Abstract
Seeds have evolved to accommodate complicated processes like senescence, dormancy, and germination. Central to these is the storage of carbohydrates and proteins derived from sugars and amino acids synthesized during photosynthesis. In the grasses, the bulk of amino acids is stored in the prolamin superfamily that specifically accumulates in seed endosperm during senescence. Their promoters contain a conserved cis-element, called prolamin-box (P-box), recognized by the trans-activator P-box binding factor (PBF). Because of the lack of null mutants in all grass species, its physiological role in storage–protein gene expression has been elusive. In contrast, a null mutant of another endosperm-specific trans-activator Opaque2 (O2) has been shown to be required for the transcriptional activation of subsets of this superfamily by binding to the O2 box. Here, we used RNAi to knockdown Pbf expression and found that only 27-kDa γ- and 22-kDa α-zein gene expression were affected, whereas the level of other zeins remained unchanged. Still, transgenic seeds had an opaque seed phenotype. Combination of PbfRNAi and o2 resulted in further reduction of α-zein expression. We also tested the interaction of promoters and constitutively expressed PBF and O2. Whereas transgenic promoters could be activated, endogenous promoters appeared to be not accessible to transcriptional activation, presumably due to differential chromatin states. Although analysis of the methylation of binding sites of PBF and O2 correlated with the expression of endogenous 22-kDa α-zein promoters, a different mechanism seems to apply to the 27-kDa γ-zein promoter, which does not undergo methylation changes.
Keywords: prolamin-box binding factor, Opaque2 transcriptional activator, prolamin gene superfamily, DNA methylation, gene amplification
ENDOSPERM is a terminal organ, which stores proteins and carbohydrates to nourish the embryo when it germinates and early seedlings when they grow. In Zea mays, the major storage proteins are alcohol-soluble prolamins called zeins, which are exclusively expressed in endosperm starting about 10 days after pollination (DAP). Transcription of the zein-gene superfamily is characteristic for three outstanding features: tissue specificity, developmental timing, and its strength. Maize seeds contain ∼10% proteins and ∼70% of them are storage proteins, of which 70% are zeins. Therefore, the zein promoters are probably among the strongest promoters in maize endosperm. Indeed, zein cDNA sequences accounted for nearly 50% of the cDNAs in a nonnormalized endosperm cDNA library, of which 30 and 15% are from α- and γ-zein genes, respectively (Hunter et al. 2002). Zeins are divided into four classes, i.e., α (19 and 22 kDa), γ (50, 27, and 16 kDa), β (15 kDa), and δ (18 and 10 kDa). In the maize B73 genome, there are 26 and 16 gene copies for 19- and 22-kDa α-zeins, respectively, whereas other sizes are only single-copy genes (Song and Messing 2003; Xu and Messing 2008; Miclaus et al. 2011).
Prolamin gene expression is synchronized during seed development. Many promoter regions retained a conserved 7-bp DNA cis-acting element (5′-TGTAAAG-3′), called the prolamin box (P-box), approximately 300 nucleotides upstream of the start codon (Figure 1) (Vicente-Carbajosa et al. 1997; Wang and Messing 1998; Xu and Messing 2009). The P-box binding factor (PBF) interacts with the P-box as an endosperm-specific transcriptional activator that belongs to the Dof class of plant zinc-finger DNA-binding proteins. Indeed, PBF was expressed about 10 DAP concomitant with activation of all zein genes, as expected for the spatial and temporal regulation of zein genes by a common transcriptional activator (Vicente-Carbajosa et al. 1997).
Figure 1 .
Alignment of the 22-kDa α-zein and 27-kDa γ-zein promoters. The P-box, O2 (or O2-like), CAAT, TATA boxes, transcription start site, and start codon are highlighted. Their positions relative to transcription start site are indicated.
Yet, another endosperm-specific transcription factor Opaque 2 (O2) co-activates transcription of a subset of zein and many other genes in maize endosperm (Hunter et al. 2002). In the zein-gene superfamily, O2 had been reported to regulate only 22-kDa α- and β-zein genes (Cord Neto et al. 1995; Schmidt et al. 1992). In the promoters of the 22-kDa α-zein gene copies, O2 recognizes the O2 box (5′-TCCACGTAGAT-3′), in which “ACGT” is the core element (Figure 1). In nonendosperm tissues, the promoters of the 22-kDa α-zein genes are highly methylated, especially at the “C” in the core motif, whereas in endosperm tissue the promoters are almost free from methylation (Locatelli et al. 2009).
P- and O2-boxes are separated by only 20 bp and it has been suggested that PBF functions in recruiting class-specific transcriptional activators like O2 for gene expression (Vicente-Carbajosa et al. 1997; Wang and Messing 1998). Although the 27-kDa γ-zein gene is not regulated by O2, an O2-like box (5′-TTTACGTAGAT-3′) was also found in its −300 element position (Figure 1). When this motif 5′-TTTACGTAGAT-3′ was modified to the conventional O2 box 5′-TCCACGTAGAT-3′ by site-directed mutagenesis, the binding affinity of O2 was dramatically increased in vitro and the transcription activity was significantly elevated compared to the wild-type promoter (Ueda et al. 1992). Interestingly, overexpression of maize PBF or O2 alone could increase the expression of rice and wheat storage protein genes and cotransfection of them resulted in an additive increase (Hwang et al. 2004).
However, because of the lack of a Pbf null mutant in any grass species, the extent of regulation of gene expression by PBF during seed development has remained unclear. Furthermore, as all zein-gene genomic sequences were available online, it was found that not all their promoters contain a P-box. To address the divergence of promoter regions of prolamin genes, we used RNA interference and constitutive overexpression of PBF and O2 in maize to study promoters of each class of zeins, where each combination of trans- and cis-acting elements was unique, demonstrating the divergence of gene regulation after amplification.
Materials and Methods
Plasmid construction, plant transformation
The method for making RNAi constructs have been described elsewhere (Wu and Messing 2010). The primers for amplification of the inverted Pbf cDNA fragments are listed in supporting information, Table S1. All primers for construction of pTF102-P4, pTF102-P5, and pTF102-P9 are listed in Table S1 and indicated in simplified diagrams (Figure S5). The binary vector pTF102 was used to build constructs for transformation and a series of intermediate constructs was also built with pTF102. The construct pTF102-P4 (P27::GFP::Tdzs10) contains a transcriptional fusion of the GFP gene with the 27-kDa γ-zein promoter (1067 bp) and the termination and polyadenylation signals 961 bp downstream from the stop codon of the 10-kDa δ-zein gene (Wu and Messing 2009). The constructs pTF102-P5 (P27::GFP::Tdzs10-P35S::PBF::T35S) and pTF102-P9 (P27::GFP::Tdzs10-P35S::PBF::T35S- P35S::O2::T35S) were derived from pTF102-P35SPBF and pTF102-P35SO2 (Figure S5A). The full-length coding sequences of Pbf and O2 genes were amplified from B73 endosperm cDNAs and cloned into the T-easy vector (Promega) for sequence verification. The CaMV 35S promoter (P35S) was amplified from the pTF102 vector with two pairs of primers to simultaneously introduce different restriction enzyme cutting sites (Figure S5A and Table S1). For inserting the fragment BamH1-P35S::PBF::T35S-AvrIIHindIII into pTF102-P4 downstream of Tdzs10, pTF102-P4-BamH1HindIII was created by simple replacement of a partial Tdzs10 with the same sequence except for a BamH1 next to the HindIII by PCR (Figure S5B).
To construct pTF102-P9, the 27-kDa γ-zein promoter in pTF102-P4-BamH1HindIII was replaced with a 22-kDa α-zein promoter (1237 bp) to create an intermediate construct, pTF102-P7. Another intermediate construct pTF102-P8 was created in the same way as pTF102-P5. Then, pTF102-P8 was digested with AvrII and HindIII and ligated with the fragment AvrII-P35S::O2::T35S-HindIII to form the final product of pTF102-P9 (Figure S5C).
Hi-II B and A lines were grown in the greenhouse at the Waksman Institute, Rutgers University. For each construct, ∼200 immature embryos at ∼10 DAP from the crosses of B × A were dissected for transformation. All the subsequent steps were performed according to known protocols (Frame et al. 2002).
Generation of the double mutant of PbfRNAi and o2
The PbfRNAi gene could be specifically amplified with a primer pair in the GFP coding and T35S regions as described recently (Wu and Messing 2010). Heterozygous and homozygous o2 genotypes could be identified with an O2 polymorphic marker umc1066 (http://www.maizegdb.org/cgi-bin/displayssrrecord.cgi?id=193779).
