Abstract
The contribution of orosensory signals, especially taste, on body mass, and feeding and drinking patterns in the rat was examined. Gustatory deafferentation was produced by bilateral transection of the chorda tympani, glossopharyngeal, and greater superficial petrosal nerves. Total calories consumed from sweetened-milk diet and oil-chow mash by the nerve-transected rats significantly decreased relative to sham-operated controls, mostly attributable to decreases in bout number, but not size. Nevertheless, caloric intake steadily increased over the postsurgical observation period, but body mass remained below both presurgical baseline and control levels and did not significantly increase over this time. After the sweetened-milk diet/oil-chow mash phase, rats received a series of sucrose preference tests. Interestingly, the nerve-transected rats preferred sucrose, and intake did not differ from controls, likely due to the stimulus sharing some nontaste chemosensory properties with the sweetened-milk diet. The neurotomized rats initiated a greater number of sucrose-licking bouts that were smaller in size and slower in licking rate, compared with control rats, and, unlike in control rats, the latter two bout parameters did not vary across concentration. Thus, in the absence of gustatory neural input, body mass is more stable compared with the progressive trajectory of weight gain seen in intact rats, and caloric intake initially decreases but recovers. The consequences of gustatory neurotomy on processes that determine meal initiation (bout number) and meal termination (bout size) are not fixed and appear to be influenced by presurgical experience with food stimuli coupled with its nongustatory chemosensory properties.
Keywords: ageusia, body mass regulation, taste loss, taste nerves
decades of research have shown that nutritional intake contributes to the development of human disease mainly by influencing the development of obesity and its associated conditions (e.g., cardiovascular disease, hypertension, Type 2 diabetes mellitus) [e.g., (8, 28, 35)]. Collectively, these studies suggest that a majority of obesity-related diseases could be prevented by the adoption of healthier eating habits. In the act of feeding, specialized chemosensory, thermosensory, and mechanosensory receptors, positioned in the nasal and oropharyngeal cavities, provide information to the brain related to the macronutrient composition, caloric density, osmolarity, and potential toxicity of food ingested (1, 43, 49, 76). Chief among these neural signals are those provided by the gustatory system, which is the final guardian of the alimentary tract and provides a chemical analysis of stimulus components that do not typically have sufficiently high vapor pressures to effectively stimulate olfactory receptors and do not appreciably stimulate mechanoreceptors in a selective fashion. It is widely believed that these orosensory inputs, especially taste, integrate with other neural and endocrine signals related to the energy and hydromineral status of the animal to ultimately influence central neural circuits controlling food and fluid intake.
Orosensory inputs, particularly signals of gustatory origin, have been implicated in the control of meal size. For example, it is well known that when postingestive inputs to the central nervous system (CNS) are substantially reduced, which can be accomplished via the use of sham feeding methodology, meal size increases considerably (e.g., 14, 46, 50). However, it is unknown what would happen to meal size and/or meal number in the absence of gustatory neural input. Thus, it is unclear whether taste input is necessary for normal feeding patterns to be observed and for the normal maintenance of body mass. Indeed, if total intake is reduced by experimentally induced ageusia, it remains unclear whether this is due to disruption in processes that terminate meals or those that initiate them or both.
Despite the large body of evidence that indicates taste can influence nutritional intake, research demonstrating a definitive role for the gustatory system in the maintenance of body mass has been sparse (16). Here, we sought to determine how combined transection of the chorda tympani nerve (CT), innervating the taste buds of the anterior tongue, the greater superficial petrosal nerve (GSP), innervating the taste buds of the palate, and the glossopharyngeal nerve (GL), innervating the taste buds of the posterior tongue, would affect feeding and body mass regulation. These nerve transections effectively remove the input from ∼90% of the taste receptor cells, which, in turn, degenerate after nerve transection (e.g., 41, 59). The remaining taste buds are found in the laryngeal epithelium (67), innervated by the superior laryngeal nerve, and are thought to be more involved in the protection of the airways (53). We reasoned that the functional consequences of this massive elimination of gustatory input would allow us to reveal whether taste signals were necessary for the maintenance of normal feeding patterns and body mass regulation.
METHODS
Subjects
Twenty-five male Sprague-Dawley rats (Charles River Breeders, Wilmington, MA) with a mean ± SE weight of 416.1 ± 6.7 g at the time of surgery, were individually housed in polycarbonate cages in a room with automatically controlled temperature (21.9–22.6°C), humidity (34–69%), and a 12:12-h dark-light cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committees at the University of Florida and at the Florida State University.
Surgery
Rats were anesthetized with a mixture of ketamine hydrochloride (125 mg/kg body wt) and xylazine hydrochloride (5 mg/kg body wt) delivered via intramuscular injection. Supplemental doses were administered as necessary. Rats received one of the following surgeries: combined bilateral transection of the CT, GSP, and GL (TRIPLEx); bilateral transection of the CT and exposure of the GSP (CTx); and combined bilateral exposure of both the CT and GL (sham group). The GL was exposed after an incision was made in the ventral neck and the sublingual and submaxillary salivary glands, the omohyoid, sternohyoid, and posterior belly of the digastric muscles were all retracted revealing the nerve running medial to the external wall of the bulla. In animals receiving sham surgery, the nerve was left uncut, and in the TRIPLEx animals ∼8–10 mm of the nerve was removed on both sides. The incision was then closed with nylon suture. In the TRIPLEx rats, an incision was also made dorsal to the pinna, transecting the external ear canal, which was retracted laterally. The muscles overlying the bone were blunt-dissected and the bony meatus was enlarged with a dental drill. The tympanic membrane and the middle ear ossicles were removed as well as a large section of the CT. The tensor tympani muscle was then avulsed exposing a ridge of temporal bone, a small piece of which was removed with microforceps revealing the GSP, which was then transected and the ends cauterized. Wound clips were used to close the incision. In animals receiving sham surgery, the pinna and ear canal were transected, and the muscle surrounding the bony meatus was blunt dissected and retracted, and the tympanic membrane was punctured. For animals in the CTx group, only the tympanic membrane, the middle ear ossicles, and a large section of the CT were removed. The inclusion of the CTx group, in addition to serving as an additional surgical trauma control group, helped to control for any putative effects of the loss of parasympathetic efferents to the sublingual and submaxillary glands on body mass. For 3 days following surgery, the animals received daily injections of penicillin G Procaine suspension (30,000 units sc) and Ketorolac Tromethamine (2 mg/kg body wt sc).
Behavioral Procedure
Body mass measurements were taken daily in TRIPLEx, sham, and CTx rats. TRIPLEx or sham group rats were placed in specially designed cages described elsewhere (55) with some modifications. These cages allowed continuous monitoring of chow and fluid ingestion in 6-s time bins over 23-h daily test sessions. Powdered laboratory chow (Purina Laboratory Chow 5001, St. Louis, MO) was provided ad libitum in a food jar placed in the feeding compartment of the cage. When the rat entered the feeding area, its head interrupted an infrared beam, and these beam-break signals were recorded by a computer. On the other side of each cage was a stainless-steel rack that held two fluid bottles. Licks on the drinking spouts activated an electronic contact circuit which was registered by a computer. The CTx rats were included in the design as a body mass control group and thus were not maintained in the testing apparatus, but had access to the same foods and fluids as the other two groups. Because there were a limited number of cages in the feeding and drinking monitor, all of the animals in the groups could not be tested at the same time, but rather, the testing was conducted in two phases with animals from both the sham and TRIPLEx groups included in each phase. All of the rats in the CTx group were tested in the second phase.
Intake of powdered food and water (deionized reverse osmosis) from the two bottles were measured for 4 days (Table 1). After this period, oil-chow mash (2 parts Canola corn oil + 5 parts powdered chow) was introduced in place of the powdered chow. After 1 wk of experience with the oil-chow mash, water in one of the fluid bottles was replaced with a sweetened-milk diet [1 part sweetened condensed milk (Borden's) + 2 parts water + 1 ml multiple vitamins (Polyvisol)], prepared fresh daily. Both nutritional regimens, the oil-chow and the milk diet, were chosen because they are lubricated, and thus, their consumption was less likely to be affected by any postsurgical complications associated with potential decreases in salivation. Moreover, these are highly palatable foods and have been shown to be effective at motivating rats to eat, as well as at maintaining the health of nerve-transected rats (e.g., 5, 24, 60, 63). The bottles were rinsed daily and then refilled with fresh solutions. The position of the bottles was switched every 24 h. On day 20 or 21 of the experiment, rats had surgery. Animals were fed the oil-chow and sweetened-milk diet for 24 days postsurgery before being moved back to oil-chow and water access for 4 days.
Table 1.
