Abstract
More than half of the known protein tyrosine phosphatases (PTPs) in the human genome are expressed in T cells, and significant progress has been made in elucidating the biology of these enzymes in T-cell development and function. Here we provide a systematic review of the current understanding of the roles of PTPs in T-cell activation, providing insight into their mechanisms of action and regulation in T-cell receptor signalling, the phenotypes of their genetically modified mice, and their possible involvement in T-cell-mediated autoimmune disease. Our projection is that the interest in PTPs as mediators of T-cell homeostasis will continue to rise with further functional analysis of these proteins, and PTPs will be increasingly considered as targets of immunomodulatory therapies.
Keywords: protein tyrosine phosphatase, T-cell activation, T-cell receptor signalling, autoimmunity, tyrosine phosphorylation
Tyrosine phosphorylation in T-cell activation: the balance between tyrosine kinases and tyrosine phosphatases
Activation of T cells is largely mediated through signal transduction downstream from the T-cell receptor (TCR) expressed on the cell surface. The TCR is a transmembrane receptor complex comprised of α and β chains, which bind ligands, and CD3 (ε, γ and δ) and ζ chains containing motifs that are phosphorylated on tyrosine, called immunoreceptor tyrosine-based activation motifs. Signal transduction through the TCR is initiated when the receptor binds to a peptide–MHC complex presented by an antigen-presenting cell. This interaction initiates a cascade of signalling events, which induces the proliferation, mobilization and differentiation of T cells. For an updated and comprehensive review of signalling through the TCR the reader is referred to recent authoritative publications.1–4
A dynamic wave of tyrosine phosphorylation phenomena is critical for ignition of intracellular signalling in T cells. Engagement of the TCR leads to the activation of the Src family protein tyrosine kinases (PTKs) LCK and FYN, which phosphorylate the immunoreceptor tyrosine-based activation motifs of the TCR. This provides docking sites for the SH2 domains of ZAP-70, a Syk family PTK, allowing ZAP-70 to be phosphorylated and activated by LCK. Once activated, ZAP-70 phosphorylates the adaptor proteins SLP-76 and LAT, which nucleate signalling complexes, leading to the phosphorylation and activation of multiple downstream effectors. This results in calcium mobilization, activation of mitogen-activated protein kinases (MAPKs), transcriptional regulation and cytoskeletal rearrangements.
Protein tyrosine phosphatases (PTPs) are the natural counterpart of PTKs. Much like PTKs, depending upon the phosphorylation site and the signalling context, they can enhance or reduce the function of their protein target(s). The modern view of phosphorylation networks is one of dynamic ‘always-on’ grids where the stoichiometry of each phosphorylation site is continuously controlled by the changing balance between the activities of kinases and phosphatases. For example, the activation state of the Src family kinases (SFKs) is balanced between the activities of CSK and CD45, which respectively phosphorylate and dephosphorylate the inhibitory C-terminal site (Y505 of LCK), and the negative regulators LYP and SHP-1, which dephosphorylate an activating tyrosine in the catalytic domain (Y394 of LCK). Acute changes in PTP activity/expression are in principle sufficient to alter the network status and even trigger true signalling waves. The recent ‘kinetic-segregation’ model postulates that PTPs are responsible for the very initiation of signalling after engagement of the TCR.5 In this model, acute removal of PTPs from membrane areas where the interactions between the T cells and antigen-presenting cells occur results in an imbalance in the phosphatase–kinase equilibrium, which is sufficient to trigger a self-amplifying wave of activation of the SFKs.
The human genome encodes more than 100 PTPs classified into four classes (see refs. 6 and 7 for reviews of PTP classifications). T cells are known to express at least 60 of these enzymes, many of which have known roles as positive or negative regulators of TCR signalling.8,9 The study of these PTPs in autoimmunity has obvious significance because TCR signalling impinges upon the pathogenesis of autoimmunity at multiple levels. For example, increased/decreased TCR signalling can alter selection at the thymic level, activation and differentiation of effector T cells, suppressive activity of regulatory T cells, and triggering and maintenance of peripheral anergy.10–12 Here, after briefly reviewing the role of each PTP in TCR signalling (summarized in Fig. 1 and Table 1), we will also summarize whether there is any evidence available of an involvement of the enzyme in autoimmunity (summarized in Table 2). The classification system adopted in this review (Fig. 2) is the one described in Alonso et al.6
Figure 1.
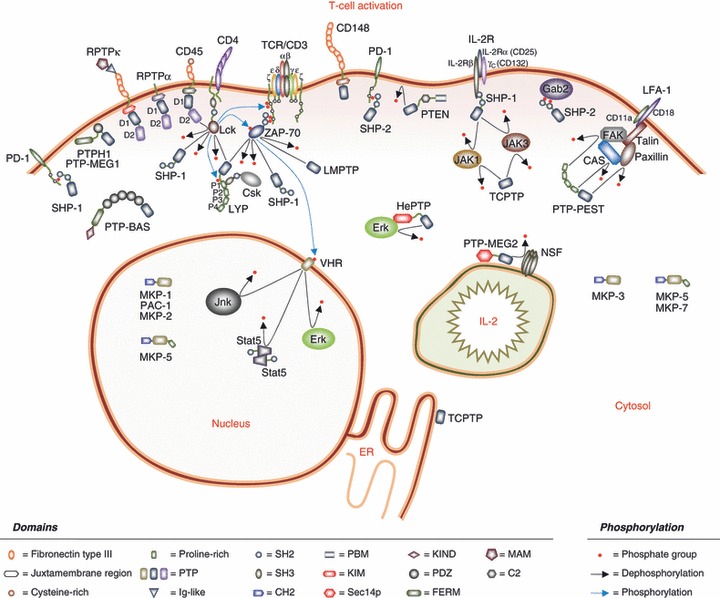
Schematic representation of the diverse functions of the protein tyrosine phosphatases (PTPs) regulating T-cell activation. The activation of a T cell involves tyrosine phosphorylation at multiple levels, and PTPs play diverse roles that work in concert together to cause the response of the T cell to the extracellular environment, to cause T-cell mobilization and to cause the production and response to cytokines such as interleukin-2 (IL-2). PTPs involved in these processes include transmembrane PTPs and intracellular membrane-proximal, cytosolic and nuclear PTPs. The initial response of a T cell to the external environment is finely tuned by PTPs regulating the early wave of phosphorylation events immediately proximal to the T-cell receptor (TCR), and many of the PTPs are found near the plasma membrane. These PTPs control the phosphorylation status of the Src family kinases (SFKs), the immunoreceptor tyrosine-based activation motifs (ITAMS) of the ζ chains and ZAP-70. Further downstream, PTPs control both membrane-proximal and cytosolic signalling effectors. Activation of mitogen-activated protein kinases (MAPKs) is spatially regulated by classical and dual-specific PTPs localized to the cytosol and/or nucleus. T-cell mobility is controlled by cytoskeletal rearrangements, involving multiple PTPs with protein–protein interaction domains and FERM (band 4.1–ezrin–radixin–moesin) domains that allow their association with complexes regulating the cytoskeleton and/or the plasma membrane. Post-transcriptionally, secretion of cytokines such as IL-2 requires phosphotyrosine-mediated vesicle formation. The autocrine response of T cells to extracellular IL-2 is mediated through tyrosine phosphorylation of signalling molecules downstream from the IL-2 receptor. An additional layer of modulation of the T-cell response is added through triggering of inhibitory receptors, whose function is mediated by cytosolic PTPs that can bind to the inhibitory motifs on these receptors.
Table 1.
Protein tyrosine phosphatases (PTPs) in T-cell activation
| PTP | Gene name | Role in T-cell activation |
|---|---|---|
| CD45 | PTPRC | Positively regulates TCR signalling by dephosphorylation of inhibitory site of LCK and FYN |
| CD148 | PTPRJ | Positively regulates TCR signalling by dephosphorylation of inhibitory site of LCK; may also negatively regulate TCR signalling by dephosphorylation of LAT and PLCγ1 |
| RPTPα | PTPRA | Positively regulates TCR signalling by dephosphorylation of inhibitory site of LCK and FYN |
| RPTPκ | PTPRK | Needed for development of CD4+ T cells; mechanism unclear |
| LAR | PTPRF | Negatively regulates TCR signalling in thymocytes by dephosphorylation of LCK and FYN |
| LYP/Pep | PTPN22 | Negatively regulates TCR signalling by dephosphorylation of inhibitory site of LCK, FYN, ZAP-70 and others; binds CSK |
| PTP-PEST | PTPN12 | Negatively regulates TCR signalling by dephosphorylation of Cas, Pyk2, FAK, paxilin; binds CSK |
| SHP-1 | PTPN6 | Negatively regulates T-cell activation through ITIM/ITSM receptors, cytokine receptors, dephosphorylates LCK, ZAP-70 and others |
| SHP-2 | PTPN11 | Positively regulates T-cell activation by increasing ERK activation; binds Gab2; may also inhibit T-cell activation through ITIM/ITSM receptors |
| TCPTP | PTPN2 | Negatively regulates T-cell activation by inhibiting IL-2 production |
| PTPH1 | PTPN3 | Inhibits TCR signalling when over-expressed by dephosphorylating immunoreceptor tyrosine-based activation motifs; dephosphorylates valosin-containing protein and interacts with TACE |
| PTP-MEG1 | PTPN4 | Inhibits T-cell activation when over-expressed |
| PTP-BAS | PTPN13 | Inhibits apoptosis by binding CD95/FAS; regulates cytokine secretion by inhibiting STAT proteins |
| PTP-MEG2 | PTPN9 | Promotes secretion – necessary for secretory vesicle formation |
| HePTP | PTPN7 | Negatively regulates TCR signalling by dephosphorylation of ERK and p38 |
| MKP-1 | DUSP1 | Negatively regulates T-cell activation by dephosphorylation of MAPKs in nucleus |
| PAC-1 | DUSP2 | Negatively regulates T-cell activation by dephosphorylation of p38 and ERK in nucleus |
| MKP-2 | DUSP4 | Negatively regulates IL-2 signalling and proliferation of CD4+ T cells through regulation of STAT5 phosphorylation |
| MKP-3 | DUSP6 | Negatively regulates T-cell activation by dephosphorylation of ERK in cytosol; may mediate TLR4-induced inhibition of TCR signalling |
| MKP-5 | DUSP10 | Negatively regulates T-cell activation by dephosphorylation of JNK in cytosol and nucleus |
| MKP-7 | DUSP16 | Regulates the balance between Th1/Th2 cells through dephosphorylation of JNK in cytosol |
| VHR | DUSP3 | Dephosphorylates p38, ERK and STAT5; promotes cell-cycle progression |
| PTEN | PTEN | Opposes PI3K activity by dephosphorylating PIP3; functions as tumour suppressor |
| LMPTP | ACP1 | Positively regulates signalling by dephosphorylation of inhibitory site of ZAP-70; may prevent TCR clustering by inhibiting cytoskeletal rearrangement through FAK |
ERK, extracellular signal-regulated kinase; IL-2, interleukin-2; ITIM/ITSM, immunoreceptor tyrosine-based inhibitory motifs/immunoreceptor tyrosine-based switch motifs; JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; STAT5, signal transducer and activator of transcription; TCR, T-cell receptor; Th1, T helper type 1; TLR4, Toll-like receptor 4.
Table 2.
T cell protein tyrosine phosphatases (PTPs) and autoimmunity
| PTP | Gene name | Autoimmune phenotype in mice | Autoimmune phenotype in humans |
|---|---|---|---|
| CD45 | PTPRC | SLE-like disease in mice with constitutively active CD45 | Genetic association with autoimmune hepatitis, myasthenia gravis and multiple sclerosis |
| LYP/Pep | PTPN22 | Genetic association with multiple autoimmune diseases, including T1D, rheumatoid arthritis, SLE, Graves’ disease, Hashimoto’s thyroiditis, myasthenia gravis, generalized vitiligo, Wegener’s granulomatosis | |
| PTP-PEST | PTPN12 | T-cell deletion reduces susceptibility to EAE | |
| SHP-1 | PTPN6 | me/me mice show systemic autoimmunity | |
| SHP-2 | PTPN11 | Located within linkage disequilibrium block that associates with coeliac disease, rheumatoid arthritis, T1D and Crohn’s disease | |
| TCPTP | PTPN2 | T-cell deletion leads to spontaneous autoimmunity | Genetic association with T1D, rheumatoid arthritis and coeliac disease |
| MKP-1 | DUSP1 | KO mice show delayed EAE development | |
| MKP-5 | DUSP10 | KO mice protected from EAE | |
| PTEN | PTEN | Autoimmunity in KO heterozygous mice | |
| LMPTP | ACP1 | Genetic association with T1D, Crohn’s disease and ulcerative colitis |
EAE, experimental autoimmune encephalitis; KO, knockout; SLE, systemic lupus erythematosus; T1D, type 1 diabetes.
Figure 2.
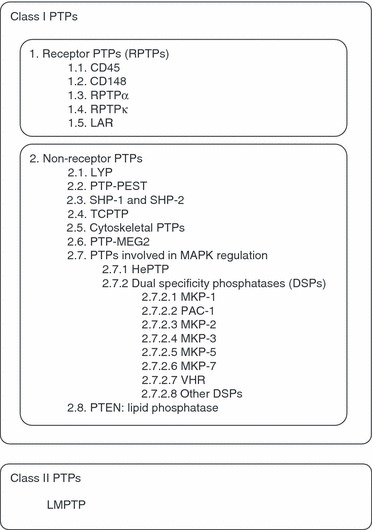
Classification scheme of the protein tyrosine phosphatases (PTPs) described in this review.
