Abstract
The gp120 CD4 binding site (CD4bs) and coreceptor binding site (CoRbs) are two functionally conserved elements of the HIV-1 envelope glycoproteins (Env). We previously defined the presence of CD4bs-neutralizing antibodies in the serum of an HIV-1-infected individual and subsequently isolated the CD4bs-specific monoclonal antibodies (MAbs) VRC01 and VRC03 from the memory B cell population. Since this donor's serum also appeared to contain neutralizing antibodies to the CoRbs, we employed a differential fluorescence-activated cell sorter (FACS)-based sorting strategy using an Env trimer possessing a CoRbs knockout mutation (I420R) to isolate specific B cells. The MAb VRC06 was recovered from these cells, and its genetic sequence allowed us to identify a clonal relative termed VRC06b, which was isolated from a prior cell sort using a resurfaced core gp120 probe and its cognate CD4bs knockout mutant. VRC06 and VRC06b neutralized 22% and 44% of viruses tested, respectively. Epitope mapping studies revealed that the two MAbs were sensitive to mutations in both the gp120 CoRbs and the CD4bs and could cross-block binding of both CD4bs and CoRbs MAbs to gp120. Fine mapping indicated contacts within the gp120 bridging sheet and the base of the third major variable region (V3), which are elements of the CoRbs. Cell surface binding assays demonstrated preferential recognition of fully cleaved Env trimers over uncleaved trimers. Thus, VRC06 and VRC06b are Env trimer precursor cleavage-sensitive neutralizing MAbs that bind to a region of gp120 that overlaps both the primary and the secondary HIV-1 receptor binding sites.
INTRODUCTION
The HIV-1 envelope glycoproteins (Env) are synthesized as a trimeric gp160 precursor protein, which is cleaved in the Golgi body by cellular furins, resulting in a heterotrimeric viral spike. The viral spike consists of the exterior envelope glycoprotein, gp120, which is noncovalently associated with the gp41 transmembrane envelope glycoprotein (39, 45). The HIV Env mediates virus entry by the initial binding of gp120 to the primary receptor, CD4, and subsequently to the major coreceptor, CCR5 (reviewed in references 2 and 50). Receptor-coreceptor interactions trigger further conformational changes in gp41 that lead to insertion of the gp41 fusion peptide into the target cell membrane to initiate fusion of the virus and target cell membranes and conclude viral entry.
The CD4 binding site (CD4bs) of gp120 consists of the functionally conserved CD4 binding loop (residues 365 to 373) and other proximal elements (19). The coreceptor binding site (CoRbs) of gp120 consists of a highly conserved bridging sheet, emanating from both the inner and outer domains, and the third major variable region (V3) (6, 31, 32). The positively charged bridging sheet and the V3 base region interact with the negatively charged CCR5 N terminus, and the tip of V3 interacts with the second extracellular loop of CCR5 during viral entry (7, 11, 14).
During natural infection, multiple forms of gp120 likely elicit a diverse and robust polyclonal antibody response. Monomeric gp120, shed from the Env spike, likely elicits both virus-neutralizing antibodies (NAbs) and nonneutralizing antibodies, with the latter being often directed against the gp120 regions occluded on the Env trimer (reviewed in references 27, 30, and 50). Both the CD4bs and CoRbs of HIV-1 gp120 are immunogenic; however, broadly reactive NAbs (bNAbs) against the CD4bs are infrequent and antibodies against the CoRbs are unable to neutralize primary viral isolates, presumably due to the fact that the CoRbs is occluded on the Env functional spike of the primary viruses prior to engagement of the primary receptor, CD4 (5, 20, 43; reviewed in references 27, 30, and 50). Prior work based on phage display or B cell transformation technology led to the isolation of CD4bs monoclonal antibodies (MAbs) b12 and HJ16, which can neutralize up to 40% of primary virus isolates (4, 8). Our previous studies and that of others revealed that broad and potent CD4bs-specific neutralizing activity could be detected in sera from a small minority of HIV-1-infected individuals (13, 23, 25, 34). From the memory B cell repertoire of one such individual, donor 45, we isolated the broadly reactive CD4bs-specific MAbs VRC01 and VRC03 (47). Subsequently, MAbs similar to VRC01 were isolated from a small set of other HIV-1-infected individuals (36, 49). In addition, in the serum of donor 45, we had previously detected a second and potentially distinct neutralizing specificity against the conserved CoRbs region of gp120 (25). This second serum antibody specificity was determined by differential protein adsorption using a wild-type (WT) gp120 and a mutant gp120 with a single point mutation in the coreceptor binding region (I420R), followed by neutralization analysis (25). In the current study, we isolated MAbs from donor 45 with binding specificity that overlaps both the HIV gp120 primary receptor CD4bs and the CoRbs. These clones coexist with VRC01 in the B cell repertoire of a single individual, conferring diverse neutralizing capacity, and likely influence the evolution of viral escape mutants within this individual (46). The existence of NAbs targeting elements of the CoRbs indicate that the human B cell repertoire can generate antibodies that are able to access this region on the primary virus Env functional spike. These dual CD4bs and CoRbs-directed bNAbs preferentially recognized fully cleaved Env functional trimers compared to uncleaved, nonfunctional trimers. These new MAbs will be valuable tools for the identification and evaluation of trimeric immunogens designed to elicit bNAbs by mimicry of the cleaved, functional Env spike.
MATERIALS AND METHODS
Human sera and PBMC samples.
The sera and peripheral blood mononuclear cells (PBMCs) described in this study were from an HIV-1-infected individual enrolled in investigational review board-approved clinical protocols at the National Institute of Allergy and Infectious Diseases. Donor 45, from whom MAbs VRC01, VRC02, VRC03 (47), VRC06, and VRC06b were isolated, has been infected with a clade B HIV-1 virus for more than 15 years. This patient is a slow progressor with CD4 T-cell counts of over 500 cells/ml and plasma HIV-1 RNA values of <15,000 copies/ml and had not initiated antiretroviral treatment at the time of PBMC sampling (23).
Probe production and isolation of CoRbs-specific memory B cells by fluorescence-activated cell sorting (FACS) and single B cell reverse transcription (RT)-PCR to amplify IgG genes.
Both expression plasmids for the HXBc2 gp120 stabilized core (2CC) and YU2 gp140-F I420R contain an Avi tag signal at the C termini. Expression of these envelope proteins was carried out by transfecting the envelope-encoding plasmid DNA into 293F FreeStyle cells as described previously (25). HXBc2 gp120 stabilized core was purified by 17b-protein A affinity column as previously described (10), and YU2 gp140-F I420R was purified by lentil lectin affinity chromatography followed by chelation chromatography (25, 26). The purified Avi-tagged proteins were biotinylated with biotin ligase Bir A (Avidity, Denver, CO) followed by conjugation with streptavidin fluorochrome as described previously (47). The 2CC core was conjugated with streptavidin-APC (Invitrogen) and YU2 gp140-F I420R with streptavidin-Qdot565 (Invitrogen). CoRbs-specific B cells were identified with a panel of ligands including fluorescently labeled antibodies for CD3, CD4, CD8, CD19, CD20, CD14, IgG, IgM, and envelope glycoprotein including 2CC core and CoRbs knockout mutant YU2 gp140-F I420R. PBMCs from donor 45 were stained with an antibody mixture consisting of anti-CD3-APC-Cy7 (BD Pharmingen), CD4-Qdot655 (VRC), CD8-Qdot655 (VRC), CD19-Qdot585 (VRC), CD20-Pacific Blue (VRC), CD14-Qdot800 (VRC), IgG-FITC (BD Pharmingen), and IgM-PE-Cy5 (BD Pharmingen). In addition, aqua blue (Invitrogen) was used to exclude dead cells. CoRbs-specific memory B cells were identified with the phenotype of CD19+, CD20+, IgG+, IgM−, CD3−, CD4−, CD8−, aqua blue−, 2CC core+, YU2 gp140-F I420R−, and single cells were sorted into 96-well PCR plates containing 20 μl of lysis buffer per well followed by single B cell RT-PCR. Subsequent sequencing and cloning were performed as previously described (47).
MAbs and CD4-Ig.