Fluorescence dissection microscopy
Immature embryos 24 hr after infection, callus, root, leaf, and seeds were monitored for GFP expression with fluorescence dissection microscope (Leica MZ16 F).
RT–PCR and SDS–PAGE
Total RNA was extracted by using TRIzol reagent (Invitrogen). A total of 5 μg RNA was digested with DNase I (Invitrogen) and then reverse transcribed. All primers for RT–PCR were listed in Table S1. The procedures for zein extraction and SDS-PAGE analysis have been described in detail elsewhere (Wu and Messing 2012).
DNA extraction and DNA methylation analysis
Genomic DNA was extracted by the cetyltrimethyl-ammonium bromide (CTAB) method. For bisulfite sequencing, genomic DNA was extracted from the leaves punched from three to five individuals for each event. A total of 2 μg DNA was subject to bisulfite treatment (Invitrogen) and 2 μl of the eluted material was applied for PCR with 55 cycles. All primers for bisulfite PCR were listed in Table S1. The common forward primer p22bisF and the specific reverse one α22bisR or GFPbisR were used to amplify the endogenous or transgenic 22-kDa α-zein promoters. Likewise, the p27bisF and the specific reverse primer γ27bisR or GFPbisR were used to amplify the endogenous or transgenic 27-kDa γ-zein promoter; the p35SbisF and the specific reverse primer PbfbisR or BarbisR were used to amplify the CaMV 35S promoter driving the Pbf or bar gene coding sequence. The PCR product was cloned into T-easy vector (Promega) and a total of 96 colonies for each sample were selected for sequencing with the ABI 3730XL sequencer in our laboratory. The sequences were aligned and the percentage of cytosine methylation was calculated by the program of DNASTAR SeqMan.
The restriction enzyme HypCH4IV cuts “ACGT” and is blocked by cytosine methylation. The leaf and endosperm genomic DNAs (1 μg of each) from P22 and P9-1 were digested with HypCH4IV overnight. Fifty nanograms of the digested DNA was applied for PCR amplification with 30 cycles. The primer pair p22F1 and GFPR2 was used to specifically amplify the transgenic 22-kDa α-zein promoter (Table S1).
Results
Effect of PbfRNAi on the expression of the zein-gene superfamily
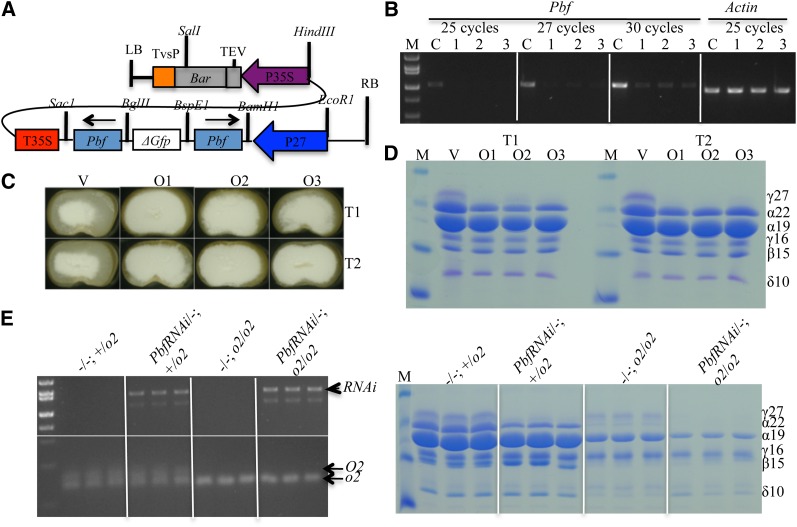
Pbf was cloned by amplification with primers based on the conservation of the Dof domain between Arabidopsis and maize (Vicente-Carbajosa et al. 1997). Because no null mutant has been found in any grass species it has been suggested that PBF could be a master regulator of endosperm development and its absence would result in a defective kernel mutant (dek). If this were to be the case, quantitative reduction of PBF should still permit us to identify target genes without preventing seed development. Therefore, we constructed an RNAi transgene for Pbf with the strong endosperm-specific 27-kDa γ-zein promoter (Figure 2A) and recovered three independent events. For each transgenic event, individual clones (PbfRNAi/-; O2/O2) were self-crossed or pollinated with W64Ao2 (o2 mutant in inbred W64A background). To evaluate the knockdown effect, six immature kernels for each event at 18 DAP were dissected from the cross PbfRNAi/-; O2/O2 × −/−; o2/o2. All progeny were heterozygous for the o2 mutant with a recessive phenotype, which would not affect zein expression, whereas half of the kernels from the crosses would inherit the dominant PbfRNAi transgene. Therefore, one-half of the progeny should have the genotype PbfRNAi/−; +/o2, and the other −/−; +/o2. The embryo and endosperm were separated individually. The embryos were saved to extract genomic DNA for transgene genotyping by PCR. Progeny from crosses of PbfRNAi/−; O2/O2 × −/−; o2/o2 inheriting and not inheriting the PbfRNAi construct were combined to extract RNA, respectively. As expected, the transcripts for Pbf were dramatically reduced in the three PbfRNAi events compared to the normal genotype (Figure 2B).
Figure 2 .
Silencing of Pbf and the expression of all zeins. (A) PbfRNAi construct. (B) RT–PCR analysis of the expression of Pbf in the three PbfRNAi events. The Actin gene was used as control. (C) PbfRNAi seed phenotype. V, vitreous kernel, not inheriting the PbfRNAi construct; O1–O3, opaque kernels, inheriting the PbfRNAi construct. (D) Zein protein accumulation pattern in PbfRNAi seeds. (E) Zein protein accumulation pattern in double mutant of PbfRNAi and o2. Left, genotyping of the progeny seeds with PCR; right, SDS-PAGE analysis of zein accumulation in the genotyped seeds from the left.
Self- and outcrossed ears of all three events segregated vitreous and opaque kernels with ratio of 1:3 and 1:1, respectively. One vitreous and three opaque kernels from events 1 and 2 were sliced in half and shown in Figure 2C. Sliced seeds were used to extract zein proteins individually and analyzed by SDS–PAGE (Figure 2D). Only the levels of 27-kDa γ- and 22-kDa α-zeins were significantly reduced in opaque seeds, whereas other zeins had no noticeable reduction (Figure 2D).
Because all genomic zein-gene sequences were now available online, the cis-elements of their promoters could be aligned for sequence homology. Indeed, in the −300 regions, the sequences of cis-acting elements in different zein genes varied considerably (Figure S1). Although the 27-kDa γ- and 22-kDa α-zein genes are the most diverged prolamin genes, one being the oldest and one being the youngest (Xu and Messing 2009), their promoters have been quite conserved in that they both contain the P-, O2-, or O2-like boxes. However, only a subset of the 22-kDa α-zein genes are regulated by O2 (Song et al. 2001). The 27-kDa and 16-kDa γ-zein genes originated from a common progenitor by allotetraploidization and share high DNA and protein sequence similarity (Xu and Messing 2008). However, the P- and O2-like boxes are missing in the 16-kDa γ-zein promoter due to a deletion (Figure S2). The 18- and 10-kDa δ-zein genes also lack a P-box in their −300 regions, consistent with their unchanged expression in PbfRNAi. Interestingly, the regulation of the 19-kDa α-zein genes is also decreased in the o2 mutant despite the absence of an O2 box and the lack of O2 binding to their promoters (Schmidt et al. 1992). On the other hand, a P-box is present in the 19-kDa α- and 15-kDa β-zein promoters, but knockdown of PBF does not reduce their expression (Figure S1 and Figure 2D). One possibility could be that even small amounts of PBF are still sufficient for expression of 19-kDa α- and 15-kDa β-zeins in the presence of O2 because the RNAi line is not a null mutant. We also cannot exclude the possibility that the 19-kDa α- and 15-kDa β-zeins are regulated by other redundant Dof-containing transcription factors. Clearly, the in-vitro binding studies cannot be confirmed in vivo, indicating interactions of several factors in addition to those that have been described.
Zein accumulation in the double mutant of PbfRNAi and o2
Double mutant of PbfRNAi and o2 was generated by backcross of PbfRNAi/−; +/o2 to W64Ao2. Kernels were collected at 36 DAP. The embryos were separated from single kernels for genotyping. We expected four genotypes among the backcrossed progeny, heterozygous o2 without PbfRNAi (−/−; +/o2) and with PbfRNAi (PbfRNAi/−; +/o2), and homozygous o2 without PbfRNAi (−/−; o2/o2) and with PbfRNAi (PbfRNAi/−; o2/o2). Three kernels for each genotype were identified for zein accumulation analysis (Figure 2E, left, see Materials and Methods for details). Kernels with genotype −/−; +/o2 showed the normal accumulation pattern of all zein proteins. In PbfRNAi/−; +/o2, kernels had significant reduced accumulation in 27-kDa γ- and 22-kDa α-zeins as observed in Figure 2D, whereas in −/−; o2/o2 kernels had dramatically decreased levels of the 22-kDa, 19-kDa α-, and 15-kDa β-zeins (Figure 2E, right).