Class I enzymes
Receptor PTPs
CD45
CD45, encoded by the PTPRC gene, is a type 1 leucocyte-specific glycoprotein and a transmembrane PTP. CD45 is highly expressed in all nucleated haematopoietic cells and comprises about 10% of lymphocyte surface proteins.13 The protein structure consists of a large extracellular domain, a short transmembrane segment and a cytoplasmic portion containing two PTP domains called D1 and D2; only the membrane-proximal domain D1 has tyrosine phosphatase activity, and it is necessary for TCR-mediated signal transduction.14
The role of CD45 in T-cell activation has been intensely studied and excellent focused reviews are available.15–17 The best-characterized substrates of CD45 in T cells are the SFKs LCK and, to a lesser extent, FYN.18–21 The negative regulatory site on SFKs (Y505 of LCK) is a bona fide substrate of CD45 in T cells, and there is substantial evidence of CD45 being a positive regulator of TCR signalling through dephosphorylation of this site.22,23 CD45-deficient T-cell lines and thymocytes from CD45−/− mice exhibit increased phosphorylation of the inhibitory sites of LCK and FYN, and the thymic phenotype of CD45−/− mice (see below) is completely rescued by the expression of the constitutively active LCK Y505F mutant.18,19,21,24–26 However, there is in vitro and in vivo evidence that CD45 is also able to dephosphorylate the positive regulatory site of LCK (Y394), and data in CD45-deficient cell lines suggest that CD45 may also behave as a negative regulator of T-cell activation.27–30
Deficiency of CD45 in both humans and mice leads to a severe-combined immunodeficiency, supporting a major positive regulatory role for CD45 in T-cell activation.31–35 CD45-deficient mice, obtained by targeting exon 6,33 exon 935 or exon 12,34 exhibit a block in the double-positive to single-positive transition due to reduced signalling through the TCR. In CD45 knockout (KO) mice reconstituted with a titration of the CD45RO transgene, rescuing just 3% of the physiological CD45 expression was able to restore T-cell development.36 When CD45 expression was increased to 30% of wild-type levels, increased CD4 and CD8 single-positive expansion was observed, suggesting a key positive role for CD45 in positive selection. However, in this system, increased levels of CD45 expression led to reduced phosphorylation of both LCK Y505 and Y394 sites, supporting the idea that CD45 can regulate both of the LCK tyrosine phosphorylation sites. A model has been postulated where high CD45 expression in T cells may be necessary to maintain the LCK Y394 site in a dephophorylated state to terminate TCR signalling.36 Recently, a mouse with a CD45 ‘lightning’ mutation was generated, in which the surface expression of CD45 is low, but the expression of all the isoforms (see below) is maintained. The authors showed that CD45 is differently required during basal and inducible TCR signalling. Once again, CD45 was found to have dual negative and positive roles in the regulation of thymic selection.37
A well-known observation is that multiple, highly conserved isoforms of CD45 are expressed on T cells at different developmental and activation stages, as the result of differential splicing of exons 4, 5 and 6.13,38 Inclusion of exons 4, 5 or 6 is indicated by the presence of the letters A, B or C, respectively, in the isoform name. The most commonly observed are the larger isoform RB (which includes only exon 5), expressed on primary naive T cells, and the shortest isoform RO (which lacks all three exons), expressed in activated and memory T cells.39 The molecular basis of this complex isoform regulation is becoming clear and the heterogeneous nuclear ribonucleoprotein L-like protein (hnRNPLL) has been recently identified as a key modulator of the expression pattern of CD45 isoforms.40–42 On the other hand, the functional significance of the changes in CD45 isoform expression during T-cell differentiation/activation remains unexplained and several apparently contrasting observations have been reported. Early biochemical experiments showed that different isoforms of CD45 have similar PTP activity in vitro.18 Studies carried out in CD45−/− mice made transgenic for various isoforms of CD45 showed that rescue of thymic development and peripheral T-cell numbers/functions was dependent on expression levels of the transgene, but was generally isoform-independent.43–45 In contrast, CD45-deficient cell lines expressing the RO isoform were found to produce more interleukin-2 (IL-2) than cells expressing the RABC isoform after TCR engagement with MHC–peptide.46 Additional studies in transgenic CD45−/− mice also found differences in phenotypic rescue between high- and low-molecular-weight isoforms.47,48 Finally, in in vitro studies using mouse T cells, Seki et al.49 showed that CD45 expressed on CD8+ T cells was less active than CD45 expressed on CD4+ T cells and correlated this difference to the distinctive expression pattern of CD45 isoforms between CD8+ (primarily expressing CD45RBC) and CD4+ (primarily expressing the RO and RB isoforms) T cells.
The phosphatase activity of CD45 is believed to be physiologically inhibited by dimerization in trans involving a specific juxtamembrane ‘wedge’ motif. Strong evidence in favour of a role for CD45 in autoimmunity was provided by the Weiss group who described the phenotype of mice carrying an inactivating point mutation (CD45E613R) in the inhibitory wedge motif. These mice express a constitutively active form of CD45 and display an autoimmune syndrome resembling human systemic lupus erythematosus (SLE).50 Although thymic development was normal in these mice, double-positive thymocytes showed enhanced TCR signalling,51 confirming a positive role for CD45 in T-cell development. In contrast, TCR activation was down-regulated in peripheral T cells.
T cells from patients with SLE show decreased CD45 expression or phosphatase activity compared with healthy controls, and show abnormal patterns of CD45 phosphorylation and isoform expression.52–54 Altered CD45 isoform expression has been also associated with infantile cholestasis.55
A C77G polymorphism of CD45 abolishes the silencing of exon 4, causing an enhanced expression of the high-molecular-weight form of CD45 in all T-cell subpopulations.56,57 This single nucleotide polymorphism (SNP) was first associated with multiple sclerosis by Jacobsen et al.,58 but the association was not confirmed in subsequent studies.59–62 The SNP is not associated with type 1 diabetes (T1D),63,64 Graves’ disease,63 SLE,65 Hashimoto’s thyroiditis66 or myasthenia gravis.67 An association was found with autoimmune hepatitis,68 and another study reported an increased frequency of the SNP in systemic sclerosis.69 Another very rare polymorphism, C59A, causes aberrant splicing of PTPRC and was found in several members of a family with multiple sclerosis.58
CD148
CD148, encoded by the PTPRJ gene, is a ubiquitous transmembrane PTP. It is structurally characterized by an extracellular domain consisting of eight fibronectin type III domains with multiple glycosylation sites, a cytoplasmic domain with a juxtamembrane wedge motif, and a single PTP domain.70,71 Naive T cells exhibit low expression of CD148 and inducibly express the phosphatase after TCR stimulation.72 Some differences in expression patterns of CD148 between human and mouse T cells have been reported.73,74 A recent expression study confirmed that in mice, the expression of CD148 is high in double-negative thymocytes, drops significantly in single-positive thymocytes, and is nearly absent in peripheral blood T cells. In contrast, human thymocytes show an opposite pattern, with high expression in single-positive thymocytes and peripheral blood T cells.75 Over-expression studies in Jurkat cells indicate that CD148 inhibits phosphorylation of phospholipase Cγ1 and LAT, causing down-regulation of TCR signalling.76 In over-expression studies in CD45-negative JS-7 T cells, CD148 was also able to dephosphorylate the negative regulatory tyrosine residue of SFKs, suggesting that in certain circumstances, CD148 might be able to promote TCR signalling.75 Interestingly, the extracellular domain of CD148 mediates the exclusion of the phosphatase from the immunological synapse. After T-cell–antigen-presenting cell disengagement, the access of CD148 to its substrates is reconstituted, causing down-regulation of TCR signalling, suggesting a role for CD148 in tempering long-term signalling in T cells.77
RPTPα
RPTPα, encoded by the PTPRA gene, is a ubiquitous transmembrane PTP expressed at low levels in lymphoid tissues.78 Like CD45, RPTPα functions to dephosphorylate SFKs on their inhibitory tyrosine residue.79 However, studies have shown that CD45 and RPTPα are not redundant PTPs.80,81 RPTPα could not compensate for loss of CD45 in dephosphorylation and activation of LCK or FYN in a CD45-deficient T-cell line, and showed lower in vitro activity than CD45 on Src-derived phosphopeptides and on recombinant LCK.80 Unlike CD45, RPTPα KO mice show a benign immune phenotype.81 Resting thymocytes from RPTPα-deficient mice showed enhanced phosphorylation of FYN on both tyrosines 417 and 528, and increased FYN activity, indicating that RPTPα may act as a negative regulator of FYN in unstimulated thymocytes. After TCR stimulation, the same thymocytes showed no differences in tyrosine phosphorylation, but had impaired proliferation and IL-2 response. A recent study suggested that RPTPα is itself regulated by CD45 through dephosphorylation at Tyr789.
RPTPκ
RPTPκ, encoded by the PTPRK gene, is a ubiquitous transmembrane PTP whose expression is induced by transforming growth factor-β.82,83 An important role for RPTPκ in T-cell development was hypothesized following the discovery that Ptprk is deleted in the LEC rat, a model that displays a monogenic recessive immunodeficiency (called T helper immunodeficiency or Thid).84,85 The Thid phenotype is characterized by hypoplasia of the thymus and spleen, reduced levels of IgG, selective deficiency in CD4 single-positive T cells, and strongly reduced T helper function.86 The CD4 single-positive T-cell deficiency is attributed to anomalous development of T cells in the thymus, and was replicated in the mouse by bone marrow reconstitution of irradiated animals with double-negative cells transduced with dominant-negative RPTPκ.84 However the role of Ptprk in the Thid phenotype has been partially called into question after the discovery that the genomic deletion of the LEC rat also inactivates a neighbouring gene encoding Themis, a recently identified key regulator of thymic development.87In vitro, a reduction of extracellular signal-regulated kinase (ERK) phosphorylation has been described in LEC thymocytes and in T cells after knock-down of Ptprk; however, the biochemical basis of this phenomenon and the substrate of RPTPκ in T cells are unclear at the moment.88
LAR
LAR, a transmembrane PTP encoded by the PTPRF gene, is expressed in thymocytes and has been suggested to negatively regulate TCR signalling in thymocytes through dephosphorylation of LCK and FYN.89,90
Non-receptor PTPs
LYP
The lymphoid phosphatase (LYP) is a cytosolic PTP encoded by the PTPN22 gene.91 The structure of LYP includes an N-terminal PTP domain, an interdomain region, and a C-terminal domain that contains four proline-rich motifs, termed P1–P4.91,92 The P4 motif is located within a C-terminal homology domain (CTH) that is also present in the phosphatases PTP-PEST and BDP1.93 LYP and its mouse orthologue Pep are expressed only in haematopoietic cells.91,92
In T cells, LYP/Pep function as potent negative regulators of T-cell activation through inhibition of early signalling downstream of the TCR. Initial over-expression studies in cell lines revealed that Pep had an inhibitory role in TCR signalling by dephosphorylation of positive regulatory tyrosines on LCK, FYN and ZAP-70.94–96 Substrate trapping experiments later identified LCK (Y394), ZAP-70 (Y493), the CD3ζ chain, VAV, CD3ε, and valosin-containing protein as substrates of LYP in T cells.97 LYP/Pep are powerful inhibitors of TCR signalling in ex vivo over-expression and inhibition systems.98–103 The phenotype of the Ptpn22 knockout mouse further supports the view of Pep as a negative regulator of TCR signalling.104 These mice display increased positive selection, an expanded CD4+ and CD8+ effector/memory T-cell compartment, and hyper-responsiveness of effector/memory T cells to TCR engagement. Interestingly, a similar enhanced response to TCR engagement was not observed in naive T cells.
In T cells, numerous studies have shown that a high stoichiometry complex is formed between the P1 motif of LYP/Pep and the SH3 domain of CSK.94,95,99,103,105–107 Due to the known inhibitory role of CSK in regulating the SFKs, it has been proposed that the constitutive Pep–CSK complex functions to synergistically repress LCK and FYN activity through combined phosphorylation of the inhibitory tyrosine by CSK and concurrent dephosphorylation of the activating phosphotyrosine by LYP.95,96 However, recent studies from our group focused on the W620 variant of the human phosphatase, which lacks association with CSK (see below), suggest that CSK might behave as an inhibitor of the phosphatase activity as well. In the proposed model, CSK recruits LCK to LYP, which leads to phosphorylation of LYP on an inhibitory tyrosine residue Y536, and consequent reduction of the phosphatase activity. Hence the CSK–LYP complex is part of a positive LCK–LYP feedback loop, which might function to maintain LCK in an activated state after TCR stimulation and so sustain signalling through the TCR.103
Recent molecular and structural analyses have provided insight into additional modes of regulation of LYP. Crystal structure analysis revealed that the catalytic domain contains a LYP-specific loop.101,102,108 In this region a serine (S35) was identified that is phosphorylated by PKC, resulting in a decrease of catalytic activity.101 Another structure solved by our group showed that the catalytic cysteine (C227) forms a disulphide bond with an additional cysteine residue (C129), which can also regulate the activity through a reversible oxidation mechanism.108 LYP also seems to be regulated by intramolecular interactions between the catalytic domain and the proximal interdomain region, suggesting a possible mechanism of action of post-translational modifications outside the catalytic domain.109
The critical role of PTPs in immune homeostasis is exemplified in the discovery of the association of PTPN22 with multiple human autoimmune diseases. This was first documented in 2004 when an SNP (C1858T) in the PTPN22 gene was reported to increase the risk of T1D,105 rheumatoid arthritis99 and SLE.110 The association with T1D, rheumatoid arthritis and SLE has been confirmed in multiple populations (reviewed in ref. 111). The T1858 allele was also found to increase the risk of juvenile idiopathic arthritis,112,113 Graves’ disease,114–117 Hashimoto’s thyroiditis,118 Addison’s disease,115,119 myasthenia gravis,120–122 generalized vitiligo,123,124 systemic sclerosis,125 alopecia areata,126 psoriatic arthritis127,128 and Wegener’s granulomatosis.129 Interestingly, the PTPN22 C1858T allele is not associated with multiple sclerosis, coeliac disease, psoriasis and ulcerative colitis,130,131 and has a protective effect against Crohn’s disease and Behçet’s disease.132–134
The presence of the C1858T SNP causes a substitution from arginine to tryptophan at amino acid 620 (R620W), which impairs the binding of LYP to CSK. The functional effect of the substitution is still somehow controversial. Some observations from our group and others suggest that LYP-W620 is a gain-of-function form of the phosphatase.100 T cells from patients with T1D carrying the T1858 allele showed decreased T-cell activation as evidenced by reduced TCR-induced IL-2 secretion100,135 and decreased calcium mobilization after TCR engagement.136 Reduced TCR-mediated calcium mobilization and IL-10 secretion was also seen in memory T cells from healthy C/T carriers compared with homozygous C/C healthy subjects. However, this pattern was only seen in memory and not naive T cells. Consistent with the data from primary T cells, over-expression studies comparing the LYP-R620 and LYP-W620 variants showed that LYP-W620 is indeed a more potent inhibitor of TCR signalling and T-cell activation.100,103 These findings are in contrast to two recent reports claiming that the W620 variant is a hypomorphic allele.137,138 In one study also based on over-expression of the two phosphatase variants, Jurkat cells co-transfected with LYP-W620 and CSK were less responsive to TCR stimulation than cells co-transfected with CSK and LYP-R620.137 In another study, a mouse carrying the W619 knock-in mutation of Pep (the homologous site to LYP-W620) was characterized. T cells from these mice showed increased proliferation and phosphorylation of ERK in response to TCR engagement compared with WT mice.138 The authors found reduced expression of Pep as a result of increased calpain and proteasome-mediated cleavage in mice carrying the W619 allele. They also show reduced expression of LYP in homozygous carriers of the T1858 allele, and increased proliferation and phosphorylation of ERK in T cells of these individuals in response to TCR stimulation.
PTP-PEST
PTP-PEST, encoded by the PTPN12 gene, is a ubiquitous cytosolic PTP expressed in most non-haematopoietic and haematopoietic cell types.139–141 The structure of PTP-PEST is characterized by an N-terminal PTP domain, a central protein–protein interaction domain containing proline-rich motifs, and a conserved C-terminal tail.
