reduced germinating efficiency (Laval et al., 2003), although VSR1 knockouts have normal germination and development (Shimada et al., 2003). This apparent controversy is probably due to the fact that insertion mutagenesis only affects the targeted gene, while antisense inhibition may additionally affect closely related VSRs as well. Despite the lack of obvious developmental phenotypes in vsr1 knockout lines, storage proteins are partially mistargeted as precursor forms to the extracellular matrix of these seeds (Shimada et al., 2003). By contrast, Arabidopsis aleurain (ALEU), at the time classified as a typical lytic vacuolar cargo protein, did not show evidence for mistargeting. This led to the proposal that VSR1 may be responsible for storage, but not lytic vacuolar transport. However, after germination of seeds, the mistargeted storage protein precursors were degraded normally, suggesting that some lytic proteases were likely to be secreted as well but may have remained below the detection limit of immunocytochemistry (Shimada et al., 2003).
To assess discriminative cargo recognition directly, the experiment was repeated with artificial heterologous cargo molecules (Craddock et al., 2008). The advantage of introducing either bean (Phaseolus vulgaris) phaseolin or the red fluorescent protein (RFP) as cargo over the analysis of endogenous cargo is that the sole experimental variable is the type of sorting signal and that variation in protein abundance and detection efficiencies are eliminated. The C-terminal hydrophobic tetrapeptide AFVY was used as a representative of the C-terminal context-dependent but sequence-independent sorting signals, while the ricin linker represented a sequence-specific sorting signal. Both were fused to RFP and both can be classified as originating from storage proteins. Using this approach, the authors observed that both types of cargo molecules showed altered proteolytic processing and partial secretion in a vsr1 mutant background. This means that the type of vacuolar sorting signal does not influence the response to the absence of VSR1 (Craddock et al., 2008). This confirmed the broad specificity of VSR substrates earlier seen with the biochemical binding studies (Kirsch et al., 1996; Miller et al., 1999; Jolliffe et al., 2004; Suen et al., 2010).
Further support for the idea of a universal role of VSRs in mediating vacuolar sorting of soluble proteins arose from a systematic study of the role of different Arabidopsis VSRs via knockout analysis (Zouhar et al., 2010). Members of the VSR1 and VSR2 groups were shown to contribute to the sorting of both storage and lytic vacuolar cargo, while the VSR3 group appeared to play no detectable role in vacuolar transport in the same experimental system. It should be noted that induced secretion of typical lytic vacuolar cargo ALEU was only detectable in a double VSR knockout covering members of the first two VSR families (Zouhar et al., 2010), which explained why in the earlier study ALEU mis-sorting was not observed in the single VSR1 knockout (Shimada et al., 2003). Even in the new double knockout, secreted ALEU represented only a minor fraction, and most of the lytic cargo was still transported to the vacuole (Zouhar et al., 2010). One interpretation is that ALEU may contain two different vacuolar sorting signals, only one of which is VSR specific. Alternatively, it could be that ALEU is simply a stronger ligand that binds with higher affinity to the same VSR. Efficient vacuolar transport may continue for strong ligands even when fewer VSRs are available at the Golgi for cargo selection, particularly if these are expressed at lower levels compared with abundant storage proteins.
Iodinated proAleurain peptide binds with an apparent Kd of 37 nM to detergent solubilized membrane fraction (Kirsch et al., 1994). The decapeptide of prosporamin, which also contains the NPIR motif, competes with the proAleurain peptide with an apparent Kd of 100 µM. Other peptides (proEP-B and barley lectin) show little or no competition in these conditions. In surface plasmon resonance experiments, the PV72 soluble domain purified from insect cells has a higher affinity for the proAleurain peptide (Kd = 100 nM) compared with the internal propeptide of pumpkin 2S albumin (Kd = 200 nM) (Watanabe et al., 2002, 2004). It should be noted that ligand binding in vivo could be influenced by the folding of the ligand, which could influence the surface exposure of the sorting determinant for the receptor. However, short peptides binding to VSRs in vitro may not be subjected to such complexity. Similarly, the TM domain could have an effect on the actual structure of the lumenal VSR ligand binding domain and thus affect binding. Consequently, absolute affinity values of a particular ligand to the receptor are not comparable between these studies (Kirsch et al., 1994; Watanabe et al., 2002, 2004). However, the published in vitro binding studies would support the idea that not all ligands have the same affinity in vivo, as mentioned above.
Interestingly, knockout mutants of the six remaining Arabidopsis VSR isoforms do not accumulate storage proteins in the extracellular matrix (Shimada et al., 2003; Zouhar et al., 2010). This is expected for those isoforms that are not expressed in seeds but unexpected for VSR2 group isoforms that are also highly transcribed in seeds (Laval et al., 2003). However, mRNA levels are by no means always correlated with protein levels, and it is possible that VSR1 would be the major protein isoform expressed in developing seeds. Although data are available for mRNA expression (Laval et al., 2003), no independent protein expression data are available for the various VSR isoforms. Indeed, if we assume that the anti-VSR1 antibody has identical affinity for all VSRs due to the high sequence homology of the lumenal domain, only the vsr1 knockout line exhibited a detectable reduction in VSR protein levels (Zouhar et al., 2010), and it will be interesting to verify protein levels also in developing seeds.
RMR, A VSR-RELATED GROUP OF TYPE I MEMBRANE SPANNING PROTEINS
The intense research into the VSR family also brought about the discovery of a different class of type 1 membrane proteins with weak homology to the N terminus of VSRs, the so-called ReMembR-H2 (RMR) protein group (Cao et al., 2000). There are six RMRs in Arabidopsis, characterized by a short lumenal domain and a longer cytosolic tail containing a RING domain. Experiments with recombinant RMR, either full length or soluble lumenal domains, revealed binding to typical storage vacuole cargo, such as bean phaseolin (Park et al., 2005) or peptides comprising vacuolar sorting signals of tobacco chitinase or barley lectin (Park et al., 2007), but not binding to lytic vacuolar cargo, such as aleurain or a peptide with the sorting signal of sweet potato sporamin. A deletion construct lacking its lumenal domain induces the secretion of phaseolin but not aleurain in transfected protoplasts (Park et al., 2005). Supporting this, a truncation of RMR1 leaving only the lumenal domain is secreted together with the cargo phaseolin and vicilin (Shen et al., 2011). Once again, aleurain is unaffected in these experiments.
When put together, these experiments suggest the appealing model that the role of RMR is to bind to proteins of the storage vacuole and not those of the lytic vacuole. However, the reciprocal specificity was not seen in vsr1 knockouts (Craddock et al., 2008) and in vitro VSR binding studies (Suen et al., 2010). In addition, the binding studies with RMR show some discrepancies in regards to the pH dependency. Bacterially expressed glutathione S-transferase–tagged lumenal domain of RMR1 has been reported to bind to the vacuolar cargo phaseolin at pH 4.0 and 6, but not pH 7. In another study, RMR expressed in insect cells was found to bind to a peptide column displaying either the vacuolar sorting signals of tobacco chitinase or barley lectin and showed binding at pH 6 to 7, but could not be eluted at pH 4.0 (Park et al., 2007). Elution was only possible using a high concentration of SDS. One of the current models for the trafficking of the RMR through the vacuolar pathway results in crystalloid structures in the storage vacuole of developing seeds via dense vesicles (Jiang et al., 2000; Wang et al., 2011). Despite this evidence for a role of RMR in storage protein transport, these results could not be confirmed by genetic RMR knockouts and an associated appraisal of storage protein transport in Arabidopsis seeds (Zouhar et al., 2010).
Potential biological functions of plant RMRs have recently been reviewed (Wang et al., 2011) and suggested to be involved in ubiquitination events as well as vacuolar sorting. This hypothesis has not been evaluated by direct experiments, but the RING domain in the C terminus of RMR is highly conserved among E3 ubiquitin protein ligases. A mammalian type I membrane spanning protein with a similar domain structure as plant RMRs (RNF13) has recently been shown to localize to endosomes where it is rapidly proteolysed to release the lumenal N-terminal domain as well as a cytoplasmic fragment that can mediate ubiquitination (Bocock et al., 2009). Lumenal release of the N-terminal domain may also happen for plant RMRs, as shown by an RFP-RMR fusion protein containing the TM domain and cytosolic tail of At-RMR1, which leads to accumulation of soluble RFP in the central vacuole lumen (Scabone et al., 2011). Further research is required to clarify the biological role of plant RMRs, and as such, we continue to focus on the VSR protein family in this review.
In conclusion, the majority of reports in the field addressing VSR function suggest that soluble proteins use a common route to plant vacuoles and that the VSR gene family is the major receptor for this pathway. This is further supported by a recent study in which vacuolar transport was inhibited at various trafficking steps with dominant-negative mutants of a Rab11 GTPase, two plant Rab5 GTPases, and a Rab7 GTPase. The results revealed no evidence for any discrimination between soluble vacuolar cargo displaying different classes of vacuolar sorting signals (Bottanelli et al., 2011), although it cannot be ruled out that RMRs use the same transport machinery as the BP80-type VSR family in tobacco leaf epidermis cells and protoplasts.
CALCIUM AND pH COOPERATE IN VSR CARGO BINDING
All VSRs studied so far have a type I single membrane span topology and can be divided into three domains: a large lumenal ligand binding domain, the TM domain, and a cytosolic tail that is thought to interact with cytosolic sorting machinery (Paris and Neuhaus, 2002). This is similar to the mammalian M6P receptor or the yeast VPS10 gene product, except for a much shorter cytosolic tail in the plant protein. The lumenal domain contains 33 conserved Cys residues, but experimental evidence for the precise arrangement of disulphide bridges remains to be gathered. The uneven number of Cys residues in the lumenal domain may indicate that VSRs could form dimers or multimers under certain conditions, and it cannot be ruled out that VSRs form heterocomplexes with other proteins. Variability of the observed molecular weight in various plant systems may be caused by different degrees of glycosylation. For instance, the Arabidopsis VSR2 group shows two consensus sites for N-linked glycosylation in the lumenal domain, but these are not conserved in all Arabidopsis VSRs. In addition, the presence of consensus sites does not guarantee that glycosylation actually takes place.