The expression of the 10-kDa δ-zein was always lower in o2 than normal seeds, but so far there is no evidence that it was directly regulated by O2. On the other hand, O2 regulates many genes and its mutant has pleiotropic effects on endosperm development (Hunter et al. 2002). Interestingly, the 10-kDa δ-zein is expressed at higher levels in the double mutant than in o2, which is consistent with our recent finding that balancing of sulfur storage can occur between the cysteine-rich γ- and β-zeins and the methionine-rich 10-kDa δ-zein. In the biosynthesis of methionine it receives the sulfur moiety from cysteine. When cysteines are not incorporated into the cysteine-rich γ- and β-zeins in the double mutant, biosynthesis of methionine can progress and be incorporated into the methionine-rich 10-kDa δ-zein (Figure 2E), which synthesis is probably regulated at the translational level (Wu et al. 2012).
The levels of 22-kDa α-zeins in −/−; o2/o2 were more decreased than in PbfRNAi/−; +/o2, but were still detectable in SDS–PAGE. In the double mutant of PbfRNAi/−; o2/o2, the effect on α-zein expression is additive (Figure 2E, right). The expression of 22-kDa α-zeins was no longer visible in SDS–PAGE. Although the accumulation of 19-kDa α-zeins was not affected by PbfRNAi alone, their expression was significantly lower in PbfRNAi/−; o2/o2 than in −/−; o2/o2. In all genotypes, the 16-kDa γ-zein expressed evenly, indicating that it has evolved to be subject to a new regulation.
Epigenetic states of transgenic and endogenous 27-kDa γ-zein promoters
Besides endosperm-specific transactivators, differential epigenetic modification between endosperm and nonendosperm tissues plays an important role in endosperm-specific expression of zein genes (Bianchi and Viotti 1988). Because of their strict tissue specificity, transgenes driven by any zein gene promoter are expected to be expressed only in endosperm. However, a positive callus generated from immature embryos transformed with the construct pTF102-P4 (abbreviated as P4), in which the GFP marker gene was driven by the promoter of the 27-kDa γ-zein gene, was GFP positive, when analyzed under a fluorescence dissection microscope. Indeed, RT–PCR and Western blot analysis showed that also the endogenous 27-kDa γ-zein gene was expressed (Wu and Messing 2009). Furthermore, RT–PCR analysis showed that the Pbf gene was also transcribed (Figure 3), consistent with the fact that the 27-kDa γ-zein gene was trans-activated by PBF (Figure 2, D and E). However, the 22-kDa α-zein and O2 genes were not activated (Figure 3).
Figure 3 .
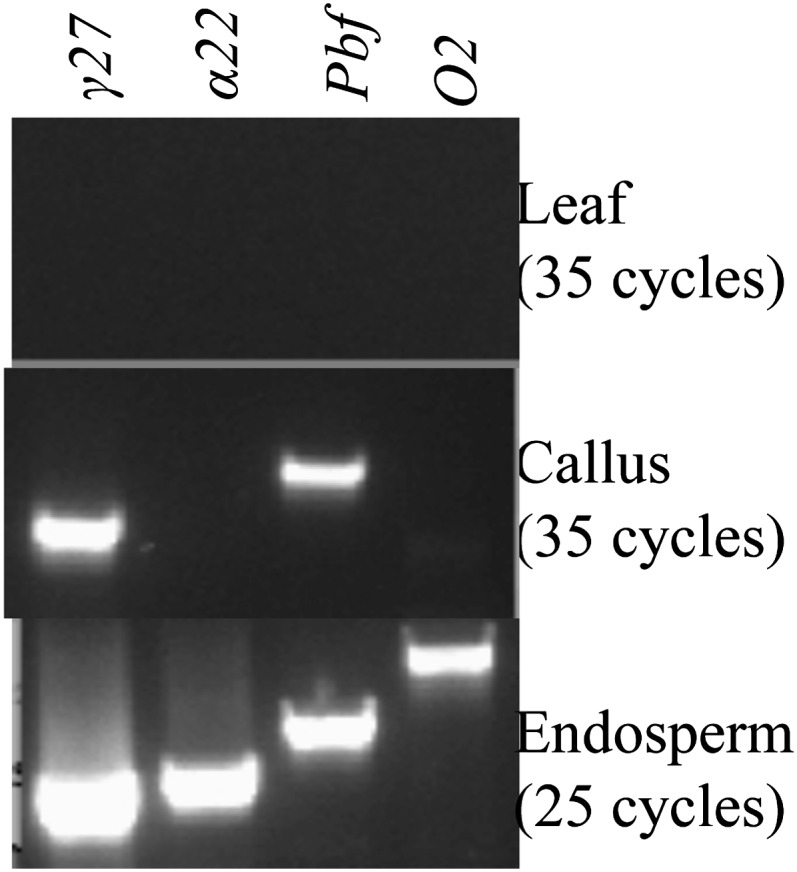
RT–PCR analysis of the expression of the 22-kDa α-zein, 27-kDa γ-zein, Pbf and O2 genes in endosperm, callus, and leaf from a regenerated control plant. PCR cycles were 25 for endosperm and 35 for callus and leaf. Top, leaf; middle, callus; bottom, endosperm.
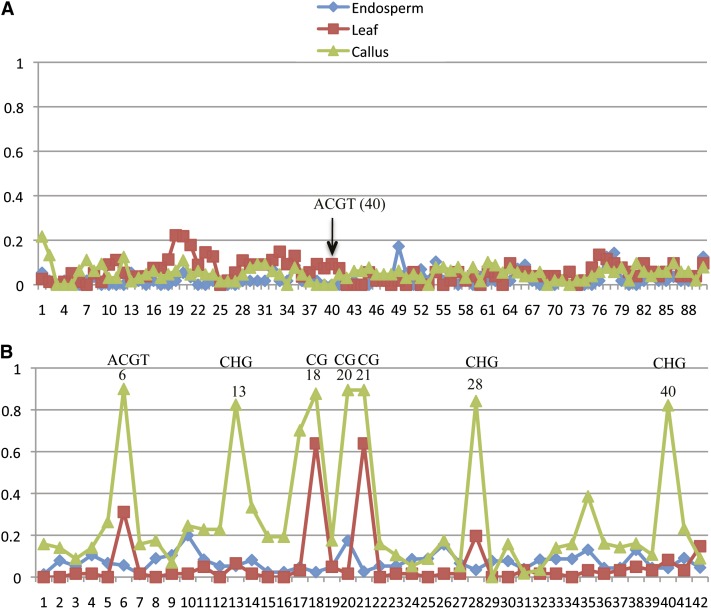
Because the 27-kDa γ-zein and Pbf genes were not expressed in nonendosperm tissues, we expected that their epigenetic states would change upon regeneration of plants from tissue culture cells. Indeed, both 27-kDa γ-zein and Pbf genes became inactivated in leaf after regeneration. Lack of expression of the 27-kDa γ-zein gene in regenerated plants could be due to the absence of Pbf gene expression or change of its epigenetic status of its promoter or both. However, DNA methylation analysis by bisulfite sequencing showed that the 421-bp fragment upstream of the transcription start site, composed of P-, O2-like, and TATA boxes with 90 cytosines in the 27-kDa γ-zein gene promoter (Figure S3), was hypomethylated throughout development in endosperm, callus, or leaf tissue (Figure 4A). If a change in its epigenetic state occurred, it would not correlate with DNA methylation. In contrast, the 22-kDa α-zein promoter regions, comprising 263-bp upstream of the transcription start site including the P-, O2-, CAAT-, and TATA boxes with 42 cytosines in this region (Figure S4), cytosines were highly methylated in the CG and CHG positions in leaf, but not in endosperm tissue. Some CG sites including the core ACGT motif were also methylated in callus, but fewer sites than in leaf (Figure 4B), indicating that callus-induced demethylation is different from developmentally induced demethylation of the 22-kDa α-zein genes in endosperm. In this respect, one must keep in mind that callus generated from immature embryos is a dedifferentiated tissue induced by the culturing conditions. It has been shown that under those conditions, transposons become demethylated and activated (Peschke et al. 1987; Huang et al. 2009; Ngezahayo et al. 2009). Therefore, a decrease in DNA methylation of 22-kDa α-zein promoters in callus is probably caused by tissue-culture-induced global demethylation (Kaeppler and Phillips 1993).