Studies in non-immune cells have shown that PTP-PEST is involved in the regulation of cell migration and cytoskeletal reorganization by dephosphorylation of focal adhesion proteins, such as Cas142 and paxillin.143 In human and mouse naive CD4+ and CD8+ T cells, PTP-PEST expression is down-regulated after TCR activation.144 In T cells early studies showed that PTP-PEST regulates signalling proteins involved in TCR activation, such as Cas, Pyk2 and FAK and behaves as a negative regulator of TCR signalling.145 Studies in CD4+ T cells from TCR transgenic mice showed that over-expressed PTP-PEST inhibited immunological synapse formation, correlating with an impaired phosphorylation of WASP.146 Over-expression studies in Jurkat T cells and primary human T cells also pointed to a possible role of PTP-PEST as a negative regulator of TCR activation.144 However, the physiological role of PTP-PEST in T-cell function in mice could not be confirmed until recently because global KO of PTP-PEST in mice causes early embryonic lethality.147
The Veillette group recently generated a conditionally deleted allele of Ptpn12 in T cells and showed that PTP-PEST plays a critical role in secondary T-cell activation without altering T-cell development and primary T-cell response.148 The phenotype was attributed to increased phosphorylation of Pyk2 and correlated with a decrease in T-cell aggregation during secondary T-cell activation. Importantly, mice deficient in PTP-PEST in T cells were less susceptible to development of experimental autoimmune encephalitis (EAE) compared with wild-type mice, providing evidence that PTP-PEST may contribute to the development of autoimmune diseases through action at the T-cell level.
SHP-1 and SHP-2
Although structurally similar, the cytoplasmic SH2-domains containing phosphatases SHP-1 and SHP-2 are distinct PTPs, both in their expression profiles and in their function. SHP-1 is encoded by the PTPN6 gene. It is expressed in all haematopoietic lineages at all stages, and at lower levels in epithelial cells and in the olfactory neuroepithelium.92,149–151 Several isoforms have been reported and two distinct transcription initiation sites regulate PTPN6 expression in haematopoietic or non-haematopoietic tissues.152,153 SHP-2, on the other hand, is ubiquitously expressed.154,155
Both SHP-1 and SHP-2 are cytosolic PTPs comprised of two tandem N-terminal SH2 domains, a central catalytic PTP domain, and a C-terminal tail. They are about 60% homologous. Both are regulated by intramolecular folding, in which the N-terminal SH2 domain binds to the catalytic pocket of the PTP domain, preventing substrate binding and reducing the phosphatase activity.156–160 Engagement of this SH2 domain by substrate binding releases this inhibition and activates the phosphatases. The C-terminal region may also be involved in intramolecular regulation of SHP-1, as truncation of the C-terminus leads to increased in vitro activity.159,161 Both SHP-1 and SHP-2 are regulated by phosphorylation in the C-terminal region. Phosphorylation on Tyr536 and Tyr564 of SHP-1 and Tyr542 and Tyr580 of SHP-2 functions to regulate the activity of the phosphatase or provide docking sites for interactors containing SH2 domains, including Grb2.162–166
SHP-1 contains a C-terminal motif (SKHKED, amino acids 557–562) that mediates a constitutive localization of about 20–30% of SHP-1 to lipid rafts.167 The localization to lipid rafts appears to be essential for SHP-1-mediated inhibition of TCR signalling. Extensive literature is already available regarding the role of SHP-1 in the immune system, and the reader is referred to several excellent reviews.168–171 In T cells, SHP-1 acts as an inhibitor of TCR signal transduction and a regulator of the T-cell activation threshold.172,173 Thymocytes and mature peripheral T cells deficient in SHP-1 show increased responses to TCR stimulation, demonstrating increased activation of SFKs and other signalling intermediates, increased IL-2 production and increased proliferation.172,174,175 After TCR engagement, SHP-1 is activated by LCK-mediated phosphorylation and is recruited to the TCR complex, where it can dephosphorylate signalling molecules such as LCK, ZAP-70, PI3K, VAV, SLP-76 and CD3ζ.170,176,177 The inhibitory role of SHP-1 is dependent upon the strength of the TCR signal.177 Weaker, antagonistic signals cause rapid recruitment of SHP-1 to the TCR. There, in a negative feedback regulation loop, SHP-1 is phosphorylated on Tyr564 by LCK. This in turn promotes interaction between SHP-1 and the SH2 domain of LCK, and subsequent dephosphorylation and inactivation of LCK by SHP-1. On the other hand, stronger, agonistic TCR signals lead to ERK activation, followed by phosphorylation of LCK on Ser. This induces a conformational change in LCK, inhibiting binding of LCK to SHP-1 and the subsequent inactivation of LCK, leading to more sustained signalling through the TCR.177 In T-cell development, SHP-1 participates in setting the thresholds for both positive and negative selection of thymocytes.172,173,178,179 SHP-1 deficiency causes hyper-responsiveness of thymocytes to TCR stimulation, and leads to increased positive and negative selection. A more recent study shows that conditional knockout of SHP-1 in mature single-positive T cells limits the production of CD8+ effector T cells, but does not affect the formation of long-lived central memory cells.180
SHP-1 inhibits T-cell activation through additional mechanisms as well. SHP-1 is a downstream mediator of signalling through inhibitory receptors in immune cells. These receptors are characterized by motifs called immunoreceptor tyrosine-based inhibitory motifs (ITIMs) or immunoreceptor tyrosine-based switch motifs (ITSMs) in their intracellular region, which become phosphorylated on tyrosine and recruit SHP-1 through its SH2 domains. Examples of such receptors expressed in T cells are CEACAM1, CD5, PD-1, BTLA and CD22.181–187 SHP-1 also regulates signalling through cytokine receptors, for example, inhibiting the IL-2 receptor (IL-2R) signalling pathway by binding to the IL-2Rβ chain and reducing phosphorylation of the IL-2Rβ and the downstream Janus PTKs JAK1 and JAK3.188
The well-described phenotype of the motheaten mouse (me/me) has demonstrated the critical role of SHP-1 as a regulator of haemopoietic cell function.169,174,175,189 A splicing mutation at the SHP-1 locus leads to a frameshift in the coding sequence of the transcript, resulting in no expressed protein. me/me mice are characterized by systemic inflammation and autoimmunity, with developmental abnormalities in macrophages, granulocytes, T cells, B cells, natural killer cells, erythrocytes and mast cells.174 The phenotype of the motheaten mouse and several other studies have suggested that SHP-1 expression levels or activity can affect autoimmunity in humans and mice, however the mechanism of action of SHP-1 in autoimmunity is probably only partially mediated by an action on TCR signalling. A subset of patients with SLE exhibit lower expression levels of SHP-1 and CD45 in B cells.39 Another study showed that T cells from psoriatic skin lesions have lower expression of SHP-1, which correlates with increased sensitivity to interferon-α through increased activation of JAK and signal transducer and activator of transcription (STAT).190 Mice with partial inhibition of SHP-1 (mev+/−) show worsened MOG-peptide-induced EAE, which includes an increased T-cell response.191
SHP-2, encoded by the PTPN11 gene, is considered a key regulator of receptor-mediated signalling in many cell types. Indeed, the importance of SHP-2 is demonstrated by the embryonic lethality of mice with homozygous deletion of the PTP192 and the requirement of SHP-2 for lymphocyte development.193 In T cells, SHP-2 has generally been regarded as a positive regulator of signalling through the TCR. Conditional deletion of SHP-2 in T cells impairs thymocyte differentiation and proliferation, reduces the expansion of CD4+ T cells, and inhibits T-cell activation as evidenced by impaired TCR-induced ERK activation, proliferation, production of activation markers and production of IL-2.194 SHP-2 has also been suggested to inhibit T-cell adhesion by dephosphorylating ADAP and VAV1 proteins through association with the LAT-Gads-SLP-76 signalling complex.195 Aside from the TCR, SHP-2 also mediates cytokine receptor signalling. Upon interleukin receptor engagement, SHP-2 binds to the adaptor protein Gab2, an association that promotes the activation of ERK.196–198 In contrast to its generally regarded role as a potentiator of T-cell activation, SHP-2, like SHP-1, is also involved in inhibitory receptor signalling in T cells and other immune cells. In T cells, for example, SHP-2 has been found to interact with and mediate the inhibitory effect of ITIM-containing and ITSM-containing receptors such as PECAM-1,199 PD-1183 and BTLA.186,187 A genome-wide association meta-analysis showed the PTPN11 gene was included within a linkage disequilibrium block with shared association for coeliac disease and rheumatoid arthritis,131 and a genome-wide association analysis showed that PTPN11 was included in a linkage disequilibrium block with T1D association and with weak Crohn’s disease association;200 however, fine mapping and characterization of the responsible variants is still under investigation.
TCPTP
T-cell protein tyrosine phosphatase (TCPTP) is encoded by PTPN2, which recently emerged as a major autoimmunity gene. This PTP is ubiquitously expressed, with the highest expression found in haematopoietic and placental tissues.201 Two different TCPTP splice variants have been identified: a 45 000 molecular weight form, which is mostly localized in the nucleus201,202 and a 48 000 molecular weight form localized in the endoplasmic reticulum.201,203
The major TCPTP substrates in T cells have been identified as JAK1 and JAK3, and TCPTP has been shown to regulate downstream phosphorylation of STAT proteins, suggesting a role for this phosphatase in cytokine receptor signalling. TCPTP−/− T cells showed decreased phosphorylation of STAT5 in response to IL-2 stimulation and of STAT1 in response to interferon-α and interferon-γ stimulation.204 It has also been suggested that TCPTP dephosphorylates nuclear STAT1, which is dependent upon arginine methylation of the STAT1 protein.205
Some insights into the role of TCPTP in T-cell activation emerged from the characterization of the global TCPTP KO mouse. These mice die between the second and third weeks after birth and show significant splenomegaly, lymphadenopathy and thymic involution, with a reduction in CD4+ CD8+ thymocytes evident by 3 weeks of age.206 A defect in T-cell proliferation after anti-CD3 or concanavalin A stimulation was also described in these mice.206,207 However, no defects in early TCR signalling (calcium mobilization and CD3 induced tyrosine phosphorylation) were found.207 These findings correlated well with over-expression studies showing that TCPTP does not affect TCR-induced IL-2 gene activation,203 suggesting that TCPTP is not involved in early TCR signalling. In contrast, a recent study with mice carrying a conditional deletion of TCPTP in T cells suggested that TCPTP is a key regulator of early TCR signalling through dephosphorylation of the active regulatory site of the SFKs LCK and FYN.208 Notably, these mice showed spontaneous development of anti-nuclear antibodies and T-cell infiltration of the lungs and liver. Transfer of CD8+ T cells was able to replicate this autoimmune phenotype in syngeneic animals. The importance of this study lies in its support of the concept that a loss-of-function of TCPTP in T cells is sufficient to trigger autoimmunity.
The first association between PTPN2 and autoimmune disease was described in a genome-wide association study in which an SNP rs2542151 5·5 kb upstream of PTPN2 was found to be associated with coeliac disease, T1D and rheumatoid arthritis.200 These associations were replicated in other studies.209,210 A new follow-up analysis confirmed the association between the rs2542151 SNP of PTPN2 and T1D and found two new SNPs in the PTPN2 gene associated with T1D.211 A recent study showed that CD4+ T cells from healthy controls carrying the rs1893217 SNP showed reduced pSTAT phosphorylation in response to IL-2 stimulation, correlating with decreased PTPN2 RNA levels. This suggests that this genetic variant functionally alters the IL-2 signalling pathway in T cells, resulting in reduced expression of FoxP3.212 However, it is currently unclear how this finding can be reconciled with the known negative role of TCPTP in STAT5 activation and the recent finding that deletion of TCPTP in T cells leads to increased numbers of regulatory T cells in mice.208
Cytoskeletal PTPs
Four cytosolic PTPs – PTPH1, PTP-MEG1, PEZ and PTP-BAS contain a FERM domain (band 4.1–ezrin–radixin–moesin), and are referred to as the cytoskeletal PTPs.203 They are expressed in T cells and all lymphoid organs but their expression varies during development. PTPH1 and PEZ are more expressed early in development, whereas PTP-MEG1 expression is higher in more mature lymphoid cells.203 The four enzymes contain an N-terminal FERM domain, a central region, and a C-terminal PTP domain. Of these, PTPH1 and PTP-MEG1 contain a central PDZ (postsynaptic density-95-discs-large-ZO-1) domain, whereas PTP-BAS contains five central PDZ domains, as well as a very N-terminal putative kinase non-catalytic C-lobe domain (KIND). Both PTPH1 and PTP-MEG1 associate with the plasma membrane through their FERM domain.203 The function of PEZ (encoded by the PTPN14 gene) in T cells has not yet been defined but the data available for the other three PTPs are summarized below.
PTPH1, encoded by the PTPN3 gene, is expressed in a variety of tissues including haematopoietic, colorectal, gastric and hepatic tissues.213 PTPH1 has been considered a negative regulator of TCR signalling through dephosphorylation of the TCRζ chain.214,215 Over-expression of PTPH1 in Jurkat cells inhibited TCR-induced activation of MAPKs and activation of an IL-2 promoter.215 The FERM domain is required for this effect, because deletion of this region inhibited the localization of PTPH1 to the plasma membrane203 and its ability to inhibit TCR signalling.215 Additional substrates/interactors of PTPH1 have been proposed, suggesting that PTPH1 may have additional roles in the function of T cells and other cell types. PTPH1 dephosphorylates the valosin-containing protein, a hexameric ATPase, which has numerous functions, including regulation of the cell cycle and membrane vesicle fusion.216 Through its PDZ domain, PTPH1 binds to the C-terminal tail of the tumour necrosis factor α-convertase (TACE), a metalloprotease-disintegrin involved in ectodomain shedding of proteins.217 PTPH1 was also recently shown to dephosphorylate p38γ and promote Ras signalling.218
PTP-MEG1, encoded by the PTPN4 gene, was first cloned from a megakaryoblastic cell line and HUVEC cDNA libraries.219 PTP-MEG1 is expressed in most tissues, including lymphoid tissue220 and was shown to inhibit TCR-induced T-cell activation when over-expressed in Jurkat cells.215,221
PTP-BAS, encoded by the PTPN13 gene, is expressed in most tissues, and with a molecular weight of about 270 000 it is the largest non-receptor PTP.222 PTP-BAS has been ascribed multiple functions, among which are inhibition of apoptosis through regulation/inhibition of the cell surface expression of FAS/CD95.223 A study in CD4+ T cells showed that PTP-BAS regulates cytokine signalling through dephosphorylation and inhibition of STAT4 and STAT6 activation.224
Surprisingly, a recent study in KO mice failed to support a role for the cytoskeletal PTPs in TCR signalling. Mice lacking the PTP domain of PTPH1 or PTP-MEG1 showed no difference in T-cell development or TCR-mediated signal transduction,221,225 although the PTPH1 KO mice did exhibit enhanced growth due to increased growth hormone signalling.226 Double-deficient PTPN3/PTPN4 and triple-mutant mice that were null for PTPN3/PTPN4 and lacking the PTP domain of PTP-BAS mice also showed no alterations in T-cell development or TCR-induced cytokine production or proliferation.220
PTP-MEG2
PTP-MEG2, encoded by the PTPN9 gene, was originally cloned from a megakaryocyte cell line227 and is expressed in many cell types, including T cells. It is unique among the PTPs, containing an N-terminal lipid-binding domain with homology to cellular retinaldehyde-binding protein and yeast protein Sec14p with phosphatidylinositol transfer activity.227 Through this domain, PTP-MEG2 is found co-localized with PIP3228 on the cytoplasmic face of secretory vesicles and regulates secretory vesicle size and fusion via dephosphorylation of N-ethylmaleimide sensitive factor on an inhibitory Y83 residue.229 Through dephosphorylation and activation of N-ethylmaleimide sensitive factor, PTP-MEG2 promotes homotypic fusion of secretory vesicles. Over-expression of PTP-MEG2 in Jurkat cells was shown to cause enlargement of the size of secretory vesicles, which required the catalytic activity of the phosphatase.230 PTP-MEG2 binds to and is activated by PIP2, PIP3 and phosphatidylserine, providing a mechanism by which phosphorylation of inositides is coupled to downstream vesicle trafficking events.228,229,231 Characterization of PTP-MEG2 knockout mice confirmed the profound effect of PTP-MEG2 on vesicle formation.232 PTP-MEG2-deficient mice are embryonic lethal, however, PTP-MEG2 deficiency in haematopoietic cells was studied by transferring haematopoietic progenitors from fetal livers into irradiated Rag2−/− mice.232 T cells isolated from the recipient mice were defective in their secretion of IL-2 and several other cytokines, although the intracellular levels of IL-2 were unaffected. Electron microscopy analysis revealed that T cells from these mice have reduced numbers of mature secretory vesicles.
