Abstract
A completely effective vaccine for malaria (one of the major infectious diseases worldwide) is not yet available; different membrane proteins involved in parasite-host interactions have been proposed as candidates for designing it. It has been found that proteins encoded by the merozoite surface protein (msp)-7 multigene family are antibody targets in natural infection; the nucleotide diversity of three Pvmsp-7 genes was thus analyzed in a Colombian parasite population. By contrast with P. falciparum msp-7 loci and ancestral P. vivax msp-7 genes, specie-specific duplicates of the latter specie display high genetic variability, generated by single nucleotide polymorphisms, repeat regions, and recombination. At least three major allele types are present in Pvmsp-7C, Pvmsp-7H and Pvmsp-7I and positive selection seems to be operating on the central region of these msp-7 genes. Although this region has high genetic polymorphism, the C-terminus (Pfam domain ID: PF12948) is conserved and could be an important candidate when designing a subunit-based antimalarial vaccine.
Introduction
Plasmodium vivax malaria should not be considered a benign disease anymore due to its wide geographical distribution [1], [2] and ability to cause severe clinical syndromes [3], [4], [5], [6]. It was estimated that 2.85 billion people were at risk of being infected by this parasite in 2009 [1]. Although several research groups have focused on developing a vaccine against P. vivax, relatively few vaccine candidate molecules have been thoroughly characterized [7], [8]; some of them are membrane proteins making attractive targets for vaccine development since they are essential for the recognition and invasion of erythrocytes, hence immune responses directed against them could inhibit parasite entry [9]. The merozoite surface protein (MSP) family is a group of surface proteins which are involved in the initial interaction between the parasite and the host cell. However, the genetic diversity of these proteins has been the main problem for developing a vaccine [10], [11], [12], [13], [14]. Proteins displaying high antigenic diversity elicit strain-specific immune responses leading to low protective efficacy upon vaccination, while proteins displaying limited variability are attractive targets for testing as vaccine candidates. Research involving proteins displaying high overall polymorphism should be focused on the conserved functional domains [15] so that strain-specific responses may be avoided.
MSP-1, MSP-6 and MSP-7 form the most abundant protein complex on the P. falciparum merozoite surface [16], [17]. msp-1 and msp-6 are both single copy genes while msp-7 has a large number of genes which seem not to have diverged functionally. Furthermore, the Plasmodium genus has dissimilar copy numbers of this gene [18], [19]. The msp-7 genes have a single exon and have been named in alphabetical order according to their position regarding the PVX_082640 flanking gene [18]. Different msp-7 genes are expressed simultaneously in the schizont stage of Plasmodium species [20], [21], [22] and several of them have been localized on the membrane surface [19], [21], [22], [23], not only forming part of the main merozoite protein complex but also binding to erythrocytes [24]. Knockout and invasion inhibition assays have also shown that MSP-7 is involved in the invasion of erythrocytes [19], [24], [25]. Moreover, Wang’s group has shown that a recombinant MSP-7 is recognized by sera from malaria-infected individuals where IgG3 subclass antibodies prevail [26]. Immunization with members of this family has been shown to protect vaccinated mice against experimental challenge [21].
P. falciparum has eight msp-7 genes and P. vivax eleven, [18], [27]; the members of this family have low genetic variability in the former specie, [28], [29] as do the P. vivax msp-7A (GenBank ID: XM_001614080.1) and msp-7K (GenBank ID: XM_001614090.1) genes [30]. A recent study has shown that P. vivax specie-specific duplicates MSP-7C (GenBank ID: XM_001614082.1) and MSP-7H (GenBank ID: XM_001614087.1) are recognized by IgG antibodies from P. vivax-infected patient sera [31]. These proteins are phylogenetically related to PvMSP-7I (GenBank ID: XM_001614088.1) [18], [27] and fragments from different MSP-7 proteins may have to be included to block this invasion route due to the biological implication that functional redundancy might have on vaccine development. Taking into account that some of these proteins are recognized by the immune system, their genetic diversity should be evaluated to determine their potential use as potent anti-malarial vaccine candidates.
Results
Forty-eight parasite samples were collected from symptomatic patients living in representative regions of Colombia for this study: Amazonian (Calamar, Guaviare department), Andean (Apartadó and El Bagre, Antioquia department), Caribbean (Tierra Alta and Puerto Libertador, Córdoba department), Orinoquia (Mapiripan, Meta department and Tauramena, Casanare department) and Pacific (Istmina, Chocó department and Tumaco, Nariño department) (Fig. S1). Forty-two samples corresponding to single P. vivax msp-1 allele infections were considered for PCR amplification even though amplicons were not detected in a few samples (Pvmsp-7C n = 37, Pvmsp-7H n = 37 and Pvmsp-7I n = 42).
Polymorphism in Pvmsp-7 Loci
1,167-bp Pvmsp-7C, 1,232-bp Pvmsp-7H and 1,182-bp Pvmsp-7I gene fragments were amplified and direct sequenced. Nucleotide sequence data here reported are available in GenBank: accession numbers JQ423957-JQ424037. Twenty-three haplotypes were found for Pvmsp-7C while twenty-eight haplotypes were found for both Pvmsp-7H and Pvmsp-7I. Haplotype diversity (Hd) was lower in the Pvmsp-7C gene than in Pvmsp-7H and Pvmsp-7I (Table 1). 148 sites from the total nucleotide sequence length analyzed were polymorphic in Pvmsp-7C and 142 sites in Pvmsp-7H, while 121 polymorphic sites were found in Pvmsp-7I (Table 1).
Table 1. Estimates of DNA diversity and neutrality test for Pvmsp-7 genes from a Colombian population.
| n | Gene | Sites | Ss | S | Ps | H | Hd (se) | θw (se) | π (se) | Tajima | Fu & Li | Fu | Zns | ZZ | RM | |
| D | D* | F* | Fs | |||||||||||||
| 37 | msp-7C | 1,098 | 148 | 1 | 147 | 23 | 0.93 (0.03) | 0.0323 (0.001) | 0.0548 (0.003) | 2.094** | 1.810* | 2.287* | 7.64 | 0.339 | 0.173* | 22 |
| 37 | msp-7H | 1,131 | 142 | 19 | 123 | 28 | 0.96 (0.02) | 0.0301 (0.002) | 0.0357 (0.003) | 0.388 | 0.952 | 0.987 | -0.57 | 0.126 | 0.343* | 27 |
| 42 | msp-7I | 1,058 | 127 | 6 | 121 | 28 | 0.97 (0.01) | 0.0280 (0.002) | 0.0430 (0.003) | 1.420 | 1.584** | 1.818* | 2.12 | 0.232 | 0.334* | 13 |
Ss: Number of segregating sites, S: number of singleton sites, Ps: number of parsimony-informative sites, H: number of haplotypes, Hd: haplotype diversity, θW: Watterson estimator, π: nucleotide diversity. (se): Standard deviation. Sites excluded from the analysis: Pvmsp-7C: nucleotides 1 to 17 (amino acids 1 to 6), 544 to 546 (amino acid 182), 616 to 618 (amino acid 206), 625 to 627 (amino acid 209), 631 to 648 (amino acids 211 to 216), 655 to 666 (amino acids 219 to 222), 676 to 684 (amino acids 226 to 228), 706 to 708 (amino acid 236), 1,105 to 1,107 (amino acid 369) and nucleotides 1,168 to 1,191 (amino acids 390 to 397); Pvmsp-7H: nucleotides 436 to 450 (amino acids 146 to 150), 448 to 486 (amino acid 162), 568 to 627 (amino acids 190 to 200) and nucleotides 772 to 774 (amino acid 258); Pvmsp-7I: nucleotides 1 to 18 (amino acids 1 to 6), 421 to 522 (amino acids 141 to 174) and nucleotides 526 to 537 (amino acids 176 to 179).
