Abstract
Sirtuins 1-7 (SIRT1-7) belong to the third class of deacetylase enzymes, which are dependent on NAD+ for activity. Sirtuins activity is linked to gene repression, metabolic control, apoptosis and cell survival, DNA repair, development, inflammation, neuroprotection and healthy aging. Because sirtuins modulation could have beneficial effects on human diseases there is a growing interest in the discovery of small molecules modifying their activity. We review here those compounds known to activate or inhibit sirtuins, discussing the data that support the use of sirtuin-based therapies. Almost all sirtuin activators have been described only for SIRT1. Resveratrol is a natural compound which activates SIRT1, and may help in the treatment or prevention of obesity, and in preventing tumorigenesis and the aging-related decline in heart function and neuronal loss. Due to its poor bioavailability, reformulated versions of resveratrol with improved bioavailability have been developed (resVida, Longevinex®, SRT501). Molecules that are structurally unrelated to resveratrol (SRT1720, SRT2104, SRT2379, among others) have been also developed to stimulate sirtuin activities more potently than resveratrol. Sirtuin inhibitors with a wide range of core structures have been identified for SIRT1, SIRT2, SIRT3 and SIRT5 (splitomicin, sirtinol, AGK2, cambinol, suramin, tenovin, salermide, among others). SIRT1 inhibition has been proposed in the treatment of cancer, immunodeficiency virus infections, Fragile X mental retardation syndrome and for preventing or treating parasitic diseases, whereas SIRT2 inhibitors might be useful for the treatment of cancer and neurodegenerative diseases.
2. Introduction
The benefits of the Fountain of Youth, able to extend human lifespan, have been a general goal, appearing in writings by the ancient Greeks and also in tales among the indigenous peoples of the Caribbean. The discovery that overexpressing the Silent information regulator (Sir2) prolonged the lifespan of Caenorhabditis elegans (1) and Drosophila melanogaster (2) attracted a lot of interest in sirtuins. This interest was even reinforced by reports that calorie restriction (CR) could extend lifespan in mammals by inducing sirtuin 1 (SIRT1) expression and promoting the long-term survival of irreplaceable cells (3). A role for sirtuins in promoting longevity is now questioned due to the recent demonstration that high-level expression of Sir2 alone was not sufficient to increase lifespan relative to the transgenic controls, both in worms and flies, and all genotypes responded similarly and normally to CR (4). However, a great interest has indeed emerged in the discovery of and in developing molecules able to regulate sirtuin activity.
Sirtuins belong to the third class of deacetylase enzymes, which require nicotinamide adenine dinucleotide (NAD+) as an essential co-factor (5). Acetylation and deacetylation is an important mechanism to regulate posttranslationally the activity of proteins. The mammalian sirtuin family is comprised by seven proteins, although deacetylase activity has not been reported for all members. However, all sirtuins contain a conserved catalytic core domain of 275 amino acids and have a stoichiometric requirement for the cofactor nicotinamide adenine dinucleotide (NAD+) to deacetylate substrates ranging from histones to transcriptional regulators (6). Promotion of longevity is perhaps the effect of sirtuins activity that has attracted most interest, although the family has been also linked to gene repression, the control of metabolic processes, apoptosis and cell survival, and to DNA repair, development, inflammation and neuroprotection (7).
In this review we begin by introducing the mammalian sirtuins and giving a brief overview of their known activities in the context of their subcellular localizations. Next, we review compounds currently known to activate or inhibit sirtuins, discussing the data that support the use of sirtuin-based therapies for the treatment of human diseases.
3. Subcellular distribution and physiological roles of sirtuins
Mammalian sirtuin proteins have been found in a variety of subcellular locations. SIRT1 is predominantly nuclear (8) and SIRT2 is located mainly in cytoplasm (9) but they can shuttle between the nucleus and cytoplasm (10, 11). SIRT3, SIRT4 and SIRT5 are mitochondrial proteins, although SIRT3 has also been identified to move from the nucleus to mitochondria during cellular stress (7). SIRT6 and SIRT7 are nuclear sirtuins (12, 13).
SIRT1 is the closest to yeast Sir2 in terms of sequence and enzymatic activity, and is also the mammalian sirtuin most extensively studied to date. SIRT1 is a key regulator of metabolism, and its activity is regulated by nutritional status, being up-regulated throughout the body during fasting and calorie restriction (3). SIRT1 up-regulates mitochondrial biogenesis in several tissues, stimulates fat and cholesterol catabolism in liver, skeletal muscle and adipose tissue, induces the gluconeogenic genes and repress glycolytic genes and activate fatty acid oxidation systemically (see (14) for a revision). SIRT1 controls the gluconeogenic/glycolytic pathways through the transcriptional co-activator PGC-1α, which leads to an increase in the mitochondrial mass and function in animal and in vitro models (15, 16). In addition to the effect of SIRT1 orchestrating key metabolic adaptations, SIRT1 is also induced in pro-opiomelanocortin neurons that are critical for normal body weight and glucose homeostasis by reducing energy intake. This hypothalamic-specific, fasting-induced SIRT1 regulation is altered in leptin-deficient, obese mice (17), and, lack of SIRT1 in these neurons causes hypersensitivity to diet-induced obesity (DIO) due to reduced energy expenditure (18). Furthermore, a recent study has reported associations of SIRT1 single nucleotide polymorphisms (SNPs) to both obesity and body mass index (BMI) (19). However, although SIRT1 overexpression was proposed to be associated to lifespan extension in some animal models (1, 2), no evidence for an association between any of the tested SNPs and exceptional human longevity in the German population was detected (20). The putative association between SIRT1 and lifespan in animal models was explained on the basis of the ability of SIRT1 to deacetylate p53 thus decreasing its activity (8), for what it has been hypothesized that SIRT1 activity may also elevate cancer risk. There are conflicting data on the role for SIRT1 in cancer development. SIRT1 knockdown accelerated tumor xenograft formation by HCT116 cells, whereas SIRT1 overexpression inhibited tumor formation. High SIRT1 levels were also detected in normal colon mucosa and benign adenomas; and SIRT1 overexpression was observed in about 25% of stage I/II/III colorectal adenocarcinomas but rarely found in advanced stage IV tumors (21). On the other hand, up-regulated SIRT1 has been described in cancer cell lines as well as in tissue samples from patients with human lung cancer, prostate cancer, colon carcinoma and chronic lymphocytic leukemia cells (22, 23). These results raise the possibility that inhibition of SIRT1 might suppress cancer cell proliferation. However, reduced SIRT1 levels have been also reported in breast cancer and hepatic cell carcinoma (HCC) compared with their normal controls, while slightly increase (thyroid) or no change (lung, colon, stomach, bladder, and skin) of SIRT1 levels were detected in other tumors (24).
