Abstract
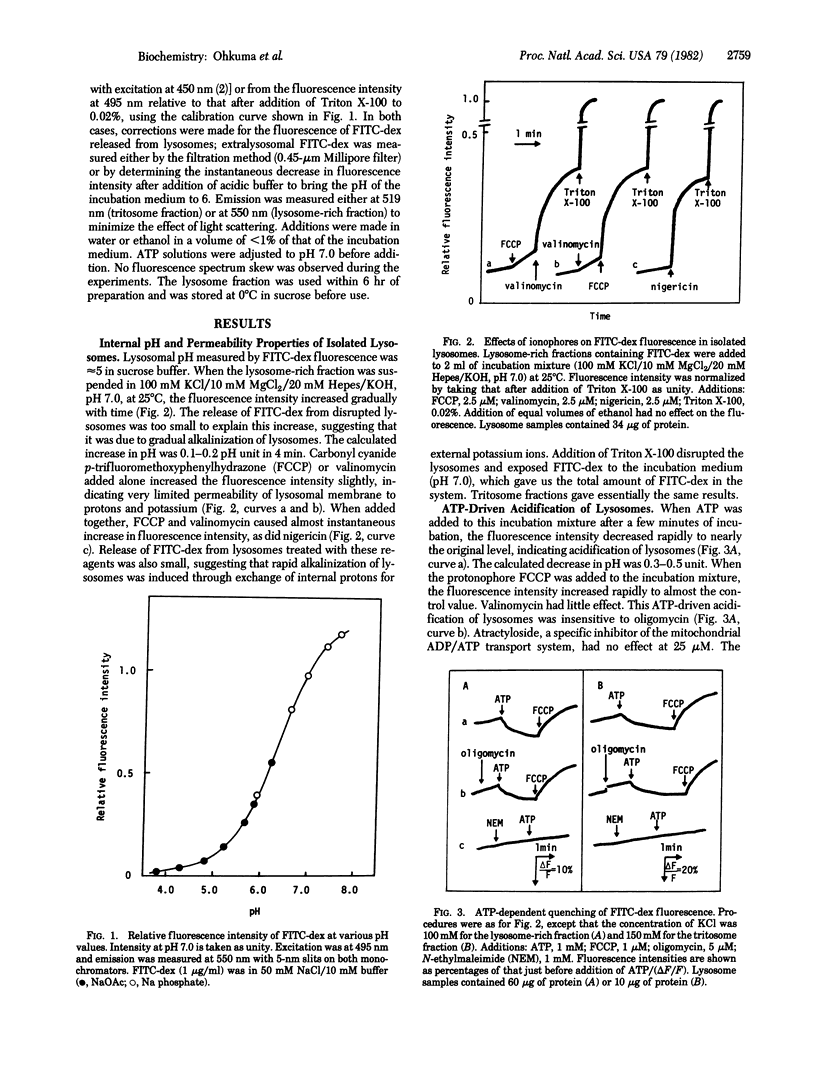
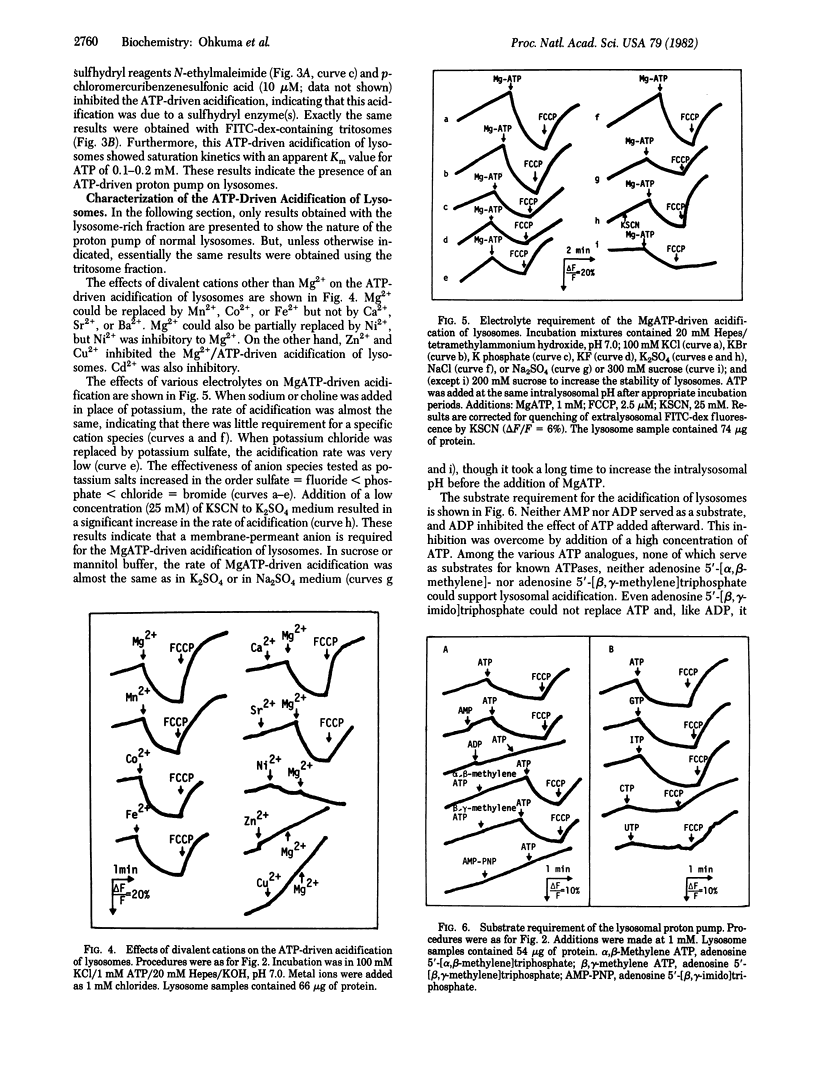
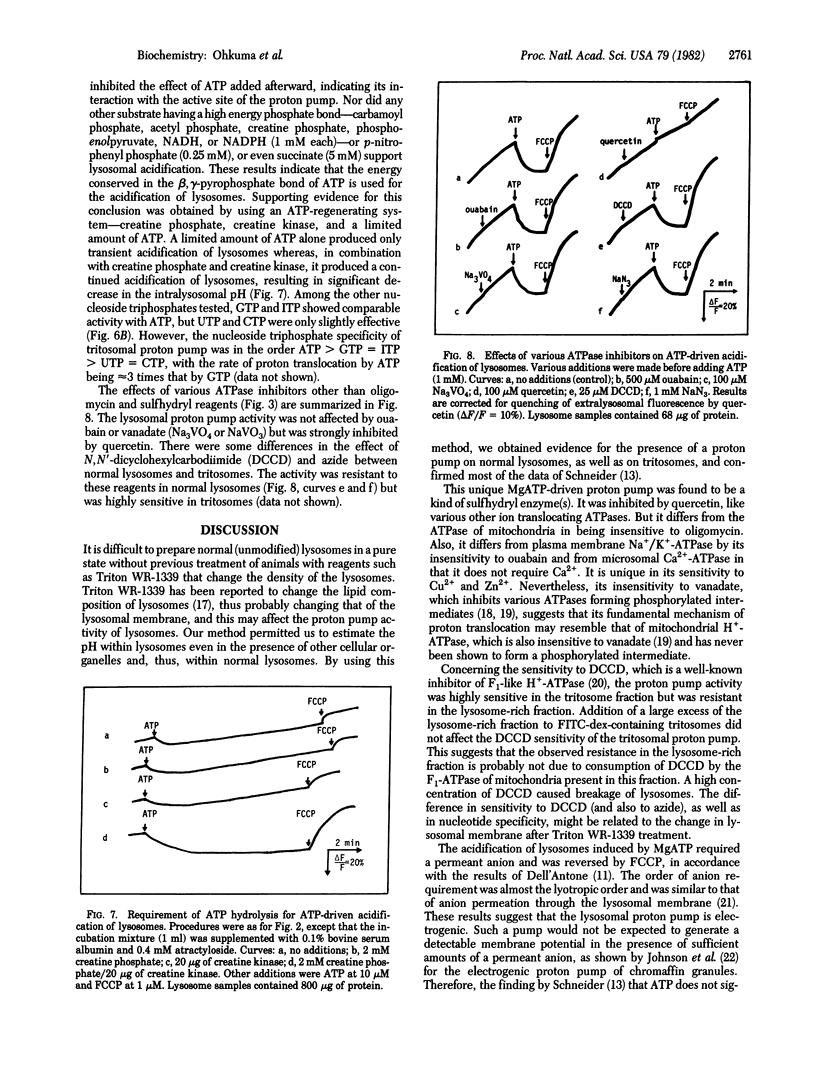
Fluorescein isothiocyanate-conjugated dextran was introduced preferentially into hepatic lysosomes by intraperitoneal injection into rats. The pH in isolated lysosomes, measured by fluorescein fluorescence, was approximately 5 and gradually increased in KCl (to 7.0) at 25 degrees C. In the presence of Mg2+, ATP caused acidification of lysosomes that was reversed by the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone. Mn2+, Co2+, and Fe2+ could replace Mg2+ but Ca2+ could not. Cu2+, Zn2+, and Cd2+ were inhibitory. A membrane-permeant anion, in practice chloride, was required for this acidification. ATP analogues, including 5'-adenylyl imidodiphosphate, could not be substituted for ATP. ATP-driven acidification was sensitive to N-ethylmaleimide and quercetin but insensitive to oligomycin, ouabain, and vanadate. There were some differences between "normal" lysosomes and tritosomes; the acidification was resistant to azide and N,N'-dicyclohexylcarbodiimide in normal lysosomes but sensitive to these reagents in tritosomes. These results provide evidence for the presence of an electrogenic proton pump driven by MgATP (H+-ATPase) on lysosomes.