: p<0.02, **: p<0.05.
Nucleotide diversity for these three genes (Pvmsp-7C π = 0.0548, Pvmsp-7H π = 0.0357 and Pvmsp-7I π = 0.0430) was higher than that previously reported for other P. vivax msp-7 family members (Pvmsp-7A π = 0 and Pvmsp-7K π = 0.0022) [30]. Furthermore, the msp-7 genes from this study were among the most polymorphic P. vivax genes reported to date (Table S1). Regarding Pvmsp-7 diversity (π value average) in the different Colombian regions, the Pacific area was the most diverse followed by the Andean, Caribbean and Orinoco regions while the Amazonian was the least polymorphic (Table S2).
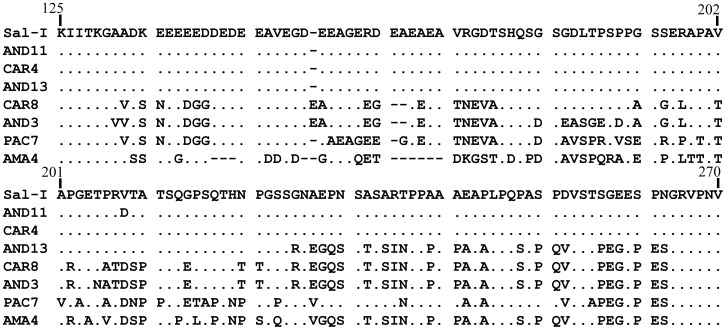
Three major allele types were found in deduced PvMSP-7C amino acid sequences (Fig. 1 and Fig. S2) between residues 134 and 238 (numbered according to the alignment in Fig. S2). Allele types had similar frequency in the Colombian parasite population.
Figure 1. Alignment of deduced PvMSP-7C amino acid sequences, including the Sal-I sequence in which the three main allele types can be observed.
Dots indicate conserved residues and dashes represent gaps introduced for alignment.
An AEAFG insertion-deletion from residue 146 to 150 and a GTG(n)GT[V/E] repeat region from residue 195 to 209 (numbered according to the alignment in Fig. S3) were revealed by protein sequence alignment in PvMSP-7H. Three regions throughout the protein sequences led to discriminating several allele types (Fig. 2 and Fig. S3). The first region from residue 153 to 170 (numbered according to the alignment given in the Fig. S3) had four different peptide sequences. Five different sequences were found in the second region (from residue 172 to 194) and three different ones were found from residue 229 to 247. These three regions were randomly associated (Fig. 2 and Fig. S3).
Figure 2. Alignment of deduced PvMSP-7H amino acid sequences, including the Sal-I sequence.
The alignment shows major allele types found in Colombian populations. Dots indicate conserved residues and dashes represent gaps introduced for alignment.
EEAVEGD and EA repeats could be observed in the deduced PvMSP-7I amino acid sequences. Similar to the genes mentioned above, several major allele types were observed between residues 131 and 219 (numbered according to the alignment in Fig. S4) characterized by two regions. The first region (between amino acids 131 and 140) had three different peptide sequences (Fig. 3 and Fig. S4). The second region ran from residue 163 to residue 219, having an extra four different peptide sequences. These regions were not randomly associated (Fig. 3 and Fig. S4). A further four peptide sequences were found from residue 221 to residue 234 and two more from residue 236 to residue 264 and, contrary to those mentioned above, these fragments were found to be associated with either of the alleles described above (Fig. 3 and Fig. S4).
Figure 3. Alignment of deduced PvMSP-7I amino acid sequences, including the Sal-I sequence in which the protein sequences differentiating major allele types can be observed.
Dots indicate conserved residues and dashes represent gaps introduced for alignment.
Neutral Evolutionary Test and Selection in the Pvmsp-7 Loci
Applying neutral evolution tests (Tajima, Fu & Li) to the Colombian P. vivax population gave significant values above 0 for Pvmsp-7C and Pvmsp-7I (p<0.05 Tajima D, p<0.02 Fu & Li D* and F*) (Table 1); these values indicated an excess of intermediate frequency alleles. The neutral evolution tests for Pvmsp-7H revealed no statistically significant differences; this gene therefore seems to be evolving under neutral expectations. However, a sliding window analysis for D, D* and F* statistics showed that different selective forces could be acting throughout the Pvmsp-7 genes. It was found that balancing selection acted on the central gene region while negative selection could be acting at the 5′-ends (significant values for Pvmsp-7C and Pvmsp-7H) and 3′-ends (no significant values) (Fig. S5).
The average number of synonymous substitutions per synonymous site (dS) and non-synonymous substitutions per non-synonymous site (dN) was estimated to determine whether natural selection was affecting the msp-7 loci. Although dN was higher than dS in Pvmsp-7C and Pvmsp-7I, dN was lower than dS in the Pvmsp-7H gene even though these values were not statistically significant (Table 2). Taking into account that a sliding window for ω (dN/dS) (Fig. S6) showed that the ω rate was higher than 1 (signal of positive selection) in the central region of the msp-7 genes analyzed here, the genes were thus split into 3 regions: 5′-end (msp-7C: between nucleotides 1 and 390, msp-7H: between nucleotides 1 and 471, msp-7I: between nucleotides 1 and 525), central (msp-7C: between nucleotides 391 and 717, msp-7H: between nucleotides 472 and 771, msp-7I: between nucleotides 526 and 789) and 3′-end (msp-7C: between nucleotide 718 and 1,191, msp-7H: between nucleotide 772 and 1,200, msp-7I: between nucleotide 790 and 1,188) (Table S3); dN and dS were then computed. dS was higher than dN for both 5′- and 3′-ends, just the 5′-end having significant statistical difference. On the other hand, the dN substitutions in the central region were significantly higher than dS substitutions for the three genes (Table 2).
Table 2. Average number of synonymous substitutions per synonymous site (dS) and non-synonymous substitutions per non-synonymous site (dN) covering all sequence pairs at the 5′-end, central region, 3′-end and complete gene.
| 5′-end | Central region | 3′-end | Full length gene | |||||
| dS (se) | dN (se) | dS (se) | dN (se) | dS (se) | dN (se) | dS (se) | dN (se) | |
| msp-7C | 0.070 (0.016)** | 0.011 (0.004) | 0.122 (0.023) | 0.287 (0.031)• | 0.004 (0.003) | 0.000 (0.000) | 0.053 (0.008) | 0.059 (0.007) |
| msp-7H | 0.059 (0.010)• | 0.010 (0.003) | 0.052 (0.016) | 0.162 (0.019)• | 0.010 (0.005) | 0.005 (0.002) | 0.038 (0.006) | 0.037 (0.005) |
| msp-7I | 0.041 (0.014)* | 0.020 (0.007) | 0.060 (0.019) | 0.186 (0.021)• | 0.017 (0.007) | 0.007 (0.003) | 0.036 (0.007) | 0.048 (0.006) |
se: standard error. 5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188).
: p<0.04, **: p<0.001, •: p<0.0001.
Furthermore, several maximum likelihood-based methods were used for identifying which codon sites were under positive or negative selection. Pvmsp-7C had 19 sites under positive selection and 21 negatively selected sites according to SCAL, FEL, IFEL and REL methods. Positive selection signatures were found for Pvmsp-7H in 50 sites while another 32 sites were negatively selected and Pvmsp-7I had 10 sites under positive selection and 30 negatively selected sites (predicted by at least one method, Tables S4 and S5). Taking into account that recombination (see below) can affect the reliability of identifying sites under selection [32], the analysis was performed again, this time considering recombination. Two sites were thus positively selected and 43 were under negative selection for Pvmsp-7C. Ten sites were positively selected and 24 were negatively selected for Pvmsp-7H, and 4 sites were under positive selection while 13 sites were under negative selection for Pvmsp-7I (Tables S6 and S7).