Domain analysis of plant VSRs revealed that a monomeric lumenal part of VSRs alone can interact with an affinity matrix as tested by specific binding of a recombinant VSR, lacking its TM domain and cytosolic tail (Sanderfoot et al., 1998; Cao et al., 2000). This was confirmed by surface plasmon resonance experiments using purified lumenal VSR domains expressed in insect cells (Watanabe et al., 2002) and secreted soluble VSR domains purified from the culture medium of tobacco Bright Yellow 2 (BY2) suspension cells (Suen et al., 2010). However, a similar construct expressed and purified from bacteria is not retained (Sanderfoot et al., 1998), which could mean that VSR lumenal domain cannot fold properly in bacteria (Yin et al., 2007). However, experimental evidence for the requirement of specific disulfide bonds or N-linked glycans remains to be obtained.
One of the basic principles in the repetitive functioning of protein sorting receptors is conditional ligand binding and release. The lumen of the donor compartment is thought to promote ligand binding, whereas cargo must dissociate from the receptor when it arrives in the acceptor compartment. This permits receptors to recycle without carrying ligands back, a necessary requirement for a full transport cycle. In the case of the mammalian KDEL receptor, ligand binding is believed to be strong in the Golgi apparatus at acidic pH, while the neutral pH of the ER lumen would stimulate ligand release (Wilson et al., 1993; Scheel and Pelham, 1996). The opposite pH dependency was observed for the M6P receptor (Kornfeld, 1992). In plants, initial reports have shown that VSR-cargo binding is optimal at pH 6 to 7, while release occurs at pH 4 (Kirsch et al., 1994; Cao et al., 2000). VSR–ligand interactions were also shown to be promoted by high (millimolar) Ca levels (Watanabe et al., 2002, 2004; Suen et al., 2010). In fact, 1 mM CaCl2 was included in the original purification binding of BP80 to aleurain-bound column (Kirsch et al., 1994).
In plants, maintenance of a low cytosolic (0.1 μM) concentration of Ca (Gilroy et al., 1987) is achieved by active transport to the ER lumen and the vacuole (Taiz, 1992; Bush, 1995). Since sorting motifs of vacuolar cargos are exposed as soon as the protein is folded in the ER, VSR–ligand interactions could occur already in the ER lumen. The recent finding that plant Golgi is also a Ca storage organelle containing active Ca importers (Ordenes et al., 2002) supports the idea that VSRs continue to select vacuolar cargo in the Golgi to mediate effective segregation from secretory cargo. A role of Ca in VSR function could be explained by the three conserved epidermal growth factor (EGF)–like repeats found near the C terminus of the lumenal VSR domain. Soluble VSR domains lacking these EGF repeats have a lower affinity to their cargo (Cao et al., 2000; Watanabe et al., 2002), supporting an indirect role of these regions in maintaining adequate conformation of the N-terminal ligand binding domain. Both calcium and pH dependence have also been reported for other EGF-containing receptors, such as the low-density lipoprotein receptor (Herz et al., 1988; Tong and Kornfeld, 1989; Arias-Moreno et al., 2008; Zhao and Michaely, 2009; Huang et al., 2010; Olson et al., 2010). Further progress could be derived from the VSR crystal structure (Rogers et al., 2004), but this approach has yet to be explored in more detail.
IS TWO BETTER THAN ONE?
Conceptually, conditional binding may be regulated by other factors or elements in addition to the properties of the aqueous environment of the ligand binding domain of the receptor. For example, other proteins, either membrane spanning or associated, or possibly the lipid composition of the membrane, could regulate conformational changes in a receptor. VSRs may interact with distinct proteins in the lipid bilayer of the donor compartment compared with the membrane of the acceptor compartment. To avoid unnecessary trafficking of unbound receptors, specific mechanisms could exist to distinguish bound receptors ready for trafficking from those that have yet to capture cargo. Likewise, recycling of receptors could be regulated so that it occurs only after ligand release. However, experimental data supporting these concepts remain to be shown for plant VSRs.
Receptors involved in either signal transduction or protein trafficking have been shown to undergo regulated dimerization, and this dimerization event is crucial for efficient receptor function (van den Eijnden et al., 2006). The human M6P receptor is found in cells as a dimer, and it also crystallizes as a dimer, indicating that this configuration is very stable (Ghosh et al., 2003). Recently, evidence for VSR complex formation was obtained by observing high molecular weight forms in gel filtration experiments (Kim et al., 2010). Coimmunoprecipitation of two differently epitope-tagged VSRs (HA and myc) provided hard evidence for direct homodimerization in vivo. Using constructs where the TM and C terminus have been replaced with similar domains of the VSR-related RMR class of membrane proteins, the authors concluded that both domains are important for dimerization of VSRs, although the C terminus is crucial. Indeed, a mutant that has a block substitution in the C-terminal tail (C2a:HA) has lower dimerization efficiency. Interestingly, binding of cargo still happens when dimerization is impaired (Kim et al., 2010), confirming earlier work suggesting that monomeric lumenal domains of plant VSRs retain ligand binding ability (Cao et al., 2000). This indicates that cargo binding could precede dimerization events. Upon cargo binding, a rearrangement of the N-terminal part of VSR was observed by proteolysis experiments (Cao et al., 2000), but it remains to be shown if crosstalk occurs between the N-terminal reorganization and the structure of the TM and C-terminal part, which would either expose motifs and/or induce dimerization as a prerequisite for VSR anterograde transport.
BIOCHEMICAL IN VIVO ASSAYS
The advantage of in vitro ligand binding assays is that it is possible to work with purified components and well-defined reaction conditions, but it is difficult to relate the results obtained to the actual reaction conditions in vivo. To overcome this problem, the retained ligand binding properties of the lumenal VSR domain alone have been exploited to construct dominant genetic effectors for in vivo experiments. Coexpression of soluble VSR domains engineered to expose the tetrapeptide HDEL at their C termini (VSR-HDEL) led to ER retention of vacuolar cargo exhibiting NPIR-containing sorting signals, establishing the first ligand binding assays in vivo based on plant expression systems (Watanabe et al., 2004; daSilva et al., 2005). When performed in protoplasts using model cargo molecules, such as secreted barley α-amylase (amy) or a vacuolar fusion protein between α-amylase and an NPIR-containing peptide from sweet potato sporamin (amy-spo), VSR-HDEL coexpression resulted in quantitative redistribution of vacuolar cargo (daSilva et al., 2005). Such experiments demonstrated that VSR–ligand interactions were highly specific because mutagenesis of the NPIR signal abolished the redistribution of amy-spo by coexpressed VSR-HDEL. Moreover, it was possible to demonstrate that the drug Wortmannin did not mediate its inhibitory effect on vacuolar sorting by inhibiting VSR–ligand interactions directly because secreted amy-spo but not the secretory cargo amy were retained by VSR-HDEL in the presence of the drug. Finally, expression of full-length VSR could restore vacuolar sorting of amy-spo (daSilva et al., 2005), demonstrating that the drug prevented VSR recycling, thus providing an in vivo binding assay with native VSRs.
An advantage of the use of engineered effectors of transport reactions in protoplasts is that effector-encoding plasmids can be used as a single experimental variable to permit dose–response analysis in a biochemically controlled manner including appropriate controls, yet within the remit of living cells. The conceptual separation of lumenal ligand binding domains from the cytosolic tails, expected to interact with sorting machinery, led to an important tool to study receptor trafficking. Replacement of most of the lumenal domain by fluorescent proteins was achieved by fusing a green fluorescent protein (GFP) or other spectral variants to the C terminus of a signal peptide for ER entry and to the N terminus of the TM domain and cytosolic tail of VSRs. These fusion proteins were shown to localize to the same compartments as the native VSRs (Tse et al., 2004) and acted as semidominant inhibitors of vacuolar sorting (daSilva et al., 2005). The inhibitory effect was dose dependent, as the fusion protein competed with endogenous VSRs for sorting machinery. It was shown that the GFP-VSR2 fusion competed mainly at the recycling step because vacuolar sorting could be restored by increased expression of wild-type VSR2, indicating that the anterograde transport step is not limiting. Independently, the drug Wortmannin caused increased GFP-VSR2 leakage to the vacuole, followed by increased degradation. Restoring vacuolar sorting with wild-type VSRs thus provided a biochemical complementation assay as it suppressed the effect of the drug or the semidominant effect of the GFP-VSR fusion (daSilva et al., 2005).
SUBCELLULAR LOCALIZATION OF VSRs
Unlike the vacuolar cargo, which only proceeds in the anterograde direction to reach the vacuole, VSRs are expected to shuttle between donor and acceptor membranes for multiple cargo selection rounds. Further to conditional ligand binding and release, VSRs must also differentially interact with cytosolic machinery for selective anterograde or retrograde transport to mediate efficient receptor recycling.
In yeast, VPS10p can be purified from CCVs together with the precursor of carboxypeptidase Y (Deloche et al., 2001). However, the TGN-to-prevacuole transport step is thought to occur by bulk flow because deletion of the VPS10 cytosolic tail does not inhibit anterograde receptor transport; instead, it leads to its degradation in the vacuole (Cereghino et al., 1995; Cooper and Stevens, 1996; Deloche et al., 2001). Steady state distributions of VPS10 between the Golgi and prevacuoles have not been quantified. In mammalian cells, the biosynthetic M6P receptor responsible for Golgi-mediated lysosomal sorting has been reported to accumulate at the cis-Golgi, the trans-Golgi, or the endosomes, depending on cell type (Kornfeld, 1992).
In plants, the localization of VSRs has been studied using immunogold electron microscopy (EM) where they have been detected in the Golgi stack (Paris et al., 1997; Sanderfoot et al., 1998; Hinz et al., 1999, 2007; Ahmed et al., 2000) and the multivesicular bodies or PVCs (Sanderfoot et al., 1998; Tse et al., 2004; Hinz et al., 2007; Foresti et al., 2010). In addition, VSRs have also been detected at a post-Golgi structure (Foresti et al., 2010; Viotti et al., 2010) that was originally termed partially coated reticulum (PCR) (Pesacreta and Lucas, 1984; Tanchak et al., 1988) but is currently called the plant TGN (Dettmer et al., 2006). However, a quantification of the relative distribution between the three possible locations is difficult due to the low statistical resolution of immunogold EM and uncertainty about the distinction between trans-Golgi cisternae and the PCR/TGN. The latter would require quantitative double labeling using anti-VSR sera together with known markers for either the trans-Golgi or the PCR/TGN, a study that has yet to be reported.