MAb 2G12 was purchased from Polymun Scientific Inc. (Vienna, Austria). CD4bs MAbs b6, b13, b12, VRC01, and VRC03 were described previously (4, 47), and F105 was provided by Marshall Posner (Dana Farber Cancer Institute). The CoRbs-specific MAbs 17b, 48d, 412d, and 2.1C were kindly provided by James Robinson (Tulane University). The CD4-Ig plasmid construct, containing the D1D2 domain of CD4 fused to the human IgG Fc domain, was provided by Joseph Sodroski (Dana Farber Cancer Institute). This fusion protein was expressed by transient transfection of 293F FreeStyle cells with plasmid DNA and purified using a recombinant protein A column (GE Healthcare). The 4-domain soluble CD4 (sCD4) was purchased from Progenics Pharmaceuticals (Tarrytown, NY).
IgG gene family analysis and amino acid residue numbering.
The nucleotide sequences of IgG heavy and light chain variable regions were analyzed with IMGT/V-Quest (http://www.imgt.org/IMGT_vquest/share/textes/) and JoinSolver (http://Joinsolver.niaid.nih.gov) (38). We followed IMGT (http://www.imgt.org/) numbering for annotation of amino acid residues in the antibodies as indicated. Kabat numbering (16) was used in the sequence alignment in Fig. S3 in the supplemental material.
Biotin labeling of MAbs.
For competition enzyme-linked immunosorbent assay (ELISA), MAbs were labeled with biotin using EZ-Link NHS-biotin reagent (Pierce, Rockford, IL). After biotin labeling, antibody-binding activity was verified by gp120 ELISA, and signals were developed with streptavidin-horseradish peroxidase (HRP) conjugate (Sigma, St. Louis, MO) at 1:1,000 dilution, followed by the addition of TMB single solution substrates to develop colorimetric reporter signals (Invitrogen, Carlsbad, CA).
ELISA binding and cross-competition assays.
Purified envelope glycoprotein ligands at 2 μg/ml with 100 μl/well were used to coat ELISA plates at 4°C overnight prior to ELISA, as described previously (47). Competition ELISA was carried out with an established protocol (28) with minor modifications (47). Briefly, gp120 was captured on ELISA plate wells precoated with sheep anti-gp120 C5 antibody D7324 (Aalto Bio Reagents, Dublin, Ireland). Biotin-labeled CoRbs MAbs with concentrations pretitrated to produce optical density at 450 nm (OD450) signals for gp120 binding ranging from 0.7 to 2 were used in the competition assay, along with competitors at 10-fold serial dilutions starting at 50 μg/ml. Since VRC06 showed greatly reduced binding activity following biotin labeling, the competition ELISA was performed in an alternative way as follows. The biotin-labeled CD4bs or CoRbs ligands were used as test antibodies and intact VRC06 as competitor at 10-fold serial dilutions starting at 50 μg/ml. To test the MAbs' gp120 binding competition with sCD4, gp120 was captured as described above followed with addition of sCD4 at concentrations starting at 50 μg/ml at 10-fold serial dilutions and incubated for 30 min. MAbs were added at concentrations pretitrated as described above and incubated for 1 h. For fine mapping, we used a full-length gp120 or YU2 gp120 Δ82ΔV1V2 protein for ELISA (32). The full-length gp120 and mutant proteins were expressed in 293F FreeStyle cells and purified as described previously (23). The gp120 Δ82ΔV1V2 protein and its selected mutants were expressed from plasmid DNA using the vector pSVIII Env (kindly provided by Joseph Sodroski, Dana Farber Cancer Institute). This vector was transfected into 293F FreeStyle cells along with plasmid pcTat at a 20:1 ratio, and cell culture supernatants containing WT and mutant gp120s were collected 4 days following transfection and stored at −80°C. For fine mapping, wells of each ELISA plate were coated with 1 μg/ml of a sheep anti-gp120 C5 antibody, D7324, followed by blocking with blocking buffer (phosphate-buffered saline [PBS]–2% milk–5% fetal bovine serum [FBS]), and 100 μl of WT or mutant gp120 supernatant was added to each well and incubated at 37°C for 1 h to capture the gp120. After washing with PBS-0.2% Tween 20, antibodies or CD4-Ig were added at 100 μl/well in blocking buffer at 5-fold serial dilutions starting at 10 μg/ml, and the wells were incubated at 37°C for 1 h. To develop ELISA signals of MAbs or CD4-Ig, goat anti-human IgG Fc-HRP conjugate (Jackson ImmunoResearch) was used at a 1:10,000 dilution in PBS containing 0.2% Tween 20. The colorimetric TMB single solution substrate (Invitrogen, Carlsbad, CA) was used to quantify the level of bound HRP. Fifty percent effective concentrations (EC50s) were determined at the antibody concentration where half-maximal binding was achieved using GraphPad Prism 5.0 curve-fitting software (GraphPad Software, La Jolla, CA) and the sigmoidal dose-response model with a variable slope. The binding affinity of the MAbs for the YU2 gp120 mutant proteins was calculated as (EC50 of WT gp120 for MAb)/(EC50 of mutant gp120 for MAb).
HIV-1 Env pseudoviruses and neutralization assays.
HIV-1 Env pseudoviruses were prepared by transfecting 293T cells with 10 μg of rev/env expression plasmids (pSVIII Env) and 30 μg of an env-deficient HIV-1 backbone vector (pSG3Δenv), using Fugene 6 transfection reagent (Roche Applied Science). Pseudovirus-containing culture supernatants were harvested 2 days after transfection, filtered with a 0.45-μm filter, and stored at −80°C or in the vapor phase of liquid nitrogen. Neutralization was measured using HIV-1 Env pseudoviruses to infect TZM-bl cells as described previously (22, 37, 48) with minor modifications. Briefly, 40 μl of virus was incubated with 10 μl of serially diluted test MAbs in duplicate wells of a 96-well flat-bottom culture plate, and the virus-MAb mixture was incubated for 30 min at 37°C. To keep assay conditions constant, sham medium was used in place of test MAbs in specified control wells. The virus input was set at a multiplicity of infection of approximately 0.01 to 0.1, which generally results in 100,000 to 400,000 relative light units (RLU) in a luciferase assay (Promega, Madison, WI). The test MAb concentrations were defined at the point of incubation with virus supernatant. Neutralization curves were fit by nonlinear regression using a 5-parameter hill slope equation as previously described (37). The 50% or 80% inhibitory concentrations (IC50 or IC80) were reported as the MAb concentrations required to inhibit infection by 50% or 80%.
Cell surface Env FACS staining.
To study VRC06 and other antibodies for recognition of the Env trimer, plasmid DNA of JR-FLgp160 cleavage(+)ΔCT (cleavage competent) or JR-FL gp160 cleavage(−)ΔCT (noncleaved, with cleavage site knocked out) was used to transfect 293T cells as described previously (29). Forty micrograms of JR-FL gp160 ΔCT DNA along with 2 μg of pcTat plasmid DNA and 150 μl of Fugene6 transfection reagent (Roche Applied Science) was used to transfect 10 million 293T cells seeded in a T225 flask. Cells were harvested 48 h after transfection. After washing with chilled PBS, the cells were resuspended with 6 ml of chilled PBS with 12 μl of Live/Dead Fixable Dead Cell violet fluorescent reactive dye (Invitrogen), distributed into a 96-well V-bottom plate at 50 μl/well, incubated at 4°C for 10 min, and covered with foil to distinguish dead and live cells. Antibodies were diluted in a 10-fold series starting at 200 μg/ml in FACS buffer (PBS–5% FBS–0.02% sodium azide) and then added to the violet-dye-stained cells at 50 μl/well to achieve a final antibody concentration starting at 100 μg/ml. Cells were then incubated at room temperature covered with foil for 1 h followed by washing once with FACS buffer. Secondary antibody, goat (Fab2′) anti-human IgG-phycoerythrin (PE) (SouthernBiotech, Birmingham, AL), was used to stain the cells in the dark at 4°C for 1 h, followed by washing 3 times with FACS buffer. Cells were fixed with 0.5% paraformaldehyde–PBS and subjected to FACS analysis on an LSR II (BD BioSciences), collecting fluorescence signals for PE as well as violet dye signal (405-nm excitation wavelength, and fluorescence emission was read at 450 nm) to exclude nonviable cells. The collected fluorescence signals were analyzed with FlowJo software (TreeStar, Cupertino, CA). Live cells were analyzed for PE fluorescence to derive antibody binding curves. Mean fluorescence intensity (MFI) of cells was used to assess the antibody recognition of the cell surface Env trimers.