Figure 4 .
DNA methylation in the 27-kDa γ- and 22-kDa α-zein promoters in callus, leaf, and endosperm. The (A) 421-bp (27-kDa γ-zein promoter) and (B) 263-bp (22-kDa α-zein promoters) fragments immediately adjacent to transcription start site covering P-box, O2-like, and TATA boxes containing 90 and 42 cytosines, respectively, were analyzed by bisulfite sequencing. The cytosine in the O2-box core motif at position 40 (γ27-zein promoter) and 6 (α-zein promoter) is indicated. Other symmetric cytosines (CG and CHG) in the 22-kDa α-zein promoters are also indicated.
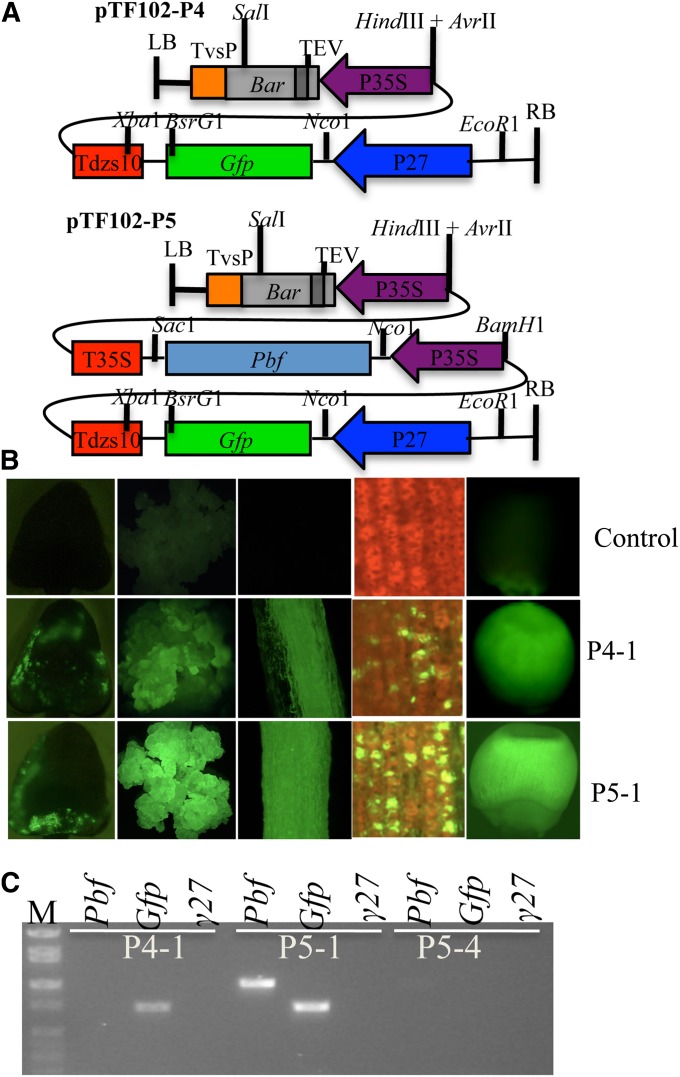
If no DNA methylation change of the 27-kDa γ-zein promoter occurs during plant development, constitutive expression of PBF should turn on the expression of the 27-kDa γ-zein gene in leaf tissue. Therefore, we constructed pTF102-P5 (abbreviated as P5) with PBF under the control of the CaMV 35S promoter (Figure 5A and Figure S5, A and B). About 200 10-DAP-old immature embryos were transformed with P4 and P5 constructs (Figure 5A). About 70% of the embryos in the two groups showed already transient expression of GFP 24 hr after infection (Figure 5B). After two rounds of bialaphos selection, independent calli for P4 (P4-1 and P4-2) and P5 (P5-1 to P5-4) grew and all strongly expressed GFP as shown with representative samples P4-1 and P5-1 (Figure 5B), consistent with our previous results (Wu and Messing 2009). When plants had been regenerated on regeneration II medium, a piece of root tissue for each event was examined for GFP expression. Whereas three events from P5 (P5-1, P5-2, and P5-3) strongly expressed GFP, this was not the case for P5-4, although the two P4 events did (Figure 5B). As expected, expression of the 27-kDa γ-zein gene itself was not detected in the two P4 transgenic plant leaves, neither in the four events of P5 (Figure 5C), indicating that the endogenous 27-kDa γ-zein promoter could not be activated by PBF alone in leaf. In contrast, the ectopic copy of the 27-kDa γ-zein promoter driving GFP expression was turned on in P4 and P5 transgenic plants with higher levels in P5-1, P5-2, and P5-3 than in P4-1 and P4-2 (Figure 5B), indicating that expression of PBF was sufficient to enhance a transgenic 27-kDa γ-zein promoter, but not the endogenous promoter. An exception was P5-4. RT–PCR results showed that the Pbf and Gfp genes were not expressed in this event (Figure 5C). Interestingly, the expression of the Bar gene in P5-4 was also significantly reduced compared to the other three events. Furthermore, although GFP expression of P5-4 was not detected in leaves, it was strongly expressed in the seed like the other three P5 and two P4 transgenic events, indicating that P5-4 underwent an epigenetic phase change whereas the other events did not. Indeed, the two 35S promoters each driving the Bar and Pbf genes were highly methylated in P5-4 not only in leaf (Figure S6, A and B) but also in endosperm (Figure S6C), whereas no significant methylation changes occurred in the transgenic 27-kDa γ-zein promoter relative to the endogenous gene (Figure S6D).
Figure 5 .
Ectopic activation of the transgenic 27-kDa γ-zein promoter. (A) Structures of constructs pTF102-P4 and -P5. (B) GFP signal under fluorescence dissection microscope in immature embryo 24 hr after cocultivation, callus, regenerated root, and adult leaf. Top, nontransgenic control; middle, P4-1; bottom, P5-1. (C) RT–PCR analysis of the expression of the Pbf, Gfp, and the 27-kDa γ-zein genes in P4-1, P5-1, and P5-4. The expression of the Gfp gene was detected in P4-1 and P5-1. In P5-4, no visible expression of the Pbf and Gfp genes was detected. M, DNA markers from top to bottom being 2, 1.55, 1.4, 1, 0.75, 0.5, 0.4, and 0.3 kb.
Reactivation of P5-4 in mop1 null mutant
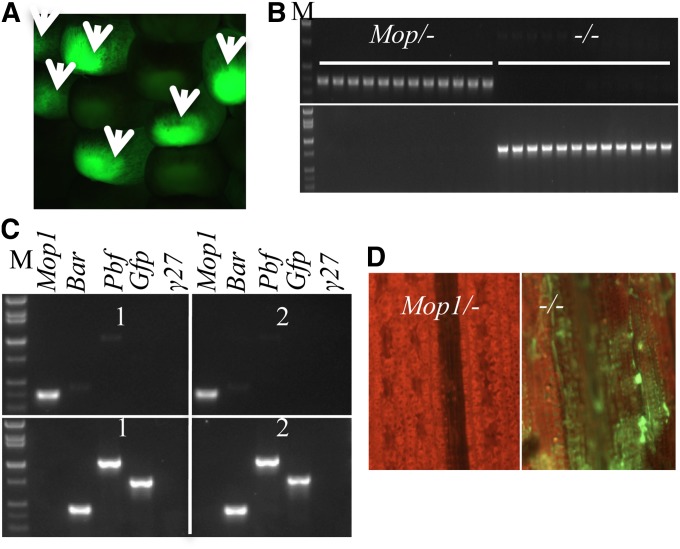
Inactivation of the CaMV35S promoter in transgenic plants has been described previously (Meyer et al. 1994; Park et al. 1996). A maize mutant with a lesion in Mop1, encoding a RNA-dependent RNA Polymerase 2 (RDR2), has been reported to suppress gene expression via a siRNA-mediated mechanism in transgene-induced silencing (McGinnis et al. 2006). To investigate whether the epigenetic state of P5-4 could be reversed in the mop1 mutant, the F1 of the P5-4 event and mop1 was backcrossed with mop1. Since P5-4 could express GFP in seeds, GFP-positive seeds were easily scored under a fluorescence dissection microscope as shown by arrows (Figure 6A). Twenty-four GFP-positive seeds were grown, about half of which should be homozygous for the mop1 mutant allele. Homozygous mop1 mutant could easily be recognized by strong pigmentation of anthocyanin in the plant stem and tassel (Alleman et al. 2006). Moreover, the mutant allele used in this work resulted from a Mu insertion in its fourth exon (Alleman et al. 2006). Therefore, a homozygous mutant could be identified using PCR amplification with specific primer pairs. Genotyping was performed at flowering time. Indeed, 12 plants that were strongly pigmented proved to be homozygous mop1 mutants (Figure 6B). Furthermore, bisulfite sequencing showed that methylation of the two 35S promoters in plants homozygous for the mop1 allele was dramatically reduced compared to nonmutated Mop1 (Figure S6, A and B), whereas the upstream transgenic 27-kDa promoter was hypomethylated both in Mop1-P5-4 and mop1-P5-4 (Figure S6D). Consistent with this result, the expression of Bar and Pbf genes was dramatically increased (Figure 6C). As a consequence of reactivation of the ectopic Pbf gene, the transgenic 27-kDa γ-zein promoter driving GFP gene was trans-activated (Figure 6, B–D). However, transcripts of the endogenous 27-kDa γ-zein could not be detected (Figure 6C), indicating that the endogenous copy is under a specific developmental control.