It should be noted here that the term TGN is easily confused with the trans-Golgi cisternae. Indeed, numerous articles have referred to the term TGN when using markers of the trans-Golgi stack, such as the well-established fluorescent sialyl-transferase fusions (i.e., ST-XFP). The origin of the new nomenclature has emerged from the use of the SNAREs SYP61 and SYP41 as markers in plants, the yeast equivalents of which (TLG1 and 2) are termed late Golgi markers, but there is no colocalization of ST-CFP with YFP-SYP61 (Foresti and Denecke, 2008). This shows that it is risky to adopt nomenclature solely based on inferences between kingdoms. For example, the RabGTPase termed Rab11 also localizes to the plant SYP61/41 compartment (Chow et al., 2008), but in mammals, rab11 is a marker for the recycling endosome, which is clearly distinct from the mammalian TGN (Maxfield and McGraw, 2004; van Ijzendoorn, 2006; Grant and Donaldson, 2009). For this reason, the post-Golgi structure of plants labeled by SYP61/41 will be referred to as the PCR/TGN in the remainder of this review to avoid confusion with the term TGN used in the mammalian and yeast literature.
Compared with immunogold EM, far more experimental results have been published on fluorescently tagged VSR fusion proteins. Since GFP-VSR fusions compete with endogenous receptors (daSilva et al., 2005) and colocalize with endogenous VSRs in situ (Tse et al., 2004), they can be used as a reagent to routinely image VSR-containing compartments in living cells. In contrast with the immunogold EM studies in root meristems, fluorescent VSR fusions have mostly been studied in leaf protoplasts, intact leaf epidermal cells, and tobacco BY2 cells. By analogy to the mammalian M6P receptors, the relative distribution between Golgi, TGN, and PVC may differ significantly between plant tissues. In leaf cells, fluorescent VSR fusions appear to show highest steady state levels at the PVC and are below the detection limit in the Golgi and the PCR/TGN (daSilva et al., 2005, 2006; Foresti et al., 2010; Saint-Jean et al., 2010). Also in BY2 cell suspensions, fluorescent VSR fusions have been proposed to label predominantly the PVC (Miao et al., 2006). This is consistent with the quantitative cell fractionation of Arabidopsis root homogenates showing cofractionation between VSRs and the PVC marker Pep12 (Sanderfoot et al., 1998). A nonoverlapping fraction containing VSR but not Pep12 remained unidentified at the time and could have either been the Golgi or the PCR/TGN. It would be interesting to repeat these cell fractionation results with leaf tissue to see if the degree of cofractionation with Pep12 is higher.
VSR TRAFFICKING INVOLVES BIOSYNTHETIC CLATHRIN-MEDIATED TRANSPORT
Unlike its functional analogs in yeast and mammals, the C terminus of all the VSR isoforms in plants is comparatively short and consists of a mere 30 to 40 residues. The search for potential targeting signals in this region was a logical consequence and emerged from different approaches and multiple groups. Inspired by the original purification of VSRs from CCVs (Kirsch et al., 1994), the presence of a putative YXXΦ motif was spotted upon cDNA cloning (Ahmed et al., 1997; Paris et al., 1997; Shimada et al., 1997) and first studied in 1998 using an elegant biochemical approach (Sanderfoot et al., 1998). In this experiment, a synthetic peptide comprising the YMPL motif of VSR1 within its natural context (IRGIMAQYMPLESQ) was used as competitor for specific interacting partners of the μ-subunit of two clathrin adaptor complexes (APs), the TGN-specific AP1 complex and the plasma membrane–specific AP2 complex. The YMPL-containing peptide weakly competed in the millimolar range for the model ligand of the μ-AP2 subunit, while 1000-fold lower peptide levels competed for the ligand of the TGN-specific μ-AP1 subunit. Replacement of the crucial Tyr residue in the peptide abolished all competition, illustrating the high specificity of the interaction (Sanderfoot et al., 1998). A similar study was later conducted with the Arabidopsis homolog of the μ-AP2 subunit, indicating that the YMPL motif of VSRs can interact with plant AP2 complexes (Happel et al., 2004). Unfortunately, the experiments did not include the two Arabidopsis genes coding for AP1 μ adaptins to compare the binding affinity. Curiously, gene-specific peptide antibodies to μ-AP2 labeled the Golgi apparatus rather than the plasma membrane (Happel et al., 2004). Further work is thus needed to distinguish between the individual roles of plant AP1 and AP2 complexes.
CCVs had previously been implicated in endocytosis from the plasma membrane (Tanchak et al., 1988) and in biosynthetic transport from the Golgi apparatus (Harley and Beevers, 1989; Griffing, 1991) and from the PCR/TGN (Pesacreta and Lucas, 1984; Tanchak et al., 1988; Dettmer et al., 2006). Expression of a dominant-negative dynamin mutant known to interfere with CCV budding led to accumulation of a fluorescent vacuolar cargo (sporamin-GFP) in the Golgi stacks as indicated by a strong colocalization with the Golgi marker ST-RFP (Jin et al., 2001). In the same study, the authors also localized the wild-type dynamin to the Golgi, which corresponds to the Golgi localization of the plant μ-Adaptin (Happel et al., 2004), albeit of the AP2 type. Evidence for biochemical interactions between EPSIN1, clathrin, AP-1, and VSR1, as well as a vacuolar sorting defect in an epsin1 T-DNA insertion line, argues that vacuolar sorting in plants is initiated by Golgi-mediated CCV budding (Song et al., 2006).
A CONSERVED YXXΦ MOTIF IS RESPONSIBLE FOR SELECTIVE ANTEROGRADE TRANSPORT OF VSRs
Having established biochemical evidence for clathrin-mediated VSR transport, it was important to obtain direct evidence for the specific role of the cytosolic tail in VSR transport. In particular, it was interesting to test if anterograde receptor transport to the PVC was signal mediated or by unspecific bulk flow as in yeast (Deloche et al., 2001). The in vivo competition assay in which GFP-VSR2 titrates endogenous sorting machinery and leads to induced secretion of vacuolar cargo (daSilva et al., 2005) offered a fast system to rapidly test the role of conserved amino acids in the short cytosolic tail. Mutations to the C terminus of GFP-VSR2 that decrease the titration effect could be scored biochemically by measuring a reduction in the induced secretion of the vacuolar cargo amy-spo. One of the strongest phenotypes was seen with the Ala substitution of the conserved Tyr residue (Y612A) within the YMPL motif, which had already proven to be crucial for interactions with AP1 (Sanderfoot et al., 1998).
Interestingly, analysis of the subcellular localization of GFP-VSR2(Y612A) by fluorescence microscopy with known organelle markers did not reveal retention in the Golgi apparatus, as may have been expected from earlier studies (Jin et al., 2001; Happel et al., 2004; Song et al., 2006). Instead, the mutant fusion protein was detected at the plasma membrane and small non-Golgi punctate structures (daSilva et al., 2006). Mistargeting of the Tyr mutant to the plasma membrane was confirmed by an independent analysis using pea BP80 as VSR model (Saint-Jean et al., 2010). The identity of the non-Golgi punctae was shown to be the SYP61-labeled PCR/TGN (Foresti et al., 2010). VSR redistribution to the TGN was observed in both leaves and roots, although more dramatic in leaves as wild-type VSR levels are below the detection limit in the TGN (Foresti et al., 2010). The anterograde sorting defect of the Y612A mutant strongly suggests that VSRs are actively selected via a clathrin-mediated process. These results are in contrast with yeast VPS10p, where deletion of either the YXXФ motif or the entire cytosolic tail has no effect on anterograde transport but lead to vacuolar receptor degradation (Cooper and Stevens, 1996).
The partial redistribution to the plasma membrane enabled another interesting observation on full-length VSR2 containing the Y612A mutation, as it led to secretion of vacuolar cargo in a dominant manner (daSilva et al., 2006; Foresti et al., 2010). This is an important discovery as it illustrates that VSRs release their ligands to the apoplast when they reach the plasma membrane and that under normal physiological conditions recycling via the plasma membrane is not a viable option for the delivery of lytic hydrolases that could damage proteins in the extracellular matrix.
The mis-sorting of the Y612A mutant to both the plasma membrane and the TGN may be interpreted as evidence for a role of the TGN in sorting vacuolar proteins from secreted proteins, but other explanations must be considered. The difference between the Y612A mutation and the effect of the dominant-negative dynamin mutant is that the former never engages with clathrin adaptors and remains amenable to bulk flow, whereas the latter probably traps VSRs in partially formed CCVs on the Golgi cisternae that are unable to bud. This would fix the fluorescent vacuolar cargo sporamin-GFP at the main exit site for CCVs, which was identified as the Golgi using ST-RFP (Jin et al., 2001). Together with the Golgi localization of a plant μ-AP2 subunit (Happel et al., 2004), the combined data indicate that secretory and vacuolar cargo segregate at the level of the Golgi stacks, rather than the TGN. In this respect, it is important to note that it has yet to be shown if secretory soluble cargo, such as reporter enzyme barley α-amylase, pass through the TGN or exit the Golgi stack directly to reach the plasma membrane. It also remains to be tested if the mis-sorting of VSR (Y612A) occurs by direct Golgi to TGN transport or if it cycles via the plasma membrane. It is plausible that both the Golgi stacks and TGN can act as export site for selective clathrin-mediated VSR transport to the PVC, and the relative proportion may depend on the cell type and physiological conditions. Perhaps in roots the level of endocytic VSR recycling is higher leading to increased steady state levels at the TGN compared with leaf tissue. Further experimental work is necessary to test these possibilities.
A PLETHORA OF SIGNALS FOR VSR TRAFFICKING
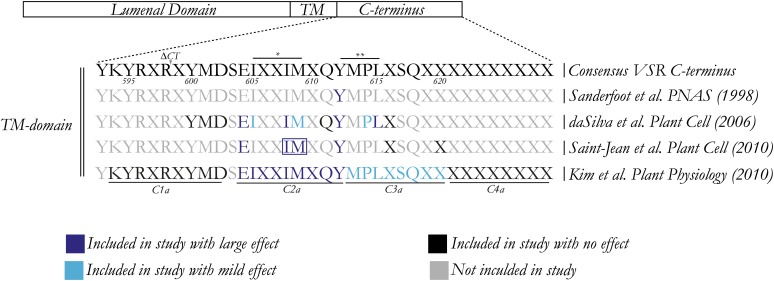
A systematic analysis of the VSR C terminus by several independent research groups revealed that VSR sorting is controlled by further signals to mediate efficient ER export, recycling from the PVC, and endocytosis. Figure 2 summarizes the critical regions contributing to VSR function as identified by independent studies. The first systematic mutant screen revealed a second mutant in the large hydrophobic residue (Φ) within the same YXXΦ motif (L615A) leading to a strong reduction in the ability to induce the secretion of vacuolar cargo in the competition assay (daSilva et al., 2006). However, vacuolar degradation of GFP-VSR2(L615A) was strongly induced, in contrast with the Y612A mutation, which stabilized the fusion protein (daSilva et al., 2006; Foresti et al., 2010). Yeast VPS10 uses the YXXΦ motif only for recycling from the PVC (Cooper and Stevens, 1996), whereas plant VSRs use this motif for both anterograde and retrograde transport. However, the Φ residue may be part of a larger motif composed of other residues because VSRs contain a conserved pair of hydrophobic amino acids (IM) prior to the YXXΦ motif (Figure 2). Single mutations I608A and M609A in GFP chimeras show a weak phenotype in the competition assay (daSilva et al., 2006), whereas the double mutant (IMAA) caused redistribution to the central vacuole (Saint-Jean et al., 2010).