Nucleotide sequence accession numbers.
The nucleotide sequences of the variable region of antibodies VRC06 and VRC06b are available in GenBank under accession numbers JX466923, JX466924, JX466925, and JX466926.
RESULTS
Isolation of CoRbs-directed MAbs from donor 45 memory B cells.
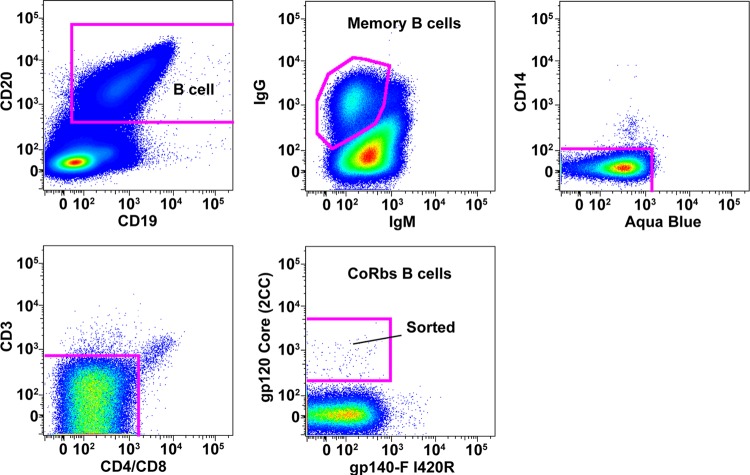
To isolate CoRbs-directed MAbs, we used peripheral blood mononuclear cells from donor 45, whose sera appeared to contain both CD4bs- and CoRbs-directed neutralizing activity (23, 25) (Fig. 1). After FACS-based identification of memory B cells (see Materials and Methods), CoRbs-specific B cells were selected based on high-level recognition of the stabilized gp120 core glycoprotein (2CC) and low-level recognition of the YU2 gp140-F glycoproteins possessing an I420R mutation. This mutation specifically eliminates or knocks out recognition by antibodies directed to the CoRbs (32, 51). The stabilized core contains a series of mutations that stabilize this molecule into the CD4-bound conformation and present the CoRbs better than the prototype gp120 core (10). Using this strategy, the CoRbs-specific memory B cells were sorted and deposited into 96-well PCR plates, followed by reverse transcription and nested PCR to amplify the matched IgG heavy and light chain coding genes (35, 42, 44, 47). Ten IgG heavy chain (HC) and kappa chain (KC) matched gene pairs were recovered, and their HC and KC genes were cotransfected into 293F FreeStyle cells to reconstitute full IgG for further analysis.
Fig 1.
Isolation of CoRbs-specific memory B cells by cell sorting. PBMCs from donor 45 were stained with a cocktail containing antibodies for cell surface markers and fluorochrome-labeled Env probes. Cells were gated as indicated: B cells, CD20+/CD19+; memory B cells, IgG+/IgM−; cells of CD14−/aqua blue−/CD3−/CD8− phenotype were retained, and dead cells (aqua blue+), monocytes (CD14+), and T cells (CD3+ or CD4+/CD8+) were gated out; CoRbs-specific memory B cells were gated as gp120 2CC core+/gp140FT I420R− and collected as single cells for RT-PCR. The I420R substitution in gp120 is a CoRbs knockout mutation.
Analysis of the putative CoRbs antibodies confirms specificity.
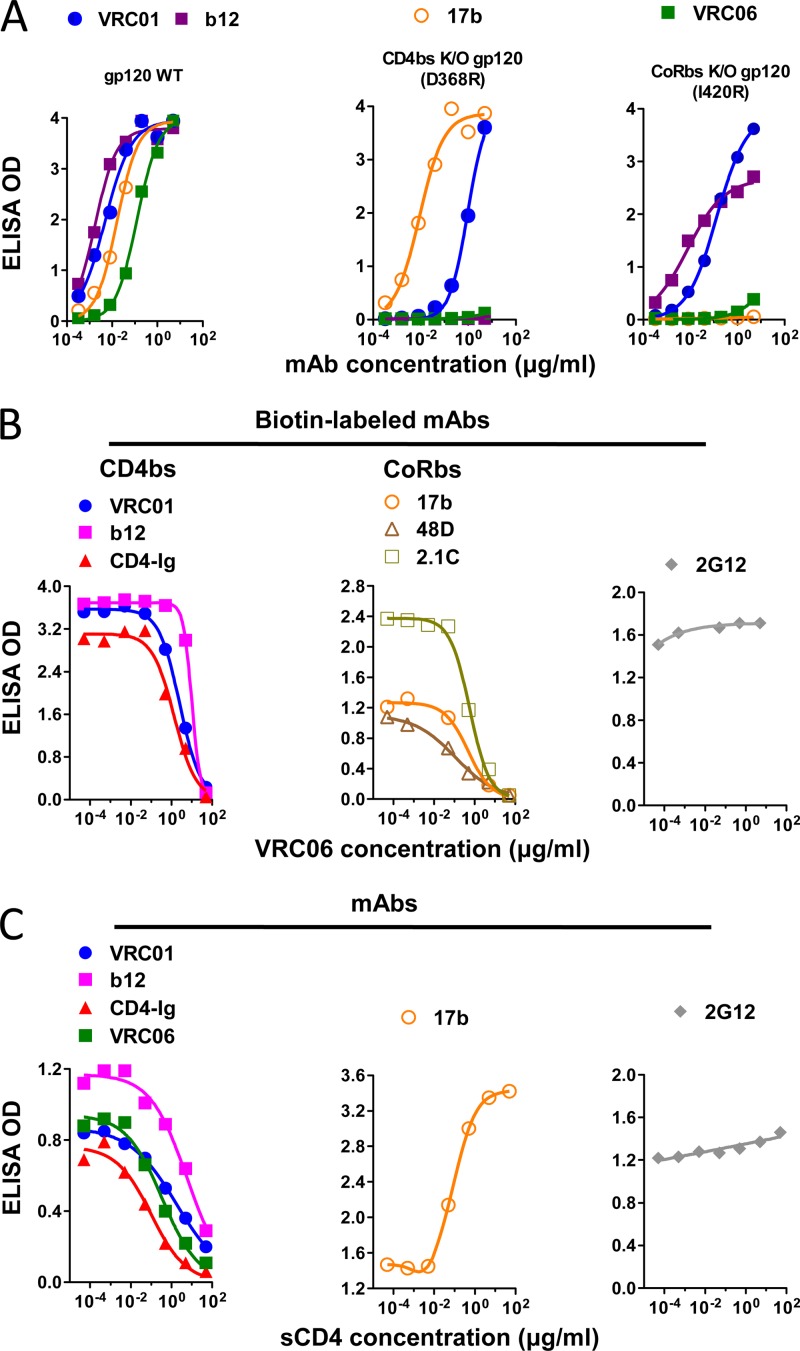
To confirm that the isolated IgG clones were specific for the CoRbs, we tested their binding specificity to gp120 variants by ELISA. As expected, each of the MAbs efficiently recognized wild-type gp120 and demonstrated markedly reduced binding to the CoRbs knockout mutant protein, gp120 I420R (Fig. 2A; see Fig. S1 in the supplemental material). However, one clone, termed VRC06, demonstrated reduced binding not only to the I420R gp120 variant but also to the CD4bs knockout variant, gp120 D368R (Fig. 2A). This binding specificity is different from that of the prototypic CD4bs ligands b12 and VRC01, which are not sensitive to the I420R gp120 mutation, and it is also different from that of the prototypic CoRbs MAb, 17b, which is not sensitive to the D368R mutation. These data suggested dual recognition of the functional receptor binding site determinants, i.e., that the VRC06 epitope spanned both primary receptor and coreceptor binding regions.