Human SIRT2 is a predominantly cytoplasmic protein that co-localizes with microtubules (9), α-tubulin being the main substrate of the SIRT2 deacetylase (25). SIRT2 also interacts with and deacetylates Lys16 on histone H4, leading to the formation of condensed chromatin during the G2/M transition (26). In addition, it has been shown to be down-regulated in gliomas, suggesting that SIRT2 deletion may permit tumor development and that its repletion may act as a tumor suppressive therapy (27). In fact, SIRT2-deficient mice develop gender-specific tumorigenesis, with females primarily developing mammary tumors, and males developing more hepatocellular carcinoma (28).
In addition to the connection between SIRT1 and the mitochondria, SIRT3, SIRT4 and SIRT5, that are located in the mitochondrion, participate in the regulation of the basic mitochondrial biology, including ATP production, metabolism, apoptosis, and intracellular signaling (see (29) for a revision). Among the seven sirtuins found in humans, SIRT3 is the only for which the existence of correlation between a polymorphism and extended human lifespan has been found. A VNTR polymorphism in intron 5 of the SIRT3 gene has an allele-specific enhancer activity. Of note, the allele completely lacking enhancer activity is virtually absent in males older than 90 years (30). Mitochondrial sirtuins appear to act in fine coordination with SIRT1, not only through transcriptional co-activator PGC-1α, but also because SIRT1 enhances SIRT6 expression and can regulate mitochondrial activity influencing the production of metabolic intermediates (31). SIRT5 was found to deacetylate cytochrome c, and thus, it could participate in the regulation of apoptosis, but also in the modulation of this protein’s function in respiration (32).
SIRT6 plays a key role in DNA repair and maintenance of genomic stability in mammalian cells integrating stress signaling to prime the DNA repair machinery in response to oxidative stress (33). SIRT6 knockout mice develop profound metabolic defects and ultimately die by four weeks of age (34). SIRT6 overexpression induces massive apoptosis in a variety of cancer cell lines but not in normal cells, making SIRT6 is an attractive target for cancer therapy (35).
SIRT7 resides in nucleoli and is associated with active rRNA genes. It regulates transcription, and is required for cell viability in mammals (12). SIRT7-deficient mice have reduced mean and maximum lifespan and develop inflammatory cardiomyopathy (36, 37). SIRT7 interacts with factors involved in RNA Pol I-and II-dependent transcriptional processes and several nucleolus-localized chromatin remodeling complexes (38).
Table 1 summarizes both subcellular and tissue-specific localization of the different sirtuins as well as some of their different substrate specificities, functions and enzyme activities (see (39) for a review).
Table 1.
Localization, substrates, functions and enzyme activities of different sirtuins
| Sirtuin | Subcellular localization | High tissue expression | Main substrates | Functions | Enzyme activity |
|---|---|---|---|---|---|
| SIRT1 | Nuclear and cytosolic | Brain, skeletal muscle, heart, kidney and uterus | Histones H1, H3 and H4 Transcripton factors p53, FOXO family, Ku70,p300, NF- κB, PGC-1α, PPAR-γ, UCP2 Acetyl-CoA synthetase 1 |
Cell survival, lifespan regulation, metabolism regulation, inflammation, oxidative stress response | NAD+-dependent deacetylase |
| SIRT2 | Cytosolic and nuclear | Brain | α-tubulin | Cell cycle regulation, nervous system development | NAD+-dependent deacetylase |
| SIRT3 | Mitochondrial, nuclear and cytosolic | Brain, heart, liver, kidney and brown adipose tissue | Acetyl-CoA synthetase 2 Isocitrate dehydrogenase 2 Ku70, FOXO 3a, MnSOD, Mitochondrial ribosomal protein L10 Long-chain acyl-CoA deshydrogenase 3-Hydroxy-3-Methylglutaryl CoA synthase 2 Succinate deshydrogenase, NADH:quinone oxidoreductase |
Regulation of mitochondrial metabolism | NAD+-dependent deacetylase |
| SIRT4 | Mitochondrial | Pancreatic β-cells, brain, liver, kidney and heart | Glutamate dehydrogenase | Regulation of mitochondrial metabolism, | ADP-ribosyl transferase |
| SIRT5 | Mitochondrial | Brain, testis, heart muscle and lymphoblast | Cytochrome c Carbamoyl phosphate synthetase 1, |
Apoptosis | NAD+-dependent deacetylase Desuccinylase Demalonylase |
| SIRT6 | Nuclear, associated to heterochromatin | Muscle, brain, heart, ovary and bone cells (absent in bone marrow) | Histone H3 TNF-α PAPR1 |
Genome stability, DNA repair | NAD+-dependent deacetylase ADP-ribosyl transferase |
| SIRT7 | Nucleolar | Peripheral blood cells, CD33+ myeloid bone marrow precursor cells | RNA polymerase I p53 |
Regulation of rRNA transcription, cell cycle regulation | NAD+-dependent deacetylase |
The wide spectrum of activities in which sirtuins participate is implicating these enzymes in the pathology of many diseases which include obesity-associated metabolic diseases, neurodegeneration diseases, inflammation, cardiovascular diseases, and cancer among others (39). For what, modulation of sirtuins activity has great promise for the development of novel therapies against a myriad of metabolic and age-related diseases. Whereas almost all sirtuin activators have been described only for SIRT1, many inhibitors have been reported for SIRT1 and SIRT2, and to a lesser extent for SIRT3 and SIRT5.
4. Sirtuin activators
CR has been known for over 75 years to improve the health and to extend the lifespan of mammals. The initial conception that dietary restriction did not extend lifespan when sirtuins were deleted (2, 40) and that CR could extend lifespan in mammals by inducing SIRT1 expression (3) prompted the investigators into sirtuin research and in the discovery and develop of molecules able to activate them. Even when the role played by sirtuins in promoting longevity by themselves is now questioned (4), on the basis of the positive health effects of CR in mammals and the associated increases in SIRT1 (3), the discovery and development of SIRT1-activating drugs has attracted great interest both in sirtuins and in the discovery and develop of molecules able to activate them.
4.1 Resveratrol
The polyphenol resveratrol (RSV), a molecule containing two phenyl rings separated by a methylene bridge (Figure 1A), was the first compound discovered able to mimic CR by stimulating sirtuins (41, 42). RSV induced gene expression patterns in multiple tissues that paralleled those induced by CR and showed a marked reduction in signs of aging without affecting the expression of any of the sirtuin genes (43, 44). Age is a major risk factor for a variety of neurodegenerative disorders. In the Tg2576 transgenic mouse strain, a model that shows Alzheimer disease (AD)-type amyloid neuropathology, SIRT1 expression causally promoted α-secretase activity and attenuated Aβ-peptides generation in primary TG2576 neuron cultures (45). Also, SIRT1 levels were upregulated in mouse models for AD, amyotrophic lateral sclerosis and in primary neurons challenged with neurotoxic insults. Of note, both RSV and ectopic expression of SIRT1 conferred significant protection against neurodegeneration (46).