Figure 2.
Summation of the Importance of Various Residues in the C-Terminal Region of VSRs.
Multiple residues analyzed in various studies have been collated to help visualize the importance of certain amino acids in trafficking and homodimerization. On the first line, a consensus C-terminal sequence is shown, generated from all VSR isoform. References to articles are given on the right. The truncation position of the C-terminal deletion (ΔCT) construct used by daSilva et al. (2006) is indicated above the consensus. The dileucine motif (single asterisk) discussed by Saint-Jean et al. (2010) and the conserved YXXΦ motif (double asterisk) are also shown above the alignment. The block substitutions (C1a to C4a) described by Kim et al. (2010) are shown below. Note that relative size between luminal, TM domain, and cytosolic tail are not to scale.
It is possible that several hydrophobic residues form a signal patch that interacts with the recycling machinery. Precedence for this is found in the cytosolic tail of the mammalian M6P receptor, which contains a hydrophobic FW motif that is thought to mediate retention in late endosomes (Schweizer et al., 1997; Díaz and Pfeffer, 1998). Another hydrophobic WLM motif was later shown to be necessary to prevent degradation of the M6P receptor (Seaman, 2007) and may act in concert with the FW motif.
An interesting observation was made with the combination of the Y612A mutation with the IMAA double mutant, which resulted in complete plasma membrane accumulation of the fluorescent VSR fusion (Saint-Jean et al., 2010). The Y612A mutation alone may be capable of endocytosis as it can rapidly redistribute from the cell surface to the vacuole upon treatment with Wortmannin (daSilva et al., 2006). The properties of the triple mutant (IMAA/Y612A) suggest that the IM motif may be responsible for interactions with endocytic transport machinery. The IM motif in plant VSRs is highly conserved and has also been compared with the so-called acidic dileucine motifs (Saint-Jean et al., 2010) sporting an acidic amino acid in position −3 relative to the dileucine. The plant sequence ExxxIM shares this feature in principle, albeit the conspicuous absence of Leu residues. Substitution of the Glu by Ala reduces in vivo competition by GFP-VSR2 (daSilva et al., 2006) and was shown to be localized to the Golgi stack (Saint-Jean et al., 2010). This would argue for a role of the ExxxIM in anterograde transport rather than retrograde transport. However, there is as yet no direct evidence for differential interaction between any of the AP complex proteins and mutant ExxxIM motifs.
Following on from these studies, another group used block substitution Ala screens to dissect four domains of the VSR C terminus (Kim et al., 2010). Interestingly the section containing both the ExxxIM and the conserved Y612 (designated C2a:HA; Figure 2) also showed relocalization to the Golgi. This is an indication that multiple signals may cooperate in the correct targeting of VSRs. Besides all the putative motifs described here, it appears as if the VSR C terminus contains other residues that are not formally recognized as being part of a sorting motif but are nevertheless important for VSR trafficking. Deletion of most of the cytosolic tail except for the three positive charges that are expected to be important for membrane orientation led to a substantial redistribution to the ER (daSilva et al., 2006), suggesting the presence of a cryptic ER export signal that remains to be identified. Due to the fact that the VSR tail is extremely short and probably contains overlapping sorting motifs, it is likely that some effects of mutations may indirectly affect other sorting signals by conformational changes, leading to unexpected experimental outcomes.
DISCOVERY OF THE LATE PREVACUOLE AND THE ELUSIVE SEGREGATION BETWEEN VSRs AND CARGO
Until recently, the prevailing view depicted the PVC or multivesicular body as the plant equivalent of the mammalian late endosome (Tse et al., 2004). The PCR/TGN is seen as an early endosome because it displays detectable steady state levels of the endocytic tracer FM4-64 before the PVC becomes labeled by the tracer molecule (Dettmer et al., 2006; Lam et al., 2007). But how can the PVC deliver the soluble cargo to the vacuole and exclude the VSRs? Work with the recycling defective L615A and IMAA mutant of VSR2 sheds light on the possible mechanism. In addition to the obvious mis-sorting to the vacuole, GFP-VSR2(L615A) was also detected in bright punctate structures that were neither Golgi, PCR/TGN, nor PVC but colocalized with the vacuolar cargo Aleu-RFP (Foresti et al., 2010). In addition, the IMAA mutant also showed increased vacuolar fluorescence and colocalization to Aleu-CFP containing punctae (Saint-Jean et al., 2010). The L615A structures were shown to be a novel organelle that is characterized by high steady state levels of soluble vacuolar cargo and both classes of Rab5 GTPases in plants (Foresti et al., 2010; Bottanelli et al., 2012). It would be interesting to see if the IMAA punctae seen by Saint-Jean and colleagues colocalize with either L615A or Rab5GTPases. The term late PVC (LPVC) was proposed for this new compartment as it is biochemically distinguished from the PVC by its protein composition (Foresti et al., 2010).
The simplest model to explain these findings is that selective retrieval of VSRs from the PVC causes organelle maturation until a new compartment is formed that is enriched in soluble cargo and other membrane components that fail to recycle. It was postulated that this could be accompanied by changes in the membrane composition that would discourage fusion of new vesicles from the Golgi/TGN when a specific cargo threshold is reached. Exactly how this could be achieved is still to be shown, but PVC maturation would culminate in the recruitment of Rab5 GTPases once VSR retrieval is completed (Foresti et al., 2010; Bottanelli et al., 2012).
Another prediction of the PVC maturation model is that LPVCs have gained fusion competence with the vacuole. If VSRs have become depleted and the organelle is enriched in cargo, it has almost the same composition as the vacuole itself, except for possibly a higher pH and some differentiating membrane markers. Further work revealed that low expression of Rab5 GTPases is essential for accurate LPVC partitioning because increased Rab5 expression causes merging of PVC, LPVC, and even TGN markers accompanied by induced secretion of soluble vacuolar cargo (Bottanelli et al., 2012). Transport from the LPVC to the vacuole is sensitive to a nucleotide-deficient mutant of a member of the Rab7 GTPases that causes accumulation of cargo-enriched LPVCs (Bottanelli et al., 2011, 2012). These results correspond well with the recent discovery of the sequential action of Rab5 and Rab7 GTPases in the retromer-dependent retrieval of M6P receptors from the endosomes to the TGN in mammals (Rojas et al., 2008; Seaman et al., 2009). Also in yeast, the rab7 homolog ypt7 is proposed to control retromer function in conjunction with late endosome to vacuole fusion (Balderhaar et al., 2010).
THE ROLE OF RETROMER IN VSR RETRIEVAL
VSR recycling is the rate-limiting step in the vacuolar transport cycle of plants and can be inhibited by the drug Wortmannin as well as by saturation with membrane displayed VSR tails (daSilva et al., 2005). In yeast and mammals, the retromer complex (Seaman et al., 1997, 1998) is thought to mediate receptor retrieval from endosomes and consists of two protein complexes. The trimeric VPS35/29/26 retromer core complex specifically interacts with hydrophobic motifs in the cytosolic domains of VPS10p in yeast or M6P receptors in mammals (Nothwehr et al., 2000; Arighi et al., 2004; Seaman, 2004, 2007). The second accessory complex, termed VPS5/17 in yeast or sortin nexin SNX1/2, has a much less defined role as it binds phosphatidylinositol 3-phosphate directly and belongs to a large gene family thought to shape tubular membrane structures (Collins, 2008; Cullen, 2008).
The first retromer component studied in plants was the VPS35/29/26 complex (Oliviusson et al., 2006). The authors could show that the complex is a stable trimer, and the highly conserved VPS35 subunit coimmunoprecipitated with VSRs and labeled the PVC together with antibodies to the syntaxin Pep12. Finally, the membranes of multivesicular bodies were consistently labeled with antibodies to all three subunits of this complex, providing a strong case for their action at the PVC membrane. In the same year, results from an elegant genetic screen for vacuolar mis-sorting in Arabidopsis seeds provided convincing genetic evidence for a role of VPS29 in vacuolar sorting in Arabidopsis seeds and possible interactions between VPS29 and VPS35 (Shimada et al., 2006; Fuji et al., 2007). The role of retromer in vacuolar sorting was later confirmed by a reverse genetic approach using combinations of knockouts in the three Arabidopsis VPS35 genes (Yamazaki et al., 2008). VPS35 could be coimmunoprecipitated with VPS29 and showed complete colocalization with antibodies to the PVC marker Pep12.
Yeast retromer mutants show increased vacuolar degradation of VPS10, as reviewed previously (Seaman, 2005). Retromer mutants in plants did not show such a clear-cut effect on VSR stability, as individual lines showing either increased or reduced degradation were found (Yamazaki et al., 2008). However, it is important to realize that VPS10 sorting was shown to be different from the equivalent process in plants. Moreover, interference with the sorting machinery itself could not only prevent VSRs from recycling but also other essential components required for anterograde transport. Precedence for this principle is the well-known inhibition of COPI-mediated recycling by Brefeldin A, which does not increase secretion of HDEL proteins, but instead causes retention of secretory proteins in the ER, accompanied by fusion of ER and Golgi membranes (Nebenführ et al., 2002).
While the role of the trimeric retromer core complex in vacuolar sorting is supported by convincing evidence from multiple research groups, the exact role of the sorting nexin dimer is less established. Evidence for a possible role of the accessory SNX1/2 dimer in plant membrane trafficking arose from the identification of a putative SNX1 homolog (Vanoosthuyse et al., 2003) and subsequent gene knockout in Arabidopsis leading to pleiotropic developmental defects and aberrant sorting of the auxin influx carrier PIN2, but not PIN1 (Jaillais et al., 2006). Moreover, Arabidopsis SNX1 was colocalized with fluorescent fusions of the canonical form of plant Rab5 (ARA7, RabF2b) and VPS29 (Jaillais et al., 2007). The Arabidopsis genome contains two other closely related sorting nexins SNX2a and SNX2b (Vanoosthuyse et al., 2003), the second of which was shown to bind directly to phosphatidylinositol 3-phosphate and caused vacuolar trafficking defects when overexpressed (Phan et al., 2008).