Solid food and fluids available
| Test Day | Food Available | Fluid (Other Than Water*) Available |
|---|---|---|
| 1–4 | Powdered chow | Nothing |
| 5–11 | chow + oil | Nothing |
| 12–44 | chow + oil | Sweetened-milk diet |
| 45–46 | chow + oil | Nothing |
| 47–48 | chow + oil | Water |
| 49–50 | chow + oil | 0.06 M Sucrose |
| 51–52 | chow + oil | 0.125 M Sucrose |
| 53–54 | chow + oil | 0.25 M Sucrose |
| 55–56 | chow + oil | 0.5 M Sucrose |
| 57–58 | chow + oil | 1.0 M Sucrose |
Water in one bottle was always available.
After day 48, rats were then tested for sucrose preference using a two-bottle test across an ascending series of concentrations of sucrose vs. water in two consecutive 23-h tests per concentration. During this phase, rats also had ad libitum access to oil-chow mash. The position of the bottles was switched every 24 h. Sucrose was presented across the days in ascending order of concentration (0.0625, 0.125, 0.25, 0.5, and 1.0 M sucrose; Sigma Aldrich, St. Louis, MO).
Data Analysis
Licks on the drinking spout and photobeam interruptions at the food jar were measured in consecutive 6-s bins. Thus, in a 23-h period, there were 13,800 bins from which to reconstruct the drinking and feeding patterns of each rat with the aid of customized software. A drinking bout (for the sweetened-milk diet and sucrose) was defined as starting with three licks in a 6-s bin and ending when 50 6-s bins (i.e., 5 min) had passed with no licking; a drinking bout had to contain a minimum of 10 licks to be included in the analysis. A feeding bout was defined as starting with a 3-s photobeam interruption in a 6-s bin and ending with 50 6-s bins (5 min) without beam breaks; a feeding bout had to last 10 s to be included in the analysis. Similar bout criteria have been successfully used in previous studies (2, 11, 19, 55, 68). Estimated fluid spillage, based on the measurement from bottles placed on empty cages, was subtracted from the intake values. We did not detect any significant spillage of food.
Caloric intake for the oil-chow mash was calculated by multiplying the amount consumed by 4.97 kcal/g. This value was based on both the oil (9 kcal/g) and chow (3.36 kcal/g; physiological fuel value) caloric contribution relative to the mixture proportions of the oil-chow mash (i.e., 2 parts oil, 5 parts chow). Caloric intake for the sweetened-milk diet was calculated by multiplying adjusted amount consumed by 1.1 kcal/g. This value was derived by adjusting the caloric value of the sweetened condensed milk (3.3 kcal/g) relative to the mixture proportions of the sweetened-milk diet (1 part sweetened condensed milk, 2 parts water). For the sweetened-milk diet and sucrose, to calculate bout size, we divided total intake by total licks to get an ml/lick value. We then converted each bout size from licks to milliliters. Food bout size was calculated by taking total intake and dividing by total beam break time to get a gram per second value and then multiplying this value by the beam break time of a given bout. The rate of ingestion during bouts was derived by dividing bout size by bout duration. Only bouts that lasted ≥1 min were included in the analysis of bout rate.
Body mass, calories ingested, as well as food intake pattern variables (e.g., bout number and bout size), were measured during the entire presurgical period and for 3 wk postsurgically. Presurgical baselines for the various measures were operationally defined as the mean dependent values 2 days before surgery for each rat and were compared across groups with the use of t-tests (see Table 2). The effects of surgery were compared by subtracting the postsurgical values for a given variable from the presurgical baseline value for each animal, resulting in a change from presurgical baseline score. This controlled for minor individual differences in the presurgical value for the variables. The mean change from presurgical baseline scores for a given variable were analyzed by performing linear regression for each surgical group over the 21-day postsurgical observation period. This produced a y-intercept that represented the initial effect of the surgery and slope that represented the change in the scores over postsurgical days. Additionally, the initial impact of surgery was also assessed between the groups through the use of t-tests that compared the mean scores for a given variable averaged over the first two postsurgical days. Recovery from surgery was assessed in a similar fashion, except the scores from the last 2 days of the postsurgical observation period were averaged and analyzed.
Table 2.
Body mass, intake, and microstructural data analyses
| Measure | Mean of the Last Two Presurgical Days (i.e., Presurgical Baseline) |
Mean Change from Presurgical Baseline on the First Two Postsurgical Days |
Mean Change from Presurgical Baseline on the Last Two Postsurgical Days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | TRIPLEx | CTx | P value | Sham | TRIPLEx | CTx | P value | Sham | TRIPLEx | CTx | P value | |
| Caloric intake | ||||||||||||
| Total calories | 127.64 ± 13.11 | 130.33 ± 9.25 | .87 | −101.52 ± 11.35 | −58.67 ± 11.82 | 0.02 | −14.54 ± 9.14 | −42.01 ± 11.99 | 0.09 | |||
| Milk diet calories | 71.09 ± 9.75 | 80.24 ± 8.03 | 0.48 | −19.24 ± 6.61 | −52.12 ± 7.76 | 0.007 | 15.45 ± 6.33 | −11.97 ± 10.41 | 0.04 | |||
| Oil-chow diet calories | 54.51 ± 11.03 | 50.87 ± 7.83 | 0.79 | −37.39 ± 12.51 | −50.13 ± 7.67 | 0.40 | −27.95 ± 10.18 | −30.82 ± 7.368 | 0.82 | |||
| Meal patterns | ||||||||||||
| Milk diet bout # | 14.14 ± 1.89 | 16.86 ± 1.40 | 0.27 | −3.43 ± 1.16 | −8.79 ± 1.84 | 0.03 | −2.93 ± 1.66 | −6.79 ± 0.93 | 0.066 | |||
| Milk diet bout size | 4.57 ± 0.41 | 4.01 ± 0.53 | 0.42 | −0.45 ± 0.44 | −0.94 ± 0.60 | 0.52 | 1.89 ± 0.48 | 2.03 ± 0.70 | 0.88 | |||
| Milk diet bout rate | 1.89 ± 0.14 | 1.65 ± 0.10 | 0.20 | −0.12 ± 0.09 | −0.11 ± 0.21 | 0.96 | 0.31 ± 0.09 | −0.15 ± 0.12 | 0.009 | |||
| Oil-chow diet bout # | 13.36 ± 1.37 | 14.14 ± 1.16 | 0.67 | −5.50 ± 1.37 | −10.50 ± 1.64 | 0.04 | −4.29 ± 1.67 | −8.00 ± 2.03 | 0.18 | |||
| Oil-chow diet bout size | 0.69 ± 0.12 | 0.63 ± 0.11 | 0.69 | −0.43 ± 0.12 | −0.59 ± 0.10 | 0.34 | −0.16 ± 0.13 | 0.13 ± 0.11 | 0.12 | |||
| Oil-chow diet bout rate | 0.25 ± 0.03 | 0.31 ± 0.03 | 0.18 | −0.07 ± 0.04 | −0.19 ± 0.06 | 0.12 | −0.04 ± 0.03 | −0.12 ± 0.05 | 0.18 | |||
| Body mass | 415.86 ± 11.85 | 415.86 ± 11.85 | 419.75 ± 8.39 | 0.95 | −2.00 ± 3.22 | −25.50 ± 7.35 | −10.25 ± 1.72 | 0.007* | 77.21 ± 9.12 | −29.07 ± 5.24 | 79.50 ± 7.85 | < 0.00001# |
Values are expressed as means ± SE.
Post hoc tests revealed that the TRIPLEx group differed from both the sham and CTx groups (both P values <0.027) and that the sham and CTx groups did not differ from one another (P = 0.21). #Post hoc tests revealed that the TRIPLEx group differed from both the sham and CTx groups (both P values <0.0001) and that the sham and CTx groups did not differ from one another (P = 0.83).
All variables from the sucrose phase of the experiment were compared using two-way ANOVAs. Post hoc analyses were conducted using t-tests and by one-way or two-way ANOVA. For all tests conducted during this experiment, P ≤ 0.05 was set as the statistical rejection criterion. Bonferroni adjustments were also performed to correct for multiple related comparisons.
One rat in the TRIPLEx group died shortly after surgery. Data from one other TRIPLEx rat had to be excluded from the analysis because of technical problems with its cage, precluding reliable measurement of behavior. We also excluded one CTx rat from the analysis of body mass because histological analysis was not conducted for this animal, but it should be noted that its trajectory of body mass gain over the course of the experiment was consistent with that of the other rats in the group. Thus, the final sample sizes, for all experiments, were as follows: TRIPLEx (n = 7), sham (n = 7), and CTx (n = 8) rats.
Histology
At the completion of behavioral testing, the rats were deeply anesthetized with pentobarbital sodium and transcardially perfused with saline followed by 10% buffered formalin. The tongue, soft palate, and nasoincisor ducts (NID) were extracted and stored in 10% buffered formalin. The anterior two-thirds of the tongue was soaked briefly in 0.5% methylene blue and then rinsed in a purified water bath. The epithelium was stripped from the underlying muscle and connective tissue and placed between two glass slides for inspection using a standard light microscope. The total number of pale staining fungiform papillae was counted as were those that possessed a taste pore indicated by a dark blue dot. The circumvallate papilla of the posterior tongue and the NID were embedded in paraffin and sectioned (10 μm) on a rotary microtome, mounted, stained with hematoxylin and eosin, and coverslipped. All tissues were coded so that taste bud counts could be conducted by an experimenter who was blind to the exact surgical treatment of a given animal.