PTPs involved in regulation of MAPKs
There are three major subfamilies of MAPKs that are expressed in the immune system: ERK, p38 and Jun N-terminal kinase (JNK) (reviewed in 233–235). All contain a TxY motif in the activation loop of the kinase that can be phosphorylated on the threonine and the tyrosine. MAPKs are activated by phosphorylation on both residues. Their inactivation is mediated by dephosphorylation by three types of phosphatases – pSer/pThr phosphatases (which do not belong to the PTP family), and two types of PTPs, the pTyr-specific PTPs, and the dual-specific PTPs (DSPs). The DSPs can dephosphorylate pTyr, pSer or pThr and include a subclass of PTPs that contain a MAPK-binding domain. A second subclass of ‘atypical’ DSPs lacks this domain, but some atypical DSPs still function to dephosphorylate MAPKs. Although regulators of the MAPKs appear to be critical for proper function of T cells and other cell types, no associations between any of these phosphatases and human autoimmunity have been reported. This review will highlight some of the MAPK regulators involved in T-cell activation.
HePTP
HePTP, encoded by the PTPN7 gene, is a cytosolic PTP containing an N-terminal kinase interaction motif (KIM) and a PTP domain.236 Two other members of this sub-class are STEP and PTP-SL. All three of these PTPs dephosphorylate MAPKs on the activating phosphotyrosine residue.237,238 Of this family, only HePTP is expressed exclusively in haematopoietic cells, in all lineages,239,240 with high expression in T cells.241. HePTP is considered a negative regulator of T-cell activation through dephosphorylation of the pY in the activation loop of the MAPKs ERK and p38.238 This inhibitory action requires the association of HePTP through the KIM with ERK1, ERK2 and p38, and provides selectivity, as HePTP does not interact with JNK.238,241–243 In resting T cells, through its KIM, HePTP associates with the inactive forms of ERK and p38 in the cytosol.238,241 This complex is disrupted by phosphorylation of HePTP on S23 by PKA (in KIM)244,245 or on T45 and S72 by MAPKs/ERK outside the KIM.238 Upon TCR stimulation, phosphorylation of HePTP on S23 causes the MAPKs to dissociate. HePTP remains in cytosol, while ERK and p38 move to the nucleus.246 An additional regulation mechanism of HePTP during TCR signalling is through phosphorylation by PKCθ. HePTP translocates to the immune synapse upon TCR stimulation, where it is phosphorylated on S225 by PKCθ. This then targets HePTP to lipid rafts, where it inhibits TCR signalling.247 HePTP−/− mice have no T-cell development, differentiation, or functional phenotype, with the exception of increased TCR-induced ERK and p38 activation.243
Dual specificity phosphatases
MKP-1
MKP-1, encoded by the DUSP1 gene, is a nuclear DSP that predominantly dephosphorylates and inactivates p38 and JNK in response to stress.248 MKP-1 has been considered a negative regulator of the innate immune response.249 A study of the Mkp-1−/− mouse recently demonstrated the importance of MKP-1 in T-cell function.250 Mkp-1-deficient mice showed normal T-cell development; however, T cells from these mice showed increased activation of JNK coupled with reduced expression of NFATc1. Both CD4+ and CD8+ T cells showed reduced IL-2 production and proliferation after TCR engagement. T cells from KO mice also demonstrated increased TCR-induced activation-induced cell death. Mkp-1 deficiency impaired effector functions of T helper type 1 (Th1), Th17 and CD8+ T cells, but not Th2 cells. Consistent with their reduced T-cell function, Mkp-1−/− mice exhibited reduced antigen-specific T-cell responses in vivo. These mice exhibited defective viral clearance when challenged with influenza virus infection, and also delayed autoimmune development in the EAE model, possibly as a result of decreased CD4+ T-cell function.
PAC-1
PAC-1, encoded by the DUSP2 gene, is a nuclear DSP cloned from human T cells. It is primarily expressed in haematopoietic cells251 and its expression is induced in activated leucocytes.252,253In vitro and over-expression studies showed that PAC-1 dephosphorylates and inactivates p38 and ERK.254,255 Hence PAC-1 is believed to be the nuclear counterpart of HePTP and leads to nuclear inactivation of MAPKs, followed by their return to the cytosol, and reassociation with HePTP.246 Unexpectedly, PAC-1-deficient mice show normal T-cell compartments.253 However, studies in these mice showed that PAC-1 rather promotes inflammatory signalling in myeloid cells by suppressing the activation of JNK while increasing the activation of p38 and ERK1/2.
MKP-2
MKP-2, encoded by the DUSP4 gene, is a nuclear DSP, expressed in both haematopoietic and non-haematopoietic tissues, which dephosphorylates ERK and JNK.255 A role for MKP-2 in IL-2 signalling and CD4+ T-cell proliferation was recently proposed by a study of Dusp4-deficient mice. CD4+ T cells from these mice exhibited increased STAT5 phosphorylation, resulting in elevated CD25 expression and IL-2 signalling, and hyperproliferation of CD4+ T cells.256
MKP-3
A role for the cytosolic MKP-3, encoded by the DUSP6 gene, in setting the threshold for thymocyte-positive selection has been proposed.257 Retroviral expression of Mkp-3 decreased ERK and JNK activation in T cells in vitro, while expression of a dominant-negative form of Mkp-3 increased their activation. In the same study, transduction of bone marrow cells with a construct encoding dominant-negative Mkp-3, followed by transfer of these cells into irradiated mice, resulted in increased positive selection of resulting thymocytes. MKP-3 may also function as a regulator of cross-talk between TLR4 and TCR signalling in CD4+ T cells, mediating an inhibitory effect of TLR4 signalling on subsequent TCR signalling by inhibition of ERK1/2 activation.258
MKP-5
MKP-5, encoded by the DUSP10 gene, is constitutively expressed in naive CD4+ T cells and is down-regulated by TCR stimulation.259 The role of this DSP in T cells has been studied in mice lacking Mkp-5.259 JNK, but not p38, was hyperactive in Th1 and Th2 cells from these mice. Naive CD4+ T cells from these mice exhibited reduced proliferation upon TCR stimulation, however Th1, Th2 and CD8+ effector T cells produced increased levels of cytokines after stimulation. These mice were protected from development of EAE, possibly through decreased T-cell proliferation.
MKP-7
Recent studies of MKP-7, encoded by the DUSP16 gene, suggest that this DSP is involved in T helper cell differentiation.260 MKP-7 shuttles between the nucleus and cytosol and preferentially dephosphorylates and inactivates JNK.261 A recent study in mouse T cells showed that Mkp-7 is expressed in CD4+ T cells, with lower expression in naive cells, increased expression in in vitro differentiated Th2 cells, and nearly absent expression in in vitro differentiated Th1 cells.260 Several data support the concept that MKP-7 regulates the balance between Th1/Th2 cells through dephosphorylation of JNK. Overexpression of Mkp-7 in vitro enhanced Th2 differentiation, as evidenced by mRNA production of GATA-3 and IL-4, while causing only modest changes in Th1 differentiation, as shown by mRNA production of interferon-γ. CD4+ T cells from transgenic mice over-expressing the active form of the phosphatase showed enhanced Th2, but not Th1, differentiation, while over-expression of inactive dominant negative Mkp-7 impaired Th2 differentiation. The authors of this study also demonstrated that upon immunization with OVA, transgenic mice over-expressing active Mkp-7 also displayed Th2-skewed production of OVA-specific IgG2a, IgG1 and IgE.260
VHR
VHR, encoded by the DUSP3 gene, is an atypical DSP constitutively expressed in central and peripheral lymphoid organs.262 VHR specifically inactivates ERK2 and JNK by dephosphorylation of the pTyr in the MAPK activation loop. Through this regulation of ERK2 and JNK, VHR acts as an inhibitor of T-cell activation.263 VHR is recruited to the immune synapse upon TCR engagement, where it is phosphorylated on tyrosine by ZAP-70, a modification that is required for the inhibition of ERK2 and JNK. VHR also has additional roles in T cells and other cell types. It is required for cell cycle progression through dephosphorylation of ERK and JNK, and unlike many other DSPs, its expression is regulated by the cell cycle rather than by TCR or mitogenic stimuli.264 VHR has also been recently reported to dephosphorylate STAT5.265
Other DSPs
Other DSPs have been implicated in the negative regulation of T-cell activation, for example MKP-6 (encoded by the DUSP14 gene) has been shown to bind to CD28 in T cells and inhibit CD28 co-stimulation.266 VHX (encoded by the DUSP22 gene) was shown to inhibit activation of ERK2 and downstream NFAT/AP-1 reporter activity when over-expressed in Jurkat cells.267
PTEN: a lipid phosphatase
PTEN (phosphatase and tensin homologue), a ubiquitous phosphoinositide lipid phosphatase, is a unique member of the PTP family. By dephosphorylating PtdIns(3,4,5)P3 (PIP3), PTEN acts as an antagonist to the activity of phosphoinositide 3-kinase (PI3K).268 PTEN is a well-known tumour suppressor that is involved in the regulation of T-cell function. Stimulation of the TCR, co-stimulatory molecules, or cytokine receptors of T cells activates PI3K, resulting in the production of PIP3, a lipid second messenger critical for the propagation of downstream signal transduction. PTEN dephosphorylates and regulates the levels of PIP3, controlling the strength and duration of signalling and activation of downstream pathways. PTEN effectively suppresses multiple T-cell functions, including cell cycle progression, adhesion, migration and survival.269,270 Knockout of Pten causes embryonic lethality, and the partial deficiency of Pten in heterozygous mice leads to a lethal autoimmunity associated with reduced Fas-mediated apoptosis.271,272 Conditional KO of Pten in T cells causes lymphadenopathy, splenomegaly, thymic enlargement, T-cell lymphoma, T-cell hyperproliferation, production of autoreactive T cells, impaired apoptosis, increased phosphorylation of ERK and AKT, and increased cytokine production.269
Class II enzymes: the LMPTP
The ubiquitously expressed low-molecular-weight PTP (LMPTP) is encoded by the ACP1 gene. Two major isoforms have been isolated, called LMPTP-A (also called the Fast isoform, or ACP1-F) and LMPTP-B (also called the Slow isoform, or ACP1-S) arising from a splicing event in which either exon 3 or exon 4 is excised. The ACP1 gene has a well-known polymorphism with three common codominant alleles, called *A, *B, and *C (reviewed in refs 273–275). These alleles affect both the total enzymatic activity and the ratio between isoforms A and B.276,277
LMPTP is involved in the regulation of growth factor signalling through dephosphorylation of a variety of growth factor receptors, which include platelet-derived growth factor receptor (PDGFR),275,278 fibroblast growth factor receptor (FGFR),279 insulin receptor (IR)280 and ephrin receptor.281 In T cells, LMPTP plays a positive regulatory role in TCR signalling through dephosphorylation of ZAP-70 on the negative regulatory tyrosine Y292.282 Phosphorylation on this site provides a binding site for the c-Cbl ubiquitin ligase complex that inhibits TCR signalling by dephosphorylation and inactivation of ZAP-70 and through internalization of the TCR. The dephosphorylation of ZAP-70 by LMPTP consequently prolongs signal transduction through the TCR. Additionally, LMPTP may regulate T-cell cytoskeletal reorganization through dephosphorylation of FAK, which participates in regulating cytoskeletal rearrangement.283 LMPTP was shown to dephosphorylate and inhibit FAK, which led to impairment of LFA-1-dependent T-cell adhesion and LFA-1 and TCR co-clustering. LMPTP may therefore control T-cell activation by preventing the cytoskeletal reorganization needed for LFA-1 and TCR clustering. The authors of this study propose a model where LMPTP enhances TCR signalling in the initial phases by dephosphorylation of ZAP-70, and then subsequently tempers signalling by reducing the cytoskeletal rearrangements needed for movement of membrane-associated signalling machinery.
The activity of LMPTP in T cells is enhanced by phosphorylation by the SFKs LCK and FYN on Y131. Y132 is also phosphorylated to a lesser extent.284,285 Studies in other cell types have shown that phosphorylation of LMPTP on Y132 has no effect on the catalytic activity, and instead provides a docking site for the recruitment of Grb2, which promotes ERK activation, suggesting that LMPTP may regulate ERK activity in T cells as well.286
The ACP1 polymorphism is associated with numerous disorders including cardiovascular, metabolic, neurological and autoimmune diseases.274 Among autoimmune diseases, ACP1 associates with inflammatory bowel diseases and T1D. Some studies suggest that ACP1 may influence Th1/Th2 orientation, in a gender-dependent manner.287 The ACP1*A allele, which is associated with low LMPTP activity, makes females more susceptible to allergy (a Th2-mediated disorder), and males more susceptible to T1D and Crohn’s disease (a Th1-mediated disorder).288 Genotypes leading to high expression of LMPTP-A are positively associated with Crohn’s disease in females and ulcerative colitis in males.287 Additionally, ACP1 genotype appears to influence the clinical manifestation of T1D.289 Females with medium-high activity genotypes have earlier age of onset of T1D, while low activity genotypes are associated with higher glycaemic levels at initial diagnosis, and increase susceptibility to T1D in offspring of older mothers.290
Future directions
In conclusion, a large amount of data support the importance of PTPs in regulation of TCR signalling. Although the emphasis is still often on single enzymes and single substrates, systems biology and proteomics are increasingly applied to the study of phosphorylation networks and PTPs. Important difficult-to-address issues which benefit from a systemic approach include the frequent redundancy between PTPs and the possible pleiotropic actions of single PTPs at several levels of a signalling pathway. Our knowledge about post-translational regulation of PTP activity also has progressed tremendously in the last few years, and in the future increasing emphasis is expected on dynamic or even real-time monitoring of PTP activity during signalling. Finally, of the large number of PTPs that are bona fide regulators of TCR signalling, only a subset has been investigated for a possible role in autoimmunity. As this subset continues to expand, we predict that an increasing number of PTPs will be identified as important autoimmunity genes, biomarkers, or drug targets.