Although SNX2 was shown to localize to the PCR/TGN and was proposed to be unrelated to the process of vacuolar sorting (Niemes et al., 2010b), other reports localize the three sorting nexins to punctate structures that colocalize with markers of the PVC (Jaillais et al., 2006, 2007; Phan et al., 2008; Pourcher et al., 2010). The observation that sorting nexins appear to be dispensable for VSR-mediated sorting (Pourcher et al., 2010) illustrates that the discrepancy between the localization of the VPS35/29/26 retromer core complex and that of the sorting nexins may not be a contradiction per se. In this study, a snx1-2 snx2a-2 snx2b-1 triple mutant showed normal localization and function of VPS29. Although the VPS29 mutant shows defects in the sorting and processing of both 12S globulin and 2S albumin, the sorting nexin triple mutant only showed defects in the processing of 12S globulin, whereas sorting of the VSR ligand 2S albumin was unaffected (Pourcher et al., 2010). This would explain why a dominant-negative SNX2a mutant lacking the coiled-coil domain for membrane deformation had no effect on the sorting of the VSR model ligand amy-spo (Niemes et al., 2010b).
THE PROBLEM OF PREVACUOLAR BIOGENESIS
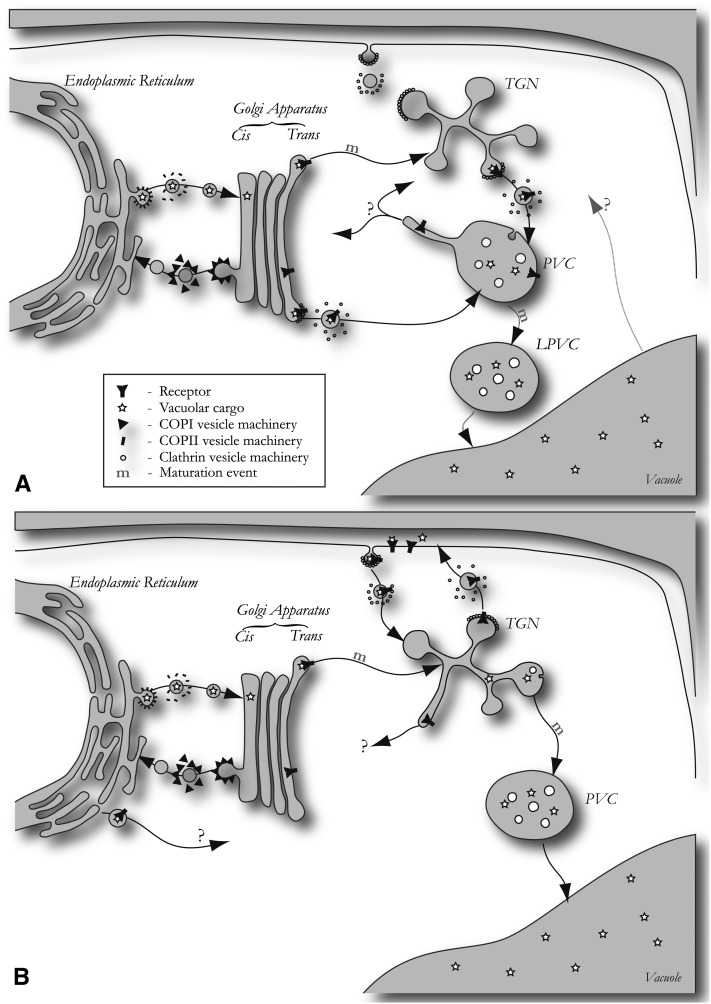
Taken together, the recent advances in the field have provided a critical mass of data to propose a transport model for late events in VSR-mediated vacuolar sorting. Figure 3A illustrates a model that combines the evidence for active signal-mediated VSR export to the PVC with the discovery of the biochemically distinct LPVC. Although PVC maturation explains how soluble vacuolar cargo can be deposited in the vacuole without losing valuable VSRs, the model does not explain how PVCs are regenerated. If PVCs gradually mature to become LPVCs and ultimately fuse with the central vacuole, how are the membranes recycled and how are PVC numbers maintained to act as acceptor membranes for new vesicles carrying VSRs and ligands? This question is just as fundamental as asking how cis-Golgi cisternae are newly formed, when they constantly mature by selective retrieval of ER residents to become medial and then trans cisternae (Hawes et al., 2010; Schoberer et al., 2010). Does it depend only on homotypic fusions of vesicles derived from the previous compartment, or is there a recycling step from a more distal compartment to form progenitors?
Figure 3.
Models of VSR Traffic.
(A) Selective VSR transport to the PVC. VSR transport to the PVC is a selective process that depends on the conserved Tyr residue in a canonical YXXФ motif present in all VSRs (Sanderfoot et al., 1998; daSilva et al., 2006; Foresti et al., 2010; Kim et al., 2010; Saint-Jean et al., 2010). Moreover, this step is likely to occur by clathrin-mediated vesicle budding for transport to the PVC (Kirsch et al., 1994; Sanderfoot et al., 1998; Jin et al., 2001; Happel et al., 2004). CCV could either bud from the Golgi, the PCR/TGN, or both. The mechanism of Golgi-to-PCR/TGN transport is currently unknown but may occur by maturation (m). Selective recycling from the PVC is thought to be mediated by the VPS35/29/26 retromer core complex to prevent receptor degradation in the vacuole (Oliviusson et al., 2006; Shimada et al., 2006; Fuji et al., 2007; Jaillais et al., 2007; Yamazaki et al., 2008). This retrieval step also depends on sorting signals on the VSR tail (Foresti et al., 2010; Saint-Jean et al., 2010), leading to gradual PVC maturation (m) to form the LPVC (Foresti et al., 2010). Changes in the delimiting membrane by microautophagy could contribute to this maturation step via the ESCRT pathway, as interference with one of its components causes reduced internalization of vesicles and induced secretion of vacuolar cargo (Haas et al., 2007; Shahriari et al., 2010). Further transport of soluble cargo from the LPVC to the vacuole is dependent on the sequential action of Rab5 and Rab7 GTPases (Bottanelli et al., 2011, 2012), which are localized to the LPVC and vacuolar membrane, respectively. The destination organelle for the recycling routes from the PVC and the vacuole are unknown (indicated with a question mark).
(B) Alternative model for VSR transport. This model represents alternative transport pathways suggested for VSR trafficking, and individual steps are not mutually dependent on each other and could be combined with aspects of the model in (A). ER export of VSRs by a COPII-independent mechanism has been proposed based on the ability of an ER-retained VSR-calnexin fusion to coretain soluble cargo (Niemes et al., 2010a). Localization of retromer components at the TGN led to the hypothesis that VSRs could be retrieved from the PCR/TGN (Niemes et al., 2010b), whereas arrival at the PVC would be by bulk flow and TGN maturation (Scheuring et al., 2011). It has also been suggested that VSR trafficking may involve a recycling step to and from the PCR/TGN via the plasma membrane to mediate endocytosis of specific cargo molecules (Saint-Jean et al., 2010). The destination organelle for the COPII-independent VSR export pathway and the retromer-mediated recycling are unknown (indicated with a question mark).
Figure 3B illustrates a recent proposal that VSRs are retrieved from the PCR/TGN instead of the PVC (Niemes et al., 2010b). Moreover, selective export of VSRs and ligands was proposed to occur from the ER in a COPII-independent manner (Niemes et al., 2010a), and passive PCR/TGN to PVC maturation was suggested from electron micrographs showing tubular extensions emanating from multivesicular bodies found in the vicinity of the Golgi (Scheuring et al., 2011). This model solves the question regarding PVC biogenesis but poses new problems. How are vacuolar proteins separated from secretory proteins? Are they sorted before the PCR/TGN in the Golgi apparatus, and if yes, how is Golgi to PCR/TGN transport mediated and how is secretory cargo excluded?
The term maturation was first coined to explain the polarity in the Golgi stack, as reviewed by Pelham and Rothman (2000). This process occurs via selective retrieval, which progressively depletes a compartment of a subset of components, leaving only those without recycling signals. In maturation, anterograde transport is therefore passive (i.e., by bulk flow). In this respect, it is interesting to note that coexpression of a trans-Golgi marker, such as ST-CFP, with PCR/TGN markers, such as YFP-SYP61, shows two distinct populations strictly labeled either by one or the other marker (Foresti and Denecke, 2008). There is no gradual distribution with intermediate stages, as known for the Golgi stack (Schoberer et al., 2010), or as observed between PVC and LPVC compartments in plants (Foresti et al., 2010). The same clear separation is observed between PCR/TGN markers and the PVC-resident VSRs, arguing against maturation models. In addition, passive bulk flow cannot explain why VSR arrival at the PVC is blocked by a single point mutation (Y612A), leading to quantitative redistribution to the PCR/TGN and the plasma membrane. If arrival at the PVC would be by bulk flow, then the point mutation should have had no effect.
OPEN QUESTIONS AND FUTURE WORK
Aside from questions regarding organelle biogenesis or the lack of such events, the main goal of research into plant VSRs must be to outline a complete receptor trafficking cycle. Although the cytosolic tail of VSRs appears to be well characterized, there are unanswered questions regarding most of the hypothesized transports steps. VPS35–VSR interactions have not been tested biochemically with recycling-defective VSR mutants, and a complete binding study of VSR tails with the entire family of plant clathrin adaptors has yet to be undertaken. Because plant vacuolar sorting signals are displayed on the surface of vacuolar proteins as soon as they are correctly folded, VSR–ligand interactions can take place in the early secretory pathway. The pH and the calcium levels in the ER lumen appear to allow early recognition between receptors and ligands (Kirsch et al., 1994; Watanabe et al., 2002, 2004; Suen et al., 2010), an event that is supported by observations that ER-retained VSR domains mediate retention of VSR ligands (Watanabe et al., 2004; daSilva et al., 2005; Niemes et al., 2010a). ER export of VSRs has not been studied directly but appears to be dependent on the cytosolic tail (daSilva et al., 2006) as well as properties of the TM domain (Brandizzi et al., 2002).