RESULTS
Histology
The histological results for the groups are summarized in Table 3. Rats in the TRIPLEx group had very few, if any, remaining taste buds in the representative taste fields examined from the oral cavity when compared with the sham group [anterior taste pores: t(12) = 36.13, P < 0.0001; circumvallate papillae: insufficient variance for test; NID: t(12) = 10.60, P < 0.0001]. The rats in the CTx group had normal numbers of taste buds in the circumvallate papillae (P = 0.25), innervated by the GL, and in the NID (P = 0.73), innervated by the GSP, but had very few intact taste pores in the fungiform papillae. The CTx and TRIPLEx did not differ in either the number of intact taste pores (P = 0.13) or the number of intact fungiform papillae (P = 0.68) found on the anterior tongue, but both groups significantly differed from the sham group (all P < 0.0001). These results indicate that the nerve transections were successful and that no significant regeneration occurred.
Table 3.
Histology results
| Group | Pores in Anterior Tongue | Number of Intact Fungiform Papillae | % Intact Fungiform Papillae Containing Pore | Number of Taste Buds in CV | Number of Taste Buds in NID |
|---|---|---|---|---|---|
| Sham | 148.2 ± 4.05 | 156.1 ± 4.03 | 94.9 ± 0.95 | 374.8 ± 10.21 | 108.8 ± 10.60 |
| CTx | 9.0 ± 3.60 | 88.3 ± 7.76 | 8.8 ± 3.15 | 399.5 ± 18.86 | 113.9 ± 9.84 |
| TRIPLEx | 3.5 ± 1.12 | 84.0 ± 6.59 | 4.1 ± 1.14 | 0.0 ± 0.00 | 2.1 ± 0.88 |
Values are expressed as means ± SE.
Body Mass
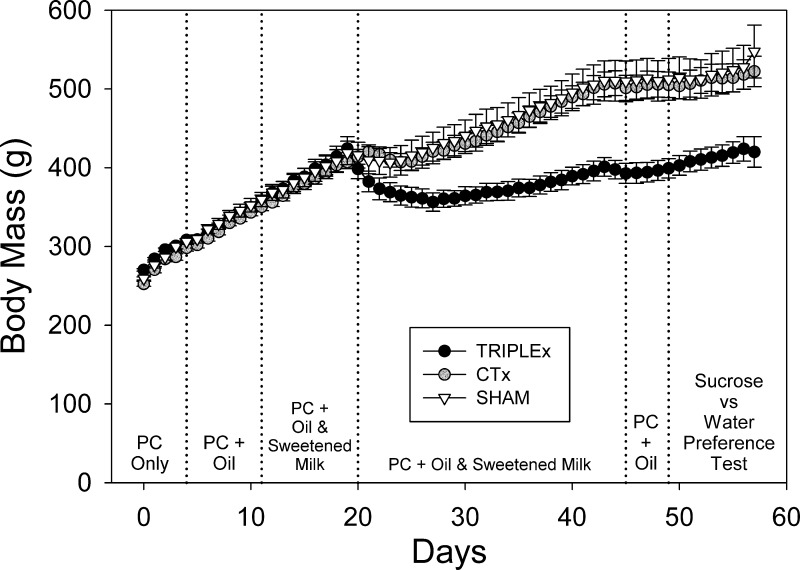
A two-way ANOVA (day × group) revealed that the groups did not differ significantly in regard to body mass during the week before surgery (P = 0.74) and that there was no interaction (P = 0.68; Table 2). However, after surgery, mean body mass values were significantly lower in TRIPLEx rats compared with both sham and CTx rats (Fig. 1). A two-way ANOVA revealed a main group effect for body mass [F(2, 19) = 9.05, P = 0.0017], an effect of postsurgical day [F(36, 684) = 135.0, P < 0.0001], as well as a significant interaction [F(72, 684) = 10.09, P < 0.0001; Fig. 1].
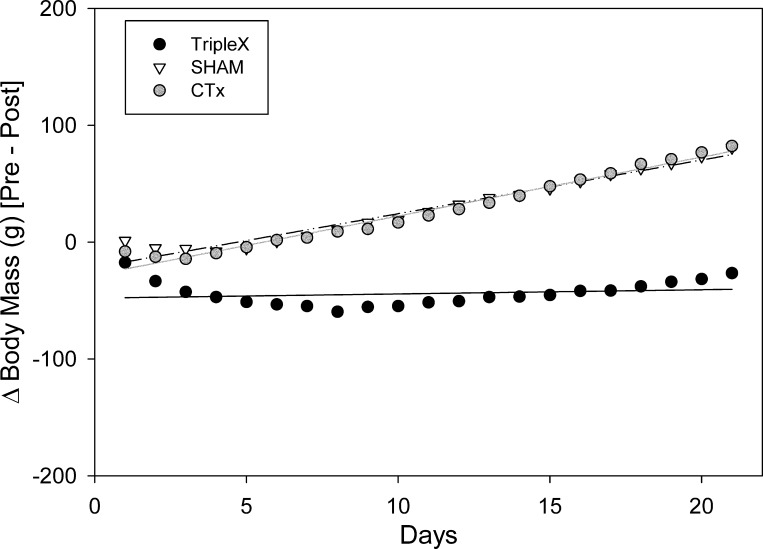
Fig. 1.
Body mass values assessed for sham, CTx, and TRIPLEx groups measured over the entire pre- and post surgical period (means ± SE).
A separate two-way ANOVA revealed that the sham and CTx groups did not differ from one another in body mass (P = 0.82). We did observe an effect of postsurgical day [F(36, 468) = 148.66, P < 0.0001; Bonferroni-adjusted α = 0.0167], but there was no significant interaction (P = 0.99). These results indicate that the main group effect observed in the plenary ANOVA were driven by the sustained reduction in body mass exhibited in the TRIPLEx rats, which significantly differed from the other groups [sham: F(1, 13) = 12.57, P = 0.004; CTx: F(1, 14) = 21.05, P = 0.0005; Bonferroni-adjusted α = 0.0167]. Indeed, TRIPLEx rats never reached sham body mass levels. Moreover, these animals never recovered presurgical baseline body mass over the entire course of the postsurgical experimental time frame (>5 wk). In fact, we fit a line to the mean body mass values for postsurgical days 10 through 25, when the same presurgical diets were offered (i.e., both the sweetened-milk and oil-chow diets) and when the rate of weight gain appeared to be relatively stable and found that the rate of gain (i.e., the slope) was 2.65 g/day (P < 0.0001) for TRIPLEx rats and was 5.32 g/day (P < 0.0001) for sham rats. At these trajectories, which appeared to be stable over the 16-day observation period, in three additional month's time, the predicted difference in body weight between the two groups would be a striking 359 g. Obviously, this observation is predicated on the assumption that these rates would be maintained over the 3-mo period.
Caloric Intake
Total calories.
The loss in body mass in the TRIPLEx group resulted, at least in part, from the fact that the animals consumed fewer calories than they did presurgically. Presurgical baseline caloric intake did not differ between the TRIPLEx and sham groups (P = 0.87; Table 2). Results from the analysis of linear regression fits of the change in caloric intake relative to presurgical baseline across postsurgical days (Fig. 2) revealed that surgery had an initial impact in both groups, as estimated by the y-intercepts (b) which significantly differed from 0 [sham: b = −46.7 kcal, t(19) = −12.8, P < 0.0001; TRIPLEx: b = −96.1 kcal, t(19) = −20.9, P < 0.0001]. Nevertheless, it is clear that the initial drop in intake was greater in the TRIPLEx group compared with the sham rats as confirmed by a t-test of the mean change total caloric intake on the first two postsurgical days relative to presurgical baseline [t(12) = 2.62, P = 0.023; Table 2].
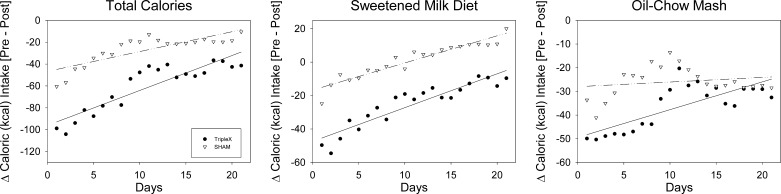
Fig. 2.
Daily total calories (left), calories from the sweetened-milk diet (middle), and calories from oil-chow mash (right) ingested by sham and TRIPLEx groups measured for 3 wk postsurgery.