Acknowledgments
This work was supported by National Institutes of Health grant R01AI070544 to N.B. The authors are deeply grateful to Dr. Massimo Bottini for help with image preparation. This manuscript is #1505 from La Jolla Institute for Allergy and Immunology.
Disclosures
The authors declare having no conflicts of interest to disclose.
References
- 1.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acuto O, Di Bartolo V, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 4.Weiss A. The right team at the right time to go for a home run: tyrosine kinase activation by the TCR. Nat Immunol. 2010;11:101–4. doi: 10.1038/ni0210-101. [DOI] [PubMed] [Google Scholar]
- 5.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–9. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 6.Alonso A, Sasin J, Bottini N, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, Tonks NK, Moller NP. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004;18:8–30. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- 8.Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- 9.Arimura Y, Yagi J. Comprehensive expression profiles of genes for protein tyrosine phosphatases in immune cells. Sci Signal. 2010;3:rs1. doi: 10.1126/scisignal.2000966. [DOI] [PubMed] [Google Scholar]
- 10.Zikherman J, Weiss A. Antigen receptor signaling in the rheumatic diseases. Arthritis Res Ther. 2009;11:202. doi: 10.1186/ar2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Tanaka S, Tanaka A, Ito Y, Maeda S, Sakaguchi N, Hashimoto M. Thymus, innate immunity and autoimmune arthritis: interplay of gene and environment. FEBS Lett. 2011;585:3633–9. doi: 10.1016/j.febslet.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Gregersen PK, Behrens TW. Genetics of autoimmune diseases – disorders of immune homeostasis. Nat Rev Genet. 2006;7:917–28. doi: 10.1038/nrg1944. [DOI] [PubMed] [Google Scholar]
- 13.Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–69. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 14.Desai DM, Sap J, Silvennoinen O, Schlessinger J, Weiss A. The catalytic activity of the CD45 membrane-proximal phosphatase domain is required for TCR signaling and regulation. EMBO J. 1994;13:4002–10. doi: 10.1002/j.1460-2075.1994.tb06716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 16.Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010;22:339–48. doi: 10.1016/j.cellsig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Irie-Sasaki J, Sasaki T, Penninger JM. CD45 regulated signaling pathways. Curr Top Med Chem. 2003;3:783–96. doi: 10.2174/1568026033452339. [DOI] [PubMed] [Google Scholar]
- 18.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989;86:8959–63. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustelin T, Coggeshall KM, Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989;86:6302–6. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustelin T, Pessa-Morikawa T, Autero M, Gassmann M, Andersson LC, Gahmberg CG, Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992;22:1173–8. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- 21.Hurley TR, Hyman R, Sefton BM. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993;13:1651–6. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seavitt JR, White LS, Murphy KM, Loh DY, Perlmutter RM, Thomas ML. Expression of the p56(Lck) Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol Cell Biol. 1999;19:4200–8. doi: 10.1128/mcb.19.6.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pingel S, Baker M, Turner M, Holmes N, Alexander DR. The CD45 tyrosine phosphatase regulates CD3-induced signal transduction and T cell development in recombinase-deficient mice: restoration of pre-TCR function by active p56(lck) Eur J Immunol. 1999;29:2376–84. doi: 10.1002/(SICI)1521-4141(199908)29:08<2376::AID-IMMU2376>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Cahir McFarland ED, Hurley TR, Pingel JT, Sefton BM, Shaw A, Thomas ML. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993;90:1402–6. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiroo M, Goff L, Biffen M, Shivnan E, Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992;11:4887–97. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone JD, Conroy LA, Byth KF, Hederer RA, Howlett S, Takemoto Y, Holmes N, Alexander DR. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ξ, and ZAP-70. J Immunol. 1997;158:5773–82. [PubMed] [Google Scholar]
- 27.D’Oro U, Ashwell JD. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–83. [PubMed] [Google Scholar]
- 28.Burns CM, Sakaguchi K, Appella E, Ashwell JD. CD45 regulation of tyrosine phosphorylation and enzyme activity of src family kinases. J Biol Chem. 1994;269:13594–600. [PubMed] [Google Scholar]
- 29.D’Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker M, Gamble J, Tooze R, et al. Development of T-leukaemias in CD45 tyrosine phosphatase-deficient mutant lck mice. EMBO J. 2000;19:4644–54. doi: 10.1093/emboj/19.17.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kung C, Pingel JT, Heikinheimo M, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–5. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 32.Tchilian EZ, Wallace DL, Wells RS, Flower DR, Morgan G, Beverley PC. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001;166:1308–13. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 33.Kishihara K, Penninger J, Wallace VA, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–56. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 34.Mee PJ, Turner M, Basson MA, Costello PS, Zamoyska R, Tybulewicz VL. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur J Immunol. 1999;29:2923–33. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Byth KF, Conroy LA, Howlett S, Smith AJ, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183:1707–18. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeill L, Salmond RJ, Cooper JC, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–37. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, Weiss A. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–54. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 39.Vang T, Miletic AV, Arimura Y, Tautz L, Rickert RC, Mustelin T. Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol. 2008;26:29–55. doi: 10.1146/annurev.immunol.26.021607.090418. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Jia X, de la Cruz L, et al. Memory T cell RNA rearrangement programmed by heterogeneous nuclear ribonucleoprotein hnRNPLL. Immunity. 2008;29:863–75. doi: 10.1016/j.immuni.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberdoerffer S, Moita LF, Neems D, Freitas RP, Hacohen N, Rao A. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science (New York, NY. 2008;321:686–91. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topp JD, Jackson J, Melton AA, Lynch KW. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA. 2008;14:2038–49. doi: 10.1261/rna.1212008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kozieradzki I, Kundig T, Kishihara K, et al. T cell development in mice expressing splice variants of the protein tyrosine phosphatase CD45. J Immunol. 1997;158:3130–9. [PubMed] [Google Scholar]
- 44.Ogilvy S, Louis-Dit-Sully C, Cooper J, Cassady RL, Alexander DR, Holmes N. Either of the CD45RB and CD45RO isoforms are effective in restoring T cell, but not B cell, development and function in CD45-null mice. J Immunol. 2003;171:1792–800. doi: 10.4049/jimmunol.171.4.1792. [DOI] [PubMed] [Google Scholar]
- 45.Salmond RJ, McNeill L, Holmes N, Alexander DR. CD4+ T cell hyper-responsiveness in CD45 transgenic mice is independent of isoform. Int Immunol. 2008;20:819–27. doi: 10.1093/intimm/dxn040. [DOI] [PubMed] [Google Scholar]
- 46.Novak TJ, Farber D, Leitenberg D, Hong SC, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–19. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 47.Tchilian EZ, Dawes R, Hyland L, et al. Altered CD45 isoform expression affects lymphocyte function in CD45 Tg mice. Int Immunol. 2004;16:1323–32. doi: 10.1093/intimm/dxh135. [DOI] [PubMed] [Google Scholar]
- 48.Dawes R, Petrova S, Liu Z, Wraith D, Beverley PC, Tchilian EZ. Combinations of CD45 isoforms are crucial for immune function and disease. J Immunol. 2006;176:3417–25. doi: 10.4049/jimmunol.176.6.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seki I, Suzuki M, Miyasaka N, Kohsaka H. Expression of CD45 isoforms correlates with differential proliferative responses of peripheral CD4+ and CD8+ T cells. Immunol Lett. 2010;129:39–46. doi: 10.1016/j.imlet.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Majeti R, Xu Z, Parslow TG, Olson JL, Daikh DI, Killeen N, Weiss A. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103:1059–70. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 51.Hermiston ML, Zikherman J, Tan AL, et al. Differential impact of the CD45 juxtamembrane wedge on central and peripheral T cell receptor responses. Proc Natl Acad Sci U S A. 2009;106:546–51. doi: 10.1073/pnas.0811647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeuchi T, Pang M, Amano K, Koide J, Abe T. Reduced protein tyrosine phosphatase (PTPase) activity of CD45 on peripheral blood lymphocytes in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1997;109:20–6. doi: 10.1046/j.1365-2249.1997.4371334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blasini AM, Alonzo E, Chacon R, Riera R, Stekman IL, Rodriguez MA. Abnormal pattern of tyrosine phosphorylation in unstimulated peripheral blood T lymphocytes from patients with systemic lupus erythematosus. Lupus. 1998;7:515–23. doi: 10.1191/096120398678920604. [DOI] [PubMed] [Google Scholar]
- 54.Neidhart M, Pataki F, Michel BA, Fehr K. CD45 isoforms expression on CD4+ and CD8+ peripheral blood T-lymphocytes is related to auto-immune processes and hematological manifestations in systemic lupus erythematosus. Schweiz Med Wochenschr. 1996;126:1922–5. [PubMed] [Google Scholar]
- 55.Socha P, Michalkiewicz J, Stachowski J, Pawlowska J, Jankowska I, Barth C, Socha J, Madalinski K. Deficiency of the expression of CD45RA isoform of CD45 common leukocyte antigen in CD4+ T lymphocytes in children with infantile cholestasis. Immunol Lett. 2001;75:179–84. doi: 10.1016/s0165-2478(00)00305-9. [DOI] [PubMed] [Google Scholar]
- 56.Thude H, Hundrieser J, Wonigeit K, Schwinzer R. A point mutation in the human CD45 gene associated with defective splicing of exon A. Eur J Immunol. 1995;25:2101–6. doi: 10.1002/eji.1830250745. [DOI] [PubMed] [Google Scholar]
- 57.Lynch KW, Weiss A. A CD45 polymorphism associated with multiple sclerosis disrupts an exonic splicing silencer. J Biol Chem. 2001;276:24341–7. doi: 10.1074/jbc.M102175200. [DOI] [PubMed] [Google Scholar]
- 58.Jacobsen M, Schweer D, Ziegler A, et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat Genet. 2000;26:495–9. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 59.Barcellos LF, Caillier S, Dragone L, et al. PTPRC (CD45) is not associated with the development of multiple sclerosis in U.S. patients. Nat Genet. 2001;29:23–4. doi: 10.1038/ng722. [DOI] [PubMed] [Google Scholar]
- 60.Vorechovsky I, Kralovicova J, Tchilian E, et al. Does 77C→G in PTPRC modify autoimmune disorders linked to the major histocompatibility locus? Nat Genet. 2001;29:22–3. doi: 10.1038/ng723. [DOI] [PubMed] [Google Scholar]
- 61.Gomez-Lira M, Liguori M, Magnani C, et al. CD45 and multiple sclerosis: the exon 4 C77G polymorphism (additional studies and meta-analysis) and new markers. J Neuroimmunol. 2003;140:216–21. doi: 10.1016/s0165-5728(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 62.Szvetko AL, Jones A, Mackenzie J, Tajouri L, Csurhes PA, Greer JM, Pender MP, Griffiths LR. An investigation of the C77G and C772T variations within the human protein tyrosine phosphatase receptor type C gene for association with multiple sclerosis in an Australian population. Brain Res. 2009;1255:148–52. doi: 10.1016/j.brainres.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 63.Wood JP, Bieda K, Segni M, Herwig J, Krause M, Usadel KH, Badenhoop K. CD45 exon 4 point mutation does not confer susceptibility to type 1 diabetes mellitus or Graves’ disease. Eur J Immunogenet. 2002;29:73–4. doi: 10.1046/j.1365-2370.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 64.Thude H, Rosenhahn S, Hunger-Dathe W, Muller UA, Barz D. A transmembrane protein-tyrosine phosphatase receptor type C (CD45) exon A point mutation (77 C to G) is not associated with the development of type 1 diabetes mellitus in a German population. Eur J Immunogenet. 2004;31:245–7. doi: 10.1111/j.1365-2370.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 65.Johanneson B, Lima G, von Salome J, Alarcon-Segovia D, Alarcon-Riquelme ME. A major susceptibility locus for systemic lupus erythemathosus maps to chromosome 1q31. Am J Hum Genet. 2002;71:1060–71. doi: 10.1086/344289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thude H, Weissenborn S, Vilser C, Muller UA, Kloos C, Wolf G, Beck J, Barz D. No association between transmembrane protein-tyrosine-phosphatase receptor type C (CD45) exon A 77C>G transversion and Hashimoto’s thyroiditis in a German population. Hum Immunol. 2010;71:220–3. doi: 10.1016/j.humimm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Ramanujam R, Pirskanen R, Hammarstrom L. The CD45 77C/G allele is not associated with myasthenia gravis – a reassessment of the potential role of CD45 in autoimmunity. BMC Res Notes. 2010;3:292. doi: 10.1186/1756-0500-3-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogel A, Strassburg CP, Manns MP. 77 C/G mutation in the tyrosine phosphatase CD45 gene and autoimmune hepatitis: evidence for a genetic link. Genes Immun. 2003;4:79–81. doi: 10.1038/sj.gene.6363918. [DOI] [PubMed] [Google Scholar]
- 69.Schwinzer R, Witte T, Hundrieser J, et al. Enhanced frequency of a PTPRC (CD45) exon A mutation (77C>G) in systemic sclerosis. Genes Immun. 2003;4:168–9. doi: 10.1038/sj.gene.6363894. [DOI] [PubMed] [Google Scholar]
- 70.Gaya A, Pirotto F, Palou E, Autschbach F, Del Pozo V, Sole J, Serra-Pages C. CD148, a new membrane tyrosine phosphatase involved in leukocyte function. Leuk Lymphoma. 1999;35:237–43. doi: 10.3109/10428199909145726. [DOI] [PubMed] [Google Scholar]
- 71.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin J, Zhu JW, Baker JE, Weiss A. Regulated expression of the receptor-like tyrosine phosphatase CD148 on hemopoietic cells. J Immunol. 2004;173:2324–30. doi: 10.4049/jimmunol.173.4.2324. [DOI] [PubMed] [Google Scholar]
- 73.Autschbach F, Palou E, Mechtersheimer G, et al. Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue Antigens. 1999;54:485–98. doi: 10.1034/j.1399-0039.1999.540506.x. [DOI] [PubMed] [Google Scholar]
- 74.Tangye SG, Phillips JH, Lanier LL, de Vries JE, Aversa G. CD148: a receptor-type protein tyrosine phosphatase involved in the regulation of human T cell activation. J Immunol. 1998;161:3249–55. [PubMed] [Google Scholar]