Figure 6 .
Reactivation of P5-4 in mop1 mutant. (A) Normal expression of GFP in P5-4 seed (section of an ear). (B) Correlation of GFP expression with mop1 zygosity. Top, zygosity test of the mop1 mutant allele in the BC1 population; bottom, RT–PCR analysis of GFP expression of the genotyped plants as shown in the top. (C) Silencing and reactivation of CaMV 35S promoters in progenies with Mop1/- and mop1 genotypes. For each genotype, two individuals were chosen for further analysis of Pbf and O2 expression. Top, genotype Mop1/+; bottom, mop1. (D) GFP expression observed under fluorescence dissection microscope; left, genotype Mop1/+; right, mop1. M, DNA markers from top to bottom being 2, 1.55, 1.4, 1, 0.75, 0.5, 0.4, and 0.3 kb.
Basal transcription of transgenic 22-kDa α-zein promoter in leaf
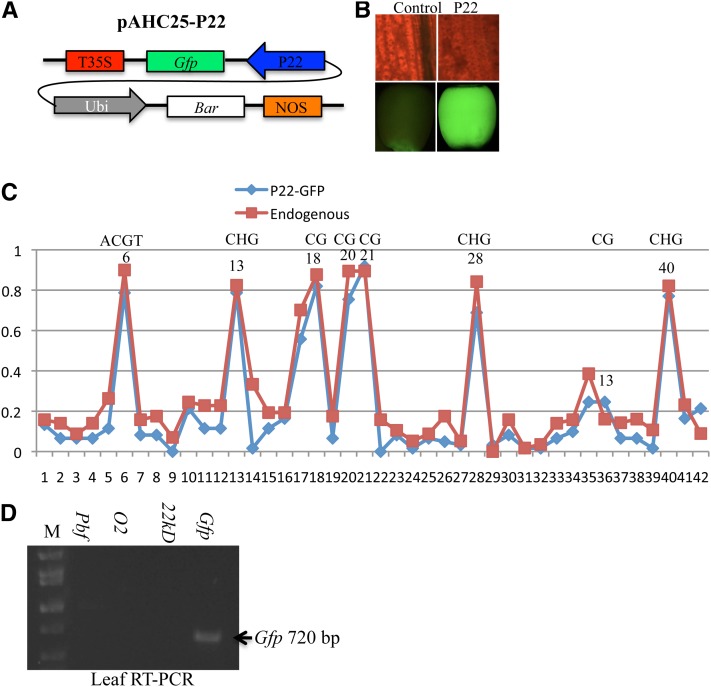
As noted above, there seems to be differences in the epigenetic state of the 27-kDa γ-zein and 22-kDa α-zein genes (Figure 4). The 22-kDa α-zein genes could not be activated in callus (Figure 3) (Wu and Messing 2009), but weak expression had been observed when a zein genomic DNA fragment Z4 comprising the entire length of a 22-kDa α-zein gene (Lewis et al. 1981; Hu et al. 1982) was transferred to sunflower callus (Matzke et al. 1984). However, different from the 27-kDa γ-zein gene, whose promoter was not significantly differentially methylated in callus, leaf, and endosperm (Figure 4A), the promoters of the 22-kDa α-zein genes were mildly methylated in callus and highly in leaf and demethylated only in endosperm (Figure 4B). Perhaps there are two classes of promoters, those that undergo developmental methylation changes (22-kDa α-zein) and those that do not (CaMV35S). When pAHC25-P22 (abbreviated as P22), a construct with the 22-kDa α-zein Zp22/6 promoter from BSSS53 driving GFP expression from an earlier particle bombardment-mediated transformation event (Figure 7A), was followed during plant development, fluorescent GFP signal was observed only in seeds (Figure 7B). Bisulfite sequencing analysis showed that the 263-bp fragment upstream of the transcription start site comprising the P-, O2-, CAAT-, and TATA boxes in the transgenic and endogenous 22-kDa α-zein promoters were highly methylated in leaf tissue (Figure 7C). As those in the endogenous α-zein promoters, cytosines were mainly methylated in the CG and CHG positions (Figure 7C, Figure 4B, and Figure S4). Still, low levels of GFP transcript levels could be detected by RT–PCR in leaf in the absence of the transcriptional activator O2, whereas no transcript of the endogenous 22-kDa α-zein genes was detectable (Figure 7D). Similarly, although expression of 22-kDa α-zein genes is dramatically reduced in the endosperm of the o2 mutant, their transcripts could still be detected by RT–PCR. The basal transcription of the GFP gene in leaf driven by the highly methylated 22-kDa α-zein promoter could also be confirmed with two other independent events generated with Agrobacterium-mediated transformation (data not shown).
Figure 7 .
Genetic behavior of the transgenic 22-kDa α-zein promoter in leaf and endosperm. (A) Structure of construct pAH25-P22. (B) GFP fluorescent signal absent in leaf (top) but strongly expressed in seed (bottom). (C) DNA methylation of the transgenic and endogenous 22-kDa α-zein promoters in leaf. Blue and red curves indicate the transgenic and endogenous 22-kDa α-zein promoters, respectively; the cytosine in the O2-box core motif at position 6 is indicated. Other symmetric cytosines (CG and CHG) are also indicated. (D) Weak expression of the transgenic 22-kDa α-zein promoter in leaf. M, DNA markers from top to bottom being 2, 1.55, 1.4, 1, 0.75, and 0.5 kb.
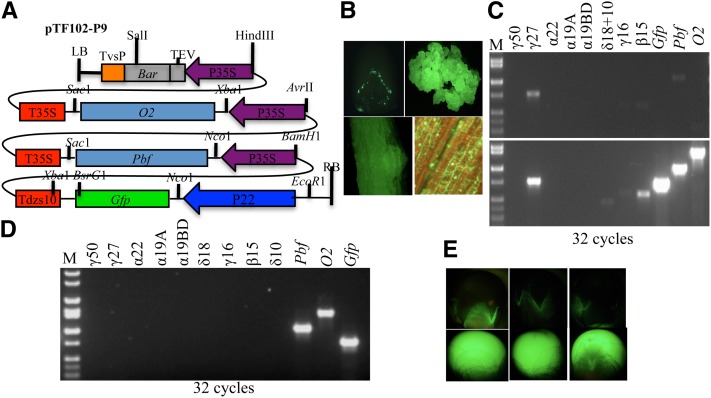
Transgenic 22-kDa α-zein promoter in the presence of PBF and O2 in leaf
If transcription was not completely suppressed by DNA methylation, could transcription be promoted in the presence of PBF and O2? In the new construct pTF102-P9 (abbreviated as P9) (Figure 8A), O2 and PBF are co-expressed by two separate CaMV 35S promoters and the GFP gene is driven by the Zp22/6 promoter (Figure S5C). Two hundred immature embryos were infected with Agrobacterium bearing this construct. Like P4 and P5 (Figure 4B), ∼70% embryos were monitored for transient GFP expression under a fluorescence dissection microscope (Figure 8B). Three growing calli had strong GFP expression. The event P9-1 was shown as an example in Figure 8B. Although the methylation level of the 22-kDa α-zein promoters is reduced in callus (Figure 4B), they were not expressed in the presence of PBF and O2, while the expression of the 27-kDa γ- and 15-kDa β-zein genes was significantly elevated compared to nontransgenic callus (Figure 8C). The regenerated root and later the adult leaf also continued to express GFP at high levels. RT–PCR analysis was performed to check expression in leaf of regenerated plants. The O2, Pbf, and Zp22/6-GFP genes were all strongly expressed in the three independent events as shown for the event P9-1 (Figure 8D) compared to low levels of expression in the absence of O2 and Pbf as shown in Figure 7D. Because of Mendelian segregation, progeny kernels inheriting the construct are entirely GFP positive. The GFP signal could even be observed in the maternal glume tissue, but not in the pericarp of progenies not inheriting the construct (Figure 8E).