The slopes (m) from the linear regression fit of change in total caloric intake relative to presurgical baseline across postsurgical days (Fig. 2) had a significant rise in both the sham and TRIPLEx groups [sham: m = 1.82 kcal/day, t(19) = 6.25, P < 0.0001; TRIPLEx: m = 3.20 kcal/day, t(19) = 8.75, P < 0.0001]. A t-test conducted of the mean values of the change in total caloric intake relative to baseline on postsurgical days 20 and 21 indicated that the significant difference between the groups that was present initially after surgery had disappeared (P = 0.09), although the TRIPLEx rats did appear to be lagging behind the sham group in their recovery of caloric intake (Table 2).
Percentage of calories ingested between the two diets.
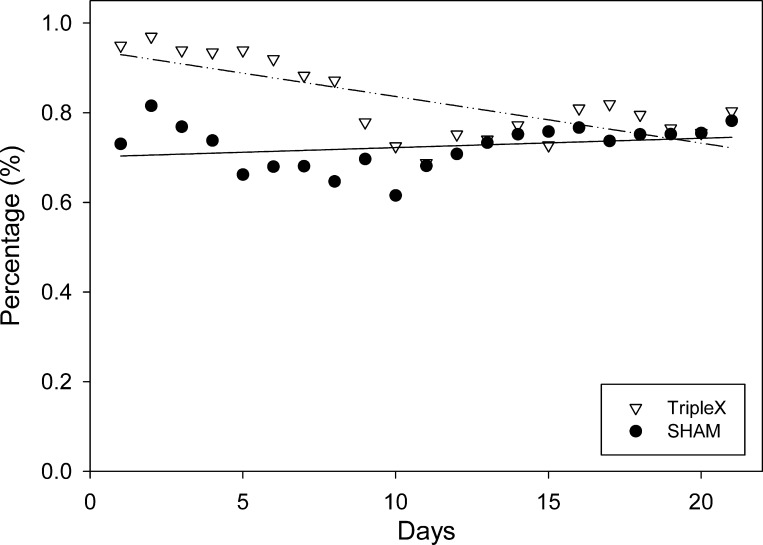
An inspection of Fig. 3 suggests that the majority of caloric intake in both groups came from the sweetened-milk diet postsurgically. For each group, more than 50% of total caloric intake came from the milk diet, presurgically, and this percentage did not differ between the groups (P = 0.49; TRIPLEx = 0.62%, sham = 0.56%). After surgery, this preference for the sweetened-milk diet increased considerably. Mean total caloric intake coming from the milk diet averaged across all 21 days, increased significantly in both groups, relative to presurgical baseline (both P < 0.02; TRIPLEx = 83%, sham = 72%).
Fig. 3.
The percentage of total calories ingested from the sweetened-milk diet measured for 3 wk postsurgery.
Calories from the sweetened-milk diet.
Presurgical baseline caloric intake from the sweetened-milk diet did not differ between the TRIPLEx and sham groups (P = 0.48; Table 2). Again, the linear regression analysis revealed that although surgery had an initial impact by decreasing caloric intake from sweetened-milk diet in both groups (Fig. 2), the drop was almost 3 times greater in the TRIPLEx animals [sham: b = −16.7 kcal, t(19) = −10.3, P < 0.0001; TRIPLEx: b = −47.45 kcal, t(19) = −19.5, P < 0.0001]. A t-test conducted on the mean change on the first two postsurgical days relative to presurgical baseline confirmed that the initial drop in caloric intake from the sweetened-milk diet was significantly greater in TRIPLEx rats relative to controls [t(12) = 3.23, P = 0.007; Table 2].
The slopes from the linear regression fit of the change in the sweetened-milk diet caloric intake relative to baseline across postsurgical days (Fig. 2) had a significant rise in both the sham and TRIPLEx groups [sham: m = 1.62 kcal/day, t(19) = 12.65, P < 0.0001; TRIPLEx: m = 2.02 kcal/day, t(19) = 10.44, P < 0.0001]. Despite the fact that sweetened-milk diet intake progressively increased across the postsurgical test period for both groups, the TRIPLEx rats still significantly differed from the sham rats in terms of their recovery of sweetened-milk diet caloric intake on postsurgical days 20 and 21 [t(12) = 2.25, P = 0.04; Table 2].
Calories from the oil-chow diet.
Presurgical baseline caloric intake from the oil-chow mash did not differ between the TRIPLEx and sham groups (P = 0.79; Table 2). The linear regression analysis of the change in caloric intake from the oil-chow mash relative to baseline across postsurgical days (Fig. 2) revealed that the y-intercepts were significantly lower than 0 in both groups [sham: b = −28.0 kcal, t(19) = −9.62, P < 0.0001; TRIPLEx: b = −49.43 kcal, t(19) = −17.4, P < 0.0001]. Although this change in oil-chow caloric intake appeared to be greater in TRIPLEx rats relative to that observed in the sham rats, a t-test run on mean values from postsurgical days 1 and 2 indicated that the groups did not significantly differ (P = 0.40; Table 2).
The postsurgical caloric intake from oil-chow significantly increased in the TRIPLEx group [m = 1.17 kcal/day, t(19) = 5.19, P < 0.0001] but stayed relatively stable in the sham rats (P = 0.41). The mean change in caloric intake from oil-chow on postsurgical days 20 and 21 relative to baseline did not significantly differ between the groups (P = 0.82; Table 2). Thus, surgery had an initial impact in both groups, and although statistically speaking, it was not more severe in the TRIPLEx rats than the sham rats, the former group, nonetheless, did statistically increase its oil-chow intake at a greater rate across the 3-wk postsurgical observation period.
Sweetened-Milk Diet Meal Patterns
Bout size.
The size of milk bouts taken by the animals presurgically did not differ between the TRIPLEx and sham groups (P = 0.42; Table 2). The analysis of the linear regression fits of the change in milk bout size relative to baseline by postsurgical day (Fig. 4) indicated that there was a significant decrease initially for both groups [sham: b = −0.73 ml, t(19) = −4.58, P = 0.0002; TRIPLEx: b = −0.96 ml, t(19) = −4.84, P = 0.0001]. A t-test conducted on the mean change in milk bout size relative to baseline comparing the two groups on days 1 and 2 was not significant (P = 0.52), indicating that the nerve transections had no additional impact on bout size relative to sham surgery (Table 2).
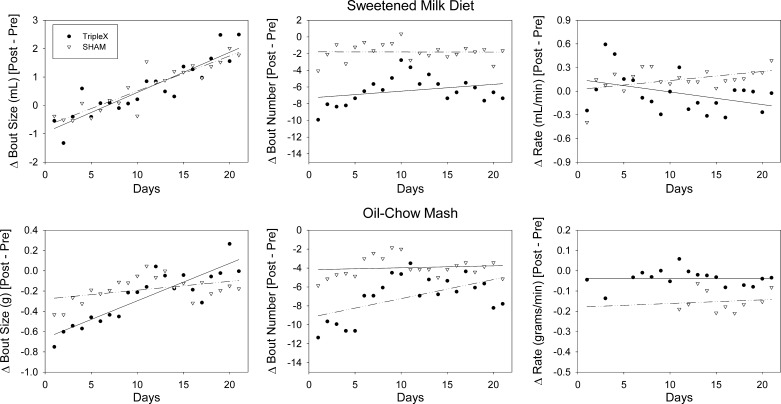
Fig. 4.
Daily bout size (left), bout number (middle), and bout rate (right) when ingesting the sweetened-milk diet (top) and the oil-chow mash (bottom) by sham and TRIPLEx groups measured for 3 wk postsurgery.
Additionally, results from this analysis revealed that milk bout size significantly increased across postsurgical days [sham: m = 0.12 ml/day, t(19) = 9.71, P < 0.0001; TRIPLEx: b = 0.14 ml/day, t(19) = 8.96, P < 0.0001]. The mean change in milk bout size on postsurgical days 20 and 21 relative to baseline did not significantly differ between the groups (P = 0.88; Table 2).
Bout number.
The number of sweetened-milk diet bouts initiated by the animals presurgically did not differ between the TRIPLEx and sham groups (P = 0.27). The analysis of the linear regression fits of the change in milk bout number relative to baseline by postsurgical day (Fig. 4) indicated that there was a significant decrease initially for both groups [sham: b = −1.79, t(19) = −3.76, P = 0.001; TRIPLEx: b = −7.32, t(19) = −9.90, P < 0.0001]. A t-test conducted on the means from the first 2 days postsurgically confirmed that the initial drop in the number of sweetened-milk diet bouts was significantly greater in TRIPLEx rats relative to controls [t(12) = 2.46, P = 0.03; Table 2].
Interestingly, the postsurgical decrease in milk bout number was relatively stable in both groups as indicated by slopes that did not significantly differ from 0 (sham: P = 0.98; TRIPLEx: P = 0.18). However, a t-test conducted on the mean change in milk bout number relative to baseline indicated that the greater decrease initially observed for the TRIPLEx rats compared with the sham rats just missed statistical significance on postsurgical days 20 and 21 (P = 0.066; Table 2).
Bout rate.