Full text
PDF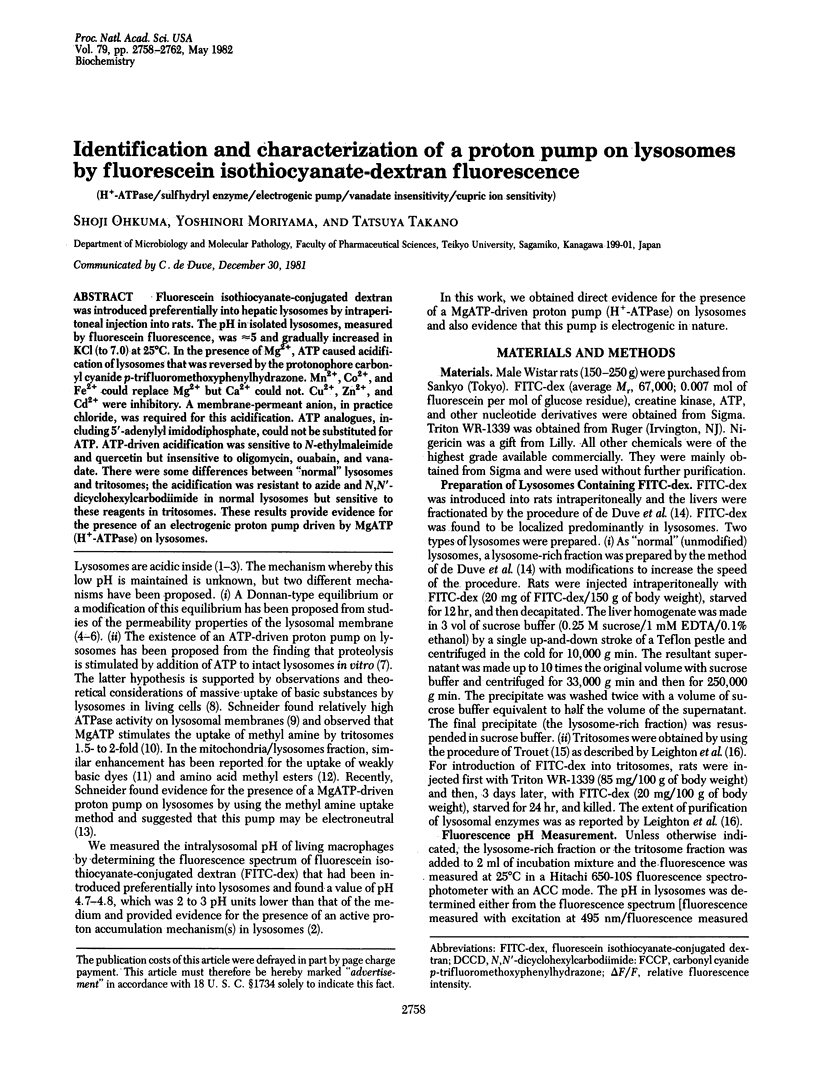

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Casey R. P., Hollemans M., Tager J. M. The permeability of the lysosomal membrane to small ions. Biochim Biophys Acta. 1978 Mar 21;508(1):15–26. doi: 10.1016/0005-2736(78)90185-2. [DOI] [PubMed] [Google Scholar]
- Chung C. H., Elliott R. L., Mego J. L. Lysosomal membrane adenosine triphosphatase; solubilization and partial characterization. Arch Biochem Biophys. 1980 Aug;203(1):251–259. doi: 10.1016/0003-9861(80)90175-7. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Antone P. Evidence for an ATP-driven "proton pump" in rat liver lysosomes by basic dyes uptake. Biochem Biophys Res Commun. 1979 Jan 15;86(1):180–189. doi: 10.1016/0006-291x(79)90398-x. [DOI] [PubMed] [Google Scholar]
- Goldman R., Rottenberg H. Ion distribution in lysosomal suspensions. FEBS Lett. 1973 Jul 1;33(2):233–238. doi: 10.1016/0014-5793(73)80200-5. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Niinobe S., Matsumoto Y., Suga T. Effects of Triton WR-1339 on lipoprotein lipolytic activity and lipid content of rat liver lysosomes. J Biochem. 1981 Feb;89(2):573–579. doi: 10.1093/oxfordjournals.jbchem.a133233. [DOI] [PubMed] [Google Scholar]