Linkage Disequilibrium (LD) and Recombination
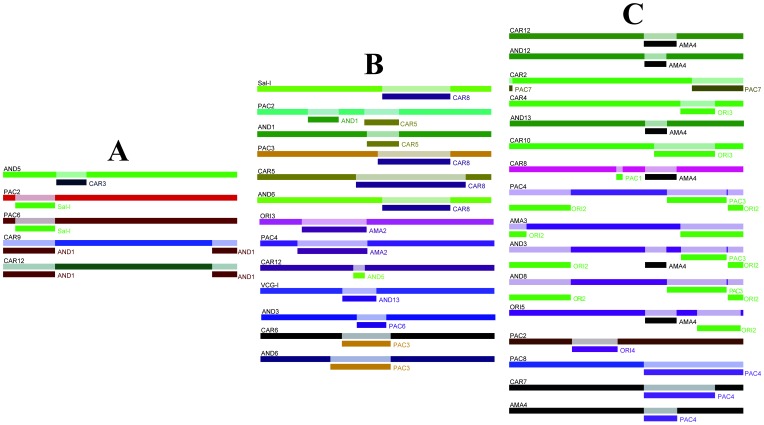
Random association was observed among Pvmsp-7 haplotypes, therefore suggesting their independent segregation. LD analysis measured by ZnS (average of R?2) for whole data revealed no statistically significant values for the three msp-7 genes (Table 1). The relationship of R?2 (linkage disequilibrium) with physical distance in regression analysis showed that LD declined as nucleotide distance increased (Fig. S7). The ZZ statistic had significant values (Table 1). Therefore, both the regression analysis and ZZ statistic suggested that intragenic recombination was taking place in Pvmsp-7C, Pvmsp-7H and Pvmsp-7I. Recombination events were detected by using DnaSP which revealed several RM events in all genes (Table 1). Figure 4 shows the recombinant regions detected with RDP v3.4 software.
Figure 4. Schematic representation of recombination fragments identified by RDP3 v.3.4 for Pvmsp-7C (A), Pvmsp-7H (B) and Pvmsp-7I (C).
The sequence names in black above the rectangles indicate the name of recombinant sequence. The rectangle with name to the right (name of the close relative minor parent) shown in different colors is a graphical representation of a sequence fragment that has potentially been derived through recombination. Only recombination events having p<0.03 were taken into account. SAL-I: Salvador strain, AMA: Amazonian, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
Clustering Analysis
Since recombination was detected for msp-7 genes, phylogeny had to be inferred taking it into account. A phylogenetic tree was inferred for each recombinant region detected (Fig. S8, S9, S10). All topologies showed no geographical clustering among the Colombian isolates; moreover, Colombian and Salvadorian sequences clustered together (Fig. S8, S9, S10).
All Pvmsp-7 sequences were aligned and trees were constructed by using ML with the TN93+G model. The tree showed three monophyletic groups (Fig. S11A); the first group clustered Pvmsp-7H sequences, close to the second group which clustered Pvmsp-7I sequences, while the third group clustered Pvmsp-7C sequences. This topology agreed with the phylogenetic relationship previously reported for Pvmsp-7 [18], [27]. However, Pvmsp-7 sequences showed recombinant fragments possibly produced by gene conversion between paralogous genes at the 5′ and 3′-ends but not in the central region (Fig. S12 and Table S8). The Pvmsp-7 genes became clustered into paraphyletic groups when trees were inferred taking gene conversion into account (Fig. S11B–E). Codons 38 and 112 in the three genes, as well as the 57, 70, 102 and 125 for Pvmsp-7C and Pvmsp-7H, and codons 48 and 86 for Pvmsp-7H and Pvmsp-7I (numbered according to the alignment in Fig. S13) were predicted as negatively selected sites but, taking into account that they lay within the gene conversion tracks detected, purifying selection at these sites could have been the result of gene conversion between paralogous gene loci.
The dS and dN rates between paralogous gene pairs were estimated to determine whether homogeneity at the Pvmsp-7 ends was caused by concerted evolution (Table 3); the dS was higher than dN at the 5′-end for the three genes. The dS rate at the 3′-end between Pvmsp-7C and the other two genes was higher than dN rate but the dS was similar to dN between Pvmsp-7H and Pvmsp-7I (Table 3).
Table 3. Synonymous nucleotide substitution per synonymous site and nonsynonymous nucleotide substitution per nonsynonymous site and the standard deviations between three Pvmsp-7 genes.
| Synonymous nucleotide substitution per synonymous site (dS) | |||||||||
| 5′-end | Central region | 3′-end | |||||||
| Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | |
| Pvmsp-7C | |||||||||
| Pvmsp-7H | 0.241 (0.050) | 1.016 (0.199) | 0.879 (0.162) | ||||||
| Pvmsp-7I | 0.468 (0.085) | 0.518 (0.092) | 0.956 (0.167) | 0.790 (0.140) | 0.788 (0.138) | 0.136 (0.031) | |||
| Non-synonymous nucleotide substitution per non-synonymous site (dN) | |||||||||
| 5′-end | Central region | 3′-end | |||||||
| Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | Pvmsp-7C | Pvmsp-7H | Pvmsp-7I | |
| Pvmsp-7C | |||||||||
| Pvmsp-7H | 0.072 (0.017) | 1.430 (0.238) | 0.234 (0.035) | ||||||
| Pvmsp-7I | 0.125 (0.028) | 0.152 (0.031) | 1.729 (0.244) | 1.841 (0.306) | 0.323 (0.047) | 0.122 (0.028) | |||
5′-end from nucleotides 1 to 384 (amino acids 1 to 128), central region from nucleotides 385 to 807 (amino acids 129 to 269) and 3′-end from nucleotides 808 to 1,248 (amino acids 270 to 416), numbered according to the alignment in Fig S13.
Discussion
At least eleven MSPs have been reported in P. falciparum and nine P. vivax orthologous genes have been described so far. Two P. vivax MSP families (msp-3 and msp-7) are particularly interesting since they have expanded differentially [18], [33]. The P. vivax msp-7 gene family has 11 functional genes [18] and at least 8 of them are transcribed during the P. vivax schizont stage [20]. MSP-7 forms part of the main protein complex interacting with host cells [16], [22]. Taking into account that this protein is localized on the parasite membrane [19], [21], [22], [23], it can be targeted by the host immune system and thus becomes an attractive vaccine candidate.
Besides being recognized by the immune system, vaccine candidates must have limited polymorphism. Results found in this study highlighted differences in allele diversity among msp-7 gene family members, at least in the two main human malaria species. By contrast with the very little polymorphism displayed by Pfmsp-7 genes [28], [29], Pvmsp-7 genes (Pvmsp-7C, Pvmsp-7H and Pvmsp-7I) have high genetic diversity. It is worth noting, however, that not all the Pvmsp-7 genes display a similar pattern. Pvmsp-7A and Pvmsp-7K, like Pfmsp-7A, Pfmsp-7B, Pfmsp-7C, Pfmsp-7D, Pfmsp-7E, Pfmsp-7F and Pfmsp-7H (GenBank ID numbers: XM_001350038.1–44.1) display very low polymorphism [28], [29], [30] suggesting that msp-7 genes might be exposed to different selective pressure (such as that exerted by the immune system) or have different biological constraints. Although low genetic polymorphism does not hold for Pvmsp-7C, Pvmsp-7H and Pvmsp-7I, vaccine development using these proteins could be focused on their conserved C-terminal domain (Pfam ID number PF12948) due to the high level of conservation it displays and, like in P. falciparum, the C-terminal domain could be involved in interaction with erythrocytes [24].
