Small organic and metal-containing molecules (MW < 1000) can catalyse synthetically useful reactions with the high levels of stereo-selectivity typically associated with macromolecular enzymatic catalysts. Whereas enzymes are generally understood to accelerate reactions and impart selectivity by stabilising specific transition structures through networks of cooperative interactions, enantioselectivity with chiral, small molecule catalysts is typically rationalised by the steric destabilisation of all but one dominant pathway. However, it is increasingly apparent that stabilising effects play an important role in small-molecule catalysis as well, although the mechanistic characterisation of such systems is rare. Here it is shown that arylpyrrolidino amido thiourea catalysts catalyse the enantioselective nucleophilic ring opening of episulfonium ions by indoles. Evidence is provided for selective transition state stabilisation of the major pathway by the thiourea catalyst in the rate- and selectivity-determining step. Enantioselectivity is achieved through a network of attractive anion binding, cation-π, and hydrogen bonding interactions between the catalyst and the reacting components in the transition structure assembly.
Multi-functional urea and thiourea derivatives have been shown to promote enantioselective reactions of cationic species in non-polar media by binding the corresponding counteranion through hydrogen bonding,1,2,3,4 with selectivity imparted through a combination of electrostatic association and additional noncovalent interactions that differentiate the diastereomeric transition structures leading to the product enantiomers.5,6,7 We sought to extend the anion-binding catalysis concept to episulfonium ions, highly reactive electrophilic species that readily undergo diastereospecific bond-forming reactions with nucleophiles.8,9,10,11 Recently, Toste and coworkers demonstrated enantioselective catalysis of the addition of alcohols to episulfonium ions using a chiral phosphoric acid catalyst.12 On the basis of our previous work in anion-binding catalysis, we envisioned that a (thio)urea could serve as a suitable host for an in situ formed episulfonium ion through interactions with the counteranion (Figure 1). High enantioselectivity might be achieved if additional interactions between the catalyst and episulfonium intermediate could be incorporated to differentially stabilise the diastereomeric pathways. This hypothesis was investigated in the context of a Friedel–Crafts-type indole alkylation reaction.13
Figure 1.
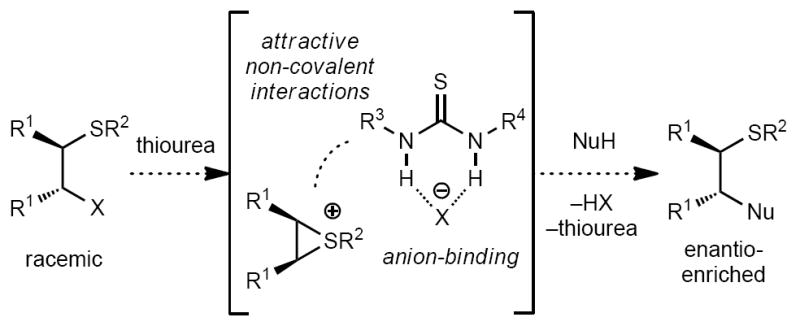
Proposed thiourea-catalysed episulfonium ion ring opening with indole via anion binding.
Results and Discussion
A. Reaction Methodology Development
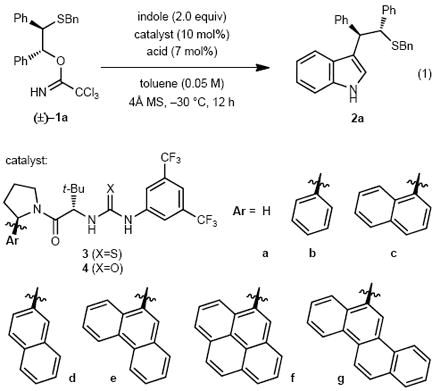
Preliminary efforts to identify a suitable episulfonium ion precursor revealed that a relatively non-nucleophilic leaving group was required in order to achieve the desired reactivity. Ultimately, we found that stable trichloroacetimidates (TCA) of type 1a12 were particularly useful substrates (eq. 1), undergoing protonolysis and substitution with a variety of strong Brønsted acids to form a meso–episulfonium ion with a counteranion that is readily varied based on the identity of the acid employed.
A wide variety of chiral urea and thiourea derivatives was evaluated in the model reaction (Table 1). Only arylpyrrolidine-derived thioureas of type 3 were found to induce reactivity above the background rate of 1a and acid alone. A broad screen of Brønsted acids revealed a pronounced counterion effect. In conjunction with thiourea 3b, mineral acids with a nucleophilic counter-anion, such as HCl, produced only trace amount of the desired indole addition product 2a (entry 1), with the corresponding chloride addition product predominating. In contrast, sulfonic acid co-catalysts afforded 2a in useful yields and varying levels of enantioselectivity, with 4-nitrobenzenesulfonic acid (4-NBSA) providing the most promising result (entry 5). The identity of the aromatic substituent on the pyrrolidino amide portion of the catalyst was also found to exert a profound effect on reaction enantioselectivity (entries 5, 7-12). Catalyst 3a, lacking an aryl group, induced little rate acceleration above the background reaction (entry 6) and afforded nearly racemic product. In contrast, catalyst 3c-3g bearing more extended aromatic substituents proved more enantioselective than catalyst 3b. Correlation between ee and either the electronic properties of the aryl substituents or the polarisability of the aromatic ring has been noted in other reactions using this family of catalysts.2,6,14 However, in the present case no such straightforward relationship was observed. Instead, ee was observed to improve upon expanding the aryl group from phenyl to phenanthryl (entries 7-10), and then to descrease slightly with more expansive aryl substituents (entries 11-12). Urea catalyst 4e induced only marginally lower ee than its thiourea counterpart (entries 10 vs 13), indicating that any mechanism wherein the thiourea sulfur is engaged productively as a Lewis base catalyst is not operative.15,16,17,18
Table 1.