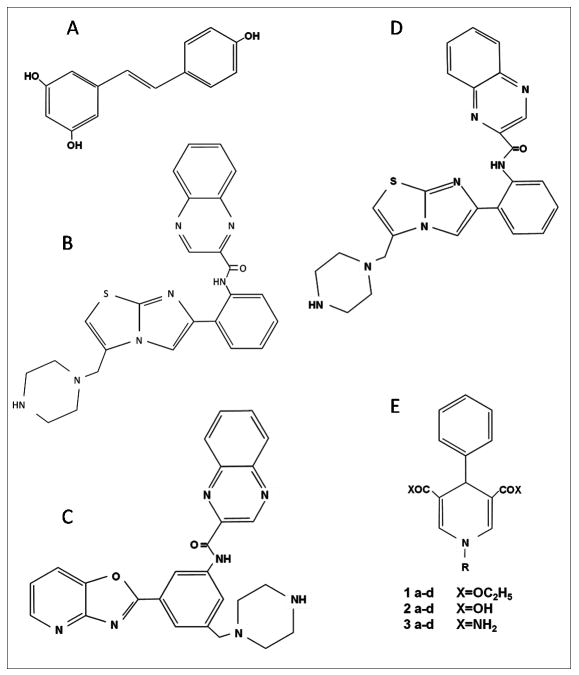
Figure 1.
Sirtuin activators. A) Resveratrol (3,5,4′-trihydroxy-trans-stilbene); B) SRT1720; C) Oxazolo[4,5-b]pyridines derivative; D) Imidazo[1,2-b]thiazole derivative; E) 1,4-Dihydropyridine (DHP) derivatives.
Revised data about bioavailability and pharmacokinetics of RSV have been previously published (47, 48). In vivo studies have reported that RSV improves the general health in mice fed with a high-calorie diet (49), showing a marked reduction in signs of aging, including reduced albuminuria and cataracts formation, decreased inflammation and apoptosis in the vascular endothelium, increased aortic elasticity, greater motor coordination, and preserved bone mineral density in mice (44). Due to its poor bioavailability, reformulated versions of RSV with improved bioavailability have been developed. A nutraceutical formulation containing 150 mg/day resveratrol (resVida) also has demonstrated beneficial effects in healthy obese men, decreasing intrahepatic lipid content, circulating glucose, triglycerides, alanine-aminotransferase, and inflammation markers, mimicking the effects of CR (50). Furthermore, oral RSV supplementation elicited an acute dose-related improvement in endothelium-dependent vasodilatation, which correlated with a dose-related increase in plasma RSV concentrations (51). RSV administrated in the nutraceutical formulation Longevinex®, improved the flow-mediated dilatation after 3 months of treatment, but this parameter returned to baseline 3 months after the discontinuation of Longevinex®, and the treatment did not modify blood pressure, insulin resistance, the lipid profile or inflammatory markers (52).
On the other hand, RSV treatment reduced tumorigenesis in SIRT1+/−;p53+/− mice which develop tumors in multiple tissues, and this protective effect was explained on the basis of SIRT1 activation (24). SRT501, another commercial micronized RSV formulation, recapitulated a molecular signature that overlaps with that of CR, such as enhanced mitochondrial biogenesis, improved metabolic signaling pathways, and blunted pro-inflammatory pathways in mice fed a high calorie diet. SRT501generated a gene expression profile that mirrored that of CR (53). This formulations has been also shown to lower blood glucose and to improve insulin sensitivity in patients with type 2 diabetes in a Phase IIa trial, warranting further clinical exploration to assess its potential clinical utility (54).
4.2. Sirtuin activators structurally unrelated to resveratrol
The implication of sirtuins in the regulation of energy expenditure and ageing-related pathologies has focused the attention to the discovery of new molecules able to stimulate sirtuin activities more potently than RSV. Milne et al. identified small molecule activators of SIRT1 that are structurally unrelated to RSV, but hundred-fold more potent. The compound named SRT1720 (Figure 1B) was the most effective, and stimulated 750% SIRT1 activity at 10 μM. It was determined that SRT1720 binds and activate the enzyme at the same molecular site as RSV, because compound combination resulted in additive SIRT1 activation, the amino acids 183–225 N-terminal to the core domain being important in defining the compound binding site (55). SRT1720 increased the deacetylation of SIRT1 substrates (e.g. p53, the transcriptional co-activator PGC-1α, and Foxo1a) in vivo and in vitro (53). The therapeutic potential of SIRT1720 to treat insulin resistance and diabetes was tested in three in vivo models of type 2 diabetes: genetically obese mice (Lep ob/ob) and DIO mice, and in the Zucker fa/fa rat, which is a genetically obese rodent model for studying insulin resistance. Administration of SRT1720 reduced fed glucose levels, partially normalizing elevated insulin levels, significantly reduced fasting blood glucose to near normal levels in mice on high-fat diet, strongly protecting mice from DIO and insulin resistance by enhancing oxidative metabolism in skeletal muscle, liver, and brown adipose tissue, through a global metabolic adaptation mimicking low energy levels (53, 55, 56). In vivo efficacy data for SRT501 and SRT1720 in multiple rodent models were similar, but SRT1720 was used at a concentration 10-fold lower than SRT501. Mitochondrial biogenesis occurs under CR, and SRT1720 induced mitochondrial biogenesis elevating mitochondrial respiration rates and ATP levels. In addition, SRT1720 accelerated recovery of mitochondrial functions after acute oxidant injury (57). Furthermore, SRT1720 treatment significantly reduced levels of liver triglyceride content and aminotransferase and the expressions of lipogenic genes in a mouse model exhibiting obesity and insulin resistance (58). Recently, it has been reported that SRT1720 extended both mean and maximum lifespan of adult mice fed a high-fat diet, and the lifespan extension was accompanied by health benefits including reduced liver steatosis, increased insulin sensitivity, enhanced locomotor activity and normalization of gene expression profiles and markers of inflammation and apoptosis, all in the absence of any observable toxicity (59). Moreover, in a preclinical evaluation for cancer treatment, SRT1720 induced apoptosis in multiple myeloma cells in vitro and significantly decreased xenografted tumor growth, by a mechanism involving the ATM-CHK2/caspase 8/9 signalling axis (60).
New small molecule activators of SIRT1 which are unrelated to RSV such as a series of oxazolo[4,5-b]pyridines have been identified (61) (Figure 1 C) and have also synthesized a series of imidazo (1,2-b) thiazole derivatives compounds bearing an oxazolopyridine core with potential new therapeutic targets to treat various disorders. The most potent analogue within this series, namely compound 29 (Figure 1D), displayed significant oral bioavailability in the mouse, as well as in the rat, and showed oral antidiabetic activity in three different models for type 2 diabetes. A dose of 100 mg/kg of compound 29 taken once daily produced a significant reduction in fasting blood glucose level in the ob/ob deficient mice after just 1 week of treatment, in the DIO model after 2 weeks of dosing, and in the genetic Zucker fa/fa rat model after 3 weeks. Even after 10 weeks of dosing in the DIO mice, compound 29 was able to maintain a significantly lower level of fed glucose and insulin without affecting the body weight, as well as the overall clinical chemistry and hematology (62).