The genetic diversity found in these three genes places them among the most diverse P. vivax genes described so far (Table S1). Different allele types were found when Colombian samples were compared with Sal-I (GenBank ID numbers: XM_001614082.1, XM_001614087.1, XM_001614088.1) and unpublished Korean sequences, (GenBank ID numbers: GU476538.1, GU476534.1, GU476518.1 and ACY66906-26) (data not shown), suggesting that such genetic diversity is distributed worldwide. Moreover, no correlation between nucleotide diversity (π) and P. vivax incidence in the geographical regions in question was observed (data not shown).
The Pvmsp-7C and Pvmsp-7I genes appear to be under balancing selection. It has been suggested that this type of selection increases variability within a population [34] maintaining alleles at intermediate frequencies, as can be observed in the alignment. Nevertheless, Tajima and Fu & Li tests are influenced not only by selection but also by the population history that can alter neutral allele frequency expectations. Therefore, the positive values in the tests could have been the result of a decrease in the population. However, a population affected by genetic drift (a mechanism that decreases the population) was not found for the Colombian population (Fs not statically significant). Moreover, the Hd and π values for both genes suggested a stable population having a long-term effective population size; allele frequency distribution would not therefore be influenced by a demographic process.
By contrast with the Pvmsp-7C and Pvmsp-7I results discussed above, Pvmsp-7H appeared to be under a standard neutral model of molecular evolution since there were no significant values in neutrality tests and no difference between dN and dS was found; consequently, high polymorphism would be expected in regions lacking functional constraints [35]. However, a deviation from neutral expectation would only be detected if the average from the whole gene was significantly greater or smaller than 0. The Nei-Gojobori method behaves similarly; positive selection can be detected only if the dN average from the whole gene is significantly greater than dS (the opposite occurs for negative selection). The sliding window test (for neutral statistics and ω) for different selective pressures appeared to be acting throughout Pvmsp-7 gene sequences, as has been described for others proteins [36], [37], [38], [39]. The tests showed that the 5′- and 3′-ends had purifying selection signals (negative values in the neutrality test and ω <1) while the central region of the three genes had balancing or positive selection signals (positive values in the neutrality test and ω >1). So, despite the loci displayed being under balancing selection or under neutrality, natural selection may have varied across codons. Two different approaches were thus followed to investigate this hypothesis. One estimated the dN and dS rates at the 5′- and 3′-ends and in the central region of the three genes. The central region was under positive selection while the 5′- and 3′-ends seemed to be under negative selection (dS higher than dN, only significant for the 5′-end). Similar results were observed when using a second approach in which several maximum likelihood methods were performed to determine positively and negatively selected codons. The central region of the Pvmsp-7C, Pvmsp-7H and Pvmsp-7I genes had codons under positive selection while sites under negative selection were preferentially located at the 5′- and 3′-ends which were relatively fully conserved in Pvmsp-7 genes.
Besides selective pressure and functional constraints, evolution of malarial antigens might be affected by recombination [13], [14], [39], [40], [41]. Several statistics and algorithms showed that intragenic recombination played an important role in generating new allele variants in individual msp-7 genes. These events affect the accuracy of detecting selected sites, increasing type I errors [32]. When sequences were screened for recombination, the positively selected sites decreased, suggesting that several of the positively selected sites were false positive. However, some were true positively selected sites (Table S6). These sites did not seem to be originated by the stochastic nature of the mutation process, since it would have been expected that dN substitutions (and/or positive selection) would have been randomly found across the genes; instead, these sites were just observed in the msp-7 central region and the non-synonymous substitutions in the positively selected sites were found as parsimonious sites and not as singleton sites. Recombination and selection thus seem to drive Pvmsp-7 genes’ antigenic variation, similar to what occurs in other pathogen antigens [42], [43], [44]. Likewise, it has been shown that both diversification by recombination and purifying selection take place in different regions of the E. coli fimA gene [45], and this also occurs in the Pvmsp-7 genes (Fig. 4 and Table S7).
On the other hand, multigene families might be evolving by recombination among paralogous genes, thereby contributing to allele diversity or the homogenization of the multigene family in the event of unequal crossover or gene conversion [46]. The gene conversion and subsequent phylogenetic analysis revealed that these genes had not evolved independently; several conversion tracks at the 5′- and 3′-ends (but not in the central region) were detected among msp-7 genes, suggesting that at least these three genes could be evolving by concerted evolution mediated by a biased gene conversion, homogenizing only the 5′ and 3′-ends. Thus, recombination would seem to affect the evolution of the msp-7 family as a whole, and individual msp-7 genes. However, protein homogeneity can also be attained by purifying selection. Under the assumption of gene conversion, it would be expected that dS between duplicated genes would be similar to dN, whereas if purifying selection is the major evolutionary force, then dS would be much higher than dN [47], According to dS and dN rate comparison, the homogeneity at the Pvmsp-7 genes’ 5′-end was apparently caused by functional constraints rather than by concerted evolution, since Pvmsp-7 genes have diverged extensively by silent nucleotide substitution at these ends. The conservation of the 3′-end in Pvmsp-7H and Pvmsp-7I but not in Pvmsp-7C seems to be maintained by gene conversion. This behavior might be a consequence of closely spaced gene duplicates being more likely to undergo gene conversion [48], [49]. Pvmsp-7H and Pvmsp-7I genes are neighbors separated by 1,086 base pairs while the Pvmsp-7C gene is separated from the others by 9,619 base pairs. Accordingly, the high Pvmsp-7s’ C-terminal conservation may have been the result of functional constraints and purifying selection, or the result of gene conversion (between Pvmsp-7H and Pvmsp-7I) possibly due to the presence of a functional domain (Pfam ID number PF12948) within this region. Therefore, the central region (the most diverse) could have been under selective pressure exerted by the immune system; consequently, the intragenic recombination and, to a lesser extent, the positively selected sites increased genetic diversity, generating different allele variants as an evasion mechanism.
Phylogenetic analysis showed that, regardless of an isolate’s origin, sequences tended to cluster without having clear geographical distribution. This pattern might indicate a constant P. vivax gene flow in Colombian regions. This result agreed with previous reports of other highly polymorphic P. vivax genes not involved in geographical clustering [13], [14], [39].
Previous studies have shown the potential role of MSP-7 in parasite invasion of erythrocytes [19], [24], [25], [50] which, added to its immunogenicity [26], [31], and following the rules for subunit-based vaccine development [51], [52], make the MSP-7 conserved domain an attractive candidate to be evaluated when designing an antimalarial vaccine.
Materials and Methods
Ethics Statement
All P. vivax-infected patients who provided us with the blood samples signed an informed consent and the purpose of the study was carefully explained to them. All procedures carried out in this study were approved by our institute’s ethics committee.
Source of Parasite DNA and Field Isolate Genotyping
Peripheral blood samples from patients proving positive for P. vivax malaria by microscope examination were collected from geographical regions of Colombia from 2007 to 2010. DNA was obtained using a Wizard Genomic DNA Purification kit (Promega) following manufacturer’s instructions.
All parasite samples were genotyped by PCR-RFLP of the Pvmsp-1 gene’s blocks 6, 7 and 8 as previously described [53] for selecting only samples having a single P. vivax msp-1 allele infection.