Figure 8 .
trans-activation of the transgenic 22-kDa α-zein promoter by overexpression of PBF and O2 in leaf. (A) Structure of construct pTF102-P9. (B) Ectopic expression of GFP in immature embryo, callus, root, and leaf; (C and D) RT–PCR analysis of construct P9 and zein genes in callus (C) and leaf (D). In C, the top and bottom are the nontransgenic control and transgenic calli, respectively. M, DNA markers from top to bottom are 4, 3, 2, 1.55, 1.4, 1, 0.75, 0.5, 0.4, and 0.3 kb. (E) GFP expression in progeny seeds not inheriting (top) or inheriting (bottom) the construct.
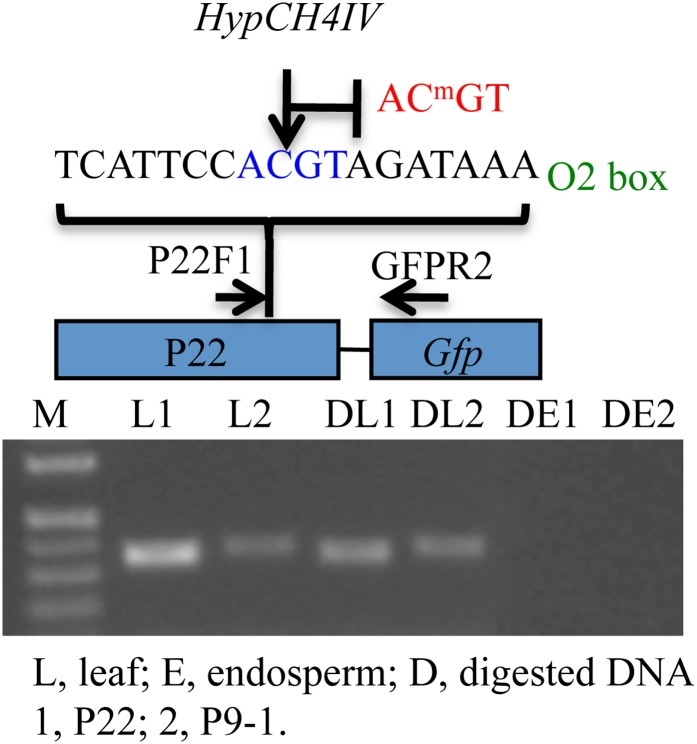
Nevertheless, similarly to the endogenous promoters of the 22-kDa α-zein genes, the cytosines in CG and CHG positions were highly methylated (Figure 9). The restriction enzyme HypCH4IV recognizes the ACGT target sequence, but is blocked by cytosine methylation. Because the ACGT element is unique in the interval from the P-box to the ATG start codon in the 22-kDa α-zein promoter (Figure S4), a methylation-sensitive PCR assay could be performed. Indeed, a primer pair as shown in Figure 10 could specifically amplify the transgenic 22-kDa α-zein promoter comprising the HypCH4IV recognition site ACGT. This specific fragment could be both amplified from undigested leaf and endosperm genomic DNA of P22 and P9-1. When DNA was digested with HypCH4IV, the fragment could only be amplified from leaf genomic DNA, in which HypCH4IV digestion was blocked by cytosine methylation (Figure 10), indicating that DNA methylation/demethylation of the transgenic and endogenous promoters were coordinatedly regulated by a common set of factors. Still, tissue-specific transcription was restricted to the endogenous promoter.
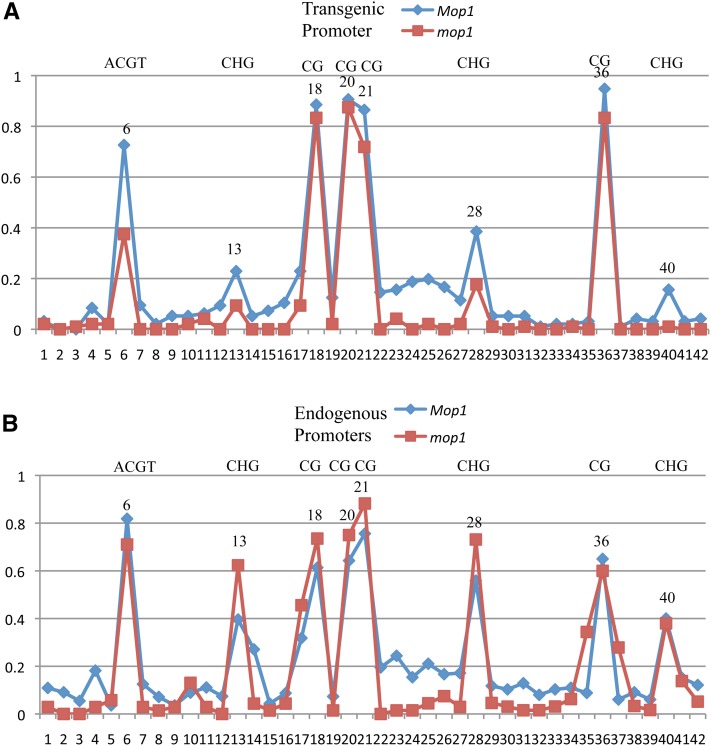
Figure 9 .
DNA methylation of the transgenic and endogenous 22-kDa α-zein promoters of pTF102-P9-1 in Mop1 wild-type and mop1 null mutant. (A) Transgenic 22-kDa α-zein promoter. (B) Endogenous 22-kDa α-zein promoters. The cytosine in the O2-box core motif at position 6 is marked. Other symmetric cytosines (CG and CHG) are also indicated.
Figure 10 .
Detection of methylation and demethylation of the transgenic 22-kDa α-zein promoter in leaf and endosperm by methylation sensitive enzyme HypCH4IV. M, DNA markers from top to bottom are 0.75, 0.5, 0.4, and 0.3 kb.
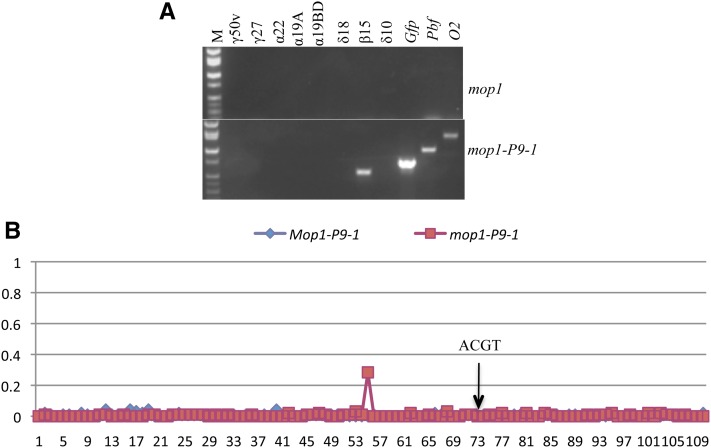
Because transcriptional silencing of the CaMV35S promoter could be reversed in the mop1 mutant concurrent with the removal of methylation (Figures 6C, Figure S6, A and B) one would wonder whether methylation of the 22-kDa α-zein promoters also required MOP1 function. Interestingly, whereas cytosines at non-CG sites were generally lower in methylation in the mop1 mutant than in wild type, those at CG sites were still highly methylated in transgenic or endogenous promoters (Figure 9), indicating that maintenance of symmetric methylation in 22-kDa α-zein promoters does not require MOP1 function in contrast to the 35S promoter, in which methylation in all contexts was lost in the mop1 mutant (Figure S6, A and B). Still, the reduction in non-CG methylation and constitutive expression of PBF and O2 in leaves did not activate the endogenous 22-kDa α-zein promoters (Figure 11A).
Figure 11 .
Activation of β-zein gene in mop1 mutant with overexpression of PBF and O2. (A) RT–PCR analysis of the expression of zein genes in mop1 (top) and mop1-P9-1 (bottom); M, DNA markers from top to bottom are 2, 1.55, 1.4, 1, 0.75, 0.5, 0.4, and 0.3 kb. (B) DNA methylation of β-zein promoter in Mop1-P9-1 and mop1-P9-1. The cytosine in the O2-box core motif at position 73 is marked.