Reaction optimisation.a
| ||||
|---|---|---|---|---|
| entry | catalyst | acid | yield (%)b | ee (%)c |
| 1 | 3b | HCl | 10 | 5 |
| 2 | 3b | HOTf | 73 | 32 |
| 3 | 3b | FSO3H | 78 | 19 |
| 4 | 3b | 2,4-diNBSA | 79 | 63 |
| 5 | 3b | 4-NBSA | 72 | 73 |
| 6 | – | 4-NBSA | 7 | n/a |
| 7 | 3a | 4-NBSA | 16 | 12 |
| 8 | 3c | 4-NBSA | 84 | 84 |
| 9 | 3d | 4-NBSA | 80 | 85 |
| 10 | 3e | 4-NBSA | 93 | 93 |
| 11 | 3f | 4-NBSA | 91 | 91 |
| 12 | 3g | 4-NBSA | 97 | 88 |
| 13 | 4e | 4-NBSA | 98 | 92 |
Optimisation reactions were performed on 0.05 mmol scale.
Isolated yields of material purified chromatographically.
Enantiomeric excesses (ee’s) determined by HPLC analysis.
MS = molecular sieves; 2,4-diNBSA = 2,4-dinitrobenzenesulfonic acid; 4-NBSA = 4-nitrobenzenesulfonic acid.
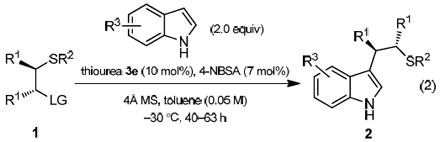
A variety of substrate combinations was evaluated in order to define the scope as well as gain insight into the mechanism of the reaction (Table 2). Substrates bearing electronically and sterically diverse sulfur substituents (R2) underwent enantioselective reactions (entries 1-9), with S-benzyl-substituted derivatives affording highest ee’s (entries 1 & 7). Electron potential maps calculated using DFT showed that the benzylic protons in the S-benzyl episulfonium ions bear a substantial amount of partial positive charge, and this may serve to enhance attractive interactions between the cationic intermediate and an electronegative functionality on the catalyst (vide infra). Various indole derivatives bearing electron-donating and -withdrawing substituents at the 2-, 4-, 5- or 6-positions all underwent the addition reaction with high levels of enantioselectivity (entries 10-16). In sharp contrast, N-methyl indole provided the desired product 2p with moderate yield and in almost racemic form (entry 17). This suggested that the indole N-H motif may be involved in a key interaction during the ee-determining transition state (vide infra). Benzotriazole also underwent the addition reaction, forming a C-N bond with synthetically useful ee (entry 18). Less nucleophilic heterocycles (i.e., π-nucleophiles with Mayr nucleophilicity parameters N < 4)19 proved unreactive. Variation of the substituents on the carbon backbone of the electrophile revealed that aryl groups with meta- and ortho- functionalities were compatible (entries 19-22); substrates bearing a para-substituent, regardless of its steric and electronic properties, resulted in substantially lower enantioselectivity (entries 23-26). Ongoing computational studies suggested that in the transition state leading to the major enantiomeric product, one of the para-C–H bonds is engaged in an attractive, electrostatic interaction with the thiourea-bound sulfonate. We reasoned that the lack of this interaction in the cases with para-substituted substrates might result in a less well-organized transition structure and reduced selectivity. Finally, three different acetimidate leaving groups displayed essentially the same reactivity and enantioselectivity, suggesting that the leaving group is not directly involved in the ee-determining step (entries 28-30).
Table 2.
Substrate scope of nucleophilic ring opening of episulfonium ions with indole derivatives.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | LG | R3 | product | yield(%) | ee (%) |
| 1 | Bn | 2a | 99 | 93 | |||
| 2 | Ph | 2b | 83 | 85 | |||
| 3b | Ph | 2b | 84 | 80 | |||
| 4 | 4-F-C6H4 |
|
2c | 73 | 81 | ||
| 5 | Ph | 4-Me-C6H4 | H | 2d | 76 | 87 | |
| 6 | 2-naphthyl | 2e | 90 | 88 | |||
| 7 | PMB | 2f | >99 | 94 | |||
| 8 | Me | 2g | 72 | 84 | |||
| 9 | t-Bu | 2h | 89 | 87 | |||
|
| |||||||
| 10 | 5-Me | 2i | 97 | 91 | |||
| 11 | 5-MeO | 2j | 93 | 93 | |||
| 12 | 5-Br | 2k | 83 | 92 | |||
| 13 | 5-F | 2l | 88 | 95 | |||
| 14 | Ph | Bn | TCA | 6-F | 2m | 92 | 85 |
| 15 | 4-MeO | 2n | 83 | 91 | |||
| 16 | 2-Me | 2o | 95 | 79 | |||
| 17 | N-Me | 2p | 54 | 3 | |||
| 18 | benzotriazolef | 2q | 92 | 80 | |||
|
| |||||||
| 19c | 3-MeO-C6H4 | 2r | 85 | 93 | |||
| 20 | 3-F-C6H4 | 2s | 97 | 95 | |||
| 21 | 3-Me-C6H4 | 2t | 95 | 93 | |||
| 22 | 2-Me-C6H4 | 2u | >99 | 79 | |||
| 23 | 4-F-C6H4 | Bn | TCA | H | 2v | 91 | 45 |
| 24 | 4-Me-C6H4 | 2w | 89 | 60 | |||
| 25c | 4-MeO-C6H4 | 2x | 67 | 6 | |||
| 26 | 4-CF3-C6H4 | 2y | 18 | 5 | |||
| 27d | —(CH2)4— | 2z | 16 | 9 | |||
|
| |||||||
| 28e | TCA | 2b | 87 | 87 | |||
| 29e | Ph | Ph |
|
H | 2b | 91 | 85 |
| 30e |
|
2b | 85 | 85 | |||
Isolated yields of material purified chromatographically are reported.