SRT2104 is a newly developed compound able to attenuate inflammation in dextran sodium sulfate (DDS)-induced colitis model. SRT2104 (1–30 mg/kg) produced a greater reduction in the colitis score compared to prednisolone, while improving the loss in body weight. Of note, the anti-inflammatory effect of SRT2104 was absent in the SIRT1 KO mice (63). SRT2104 has been also tested in a Phase IIa trial in patients with metabolic, inflammatory and cardiovascular diseases. It was found to be safe and well tolerated in Phase I trials in healthy volunteers. In a double-blind placebo-controlled study, SRT2104 was shown to significantly attenuate LPS-induced IL-6 and IL-8 release and activation of coagulation (64). Different clinical trials have been completed to evaluate safety, tolerability, the pharmacokinetics and the bioavailability of SRT2104 and SRT2379, another SIRT activator (see in http://clinicaltrials.gov/).
Also, pyrrolo[3,2-b]quinoxalines have been described as potent lipolytic agents which also showed anti-inflammatory properties in vitro, but their putative utility in animal models has been not reported (65). Finally, Mai et al. prepared new 1,4-dihydropyridine (DHP) derivatives compounds and they found that the DHPs bearing a benzyl group at the N1 position behaved as potent, dose-dependent human SIRT1, SIRT2 and SIRT3 activators, leading to a significant increase in mitochondrial density and reduced senescence in murine C2C12 myoblasts (66). This constitutes the first report of small molecule activators of sirtuins different than SIRT1. However, modulation of sirtuin activity by these compounds was assessed using the Fluor de Lys fluorescent biochemical assay (BioMol) (66), which validity has been seriously questioned (67–71).
It must be mentioned that, to date, none of sirtuin activators have been demonstrated to increase lifespan of mice fed a normal diet. However, the effects of sirtuin activation on overall health and age-related diseases (healthspan) may be as interesting as a putative effect on lifespan.
4.3. The controversy of sirtuin activation by small molecules
Originally, it was proposed that RSV might bind to the non-catalytic N-terminus of SIRT1 to induce a conformational change that lowers the Michaelis constant for both the acetylated substrate and NAD+ (72). Maximal SIRT1 activation (780% of control) was achieved at a RSV concentration of 200 μM (41). However, due to its poor bioavailability, the peak plasma levels of RSV and metabolites only reached about 2 μM after an oral dose of 25 mg in humans (73), making it controversial that in vivo effects of RSV were based on direct activation of SIRT1. Later it was seriously questioned that RSV was a direct activator of SIRT1, because RSV could activate deacetylation of Ac-Arg-His-Lys-Lys-Ac-AMC catalyzed by SIRT1, but not of the simple amide or acid of this peptide (68, 70). Not only RSV, but also the second-generation sirtuin activators, SRT1460, SRT1720, and SRT2183, were reported to activate SIRT1 when fluorescent substrates were used in the deacetylase assay, but not when unmodified peptides or the native protein substrates were used (71). Another paper has recently published the kinetic and biophysical evidence for direct interaction of enzyme and activators, confirming that SRT1460 and SRT1720 can bind to fluorescently labeled peptides (74). However, they were one step beyond to demonstrate that selected peptide sequences composed of only natural amino acids could be competent substrates for activation of SIRT1, suggesting that SIRT1-catalyzed deacetylation is accelerated by the activators only when the peptide bears specific ring systems, designated as “activation cofactors”. Striking inconsistencies between in vivo effects of sirtuin activators found in two recent studies, one reporting that SRT1720 neither lowered plasma glucose nor improved mitochondrial capacity in mice fed a high fat diet (71) and the other showing an increase on health and lifespan of mice treated with two different doses of SRT1720 for 2 years on a high fat diet (59) warrant further research using comparable experimental designs.
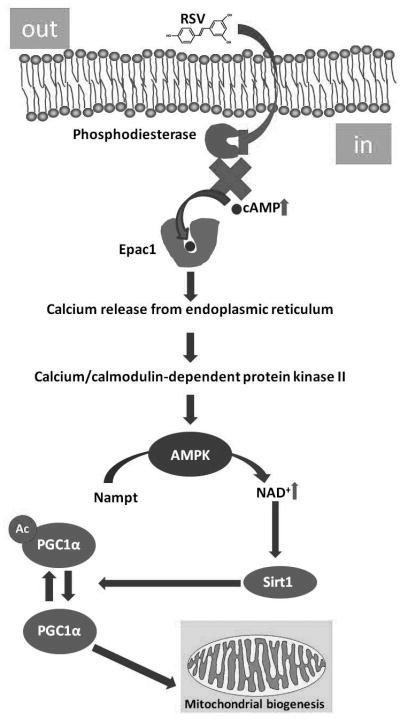
More recently, new evidences support that the indirect activation of SIRT1 by RVS is mediated by the activation of AMPK. AMPK acts as a primary initial sensor that increases NAD+ levels, thus inducing a higher deacetylation of SIRT1 targets, PGC-1α and FOXO1, due to concomitant increases of SIRT1 activity (75, 76). One strong mechanistic study has demonstrated that metabolic effects of RSV can be accounted by elevation of cAMP levels both in skeletal muscle and white adipose tissue by inhibiting phosphodiesterases, because RSV competes with cAMP in its binding site. The elevation of citosolyc cAMP activates the cAMP effector protein Epac1, which increases levels of cytosolic Ca2+, thus inducing AMPK phosphorylation via calcium/calmodulin-dependent protein kinase II. The activation of AMPK increases NAD+ levels, thus leading to SIRT1 activation (77). A diagram of this mechanism is shown in Figure 2. This study unequivocally establishes that RSV is at least an indirect activator of SIRT1, and poses a plausible mechanism to explain its metabolic effects. Similar mechanistic studies for SIRT1 activators structurally unrelated to RSV are needed.
Figure 2.
Mechanism of Sirt1activation by RSV. The metabolic effects of RSV can be accounted for competitive inhibition of cAMP-degrading phosphodiesterases and the elevation of cytosolic calcium via the cAMP effector protein Epac1. This pathway activates the calcium/calmodulin-dependent protein kinase II which phosphorylates AMPK, which in turn increases of NAD+ levels, thus leading ultimately to SIRT1 activation.
5. Sirtuin inhibitors
All sirtuins require nicotinamide adenine dinucleotide (NAD+) as an essential cofactor (5). The initial step of the proposed reaction mechanism is the cleavage of nicotinamide (NIC) from NAD+ whereas ADP-ribose binds the acetyl-peptide with the formation of a 0-alkylamidate intermediate. NIC is a potent product inhibitor of the reaction because it can rebind to the 0-alkylamidate intermediate form of the enzyme and attack the intermediate (78). Recently it has been reported that NIC increases megakariocyte maturation and ploidy without altering normal megakariocyte ultrastructure at least in part through SIRT1 inhibition, by increasing the p53 binding to its consensus DNA binding sequence (79).