- 75.Stepanek O, Kalina T, Draber P, et al. Regulation of Src family kinases involved in T cell receptor signaling by protein-tyrosine phosphatase CD148. J Biol Chem. 2011;286:22101–12. doi: 10.1074/jbc.M110.196733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cγ1 phosphorylation. Mol Cell Biol. 2001;21:2393–403. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin J, Weiss A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J Cell Biol. 2003;162:673–82. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sap J, D’Eustachio P, Givol D, Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci U S A. 1990;87:6112–6. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su J, Muranjan M, Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol. 1999;9:505–11. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 80.Ng DH, Jabali MD, Maiti A, et al. CD45 and RPTPalpha display different protein tyrosine phosphatase activities in T lymphocytes. Biochem J. 1997;327(Pt 3):867–76. doi: 10.1042/bj3270867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maksumova L, Le HT, Muratkhodjaev F, Davidson D, Veillette A, Pallen CJ. Protein tyrosine phosphatase alpha regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. J Immunol. 2005;175:7947–56. doi: 10.4049/jimmunol.175.12.7947. [DOI] [PubMed] [Google Scholar]
- 82.Yang Y, Gil M, Byun SM, Choi I, Pyun KH, Ha H. Transforming growth factor-β1 inhibits human keratinocyte proliferation by upregulation of a receptor-type tyrosine phosphatase R-PTP-κ gene expression. Biochem Biophys Res Commun. 1996;228:807–12. doi: 10.1006/bbrc.1996.1736. [DOI] [PubMed] [Google Scholar]
- 83.Wang SE, Wu FY, Shin I, Qu S, Arteaga CL. Transforming growth factor β (TGF-β)-Smad target gene protein tyrosine phosphatase receptor type κ is required for TGF-β function. Mol Cell Biol. 2005;25:4703–15. doi: 10.1128/MCB.25.11.4703-4715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asano A, Tsubomatsu K, Jung CG, Sasaki N, Agui T. A deletion mutation of the protein tyrosine phosphatase κ (Ptprk) gene is responsible for T-helper immunodeficiency (thid) in the LEC rat. Mamm Genome. 2007;18:779–86. doi: 10.1007/s00335-007-9062-0. [DOI] [PubMed] [Google Scholar]
- 85.Kose H, Sakai T, Tsukumo S, Wei K, Yamada T, Yasutomo K, Matsumoto K. Maturational arrest of thymocyte development is caused by a deletion in the receptor-like protein tyrosine phosphatase κ gene in LEC rats. Genomics. 2007;89:673–7. doi: 10.1016/j.ygeno.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Agui T, Oka M, Yamada T, Sakai T, Izumi K, Ishida Y, Himeno K, Matsumoto K. Maturational arrest from CD4+8+ to CD4+8− thymocytes in a mutant strain (LEC) of rat. J Exp Med. 1990;172:1615–24. doi: 10.1084/jem.172.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwata R, Sasaki N, Agui T. Contiguous gene deletion of Ptprk and Themis causes T-helper immunodeficiency (thid) in the LEC rat. Biomed Res. 2010;31:83–7. doi: 10.2220/biomedres.31.83. [DOI] [PubMed] [Google Scholar]
- 88.Erdenebayar N, Maekawa Y, Nishida J, Kitamura A, Yasutomo K. Protein-tyrosine phosphatase-κ regulates CD4+ T cell development through ERK1/2-mediated signaling. Biochem Biophys Res Commun. 2009;390:489–93. doi: 10.1016/j.bbrc.2009.09.117. [DOI] [PubMed] [Google Scholar]
- 89.Tsujikawa K, Ichijo T, Moriyama K, et al. Regulation of Lck and Fyn tyrosine kinase activities by transmembrane protein tyrosine phosphatase leukocyte common antigen-related molecule. Mol Cancer Res. 2002;1:155–63. [PubMed] [Google Scholar]
- 90.Kondo S, Kishi H, Muraguchi A. Regulatory role of leukocyte-common-antigen-related molecule (LAR) in thymocyte differentiation. Eur J Immunol. 2010;40:1296–302. doi: 10.1002/eji.200939743. [DOI] [PubMed] [Google Scholar]
- 91.Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood. 1999;93:2013–24. [PubMed] [Google Scholar]
- 92.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veillette A, Rhee I, Souza CM, Davidson D. PEST family phosphatases in immunity, autoimmunity, and autoinflammatory disorders. Immunol Rev. 2009;228:312–24. doi: 10.1111/j.1600-065X.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 94.Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–18. [PMC free article] [PubMed] [Google Scholar]
- 95.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–21. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gjorloff-Wingren A, Saxena M, Williams S, Hammi D, Mustelin T. Characterization of TCR-induced receptor-proximal signaling events negatively regulated by the protein tyrosine phosphatase PEP. Eur J Immunol. 1999;29:3845–54. doi: 10.1002/(SICI)1521-4141(199912)29:12<3845::AID-IMMU3845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 97.Wu J, Katrekar A, Honigberg LA, et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006;281:11002–10. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 98.Hill RJ, Zozulya S, Lu YL, Ward K, Gishizky M, Jallal B. The lymphoid protein tyrosine phosphatase Lyp interacts with the adaptor molecule Grb2 and functions as a negative regulator of T-cell activation. Exp Hematol. 2002;30:237–44. doi: 10.1016/s0301-472x(01)00794-9. [DOI] [PubMed] [Google Scholar]
- 99.Begovich AB, Carlton VE, Honigberg LA, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–7. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vang T, Congia M, Macis MD, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–9. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 101.Yu X, Sun JP, He Y, Guo X, Liu S, Zhou B, Hudmon A, Zhang ZY. Structure, inhibitor, and regulatory mechanism of Lyp, a lymphoid-specific tyrosine phosphatase implicated in autoimmune diseases. Proc Natl Acad Sci U S A. 2007;104:19767–72. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orru V, Tsai SJ, Rueda B, et al. A loss-of-function variant of PTPN22 is associated with reduced risk of systemic lupus erythematosus. Hum Mol Genet. 2009;18:569–79. doi: 10.1093/hmg/ddn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiorillo E, Orru V, Stanford SM, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010;285:26506–18. doi: 10.1074/jbc.M110.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science (New York, NY. 2004;303:685–9. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 105.Bottini N, Musumeci L, Alonso A, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 106.Gregorieff A, Cloutier JF, Veillette A. Sequence requirements for association of protein-tyrosine phosphatase PEP with the Src homology 3 domain of inhibitory tyrosine protein kinase p50(csk) J Biol Chem. 1998;273:13217–22. doi: 10.1074/jbc.273.21.13217. [DOI] [PubMed] [Google Scholar]
- 107.Ghose R, Shekhtman A, Goger MJ, Ji H, Cowburn D. A novel, specific interaction involving the Csk SH3 domain and its natural ligand. Nat Struct Biol. 2001;8:998–1004. doi: 10.1038/nsb1101-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai SJ, Sen U, Zhao L, et al. Crystal structure of the human lymphoid tyrosine phosphatase catalytic domain: insights into redox regulation. Biochemistry. 2009;48:4838–45. doi: 10.1021/bi900166y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y, Stanford SM, Jog SP, Fiorillo E, Orru V, Comai L, Bottini N. Regulation of lymphoid tyrosine phosphatase activity: inhibition of the catalytic domain by the proximal interdomain. Biochemistry. 2009;48:7525–32. doi: 10.1021/bi900332f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kyogoku C, Langefeld CD, Ortmann WA, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–7. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stanford SM, Mustelin TM, Bottini N. Lymphoid tyrosine phosphatase and autoimmunity: human genetics rediscovers tyrosine phosphatases. Semin Immunopathol. 2010;32:127–36. doi: 10.1007/s00281-010-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viken MK, Amundsen SS, Kvien TK, et al. Association analysis of the 1858C>T polymorphism in the PTPN22 gene in juvenile idiopathic arthritis and other autoimmune diseases. Genes Immun. 2005;6:271–3. doi: 10.1038/sj.gene.6364178. [DOI] [PubMed] [Google Scholar]
- 113.Hinks A, Barton A, John S, et al. Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum. 2005;52:1694–9. doi: 10.1002/art.21049. [DOI] [PubMed] [Google Scholar]
- 114.Smyth D, Cooper JD, Collins JE, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 115.Velaga MR, Wilson V, Jennings CE, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89:5862–5. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 116.Skorka A, Bednarczuk T, Bar-Andziak E, Nauman J, Ploski R. Lymphoid tyrosine phosphatase (PTPN22/LYP) variant and Graves’ disease in a Polish population: association and gene dose-dependent correlation with age of onset. Clin Endocrinol. 2005;62:679–82. doi: 10.1111/j.1365-2265.2005.02279.x. [DOI] [PubMed] [Google Scholar]
- 117.Heward JM, Brand OJ, Barrett JC, Carr-Smith JD, Franklyn JA, Gough SC. Association of PTPN22 haplotypes with Graves’ disease. J Clin Endocrinol Metab. 2007;92:685–90. doi: 10.1210/jc.2006-2064. [DOI] [PubMed] [Google Scholar]
- 118.Criswell LA, Pfeiffer KA, Lum RF, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–71. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skinningsrud B, Husebye ES, Gervin K, et al. Mutation screening of PTPN22: association of the 1858T-allele with Addison’s disease. Eur J Hum Genet. 2008;16:977–82. doi: 10.1038/ejhg.2008.33. [DOI] [PubMed] [Google Scholar]
- 120.Vandiedonck C, Capdevielle C, Giraud M, et al. Association of the PTPN22*R620W polymorphism with autoimmune myasthenia gravis. Ann Neurol. 2006;59:404–7. doi: 10.1002/ana.20751. [DOI] [PubMed] [Google Scholar]
- 121.Greve B, Hoffmann P, Illes Z, Rozsa C, Berger K, Weissert R, Melms A. The autoimmunity-related polymorphism PTPN22 1858C/T is associated with anti-titin antibody-positive myasthenia gravis. Hum Immunol. 2009;70:540–2. doi: 10.1016/j.humimm.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 122.Chuang WY, Strobel P, Belharazem D, et al. The PTPN22(gain-of-function)+1858T(+) genotypes correlate with low IL-2 expression in thymomas and predispose to myasthenia gravis. Genes Immun. 2009;10:667–72. doi: 10.1038/gene.2009.64. [DOI] [PubMed] [Google Scholar]
- 123.Canton I, Akhtar S, Gavalas NG, Gawkrodger DJ, Blomhoff A, Watson PF, Weetman AP, Kemp EH. A single-nucleotide polymorphism in the gene encoding lymphoid protein tyrosine phosphatase (PTPN22) confers susceptibility to generalised vitiligo. Genes Immun. 2005;6:584–7. doi: 10.1038/sj.gene.6364243. [DOI] [PubMed] [Google Scholar]
- 124.LaBerge GS, Bennett DC, Fain PR, Spritz RA. PTPN22 is genetically associated with risk of generalized vitiligo, but CTLA4 is not. J Investig Dermatol. 2008;128:1757–62. doi: 10.1038/sj.jid.5701233. [DOI] [PubMed] [Google Scholar]
- 125.Gourh P, Tan FK, Assassi S, Ahn CW, McNearney TA, Fischbach M, Arnett FC, Mayes MD. Association of the PTPN22 R620W polymorphism with anti-topoisomerase I- and anticentromere antibody-positive systemic sclerosis. Arthritis Rheum. 2006;54:3945–53. doi: 10.1002/art.22196. [DOI] [PubMed] [Google Scholar]
- 126.Betz RC, Konig K, Flaquer A, et al. The R620W polymorphism in PTPN22 confers general susceptibility for the development of alopecia areata. Br J Dermatol. 2008;158:389–91. doi: 10.1111/j.1365-2133.2007.08312.x. [DOI] [PubMed] [Google Scholar]
- 127.Huffmeier U, Reis A, Steffens M, et al. Male restricted genetic association of variant R620W in PTPN22 with psoriatic arthritis. J Investig Dermatol. 2006;126:932–5. doi: 10.1038/sj.jid.5700179. [DOI] [PubMed] [Google Scholar]
- 128.Butt C, Peddle L, Greenwood C, Hamilton S, Gladman D, Rahman P. Association of functional variants of PTPN22 and tp53 in psoriatic arthritis: a case–control study. Arthritis Res Ther. 2006;8:R27. doi: 10.1186/ar1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jagiello P, Aries P, Arning L, Wagenleiter SE, Csernok E, Hellmich B, Gross WL, Epplen JT. The PTPN22 620W allele is a risk factor for Wegener’s granulomatosis. Arthritis Rheum. 2005;52:4039–43. doi: 10.1002/art.21487. [DOI] [PubMed] [Google Scholar]
- 130.Rueda B, Nunez C, Orozco G, Lopez-Nevot MA, de la Concha EG, Martin J, Urcelay E. C1858T functional variant of PTPN22 gene is not associated with celiac disease genetic predisposition. Hum Immunol. 2005;66:848–52. doi: 10.1016/j.humimm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 131.Zhernakova A, Stahl EA, Trynka G, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7:e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baranathan V, Stanford MR, Vaughan RW, et al. The association of the PTPN22 620W polymorphism with Behcet’s disease. Ann Rheum Dis. 2007;66:1531–3. doi: 10.1136/ard.2007.073866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diaz-Gallo LM, Espino-Paisan L, Fransen K, et al. Differential association of two PTPN22 coding variants with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2011;17:2287–94. doi: 10.1002/ibd.21630. [DOI] [PubMed] [Google Scholar]
- 135.Aarnisalo J, Treszl A, Svec P, et al. Reduced CD4+ T cell activation in children with type 1 diabetes carrying the PTPN22/Lyp 620Trp variant. J Autoimmun. 2008;31:13–21. doi: 10.1016/j.jaut.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 136.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179:4704–10. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 137.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J, Zahir N, Jiang Q, et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–7. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 139.Yang Q, Co D, Sommercorn J, Tonks NK. Cloning and expression of PTP-PEST. A novel, human, nontransmembrane protein tyrosine phosphatase. J Biol Chem. 1993;268:17650. [PubMed] [Google Scholar]
- 140.Davidson D, Cloutier JF, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997;272:23455–62. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- 141.Charest A, Wagner J, Shen SH, Tremblay ML. Murine protein tyrosine phosphatase-PEST, a stable cytosolic protein tyrosine phosphatase. Biochem J. 1995;308(Pt 2):425–32. doi: 10.1042/bj3080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garton AJ, Flint AJ, Tonks NK. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–18. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen Y, Lyons P, Cooley M, Davidson D, Veillette A, Salgia R, Griffin JD, Schaller MD. The noncatalytic domain of protein-tyrosine phosphatase-PEST targets paxillin for dephosphorylation in vivo. J Biol Chem. 2000;275:1405–13. doi: 10.1074/jbc.275.2.1405. [DOI] [PubMed] [Google Scholar]