Although there is no direct evidence comparing the trafficking or function of proteins with and without the lumenal domain, the presence or absence of such a domain does not seem to play an important part in receptor trafficking, as the GFP-VSR variants are still competent for anterograde and retrograde transport. This is surprising because transport of unloaded VSRs would seem futile, but perhaps the presence of a lumenal domain-dependent trafficking mechanism would decrease the efficiency of the anterograde sorting mechanism. It has also been proposed that ER export of VSRs occurs in a COPII-independent vesicular pathway (Niemes et al., 2010a). The observation that VSR ligands are retained in the ER via inhibition of COPII-mediated transport (Bottanelli et al., 2011) would argue against that, but direct studies using COPII transport inhibitors on the targeting of VSRs remain to be performed.
The open issue of ER export is minor compared with the uncertainties regarding further transport steps from the Golgi apparatus. This is mainly due to technical limitations that prevent us from uncovering the exact sequence of transport events. We currently have no direct evidence for vectorial transport from the Golgi stacks to the PCR/TGN. Although polysaccharides and proteins detected in the PCR/TGN were believed to be biosynthetic by nature (Toyooka et al., 2009; Viotti et al., 2010; Kang et al., 2011), these observations were not accompanied by experimental proof that the antigens were derived directly from the Golgi stacks and not the plasma membrane. It also remains to be shown if secretory cargo segregates from vacuolar cargo at the level of the Golgi stack or at the PCR/TGN. This is an experimental issue; one cannot easily watch a vesicle budding from one compartment and then observe how it fuses to another in a live cell, while in fixed cells one can only observe structures, the potential origins and destinations of which remain unknown. Finally, it remains to be shown if SYP61/41 labeled structures fall into two categories that can be distinguished biochemically (Kang et al., 2011; Drakakaki et al., 2012). We can only infer indirectly from combinations of experiments when these are not only reproducible, but pointing in the same direction.
It is possible that protein sorting can occur via mechanisms that fall neither into the vesicle transport or maturation classes. Organelle stratification could lead to the formation of subdomains followed by asymmetrical fission, as shown recently for the transport of storage globulins (Wang et al., 2012). Such a mechanism would cause signal-mediated anterograde transport in the form of an entire organelle budding off from the stratified donor rather than within a small transport vesicle. Further work is necessary to test if this is applicable to TGN/PCR-mediated transport to the PVC.
In addition to their presence in VSR tails, YXXΦ motifs have also been implicated in transport from the plasma membrane in plants and mammals. Of note, endocytosis of the B transporter was shown to be dependent on a Tyr residue in a putative YXXΦ motif (Takano et al., 2010). A repetition of the elegant AP1 versus AP2 interaction studies by Sanderfoot et al. (1998) with peptides displaying the YXXΦ motif of the B transporter in its unique peptide context would help to determine if the AP binding preference is similar or opposite to that of the VSR tail. Another example can be raised regarding the use of glycan modifications to test specific transport steps. Resistance to endoglycosidase H is commonly used to test for passage through the Golgi apparatus, and VSRs have been shown to be largely insensitive to the enzyme, suggesting that VSR traffic does indeed proceed via the Golgi stack (daSilva et al., 2006; Kim et al., 2010). However, the experimental evidence does not show in any way that passage through the Golgi occurred in a classical anterograde manner from the ER. It cannot be ruled out that VSRs visit the Golgi stack as part of the recycling from the PVC and acquire their glycan modifications after the first recycling. It is important to realize that the target organelle of the recycling carriers for the mammalian M6P receptors, yeast VPS10p, and plant VSRs is currently unknown, although popular belief only considers the PCR/TGN for this role.
In conclusion, there are currently still far more unknowns in the pathway and insufficient equations to reach a consensus with respect to the full transport cycle for the VSR family of sorting receptors. That is not to say that we have not made significant progress in the field, and with careful evaluation of transport models in the light of all the research data, further experiments can be designed to shed more light on the most elusive steps.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Data Set 1. Alignment and Accession Numbers of Sequences Used to Produce the Phylogenetic Tree Shown in Figure 1.
Supplementary Material
Acknowledgments
D.C.G. thanks the Biotechnology and Biological Sciences Research Council for a doctoral training grant. We thank John Grahame for scientific discussion regarding evolutionary theory and the calculation of phylogenetic trees. A research grant from the Leverhume Trust (F/10 105/E) is gratefully acknowledged for funding C.D.M.L. and a great deal of the current research by the Denecke group.
AUTHOR CONTRIBUTIONS
All authors contributed equally to this article.
References
- Ahmed S.U., Bar-Peled M., Raikhel N.V. (1997). Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol. 114: 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.U., Rojo E., Kovaleva V., Venkataraman S., Dombrowski J.E., Matsuoka K., Raikhel N.V. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149: 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Moreno X., Velazquez-Campoy A., Rodríguez J.C., Pocoví M., Sancho J. (2008). Mechanism of low density lipoprotein (LDL) release in the endosome: implications of the stability and Ca2+ affinity of the fifth binding module of the LDL receptor. J. Biol. Chem. 283: 22670–22679 [DOI] [PubMed] [Google Scholar]
- Arighi C.N., Hartnell L.M., Aguilar R.C., Haft C.R., Bonifacino J.S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165: 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila E.L., Brown M., Pan S., Desikan R., Neill S.J., Girke T., Surpin M., Raikhel N.V. (2008). Expression analysis of Arabidopsis vacuolar sorting receptor 3 reveals a putative function in guard cells. J. Exp. Bot. 59: 1149–1161 [DOI] [PubMed] [Google Scholar]
- Balderhaar H.J., Arlt H., Ostrowicz C., Bröcker C., Sündermann F., Brandt R., Babst M., Ungermann C. (2010). The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J. Cell Sci. 123: 4085–4094 [DOI] [PubMed] [Google Scholar]
- Beckers C.J., Keller D.S., Balch W.E. (1987). Semi-intact cells permeable to macromolecules: Use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell 50: 523–534 [DOI] [PubMed] [Google Scholar]
- Bednarek S.Y., Wilkins T.A., Dombrowski J.E., Raikhel N.V. (1990). A carboxyl-terminal propeptide is necessary for proper sorting of barley lectin to vacuoles of tobacco. Plant Cell 2: 1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocock J.P., Carmicle S., Chhotani S., Ruffolo M.R., Chu H., Erickson A.H. (2009). The PA-TM-RING protein RING finger protein 13 is an endosomal integral membrane E3 ubiquitin ligase whose RING finger domain is released to the cytoplasm by proteolysis. FEBS J. 276: 1860–1877 [DOI] [PubMed] [Google Scholar]
- Bonifacino J.S., Glick B.S. (2004). The mechanisms of vesicle budding and fusion. Cell 116: 153–166 [DOI] [PubMed] [Google Scholar]
- Bottanelli F., Foresti O., Hanton S., Denecke J. (2011). Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell 23: 3007–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottanelli F., Gershlick D.C., Denecke J. (2012). Evidence for sequential action of Rab5 and Rab7 GTPases in prevacuolar organelle partitioning. Traffic 13: 338–354 [DOI] [PubMed] [Google Scholar]
- Brandizzi F., Frangne N., Marc-Martin S., Hawes C., Neuhaus J.M., Paris N. (2002). The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 14: 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.C., Jolliffe N.A., Frigerio L., Roberts L.M. (2003). Sequence-specific, Golgi-dependent vacuolar targeting of castor bean 2S albumin. Plant J. 36: 711–719 [DOI] [PubMed] [Google Scholar]
- Brown W.J., Farquhar M.G. (1984). The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell 36: 295–307 [DOI] [PubMed] [Google Scholar]
- Bush D.S. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 95–122 [Google Scholar]
- Campbell C.H., Fine R.E., Squicciarini J., Rome L.H. (1983). Coated vesicles from rat liver and calf brain contain cryptic mannose 6-phosphate receptors. J. Biol. Chem. 258: 2628–2633 [PubMed] [Google Scholar]
- Campbell C.H., Rome L.H. (1983). Coated vesicles from rat liver and calf brain contain lysosomal enzymes bound to mannose 6-phosphate receptors. J. Biol. Chem. 258: 13347–13352 [PubMed] [Google Scholar]
- Cao X., Rogers S.W., Butler J., Beevers L., Rogers J.C. (2000). Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12: 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghino J.L., Marcusson E.G., Emr S.D. (1995). The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell 6: 1089–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C.M., Neto H., Foucart C., Moore I. (2008). Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.M. (2008). The structure and function of the retromer protein complex. Traffic 9: 1811–1822 [DOI] [PubMed] [Google Scholar]
- Cooper A.A., Stevens T.H. (1996). Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133: 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C.P., Hunter P.R., Szakacs E., Hinz G., Robinson D.G., Frigerio L. (2008). Lack of a vacuolar sorting receptor leads to non-specific missorting of soluble vacuolar proteins in Arabidopsis seeds. Traffic 9: 408–416 [DOI] [PubMed] [Google Scholar]
- Cullen P.J. (2008). Endosomal sorting and signalling: An emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9: 574–582 [DOI] [PubMed] [Google Scholar]
- daSilva L.L., Foresti O., Denecke J. (2006). Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18: 1477–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva L.L., Taylor J.P., Hadlington J.L., Hanton S.L., Snowden C.J., Fox S.J., Foresti O., Brandizzi F., Denecke J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C. (1975). The role of lysosomes in the pathogeny of disease. Scand. J. Rheumatol. Suppl. 12: 63–66 [PubMed] [Google Scholar]
- Deloche O., Yeung B.G., Payne G.S., Schekman R. (2001). Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell 12: 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J., Botterman J., Deblaere R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2: 51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E., Pfeffer S.R. (1998). TIP47: A cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93: 433–443 [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano G.P., Paris N., Marc-Martin S., Neuhaus J.M. (1998). Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J. 15: 449–457 [DOI] [PubMed] [Google Scholar]
- Dombrowski J.E., Schroeder M.R., Bednarek S.Y., Raikhel N.V. (1993). Determination of the functional elements within the vacuolar targeting signal of barley lectin. Plant Cell 5: 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C., Voelker T.A., Herman E.M., Chrispeels M.J. (1989). Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J. Cell Biol. 108: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G., van de Ven W., Pan S.Q., Miao Y.S., Wang J.Q., Keinath N.F., Weatherly B., Jiang L.W., Schumacher K., Hicks G., Raikhel N. (2012). Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res. 22: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M.G. (1985). Progress in unraveling pathways of Golgi traffic. Annu. Rev. Cell Biol. 1: 447–488 [DOI] [PubMed] [Google Scholar]