- Henning R. pH gradient across the lysosomal membrane generated by selective cation permeability and Donnan equilibrium. Biochim Biophys Acta. 1975 Aug 20;401(2):307–316. doi: 10.1016/0005-2736(75)90314-4. [DOI] [PubMed] [Google Scholar]
- Hollemans M., Elferink R. O., De Groot P. G., Strijland A., Tager J. M. Accumulation of weak bases in relation to intralysosomal pH in cultured human skin fibroblasts. Biochim Biophys Acta. 1981 Apr 22;643(1):140–151. doi: 10.1016/0005-2736(81)90226-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Pfister D., Carty S. E., Scarpa A. Biological amine transport in chromaffin ghosts. Coupling to the transmembrane proton and potential gradients. J Biol Chem. 1979 Nov 10;254(21):10963–10972. [PubMed] [Google Scholar]
- Kagawa Y., Sone N., Hirata H., Yoshida M. Structure and function of H+-ATPase. J Bioenerg Biomembr. 1979 Aug;11(3-4):39–78. doi: 10.1007/BF00743196. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L., Farb R. M., Barnes J. An adenosine triphosphate-dependent stabilization of proteolytic activity in heterolysosomes. Evidence for a proton pump. Biochem J. 1972 Jul;128(4):763–769. doi: 10.1042/bj1280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Reames T. ATP stimulates amino acid accumulation by lysosomes incubated with amino acid methyl esters. Evidence for a lysosomal proton pump. J Biol Chem. 1981 Jun 25;256(12):6047–6053. [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. The permeability properties of the lysosomal membrane. Biochim Biophys Acta. 1977 Nov 14;472(3-4):419–449. doi: 10.1016/0304-4157(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Riejngoud D. J., Tager J. M. Measurement of intralysosomal pH. Biochim Biophys Acta. 1973 Jan 24;297(1):174–178. doi: 10.1016/0304-4165(73)90061-5. [DOI] [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Schneider D. L. Membranous localization and properties of ATPase of rat liver lysosomes. J Membr Biol. 1977 Jun 6;34(2-3):247–261. doi: 10.1007/BF01870302. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. The acidification of rat liver lysosomes in vitro: a role for the membranous ATPase as a proton pump. Biochem Biophys Res Commun. 1979 Mar 30;87(2):559–565. doi: 10.1016/0006-291x(79)91831-x. [DOI] [PubMed] [Google Scholar]
- Trouet A. Immunisation de lapins par des lysosomes hépatiques de rats traités au Triton WR 1339. Arch Int Physiol Biochim. 1964 Sep;72(4):698–700. [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