While the beneficial impact of increased SIRT1 activity observed in several animal models favored the discovery and design of pharmacological activators of sirtuins, presumably as CR mimetics, sirtuin inhibitors can also be potentially useful as therapeutic agents because up-regulated SIRT1 has been described in cancer cell lines (22–24), raising the possibility that SIRT1 inhibition might suppress cancer cell proliferation. In addition to cancer therapy, sirtuin inhibitors have also been proposed in the treatment of Parkinson’s disease (80), leishmaniosis (81), and human immunodeficiency virus (82), among others. Furthermore, it is important to consider that SIRT3, SIRT4, and SIRT5 are located into the mitochondria, where a high number of mitochondrial proteins suffer cycles of acetylation/deacetylation processes important for energy metabolism and apoptosis initiation, but also in the context of various diseases such as metabolic syndrome and cancer (83).
In contrast with the activators field, where only SIRT1 activators have been developed, sirtuin inhibitors with a wide range of core structures have been identified for SIRT1, SIRT2, SIRT3 and SIRT5 through high-throughput and in silico screenings. Most of the sirtuin inhibitors characterized so far only inhibit SIRT1 and/or SIRT2, but also some of them inhibit SIRT3 and SIRT5 with a lower affinity. This review outlines biological evaluations of sirtuin inhibitors. Two recent publications have reviewed the development of pharmacological small molecule inhibitors of the sirtuins and their chemical modifications, highlighting strategies to enhance inhibitory activity and selectivity among sirtuin isoforms (84, 85).
5.1. Splitomicin and its derivatives
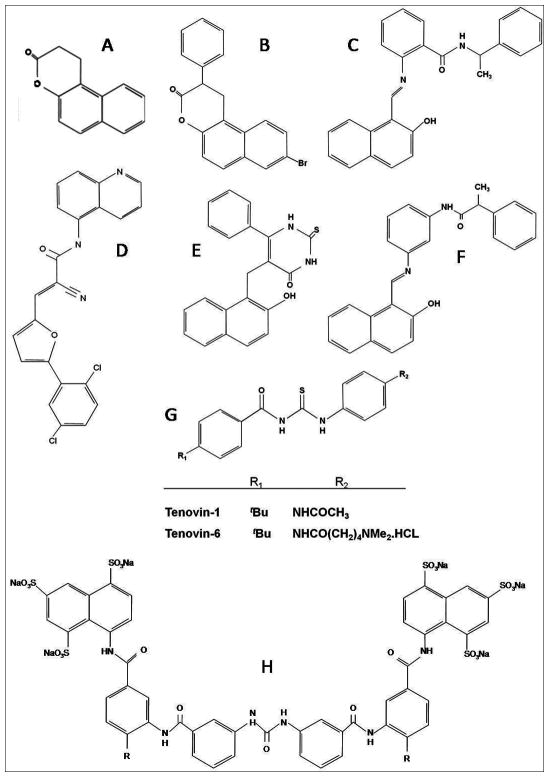
To provide a new tool to dissect the functional role of Sir2 in vivo, Bedalov et al identified a compound, splitomicin (Figure 3A), which creates a conditional phenocopy of a Sir2 deletion mutant in Saccharomyces cerevisiae. Splitomicin inhibits Sir2 with an IC50 of 60 μM (86). However, splitomicin showed rather weak inhibition on human SIRT1. Based on the docking and free energy calculations, it was demonstrated a clear preference for one of the two β-phenylsplitomicin stereoisomers, and a link between increased enzyme inhibition and antiproliferative effect in MCF-7 breast cancer cells was established (87). SIRT1 plays an important role in the expansion of the CGG•CCG-repeat tract resulting in Fragile X syndrome, the most common heritable form of mental retardation, by the heterochromatinization of the promoter and gene silencing. Interestingly, splitomicin-mediated inhibition of SIRT1 activity attenuates gene silencing in Fragile X mental retardation syndrome (88).
Figure 3.
Sirtuin inhibitors. A) Splitomicin; B) HR73; C) Sirtinol; D) AGK2; E) Cambinol; F) Salermide; G) Tenovin; H) Suramin.
Cycles of acetylation and deacetylation regulate the human immunodeficiency virus (HIV) Tat protein. Acetylated Tat, bound to the elongating polymerase, recruits the transcriptional coactivator PCAF via its acetyl group and the bromodomain of PCAF. Deacetylation of Tat by SIRT1 could lead to its dissociation from the elongating polymerase and from PCAF. Pagans et al. have identified a splitomicin derivative, called HR73 (Figure 3B), which inhibited the activity of SIRT1 in vitro with an IC50 lower than 5 μM, validating SIRT1 as a novel therapeutic target for HIV infection (82).
5.2. Sirtinol
In a cell-based screen, Grozinger et al. (2001) concurrently discovered another sirtuin inhibitor, sirtinol (2-[(2-hydroxy-naphthalen-1-ylmethylene)- amino]-N-(1-phenyl-ethyl)-benzamide) (Figure 3C) that inhibited both yeast Sir2 and human SIRT2 activity in vitro. The 2-hydroxyl-1-napthol moiety was demonstrated to be sufficient for inhibition (89). Two analogues, m- and p-sirtinol, were 2- to 10-fold more potent than sirtinol against human SIRT1 and SIRT2 (90). SIRT1 expression may play an important role in promoting cell growth. Downregulation of p53 activity by deacetylation may result in reduced apoptosis in response to various genotoxic stimuli (8) and thus, inhibitors of SIRT1 such as sirtinol may have anticancer potential. Sirtinol induced senescence-like growth arrest in human breast cancer MCF-7 and lung cancer H1299 cells (91), and enhanced chemosensitivity to camptothecin and cisplatin in PC3, DU145 and HeLa cells, resulting in a significant reduction of viable cells due to enhanced apoptotic cell death (92–94). Moreover, it has been reported that sirtinol, nicotinamide and SIRT3 down-regulation inhibited cell growth and proliferation and induced apoptosis in oral squamous cell carcinoma (OSCC) cell lines in vitro and in vivo where SIRT3 is overexpressed compared with other sirtuins. SIRT3 down-regulation also enhanced the sensitivity of OSCC cells to radiation and cis-platin-induced cytotoxicity (95).
Furthermore, increasing the dosage of Sir2/SIRT1 exacerbated muscle pathology in a nematode model of the oculopharyngeal muscular dystrophy, a disease caused by polyalanine expansion in the nuclear protein PABPN1, and the treatment with sirtinol was protective (96, 97). Sirtinol treatment also reduced inflammation in human dermal microvascular endothelial cells, modulating the expression of adhesion molecules and monocyte adhesion in primary human dermal microvascular endothelial cells (98), and induced apoptosis in Leishmania infontum, significantly inhibiting the in vitro proliferation of this axenic amastigote (81).