Amplification and Sequencing
Primers were designed to amplify Pvmsp-7C, Pvmsp-7H and Pvmsp-7I DNA fragments based on the Sal-I reference sequences (GenBank IDs: XM_001614082.1, XM_001614087.1 and XM_001614088.1, respectively). The DNA fragment from Pvmsp-7C was amplified with 7Cdto 5′ ACCACAAAGATGAATAAAACG 3′ and 7Crev 5′ CACCTCAATCGTGTTCAGC 3′ primers. Pvmsp-7H was amplified by using 7Hdto 5′ GTGTGCATCAGTATAGCGAC 3′ and 7Hrev 5′ AAGAAGGTTAGCCATAAATGC 3′ primers and Pvmsp-7I was amplified with 7Idto 5′ ACAATGAGGGGCAAGTACG 3′ and 7Irev 5′ TTCATTCGTTGCTCACTTCG 3′ primers. All PCR reactions were performed using KAPA HiFi HotStart Readymix containing 0.3 µM of each primer in a final 25 µL volume. Thermal conditions were set as follows: one cycle of 5 min at 95°C, 25 cycles of 20 sec at 98°C, 15 sec at 62°C for Pvmsp-7C and Pvmsp-7I and 60°C for Pvmsp-7H, 30 sec at 72°C, followed by a 5 min final extension at 72°C. PCR products were purified using the Wizard PCR preps kit (Promega), and then sequenced with a BigDye Terminator kit (MACROGEN, Seoul, South Korea) in both directions. Internal primers were used for sequencing (intdc 5′ CTGTTGGACCCGGTGGAG3′, intrc 5′ CTTGTTGATTCGCTCCTGG 3′; intdh 5′ TCAAATACAGCACAGACTTCC, intrh 5′ CCTCAGGACAACCCGAAAG 3′; intdi 5′ TCACAAACGCACAACCCAGG 3′ and 5′ GCTCCATTACCACAACCGG 3′). Two PCR products obtained from independent PCR amplifications were sequenced per isolate to discard errors.
Statistical Analysis for the msp-7 Sequences
Electropherograms were assembled using CLC DNA workbench 6 (CLC bio, Cambridge, MA, USA) and Clustal W [54] was used for aligning the deduced amino acid sequences, followed by manual editing. Additionally, repeats with 90% similarity in the deduced msp-7 amino acid sequences were detected by using the T-REKS algorithm [55]. The PAL2NAL program [56] was used for constructing codon alignments from the corresponding aligned amino acid sequences. Colombian P. vivax msp-7C, msp-7H and msp-7I sequences were compared against the previously described Sal-I strain sequences available in GenBank (ID numbers: XM_001614082.1, XM_001614087.1, and XM_001614088.1).
DNA polymorphism was calculated with DnaSP v.5 software [57]. Tests to assess departure from the neutral model were applied using Tajima’s D and Fu & Li’s D* and F* statistics. The former statistic compares the differences between the total number of segregating sites and the average number of nucleotide differences between sequence pairs. The Fu & Li test calculates the D* statistic which is based on the difference between the number of singletons and the total number of mutations, as well as the F* statistic which is based on the difference between the number of singletons and the average number of nucleotide differences between sequence pairs. Positive and negative values from both tests correspond to departures from neutrality. The Fs statistic [58] was used; it is based on gene frequency distribution. All tests were applied using DnaSP v.5 software, considering coalescent simulations for obtaining confidence intervals [57]. Natural selection was estimated using the modified Nei-Gojobori method [59] to calculate the average number of non-synonymous (dN) and synonymous (dS) substitutions. Differences between dN and dS were assessed by applying the Z-test using MEGA software v.5 [60]. Additionally, codon sites under positive or negative selection at the population level were assessed by using Datamonkey web server [61] with IFEL, a codon-based maximum likelihood method [62]. This method infers selective pressure at population level; positive or negatively selected sites were assessed by FEL, SLAC and REL methods [63]. All algorithms estimated the ω (dN/dS ratio) at every codon in the alignment. A ≤0.1 p-value was considered significant for IFEL, FEL and SLAC methods and ≥50 Bayes factor for REL. Only Colombian sequences were considered for all analyses performed; positions containing gaps or repeats in the alignment were not taken into account (Table S9).
Linkage disequilibrium (LD) was evaluated by calculating the ZnS statistic [64] which is the average of R?2 over all pairwise comparisons. A lineal regression between LD (R?2) and nucleotide distances was performed to evaluate whether recombination was taking place in Pvmsp-7 genes. Recombination events were assessed using DnaSP v.5 software [57] applying the ZZ statistic [65] and RM parameter [66]. The latter statistic incorporates the effective population size and probability of recombination between adjacent nucleotides per generation. RDP3 v3.4 software [67] was used for detecting recombination regions in msp-7 genes. This tool looks for evidence of recombination among aligned sequences by examining all possible triplet combinations following a scanning approach with a range of different recombination detection algorithms. Additionally, the algorithm developed by Betrán et al. (1997) [68] incorporated in DnaSP [57] and the GENECONV program [69] incorporated in RDP3 v3.4 software [67] were used to detect gene conversion among paralogous genes.
Geographical Clustering
Maximum Likelihood (ML) trees describing the phylogenetic consequences of the recombination events were constructed using RDP3 v3.4 software [67] with the HKY model (selected by the ModelTest algorithm [70]) to evaluate relationships between polymorphism and the geographical distribution of the isolates. Additionally, Pvmsp-7C, Pvmsp-7H and Pvmsp-7I sequences were aligned and trees were constructed by using ML with the TN93+G model selected by the ModelTest algorithm [70]. Bootstrap analysis (with 1,000 replicates each) was used for assigning confidence levels to branch nodes. Positions containing gaps as well as regions in the alignment that contained repeats were not taken into account in the phylogenetic analysis (Table S9).
Supporting Information
Geographical location of the study regions within Colombia. Amazonian region (samples from Calamar in the Guaviare department), Andean region (samples from Apartadó and El Bagre in the Antioquia department), Caribbean region (samples from Tierra Alta and Puerto Libertador in the Córdoba department), Orinoco region (samples from Tauramena in the Casanare department and Mapiripán in the Meta department), and Pacific region (samples from Istmina in the Chocó department and Tumaco in the Nariño department). Black dots on the map represent the areas from which patients came who donated the infected blood samples. 1: Puerto Libertador, 2: Tierra Alta, 3: Istmina, 4: Tumaco, 5: Apartado, 6: El Bagre, 7: Tauramena, 8: Mapiripan, 9: Calamar.
(TIF)
Sliding window analysis of Tajima D and Fu & Li D* and F* statistics along the Pvmsp-7C (A), Pvmsp-7H (B) and Pvmsp-7I (C) genes. Bars [Tajima’s D (blue), Fu & Li’s D* (red) and F* (green)] below each figure represent the regions where the tests showed a significant deviation from the neutral expectation. 5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188).
(PDF)
Sliding window analysis for ω rates (dN/dS) throughout the Pvmsp-7C (Blue), Pvmsp-7H (Red) and Pvmsp-7I (Green) genes. Discontinuity of the curves is due to gaps within the alignments which were not considered for the analysis. 5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188).
(PDF)
The linkage disequilibrium (LD) plot for P. vivax Pvmsp-7C (A), Pvmsp-7H (B) and Pvmsp-7I (C). Trace line represents the regression line which declined as nucleotide distance increased suggesting that intragenic recombination was taking place in msp-7 genes.