Activation of β-zein in mop1 in the presence of PBF and O2
Although the O2 box is located 124 bp distal to the P-box of the 15-kDa β-zein (Figure S7), it is also regulated by O2 (Cord Neto et al. 1995). An elite hybrid cDNA database also suggested expression in leaf (accession no. BM337640) (Swanson-Wagner et al. 2006), but we could not detect its expression in leaf of the Hi-II B × A hybrid even in the presence of PBF and O2 (Figure 8D). However, when constitutive expression of O2 and PBF in the mop1 mutant was used to investigate expression of the 15-kDa β-zein gene by RT–PCR, it was expressed (Figure 11A), whereas other zein genes were not. Moreover, bisulfite sequencing results showed that the 532-bp fragment upstream of the start codon, comprising the P-, O2-, and TATA boxes with 110 cytosines, was hypomethylated in every context including the ACGT motif regardless whether MOP1 was present or not (Figures 11B and Figure S7), suggesting that DNA methylation of the target gene is not essential for RDR2’s mode of function. Still, there could be an element further upstream of the 532 bp that was responsive to mop1 and served as an enhancer for the expression of the 15-kDa β-zein.
Discussion
We have investigated here the genetic interactions of two transcription factors, O2 and PBF, with promoters of the prolamin gene family in maize. Because no null mutant was available, we used RNAi to knock down the expression of PBF. We examined tissue specificity of transgenic and endogenous zein gene expression by constitutively expressing PBF and O2. Because of differences in their capacity to respond to PBF and O2, we also examined the methylation status of target promoters during plant development. As methylation changes in target promoter regions appear to be critical for transcriptional activation of promoters, we also examined whether methylation changes were controlled by RDR2, which was facilitated through the maize mop1 mutation.
Endosperm-specific transcriptional factors
O2 is endosperm-specifically expressed about 10 DAP, containing the basic leucine-zipper (bZIP) DNA binding motif (Schmidt et al. 1992). O2 forms a homodimer that transactivates transcription of many genes in maize endosperm (Hunter et al. 2002). However, O2 target elements are highly variable among different grass species. In the zein-gene superfamily, only a subset of α- and the β-zein genes are regulated by O2. In the o2 mutant, γ-zeins are not affected, suggesting that O2 is not the common factor, regulating all zein-gene expression.
Compared to O2 target elements, the PBF target element, the P-box, is conserved across the grass family (Xu and Messing 2009). In addition, PBF is also expressed in endosperm from around 10 DAP, concomitant with trans-activation of all zein genes, leading to the hypothesis that the spatial and temporal regulation of zein genes might be synchronized by PBF. However, when all zein-gene genomic sequences are compared, some of them like the 16-kDa γ- and two δ-zein genes do not have a P-box in their −300 promoter regions (Figure S1 and Figure S2). Moreover, in PbfRNAi, only the synthesis of the 27-kDa γ- and 22-kDa α-zeins are dramatically reduced, while other zeins appear to be insensitive to the reduction of PBF (Figure 2D). Although the 19-kDa α-zein promoters do have a P-box (Figure S1), their expression is not significantly affected in contrast to the 22-kDa α-zeins. Similarly, the 19-kDa α-zeins are not reduced as much as the 22-kDa α-zeins in o2 mutant, indicating that besides γ-zeins, the two subclasses of α-zeins have also undergone divergent gene regulation. Expression of PBF and O2 failed to trans-activate any of the endogenous zein genes in leaf, but did enhance the expression of transgenic 27-kDa γ- and 22-kDa α-zein promoters, which have already basal transcription in nonendosperm tissues (Figures 5–8).
In summary, the expression of all zein genes except the 16-kDa γ-zein was affected either by PbfRNAi (27-kDa γ- and 22-kDa α-zein genes) or o2 (α-, β-, and δ-zein genes), and the additive effect of the double mutant was observed only on the expression of 19- and 22-kDa α-zeins (Table 1).
Table 1 . Summary of cis- and trans-regulations of zein genes.
| Zein genes |
Cis-elements |
Gene expressiona |
|||
|---|---|---|---|---|---|
| P-box | O2 or O2-like box | PbfRNAi | o2 | PbfRNAi/−; o2/o2 | |
| γ-27 | + | + | Reduced | Normal | Reduced |
| α-22 | + | + | Reduced | Reduced | Additive effect |
| α-19 | + | − | Normal | Reduced | Additive effect |
| γ-16 | − | − | Normal | Normal | Normal |
| β-15 | + | + | Normal | Reduced | Reduced |
| δ-10 | − | − | Normal | Reduced | Reduced |
Gene expression in mutants compared to normal background.
Developmental change of epigenetic state of zein promoters
We have investigated the interplay between trans-acting factors and their target genes by varying the timing of the expression of the trans-acting factors. The interesting aspect of this approach is that we can unveil epigenetic properties of developmental regulated genes. Because it had been shown that the 22-kDa α-zein genes are methylated in leaf tissue and demethylated in endosperm, we could investigate whether such methylation would be sufficient to block the trans-acting factors in their function, even if expressed at sufficient levels. We also investigated how in the same circumstances the same promoters would behave when inserted into the genome by transformation. Indeed, when Pbf and O2 are expressed in leaf, the de novo methylated transgenic 22-kDa promoter could be activated whereas the endogenous methylated ones could not. Therefore, trans-activation is not blocked or completely blocked by any methylation of the transgenic promoter.
We also took advantage of the RDR2 mutation in maize (mop1) that blocks the siRNA-directed DNA methylation and confirmed the canonical belief that transgenic promoters could be silenced through this pathway. Although the 22-kDa promoters undergo methylation changes correlating with their tissue-specific expression in endosperm (Figure 4B), we could corroborate that the methylation changes for tissue specificity were different from those caused by RDR2, like silencing of the 35S promoter (Figure 4B, Figure 9, and Figure S6, A–C). Although both of the transgenic 22-kDa and 35S promoters had been subjected to de novo methylation, they underwent different developmental changes. Methylation of the former was sustained without RDR2 (Figure 9A) and only developmentally demethylated in endosperm (Figure 10), whereas the latter could not be demethylated in endosperm (Figure S6C) and required RDR2 for maintenance of methylation (Figure S6, A and B). In contrast to the 22-kDa α-zein genes, the endogenous 27-kDa γ-zein promoter would not change its methylation status regardless whether it was expressed or not (Figure 4A and Figure S6D).
In the presence of PBF and O2 and in the absence of MOP1, the only transactivated zein gene in leaf is β-zein (Figure 11A). Although the ectopic expression of β-zein was not correlated with any methylation changes, there could be an enhancer element regulated by MOP1 further upstream in the promoter region of the β-zein gene.
Coordinated regulation of DNA methylation in transgenic and endogenous 22-kDa α-zein promoters
The transgenic 22-kDa α-zein promoter was methylated in regenerated plants regardless whether it was transformed by Agrobacterium or particle bombardment (Figures 7C and 9A). The de novo DNA methylation of cytosines in all sequence contexts requires DRM1 and DRM2, which is mediated by siRNAs (Cao and Jacobsen 2002; Chan et al. 2005), a process termed RNA-directed DNA methylation (RdDM). In flowering plants, the most abundant siRNAs originate from transposon loci and DNA repeats. The siRNA precursor transcripts are transcribed by DNA-dependent RNA polymerase IV (Erhard et al. 2009; Hale et al. 2009) and then serve as the template for an RNA-dependent RNA polymerase, RDR2, to generate double-stranded RNAs (dsRNAs), which are further processed by Dicer-like 3 (DCL3) into 24-nt siRNAs. Besides direction of de novo DNA methylation in heterochromatic regions, RDR2 is also required for transcriptional silencing of transgenes. The lack of MOP1 (RDR2 in maize) can reverse silencing of the 35S promoter by removal of methylation in all contexts (Figure S6, A and B) (McGinnis et al. 2006). Although a decrease of overall non-CG methylation was observed in the mop1-P9-1 event for both endogenous and transgenic 22-kDa α-zein promoters, the methylation in CG context was still high (Figure 9). In contrast to fungi and animals, in which methylation is predominantly in the CG sequence context, plants have 5-methylcytosine at CG, CHG, and even asymmetric CNN sites. In A. thaliana, maintenance and de novo CG methylation depends on MET1, a homolog of the mammalian DNA methyltransferase 1, whereas non-CG methylation is regulated by two members of DOMAINS REARRANGED METHYLTRANSFERASE (DRM1 and DRM2) and CHROMOMETHYLASE3 (CMT3) (Chan et al. 2005). It seems that the symmetric methylation in the 22-kDa α-zein promoters can be maintained in a DNA replication-coupled way by MET1.