- Foresti O., Denecke J. (2008). Intermediate organelles of the plant secretory pathway: Identity and function. Traffic 9: 1599–1612 [DOI] [PubMed] [Google Scholar]
- Foresti O., Gershlick D.C., Bottanelli F., Hummel E., Hawes C., Denecke J. (2010). A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in tobacco. Plant Cell 22: 3992–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L., Jolliffe N.A., Di Cola A., Felipe D.H., Paris N., Neuhaus J.M., Lord J.M., Ceriotti A., Roberts L.M. (2001). The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant Physiol. 126: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji K., Shimada T., Takahashi H., Tamura K., Koumoto Y., Utsumi S., Nishizawa K., Maruyama N., Hara-Nishimura I. (2007). Arabidopsis vacuolar sorting mutants (green fluorescent seed) can be identified efficiently by secretion of vacuole-targeted green fluorescent protein in their seeds. Plant Cell 19: 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H.J., Slot J.W., Strous G.J., Hasilik A., von Figura K. (1985). Possible pathways for lysosomal enzyme delivery. J. Cell Biol. 101: 2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Dahms N.M., Kornfeld S. (2003). Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 4: 202–212 [DOI] [PubMed] [Google Scholar]
- Gilroy S., Blowers D.P., Trewavas A.J. (1987). Calcium: A regulation system emerges in plant cells. Development 100: 181–184 [Google Scholar]
- Grant B.D., Donaldson J.G. (2009). Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10: 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffing L.R. (1991). Comparisons of Golgi structure and dynamics in plant and animal cells. J. Electron Microsc. Tech. 17: 179–199 [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. (1986). The trans Golgi network: Sorting at the exit site of the Golgi complex. Science 234: 438–443 [DOI] [PubMed] [Google Scholar]
- Haas T.J., Sliwinski M.K., Martínez D.E., Preuss M., Ebine K., Ueda T., Nielsen E., Odorizzi G., Otegui M.S. (2007). The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 19: 1295–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel N., Höning S., Neuhaus J.M., Paris N., Robinson D.G., Holstein S.E. (2004). Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. 37: 678–693 [DOI] [PubMed] [Google Scholar]
- Harley S.M., Beevers L. (1989). Coated vesicles are involved in the transport of storage proteins during seed development in Pisum sativum L. Plant Physiol. 91: 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselbeck A., Schekman R. (1986). Interorganelle transfer and glycosylation of yeast invertase in vitro. Proc. Natl. Acad. Sci. USA 83: 2017–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C., Schoberer J., Hummel E., Osterrieder A. (2010). Biogenesis of the plant Golgi apparatus. Biochem. Soc. Trans. 38: 761–767 [DOI] [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K.K. (1988). Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 7: 4119–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G., Colanesi S., Hillmer S., Rogers J.C., Robinson D.G. (2007). Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8: 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hinz G., Hillmer S., Baumer M., Hohl I.I. (1999). Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11: 1509–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflack B., Kornfeld S. (1985). Lysosomal enzyme binding to mouse P388D1 macrophage membranes lacking the 215-kDa mannose 6-phosphate receptor: Evidence for the existence of a second mannose 6-phosphate receptor. Proc. Natl. Acad. Sci. USA 82: 4428–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda B.C., Padgett H.S., Rogers J.C. (1992). Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell 4: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Henry L., Ho Y.K., Pownall H.J., Rudenko G. (2010). Mechanism of LDL binding and release probed by structure-based mutagenesis of the LDL receptor. J. Lipid Res. 51: 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P.R., Craddock C.P., Di Benedetto S., Roberts L.M., Frigerio L. (2007). Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol. 145: 1371–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov S., Isner J.C., Maathuis F.J. (2011). Rice two-pore K+ channels are expressed in different types of vacuoles. Plant Cell 23: 756–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Rollin C., Gaude T. (2006). AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Santambrogio M., Rozier F., Fobis-Loisy I., Miège C., Gaude T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Jiang L.W., Phillips T.E., Rogers S.W., Rogers J.C. (2000). Biogenesis of the protein storage vacuole crystalloid. J. Cell Biol. 150: 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.B., Kim Y.A., Kim S.J., Lee S.H., Kim D.H., Cheong G.W., Hwang I. (2001). A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13: 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe N.A., Brown J.C., Neumann U., Vicré M., Bachi A., Hawes C., Ceriotti A., Roberts L.M., Frigerio L. (2004). Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. Plant J. 39: 821–833 [DOI] [PubMed] [Google Scholar]
- Jones D.T., Taylor W.R., Thornton J.M. (1992). The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8: 275–282 [DOI] [PubMed] [Google Scholar]
- Kanaseki T., Kadota K. (1969). The “vesicle in a basket”. A morphological study of the coated vesicle isolated from the nerve endings of the guinea pig brain, with special reference to the mechanism of membrane movements. J. Cell Biol. 42: 202–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Nielsen E., Preuss M.L., Mastronarde D., Staehelin L.A. (2011). Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329 [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D.T., Sly W.S. (1977). Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc. Natl. Acad. Sci. USA 74: 2026–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Kang H., Jang M., Chang J.H., Miao Y., Jiang L., Hwang I. (2010). Homomeric interaction of AtVSR1 is essential for its function as a vacuolar sorting receptor. Plant Physiol. 154: 134–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., Paris N., Butler J.M., Beevers L., Rogers J.C. (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA 91: 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., Saalbach G., Raikhel N.V., Beevers L. (1996). Interaction of a potential vacuolar targeting receptor with amino- and carboxyl-terminal targeting determinants. Plant Physiol. 111: 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H.J. (1993). Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 121: 997–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. (1992). Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61: 307–330 [DOI] [PubMed] [Google Scholar]
- Lam S.K., Siu C.L., Hillmer S., Jang S., An G., Robinson D.G., Jiang L. (2007). Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19: 296–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval V., Masclaux F., Serin A., Carrière M., Roldan C., Devic M., Pont-Lezica R.F., Galaud J.P. (2003). Seed germination is blocked in Arabidopsis putative vacuolar sorting receptor (atbp80) antisense transformants. J. Exp. Bot. 54: 213–221 [DOI] [PubMed] [Google Scholar]
- Lemansky P., Hasilik A., von Figura K., Helmy S., Fishman J., Fine R.E., Kedersha N.L., Rome L.H. (1987). Lysosomal enzyme precursors in coated vesicles derived from the exocytic and endocytic pathways. J. Cell Biol. 104: 1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A. (2011). A brief history of the cisternal progression-maturation model. Cell. Logist. 1: 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson E.G., Horazdovsky B.F., Cereghino J.L., Gharakhanian E., Emr S.D. (1994). The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77: 579–586 [DOI] [PubMed] [Google Scholar]
- Marty F. (1999). Plant vacuoles. Plant Cell 11: 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K. (2000). C-terminal propeptides and vacuolar sorting by BP-80-type proteins: Not all C-terminal propeptides are equal. Plant Cell 12: 181–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Bassham D.C., Raikhel N.V., Nakamura K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130: 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Nakamura K. (1991). Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc. Natl. Acad. Sci. USA 88: 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Neuhaus J.-M. (1999). Cis-elements of protein transport to the plant vacuoles. J. Exp. Bot. 50: 165–174 [Google Scholar]
- Maxfield F.R., McGraw T.E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5: 121–132 [DOI] [PubMed] [Google Scholar]
- Miao Y., Yan P.K., Kim H., Hwang I., Jiang L. (2006). Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol. 142: 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.A., Lee M.C., Anderson M.A. (1999). Identification and characterization of a prevacuolar compartment in stigmas of Nicotiana alata. Plant Cell 11: 1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Matsuoka K. (1993). Protein targeting to the vacuole in plant cells. Plant Physiol. 101: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A., Ritzenthaler C., Robinson D.G. (2002). Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J.M., Paris N. (2005). Plant Vacuoles: From Biogenesis to Function. In Plant Endocytosis, J. Samaj, F. Baluska, and D. Menzel, eds (Berlin, Heidelberg: Springer-Verlag), pp. 63–82 [Google Scholar]
- Neuhaus J.M., Sticher L., Meins F., Jr, Boller T. (1991). A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 88: 10362–10366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S., Labs M., Scheuring D., Krueger F., Langhans M., Jesenofsky B., Robinson D.G., Pimpl P. (2010a). Sorting of plant vacuolar proteins is initiated in the ER. Plant J. 62: 601–614 [DOI] [PubMed] [Google Scholar]
- Niemes S., Langhans M., Viotti C., Scheuring D., San Wan Yan M., Jiang L., Hillmer S., Robinson D.G., Pimpl P. (2010b). Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Nothwehr S.F., Ha S.A., Bruinsma P. (2000). Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151: 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. (1979). Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76: 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviusson P., Heinzerling O., Hillmer S., Hinz G., Tse Y.C., Jiang L., Robinson D.G. (2006). Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L.J., Sun G., Bohnsack R.N., Peterson F.C., Dahms N.M., Kim J.J. (2010). Intermonomer interactions are essential for lysosomal enzyme binding by the cation-dependent mannose 6-phosphate receptor. Biochemistry 49: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Louvard D., Perrelet A. (1985). Clathrin-immunoreactive sites in the Golgi apparatus are concentrated at the trans pole in polypeptide hormone-secreting cells. Proc. Natl. Acad. Sci. USA 82: 5385–5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordenes V.R., Reyes F.C., Wolff D., Orellana A. (2002). A thapsigargin-sensitive Ca(2+) pump is present in the pea Golgi apparatus membrane. Plant Physiol. 129: 1820–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. (1975). Intracellular aspects of the process of protein synthesis. Science 189: 347–358 [DOI] [PubMed] [Google Scholar]
- Paris N., Neuhaus J.M. (2002). BP-80 as a vacuolar sorting receptor. Plant Mol. Biol. 50: 903–914 [DOI] [PubMed] [Google Scholar]