5.3. AGK2
The inhibition of SIRT2 activity has been reported to confer neuroprotection. The most common histopathological feature in Parkinson disease (PD) is the presence of concentric hyaline cytoplasmic inclusions, called Lewy bodies, which contain the protein α-synuclein (α-syn). SIRT2 inhibition via small interfering RNA or by addition of AGK2 (Figure 3D) rescued α-syn toxicity and modified inclusion morphology in a cellular model of PD, ameliorated mutant α-syn neurotoxicity in rat primary dopamine-positive neurons, and rescued degeneration of dopaminergic neurons from α-syn toxicity in a Drosophila PD model (80). Treatment with AGK2 led to an increase in acetylated tubulin (80), but also exacerbated H2O2-induced decreases in the intracellular ATP levels, increasing necrosis of PC12 cells without affecting autophagy, and induced caspase-3-dependent apoptosis in C-6 glioma cells (99, 100). Also, inhibition of SIRT2 achieved neuroprotection in cellular and invertebrate models of Huntington’s disease (HD) down-regulating the RNAs responsible for sterol biosynthesis (101). In addition to the neuroprotective effect of SIRT2 inhibition in PD and HD models, pharmacological inhibition of SIRT1/2 by nicotinamide, AGK2 or cambinol, also augmented ploidy in cultured megakaryocytic cells increasing acetylation of nucleosomes and p53 (79).
5.4. Cambinol
Cambinol (Figure 3E) is a chemically stable compound that shares a β-naphtol pharmacophore with sirtinol and splitomicin, and inhibits both SIRT1 and SIRT2 in vitro with IC50 values of 56 and 59 μM respectively. Cambinol displayed only weak inhibitory activity against SIRT5 (42% inhibition at 300 μM) (102). Cambinol was well tolerated in mice and inhibited growth of Burkitt lymphoma xenografts, inducing apoptosis via hyperacetylation of BCL6 oncoprotein and p53 (102). Changes in the phenyl ring of cambinol improved activity and increased selectivity for SIRT1, whereas incorporation of substitutents at the N1-position led to an increase in potency against SIRT2 in vitro that was not seen for SIRT1, resulting in the identification of a range of SIRT2 selective analogs (103).
5.5. Suramin
Unlike cambinol, suramin (Figure 3H) is a more potent inhibitor towards SIRT5 NAD+-dependent deacetylase activity, with an IC50 value of 22 μM (104). Suramin is also a potent inhibitor of SIRT1 (IC50=0.297 μM) and SIRT2 (IC50= 1.15 μM) (105). Suramin is a polyanionic naphthylurea that was originally used for the treatment of trypanosomiasis, but also shows antiproliferative and antiviral activity (106).
5.6. Tenovin
Using a cell-based screen for small molecules able to activate p53 and decrease tumor growth, Lain et al. (2008) found two SIRT1 inhibitors: tenovin-1 and its more water soluble analog tenovin-6 (Figure 3G). Both compounds decreased tumor growth in vitro at one-digit micromolar concentrations, and delayed tumor growth in vivo without significant general toxicity (107). SIRT1 activation promotes cell survival in chronic myelogenous leukemia (CML) and proliferation was associated with deacetylation of multiple SIRT1 substrates, including FOXO1, p53 and KU70. Treatment of mice with the SIRT1 inhibitor tenovin-6 prevented the disease progression (108). Recently, a series of more water soluble analogues of tenovin-1 and tenovin-6 that, in general, retained the desired biological activity has been developed. Tenovin-1 analogues adopt a preferred conformation in solution that contains an intramolecular hydrogen bond that is essential for SIRT1 binding. Also, the replacement of the 4-tert-butyl substituent in tenovin-6 with shorter alkyl chains (4-propyl or 4-iso-propyl substituent) showed the initially expected activity in cells, but analogues with longer n-alkyl chains (4-n-butyl or 4-n-pentyl substituent) proved either toxic or inactive in cells (109).
5.7. Salermide
Salermide (N-{3-[(2-hydroxy-1-naphthalenylmethylene)-amino]-phenyl 2}-phenyl-propionamide) (Figure 3F) is a reverse amide with a strong in vitro inhibitory effect on SIRT1 and SIRT2. Salermide was well tolerated by mice at concentrations up to 100 μM and induced p53-independent apoptosis in cancer but not in normal cells, by reactivation of proapoptotic genes that had been epigenetically repressed exclusively in cancer cells by SIRT1 (110). Another study using breast carcinoma cell lines and p53-deficient mouse fibroblasts reported that p53 is essential for salermide-induced apoptosis, as well as for sirtinol, another SIRT1 and SIRT2 inhibitor (94). Salermide effect can be mediated by the up-regulation of death receptor 5 expression in human non-small cell lung cancer cells (111).
5.8. Other inhibitors of human sirtuins
Other inhibitors of SIRT1 and SIRT2 have been described, and they have been previously reviewed (22, 39, 84, 85). They include lysine-based tripeptide analogues, non-peptide containing N-thioacetyl lysine, thiobarbiturates, indol derivatives (EX-527), and other inhibitors based on diverse structural cores.
6. Conclusions
Since they hold sway over such a wide range of cellular targets and locations, mammalian sirtuins are linked to healthy ageing, and the search for sirtuin modulators is a hot topic because of their possible therapeutic use in age-related diseases, metabolic and cardiovascular diseases, and inflammation. There are well documented activators for SIRT1 and inhibitors for SIRT1 and SIRT2. Despite the original controversy about whether or not RSV and other structurally unrelated compounds are direct activators of SIRT1, recent mechanistic data demonstrates that, at least indirectly, RSV indeed activates SIRT1 activity in vivo by increasing NAD+ levels. SIRT1 activators have been proposed for treating and/or preventing a wide variety of diseases and disorders including those related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders and inflammation. Both antagonistic mechanisms, i.e. SIRT1 activation and inhibition, have been proposed in cancer therapy. SIRT1 inhibition has also been proposed in the treatment of immunodeficiency virus infections, Fragile X mental retardation syndrome and even for the prevention or the treatment of parasitic diseases, whereas SIRT2 inhibitors might be useful for the treatment of cancer and neurodegenerative diseases such as PD or HD.
Acknowledgments
Authors′ work is supported by Grants: Universidad de Castilla la Mancha GE20112221, Junta de Comunidades de Castilla la Mancha PPII11 -0318-2669 (to FJA) and NIH 1R01AG028125-01A1, Ministerio de Economía y Competitividad BFU2011-23578, Junta de Andalucía Proyectos de Excelencia P09-CVI-4887, Junta de Andalucía Proyectos Internacionales, and BIO-276 (Junta de Andalucía and University of Córdoba) (to JMV).