The rate of the sweetened-milk diet ingestion within bouts did not appear to be affected by surgery. The presurgical baseline milk bout ingestion rate did not differ between the TRIPLEx and sham groups (P = 0.20; Table 2), and there was no significant change from baseline milk bout ingestion rate after surgery, as indicated by the fact that the y-intercepts from the linear regression analysis (Fig. 4) did not significantly differ from 0 (sham: P = 0.82; TRIPLEx: P = 0.56). Likewise, a t-test conducted on the mean change in milk bout ingestion rate on postsurgical days 1 and 2 relative to baseline did not detect any significant difference between the groups (P = 0.96; Table 2).
The slopes of the linear regression fits of the change in milk bout ingestion rate by postsurgical day did not significantly differ from 0 in either the sham or the TRIPLEx group (sham: P = 0.05; TRIPLEx: P = 0.21; Fig. 4), suggesting that bout ingestion rate remained relatively stable. However, the mean change in milk bout ingestion rate relative to baseline on postsurgical days 20 and 21 was significantly lower in the TRIPLEx group compared with controls [t(12) = 3.11, P = 0.009; Table 2].
Oil-Chow Diet Meal Patterns
Bout size.
The size of oil-chow bouts taken by the animals presurgically did not differ between the TRIPLEx and sham groups (P = 0.69; Table 2). The analysis of the linear regression fits of the change in oil-chow bout size by postsurgical day (Fig. 4) indicated that there was a significant decrease initially for both groups [sham: b = −0.24 g, t(19) = −3.13, P = 0.008; TRIPLEx: b = −0.59 g, t(19) = −6.71, P < 0.0001]. However, a t-test did not detect any significant group difference in the mean change in oil-chow bout size based on the first two postsurgical days [t(12) = 1.00, P = 0.34; Table 2].
Although the TRIPLEx group progressively increased oil-chow bout size [m = 0.03 g, t(19) = 5.11, P = 0.0002] across the postsurgical observation period and the sham group did not (P = 0.27), there was also no difference between the groups in the mean change in bout size on postsurgical days 20 and 21 relative to baseline (P = 0.12; Table 2).
Bout number.
Presurgical baseline oil-chow bout number did not differ between the TRIPLEx and sham groups (P = 0.67: Table 2). The analysis of the linear regression fits of the change in oil-chow bout number relative to baseline by postsurgical day (Fig. 4) indicated that there was a significant decrease initially for both groups [sham: b = −4.18, t(19) = −8.52, P < 0.0001; TRIPLEx: b = −9.25, t(19) = −10.4, P < 0.0001]. The initial drop in bout number was about twice as great in the TRIPLEx rats compared with sham controls as assessed on postsurgical days 1 and 2 [t(12) = 2.342, P = 0.037: Table 2].
Furthermore, the results from the linear regression analysis revealed that oil-chow bout number significantly increased across postsurgical days in the TRIPLEx group [m = 0.20, t(19) = 2.85, P = 0.01] but was relatively stable in the sham controls (P = 0.60). Accordingly, there was also no difference between the groups in the mean change in bout number on postsurgical days 20 and 21 relative to baseline (P = 0.18; Table 2).
Bout rate.
Presurgical baseline oil-chow ingestion rate did not differ between the TRIPLEx and sham groups (P = 0.18), and there was no significant change from baseline oil-chow ingestion rate after surgery as indicated by the fact that the y-intercepts from the linear regression analysis (Fig. 4) did not significantly differ from 0 (sham: P = 0.15; TRIPLEx: P = 0.08). This was confirmed by a lack of difference between the groups in the postsurgical change in oil-chow ingestion rate, as assessed on postsurgical days 1 and 2 (P = 0.12). Furthermore, the slopes of the linear regression fits of the change in oil-chow ingestion rate relative to baseline by postsurgical day did not significantly differ from 0 in either the sham or the TRIPLEx group (sham: P = 0.99; TRIPLEx: P = 0.75), suggesting that, like what was observed with the milk diet, ingestion rate remained relatively stable. There was also no difference between the groups in the postsurgical change in oil-chow ingestion rate relative to baseline as assessed on postsurgical days 20 and 21 (P = 0.18).
Body Mass Regression Analysis
To facilitate comparison of the body mass and microstructural data analyses, we conducted a regression analysis of body mass on the same 21 postsurgical days for which microstructural data were collected. Presurgical baseline body mass values did not differ between the groups (P = 0.95; Table 2). The analysis of the linear regression fits of the change in body mass relative to baseline by postsurgical day (Fig. 5) indicated that there was a significant decrease initially for all groups [sham: b = −21.6 g, t(19) = −8.19, P < 0.0001; CTx: b = −28.1 g, t(19) = −12.3, P < 0.0001; TRIPLEx: b = −47.9 g, t(19) = −9.85, P < 0.0001]. The initial drop in body mass was more than twice as great in the TRIPLEx rats compared with sham and CTx controls as assessed on postsurgical days 1 and 2 [F(2,19) = 6.60, P = 0.007; Table 2]; post hoc tests revealed that the TRIPLEx group differed from both the sham and CTx groups (both P < 0.027) and that the sham and CTx groups did not differ from one another (P = 0.21).
Fig. 5.
Body mass values assessed for sham and TRIPLEx groups measured for 3 wk postsurgery.
The results from the linear regression analysis revealed that body mass significantly increased across postsurgical days in both the sham and CTx groups [sham: m = 4.59 g/day, t(19) = 21.85, P < 0.0001; CTx: m = 5.04 g/day, t(19) = 27.85, P < 0.0001] but not in the TRIPLEx cut group [m = 0.36 g/day, t(19) = 0.92, P = 0.37]. Indeed, there was a significant difference between the groups in the change in body mass relative to presurgical baseline on postsurgical days 20 and 21 [F(2, 19) = 64.78, P < 0.0001; Table 2]; post hoc tests revealed that the TRIPLEx group differed from both the sham and CTx groups (both P < 0.0001) and that the sham and CTx groups did not differ from one another (P = 0.83).
Sucrose
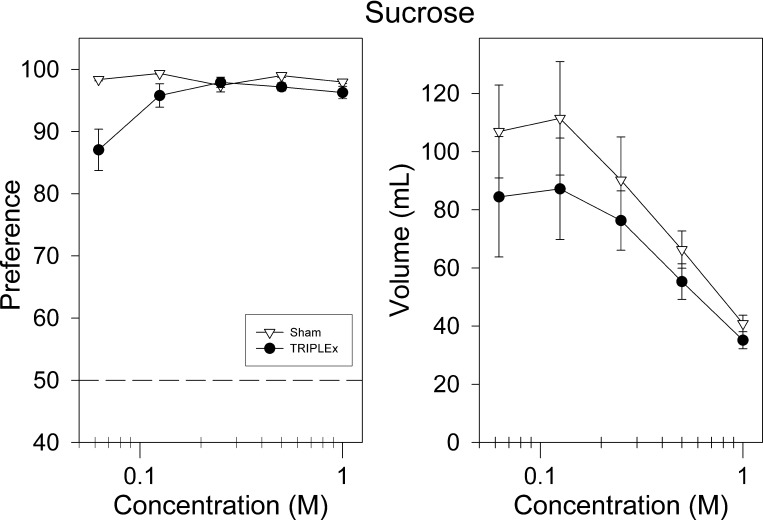
Surprisingly, both groups of animals displayed robust preference for sucrose in two-bottle intake tests. Although the sham group exhibited slightly greater preference than did the TRIPLEx rats, the latter group still chose to drink sucrose primarily over water at all concentrations. A two-way ANOVA revealed a main group effect for sucrose preference [F(1,12) = 10.16, P = 0.0078; Fig. 6], an effect of sucrose concentration [F(4, 48) = 6.75, P = 0.0002], as well as a significant interaction [F(4, 48) = 7.17, P = 0.0001]. Post hoc analysis revealed that rats in the sham group displayed significantly greater preference for sucrose at the 0.0625 and 0.5 M concentrations with the latter not surviving Bonferroni correction [0.0625: t(12) = 3.40, P = 0.0053, 0.5: t(12) = 2.56, P = 0.03; Fig. 6; Bonferroni-adjusted α = 0.01].
Fig. 6.
Sucrose preference vs. water (left) and intake (right) as a function of concentration for sham and TRIPLEx groups (means ± SE).
A two-way ANOVA revealed that the groups did not differ in their total intake of sucrose (P = 0.33), although the TRIPLEx rats trended toward lower levels of ingestion (Fig. 6). There was an effect of concentration [F(4, 48) = 16.85, P < 0.0001] on sucrose intake, but no significant interaction was observed (P = 0.82).