With urea 4e.
Yields determined by 1H NMR are reported. Product isolated via flash column chromatography usually contains a small amount of 3e.
R2 = Ph, with HOTf.
With 20 mol% 3e and 10 mol% 4-NBSA.
Benzotriazole as nucleophile instead of indole.
B. Determination of the Rate- and Enantio- Determining Step
Thiourea derivatives of type 3 bearing specific arylpyrrolidino residues have been identified as highly enantioselective catalysts for nucleophilic additions to a remarkable variety of cationic electrophilic intermediates, including oxocarbenium ions,2 acyliminium ions,6 acylpyridinium ions,20 and now episulfonium ions. Elucidation of the mechanisms that underlie catalytic activity and stereoinduction by these thioureas may potentially serve as a foundation for new reaction discovery and provide broader insights into cooperative non-covalent pathways. We therefore undertook a detailed experimental and computational investigation of the indole addition to episulfonium ions promoted by 3e and related catalysts. On the basis of the qualitative observations described above and previous studies involving thiourea anion-binding pathways, a simple catalytic cycle for the reaction of indoles with 1 mediated by 3e can be advanced (Figure 2). The absence of dependence of enantioselectivity on the identity of the acetimidate group suggests that the reaction begins with protonation of the trichloroacetimidate substrate, followed by episulfonium ion formation in a step that may or may not be under the influence of the thiourea catalyst. A series of 1H NMR studies of pre-formed episulfonium ion derivatives17 revealed that the episulfonium sulfonate most likely exists as a covalent adduct both in the presence or absence of the thiourea catalyst, so an endothermic ionization to the episulfonium ion complex is presumably taking place before addition of the indole nucleophile. Finally re-aromatisation to form the product and regenerate the acid closes the catalytic cycle. In the thiourea-promoted pathway, the chiral catalyst must therefore induce enantioselectivity through association with the charged intermediates and transition structures, indicated in Figure 2 as taking place via direct binding to the sulfonate counterion.
Figure 2.
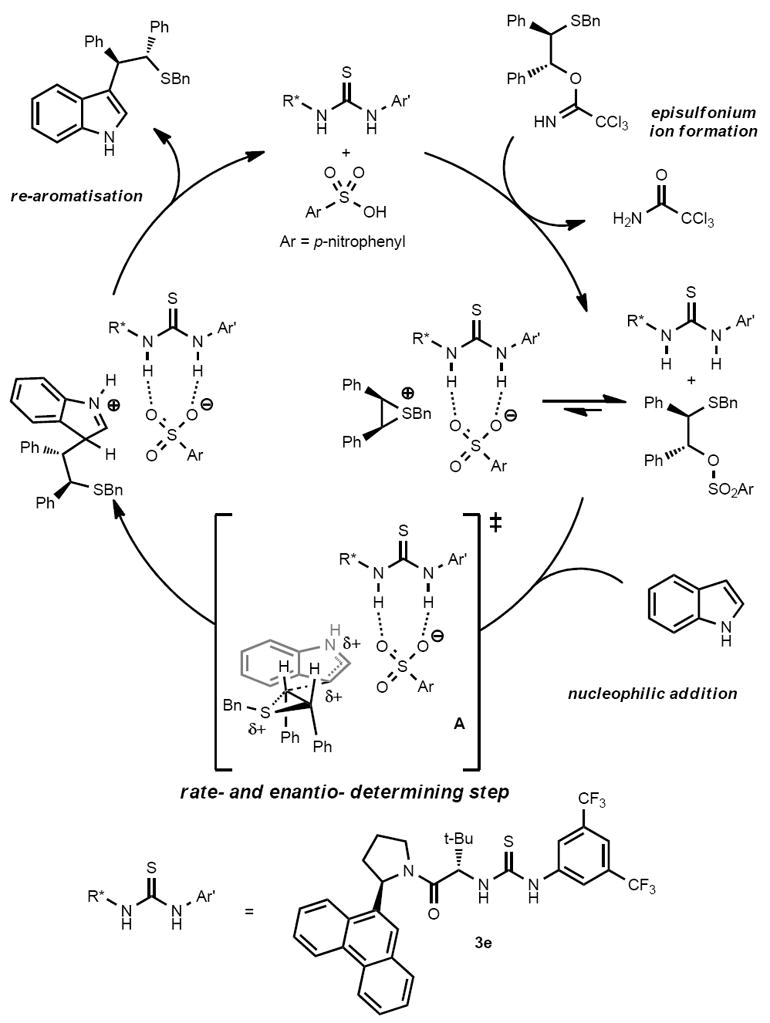
Proposed catalytic cycle for thiourea-catalysed episulfonium ion ring opening with indole.
In order to identify the rate- and enantioselectivity-determining step in the catalytic mechanism, reaction progress kinetic analysis21 of the reaction employing substrate 1a, indole, thiourea 3e, and 4-NBSA in toluene at 0 °C was conducted with in situ IR spectroscopy.22,23 Using reaction rates measured over 10% to 60% conversion with “different excess” experiments,22 the empirical rate equations were determined for both the racemic reaction catalysed by only 4-NBSA (equation 3) and the asymmetric reaction co-catalysed by 4-NBSA and thiourea (equation 4).
| (3) |
| (4) |
Here, krac is the second-order rate constant for the racemic reaction, and kasym is the third-order rate constant for the asymmetric reaction, and [4-NBSA]T and [3e]T are the total initial concentrations of acid and thiourea, respectively.