(PDF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7C . A topology is inferred for each recombinant fragment, (A) from nucleotides 685 to 855, (B) from nucleotides 1,060 to 1,167 (excluding nucleotides 1,105 to 1,107) and from 18 to 266, and (C) from nucleotides 62 to 266. Isolates clustered without a clear geographical distribution. Sal-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7H . A topology is inferred for each recombinant fragment, (A) nucleotides 189 to 543 (excluding nucleotides 436 to 450 and nucleotides 484 to 486), (B) nucleotides 353 to 661 (excluding nucleotides 436 to 450 and 568 to 627), (C) nucleotides 425 to 655 (excluding nucleotides 436 to 450, 484 to 486 and 568 to 627), (D) nucleotides 500 to 1,057 (excluding nucleotides 568 to 627 and 772 to 774), (E) nucleotides 628 to 977 (excluding nucleotides 772 to 774), (F) nucleotides 630 to 997, (G) nucleotides 416 to 590 (excluding nucleotides 436 to 450 and 484 to 486), (H) nucleotides 472 to 531 (excluding nucleotides 484 to 486), and (I) nucleotides 482 to 646 (excluding nucleotides 568 to 627). Isolates clustered without a clear geographical distribution. Sal-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7I . A topology is inferred for each recombinant fragment identified, (A) nucleotides 19 to 187 and nucleotides 683 to 1,188, (B) nucleotides 683 to 849, (C) nucleotides 684 to 797, (D) nucleotides 19 to 358 and nucleotides 796 to 1,188, (E) nucleotides 798 to 1,105, (F) nucleotides 868 to 1,188, (G) nucleotides 920 to 1,188, (H) nucleotides 733 to 1,044, (I) nucleotides 688 to 797, and (J) nucleotides 683 to 849. Isolates clustered without a clear geographical distribution. SAL-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
(A) Phylogenetic tree obtained by ML for Pvmsp-7 sequences based on the TN93+G model, ignoring recombination. Three monophyletic groups are shown; the first groups clustered Pvmsp-7H (red) sequences, the second group clustered Pvmsp-7I (green) sequences and the third group clustered Pvmsp-7C (blue) sequences. (B-E) Trees describing phylogenetic consequences of some gene conversion tracks identified. (B) nucleotides 71 to 265 (amino acids 24 to 89), (C) nucleotides 913 to 1,090 (amino acids 305 to 390), (D) nucleotides 913 to 1,090 (amino acids 305 to 354), (E) nucleotides 913 to 1,175 (amino acids 305 to 392). Positions are numbered according to the alignment in Fig. S13. These topologies suggest that at least Pvmsp-7C, Pvmsp-7H and Pvmsp-7I genes did not evolve independently. Numbers represent bootstrap values with 1,000 replicates.
(PDF)
Schematic representation of gene conversion tracks identified by DnaSP and GENECONV for Pvmsp-7C (blue), Pvmsp-7H (red) and Pvmsp-7I (green) as a combined data set. Rectangles in a different color are graphical representations of sequence fragments that have potentially been originated by gene conversion and are localized at the conserved 5′- and 3′-ends. The black bars delimit the 5′-end, the central region and the 3′-end, [5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188)].
(PDF)
Alignment of deduced PvMSP-7C amino acid sequences.
(PDF)
Alignment of deduced PvMSP-7H amino acid sequences.
(TIF)
Alignment of deduced PvMSP-7C amino acid sequences.
(TIF)
Alignment of the paralogous PvMSP-7deduced amino acid sequences.
(PDF)
Nucleotide diversity for P. vivax antigens. n: number of isolates, π: nucleotide diversity.
(PDF)
Nucleotide diversity (π) values for subpopulations within Colombia.
(PDF)
Nucleotide and amino acid positions within the 5′- end, central region and 3′-end.
(PDF)
Positively selected sites detected for Pvmsp-7 genes without taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Negatively selected sites detected for Pvmsp-7 genes without taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Positively selected sites detected for Pvmsp-7 genes taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Negatively selected sites detected for Pvmsp-7 genes taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Conversion tracks identified by DnaSP and GENECONV, between Pvmsp-7 genes. Nucleotides and amino acids based in alignment of the Fig. S13.
(PDF)
Nucleotides and amino acid positions excluded from the analysis.
(PDF)
Acknowledgments
We would like to thank the Instituto Nacional de Salud (INS), Bogotá, Colombia, for supplying the samples. We would like to thank Jason Garry for translating the manuscript. Our special gratitude goes to Francy Homez Homez and Pamela Jory Rios for their technical assistance. We would also like to thank Professor Manuel Elkin Patarroyo for his invaluable comments and suggestions. In loving memory of Doctor Carlos Fernando Cortés, who passed away recently.
Funding Statement
The authors have no support or funding to report.
References
- 1. Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, et al. (2010) The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis 4: e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lysenko AJ, Semashko IN (1968) Medical geography: a medical-geographical study of an ancient disease. WHO Publisher’s Code 911 (3) 616.936 (100).. [Google Scholar]
- 3. Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, et al. (2009) Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis 48: 1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, et al. (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, et al. (2007) Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77: 79–87. [PMC free article] [PubMed] [Google Scholar]
- 6. Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, et al. (2009) Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80: 194–198. [PubMed] [Google Scholar]
- 7. Carvalho LJ, Daniel-Ribeiro CT, Goto H (2002) Malaria vaccine: candidate antigens, mechanisms, constraints and prospects. Scand J Immunol 56: 327–343. [DOI] [PubMed] [Google Scholar]
- 8. Galinski MR, Barnwell JW (2008) Plasmodium vivax: who cares? Malar J 7 Suppl 1 S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Donnell RA, de Koning-Ward TF, Burt RA, Bockarie M, Reeder JC, et al. (2001) Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J Exp Med 193: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Genton B, Reed ZH (2007) Asexual blood-stage malaria vaccine development: facing the challenges. Curr Opin Infect Dis 20: 467–475. [DOI] [PubMed] [Google Scholar]
- 11. Takala SL, Plowe CV (2009) Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol 31: 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figtree M, Pasay CJ, Slade R, Cheng Q, Cloonan N, et al. (2000) Plasmodium vivax synonymous substitution frequencies, evolution and population structure deduced from diversity in AMA 1 and MSP 1 genes. Mol Biochem Parasitol 108: 53–66. [DOI] [PubMed] [Google Scholar]
- 13. Gomez A, Suarez CF, Martinez P, Saravia C, Patarroyo MA (2006) High polymorphism in Plasmodium vivax merozoite surface protein-5 (MSP5). Parasitology 133: 661–672. [DOI] [PubMed] [Google Scholar]
- 14. Putaporntip C, Udomsangpetch R, Pattanawong U, Cui L, Jongwutiwes S (2010) Genetic diversity of the Plasmodium vivax merozoite surface protein-5 locus from diverse geographic origins. Gene 456: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richie TL, Saul A (2002) Progress and challenges for malaria vaccines. Nature 415: 694–701. [DOI] [PubMed] [Google Scholar]
- 16. Pachebat JA, Ling IT, Grainger M, Trucco C, Howell S, et al. (2001) The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol Biochem Parasitol 117: 83–89. [DOI] [PubMed] [Google Scholar]
- 17. Trucco C, Fernandez-Reyes D, Howell S, Stafford WH, Scott-Finnigan TJ, et al. (2001) The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasitol 112: 91–101. [DOI] [PubMed] [Google Scholar]
- 18. Garzon-Ospina D, Cadavid LF, Patarroyo MA (2010) Differential expansion of the merozoite surface protein (msp)-7 gene family in Plasmodium species under a birth-and-death model of evolution. Mol Phylogenet Evol 55: 399–408. [DOI] [PubMed] [Google Scholar]
- 19. Tewari R, Ogun SA, Gunaratne RS, Crisanti A, Holder AA (2005) Disruption of Plasmodium berghei merozoite surface protein 7 gene modulates parasite growth in vivo. Blood 105: 394–396. [DOI] [PubMed] [Google Scholar]
- 20. Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, et al. (2008) The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci U S A 105: 16290–16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mello K, Daly TM, Long CA, Burns JM, Bergman LW (2004) Members of the merozoite surface protein 7 family with similar expression patterns differ in ability to protect against Plasmodium yoelii malaria. Infect Immun 72: 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mello K, Daly TM, Morrisey J, Vaidya AB, Long CA, et al. (2002) A multigene family that interacts with the amino terminus of plasmodium MSP-1 identified using the yeast two-hybrid system. Eukaryot Cell 1: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mongui A, Perez-Leal O, Soto SC, Cortes J, Patarroyo MA (2006) Cloning, expression, and characterisation of a Plasmodium vivax MSP7 family merozoite surface protein. Biochem Biophys Res Commun 351: 639–644. [DOI] [PubMed] [Google Scholar]