References
- 1.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 2.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen HY, Miller C, Bitterman KJ, Wall NR, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 4.Burnett C, Valentini S, Cabreiro F, Goss M, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landry J, Sutton A, Tafrov ST, Heller RC, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittain DWH, Ottow E. Recent advances in the medicinal chemistry of histone deacetylase inhibitors. Ann Rep Med Chem. 2007;42:337–348. [Google Scholar]
- 7.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 9.North BJ, Marshall BL, Borra MT, Denu JM, et al. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 10.North BJ, Verdin E. Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS One. 2007;2:e784. doi: 10.1371/journal.pone.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanno M, Sakamoto J, Miura T, Shimamoto K, et al. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 12.Ford E, Voit R, Liszt G, Magin C, et al. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahlknecht U, Ho AD, Voelter-Mahlknecht S. Chromosomal organization and fluorescence in situ hybridization of the human Sirtuin 6 gene. Int J Oncol. 2006;28:447–456. [PubMed] [Google Scholar]
- 14.Silva JP, Wahlestedt C. Role of Sirtuin 1 in metabolic regulation. Drug Discov Today. 2010;15:781–791. doi: 10.1016/j.drudis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers JT, Lerin C, Haas W, Gygi SP, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 17.Ramadori G, Lee CE, Bookout AL, Lee S, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramadori G, Fujikawa T, Fukuda M, Anderson J, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark SJ, Falchi M, Olsson B, Jacobson P, et al. Association of sirtuin 1 (SIRT1) gene SNPs and transcript expression levels with severe obesity. Obesity (Silver Spring) 2012;20:178–185. doi: 10.1038/oby.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, et al. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Kabra N, Li Z, Chen L, Li B, et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem. 2009;284:18210–18217. doi: 10.1074/jbc.M109.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcain FJ, Villalba JM. Sirtuin inhibitors. Expert Opin Ther Pat. 2009;19:283–294. doi: 10.1517/13543770902755111. [DOI] [PubMed] [Google Scholar]
- 23.Audrito V, Vaisitti T, Rossi D, Gottardi D, et al. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011;71:4473–4483. doi: 10.1158/0008-5472.CAN-10-4452. [DOI] [PubMed] [Google Scholar]
- 24.Wang RH, Sengupta K, Li C, Kim HS, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Zhang B, Tang J, Cao Q, et al. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating alpha-tubulin. J Neurosci. 2007;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, et al. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiratsuka M, Inoue T, Toda T, Kimura N, et al. Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys Res Commun. 2003;309:558–566. doi: 10.1016/j.bbrc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellizzi D, Rose G, Cavalcante P, Covello G, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhong L, Mostoslavsky R. Fine tuning our cellular factories: sirtuins in mitochondrial biology. Cell Metab. 2011;13:621–626. doi: 10.1016/j.cmet.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gertz M, Steegborn C. Function and regulation of the mitochondrial sirtuin isoform Sirt5 in Mammalia. Biochim Biophys Acta. 2010;1804:1658–1665. doi: 10.1016/j.bbapap.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Mao Z, Hine C, Tian X, Van Meter M, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Van Meter M, Mao Z, Gorbunova V, Seluanov A. SIRT6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell Cycle. 2011;10:3153–3158. doi: 10.4161/cc.10.18.17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vakhrusheva O, Braeuer D, Liu Z, Braun T, et al. Sirt7-dependent inhibition of cell growth and proliferation might be instrumental to mediate tissue integrity during aging. J Physiol Pharmacol. 2008;59(Suppl 9):201–212. [PubMed] [Google Scholar]
- 37.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 38.Tsai YC, Greco TM, Boonmee A, Miteva Y, et al. Functional Proteomics Establishes the Interaction of SIRT7 with Chromatin Remodeling Complexes and Expands Its Role in Regulation of RNA Polymerase I Transcription. Mol Cell Proteomics. 2012;11:M111 015156. doi: 10.1074/mcp.M111.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alcain FJ, Minor RK, Villalba JM, de Cabo R. The Future of Aging. In: Fahy GM, West MD, Harris SB, editors. Small molecule modulators of sirtuin activity. 2010. Springer; 2010. pp. 331–356. [Google Scholar]
- 40.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 41.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 42.Wood JG, Rogina B, Lavu S, Howitz K, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 43.Barger JL, Kayo T, Pugh TD, Prolla TA, et al. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol. 2008;43:859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Pearson KJ, Baur JA, Lewis KN, Peshkin L, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin W, Yang T, Ho L, Zhao Z, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 46.Kim D, Nguyen MD, Dobbin MM, Fischer A, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alcain FJ, Villalba JM. Sirtuin activators. Expert Opin Ther Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- 48.Vang O, Ahmad N, Baile CA, Baur JA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baur JA, Pearson KJ, Price NL, Jamieson HA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timmers S, Konings E, Bilet L, Houtkooper RH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong RH, Howe PR, Buckley JD, Coates AM, et al. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Fujitaka K, Otani H, Jo F, Jo H, et al. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res. 2011;31:842–847. doi: 10.1016/j.nutres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, et al. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howells LM, Berry DP, Elliott PJ, Jacobson EW, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milne JC, Lambert PD, Schenk S, Carney DP, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feige JN, Lagouge M, Canto C, Strehle A, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamazaki Y, Usui I, Kanatani Y, Matsuya Y, et al. Treatment with SRT1720, a SIRT1 activator, ameliorates fatty liver with reduced expression of lipogenic enzymes in MSG mice. Am J Physiol Endocrinol Metab. 2009;297:E1179–1186. doi: 10.1152/ajpendo.90997.2008. [DOI] [PubMed] [Google Scholar]
- 59.Minor RK, Baur JA, Gomes AP, Ward TM, et al. SRT1720 suppresses apoptosis and restores a more normal gene expression profile indicative of reduced inflammation in the livers of mice fed a high-fat diet. Scientific Reports. 2011;1 doi: 10.1038/srep00070. [DOI] [Google Scholar]
- 60.Chauhan D, Bandi M, Singh AV, Ray A, et al. Preclinical evaluation of a novel SIRT1 modulator SRT1720 in multiple myeloma cells. Br J Haematol. 2011;155:588–598. doi: 10.1111/j.1365-2141.2011.08888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bemis JE, Vu CB, Xie R, Nunes JJ, et al. Discovery of oxazolo[4,5-b]pyridines and related heterocyclic analogs as novel SIRT1 activators. Bioorg Med Chem Lett. 2009;19:2350–2353. doi: 10.1016/j.bmcl.2008.11.106. [DOI] [PubMed] [Google Scholar]
- 62.Vu CB, Bemis JE, Disch JS, Ng PY, et al. Discovery of imidazo[1,2-b]thiazole derivatives as novel SIRT1 activators. J Med Chem. 2009;52:1275–1283. doi: 10.1021/jm8012954. [DOI] [PubMed] [Google Scholar]
- 63.Ellis DJGJL, Suri V, Cermak JM, Lyng GD, Guarente LP, Vlasuk GP. SRT2104, a novel and selective small molecule SIRT1 activator, inhibits DSS-induced colitis in a SIRT1-dependent manner. 10th World Congress on Inflammation; Paris. 2011. [Google Scholar]