The rate of the asymmetric reaction was accelerated by the chiral thiourea catalyst relative to the reaction catalysed by 4-NBSA alone. For instance, at 273 K, [3e]T = 50 mmol/L, rasym/rrac at 10% conversion of 1a is 43.4 ± 5.0. This rate acceleration corresponds to a lowering of the free energy of activation of the reaction by 3e by 2.0 ± 0.1 kcal/mol.
The 0th-order rate dependence on 1a and first-order rate dependence on 4-NBSA indicate that quantitative protonation of the substrate occurs under the reaction conditions prior to the rate-determining step (pKa (4-NBSA) ~ −7, pKa (1a) ~ 2).24 In support of this conclusion, treatment of substrate 1a with 1 equiv of 4-NBSA resulted in instantaneous, complete consumption of 1a and concomitant, quantitative formation of trichloroacetamide (the by-product generated during episulfonium ion formation) as determined by in-situ IR spectroscopy. The first-order dependence on indole in both the reaction catalysed by 4-NBSA alone or by 4-NBSA/3e reveals that indole is present in the rate-determining transition structure and that episulfonium•4-nitrobenzenesulfonate (existing predominantly as the covalent adduct) is the resting state of the substrate in the reaction. The kinetic data are consistent with the reaction with indole involving either addition or reversible addition followed by slow re-aromatisation as the rate-determining step (Figure 2).
To distinguish between these two possibilities, linear free energy relationship and kinetic isotope effect studies were carried out. In the racemic reaction catalysed by 4-NBSA alone, a linear correlation was observed between reaction rate and Mayr’s nucleophilicity parameter N for five different 5-substituted indoles (log(krac) vs. sNN, R2 = 0.997), consistent with the indole addition step resulting in nucleophilic ring opening of episulfonium ion being the rate-limiting step.25 A correlation between indole nucleophilicity and rate was also obtained in the thiourea-catalysed reaction (log(kasym) vs. sNN, R2 = 0.757). As discussed below, a strict linear correlation is not observed in this case because the Brønsted acidity of the indole N-H group also influences reaction rate in the thiourea-catalysed reaction (see Section C2). Evaluation of 3-deuterioindole in the thiourea-catalysed addition reaction revealed a very small effect of isotopic substitution (kH/kD = 0.93 ± 0.12). This rules out re-aromatisation as the rate-determining step, which would be expected to display a significant primary isotope effect (kH/kD > 2.5),26 and is fully consistent with rate-determining indole addition. It can be concluded that indole addition is enantio-determining as well, since the product’s stereogenic centers are generated in this rate-determining step.27
C. Elucidation of the Catalyst–Substrate Interactions in the Enantio-Determining Step
While the kinetic studies served to define the stoichiometry of the transition structure in the rate- and ee-determining addition of indoles to 1, they do not provide any direct insight into the specific manner by which the thiourea catalyst induces rate acceleration and enantioselectivity. This proved attainable, however, through the series of structure-reactivity and structure-enantioselectivity studies detailed below.
C.1 Thiourea Dual Hydrogen Bond Donation to the Sulfonate Anion
Anion-binding catalysis has been recognised as the primary mode of substrate activation in a variety of asymmetric reactions involving H-bond donors.1 In studies relevant to the system described here, sulfonate ion association to urea or thiourea derivatives via dual hydrogen bond donation interactions has been identified in both binding and reactivity studies.28,29 To test whether such sulfonate binding is operative in the current reaction, model binding studies and catalyst structure–activity studies were conducted. Titration of solutions of thiourea 3e in d8-toluene with dibenzylmethylsulfonium triflate [(Bn2MeS)+(OTF)−] (5) revealed formation of a 1:1 complex as determined by 1H NMR. Resonances assigned to each of the thiourea N-H protons sharpened and shifted downfield upon complexation, consistent with a dual hydrogen bonding interaction.29,30 Perturbations to the chiral catalyst that diminished its ability to act as an H-bond donor, either by reduction of its acidity through substituent effects or by excision of one of the donor groups, led to strong or complete decreases in reactivity and enantioselectivity in the indole addition reaction (see Supplementary Information, Section 4). These data, taken together with the strong ee-dependence on the sulfonate counterion of the Brønsted acid (Table 1) suggests strongly that the thiourea–sulfonate interaction is a key element in the mechanism for catalysis and stereoinduction.
C.2 Hydrogen Bonding Interaction with the Indole N-H
As noted above, a striking difference in enantioselectivity was observed between N-H indole and N-methyl indole analogs in the addition reaction (93% vs 3% ee using catalyst 3e in additions to 1a). This suggests an important organisation role of the indole N-H in the stereoinduction mechanism, likely through hydrogen bonding to a Lewis basic functionality on the catalyst. To define the catalyst-indole interactions, a structure-reactivity and structure-enantioselectivity relationship study was undertaken with a series of π-nucleophiles (Figure 3a). In general, the absence of an N-H motif in a (1,3) relationship with the reactive nucleophilic site leads to very low levels of rate acceleration and enantioselectivity (entries 1-2 vs. entries 3-5).
Figure 3. Reactivity- and enantioselectivity- dependence on the presence and the acidity of a N-H bond in the nucleophile.