Fig 2.
Binding specificity of VRC06 to gp120 defined by ELISA. (A) VRC06 binding to YU2 gp120 was sensitive to gp120 mutations in both the CD4bs (D368R) and the CoRbs (I420R). Control MAbs included CD4bs ligands (VRC01 and b12) and CoRbs MAb 17b. (B) VRC06 competed with biotin-labeled CD4bs and CoRbs MAb binding to YU2 gp120. (C) sCD4 competed with CD4bs MAbs and enhanced CoRbs MAb binding to gp120. The CD4bs ligands were VRC01, b12, and CD4-Ig; the CoRbs MAbs were 17b, 48d, and 2.1C. The MAb 2G12 was used as negative control.
To further confirm the binding specificity of VRC06, we performed competition ELISAs with relevant ligands, using VRC06 as the competitor. VRC06 competed with all CD4bs ligands tested, including VRC01, b12, and CD4-Ig, and, additionally, with the CoRbs MAbs 17b, 48d, and 2.1C (Fig. 2B). In the reverse orientation, sCD4 competed with VRC06 as well as other CD4bs MAbs for binding to gp120. As expected, sCD4 enhanced the binding of the CoRbs MAb 17b to gp120 (Fig. 2C). No competition was observed for the glycan-specific MAb 2G12, which served as a negative control. Taken together, these data are consistent with the observation that the epitope of VRC06 recognized on gp120 overlaps both CD4bs and CoRbs.
The other CoRbs clones isolated from donor 45 displayed a more typical cross-competition pattern (28) in that they competed with the CoRbs MAb 17b and their recognition of gp120 was enhanced in the presence of CD4-Ig and VRC01, as described previously (47). In addition, these CoRbs MAbs moderately competed with CD4bs-directed MAbs, such as b12, for binding to full-length gp120 (see Fig. S2 in the supplemental material).
Analysis of the CoRbs antibody IgG gene family suggests related somatic variants.
The IgG gene families of the isolated antibodies were analyzed using IMGT/V-Quest http://www.imgt.org/IMGT_vquest/share/textes/) and Joinsolver (http://Joinsolver.niaid.nih.gov) (Table 1). The heavy chain gene usage was skewed toward the VH1 family, with several MAbs originating from VH1-69 or VH1-2*02 lineages. There was less-frequent usage of VH3 or VH4 family members, even though those are more frequent alleles in the human heavy chain germ line immunoglobulin locus (1, 12). Four of the nine MAbs use VH1-69, a heavy chain gene used by typical CoRbs antibodies, as reported previously (15, 21). Three of the nine MAbs use VH1-2*02, which is the same VH gene used by VRC01, VRC02, and VRC03. Based on heavy and light chain gene family assignments, the MAbs VcT39 and Vc813 were determined to be clonally related variants, whereas VRC06 possessed a different heavy chain/light chain usage.
Table 1.
Inferred MAb IgG gene family usagea
| MAb | Genes | CDR3 length (aa) | Mutation frequency (%) | Note | ||
|---|---|---|---|---|---|---|
| Heavy chain | VH family | D family | JH family | VH | ||
| VcT16 | IGHV3-7*01 | IGHD3-10*01 | IGHJ6*02 | 18 | 8 | |
| VcT39 | IGHV1-2*02 | IGHD3-16*01b | IGHJ6*02 | 25 | 7 | |
| Vc813 | IGHV1-2*02 | IGHD3-16*01b | IGHJ6*02 | 25 | 6 | |
| Vc822 | IGHV1-69*09 | IGHD3-22*01 | IGHJ3*02 | 22 | 11 | |
| Vc827 | IGHV4-4*07 | IGHD3-10*01 | IGHJ4*02 | 15 | 14 | |
| Vc908 | IGHV1-69*01 | IGHD1-26*01 | IGHJ6*03 | 25 | 5 | |
| Vc932 | IGHV1-69*01 | IGHD3-22*01 | IGHJ6*03 | 23 | 7 | |
| Vc949 | IGHV1-69*01 | IGHD4-17*01 | IGHJ6*03 | 19 | 6 | |
| VRC06 | IGHV1-2*02 or IGHV1-2*04 | IGHD4-17*01b | IGHJ1*01 | 17 | 29 | 21-bp insertion in FR3 |
| VRC06b | IGHV1-2*02 or IGHV1-2*04 | IGHD4-17*01b | IGHJ1*01 | 17 | 32 | 21-bp insertion in FR3 |
| VRC03 | IGHV1-2*02 or IGHV1-2*04 | IGHD3-22*01b | IGHJ1*01 | 16 | 29 | 21-bp insertion in FR3 |
| Light chain | VK family | JK family | VK | |||
| VcT16 | IGKV3-11*01 | IGKJ3*01 | 10 | 2 | ||
| VcT39 | IGKV1-39*01 or IGKV1D-39*01 | IGKJ4*01 | 8 | 5 | ||
| Vc813 | IGKV1-39*01 or IGKV1D-39*01 | IGKJ4*01 | 8 | 6 | ||
| Vc822 | IGKV1-5*03 | IGKJ1*01 | 9 | 3 | ||
| Vc827 | IGKV1-13*02 or IGKV1D13*01 | IGKJ4*03 | 9 | 11 | ||
| Vc908 | IGKV3-15*01 | IGKJ2*01 | 10 | 2 | ||
| Vc932 | IGKV3-20*01 | IGKJ3*01 | 9 | 1 | ||
| Vc949 | IGKV4-1*01 | IGKJ1*01 | 9 | 4 | ||
| VRC06 | IGKV3-20*01c | IGKJ2*01 | 5 | 16 | 6-bp deletion in CDR1 | |
| VRC06b | IGKV3-20*01c | IGKJ2*01 | 5 | 18 | 6-bp deletion in CDR1 | |
| VRC03 | IGKV3-20*01c | IGKJ2*01 | 5 | 20 | 6-bp deletion in CDR1 | |
JoinSolver (38) and IMGT were used for determining the gene utilization in this table.
Putative D segments assigned with less than 95% confidence due to the small number of consecutively matching residues compared to the V-to-J distance.
IGKV3-20*01 was selected over IGKV3-NL5 in order to use genes mapped to the IGK locus on chromosome 2.
When we compared the gene usage of VRC06 with that of previously isolated MAbs, surprisingly, we found that VRC06 shared homology with that of previously described CD4bs MAb VRC03 (47). In addition to VRC03, one MAb, termed VRC06b, had been also isolated from the same single B cell sorting experiment. These B cells and MAbs were isolated using the RSC3 modified gp120 core as a probe (47). Gene family analysis strongly suggested that VRC03, VRC06, and VRC06b were clonal variants, each arising from the same heavy chain V and J genes and the same light chain genes (Table 1). Each MAb harbored a 21-bp insertion in framework 3 (FR3) of their heavy chains and a 6-bp deletion in their common kappa chain allele, IGKV3-20*01. Consistent with a common lineage, VRC06, VRC06b, and VRC03 also used the same IGKJ2*01 J region element.
We aligned the VRC03, VRC06, and VRC06b amino acid sequences with the previously published liganded VRC03 structure (49) and assessed diversity at known VRC03 contact residues. The observed substantial differences in amino acid sequence, particularly within the heavy chain (see Fig. S3 in the supplemental material), suggest that VRC06/06b might display distinct binding patterns compared to the genetically related VRC03 MAb.
VRC06 and VRC06b display a unique binding specificity across functional determinants.