- 144.Arimura Y, Vang T, Tautz L, Williams S, Mustelin T. TCR-induced downregulation of protein tyrosine phosphatase PEST augments secondary T cell responses. Mol Immunol. 2008;45:3074–84. doi: 10.1016/j.molimm.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001;20:3414–26. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott–Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sirois J, Cote JF, Charest A, Uetani N, Bourdeau A, Duncan SA, Daniels E, Tremblay ML. Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech Dev. 2006;123:869–80. doi: 10.1016/j.mod.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davidson D, Shi X, Zhong MC, Rhee I, Veillette A. The phosphatase PTP-PEST promotes secondary T cell responses by dephosphorylating the protein tyrosine kinase Pyk2. Immunity. 2010;33:167–80. doi: 10.1016/j.immuni.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 149.Yi TL, Cleveland JL, Ihle JN. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992;12:836–46. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci U S A. 1992;89:1123–7. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Walton KM, Martell KJ, Kwak SP, Dixon JE, Largent BL. A novel receptor-type protein tyrosine phosphatase is expressed during neurogenesis in the olfactory neuroepithelium. Neuron. 1993;11:387–400. doi: 10.1016/0896-6273(93)90193-u. [DOI] [PubMed] [Google Scholar]
- 152.Banville D, Stocco R, Shen SH. Human protein tyrosine phosphatase 1C (PTPN6) gene structure: alternate promoter usage and exon skipping generate multiple transcripts. Genomics. 1995;27:165–73. doi: 10.1006/geno.1995.1020. [DOI] [PubMed] [Google Scholar]
- 153.Jin YJ, Yu CL, Burakoff SJ. Human 70-kDa SHP-1L differs from 68-kDa SHP-1 in its C-terminal structure and catalytic activity. J Biol Chem. 1999;274:28301–7. doi: 10.1074/jbc.274.40.28301. [DOI] [PubMed] [Google Scholar]
- 154.Feng GS, Hui CC, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science (New York, NY) 1993;259:1607–11. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 155.Freeman RM, Jr, Plutzky J, Neel BG. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992;89:11239–43. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–50. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 157.Pei D, Wang J, Walsh CT. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci U S A. 1996;93:1141–5. doi: 10.1073/pnas.93.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dechert U, Adam M, Harder KW, Clark-Lewis I, Jirik F. Characterization of protein tyrosine phosphatase SH-PTP2. Study of phosphopeptide substrates and possible regulatory role of SH2 domains. J Biol Chem. 1994;269:5602–11. [PubMed] [Google Scholar]
- 159.Pei D, Lorenz U, Klingmuller U, Neel BG, Walsh CT. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry. 1994;33:15483–93. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- 160.Townley R, Shen SH, Banville D, Ramachandran C. Inhibition of the activity of protein tyrosine phosphate 1C by its SH2 domains. Biochemistry. 1993;32:13414–8. doi: 10.1021/bi00212a006. [DOI] [PubMed] [Google Scholar]
- 161.Zhao Z, Bouchard P, Diltz CD, Shen SH, Fischer EH. Purification and characterization of a protein tyrosine phosphatase containing SH2 domains. J Biol Chem. 1993;268:2816–20. [PubMed] [Google Scholar]
- 162.Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor β to Ras. Proc Natl Acad Sci U S A. 1994;91:7335–9. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lorenz U, Ravichandran KS, Pei D, Walsh CT, Burakoff SJ, Neel BG. Lck-dependent tyrosyl phosphorylation of the phosphotyrosine phosphatase SH-PTP1 in murine T cells. Mol Cell Biol. 1994;14:1824–34. doi: 10.1128/mcb.14.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uchida T, Matozaki T, Noguchi T, et al. Insulin stimulates the phosphorylation of Tyr538 and the catalytic activity of PTP1C, a protein tyrosine phosphatase with Src homology-2 domains. J Biol Chem. 1994;269:12220–8. [PubMed] [Google Scholar]
- 165.Yoshida K, Kharbanda S, Kufe D. Functional interaction between SHPTP1 and the Lyn tyrosine kinase in the apoptotic response to DNA damage. J Biol Chem. 1999;274:34663–8. doi: 10.1074/jbc.274.49.34663. [DOI] [PubMed] [Google Scholar]
- 166.Zhang T, Ma J, Cao X. Grb2 regulates Stat3 activation negatively in epidermal growth factor signalling. Biochem J. 2003;376(Pt 2):457–64. doi: 10.1042/BJ20030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sankarshanan M, Ma Z, Iype T, Lorenz U. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. J Immunol. 2007;179:483–90. doi: 10.4049/jimmunol.179.1.483. [DOI] [PubMed] [Google Scholar]
- 168.Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–32. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 169.Tsui FW, Martin A, Wang J, Tsui HW. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase, SHP-1. Immunol Res. 2006;35:127–36. doi: 10.1385/IR:35:1:127. [DOI] [PubMed] [Google Scholar]
- 170.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–59. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu Rev Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 172.Johnson KG, LeRoy FG, Borysiewicz LK, Matthews RJ. TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J Immunol. 1999;162:3802–13. [PubMed] [Google Scholar]
- 173.Carter JD, Neel BG, Lorenz U. The tyrosine phosphatase SHP-1 influences thymocyte selection by setting TCR signaling thresholds. Int Immunol. 1999;11:1999–2014. doi: 10.1093/intimm/11.12.1999. [DOI] [PubMed] [Google Scholar]
- 174.Shultz LD, Rajan TV, Greiner DL. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol. 1997;15:302–7. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–78. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 176.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science (New York, NY) 1996;272:1173–6. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 177.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Somani AK, Yuen D, Yang Y, Love PE, Siminovitch KA. Involvement of the SHP-1 tyrosine phosphatase in regulation of T cell selection. J Immunol. 1999;163:3012–21. [PubMed] [Google Scholar]
- 179.Plas DR, Williams CB, Kersh GJ, et al. Cutting edge: the tyrosine phosphatase SHP-1 regulates thymocyte positive selection. J Immunol. 1999;162:5680–4. [PubMed] [Google Scholar]
- 180.Fowler CC, Pao LI, Blattman JN, Greenberg PD. SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol. 2010;185:3256–67. doi: 10.4049/jimmunol.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–93. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee HS, Ostrowski MA, Gray-Owen SD. CEACAM1 dynamics during neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J Immunol. 2008;180:6827–35. doi: 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- 183.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 184.Sathish JG, Walters J, Luo JC, et al. CD22 is a functional ligand for SH2 domain-containing protein-tyrosine phosphatase-1 in primary T cells. J Biol Chem. 2004;279:47783–91. doi: 10.1074/jbc.M402354200. [DOI] [PubMed] [Google Scholar]
- 185.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–12. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–9. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 187.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236–43. doi: 10.1016/j.bbrc.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 188.Migone TS, Cacalano NA, Taylor N, Yi T, Waldmann TA, Johnston JA. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci U S A. 1998;95:3845–50. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–9. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 190.Eriksen KW, Woetmann A, Skov L, et al. Deficient SOCS3 and SHP-1 expression in psoriatic T cells. J Investig Dermatol. 2010;130:1590–7. doi: 10.1038/jid.2010.6. [DOI] [PubMed] [Google Scholar]
- 191.Deng C, Minguela A, Hussain RZ, Lovett-Racke AE, Radu C, Ward ES, Racke MK. Expression of the tyrosine phosphatase SRC homology 2 domain-containing protein tyrosine phosphatase 1 determines T cell activation threshold and severity of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4511–8. doi: 10.4049/jimmunol.168.9.4511. [DOI] [PubMed] [Google Scholar]
- 192.Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–64. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qu CK, Nguyen S, Chen J, Feng GS. Requirement of Shp-2 tyrosine phosphatase in lymphoid and hematopoietic cell development. Blood. 2001;97:911–4. doi: 10.1182/blood.v97.4.911. [DOI] [PubMed] [Google Scholar]
- 194.Nguyen TV, Ke Y, Zhang EE, Feng GS. Conditional deletion of Shp2 tyrosine phosphatase in thymocytes suppresses both pre-TCR and TCR signals. J Immunol. 2006;177:5990–6. doi: 10.4049/jimmunol.177.9.5990. [DOI] [PubMed] [Google Scholar]
- 195.Kwon J, Qu CK, Maeng JS, Falahati R, Lee C, Williams MS. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005;24:2331–41. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–40. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 197.Arnaud M, Crouin C, Deon C, Loyaux D, Bertoglio J. Phosphorylation of Grb2-associated binder 2 on serine 623 by ERK MAPK regulates its association with the phosphatase SHP-2 and decreases STAT5 activation. J Immunol. 2004;173:3962–71. doi: 10.4049/jimmunol.173.6.3962. [DOI] [PubMed] [Google Scholar]
- 198.Arnaud M, Mzali R, Gesbert F, et al. Interaction of the tyrosine phosphatase SHP-2 with Gab2 regulates Rho-dependent activation of the c-fos serum response element by interleukin-2. Biochem J. 2004;382(Pt 2):545–56. doi: 10.1042/BJ20040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Newman DK, Hamilton C, Newman PJ. Inhibition of antigen-receptor signaling by Platelet Endothelial Cell Adhesion Molecule-1 (CD31) requires functional ITIMs, SHP-2, and p56(lck) Blood. 2001;97:2351–7. doi: 10.1182/blood.v97.8.2351. [DOI] [PubMed] [Google Scholar]
- 200.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mosinger B, Jr, Tillmann U, Westphal H, Tremblay ML. Cloning and characterization of a mouse cDNA encoding a cytoplasmic protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1992;89:499–503. doi: 10.1073/pnas.89.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tillmann U, Wagner J, Boerboom D, Westphal H, Tremblay ML. Nuclear localization and cell cycle regulation of a murine protein tyrosine phosphatase. Mol Cell Biol. 1994;14:3030–40. doi: 10.1128/mcb.14.5.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gjorloff-Wingren A, Saxena M, Han S, et al. Subcellular localization of intracellular protein tyrosine phosphatases in T cells. Eur J Immunol. 2000;30:2412–21. doi: 10.1002/1521-4141(2000)30:8<2412::AID-IMMU2412>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 204.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr Biol. 2002;12:446–53. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 205.Zhu W, Mustelin T, David M. Arginine methylation of STAT1 regulates its dephosphorylation by T cell protein tyrosine phosphatase. J Biol Chem. 2002;277:35787–90. doi: 10.1074/jbc.C200346200. [DOI] [PubMed] [Google Scholar]
- 206.You-Ten KE, Muise ES, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp WS, Tremblay ML. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–93. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dupuis M, De Jesus Ibarra-Sanchez M, Tremblay ML, Duplay P. Gr-1+ myeloid cells lacking T cell protein tyrosine phosphatase inhibit lymphocyte proliferation by an IFN-γ- and nitric oxide-dependent mechanism. J Immunol. 2003;171:726–32. doi: 10.4049/jimmunol.171.2.726. [DOI] [PubMed] [Google Scholar]
- 208.Wiede F, Shields BJ, Chew SH, et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Investig. 2011;121:4758–74. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Parkes M, Barrett JC, Prescott NJ, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–2. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Franke A, Balschun T, Karlsen TH, et al. Replication of signals from recent studies of Crohn’s disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet. 2008;40:713–5. doi: 10.1038/ng.148. [DOI] [PubMed] [Google Scholar]
- 211.Todd JA, Walker NM, Cooper JD, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–64. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Long SA, Cerosaletti K, Wan JY, Ho JC, Tatum M, Wei S, Shilling HG, Buckner JH. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun. 2011;12:116–25. doi: 10.1038/gene.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Itoh F, Ikuta S, Hinoda Y, et al. Expression and chromosomal assignment of PTPH1 gene encoding a cytosolic protein tyrosine phosphatase homologous to cytoskeletal-associated proteins. Int J Cancer. 1993;55:947–51. doi: 10.1002/ijc.2910550612. [DOI] [PubMed] [Google Scholar]
- 214.Sozio MS, Mathis MA, Young JA, et al. PTPH1 is a predominant protein-tyrosine phosphatase capable of interacting with and dephosphorylating the T cell receptor zeta subunit. J Biol Chem. 2004;279:7760–9. doi: 10.1074/jbc.M309994200. [DOI] [PubMed] [Google Scholar]
- 215.Han S, Williams S, Mustelin T. Cytoskeletal protein tyrosine phosphatase PTPH1 reduces T cell antigen receptor signaling. Eur J Immunol. 2000;30:1318–25. doi: 10.1002/(SICI)1521-4141(200005)30:5<1318::AID-IMMU1318>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 216.Zhang SH, Liu J, Kobayashi R, Tonks NK. Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1. J Biol Chem. 1999;274:17806–12. doi: 10.1074/jbc.274.25.17806. [DOI] [PubMed] [Google Scholar]
- 217.Zheng Y, Schlondorff J, Blobel CP. Evidence for regulation of the tumor necrosis factor alpha-convertase (TACE) by protein-tyrosine phosphatase PTPH1. J Biol Chem. 2002;277:42463–70. doi: 10.1074/jbc.M207459200. [DOI] [PubMed] [Google Scholar]
- 218.Hou SW, Zhi HY, Pohl N, Loesch M, Qi XM, Li RS, Basir Z, Chen G. PTPH1 dephosphorylates and cooperates with p38γ MAPK to increase ras oncogenesis through PDZ-mediated interaction. Cancer Res. 2010;70:2901–10. doi: 10.1158/0008-5472.CAN-09-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gu MX, York JD, Warshawsky I, Majerus PW. Identification, cloning, and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to cytoskeletal protein 4.1. Proc Natl Acad Sci U S A. 1991;88:5867–71. doi: 10.1073/pnas.88.13.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bauler TJ, Hendriks WJ, King PD. The FERM and PDZ domain-containing protein tyrosine phosphatases, PTPN4 and PTPN3, are both dispensable for T cell receptor signal transduction. PLoS ONE. 2008;3:e4014. doi: 10.1371/journal.pone.0004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Young JA, Becker AM, Medeiros JJ, et al. The protein tyrosine phosphatase PTPN4/PTP-MEG1, an enzyme capable of dephosphorylating the TCR ITAMs and regulating NF-κB, is dispensable for T cell development and/or T cell effector functions. Mol Immunol. 2008;45:3756–66. doi: 10.1016/j.molimm.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abaan OD, Toretsky JA. PTPL1: a large phosphatase with a split personality. Cancer Metastasis Rev. 2008;27:205–14. doi: 10.1007/s10555-008-9114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–52. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–76. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 225.Bauler TJ, Hughes ED, Arimura Y, Mustelin T, Saunders TL, King PD. Normal TCR signal transduction in mice that lack catalytically active PTPN3 protein tyrosine phosphatase. J Immunol. 2007;178:3680–7. doi: 10.4049/jimmunol.178.6.3680. [DOI] [PubMed] [Google Scholar]