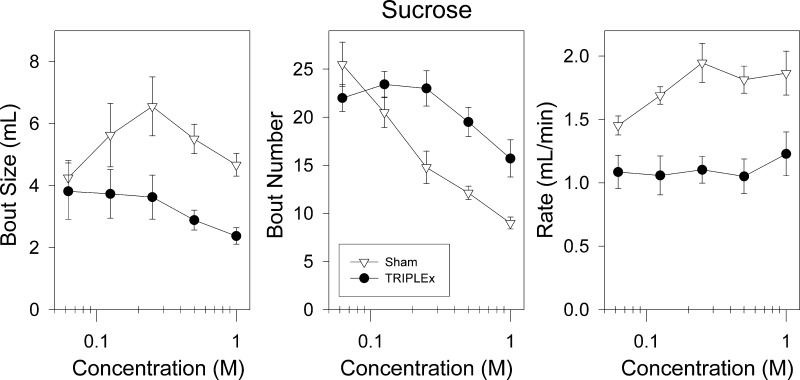
Although both groups of rats displayed robust preference for and intake of sucrose, sham rats consumed the sugar solutions in an entirely different manner. Rats in the sham group took significantly larger sucrose bouts, relative to rats in the TRIPLEx group. A two-way ANOVA revealed a main effect of group for sucrose bout size [F(1,12) = 6.901, P = 0.022; Fig. 7] and a main effect of concentration [F(4, 48) = 3.375, P = 0.016]. No significant interaction was observed (P = 0.09). Consistent with the effect of the nerve transections on sucrose bout size, a two-way ANOVA revealed that the TRIPLEx rats had a significantly slower rate of sucrose drinking (ml/min) within bouts compared with the sham group [F(1, 12) = 15.5, P = 0.002; Fig. 7]. There was also a significant effect of concentration on rate of drinking within sucrose bouts [F(4, 48) = 5.1, P = 0.0016], as well as a significant interaction [F(4, 48) = 3.2, P = 0.0196]. Interestingly, the within-bout drinking rate for sham rats increased as a function of concentration [one-way ANOVA: F(4, 24) = 5.69, P = 0.0023; Bonferroni-adjusted α = 0.025] and the higher concentrations approached twice the value seen for TRIPLEx rats, which, in turn, stayed relatively constant across the concentration range (one-way ANOVA: P = 0.25; Fig. 7; Bonferroni-adjusted α = 0.025).
Fig. 7.
Bout size (left), bout number (middle), and bout rate (right) as a function of concentration for sham and TRIPLEx groups when ingesting sucrose during preference testing (means ± SE).
Apparently, the TRIPLEx rats compensated for their significantly decreased sucrose bout size by initiating a significantly larger number of sucrose bouts compared with the sham group. A two-way ANOVA for bout number revealed main effects of group [F(1,12) = 10.641, P = 0.0068; Fig. 7] and concentration [F(4, 48) = 21.55, P < 0.0001], as well as a significant interaction [F(4, 48) = 5.93, P = 0.00058]. Post hoc analysis revealed that the TRIPLEx group took more bouts when sampling the three highest concentrations of sucrose tested (all P values <0.006; Fig. 6; Bonferroni-adjusted α = 0.01).
DISCUSSION
There were four principal outcomes in this experiment. First, rats that had the gustatory branches of the seventh and ninth cranial nerves transected lost body mass initially, but then stabilized, even though total caloric intake steadily increased over the postsurgical observation period. Second, the absence of lingual and palatal gustatory input resulted in an initial drop in caloric intake that was effected primarily through decreases in bout number, not bout size, when rats were offered a sweetened-milk and oil-chow diet to which they had been presurgically exposed. Third, despite the loss of histologically confirmed oral gustatory input, TRIPLEx rats displayed elevated intake of and preference for sucrose solution similar to that observed in sham-operated controls. Finally, although the TRIPLEx rats did not differ from sham group of rats in their sucrose intake, the manner by which the two groups ingested the sugar solution varied remarkably and did not emulate what was observed with the milk diet. The conceptual ramifications of each of these findings are discussed below.
Impact of Gustatory Neurotomy on Body Mass Regulation and Caloric Intake
Rats in the TRIPLEx group lost more body mass after surgery and stabilized at a lower level compared with the two other surgical control groups. The greater initial loss of body mass by the TRIPLEx rats can be explained by the greater drop in total caloric intake postsurgically relative to the rats in the sham group. In the absence of lingual and palatal taste input, accounting for 90% of the taste bud population, body mass was more stable compared with the more progressive course of weight gain observed in controls. What is noteworthy about the relatively lower body mass maintained during the postsurgical observation period in TRIPLEx rats is that it occurred even though total caloric intake steadily increased (Fig. 2). Thus, we speculate that, in addition to having effects on feeding, the loss of oral gustatory signals, at least those provided by the seventh and ninth cranial nerves, has additional effects on nutrient absorption and/or energy expenditure. It would be instructive to explicitly test this in future studies.
Our results are consistent with the literature regarding the effects of gustatory neurotomy on body mass and food intake. For example, previous research has shown that when the CT, GL, and the pharyngeal branch of the vagus (PBV) nerves are bilaterally transected, rats do indeed lose significant levels of body mass, and depending on the nature and composition of the diet to which the animals have access, these rats do not recover their baseline levels of body mass until anywhere between 1 and 5 wk (29, 42). Similar results were seen when these nerves were cut in addition to the anterior palatine nerve, which supplies the taste buds of the incisive papilla in the rat (71). The interpretation of the findings from these studies must be tempered by the fact that there is no evidence that the PBV innervates taste buds, but it does provide substantial motor innervation to the pharynx (12, 32), which could have a significant impact upon feeding behavior and body mass. The possibility of a motor component is supported by the observation that the “control” group of rats in the latter study, which only had the PBV nerve bilaterally transected, lost more weight than any of the “gustatory” control groups (rats that had either the CT, GL, or anterior palatine nerve bilaterally transected) and had a similar, but significantly different, weight loss as rats with combined transections (71).
In the current report, the TRIPLEx group did not recover baseline levels of body mass over the entire course of the 5-wk experiment. Jacquin (29) reported that after having the CT, GL, and the PBV nerves bilaterally transected, the rats, on a “cafeteria” diet, recovered to baseline levels at a mean of 34 days. Moreover, in this same report, a small number of nerve-transected rats, given access to pablum, displayed hyperphagia and recovered normal intake and body mass within 10 days, suggesting that the characteristics of the diet (e.g., caloric content, nutrient content, palatability level, texture, viscosity) can interact with the nature of the neural deficit to influence body mass regulation. It should be noted, however, that a post mortem histological analysis of oral tissue was not performed in this or in any of the other reports on the effects of gustatory deafferentation on body mass (29, 42, 71). Thus, it is uncertain whether the observed outcomes (e.g., return to baseline/control body mass levels) were the result of compensatory physiological mechanisms, remaining gustatory sensory innervation of the oral cavity (i.e., incomplete nerve transections, GSP, superior laryngeal branch of the vagus), or simply the regeneration of the transected nerves, which would result in the reestablishment of some gustatory function (e.g., 23, 59, 63). That said, in a report by Grill and Schwartz (25), in which the neurotomies were histologically confirmed, rats that had bilateral CT and GL nerve transections, maintained a significant weight loss, relative to controls, 20 days postsurgery (25). These rats, however, only had access to standard laboratory chow. As alluded to above, it has been demonstrated that rats deprived of gustatory neural input that are given standard chow, lose substantially greater amounts of body mass than do rats maintained on a more palatable, calorically dense diet. Yet, rats in the current report were given a very palatable diet and still did not return to baseline weight levels, highlighting the importance of both diet and neurophysiology in the maintenance of body mass.
In addition to innervating the taste buds of the fungiform papillae on the anterior two-thirds of the tongue, the CT nerve also contains parasympathetic fibers that innervate the submaxillary, sublingual, and accessory salivary glands (12, 57). Likewise, the GL contains parasympathetic fibers that innervate the von Ebner's gland (7, 33). Thus, transection of the CT and GL could affect the salivary content of the oral cavity, and, in turn, possibly influence food intake and body mass. However, both the sublingual and submaxillary salivary glands receive sympathetic innervation via other nerves, and the lingual branch of the trigeminal nerve, which was left intact, also supplies some parasympathetic innervation (22, 27). Moreover, the rats in the CTx group did not lose body mass relative to sham controls. Nevertheless, parasympathetic fibers from the GSP, in part, innervate the mucosal glands of the palate (32) and when transection of this nerve is combined with that of the other gustatory nerves, the functional status of the oral epithelium could be adversely affected and we cannot entirely dismiss that this contributed to the effects observed here. That said, there are multiple reports that changes in the saliva content of the oral cavity, via the complete removal of some or all of these salivary glands, while increasing chow bout duration, has no impact upon daily food and fluid consumption or body mass gain (20, 56, 65, 69).
It is important to acknowledge that the GSP and GL possess somatosensory afferent fibers innervating the palate and posterior tongue, respectively. It has been shown that trigeminal deafferentation of the anterior tongue leads to significant decreases in intake and body mass (30). Accordingly, we cannot entirely dismiss the possibility that a decrease in oral somatosensory signals in the TRIPLEx rats contributed to the outcomes observed here. That said, the lingual branch of the trigeminal nerve was left intact and thus somatosenory input from the anterior two-thirds of the tongue was maintained in the TRIPLEx rats. Another consideration is that although the combined transection of the CT, GSP, and GL results in loss of 90% of taste bud population, the superior laryngeal branch of the vagus was left intact; this nerve innervates the few taste buds residing in the laryngeal epithelium. However, these taste buds are thought to be involved with protection of the airways (6, 15, 53).