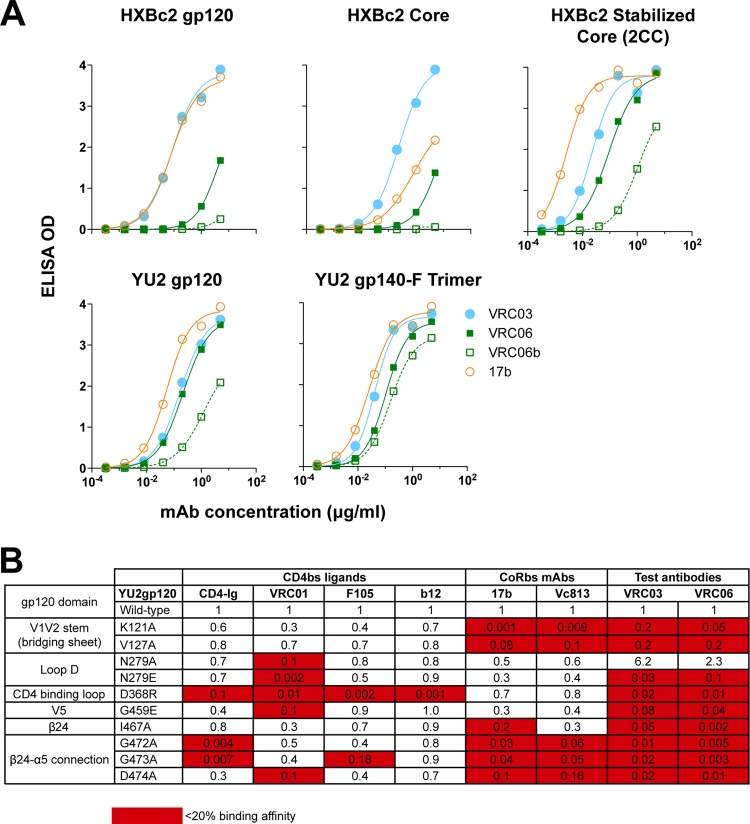
Given the similar germ line assignments but divergent amino acid sequences for VRC06 and VRC06b relative to VRC03, we sought to further characterize the binding specificity of these MAbs. Compared to VRC03, VRC06 and VRC06b displayed weak binding to HXBc2 monomeric gp120 or gp120 core (Fig. 3A). Notably, VRC06 and VRC06b showed improved binding affinity to the HXBc2 2CC core (Fig. 3A, right panel), which is stabilized in a CD4-bound conformation. Also note that this stabilization also leads to enhanced formation of the bridging sheet region of Env. The improved binding to the 2CC core suggests that VRC06 and VRC06b might have significant contact with the CoRbs, perhaps including the beta strands that compose the bridging sheet subdomain of gp120.
Fig 3.
VRC06 and VRC06b displayed unique binding properties for Env gp120. (A) VRC06 and VRC06b preferred recognition of Env of CD4-bound conformation (stabilized core, 2CC) and of trimeric context YU2 gp140-F trimer. The CoRbs MAb 17b was used as control. (B) VRC06 and VRC03 binding specificities to point mutations in various gp120 regions including the CD4 binding loop, loop D, and V5 regions are shown. The MAb binding affinity for the mutant gp120 proteins relative to the WT gp120 was calculated as (EC50 of WT gp120 for MAb)/(EC50 of Mut gp120 for MAb).
VRC06 and VRC06b displayed an increased binding affinity for a gp120 derived from the tier 2 primary isolate YU2 compared with that derived from HXBc2, which is a lab-adapted virus (Fig. 3A). In addition, their affinities for a soluble trimeric version of Env, YU2 gp140-F, were also higher than that for the monomeric gp120. These data suggested that VRC06 and VRC06b prefer to recognize Env stabilized in the CD4-bound conformation and presented in a trimeric context. Since VRC06 failed to recognize gp120 possessing the CD4bs knockout mutation D368R (Fig. 2A), similar to the CD4bs MAbs such as VRC01 and b12, we further tested its binding specificity against a panel of gp120 mutant proteins possessing individual mutations in selected regions of gp120, including the CD4 binding loop. We compared the VRC06 binding pattern to those of other CD4bs MAbs. VRC06b was not included in these assays due to its low affinity for soluble, monomeric gp120. We found that VRC06, along with VRC03, displayed decreased affinity for gp120 mutants in the CD4 binding loop, loop D, V5, β24, and β24-α5 connection (Fig. 3B). Such recognition specificity is similar to that of VRC01 (24) but is distinctive from that of other CD4bs MAbs, such as b12 and F105, and CoRbs MAb 17b. In addition, we observed that VRC06 and VRC03 also failed to recognize gp120 variants possessing mutations in the V1V2 stem region in a manner similar to that of the prototypical CoRbs MAb, 17b (Fig. 3B). Collectively, the binding specificity data suggested that VRC06 and VRC03 display dual recognition of elements of the two most functionally important determinants on gp120, namely, the CD4 and coreceptor binding regions.
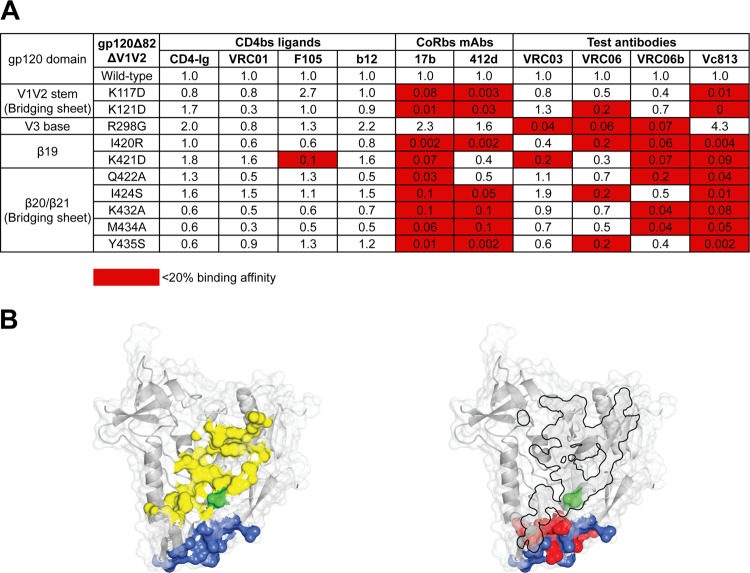
To more precisely map VRC06 and VRC06b recognition of the CoRbs, we assessed VRC06 and VRC06b binding affinity for another panel of gp120 mutants. These mutants were analyzed in the context of a gp120 possessing deletions of both the N terminus (Δ82) and the first and second major variable regions (ΔV1V2). We also analyzed MAb binding to single mutations in the CoRbs region including the bridging sheets, β19 strand, or V3 base in this gp120 Δ82ΔV1V2 context (Fig. 4A). We used the N terminus- and V1V2-deleted YU2 gp120 variant, as this modified Env is more efficiently recognized by VRC06b than is the full-length YU2 gp120 used above, thereby facilitating fine mapping of the epitope. The mapping revealed that, similar to the conventional CoRbs MAbs such as 17b, 412d, and Vc813, VRC06b and VRC06 exhibited abrogated binding to gp120 Δ82ΔV1V2 proteins possessing mutations in the bridging sheet region, the β19 strand, or the base of V3 (Fig. 4A). These results are consistent with a model in which VRC06 and VRC06b directly contact the CoRbs. In contrast, binding affinity of CD4bs ligands including VRC01 and b12 for gp120 Δ82ΔV1V2 CoRbs mutants was not affected, while VRC03 recognition of these mutants was moderately affected. The fine mapping results strongly support the notion that the VRC06 and VRC06b epitopes functionally or/and structurally overlap the CoRbs, as well as the CD4bs, as shown in Fig. 4B.
Fig 4.
VRC06 and related MAbs' binding specificity for the CoRbs. (A) Fine mapping of VRC06 and VRC06b binding specificity for point mutations in the CoRbs, including the bridging sheets and V3 base, in the context of the YU2 gp120 Δ82ΔV1V2 glycoprotein by ELISA. The binding affinity of MAbs for the YU2 gp120 Δ82ΔV1V2 mutant proteins compared to that for the wild-type protein was calculated as described in the legend for Fig. 3. Mutations in the CoRbs regions affected VRC06 and VRC06b binding, similar to other CoRbs MAbs, but had no effect on CD4bs ligands, suggesting that the epitopes of VRC06 and VRC06b were functionally or structurally related to the CoRbs of gp120. (B) CD4bs and CoRbs on the gp120 core surface. Left, CD4 footprint (yellow) and CoRbs MAb 17b footprint (blue) on the gp120 core surface (Protein Data Bank [PDB], 2NY3). The prototypic residue in the CD4bs, D368, is in green, and VRC06 and VRC06b are sensitive to mutations at this residue. Right, the VRC03 footprint from the published structure (PDB, 3SE8) shown on the molecular surface of the gp120 core (PDB, 2NY3) as a black-bordered area. The residues identified in panel A affecting VRC06 and VRC06b binding to the YU2 gp120 Δ82ΔV1V2 that are located in the bridging sheet area are shown in red. Note that the VRC03 footprint has some limited overlap with the footprint of 17b (blue), while the Ala scan-inferred footprints of VRC06 and VRC06b in the bridging sheet demonstrate more overlap with the 17b footprint. The Ala scan-inferred footprints of VRC06 and VRC06b contain the prototypic CD4bs residue, D368, as does the footprint of VRC03.