- 226.Pilecka I, Patrignani C, Pescini R, et al. Protein-tyrosine phosphatase H1 controls growth hormone receptor signaling and systemic growth. J Biol Chem. 2007;282:35405–15. doi: 10.1074/jbc.M705814200. [DOI] [PubMed] [Google Scholar]
- 227.Gu M, Warshawsky I, Majerus PW. Cloning and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to retinaldehyde-binding protein and yeast SEC14p. Proc Natl Acad Sci U S A. 1992;89:2980–4. doi: 10.1073/pnas.89.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huynh H, Wang X, Li W, et al. Homotypic secretory vesicle fusion induced by the protein tyrosine phosphatase MEG2 depends on polyphosphoinositides in T cells. J Immunol. 2003;171:6661–71. doi: 10.4049/jimmunol.171.12.6661. [DOI] [PubMed] [Google Scholar]
- 229.Huynh H, Bottini N, Williams S, et al. Control of vesicle fusion by a tyrosine phosphatase. Nat Cell Biol. 2004;6:831–9. doi: 10.1038/ncb1164. [DOI] [PubMed] [Google Scholar]
- 230.Wang X, Huynh H, Gjorloff-Wingren A, Monosov E, Stridsberg M, Fukuda M, Mustelin T. Enlargement of secretory vesicles by protein tyrosine phosphatase PTP-MEG2 in rat basophilic leukemia mast cells and Jurkat T cells. J Immunol. 2002;168:4612–9. doi: 10.4049/jimmunol.168.9.4612. [DOI] [PubMed] [Google Scholar]
- 231.Zhao R, Fu X, Li Q, Krantz SB, Zhao ZJ. Specific interaction of protein tyrosine phosphatase-MEG2 with phosphatidylserine. J Biol Chem. 2003;278:22609–14. doi: 10.1074/jbc.M301560200. [DOI] [PubMed] [Google Scholar]
- 232.Wang Y, Vachon E, Zhang J, et al. Tyrosine phosphatase MEG2 modulates murine development and platelet and lymphocyte activation through secretory vesicle function. J Exp Med. 2005;202:1587–97. doi: 10.1084/jem.20051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lang R, Hammer M, Mages J. DUSP meet immunology: dual specificity MAPK phosphatases in control of the inflammatory response. J Immunol. 2006;177:7497–504. doi: 10.4049/jimmunol.177.11.7497. [DOI] [PubMed] [Google Scholar]
- 234.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 235.Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–89. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 236.Barr AJ, Knapp S. MAPK-specific tyrosine phosphatases: new targets for drug discovery? Trends Pharmacol Sci. 2006;27:525–30. doi: 10.1016/j.tips.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 237.Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–50. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Saxena M, Williams S, Brockdorff J, Gilman J, Mustelin T. Inhibition of T cell signaling by mitogen-activated protein kinase-targeted hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1999;274:11693–700. doi: 10.1074/jbc.274.17.11693. [DOI] [PubMed] [Google Scholar]
- 239.Adachi M, Sekiya M, Isobe M, Kumura Y, Ogita Z, Hinoda Y, Imai K, Yachi A. Molecular cloning and chromosomal mapping of a human protein-tyrosine phosphatase LC-PTP. Biochem Biophys Res Commun. 1992;186:1607–15. doi: 10.1016/s0006-291x(05)81592-x. [DOI] [PubMed] [Google Scholar]
- 240.Zanke B, Suzuki H, Kishihara K, Mizzen L, Minden M, Pawson A, Mak TW. Cloning and expression of an inducible lymphoid-specific, protein tyrosine phosphatase (HePTPase) Eur J Immunol. 1992;22:235–9. doi: 10.1002/eji.1830220134. [DOI] [PubMed] [Google Scholar]
- 241.Saxena M, Williams S, Gilman J, Mustelin T. Negative regulation of T cell antigen receptor signal transduction by hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1998;273:15340–4. doi: 10.1074/jbc.273.25.15340. [DOI] [PubMed] [Google Scholar]
- 242.Oh-hora M, Ogata M, Mori Y, Adachi M, Imai K, Kosugi A, Hamaoka T. Direct suppression of TCR-mediated activation of extracellular signal-regulated kinase by leukocyte protein tyrosine phosphatase, a tyrosine-specific phosphatase. J Immunol. 1999;163:1282–8. [PubMed] [Google Scholar]
- 243.Gronda M, Arab S, Iafrate B, Suzuki H, Zanke BW. Hematopoietic protein tyrosine phosphatase suppresses extracellular stimulus-regulated kinase activation. Mol Cell Biol. 2001;21:6851–8. doi: 10.1128/MCB.21.20.6851-6858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Saxena M, Williams S, Tasken K, Mustelin T. Crosstalk between cAMP-dependent kinase and MAP kinase through a protein tyrosine phosphatase. Nat Cell Biol. 1999;1:305–11. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- 245.Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, Lombroso PJ, Mustelin T. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T-cells: dynamics and subcellular location. Biochem J. 2004;378(Pt 2):335–42. doi: 10.1042/BJ20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mustelin T, Alonso A, Bottini N, et al. Protein tyrosine phosphatases in T cell physiology. Mol Immunol. 2004;41:687–700. doi: 10.1016/j.molimm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 247.Nika K, Charvet C, Williams S, et al. Lipid raft targeting of hematopoietic protein tyrosine phosphatase by protein kinase Cθ-mediated phosphorylation. Mol Cell Biol. 2006;26:1806–16. doi: 10.1128/MCB.26.5.1806-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–6. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 249.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases – regulating the immune response. Nat Rev. 2007;7:202–12. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Y, Reynolds JM, Chang SH, Martin-Orozco N, Chung Y, Nurieva RI, Dong C. MKP-1 is necessary for T cell activation and function. J Biol Chem. 2009;284:30815–24. doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rohan PJ, Davis P, Moskaluk CA, Kearns M, Krutzsch H, Siebenlist U, Kelly K. PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science (New York, NY) 1993;259:1763–6. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- 252.Grumont RJ, Rasko JE, Strasser A, Gerondakis S. Activation of the mitogen-activated protein kinase pathway induces transcription of the PAC-1 phosphatase gene. Mol Cell Biol. 1996;16:2913–21. doi: 10.1128/mcb.16.6.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jeffrey KL, Brummer T, Rolph MS, et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol. 2006;7:274–83. doi: 10.1038/ni1310. [DOI] [PubMed] [Google Scholar]
- 254.Ward Y, Gupta S, Jensen P, Wartmann M, Davis RJ, Kelly K. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature. 1994;367:651–4. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- 255.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 256.Huang CY, Lin YC, Hsiao WY, Liao FH, Huang PY, Tan TH. DUSP4 deficiency enhances CD25 expression and CD4+ T-cell proliferation without impeding T-cell development. Eur J Immunol. 2012;42:476–88. doi: 10.1002/eji.201041295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bettini ML, Kersh GJ. MAP kinase phosphatase activity sets the threshold for thymocyte positive selection. Proc Natl Acad Sci U S A. 2007;104:16257–62. doi: 10.1073/pnas.0705321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gonzalez-Navajas JM, Fine S, Law J, et al. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Investig. 2010;120:570–81. doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang Y, Blattman JN, Kennedy NJ, et al. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–7. doi: 10.1038/nature02764. [DOI] [PubMed] [Google Scholar]
- 260.Musikacharoen T, Bandow K, Kakimoto K, Kusuyama J, Onishi T, Yoshikai Y, Matsuguchi T. Functional involvement of dual specificity phosphatase 16 (DUSP16), a c-Jun N-terminal kinase-specific phosphatase, in the regulation of T helper cell differentiation. J Biol Chem. 2011;286:24896–905. doi: 10.1074/jbc.M111.245019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Masuda K, Shima H, Watanabe M, Kikuchi K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J Biol Chem. 2001;276:39002–11. doi: 10.1074/jbc.M104600200. [DOI] [PubMed] [Google Scholar]
- 262.Alonso A, Saxena M, Williams S, Mustelin T. Inhibitory role for dual specificity phosphatase VHR in T cell antigen receptor and CD28-induced Erk and Jnk activation. J Biol Chem. 2001;276:4766–71. doi: 10.1074/jbc.M006497200. [DOI] [PubMed] [Google Scholar]
- 263.Alonso A, Rahmouni S, Williams S, et al. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nat Immunol. 2003;4:44–8. doi: 10.1038/ni856. [DOI] [PubMed] [Google Scholar]
- 264.Rahmouni S, Cerignoli F, Alonso A, et al. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nat Cell Biol. 2006;8:524–31. doi: 10.1038/ncb1398. [DOI] [PubMed] [Google Scholar]
- 265.Hoyt R, Zhu W, Cerignoli F, Alonso A, Mustelin T, David M. Cutting edge: selective tyrosine dephosphorylation of interferon-activated nuclear STAT5 by the VHR phosphatase. J Immunol. 2007;179:3402–6. doi: 10.4049/jimmunol.179.6.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Marti F, Krause A, Post NH, Lyddane C, Dupont B, Sadelain M, King PD. Negative-feedback regulation of CD28 costimulation by a novel mitogen-activated protein kinase phosphatase, MKP6. J Immunol. 2001;166:197–206. doi: 10.4049/jimmunol.166.1.197. [DOI] [PubMed] [Google Scholar]
- 267.Alonso A, Merlo JJ, Na S, et al. Inhibition of T cell antigen receptor signaling by VHR-related MKPX (VHX), a new dual specificity phosphatase related to VH1 related (VHR) J Biol Chem. 2002;277:5524–8. doi: 10.1074/jbc.M107653200. [DOI] [PubMed] [Google Scholar]
- 268.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–79. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 269.Suzuki A, Yamaguchi MT, Ohteki T, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–34. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 270.Seminario MC, Wange RL. Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial. Immunol Rev. 2003;192:80–97. doi: 10.1034/j.1600-065x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 271.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 272.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science (New York, NY) 1999;285:2122–5. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 273.Souza AC, Azoubel S, Queiroz KC, Peppelenbosch MP, Ferreira CV. From immune response to cancer: a spot on the low molecular weight protein tyrosine phosphatase. Cell Mol Life Sci. 2009;66:1140–53. doi: 10.1007/s00018-008-8501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp (Warsz) 2002;50:95–104. [PubMed] [Google Scholar]
- 275.Chiarugi P, Cirri P, Taddei ML, et al. Insight into the role of low molecular weight phosphotyrosine phosphatase (LMW-PTP) on platelet-derived growth factor receptor (PDGF-r) signaling. LMW-PTP controls PDGF-r kinase activity through TYR-857 dephosphorylation. J Biol Chem. 2002;277:37331–8. doi: 10.1074/jbc.M205203200. [DOI] [PubMed] [Google Scholar]
- 276.Spencer N, Hopkinson DA, Harris H. Quantitative differences and gene dosage in the human red cell acid phosphatase polymorphism. Nature. 1964;201:299–300. doi: 10.1038/201299a0. [DOI] [PubMed] [Google Scholar]
- 277.Dissing J. Immunochemical characterization of human red cell acid phosphatase isozymes. Biochem Genet. 1987;25:901–18. doi: 10.1007/BF00502609. [DOI] [PubMed] [Google Scholar]
- 278.Chiarugi P, Cirri P, Raugei G, Camici G, Dolfi F, Berti A, Ramponi G. PDGF receptor as a specific in vivo target for low Mr phosphotyrosine protein phosphatase. FEBS Lett. 1995;372:49–53. doi: 10.1016/0014-5793(95)00947-8. [DOI] [PubMed] [Google Scholar]
- 279.Park EK, Warner N, Mood K, Pawson T, Daar IO. Low-molecular-weight protein tyrosine phosphatase is a positive component of the fibroblast growth factor receptor signaling pathway. Mol Cell Biol. 2002;22:3404–14. doi: 10.1128/MCB.22.10.3404-3414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pandey SK, Yu XX, Watts LM, et al. Reduction of low molecular weight protein-tyrosine phosphatase expression improves hyperglycemia and insulin sensitivity in obese mice. J Biol Chem. 2007;282:14291–9. doi: 10.1074/jbc.M609626200. [DOI] [PubMed] [Google Scholar]
- 281.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J Biol Chem. 2002;277:39274–9. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 282.Bottini N, Stefanini L, Williams S, Alonso A, Jascur T, Abraham RT, Couture C, Mustelin T. Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP) J Biol Chem. 2002;277:24220–4. doi: 10.1074/jbc.M202885200. [DOI] [PubMed] [Google Scholar]
- 283.Giannoni E, Chiarugi P, Cozzi G, et al. Lymphocyte function-associated antigen-1-mediated T cell adhesion is impaired by low molecular weight phosphotyrosine phosphatase-dependent inhibition of FAK activity. J Biol Chem. 2003;278:36763–76. doi: 10.1074/jbc.M302686200. [DOI] [PubMed] [Google Scholar]
- 284.Tailor P, Gilman J, Williams S, Couture C, Mustelin T. Regulation of the low molecular weight phosphotyrosine phosphatase by phosphorylation at tyrosines 131 and 132. J Biol Chem. 1997;272:5371–4. doi: 10.1074/jbc.272.9.5371. [DOI] [PubMed] [Google Scholar]
- 285.Bucciantini M, Chiarugi P, Cirri P, Taddei L, Stefani M, Raugei G, Nordlund P, Ramponi G. The low Mr phosphotyrosine protein phosphatase behaves differently when phosphorylated at Tyr131 or Tyr132 by Src kinase. FEBS Lett. 1999;456:73–8. doi: 10.1016/s0014-5793(99)00828-5. [DOI] [PubMed] [Google Scholar]
- 286.Giannoni E, Raugei G, Chiarugi P, Ramponi G. A novel redox-based switch: LMW-PTP oxidation enhances Grb2 binding and leads to ERK activation. Biochem Biophys Res Commun. 2006;348:367–73. doi: 10.1016/j.bbrc.2006.07.091. [DOI] [PubMed] [Google Scholar]
- 287.Bottini N, Gloria-Bottini F, Lucarini N, Ronchetti PG, Fontana L. Inflammatory bowel disease: are there gender differences in the genetics of signal transduction? A preliminary study of cytosolic low molecular weight protein tyrosine phosphatase. Dis Markers. 2000;16:163–6. doi: 10.1155/2000/101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gloria-Bottini F, Bottini N, Renzetti G, Bottini E. ACP1 and Th class of immunological disease: evidence of interaction with gender. Int Arch Allergy Immunol. 2007;143:170–6. doi: 10.1159/000099308. [DOI] [PubMed] [Google Scholar]
- 289.Bottini N, Meloni GF, Lucarelli P, Amante A, Saccucci P, Gloria-Bottini F, Bottini E. Risk of type 1 diabetes in childhood and maternal age at delivery, interaction with ACP1 and sex. Diabetes Metab Res Rev. 2005;21:353–8. doi: 10.1002/dmrr.521. [DOI] [PubMed] [Google Scholar]
- 290.Meloni G, Bottini N, Borgiani P, Lucarelli P, Meloni T, Bottini E. Association of the ACP1 genotype with metabolic parameters upon initial diagnosis of type 1 diabetes. Med Sci Monit. 2003;9:CR105–8. [PubMed] [Google Scholar]