Impact of gustatory neurotomy on feeding and drinking patterns.
After surgery, the majority of total calories ingested, from both surgical groups, came from sweetened-milk diet consumption (see Fig. 2). The neurotomy-induced decrease in sweetened-milk diet intake can be explained by a reduction in bout frequency, but not in bout size (see Fig. 4). A similar outcome was observed for the oil-chow mash diet.
In the context of feeding patterns, bout initiation can be considered as governed by purely appetitive processes, defined as the actions that bring the animal to the goal. Bout size can be considered to be controlled by both appetitive and consummatory processes, with, perhaps, a bias toward the latter, which can be defined as the final behavior of the appetitive sequence stimulated by contact of the stimulus with the receptors. This distinction is important because it has been shown through a variety of pharmacological, endocrine, and neural manipulations that mechanisms controlling bout initiation are dissociable from those controlling bout size (39, 40, 54). Given that gustatory signals have been implicated in the control of meal size as clearly shown by the concentration-dependent increases in sucrose intake in sham-feeding preparations (46, 72), it was surprising that bout size of neither the sweetened-milk diet or oil-chow mash was affected by the neurotomy. It was also especially surprising that the drinking rate during sweetened-milk diet bouts did not differ between the TRIPLEx and control rats, at least initially after surgery, given that ingestion rate has been shown to be influenced by stimulus palatability (e.g., 13, 62, 74). We speculate that these outcomes are related to the presurgical experience that the animals had with these diets. As such, the animals had ample opportunity to learn about the nongustatory features of the sweetened-milk and oil-chow mash (olfactory, textural, visual) and associate them with the beneficial nutritive properties of diets. In other words, it is possible that the conditioned palatability based on the nongustatory cues of the diets were sufficient to maintain bout size and bout rate. In contrast, bout number was appreciably affected initially by the neurotomy, indicating a significant consequence of the removal of gustatory signals on the appetitive drive to approach nutritive sources, and this resulted in a decrease in caloric consumption. However, bout number tended to increase over the 21-day postsurgical observation period, suggesting a recovery of appetitive drive, as did bout size in the TRIPLEx rats, and this led to a steady increase in caloric intake that could not be based on oral gustatory signals. Regardless of the underlying mechanism, it is clear, that under some conditions, a normal meal size can be maintained in the absence of gustatory neural input from the tongue and palate.
Impact of gustatory neurotomy on sucrose intake, preference, and drinking patterns.
Surprisingly, despite histologically confirmed massive gustatory deafferentation, the TRIPLEx group displayed a robust, albeit slightly attenuated, preference for sucrose and did not differ from sham rats in their absolute sucrose intake. High intake of (34, 42, 70, 71) or preference for (25, 42, 70) sucrose and other sweeteners by rats with various combinations of gustatory neurotomies has been reported previously. However, in prior work, we have demonstrated that after the bilateral transection of the CT, GL, and GSP, behavioral responsiveness to sucrose across a broad range of concentrations is effectively eliminated (61). These prior findings were based on rats that had the same gustatory neurotomy performed using the same surgical procedures as the rats described in the current report, but sucrose responsiveness was assayed using a brief-access taste test that minimizes postingestive influences on concentration-dependent licking during a given trial (see Ref. 58 for a detailed explanation). Thus, these data suggest that postingestive factors, possibly interacting with the substantial experience of the animals with sweet-tasting stimuli (i.e., sweetened-condensed milk), and not residual gustatory input from superior laryngeal nerve, drove the sucrose preference observed here. This hypothesis is supported by data gathered with T1R3 knockout mice. T1R3 is a protein that serves as a component of the canonical sweet taste receptor, which mediates the perception of sweeteners in mammals (75). In long-term intake tests, these animals initially show greatly reduced preference for sucrose across a broad range of concentrations, relative to wild-type controls, but with repeated testing, eventually display hearty preferences for the sugar (75, 77, 78). Indeed, other types of knockout mice missing critical taste transduction components can display significant preference for sweeteners, including sucrose, if they have had prior testing experience with the compound (e.g., 51). Thus, it would appear that normal gustatory function is not necessary for sucrose preferences, as measured in long-term intake tests, to be expressed by rodents.
Interestingly, despite having relatively normal preference and intake of sucrose, the manner in which the TRIPLEx rats ingested this taste stimulus was entirely different from the way that sham rats did. Relative to sham rats, TRIPLEx rats had significantly smaller sucrose drinking bouts and compensated for this decrease by initiating a significantly greater number of bouts over the 23-h period. Apparently, the loss of gustatory neural input influences the ingestion of sucrose by decreasing the maintenance of licking during bouts likely because of the absence of excitatory signals. This could be viewed as a decrease in the orosensory-based hedonic effectiveness of the stimulus (55). In support of this interpretation, the bout drinking rate, thought to reflect the motivational state of the animal (e.g., 13, 55, 62), was significantly lower in TRIPLEx rats relative to sham controls and was not dependent on sucrose concentration. Thus, the prior experience with the sweetened condensed-milk diet presurgically and postsurgically, during which animals could learn that calories were available from the fluid source that was distinguishable from water (not necessarily based on taste), was likely responsible for increasing the probability at which the animals returned to the sucrose drinking spout to initiate a bout. It is noteworthy that the sweetened-milk diet contained about 18% sugar, and there is evidence that sucrose has a detectable odor starting at concentrations of 0.03 M (∼1.3%; Ref. 47). One possible reason that sham animals had a lower frequency of bout initiation could arguably be due to the greater postingestive inhibition that they experienced from the larger bouts they had that maintained satiety longer.
Although it is possible that the nerve transections affected the oromotor competence of the animals, Spector et al. (61), found that TRIPLEx rats had interlick intervals, a measure often used to assess the status of the putative central pattern generator for licking, that were not significantly different from controls. Moreover, while the animals in that study displayed virtually flat sucrose concentration-response functions in a brief-access test, their maximum licks across all of the trials was no different from sham-operated animals. Finally, the bout drinking rate observed for the sweetened-milk diet was greater than that for sucrose in the TRIPLEx rats, demonstrating that these animals had the capacity to display higher rates of ingestion.
The profile of sucrose drinking patterns in TRIPLEx rats is opposite to what we observed when the animals were presented with the sweetened-milk diet after surgery (i.e., no difference in bout size and a decrease in bout number). The differences appear to relate to the chemical composition of the stimuli (pure sugar solution vs. liquid mixture of fat, protein, sugar, minerals, and vitamins) and the extensive presurgical experience the rats had with the sweetened-milk diet vs. the lack of prior experience with pure sucrose solutions. The nongustatory cue of the sucrose is only one component of the complex milk diet, which could, in some sense, be considered a conditioned stimulus, and while it is sufficient to drive choice and intake, it is apparently insufficient to drive drinking bout size and rate in marked contrast to the control rats. Such results highlight the important distinction between measures of preference and intake and measures of the organization of licking itself.
Perspectives and Significance
On a final note, it is worth considering some clinical ramifications of these findings. First, there is the well-documented decrease in appetite associated with cancer treatments. This decrease in food intake can impact upon a broad number of factors, including tissue healing, energy levels, mood, social interactions, and overall quality of life (17, 38). Although it is difficult to completely disambiguate the contributions of the disease, as well as the nonspecific consequences of the therapy, including effects on salivation, a growing body of current research suggests that taste loss is a contributing factor to the decrease in appetite observed in cancer patients (4, 37, 38, 66). For example, it has been demonstrated by numerous laboratories that alterations in chemosensory function in cancer patients predict dietary intake patterns, as well as quality of life (e.g., 9, 21). The use of radiation and/or cytotoxic agents to combat the cancer is thought to be the major cause of taste dysfunction in cancer patients (17, 18, 31, 64, 73) via influences on taste bud cell survival, replacement, and proliferation (3, 44, 45, 48). Collectively, these data provide powerful support for the role of taste in maintaining eating habits and quality of life. Current treatment for taste loss due to cancer treatment consists primarily of dietary manipulation focused on enhancing the flavor of food with more intense tastes (17, 18). The results presented here would suggest that, in addition to presenting more intense flavors, giving these patients a familiar palatable caloric supplement, particularly a liquid diet such as Ensure, before treatment begins, may help to mitigate the influence of cytotoxic cancer treatments on the subsequent intake of the caloric supplement during treatment.