- 64.Van Der Meer A, Scicluna B, Lin J, Jacobson E, et al. The first demonstration of clinical activity by a small molecule sirt1 activator: srt2104 reduces cytokine release and coagulation activation in a human endotoxemia model. 10th World Congress on Inflammation; Paris. 2011. [Google Scholar]
- 65.Nayagam VM, Wang X, Tan YC, Poulsen A, et al. SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents. J Biomol Screen. 2006;11:959–967. doi: 10.1177/1087057106294710. [DOI] [PubMed] [Google Scholar]
- 66.Mai A, Valente S, Meade S, Carafa V, et al. Study of 1,4-dihydropyridine structural scaffold: discovery of novel sirtuin activators and inhibitors. J Med Chem. 2009;52:5496–5504. doi: 10.1021/jm9008289. [DOI] [PubMed] [Google Scholar]
- 67.Beher D, Wu J, Cumine S, Kim KW, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 68.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 69.Huber JL, Mcburney MW, Distefano PS, Mcdonagh T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med Chem. 2010;2:1751–1759. doi: 10.4155/fmc.10.257. [DOI] [PubMed] [Google Scholar]
- 70.Kaeberlein M, Mcdonagh T, Heltweg B, Hixon J, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 71.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 73.Walle T, Hsieh F, Delegge MH, Oatis JE, Jr, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 74.Dai H, Kustigian L, Carney D, Case A, et al. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canto C, Jiang LQ, Deshmukh AS, Mataki C, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SJ, Ahmad F, Philp A, Baar K, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003;42:9249–9256. doi: 10.1021/bi034959l. [DOI] [PubMed] [Google Scholar]
- 79.Giammona LM, Panuganti S, Kemper JM, Apostolidis PA, et al. Mechanistic studies on the effects of nicotinamide on megakaryocytic polyploidization and the roles of NAD+ levels and SIRT inhibition. Exp Hematol. 2009;37:1340–1352. e1343. doi: 10.1016/j.exphem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 81.Vergnes B, Vanhille L, Ouaissi A, Sereno D. Stage-specific antileishmanial activity of an inhibitor of SIR2 histone deacetylase. Acta Trop. 2005;94:107–115. doi: 10.1016/j.actatropica.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Pagans S, Pedal A, North BJ, Kaehlcke K, et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira CV, Lebiedzinska M, Wieckowski MR, Oliveira PJ. Regulation and protection of mitochondrial physiology by sirtuins. Mitochondrion. 2012;12:66–76. doi: 10.1016/j.mito.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Chen L. Medicinal chemistry of sirtuin inhibitors. Curr Med Chem. 2011;18:1936–1946. doi: 10.2174/092986711795590057. [DOI] [PubMed] [Google Scholar]
- 85.Mahajan VLSS, Simon JA, Bedalov Antonio. Handbook of Experimental Pharmacology. In: Yao T-P, Seto E, editors. Histone Deacetylases: the Biology and Clinical Implication. 2001. pp. 241–255. [Google Scholar]
- 86.Bedalov A, Gatbonton T, Irvine WP, Gottschling DE, et al. Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci U S A. 2001;98:15113–15118. doi: 10.1073/pnas.261574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neugebauer RC, Uchiechowska U, Meier R, Hruby H, et al. Structure-activity studies on splitomicin derivatives as sirtuin inhibitors and computational prediction of binding mode. J Med Chem. 2008;51:1203–1213. doi: 10.1021/jm700972e. [DOI] [PubMed] [Google Scholar]
- 88.Biacsi R, Kumari D, Usdin K. SIRT1 inhibition alleviates gene silencing in Fragile X mental retardation syndrome. PLoS Genet. 2008;4:e1000017. doi: 10.1371/journal.pgen.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grozinger CM, Chao ED, Blackwell HE, Moazed D, et al. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- 90.Mai A, Massa S, Lavu S, Pezzi R, et al. Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors. J Med Chem. 2005;48:7789–7795. doi: 10.1021/jm050100l. [DOI] [PubMed] [Google Scholar]
- 91.Ota H, Tokunaga E, Chang K, Hikasa M, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 92.Jin KL, Park JY, Noh EJ, Hoe KL, et al. The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol. 2010;21:262–268. doi: 10.3802/jgo.2010.21.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kojima K, Ohhashi R, Fujita Y, Hamada N, et al. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun. 2008;373:423–428. doi: 10.1016/j.bbrc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 94.Peck B, Chen CY, Ho KK, Di Fruscia P, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 95.Alhazzazi TY, Kamarajan P, Joo N, Huang JY, et al. Sirtuin-3 (SIRT3), a novel potential therapeutic target for oral cancer. Cancer. 2011;117:1670–1678. doi: 10.1002/cncr.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Catoire H, Pasco MY, Abu-Baker A, Holbert S, et al. Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Hum Mol Genet. 2008;17:2108–2117. doi: 10.1093/hmg/ddn109. [DOI] [PubMed] [Google Scholar]
- 97.Pasco MY, Rotili D, Altucci L, Farina F, et al. Characterization of sirtuin inhibitors in nematodes expressing a muscular dystrophy protein reveals muscle cell and behavioral protection by specific sirtinol analogues. J Med Chem. 2010;53:1407–1411. doi: 10.1021/jm9013345. [DOI] [PubMed] [Google Scholar]
- 98.Orecchia A, Scarponi C, Di Felice F, Cesarini E, et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS One. 2011;6:e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He X, Nie H, Hong Y, Sheng C, et al. SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun. 2012;417:468–472. doi: 10.1016/j.bbrc.2011.11.141. [DOI] [PubMed] [Google Scholar]
- 100.Nie H, Chen H, Han J, Hong Y, et al. Silencing of SIRT2 induces cell death and a decrease in the intracellular ATP level of PC12 cells. Int J Physiol Pathophysiol Pharmacol. 2011;3:65–70. [PMC free article] [PubMed] [Google Scholar]
- 101.Luthi-Carter R, Taylor DM, Pallos J, Lambert E, et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heltweg B, Gatbonton T, Schuler AD, Posakony J, et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006;66:4368–4377. doi: 10.1158/0008-5472.CAN-05-3617. [DOI] [PubMed] [Google Scholar]
- 103.Medda F, Russell RJ, Higgins M, Mccarthy AR, et al. Novel cambinol analogs as sirtuin inhibitors: synthesis, biological evaluation, and rationalization of activity. J Med Chem. 2009;52:2673–2682. doi: 10.1021/jm8014298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuetz A, Min J, Antoshenko T, Wang CL, et al. Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure. 2007;15:377–389. doi: 10.1016/j.str.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Trapp J, Meier R, Hongwiset D, Kassack MU, et al. Structure-activity studies on suramin analogues as inhibitors of NAD+-dependent histone deacetylases (sirtuins) ChemMedChem. 2007;2:1419–1431. doi: 10.1002/cmdc.200700003. [DOI] [PubMed] [Google Scholar]
- 106.Perabo FG, Muller SC. New agents in intravesical chemotherapy of superficial bladder cancer. Scand J Urol Nephrol. 2005;39:108–116. doi: 10.1080/00365590510007676. [DOI] [PubMed] [Google Scholar]
- 107.Lain S, Hollick JJ, Campbell J, Staples OD, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13:454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan H, Wang Z, Li L, Zhang H, et al. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119:1904–1914. doi: 10.1182/blood-2011-06-361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mccarthy AR, Pirrie L, Hollick JJ, Ronseaux S, et al. Synthesis and biological characterisation of sirtuin inhibitors based on the tenovins. Bioorg Med Chem. 2012;20:1779–1793. doi: 10.1016/j.bmc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Lara E, Mai A, Calvanese V, Altucci L, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009;28:781–791. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 111.Liu G, Su L, Hao X, Zhong N, et al. Salermide upregulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]