Abstract
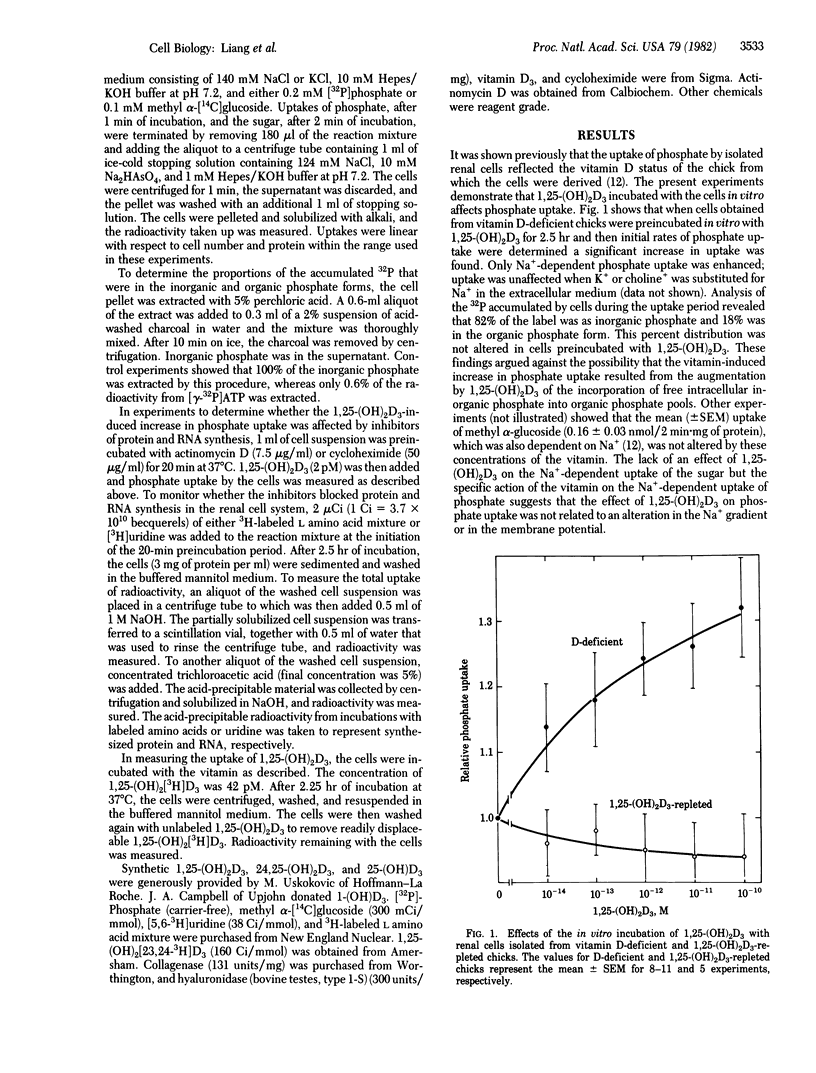
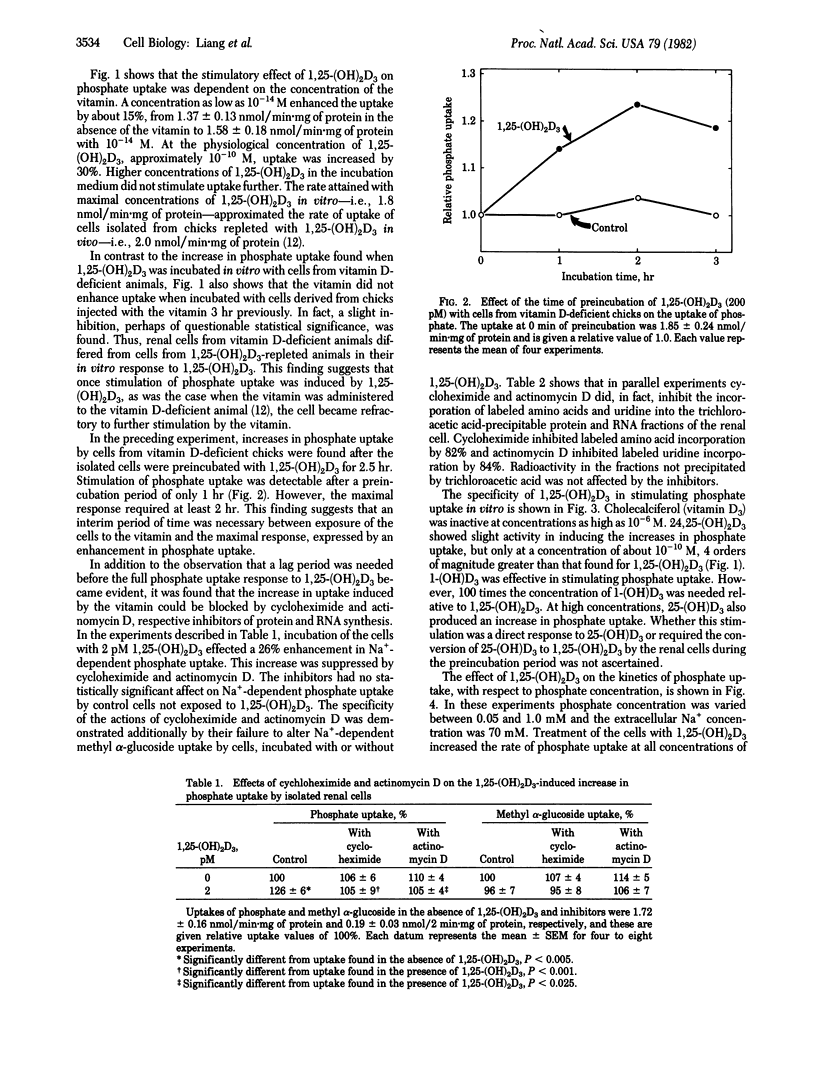
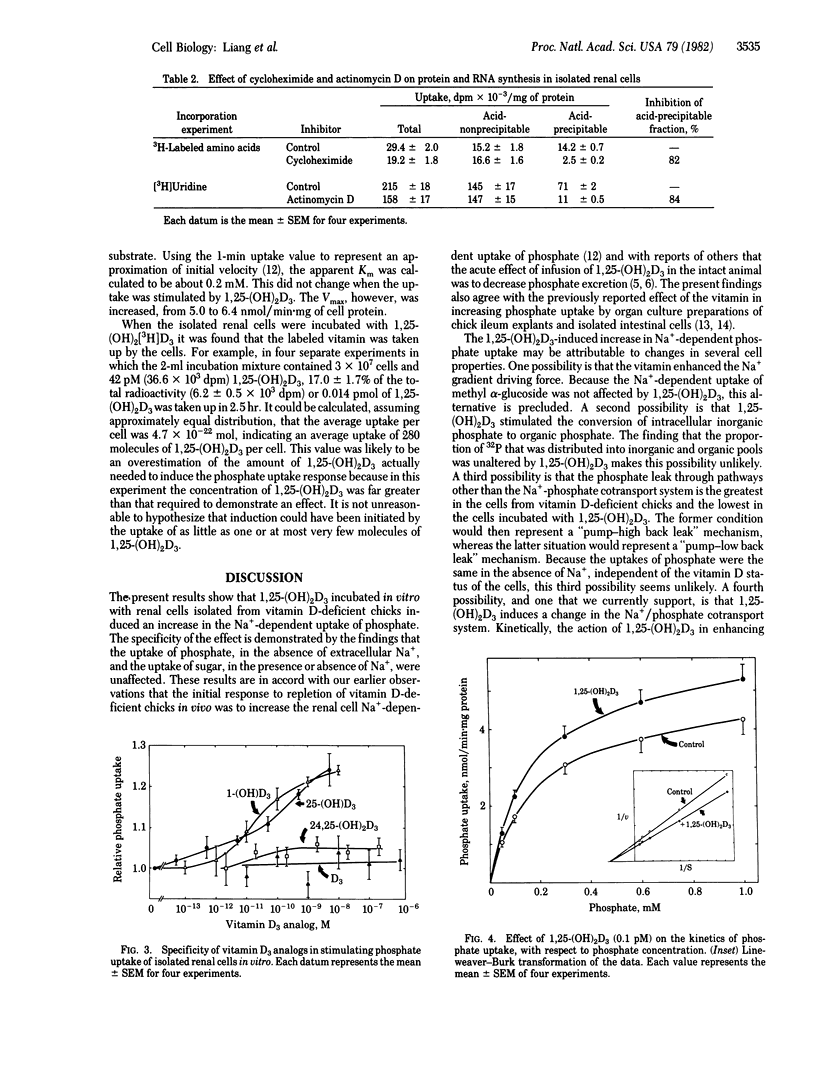
Renal cells isolated from vitamin D-deficient chicks had an increased Na+-dependent phosphate uptake when preincubated with 1,25-dihydroxycholecalciferol [1,25-(OH)2D3]. Phosphate uptake in the absence of Na+ and methyl alpha-glucoside uptake dependent on Na+ were not affected. Phosphate uptake was stimulated 15% by 0.010 pM 1,25-(OH)2D3. Maximal enhancement of 30% was obtained with 100 pM. The uptake when fully stimulated by preincubation in vitro approximated the uptake of cells isolated from chicks that were previously repleted with 1,25-(OH)2D3 in vivo. Cells from repleted chicks were not stimulated additionally when preincubated with 1,25-(OH)2D3 in vitro. The increase in phosphate uptake could be measured after a 1-hr preincubation period; full response required at least 2 hr. Phosphate uptake induced by 1,25-(OH)2D3 was blocked by cycloheximide and actinomycin D. Enhancement of phosphate uptake was relatively specific for the 1,25-(OH)2D3 analog of vitamin D3. The potency order was 1,25-(OH)2D3 greater than 25-(OH)D3 = 1-(OH)D3 greater than 24,25-(OH)2D3 greater than D3. Kinetically, 1,25-(OH)2D3 increased the Vmax of the phosphate uptake system; the affinity for phosphate was unaffected. 3H-Labeled 1,25-(OH)2D3 was taken up by the isolated renal cells. It was estimated that the stimulation of phosphate uptake might be initiated by very few molecules of 1,25-(OH)2D3 per cell. It is proposed that 1,25-(OH)2D3 contributes importantly to the mechanisms by which phosphate transport is regulated in the kidney.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K., de Rouffignac C., Roinel N., Rumrich G., Ullrich K. J. Renal phosphate transport: inhomogeneity of local proximal transport rates and sodium dependence. Pflugers Arch. 1975;356(4):287–298. doi: 10.1007/BF00580003. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Avioli R. C. Intestinal phosphate transport and alkaline phosphatase activity in the chick. Am J Physiol. 