VRC06 displays increased breadth and potency of neutralization against primary virus isolates compared to conventional CoRbs antibodies.
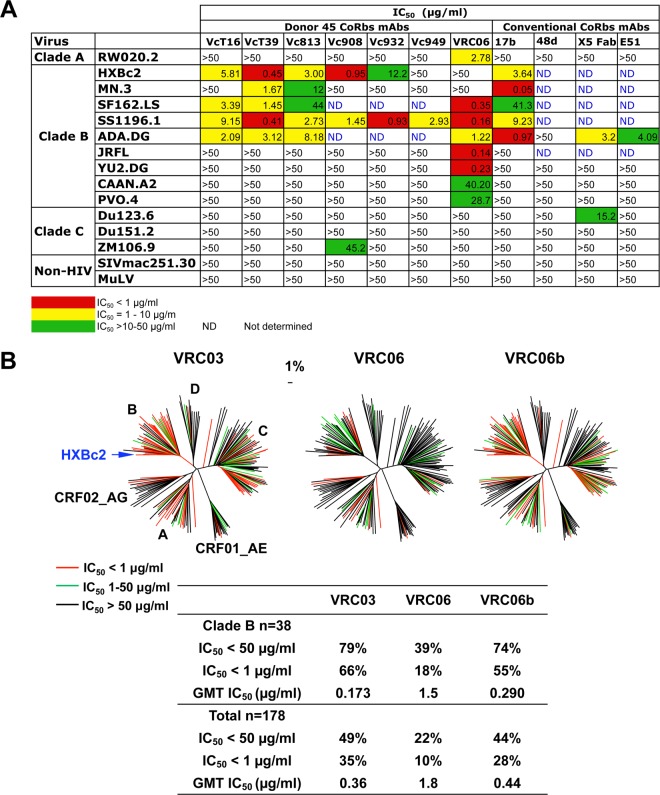
Our initial neutralization analysis of the CoRbs MAbs isolated from donor 45, including VRC06, was performed with a panel of 13 isolates that included several highly sensitive tier 1 strains of HIV-1 (HXBc2, MN, SF162, and SS1196) (Fig. 5A). Most of the antibodies neutralized a subset of these tier 1 viruses (Fig. 5A) but could not neutralize any of the tier 2 primary isolates. These properties are consistent with the neutralization profile of conventional CoRbs MAbs, such as 17b. In contrast to the more conventional CoRbs-directed MAbs, VRC06 neutralized several of the clade B tier 2 primary isolates.
Fig 5.
Virus neutralization data. (A) The 50% inhibitory concentrations (IC50s) of VRC06 and other CoRbs MAbs against a limited panel of HIV-1 isolates, including both tier 1 and tier 2 viruses. (B) Analysis of neutralization by MAbs VRC03, VRC06, and VRC06b against a panel of 178 Env pseudoviruses consisting of representatives from the major circulating clades of HIV-1. Dendrograms made by the neighbor-joining method show the protein distances of the Env gp160 sequences derived from 178 HIV-1 isolates. The clade B reference strain HXBc2 was used to root the tree, and the amino acid distance scale is indicated with a value of 1% distance. The clades of HIV-1 group M, including circulating recombinant forms (CRFs), are indicated. Neutralization potency of VRC03, VRC06, and VRC06b is indicated by the color of the branch for each virus. The data under the dendrograms show the percentages of viruses neutralized with an IC50 of <50 μg/ml and <1 μg/ml, and the geometric mean titer (GMT) of IC50 for viruses neutralized with an IC50 of <50 μg/ml.
We then extended this analysis to a panel of 178 primary viruses and included the somatic variant VRC06b, as well as VRC03 (Fig. 5B and Table 2; see Tables S1 to S7 in the supplemental material). VRC06 and VRC06b neutralized 22% and 44% of circulating primary virus isolates, respectively. The order of VRC06 and VRC06b neutralizing breadth for viruses from different clades was as follows: clade B > clade A > clade C > clade D. VRC06b had neutralizing breadth and potency similar to those of VRC03, while VRC06 had lower potency and breadth for these circulating virus isolates. Most autologous envelope viral variants from donor 45 demonstrated neutralization escape from VRC01 and VRC03 (46), yet some of these escape variants remained sensitive to VRC06 and VRC06b. The data suggest that the diversity of recognition displayed by these related antibodies may represent an archiving of a series of immune selective forces, followed by escape of the differentially sensitive viruses.
Table 2.
Summary of the breadth and potency of MAb neutralization against 178 HIV-1 Env pseudoviruses
| Virus clade | Titer (μg/ml) | Measured by IC50 |
Measured by IC80 |
||||
|---|---|---|---|---|---|---|---|
| VRC03a | VRC06 | VRC06b | VRC03a | VRC06 | VRC06b | ||
| All clades | <50 | 49% | 22% | 44% | 41% | 16% | 37% |
| (n = 178) | <1 | 35% | 10% | 28% | 24% | 3% | 20% |
| GMTb | 0.361 | 1.8 | 0.443 | 0.763 | 3.8 | 1.1 | |
| A | <50 | 56% | 37% | 56% | 41% | 30% | 41% |
| (n = 27) | <1 | 37% | 15% | 26% | 19% | 4% | 19% |
| GMT | 0.510 | 1.7 | 0.899 | 0.775 | 6.2 | 1.7 | |
| B | <50 | 79% | 39% | 74% | 71% | 26% | 66% |
| (n = 38) | <1 | 66% | 18% | 55% | 53% | 11% | 34% |
| GMT | 0.173 | 1.5 | 0.290 | 0.504 | 2.0 | 0.831 | |
| C | <50 | 48% | 19% | 44% | 35% | 11% | 31% |
| (n = 54) | <1 | 30% | 6% | 24% | 19% | 0% | 17% |
| GMT | 0.652 | 3.2 | 0.778 | 0.992 | 7.3 | 1.2 | |
| D | <50 | 22% | 11% | 11% | 11% | 0% | 11% |
| (n = 9) | <1 | 11% | 0% | 11% | 11% | 0% | 11% |
| GMT | 1.9 | 36.9 | 0.055 | 0.286 | >50 | 0.2 | |
| CRF01_AE | <50 | 25% | 6% | 13% | 25% | 6% | 13% |
| (n = 16) | <1 | 13% | 6% | 6% | 6% | 0% | 6% |
| GMT | 0.774 | 0.232 | 0.291 | 4.6 | 1.0 | 2.6 | |
| CRF02_AG | <50 | 19% | 13% | 25% | 19% | 13% | 25% |
| (n = 16) | <1 | 19% | 13% | 19% | 13% | 0% | 19% |
| GMT | 0.100 | 0.633 | 0.100 | 0.955 | 3.6 | 0.474 | |
| Other | <50 | 39% | 6% | 28% | 33% | 6% | 28% |
| (n = 18) | <1 | 33% | 6% | 22% | 17% | 0% | 17% |
| GMT | 0.313 | 0.305 | 0.224 | 0.770 | 2.4 | 1.4 | |
Data for VRC03 were published previously (47). However, the data used in this paper are from a completely independent experimental run and are consistent with those generated from the previous study.
Geometric mean titers (GMT) were calculated for neutralization-sensitive viruses with an IC50 or IC80 value of <50 μg/ml.
VRC03, VRC06, and especially VRC06b recognize cleaved cell surface Env trimers better than uncleaved ones.