- 24. Garcia Y, Puentes A, Curtidor H, Cifuentes G, Reyes C, et al. (2007) Identifying merozoite surface protein 4 and merozoite surface protein 7 Plasmodium falciparum protein family members specifically binding to human erythrocytes suggests a new malarial parasite-redundant survival mechanism. J Med Chem 50: 5665–5675. [DOI] [PubMed] [Google Scholar]
- 25. Kadekoppala M, O’Donnell RA, Grainger M, Crabb BS, Holder AA (2008) Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes. Eukaryot Cell 7: 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Crouch L, Richie TL, Nhan DH, Coppel RL (2003) Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol 25: 403–412. [DOI] [PubMed] [Google Scholar]
- 27. Kadekoppala M, Holder AA (2010) Merozoite surface proteins of the malaria parasite: the MSP1 complex and the MSP7 family. Int J Parasitol 40: 1155–1161. [DOI] [PubMed] [Google Scholar]
- 28. Tetteh KK, Stewart LB, Ochola LI, Amambua-Ngwa A, Thomas AW, et al. (2009) Prospective identification of malaria parasite genes under balancing selection. PLoS One 4: e5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roy SW, Weedall GD, da Silva RL, Polley SD, Ferreira MU (2009) Sequence diversity and evolutionary dynamics of the dimorphic antigen merozoite surface protein-6 and other Msp genes of Plasmodium falciparum. Gene 443: 12–21. [DOI] [PubMed] [Google Scholar]
- 30. Garzon-Ospina D, Romero-Murillo L, Tobon LF, Patarroyo MA (2011) Low genetic polymorphism of merozoite surface proteins 7 and 10 in Colombian Plasmodium vivax isolates. Infect Genet Evol 11: 528–531. [DOI] [PubMed] [Google Scholar]
- 31. Chen JH, Jung JW, Wang Y, Ha KS, Lu F, et al. (2010) Immunoproteomics profiling of blood stage Plasmodium vivax infection by high-throughput screening assays. J Proteome Res 9: 6479–6489. [DOI] [PubMed] [Google Scholar]
- 32. Anisimova M, Nielsen R, Yang Z (2003) Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics 164: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, et al. (2008) Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature 455: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nielsen R (2005) Molecular signatures of natural selection. Annu Rev Genet 39: 197–218. [DOI] [PubMed] [Google Scholar]
- 35.Kimura M (1983) The neutral theory of molecular evolution. Cambridge Cambridgeshire; New York: Cambridge University Press. xv, 367 p.
- 36. Suarez CF, Patarroyo ME, Trujillo E, Estupinan M, Baquero JE, et al. (2006) Owl monkey MHC-DRB exon 2 reveals high similarity with several HLA-DRB lineages. Immunogenetics 58: 542–558. [DOI] [PubMed] [Google Scholar]
- 37. Martinez P, Suarez CF, Gomez A, Cardenas PP, Guerrero JE, et al. (2005) High level of conservation in Plasmodium vivax merozoite surface protein 4 (PvMSP4). Infect Genet Evol 5: 354–361. [DOI] [PubMed] [Google Scholar]
- 38. Putaporntip C, Jongwutiwes S, Ferreira MU, Kanbara H, Udomsangpetch R, et al. (2009) Limited global diversity of the Plasmodium vivax merozoite surface protein 4 gene. Infect Genet Evol 9: 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez P, Suarez CF, Cardenas PP, Patarroyo MA (2004) Plasmodium vivax Duffy binding protein: a modular evolutionary proposal. Parasitology 128: 353–366. [DOI] [PubMed] [Google Scholar]
- 40. Mascorro CN, Zhao K, Khuntirat B, Sattabongkot J, Yan G, et al. (2005) Molecular evolution and intragenic recombination of the merozoite surface protein MSP-3alpha from the malaria parasite Plasmodium vivax in Thailand. Parasitology 131: 25–35. [DOI] [PubMed] [Google Scholar]
- 41. Putaporntip C, Jongwutiwes S, Seethamchai S, Kanbara H, Tanabe K (2000) Intragenic recombination in the 3′ portion of the merozoite surface protein 1 gene of Plasmodium vivax. Mol Biochem Parasitol 109: 111–119. [DOI] [PubMed] [Google Scholar]
- 42. Orsi RH, Ripoll DR, Yeung M, Nightingale KK, Wiedmann M (2007) Recombination and positive selection contribute to evolution of Listeria monocytogenes inlA. Microbiology 153: 2666–2678. [DOI] [PubMed] [Google Scholar]
- 43. Andrews TD, Gojobori T (2004) Strong positive selection and recombination drive the antigenic variation of the PilE protein of the human pathogen Neisseria meningitidis. Genetics 166: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Polley SD, Conway DJ (2001) Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics 158: 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peek AS, Souza V, Eguiarte LE, Gaut BS (2001) The interaction of protein structure, selection, and recombination on the evolution of the type-1 fimbrial major subunit (fimA) from Escherichia coli. J Mol Evol 52: 193–204. [DOI] [PubMed] [Google Scholar]
- 46. Nei M, Rooney AP (2005) Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 39: 121–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nei M, Rogozin IB, Piontkivska H (2000) Purifying selection and birth-and-death evolution in the ubiquitin gene family. Proc Natl Acad Sci U S A 97: 10866–10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Semple C, Wolfe KH (1999) Gene duplication and gene conversion in the Caenorhabditis elegans genome. J Mol Evol 48: 555–564. [DOI] [PubMed] [Google Scholar]
- 49. Katju V, Lynch M (2003) The structure and early evolution of recently arisen gene duplicates in the Caenorhabditis elegans genome. Genetics 165: 1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Woehlbier U, Epp C, Hackett F, Blackman MJ, Bujard H (2010) Antibodies against multiple merozoite surface antigens of the human malaria parasite Plasmodium falciparum inhibit parasite maturation and red blood cell invasion. Malar J 9: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cifuentes G, Bermudez A, Rodriguez R, Patarroyo MA, Patarroyo ME (2008) Shifting the polarity of some critical residues in malarial peptides’ binding to host cells is a key factor in breaking conserved antigens’ code of silence. Med Chem 4: 278–292. [DOI] [PubMed] [Google Scholar]
- 52. Patarroyo ME, Patarroyo MA (2008) Emerging rules for subunit-based, multiantigenic, multistage chemically synthesized vaccines. Acc Chem Res 41: 377–386. [DOI] [PubMed] [Google Scholar]
- 53. Imwong M, Pukrittayakamee S, Gruner AC, Renia L, Letourneur F, et al. (2005) Practical PCR genotyping protocols for Plasmodium vivax using Pvcs and Pvmsp1. Malar J 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jorda J, Kajava AV (2009) T-REKS: identification of Tandem REpeats in sequences with a K-meanS based algorithm. Bioinformatics 25: 2632–2638. [DOI] [PubMed] [Google Scholar]
- 56. Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34: W609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- 58. Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang J, Rosenberg HF, Nei M (1998) Positive Darwinian selection after gene duplication in primate ribonuclease genes. Proc Natl Acad Sci U S A 95: 3708–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Delport W, Poon AF, Frost SD, Kosakovsky Pond SL (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26: 2455–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pond SL, Frost SD, Grossman Z, Gravenor MB, Richman DD, et al. (2006) Adaptation to different human populations by HIV-1 revealed by codon-based analyses. PLoS Comput Biol 2: e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kosakovsky Pond SL, Frost SD (2005) Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol 22: 1208–1222. [DOI] [PubMed] [Google Scholar]
- 64. Kelly JK (1997) A test of neutrality based on interlocus associations. Genetics 146: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rozas J, Gullaud M, Blandin G, Aguade M (2001) DNA variation at the rp49 gene region of Drosophila simulans: evolutionary inferences from an unusual haplotype structure. Genetics 158: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hudson RR, Kaplan NL (1985) Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111: 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martin DP, Lemey P, Lott M, Moulton V, Posada D, et al. (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26: 2462–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Betran E, Rozas J, Navarro A, Barbadilla A (1997) The estimation of the number and the length distribution of gene conversion tracts from population DNA sequence data. Genetics 146: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sawyer S (1989) Statistical tests for detecting gene conversion. Mol Biol Evol 6: 526–538. [DOI] [PubMed] [Google Scholar]
- 70. Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Geographical location of the study regions within Colombia. Amazonian region (samples from Calamar in the Guaviare department), Andean region (samples from Apartadó and El Bagre in the Antioquia department), Caribbean region (samples from Tierra Alta and Puerto Libertador in the Córdoba department), Orinoco region (samples from Tauramena in the Casanare department and Mapiripán in the Meta department), and Pacific region (samples from Istmina in the Chocó department and Tumaco in the Nariño department). Black dots on the map represent the areas from which patients came who donated the infected blood samples. 1: Puerto Libertador, 2: Tierra Alta, 3: Istmina, 4: Tumaco, 5: Apartado, 6: El Bagre, 7: Tauramena, 8: Mapiripan, 9: Calamar.