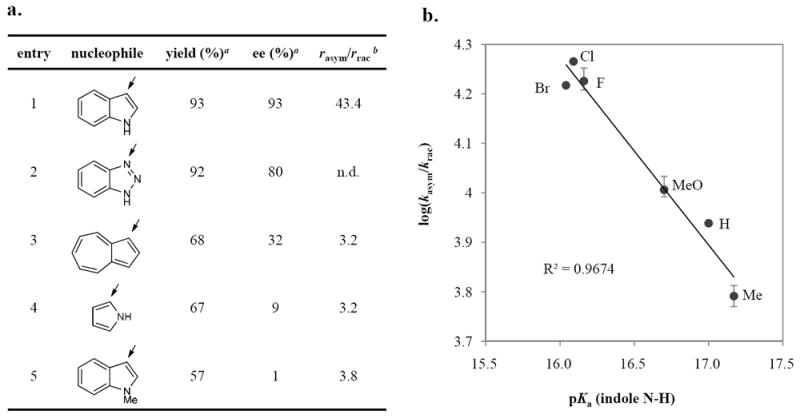
a. Structure-reactivity and -enantioselectivity relationship of p-nucleophiles. a Yields and enantiomeric excesses were obtained under reaction conditions described in eq. 2. b The initial reaction rates with 4-NBSA alone (rrac) and with 4-NBSA/3e (rasym) were determined directly by in situ IR spectroscopy. The (rasym/rrac) value was not determined for benzotriazole (entry 2) because the kinetic analysis was complicated by the poor solubility of the nucleophile in the reaction medium. b. Correlation between the degree of rate acceleration by 3e over the background racemic reaction (kasym/krac) and the acidity of the N-H motif of 5-substituted indole derivatives (pKa). The rate data were obtained by in situ IR and 1H NMR spectroscopy (see Supplementary Information, Section 14 for detailed experimental procedure and data analysis). Error bars reflect the range of experimental data from 2-3 individual measurements, and the line represents the least-squares fit.
To further probe the role of H-bonding interactions between indole nucleophile and catalyst, various 5-substituted indoles were evaluated in competition experiments under both racemic and thiourea-catalysed reaction conditions. The degree of rate acceleration induced by thiourea 3e was found to be linked directly to the acidity of the indoles, and a linear correlation between log(kasym/krac) and the pKa of indoles was observed (Figure 3b). Therefore, in the presence of thiourea, the rate of the reaction is correlated not only to the intrinsic nucleophilicity of the indole, but also to its H-bond donor properties. No correlation was observed between reaction enantioselectivity and the acidity of the indoles, however, indicating that this interaction is likely to exist to a similar degree in both the major and the minor pathways. The kinetic data are thus consistent with general base activation of indole through an indole N-H–catalyst hydrogen bonding interaction in the rate-determining addition to episulfonium ions.31,32,33,34 Catalyst 3e possesses few Lewis basic functionalities – namely the thiourea, the amide, and perhaps the extended arene substituent – and therefore the possible catalyst H-bond acceptor sites are limited in number. The similar reactivity and enantioselectivity displayed with urea 4e and thiourea 3e would appear to rule out a direct role of the thiourea sulfur atom. As discussed below, the extended aromatic plays a key role that can be tied to interactions with the episulfonium ion. By this simple process of elimination, we therefore propose that the amide oxygen is the most likely H-bond acceptor site for activation of the indole.35,36 Consistent with this hypothesis, catalyst 3a was found to be more reactive than the corresponding thioamide analog in the model reaction (eq 2). In addition, the reaction catalyzed by 3a is ca. 4 times as fast as the reaction with Schreiner’s thiourea [1,3-bis(3,5-bis(trifluoromethyl)phenyl)thiourea], which is a stronger H-bond donor but lacks an amide appendage.
C.3 Stabilisation of the Cationic Transition State Through Cation-π Interactions
The strong correlation between reaction enantioselectivity and the identity of the arene on the catalyst suggests a direct role of the extended π-system in the mechanism of stereoinduction. In principle, this arene effect may be due primarily either to acceleration of the major pathway through transition state stabilisation, or to inhibition of pathways leading to the minor enantiomer through destabilising interactions. This question was addressed through kinetic analysis of the reaction, taking advantage of the fact that the indole addition step is both rate- and ee- determining. The rate constant corresponding to the major pathway (kasym,major) could be deduced for catalysts 3a-3g from in situ IR-based kinetic measurements combined with er (enantiomeric ratio) determinations. A strong correlation between this rate and reaction enantioselectivity was observed, with plots of ln(kasym,major) vs ln(er) providing a good linear fit (Figure 4a). This provides unambiguous evidence that enantioselectivity increases due to variation of the aryl component of the catalyst 3 are indeed tied to stabilisation of the major transition structure.14 The rate of the pathway leading to the minor enantiomer also displays a linear, positive correlation with reaction er, indicating that the minor transition structure is also stabilised selectively by the more enantioselective catalysts, albeit to a substantially lesser extent.
Figure 4. Enantioinduction is achieved by the thiourea catalysts through a selective, attractive cation-π interaction between the extended aromatic residue on the catalyst and the acidic a-protons in the episulfonium ion.