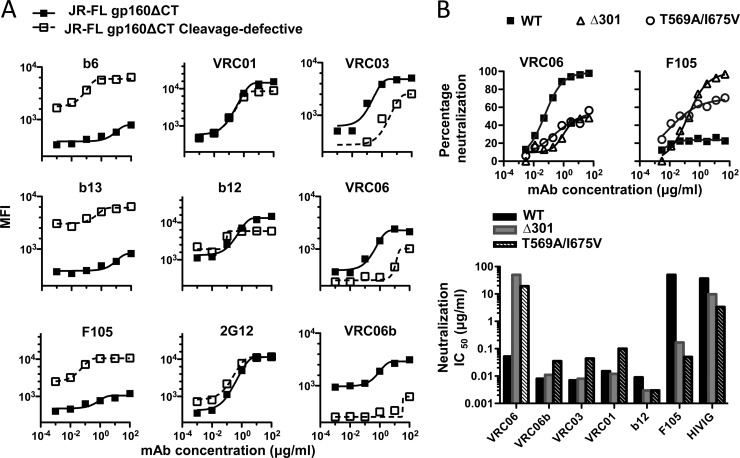
A noncovalent association between gp120 and gp41 subunits of HIV Env is also a property shared by all the lentivirus envelope glycoproteins. For HIV Env, these subunits are derived from the gp160 precursor glycoprotein, which is cleaved into gp120 and gp41 by cellular proteases, likely furins. A previous study demonstrated that when Env trimers were expressed on cell surfaces by transient transfection, well-cleaved Env trimers derived from primary isolate JR-FL were efficiently recognized by neutralizing antibodies, but not by nonneutralizing antibodies (29). In contrast, both neutralizing and nonneutralizing antibodies recognize noncleaved Env trimer (29, 33). These data suggest that cleavage imposes significant changes in the Env spike architecture. To characterize how VRC03, VRC06, and VRC06b recognize Env trimer, we tested their binding with cleaved and noncleaved JR-FL cell surface Env. Consistent with the previous data, we observed that nonneutralizing antibodies such as b6, b13, and F105 displayed little or no recognition of the JR-FL cleaved trimer and recognized only the noncleaved trimer. The neutralizing antibodies VRC01, b12, and 2G12 recognized both forms of these cell surface trimers equivalently (Fig. 6A). In contrast, VRC03, VRC06, and especially VRC06b bound the cleaved trimer much better than the noncleaved trimer (Fig. 6A). These data suggest that VRC03, VRC06, and VRC06b can differentiate the architectural difference between the two forms of trimers and preferentially recognize the cleaved, functional Env spike.
Fig 6.
VRC06 and related MAbs recognized quaternary epitopes on Env functional trimer. (A) Binding of VRC03, VRC06, and VRC06b to JR-FL gp160ΔCT-transfected 293T cell surface by FACS assay, represented by MFI (mean of fluorescence intensity). JR-FL gp160ΔCT is the cleaved trimer; JR-FL gp160ΔCT cleavage-defective is the uncleaved trimer, which contains two mutations that render it cleavage defective (29). (B) VRC06 neutralization potency against JR-FL gp160ΔCT virus was affected by Env mutations leading to an alteration of neutralization sensitivity. Top graphs, Env mutants Δ301 and T569A/I675V display enhanced neutralization sensitivity to CD4bs MAb F105 but substantially decreased neutralization sensitivity to VRC06; the bar graphs on the bottom depict the effects of the Env mutations Δ301 and T569A/I675V on the HIV sensitivity (IC50 values) to VRC06 and other CD4bs ligands.
To extend our analysis of VRC06 recognition of Env functional trimers, we tested VRC06 neutralization capacity against viruses pseudotyped with selected mutant Env trimers displaying altered neutralization sensitivity to CD4bs-directed MAbs. The Δ301 Env variant contains a point mutation (T303A) that results in a removal of a V3 loop N-linked glycan at position 301 (17), and the gp41 T569A/I675V mutant (3) both display increased neutralization sensitivity to CD4bs ligands including b12 and F105 (3, 17, 24), presumably due to a globally “opened” Env architecture as a result of these mutations. VRC06 neutralized both Δ301 and T569A/I675V mutant viruses less potently than the WT virus (Fig. 6B). In contrast, many CD4bs ligands, including nonbroad CD4bs MAb F105, neutralized these mutant viruses more potently (Fig. 6B), as previously observed. Taken together, VRC06 and related MAbs appear to be highly sensitive to the mode of packing of the functional Env trimer, suggesting that these types of antibodies recognize their epitopes more efficiently in the context of a tightly packed, quaternary spike architecture.
DISCUSSION
The accessibility of conserved neutralizing determinants on the HIV-1 Env is an important issue for immunogen design, and tools for assessing these epitopes may help to identify and prioritize targets for B cell responses as part of a broadly effective vaccine. Here, using PBMCs from donor 45, from whom the broadly neutralizing CD4bs antibody VRC01 was isolated (47) and in whose serum we mapped some neutralization to the CoRbs (25), we isolated several additional antibody clones that recognized elements of the CoRbs. We demonstrated that two of these MAbs displayed epitope recognition overlapping both the CD4bs and the CoRbs. In addition, these antibodies displayed modest potency and breadth of neutralization against circulating primary isolates, an unusual capability compared to the very limited neutralization capacity of typical CoRbs MAbs (e.g., 17b, 48d, or X5). This study provides evidence that the human B cell repertoire can access elements of the gp120 CoRbs on the primary functional virus spike and that neutralizing antibodies with diverse specificities focused on conserved elements of Env have been elicited or have evolved in a single HIV-1-infected individual. Interestingly, when we assessed recognition of cleaved trimeric spikes by the CD4bs/CoRbs VRC06/6b MAbs, we observed that they also preferentially recognized the fully cleaved, primary isolate Env functional spike rather than spikes lacking Env precursor cleavage or with a more open configuration.
The HIV-1 Env CoRbs is a conserved functional element critical for Env-mediated virus entry, and the accessibility to this region is limited on the HIV-1 primary virus Env functional spike prior to receptor CD4 engagement (5, 9, 20, 43). Antibodies against the HIV-1 Env CoRbs exist (40, 41, 51, 53) but normally display limited neutralizing capacity against primary virus isolates, consistent with the current model whereby accessibility by antibody to this region is often sterically restricted on circulating isolates. The conventional CoRbs MAbs are also termed “CD4-induced (CD4i) MAbs,” as their epitope recognition of gp120 is enhanced in the presence of soluble CD4, which induces Env conformational changes that lead to the better exposure of the CoRbs or formation of this binding site. Here, we isolated a series of genetically related bNAbs, which targeted both the CD4bs and the CoRbs of HIV Env and which neutralized up to 44% of circulating primary virus isolates. The neutralizing specificity of these bNAbs was directed in part to the Env CoRbs and is in contrast to the usually fully occluded nature of CoRbs in the context of the prereceptor engaged functional virus spike. One model to account for the unique recognition specificity of these neutralizing antibodies is that they initially interact with the gp120 CD4bs and then, following conformational rearrangements, are able to access elements of the CoRbs.
In addition to isolation of the CD4bs bNAbs VRC01 and VRC03 from donor 45, we have now isolated the MAbs VRC06 and VRC06b. These MAbs each displayed a recognition specificity, which mapped to both CD4bs and CoRbs. We also found that although the neutralization breadth of VRC06 and VRC06b is narrower than that of VRC01, VRC06 and VRC06b neutralized some VRC01 escape viral variants from donor 45 (46). Thus, neutralizing antibody responses with diversified yet overlapping and complementary specificities in a single individual can be coelicited and might be generated by selection and virus escape followed by a subsequent adaptive humoral response by the host immune system. Furthermore, broad and potent neutralizing antibody responses specific for the Env CoRbs have been reported in individuals other than donor 45 (25, 36), suggesting that such neutralizing specificity may exist more commonly than is currently appreciated. This raises the possibility of targeting broadly conserved Env-neutralizing epitopes that may include elements of the CoRbs through immunogen design.