(TIF)
Sliding window analysis of Tajima D and Fu & Li D* and F* statistics along the Pvmsp-7C (A), Pvmsp-7H (B) and Pvmsp-7I (C) genes. Bars [Tajima’s D (blue), Fu & Li’s D* (red) and F* (green)] below each figure represent the regions where the tests showed a significant deviation from the neutral expectation. 5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188).
(PDF)
Sliding window analysis for ω rates (dN/dS) throughout the Pvmsp-7C (Blue), Pvmsp-7H (Red) and Pvmsp-7I (Green) genes. Discontinuity of the curves is due to gaps within the alignments which were not considered for the analysis. 5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188).
(PDF)
The linkage disequilibrium (LD) plot for P. vivax Pvmsp-7C (A), Pvmsp-7H (B) and Pvmsp-7I (C). Trace line represents the regression line which declined as nucleotide distance increased suggesting that intragenic recombination was taking place in msp-7 genes.
(PDF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7C . A topology is inferred for each recombinant fragment, (A) from nucleotides 685 to 855, (B) from nucleotides 1,060 to 1,167 (excluding nucleotides 1,105 to 1,107) and from 18 to 266, and (C) from nucleotides 62 to 266. Isolates clustered without a clear geographical distribution. Sal-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7H . A topology is inferred for each recombinant fragment, (A) nucleotides 189 to 543 (excluding nucleotides 436 to 450 and nucleotides 484 to 486), (B) nucleotides 353 to 661 (excluding nucleotides 436 to 450 and 568 to 627), (C) nucleotides 425 to 655 (excluding nucleotides 436 to 450, 484 to 486 and 568 to 627), (D) nucleotides 500 to 1,057 (excluding nucleotides 568 to 627 and 772 to 774), (E) nucleotides 628 to 977 (excluding nucleotides 772 to 774), (F) nucleotides 630 to 997, (G) nucleotides 416 to 590 (excluding nucleotides 436 to 450 and 484 to 486), (H) nucleotides 472 to 531 (excluding nucleotides 484 to 486), and (I) nucleotides 482 to 646 (excluding nucleotides 568 to 627). Isolates clustered without a clear geographical distribution. Sal-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
ML trees describing the phylogenetic consequences of the intragenic recombination in Pvmsp-7I . A topology is inferred for each recombinant fragment identified, (A) nucleotides 19 to 187 and nucleotides 683 to 1,188, (B) nucleotides 683 to 849, (C) nucleotides 684 to 797, (D) nucleotides 19 to 358 and nucleotides 796 to 1,188, (E) nucleotides 798 to 1,105, (F) nucleotides 868 to 1,188, (G) nucleotides 920 to 1,188, (H) nucleotides 733 to 1,044, (I) nucleotides 688 to 797, and (J) nucleotides 683 to 849. Isolates clustered without a clear geographical distribution. SAL-I: Salvador strain, AMA: Amazon, AND: Andean, CAR: Caribbean, ORI: Orinoco, PAC: Pacific.
(TIF)
(A) Phylogenetic tree obtained by ML for Pvmsp-7 sequences based on the TN93+G model, ignoring recombination. Three monophyletic groups are shown; the first groups clustered Pvmsp-7H (red) sequences, the second group clustered Pvmsp-7I (green) sequences and the third group clustered Pvmsp-7C (blue) sequences. (B-E) Trees describing phylogenetic consequences of some gene conversion tracks identified. (B) nucleotides 71 to 265 (amino acids 24 to 89), (C) nucleotides 913 to 1,090 (amino acids 305 to 390), (D) nucleotides 913 to 1,090 (amino acids 305 to 354), (E) nucleotides 913 to 1,175 (amino acids 305 to 392). Positions are numbered according to the alignment in Fig. S13. These topologies suggest that at least Pvmsp-7C, Pvmsp-7H and Pvmsp-7I genes did not evolve independently. Numbers represent bootstrap values with 1,000 replicates.
(PDF)
Schematic representation of gene conversion tracks identified by DnaSP and GENECONV for Pvmsp-7C (blue), Pvmsp-7H (red) and Pvmsp-7I (green) as a combined data set. Rectangles in a different color are graphical representations of sequence fragments that have potentially been originated by gene conversion and are localized at the conserved 5′- and 3′-ends. The black bars delimit the 5′-end, the central region and the 3′-end, [5′-end (Pvmsp-7C: nucleotides 1–390, Pvmsp-7H: nucleotides 1–471, Pvmsp-7I: nucleotides 1–525), central (Pvmsp-7C: nucleotides 391–717, Pvmsp-7H: nucleotides 472–771, Pvmsp-7I: nucleotides 526–789) and 3′-end (Pvmsp-7C: nucleotides 718–1,191, Pvmsp-7H: nucleotides 772–1,200, Pvmsp-7I: nucleotides 790–1,188)].
(PDF)
Alignment of deduced PvMSP-7C amino acid sequences.
(PDF)
Alignment of deduced PvMSP-7H amino acid sequences.
(TIF)
Alignment of deduced PvMSP-7C amino acid sequences.
(TIF)
Alignment of the paralogous PvMSP-7deduced amino acid sequences.
(PDF)
Nucleotide diversity for P. vivax antigens. n: number of isolates, π: nucleotide diversity.
(PDF)
Nucleotide diversity (π) values for subpopulations within Colombia.
(PDF)
Nucleotide and amino acid positions within the 5′- end, central region and 3′-end.
(PDF)
Positively selected sites detected for Pvmsp-7 genes without taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Negatively selected sites detected for Pvmsp-7 genes without taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Positively selected sites detected for Pvmsp-7 genes taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Negatively selected sites detected for Pvmsp-7 genes taking recombination into account. Numbers according to the reference Sal-I protein sequence Pvmsp-7C: XP_001614132.1, Pvmsp-7H: XP_001614137.1 and Pvmsp-7I: XP_001614138.1.
(PDF)
Conversion tracks identified by DnaSP and GENECONV, between Pvmsp-7 genes. Nucleotides and amino acids based in alignment of the Fig. S13.
(PDF)
Nucleotides and amino acid positions excluded from the analysis.
(PDF)