a. Correlation between rate and enantioselectivity of reactions catalysed by thioureas 3a-3g. Each data point represents the average rate determined from two individual kinetic experiments, with the error bar showing the range of the measurements. The rate constants for the major and the minor pathways (kasym,major and kasym,minor, respectively) are calculated on the basis of the rate equations (eqs. 3 & 4) and the following equations: (a) rasym = rasym,major + rasym,minor, (b) er = rasym,major/rasym,minor. Lines represent least-squares fits. b. 1H NMR binding study of thiourea and 5 in d8-toluene, showing attractive interactions between the aromatic group in 3e and the a-protons in 5. The resonances of the benzylic protons and the methyl protons in 5 are labled with blue and green dots, respectively. In the two bottom spectra, the methyl resonance overlaps with the solvent peak, but can be identified when zoomed-in c. Electrostatic potential maps for fully optimized structures (B3LYP/6-31G(d)) of the episulfonium ion derived from 1a and the sulfonium ion in 5, revealing a similar distribution of positive charge over the benzylic protons. Negative potentials are shown in red and positive potentials in blue.
Insight into the nature of the transition state stabilising interactions that may be at play was provided through spectroscopic analysis of thiourea derivatives 3a and 3e complexed to a sulfonium ion model system. The dibenzylmethylsulfonium ion triflate 5 was selected for these studies because episulfonium sulfonate could not be examined directly (see section B), and the cation in 5 was found by computational methods to have a very similar charge distribution to the episulfonium ion (Figure 4 c). With thiourea 3e, the resonances of the benzylic and the methyl protons of 5 underwent a significant upfield shift (0.6-0.8 ppm) upon formation of the 1:1 complex (Figure 4b).37 In contrast, the chemical shift of those protons was bearly perturbed to any measurable extent (Δδ < 0.05 ppm) upon complexation of 5 with the thiourea derivative 3a, which lacks an aryl substituent. This points to an attractive cation-π interaction between the π-face of the arene in catalyst 3e and the sulfonium ion in the model system.38,39,40,41,42 Such interaction in the transition structure of the indole addition to episulfonium ions could underlie the observed enantioselectivity effects, as the cation-π interaction would be expected to increase in magnitude with more extended aromatic substituents.43,44 Based on the kinetic and enantioselectivity data, we therefore propose that the difference in the strength of the cation-π interaction between the major and minor pathways lies at the origin of the observed high reaction enantioselectivity.6,14,45 It should be noted that on the basis of polarisability effects alone, which are known to correlate directly with cation-π binding ability,46 the most expansive aryl-substituted catalysts 3f and 3g might be expected to be most enantioselective. However, as noted above (Table 1) these two thiourea derivatives afford slightly lower ee’s in the model reaction than the smaller, phenanthryl catalyst 3e. The reason for the lack of an exact correlation between the polarisability of the aromatic substituent and reaction enantioselectivity has not yet been established; however, it seems reasonable to expect that any number of minor steric or conformational factors could attenuate the ability of the largest substituents to engage fully in the stabilising cation-π interaction.
Taken together, the data presented above allow construction of a detailed mechanistic model for the enantioselective reaction, wherein rate-acceleration and enantioselectivity are induced by the thiourea catalyst through a network of attractive non-covalent interactions. In particular, we propose that the transition structure for the rate-determining addition of indole to the episulfonium ion is stabilised by a combination of anion binding of the thiourea to the sulfonate, general base activation of the indole via a catalyst amide–indole N-H interaction, and a cation-π interaction between the arene of the catalyst and the benzylic protons of the episulfonium ion (Figure 5). We anticipate that characterisation of these enzyme-like non-covalent stabilising elements with small molecule catalysts such as 3e may enable the future design and application of such biomimetic strategies in organic asymmetric synthesis.
Figure 5.

Proposed transition structure model. The transition structure for the rate-determining addition of indole to the episulfonium ion is stabilised by a combination of attractive, non-covalent interactions, including anion binding of the thiourea to the sulfonate, general base activation of the indole via a catalyst amide–indole N-H interaction, and a cation-p interaction between the arene of the catalyst and the benzylic protons of the episulfonium ion.
Methods
General procedure for thiourea 3e-catalysed nucleophilic ring opening of episulfonium ions with indole derivatives
An oven-dried 1.5 dram vial was charged with substrate 1 (0.05 mmol, 1.0 equiv), 3e (3.2 mg, 0.0050 mmol, 0.10 equiv), indole (11.7 mg, 0.10 mmol, 2.0 equiv) and 4Å molecular sieves (25 mg, powder, activated) under an atmosphere of N2. The vial was cooled to −30 °C, and toluene (1 mL) was added with stirring. Once the reactants and catalyst were fully dissolved, the mixture was cooled to −78 °C, and solid 4-NBSA (0.7 mg, 0.0035 mmol, 0.07 equiv) was added at once against a counterflow of N2. The resulting solution was stirred at −30 °C and the progress of the reaction was monitored by thin layer chromatography (TLC) (see Supplementary Information, Section 3). When the progress of the reaction was determined to be complete, triethylamine (~10 μL) was added at −30 °C. The resulting mixture was applied directly to a pipette column containing 4-5 cm of silica gel, and product was isolated by eluting hexanes/ethyl acetate (20:1 to 10:1) and solvent removal.
The Supplementary Information contains: detailed experimental procedures, synthesis of substrates and catalysts, characterisation data for all new compounds, procedures and data for mechanistic investigations (including reaction progress kinetic analysis, linear free energy relationship studies with Mayr’s reactivity parameters, kinetic isotope effect studies, model binding studies by 1H NMR, and other experimental kinetic studies). Crystallographic information for compounds 2b, 2g and 2q have been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition numbers (CCDC 862750, 862751 and 862752, respectively).
Supplementary Material
Acknowledgments
This work was supported by the NIGMS (P50 GM-69721 and RO1 GM-43214) and by a predoctoral fellowship to S.L. from Eli Lilly. We thank Dr. Robert Knowles, Dr. Katrien Brak and Prof. Dr. Herbert Mayr for helpful discussions, Adam Brown, Dr. Dan Lehnherr and Dr. Alan Hyde for the use of catalysts, and Dr. Shao-Liang Zheng for crystal structure determinations.