Somewhat unexpectedly, VRC03, VRC06, and especially VRC06b preferentially recognize the more tightly packed Env functional trimer on primary isolates over Env trimers with a more open configuration. VRC06 and VRC06b can weakly recognize monomeric gp120 harboring deletions in the V1V2 variable region and the N terminus (the gp120 core), although these MAbs poorly recognize WT gp120. If the variable region-deleted cores are stabilized in the CD4-bound conformation, recognition by VRC06 and VRC06b is further improved over the nonstabilized cores. Thus, it is likely that the influence of the cleavage-dependent, quaternary packing of the functional Env spike on VRC06 and VRC06b recognition is an indirect effect, as these MAbs can bind the “loop-deleted” monomeric gp120 variants. This may not be too surprising since, indeed, these MAbs are directed to the conserved, gp120 receptor binding sites and not the variable elements of the Env themselves. These results are consistent with a recent report showing that the variable regions inhibit gp120 from assuming the CD4-bound conformation (18). That is, variable region removal may allow monomeric gp120 to sample other conformations that can then be recognized by VRC06/VRC06b. The results also suggest either that the VRC06- and VRC06b-bound conformation is sampled on the functional, cleaved spike or that these MAbs can induce and trap this conformation in this relevant context. Perhaps the Env CD4bs and CoRbs can sample a quaternary conformation on the precisely packed Env functional trimer that cannot be sampled by the variant and more open Env spikes. The unique VRC06/VRC06b pattern of dual recognition of the functionally conserved CD4bs and CoRbs, coupled with the cleavage sensitivity of recognition displayed by these modestly broadly reactive Nabs, distinguishes these MAbs from other “classical” CD4bs-directed MAbs. Their recognition properties may be valuable for screening Env immunogens designed to reconstitute the cleaved conformation of the Env functional trimer. If soluble cleaved trimeric proteins can be identified, their antigenic profile should more closely mimic that of the functional spike. That is, cleaved primary isolate spikes are recognized efficiently by only the broadly neutralizing antibodies such as VRC01, b12, 2F5, PG9/16, and the PGTs. Such soluble mimetics, selected by VRC06/VRC06b, may more efficiently elicit such bNAbs. Recent antigenicity studies on the HIV Env trimer suggest that the gp120 CoRbs, including the elements of V3, can sample distinct quaternary packing modes in the unliganded and CD4-bound states (52, 54). Thus, ongoing studies focused on defining the quaternary packing modes and the influence of such packing on recognition by VRC06, VRC06b, and VRC03 may help with the design of immunogens to better elicit bNAbs directed at elements of the conserved CoRbs and CD4bs.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the International AIDS Vaccine Initiative and the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We are grateful to James Robinson (Tulane University) for providing antibodies including 17b, 48d, 412d, and 2.1C; Joseph Sodroski (Dana Farber Cancer Institute) for providing the plasmid encoding CD4-Ig and CoRbs mutant gp120 Δ82ΔV1V2 glycoprotein; Marshall Posner (Dana Farber Cancer Institute) for providing antibody F105; and Dennis Burton (The Scripps Research Institute) for providing antibodies b6, b12, and b13.
Footnotes
Published ahead of print 8 August 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Arnaout R, et al. 2011. High-resolution description of antibody heavy-chain repertoires in humans. PLoS One 6:e22365 doi:10.1371/journal.pone.0022365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger EA, Murphy PM, Farber JM. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657–700 [DOI] [PubMed] [Google Scholar]
- 3. Blish CA, Nguyen MA, Overbaugh J. 2008. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med. 5:e9 doi:10.1371/journal.pmed.0050009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burton DR, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 5. Chakrabarti BK, et al. 2011. HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies. AIDS Res. Hum. Retroviruses 27:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cormier EG, Dragic T. 2002. The crown and stem of the V3 loop play distinct roles in human immunodeficiency virus type 1 envelope glycoprotein interactions with the CCR5 coreceptor. J. Virol. 76:8953–8957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cormier EG, Tran DN, Yukhayeva L, Olson WC, Dragic T. 2001. Mapping the determinants of the CCR5 amino-terminal sulfopeptide interaction with soluble human immunodeficiency virus type 1 gp120-CD4 complexes. J. Virol. 75:5541–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corti D, et al. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805 doi:10.1371/journal.pone.0008805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decker JM, et al. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dey B, et al. 2009. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS Pathog. 5:e1000445 doi:10.1371/journal.ppat.1000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farzan M, et al. 1999. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96:667–676 [DOI] [PubMed] [Google Scholar]
- 12. Glanville J, et al. 2009. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc. Natl. Acad. Sci. U. S. A. 106:20216–20221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gray ES, et al. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang CC, et al. 2007. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317:1930–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang CC, et al. 2004. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc. Natl. Acad. Sci. U. S. A. 101:2706–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. 1991. Sequences of proteins of immunological interest, 5th ed U.S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD [Google Scholar]
- 17. Koch M, et al. 2003. Structure-based, targeted deglycosylation of HIV-1 gp120 and effects on neutralization sensitivity and antibody recognition. Virology 313:387–400 [DOI] [PubMed] [Google Scholar]
- 18. Kwon YD, et al. 2012. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc. Natl. Acad. Sci. U. S. A. 109:5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwong PD, et al. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labrijn AF, et al. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77:10557–10565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewis GK, et al. 2011. Identification and characterization of an immunogenic hybrid epitope formed by both HIV gp120 and human CD4 proteins. J. Virol. 85:13097–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, et al. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13:1032–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, et al. 2011. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J. Virol. 85:8954–8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, et al. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, et al. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 28:413–444 [DOI] [PubMed] [Google Scholar]
- 28. Moore JP, Sodroski J. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pancera M, Wyatt R. 2005. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology 332:145–156 [DOI] [PubMed] [Google Scholar]
- 30. Pantophlet R, Burton DR. 2006. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24:739–769 [DOI] [PubMed] [Google Scholar]
- 31. Rizzuto C, Sodroski J. 2000. Fine definition of a conserved CCR5-binding region on the human immunodeficiency virus type 1 glycoprotein 120. AIDS Res. Hum. Retroviruses 16:741–749 [DOI] [PubMed] [Google Scholar]
- 32. Rizzuto CD, et al. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949–1953 [DOI] [PubMed] [Google Scholar]
- 33. Roben P, et al. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sather DN, et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scheid JF, et al. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640 [DOI] [PubMed] [Google Scholar]
- 36. Scheid JF, et al. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seaman MS, et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Souto-Carneiro MM, Longo NS, Russ DE, Sun HW, Lipsky PE. 2004. Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J. Immunol. 172:6790–6802 [DOI] [PubMed] [Google Scholar]
- 39. Stein BS, Engleman EG. 1990. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J. Biol. Chem. 265:2640–2649 [PubMed] [Google Scholar]
- 40. Sullivan N, et al. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thali M, et al. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tiller T, et al. 2008. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 329:112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trkola A, et al. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187 [DOI] [PubMed] [Google Scholar]
- 44. Wardemann H, et al. 2003. Predominant autoantibody production by early human B cell precursors. Science 301:1374–1377 [DOI] [PubMed] [Google Scholar]
- 45. Willey RL, Bonifacino JS, Potts BJ, Martin MA, Klausner RD. 1988. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc. Natl. Acad. Sci. U. S. A. 85:9580–9584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu X, et al. 2012. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J. Virol. 86:5844–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu X, et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu X, et al. 2009. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. J. Virol. 83:10892–10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu X, et al. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888 [DOI] [PubMed] [Google Scholar]
- 51. Xiang SH, Doka N, Choudhary RK, Sodroski J, Robinson JE. 2002. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res. Hum. Retroviruses 18:1207–1217 [DOI] [PubMed] [Google Scholar]
- 52. Xiang SH, et al. 2010. A V3 loop-dependent gp120 element disrupted by CD4 binding stabilizes the human immunodeficiency virus envelope glycoprotein trimer. J. Virol. 84:3147–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiang SH, et al. 2003. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 315:124–134 [DOI] [PubMed] [Google Scholar]
- 54. Yuan W, Bazick J, Sodroski J. 2006. Characterization of the multiple conformational states of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J. Virol. 80:6725–6737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.