- Paris N., Rogers S.W., Jiang L., Kirsch T., Beevers L., Phillips T.E., Rogers J.C. (1997). Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 115: 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris N., Stanley C.M., Jones R.L., Rogers J.C. (1996). Plant cells contain two functionally distinct vacuolar compartments. Cell 85: 563–572 [DOI] [PubMed] [Google Scholar]
- Park J.H., Oufattole M., Rogers J.C. (2007). Golgi-mediated vacuolar sorting in plant cells: RMR proteins are sorting receptors for the protein aggregation/membrane internalization pathway. Plant Sci. 172: 728–745 [Google Scholar]
- Park M., Lee D., Lee G.J., Hwang I. (2005). AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J. Cell Biol. 170: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G.S., Schekman R. (1985). A test of clathrin function in protein secretion and cell growth. Science 230: 1009–1014 [DOI] [PubMed] [Google Scholar]
- Pearse B.M. (1975). Coated vesicles from pig brain: purification and biochemical characterization. J. Mol. Biol. 97: 93–98 [DOI] [PubMed] [Google Scholar]
- Pearse B.M. (1976). Clathrin: A unique protein associated with intracellular transfer of membrane by coated vesicles. Proc. Natl. Acad. Sci. USA 73: 1255–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B.M., Bretscher M.S. (1981). Membrane recycling by coated vesicles. Annu. Rev. Biochem. 50: 85–101 [DOI] [PubMed] [Google Scholar]
- Pelham H.R., Roberts L.M., Lord J.M. (1992). Toxin entry: How reversible is the secretory pathway? Trends Cell Biol. 2: 183–185 [DOI] [PubMed] [Google Scholar]
- Pelham H.R., Rothman J.E. (2000). The debate about transport in the Golgi—Two sides of the same coin? Cell 102: 713–719 [DOI] [PubMed] [Google Scholar]
- Pesacreta T.C., Lucas W.J. (1984). Plasma membrane coat and a coated vesicle-associated reticulum of membranes: Their structure and possible interrelationship in Chara corallina. J. Cell Biol. 98: 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan N.Q., Kim S.J., Bassham D.C. (2008). Overexpression of Arabidopsis sorting nexin AtSNX2b inhibits endocytic trafficking to the vacuole. Mol. Plant 1: 961–976 [DOI] [PubMed] [Google Scholar]
- Pimpl P., Hanton S.L., Taylor J.P., Pinto-daSilva L.L., Denecke J. (2003). The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15: 1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcher M., Santambrogio M., Thazar N., Thierry A.M., Fobis-Loisy I., Miège C., Jaillais Y., Gaude T. (2010). Analyses of sorting nexins reveal distinct retromer-subcomplex functions in development and protein sorting in Arabidopsis thaliana. Plant Cell 22: 3980–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes F.C., Buono R., Otegui M.S. (2011). Plant endosomal trafficking pathways. Curr. Opin. Plant Biol. 14: 666–673 [DOI] [PubMed] [Google Scholar]
- Rogers S.W., Youn B., Rogers J.C., Kang C. (2004). Purification, crystallization and preliminary crystallographic studies of the ligand-binding domain of a plant vacuolar sorting receptor. Acta Crystallogr. D Biol. Crystallogr. 60: 2028–2030 [DOI] [PubMed] [Google Scholar]
- Rojas R., van Vlijmen T., Mardones G.A., Prabhu Y., Rojas A.L., Mohammed S., Heck A.J., Raposo G., van der Sluijs P., Bonifacino J.S. (2008). Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J. Cell Biol. 183: 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.F., Porter K.R. (1964). Yolk protein uptake in the oocyte of the mosquito Aedes aegypti L. J. Cell Biol. 20: 313–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jean B., Seveno-Carpentier E., Alcon C., Neuhaus J.M., Paris N. (2010). The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis. Plant Cell 22: 2825–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Sanderfoot A.A., Ahmed S.U., Marty-Mazars D., Rapoport I., Kirchhausen T., Marty F., Raikhel N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95: 9920–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scabone C.M., Frigerio L., Petruccelli S. (2011). A fluorescent reporter protein containing AtRMR1 domains is targeted to the storage and central vacuoles in Arabidopsis thaliana and tobacco leaf cells. Plant Cell Rep. 30: 1823–1833 [DOI] [PubMed] [Google Scholar]
- Scheel A.A., Pelham H.R. (1996). Purification and characterization of the human KDEL receptor. Biochemistry 35: 10203–10209 [DOI] [PubMed] [Google Scholar]
- Schekman R. (1985). Protein localization and membrane traffic in yeast. Annu. Rev. Cell Biol. 1: 115–143 [DOI] [PubMed] [Google Scholar]
- Scheuring D., Viotti C., Krüger F., Künzl F., Sturm S., Bubeck J., Hillmer S., Frigerio L., Robinson D.G., Pimpl P., Schumacher K. (2011). Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J., Runions J., Steinkellner H., Strasser R., Hawes C., Osterrieder A. (2010). Sequential depletion and acquisition of proteins during Golgi stack disassembly and reformation. Traffic 11: 1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Kornfeld S., Rohrer J. (1997). Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc. Natl. Acad. Sci. USA 94: 14471–14476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N. (2004). Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165: 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15: 68–75 [DOI] [PubMed] [Google Scholar]
- Seaman M.N. (2007). Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J. Cell Sci. 120: 2378–2389 [DOI] [PubMed] [Google Scholar]
- Seaman M.N., Harbour M.E., Tattersall D., Read E., Bright N. (2009). Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 122: 2371–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N., Marcusson E.G., Cereghino J.L., Emr S.D. (1997). Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 137: 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman M.N., McCaffery J.M., Emr S.D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142: 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahriari M., Keshavaiah C., Scheuring D., Sabovljevic A., Pimpl P., Häusler R.E., Hülskamp M., Schellmann S. (2010). The AAA-type ATPase AtSKD1 contributes to vacuolar maintenance of Arabidopsis thaliana. Plant J. 64: 71–85 [DOI] [PubMed] [Google Scholar]
- Shen Y., Wang J., Ding Y., Lo S.W., Gouzerh G., Neuhaus J.M., Jiang L. (2011). The rice RMR1 associates with a distinct prevacuolar compartment for the protein storage vacuole pathway. Mol. Plant 4: 854–868 [DOI] [PubMed] [Google Scholar]
- Shimada T., Fuji K., Tamura K., Kondo M., Nishimura M., Hara-Nishimura I. (2003). Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100: 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Koumoto Y., Li L., Yamazaki M., Kondo M., Nishimura M., Hara-Nishimura I. (2006). AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 47: 1187–1194 [DOI] [PubMed] [Google Scholar]
- Shimada T., Kuroyanagi M., Nishimura M., Hara-Nishimura I. (1997). A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 38: 1414–1420 [DOI] [PubMed] [Google Scholar]
- Shinshi H., Wenzler H., Neuhaus J.M., Felix G., Hofsteenge J., Meins F. (1988). Evidence for N- and C-terminal processing of a plant defense-related enzyme: Primary structure of tobacco prepro-beta-1,3-glucanase. Proc. Natl. Acad. Sci. USA 85: 5541–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R.C., Polzin K., Labate J., Specht J., Brummer E.C., Olson T., Young N., Concibido V., Wilcox J., Tamulonis J.P., Kochert G., Boerma H.R. (1996). Genome duplication in soybean (Glycine subgenus soja). Genetics 144: 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Lee M.H., Lee G.J., Yoo C.M., Hwang I. (2006). Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell 18: 2258–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen P.K., Shen J., Sun S.S.M., Jiang L. (2010). Expression and characterization of two functional vacuolar sorting receptor (VSR) proteins, BP-80 and AtVSR4 from culture media of transgenic tobacco BY-2 cells. Plant Sci. 179: 68–76 [Google Scholar]
- Taiz L. (1992). The plant vacuole. J. Exp. Biol. 172: 113–122 [DOI] [PubMed] [Google Scholar]
- Takano J., Tanaka M., Toyoda A., Miwa K., Kasai K., Fuji K., Onouchi H., Naito S., Fujiwara T. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchak M.A., Rennie P.J., Fowke L.C. (1988). Ultrastructure of the partially coated reticulum and dictyosomes during endocytosis by soybean protoplasts. Planta 175: 433–441 [DOI] [PubMed] [Google Scholar]
- Tong P.Y., Kornfeld S. (1989). Ligand interactions of the cation-dependent mannose 6-phosphate receptor. Comparison with the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 264: 7970–7975 [PubMed] [Google Scholar]
- Toyooka K., Goto Y., Asatsuma S., Koizumi M., Mitsui T., Matsuoka K. (2009). A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse Y.C., Mo B., Hillmer S., Zhao M., Lo S.W., Robinson D.G., Jiang L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eijnden M.J., Lahaye L.L., Strous G.J. (2006). Disulfide bonds determine growth hormone receptor folding, dimerisation and ligand binding. J. Cell Sci. 119: 3078–3086 [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn S.C. (2006). Recycling endosomes. J. Cell Sci. 119: 1679–1681 [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse V., Tichtinsky G., Dumas C., Gaude T., Cock J.M. (2003). Interaction of calmodulin, a sorting nexin and kinase-associated protein phosphatase with the Brassica oleracea S locus receptor kinase. Plant Physiol. 133: 919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C., et al. (2010). Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T.A., Herman E.M., Chrispeels M.J. (1989). In vitro mutated phytohemagglutinin genes expressed in tobacco seeds: Role of glycans in protein targeting and stability. Plant Cell 1: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Rogers J.C., Jiang L. (2011). Plant RMR proteins: Unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. FEBS J. 278: 59–68 [DOI] [PubMed] [Google Scholar]
- Wang J.Q., Tse Y.C., Hinz G., Robinson D.G., Jiang L.W. (2012). Storage globulins pass through the Golgi apparatus and multivesicular bodies in the absence of dense vesicle formation during early stages of cotyledon development in mung bean. J. Exp. Bot. 63: 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E., Shimada T., Kuroyanagi M., Nishimura M., Hara-Nishimura I. (2002). Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J. Biol. Chem. 277: 8708–8715 [DOI] [PubMed] [Google Scholar]
- Watanabe E., Shimada T., Tamura K., Matsushima R., Koumoto Y., Nishimura M., Hara-Nishimura I. (2004). An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol. 45: 9–17 [DOI] [PubMed] [Google Scholar]
- Wieland F.T., Gleason M.L., Serafini T.A., Rothman J.E. (1987). The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50: 289–300 [DOI] [PubMed] [Google Scholar]
- Wilson D.W., Lewis M.J., Pelham H.R. (1993). pH-dependent binding of KDEL to its receptor in vitro. J. Biol. Chem. 268: 7465–7468 [PubMed] [Google Scholar]
- Wink M. (1993). The plant vacuole: A multifunctional compartment. J. Exp. Bot. 44: 231–246 [Google Scholar]
- Yamazaki M., Shimada T., Takahashi H., Tamura K., Kondo M., Nishimura M., Hara-Nishimura I. (2008). Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 49: 142–156 [DOI] [PubMed] [Google Scholar]
- Yin J., Li G., Ren X., Herrler G. (2007). Select what you need: A comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol. 127: 335–347 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Michaely P. (2009). The role of calcium in lipoprotein release by the low-density lipoprotein receptor. Biochemistry 48: 7313–7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhar J., Muñoz A., Rojo E. (2010). Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. Plant J. 64: 577–588 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.