Footnotes
Author Contributions
S.L. conducted the experiments; S.L. and E.N.J. wrote the manuscript; E.N.J. guided the research.
Supplementary information and chemical compound information accompany this paper at www.nature.com/naturechemistry.
The authors declare no competing financial interests.
References
- 1.Zhang ZG, Schreiner PR. (Thio)urea organocatalysis—What can be learnt from anion recognition? Chem Soc Rev. 2009;38:1187–1198. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- 2.Reisman SE, Doyle AG, Jacobsen EN. Enantioselective thiourea-catalyzed additions to oxocarbenium ions. J Am Chem Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AR, Kuo W-H, Jacobsen EN. Enantioselective catalytic α-alkylation of aldehydes via an SN1 pathway. J Am Chem Soc. 2010;132:9286–9288. doi: 10.1021/ja103618r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De CK, Klauber EG, Seidel D. Merging nucleophilic and hydrogen bonding catalysis: an anion binding approach to the kinetic resolution of amines. J Am Chem Soc. 2009;131:17060–17061. doi: 10.1021/ja9079435. [DOI] [PubMed] [Google Scholar]
- 5.Knowles RR, Jacobsen EN. Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts. Proc Natl Acad Sci U S A. 2010;107:20678–20685. doi: 10.1073/pnas.1006402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles RR, Lin S, Jacobsen EN. Enantioselective thiourea-catalyzed cationic polycyclizations. J Am Chem Soc. 2010;132:5030–5032. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuend SJ, Jacobsen EN. Mechanism of amido-thiourea catalyzed enantioselective imine hydrocyanation: transition state stabilization via multiple non-covalent interactions. J Am Chem Soc. 2009;131:15358–15374. doi: 10.1021/ja9058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox DJ, House D, Warren S. Mechanisms of sulfanyl (RS) migrations: synthesis of heterocycles. Angew Chem Int Ed. 2002;41:2462–2482. doi: 10.1002/1521-3773(20020715)41:14<2462::AID-ANIE2462>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Smit WA, Caple R, Smoliakova IP. Stepwise electrophilic addition. Some novel synthetic ramifications of an old concept. Chem Rev. 1994;94:2359–2382. [Google Scholar]
- 10.Fachini M, Lucchini V, Modena G, Pasi M, Pasquato L. Nucleophilic reactions at the sulfur of thiiranium and thiirenium ions. New insight in the electrophilic additions to alkenes and alkynes. Evidence for an episulfurane intermediate. J Am Chem Soc. 1999;121:3944–3950. [Google Scholar]
- 11.Toshimitsu A, Hirosawa C, Tamao K. Retention of configuration in the Ritter-type substitution reaction of chiral/3-arylthio alcohols through the anchimeric assistance of the arylthio group. Tetrahedron. 1994;50:8997–9008. [Google Scholar]
- 12.Hamilton GL, Kanai T, Toste FD. Chiral anion-mediated asymmetric ring opening of meso-aziridinium and episulfonium ions. J Am Chem Soc. 2008;130:14984–14986. doi: 10.1021/ja806431d. [DOI] [PubMed] [Google Scholar]
- 13.Wu YJ. In: Heterocyclic Scaffolds II: Topics in Heterocyclic Chemistry. Gribble GW, editor. Vol. 26. Springer Berlin; 2011. pp. 1–29. [Google Scholar]
- 14.Uyeda C, Jacobsen EN. Transition-state charge stabilization through multiple non-covalent interactions in the guanidinium-catalyzed enantioselective claisen rearrangement. J Am Chem Soc. 2011;133:5062–5075. doi: 10.1021/ja110842s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denmark SE, Kornfilt DJP, Vogler T. Catalytic asymmetric thiofunctionalization of unactivated alkenes. J Am Chem Soc. 2011;133:15308–15311. doi: 10.1021/ja2064395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denmark SE, Collins WR, Cullen MD. Observation of direct sulfenium and selenenium group transfer from thiiranium and seleniranium ions to alkenes. J Am Chem Soc. 2009;131:3490–3492. doi: 10.1021/ja900187y. [DOI] [PubMed] [Google Scholar]
- 17.Denmark SE, Vogler T. Synthesis and reactivity of enantiomerically enriched thiiranium ions. Chem Eur J. 2009;15:11737–11745. doi: 10.1002/chem.200901377. [DOI] [PubMed] [Google Scholar]
- 18.Lucchini V, Modena G, Pasquato L. Enantiopure thiosulfonium salts in asymmetric synthesis. Face selectivity in electrophilic additions to unfunctionalised olefins. J Chem Soc Chem Commun. 1994:1565–1566. [Google Scholar]
- 19.Mayr H, Kempf B, Ofial AR. Π-nucleophilicity in carbon–carbon bond-forming reactions. Acc Chem Res. 2003;36:66–77. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]
- 20.Birrell JA, Desrosiers J-N, Jacobsen EN. Enantioselective acylation of silyl ketene acetals through fluoride anion-binding catalysis. J Am Chem Soc. 2011;133:13872–13875. doi: 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackmond DG. Reaction progress kinetic analysis: a powerful methodology for mechanistic sudies of complex catalytic reactions. Angew Chem Int Ed. 2005;44:4302–4320. doi: 10.1002/anie.200462544. [DOI] [PubMed] [Google Scholar]
- 22.Blackmond DG, Ropic M, Stefinovic M. Kinetic studies of the asymmetric transfer hydrogenation of imines with formic acid catalyzed by Rh-diamine catalysts. Org Process Res & Dev. 2006;10:457–463. [Google Scholar]