1981 Apr;240(4):E384–E390. doi: 10.1152/ajpendo.1981.240.4.E384. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Miller R. The role of phosphate in the action of vitamin D on the intestine. J Clin Invest. 1977 Nov;60(5):980–988. doi: 10.1172/JCI108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour J. P., Preston C., Fleisch H. Effect of 1,25-dihydroxyvitamin D3 on the renal handling of Pi in thyroparathyroidectomized rats. J Clin Invest. 1977 Dec;60(6):1419–1428. doi: 10.1172/JCI108903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Deluca H. F. Stimulation of (3H)uridine incorporation into nuclear RNA of rat kidney by vitamin D metabolites. Arch Biochem Biophys. 1973 May;156(1):321–327. doi: 10.1016/0003-9861(73)90370-6. [DOI] [PubMed] [Google Scholar]
- Cheng L., Sacktor B. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. J Biol Chem. 1981 Feb 25;256(4):1556–1564. [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Studies on the mode of action of calciferol. XVIII. Evidence for a specific high affinity binding protein for 1,25 dihydroxyvitamin D3 in chick kidney and pancreas. Biochem Biophys Res Commun. 1979 Jul 12;89(1):56–63. doi: 10.1016/0006-291x(79)90942-2. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D system in the regulation of calcium and phosphorus metabolism. Nutr Rev. 1979 Jun;37(6):161–193. doi: 10.1111/j.1753-4887.1979.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978 Aug;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi R. T., Simpson R. U., DeLuca H. F. Binding proteins for vitamin D metabolites: serum carriers and intracellular receptors. Arch Biochem Biophys. 1981 Aug;210(1):1–13. doi: 10.1016/0003-9861(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Peterlik M. Vitamin D-induced phosphate transport in intestinal brush border membrane vesicles. Biochem Biophys Res Commun. 1980 Mar 13;93(1):87–92. doi: 10.1016/s0006-291x(80)80249-x. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Transfer of Ca45 across intestinal wall in vitro in relation to action of vitamin D and cortisol. Am J Physiol. 1960 Aug;199:265–271. doi: 10.1152/ajplegacy.1960.199.2.265. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Kowarski S., Schachter D. Effects of vitamin D on phosphate transport and incorporation into mucosal constituents of rat intestinal mucosa. J Biol Chem. 1969 Jan 10;244(1):211–217. [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D-3 on phosphate uptake into chick intestinal brush border membrane vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):13–23. doi: 10.1016/0005-2736(80)90052-8. [DOI] [PubMed] [Google Scholar]
- Peterlik M., Wasserman R. H. Effect of vitamin D on transepithelial phosphate transport in chick intestine. Am J Physiol. 1978 Apr;234(4):E379–E388. doi: 10.1152/ajpendo.1978.234.4.E379. [DOI] [PubMed] [Google Scholar]
- Popovtzer M. M., Robinette J. B., DeLuca H. F., Holick M. F. The acute effect of 25-hydroxycholecalciferol on renal handling of phosphorus. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest. 1974 Mar;53(3):913–921. doi: 10.1172/JCI107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Fernandez P. C., Boyle I. T., Gray R. W., Omdahl J. L., DeLuca H. F. The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1972 Oct;141(1):379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- Simpson R. U., Franceschi R. T., DeLuca H. F. Characterization of a specific, high affinity binding macromolecule for 1 alpha, 25-dihydroxyvitamin D3 in cultured chick kidney cells. J Biol Chem. 1980 Nov 10;255(21):10160–10166. [PubMed] [Google Scholar]
- Steele T. H., Engle J. E., Tanaka Y., Lorenc R. S., Dudgeon K. L., DeLuca H. F. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975 Aug;229(2):489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- Stoll R., Kinne R., Murer H., Fleisch H., Bonjour J. P. Phosphate transport by rat renal brush border membrane vesicles: influence of dietary phosphate, thyroparathyroidectomy, and 1,25-dihydroxyvitamin D3. Pflugers Arch. 1979 May 15;380(1):47–52. doi: 10.1007/BF00582611. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H. Intestinal absorption of calcium and phosphorus. Fed Proc. 1981 Jan;40(1):68–72. [PubMed] [Google Scholar]