Still, both the endogenous and the transgenic zein promoters were specifically demethylated in endosperm (Figures 10 and 4B), indicating that they were subject to synchronized epigenetic regulation different from a siRNA-mediated mechanism. However, due to lack of mutants that affect demethylation of the 22-kDa α-zein promoters in endosperm and methylation in leaf, it is not clear whether the switch of the methylation status is sufficient to mediate the specific expression of the 22-kDa α-zein genes. In addition, O2 regulates many genes in endosperm (Hunter et al. 2002) and can autoregulate the expression of its own promoter by recognition of its target sequence also comprising an ACGT core element. Like the 22-kDa α-zein promoters, O2 promoter was less methylated in endosperm than in leaf. However, comparison of the methylation patterns in endosperm and leaf revealed that endosperm-specific expression of the O2 gene was not mediated by demethylation of a specific cytosine. Further, gel-shift experiments showed that O2 binding activity was not impaired by CpG methylation (Rossi et al. 1997). Although the methylation in ACGT was shown to reduce the O2 affinity for the 22-kDa promoter, a detectable binding activity was still observed (Sturaro and Viotti 2001), indicating that DNA methylation alone was not sufficient to completely silence the transcription of the 22-kDa α-zein genes. On the other hand, when methylation levels were reduced in callus, expression of the 22-kDa α-zeins was not detected in the presence of PBF and O2 (Figure 8C).
These results indicate a rapid divergence of prolamin promoter structures and corresponding DNA binding properties probably due to amplification and dispersal of gene copies into a different chromosomal epigenetic context, which is consistent with our previous observations (Miclaus et al. 2011).
Supplementary Material
Acknowledgments
We thank Gregorio Segal for his invaluable work on particle bombardment-mediated transformation of P22 and Yubin Li and Hugo Dooner for their generous provision of their transgenic events. The research described in this article was supported by the Selman A. Waksman Chair in Molecular Genetics.
Footnotes
Communicating editor: S. Poethig
Literature Cited
- Alleman M., Sidorenko L., McGinnis K., Seshadri V., Dorweiler J. E., et al. , 2006. An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442: 295–298 [DOI] [PubMed] [Google Scholar]
- Bianchi M. W., Viotti A., 1988. DNA methylation and tissue-specific transcription of the storage protein genes of maize. Plant Mol. Biol. 11: 203–214 [DOI] [PubMed] [Google Scholar]
- Cao X., Jacobsen S. E., 2002. Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12: 1138–1144 [DOI] [PubMed] [Google Scholar]
- Chan S. W., Henderson I. R., Jacobsen S. E., 2005. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6: 351–360 [DOI] [PubMed] [Google Scholar]
- Cord Neto G., Yunes J. A., da Silva M. J., Vettore A. L., Arruda P., et al. , 1995. The involvement of Opaque 2 on beta-prolamin gene regulation in maize and Coix suggests a more general role for this transcriptional activator. Plant Mol. Biol. 27: 1015–1029 [DOI] [PubMed] [Google Scholar]
- Erhard K. F., Jr, Stonaker J. L., Parkinson S. E., Lim J. P., Hale C. J., et al. , 2009. RNA polymerase IV functions in paramutation in Zea mays. Science 323: 1201–1205 [DOI] [PubMed] [Google Scholar]
- Frame B. R., Shou H., Chikwamba R. K., Zhang Z., Xiang C., et al. , 2002. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 129: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C. J., Erhard K. F., Jr, Lisch D., Hollick J. B., 2009. Production and processing of siRNA precursor transcripts from the highly repetitive maize genome. PLoS Genet. 5: e1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N. T., Peifer M. A., Heidecker G., Messing J., Rubenstein I., 1982. Primary structure of a genomic zein sequence of maize. EMBO J. 1: 1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhang K., Shen Y., Huang Z., Li M., et al. , 2009. Identification of a high frequency transposon induced by tissue culture, nDaiZ, a member of the hAT family in rice. Genomics 93: 274–281 [DOI] [PubMed] [Google Scholar]
- Hunter B. G., Beatty M. K., Singletary G. W., Hamaker B. R., Dilkes B. P., et al. , 2002. Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell 14: 2591–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. S., Ciceri P., Parsons R. L., Moose S. P., Schmidt R. J., et al. , 2004. The maize O2 and PBF proteins act additively to promote transcription from storage protein gene promoters in rice endosperm cells. Plant Cell Physiol. 45: 1509–1518 [DOI] [PubMed] [Google Scholar]
- Kaeppler S. M., Phillips R. L., 1993. Tissue culture-induced DNA methylation variation in maize. Proc. Natl. Acad. Sci. USA 90: 8773–8776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. D., Hagen G., Mullins J. I., Mascia P. N., Park W. D., et al. , 1981. Cloned genomic segments of Zea mays homologous to zein mRNAs. Gene 14: 205–215 [DOI] [PubMed] [Google Scholar]
- Locatelli S., Piatti P., Motto M., Rossi V., 2009. Chromatin and DNA modifications in the Opaque2-mediated regulation of gene transcription during maize endosperm development. Plant Cell 21: 1410–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M. A., Susani M., Binns A. N., Lewis E. D., Rubenstein I., et al. , 1984. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 3: 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis K. M., Springer C., Lin Y., Carey C. C., Chandler V., 2006. Transcriptionally silenced transgenes in maize are activated by three mutations defective in paramutation. Genetics 173: 1637–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P., Niedenhof I., ten Lohuis M., 1994. Evidence for cytosine methylation of non-symmetrical sequences in transgenic Petunia hybrida. EMBO J. 13: 2084–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclaus M., Xu J. H., Messing J., 2011. Differential gene expression and epiregulation of alpha zein gene copies in maize haplotypes. PLoS Genet. 7: e1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngezahayo F., Xu C., Wang H., Jiang L., Pang J., et al. , 2009. Tissue culture-induced transpositional activity of mPing is correlated with cytosine methylation in rice. BMC Plant Biol. 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. D., Papp I., Moscone E. A., Iglesias V. A., Vaucheret H., et al. , 1996. Gene silencing mediated by promoter homology occurs at the level of transcription and results in meiotically heritable alterations in methylation and gene activity. Plant J. 9: 183–194 [DOI] [PubMed] [Google Scholar]
- Peschke V. M., Phillips R. L., Gengenbach B. G., 1987. Discovery of transposable element activity among progeny of tissue culture–derived maize plants. Science 238: 804–807 [DOI] [PubMed] [Google Scholar]
- Rossi V., Motto M., Pellegrini L., 1997. Analysis of the methylation pattern of the maize opaque-2 (O2) promoter and in vitro binding studies indicate that the O2 B-Zip protein and other endosperm factors can bind to methylated target sequences. J. Biol. Chem. 272: 13758–13765 [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Ketudat M., Aukerman M. J., Hoschek G., 1992. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell 4: 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Messing J., 2003. Gene expression of a gene family in maize based on noncollinear haplotypes. Proc. Natl. Acad. Sci. USA 100: 9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R., Llaca V., Linton E., Messing J., 2001. Sequence, regulation, and evolution of the maize 22-kD alpha zein gene family. Genome Res. 11: 1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturaro M., Viotti A., 2001. Methylation of the Opaque2 box in zein genes is parent-dependent and affects O2 DNA binding activity in vitro. Plant Mol. Biol. 46: 549–560 [DOI] [PubMed] [Google Scholar]
- Swanson-Wagner R. A., Jia Y., DeCook R., Borsuk L. A., Nettleton D., et al. , 2006. All possible modes of gene action are observed in a global comparison of gene expression in a maize F1 hybrid and its inbred parents. Proc. Natl. Acad. Sci. USA 103: 6805–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Waverczak W., Ward K., Sher N., Ketudat M., et al. , 1992. Mutations of the 22- and 27-kD zein promoters affect transactivation by the Opaque-2 protein. Plant Cell 4: 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Carbajosa J., Moose S. P., Parsons R. L., Schmidt R. J., 1997. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc. Natl. Acad. Sci. USA 94: 7685–7690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Messing J., 1998. Modulation of gene expression by DNA-protein and protein-protein interactions in the promoter region of the zein multigene family. Gene 223: 333–345 [DOI] [PubMed] [Google Scholar]
- Wu Y., Messing J., 2009. Tissue-specificity of storage protein genes has evolved with younger gene copies. Maydica 54: 409–415 [Google Scholar]
- Wu Y., Messing J., 2010. RNA interference-mediated change in protein body morphology and seed opacity through loss of different zein proteins. Plant Physiol. 153: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Messing J., 2012. RNA interference can rebalance the nitrogen sink of maize seeds without losing hard endosperm. PLoS ONE 7: e32850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang W., Messing J., 2012. Balancing of sulfur storage in maize seed. BMC Plant Biol. 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. H., Messing J., 2008. Organization of the prolamin gene family provides insight into the evolution of the maize genome and gene duplications in grass species. Proc. Natl. Acad. Sci. USA 105: 14330–14335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. H., Messing J., 2009. Amplification of prolamin storage protein genes in different subfamilies of the Poaceae. Theor. Appl. Genet. 119: 1397–1412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.