- 23.Denmark SE, Burk MT. Lewis base catalysis of bromo- and iodolactonization, and cycloetherification. Proc Natl Acad Sci. 2010;107:20655–20660. doi: 10.1073/pnas.1005296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French DC, Crumrine DS. Improved correlation of 33S chemical shifts with pKa’s of arenesulfonic acids: use of 33S NMR for pKa determination. J Org Chem. 1990;55:5494–5496. [Google Scholar]
- 25.Phan TB, Breugst M, Mayr H. Towards a general scale of nucleophilicity? Angew Chem Int Ed. 2006;45:3869–3874. doi: 10.1002/anie.200600542. [DOI] [PubMed] [Google Scholar]
- 26.Maresh JJ, et al. Strictosidine synthase : mechanism of a Pictet–Spengler catalyzing enzyme. J Am Chem Soc. 2008;130:710–723. doi: 10.1021/ja077190z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosnich B. In: Asymmetric Catalysis. Bosnich B, editor. Marinus Nijhoff; 1986. p. 14. [Google Scholar]
- 28.Kelly TR, Kim MH. Relative binding affinity of carboxylate and its isosteres: nitro, phosphate, phosphonate, sulfonate, and δ-lactone. J Am Chem Soc. 1994;116:7072–7080. [Google Scholar]
- 29.Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN. Asymmetric cooperative catalysis of strong Brønsted acid–promoted reactions using chiral ureas. Science. 2010;327:986–990. doi: 10.1126/science.1182826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheerder J, Engbersen JFJ, Casnati A, Ungaro R, Reinhoudt DN. Complexation of halide anions and tricarboxylate anions by neutral urea-derivatized p-tert-butylcalix[6]arenes. J Org Chem. 1995;60:6448–6454. [Google Scholar]
- 31.Lan T, McLaughlin LW. The energetic contribution of a bifurcated hydrogen bond to the binding of DAPI to dA-dT rich sequences of DNA. J Am Chem Soc. 2001;123:2064–2065. doi: 10.1021/ja003451s. [DOI] [PubMed] [Google Scholar]
- 32.Braun J, Neusser HJ, Hobza P. N-H···π interactions in indole···benzene-h6,d6 and indole···benzene-h6,d6 radical cation complexes. Mass analyzed threshold ionization experiments and correlated ab initio quantum chemical calculations. J Phys Chem A. 2003;107:3918–3924. [Google Scholar]
- 33.Biswal HS, Wategaonkar S. Nature of the N–H···S hydrogen bond. J Phys Chem A. 2009;113:12763–12773. doi: 10.1021/jp907658w. [DOI] [PubMed] [Google Scholar]
- 34.Herrera RP, Sgarzani V, Bernardi L, Ricci A. Catalytic enantioselective Friedel–Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew Chem Int Ed. 2005;44:6576–6579. doi: 10.1002/anie.200500227. [DOI] [PubMed] [Google Scholar]
- 35.James WH, III, et al. Evolution of amide stacking in larger γ-peptides: triamide H-bonded cycles. J Phys Chem A. 2011;115:13783–13798. doi: 10.1021/jp205527e. [DOI] [PubMed] [Google Scholar]
- 36.Gustafson J, Lim D, Miller SJ. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepodd TJ, Petti MA, Dougherty DA. Tight, oriented binding of an aliphatic guest by a new class of water-soluble molecules with hydrophobic binding sites. J Am Chem Soc. 1986;108:6085–6087. doi: 10.1021/ja00279a093. [DOI] [PubMed] [Google Scholar]
- 38.Kearney PC, Mizoue LS, Kumpf RA, Forman JE, McCurdy A, Dougherty DA. Molecular recognition in aqueous media. New binding studies provide further insights into the cation-π interaction and related phenomena. J Am Chem Soc. 1993;115:9907–9919. [Google Scholar]
- 39.Ma JC, Dougherty DA. The cation-π interaction. Chem Rev. 1997;97:1303–1324. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]
- 40.Dougherty DA. Cation-π interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Scienc. 1996;271:163–168. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]
- 41.Raju RK, Bloom JWG, An Y, Wheeler SE. Substituent effects on non-covalent interactions with aromatic rings: insights from computational chemistry. ChemPhysChem. 2011;12:3116–3130. doi: 10.1002/cphc.201100542. [DOI] [PubMed] [Google Scholar]
- 42.Xiu X, Puskar NL, Shanata JAP, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation–π interaction. Nature. 2009;458:534–538. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vijay D, Sastry GN. Exploring the size dependence of cyclic and acyclic π-systems on cation–π binding. Phys Chem Chem Phys. 2008;10:582–590. doi: 10.1039/b713703f. [DOI] [PubMed] [Google Scholar]
- 44.Gal J-F, Maria P-C, Decouzon M, Mo O, Yanez M, Abboud JLM. Lithium-cation/π complexes of aromatic systems. The effect of increasing the number of fused rings. J Am Chem Soc. 2003;125:10394–10401. doi: 10.1021/ja029843b. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Liu P, Houk KN, Birman VB. Origin of enantioselectivity in CF3–PIP-catalyzed kinetic resolution of secondary benzylic alcohols. J Am Chem Soc. 2008;130:13836–13837. doi: 10.1021/ja805275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngola SM, Dougherty DA. Evidence for the importance of polarizability in biomimetic catalysis involving cyclophane receptors. J Org Chem. 1996;61:4355–4360. doi: 10.1021/jo960521y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