Another clinically relevant observation is that the postsurgical difference in body mass between the sham and TRIPLEx rats resembled the magnitude of the difference seen between control rats and those that have received Roux-en-Y gastric bypass (e.g., 10, 26, 36, 52). Currently, gastric bypass is considered the most effective treatment to cause long-term weight loss far surpassing what is seen with pharmacological interventions or dietary restriction regimens. These data provide strong support for the important contribution of the gustatory system in the regulation of body mass and although, obviously, gustatory neurotomy would not be a viable treatment option for obesity, the development of more selective pharmacological interventions targeting the gustatory pathways could have some potential utility in curbing hyperphagia and promoting body mass regulation.
GRANTS
This work was supported by the National Institutes of Health Grant R01-DC01628 to A. C. Spector.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.D.D., C.L.C., J.C.S., and A.C.S. analyzed data; C.D.D. and A.C.S. interpreted results of experiments; C.D.D. prepared figures; C.D.D. drafted manuscript; C.D.D. and A.C.S. edited and revised manuscript; C.D.D., C.L.C., M.G., J.C.S., and A.C.S. approved final version of manuscript; C.L.C. and M.G. performed experiments; J.C.S. and A.C.S. conception and design of research.
ACKNOWLEDGMENTS
All authors read and approved the final manuscript. The authors thank M. Denbleyker, K. Ferrence, and P. Maras for assistance in data collection.
REFERENCES
- 1. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am 37: 811– 823, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol 280: R1613– R1619, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bergdahl M, Bergdahl J. Perceived taste disturbance in adults: prevalence and association with oral and psychological factors and medication. Clin Oral Investig 6: 145– 149, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bernhardson BM, Olson K, Baracos VE, Wismer WV. Reframing eating during chemotherapy in cancer patients with chemosensory alterations. Eur J Oncol Nurs In press [DOI] [PubMed] [Google Scholar]
- 5. Blonde GD, Garcea M, Spector AC. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci 120: 580– 589, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bradley RM. Sensory receptors of the larynx. Am J Med 108 Suppl 4A: 47S– 50S, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bradley RM, Mistretta CM, Bates CA, Killackey HP. Transganglionic transport of HRP from the circumvallate papilla of the rat. Brain Res 361: 154– 161, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr 90: 23– 32, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV. Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 41: 673– 683, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Bueter M, Ashrafian H, Frankel AH, Tam FW, Unwin RJ, le Roux CW. Sodium and water handling after gastric bypass surgery in a rat model. Surg Obes Relat Dis 7: 68– 73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res Bull 17: 439– 443, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Contreras RJ, Gomez MM, Norgren R. Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190: 373– 394, 1980 [DOI] [PubMed] [Google Scholar]
- 13. Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav 11: 39– 45, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Davis JD, Campbell CS. Peripheral control of meal size in the rat. Effect of sham feeding on meal size and drinking rate. J Comp Physiol Psychol 83: 379– 387, 1973 [DOI] [PubMed] [Google Scholar]
- 15. Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res 450: 25– 38, 1988 [DOI] [PubMed] [Google Scholar]
- 16. Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 90: 800S– 803S, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Epstein JB, Barasch A. Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol 46: 77– 81, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Epstein JB, Huhmann MB. Dietary and nutritional needs of patients after therapy for head and neck cancer. J Am Dent Assoc 143: 588– 592, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Fetissov SO, Meguid MM. Serotonin delivery into the ventromedial nucleus of the hypothalamus affects differently feeding pattern and body weight in obese and lean Zucker rats. Appetite 54: 346– 353, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Galili D, Shpack N, Steiner JE. Food preference in surgically desalivated rats-texture versus taste. Chem Senses 10: 483– 489, 1985 [Google Scholar]
- 21. Gamper EM, Giesinger JM, Oberguggenberger A, Kemmler G, Wintner LM, Gattringer K, Sperner-Unterweger B, Holzner B, Zabernigg A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: prevalence, course of severity, and quality of life correlates. Acta Oncol 51: 490– 496, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Garrett JR, Kidd A. The innervation of salivary glands as revealed by morphological methods. Microsc Res Tech 26: 75– 91, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Geran LC, Garcea M, Spector AC. Nerve regeneration-induced recovery of quinine avoidance after complete gustatory deafferentation of the tongue. Am J Physiol Regul Integr Comp Physiol 287: R1235– R1243, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Geran LC, Garcea M, Spector AC. Transecting the gustatory branches of the facial nerve impairs NH(4)Cl vs. KCl discrimination in rats. Am J Physiol Regul Integr Comp Physiol 283: R739– R747, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Grill HJ, Schwartz GJ. The contribution of gustatory nerve input to oral motor behavior and intake-based preference. II. Effects of combined chorda tympani and glossopharyngeal nerve section in the rat. Brain Res 573: 105– 113, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967– G979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellekant G, Kasahara Y. Secretory fibres in the trigeminal part of the lingual nerve to the mandibular salivary gland of the rat. Acta Physiol Scand 89: 198– 207, 1973 [DOI] [PubMed] [Google Scholar]
- 28. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345: 790– 797, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Jacquin MF. Gustation and ingestive behavior in the rat. Behav Neurosci 97: 98– 109, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Jacquin MF, Zeigler HP. Trigeminal orosensation and ingestive behavior in the rat. Behav Neurosci 97: 62– 97, 1983 [DOI] [PubMed] [Google Scholar]
- 31. Jansen L, Hoffmeister M, Chang-Claude J, Koch M, Brenner H, Arndt V. Age-specific administration of chemotherapy and long-term quality of life in stage II and III colorectal cancer patients: a population-based prospective cohort. Oncologist 16: 1741– 1751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jinkins JR. Atlas of neuroradiologic embryology, anatomy, and variants. Philadelphia, PA: Lippincott Williams & Wilkins, 2000, p. xvi, 732 p [Google Scholar]
- 33. Kim M, Chiego DJ, Bradley RM. Morphology of parasympathetic neurons innervating rat lingual salivary glands. Auton Neurosci 111: 27– 36, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Krimm RF, Nejad MS, Smith JC, Miller IJ, Beidler LM. The effect of bilateral sectioning of the chorda tympani and the greater superficial petrosal nerves on the sweet taste in the rat. Physiol Behav 41: 495– 501, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Kris-Etherton PM, Etherton TD, Carlson J, Gardner C. Recent discoveries in inclusive food-based approaches and dietary patterns for reduction in risk for cardiovascular disease. Curr Opin Lipidol 13: 397– 407, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057– R1066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahmoud FA, Aktas A, Walsh D, Hullihen B. A pilot study of taste changes among hospice inpatients with advanced cancer. Am J Hosp Palliat Care 28: 487– 492, 2011 [DOI] [PubMed] [Google Scholar]
- 38. McLaughlin L, Mahon SM. Understanding taste dysfunction in patients with cancer. Clin J Oncol Nurs 16: 171– 178, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Meguid MM, Laviano A, Rossi-Fanelli F. Food intake equals meal size times mean number. Appetite 31: 404, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Meguid MM, Yang ZJ, Laviano A. Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am J Physiol Regul Integr Comp Physiol 272: R1925– R1930, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Miller IJ., Jr Gustatory receptors of the palate In: Food intake and Chemical Senses, edited by Katsuki Y, Sato M, Takagi S, and O, omura Y. Tokyo, Japan: University of Tokyo Press, 1977, p. 173– 186 [Google Scholar]
- 42. Miller MG, Teates JF. The role of taste in dietary self-selection in rats. Behav Neurosci 100: 399– 409, 1986 [PubMed] [Google Scholar]
- 43. Morrison C, Berthoud H. Neurobiology of nutrition and obesity. Nutr Rev 65: 517– 534, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Nelson GM. Biology of taste buds and the clinical problem of taste loss. Anat Rec 253: 70– 78, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci 32: 3474– 3484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nissenbaum JW, Sclafani A. Sham-feeding response of rats to Polycose and sucrose. Neurosci Biobehav Rev 11: 215– 222, 1987 [DOI] [PubMed] [Google Scholar]
- 47. Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses 19: 425– 431, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Ripamonti C, Fulfaro F. Taste alterations in cancer patients. J Pain Symptom Manage 16: 349– 351, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199– 211, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Sclafani A, Nissenbaum JW. Oral versus postingestive origin of polysaccharide appetite in the rat. Neurosci Biobehav Rev 11: 169– 172, 1987 [DOI] [PubMed] [Google Scholar]
- 51. Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol 293: R1504– R1513, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588– 1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol 65: 1098– 1114, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Smith GP. The controls of eating: brain meanings of food stimuli. Prog Brain Res 122: 173– 186, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Smith JC. Microstructure of the rat's intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev 24: 199– 212, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Smith JC, Miller IJ, Jr, Krimm RF, Nejad MS, Beidler LM. A comparison of the effects of bilateral sections of the chorda tympani nerve and extirpation of the submaxillary and sublingual salivary glands on the eating and drinking patterns of the rat. Physiol Behav 44: 435– 444, 1988 [DOI] [PubMed] [Google Scholar]
- 57. Smith JJ, Breathnach CS. Functions of the seventh cranial nerve. Ear Nose Throat J 69: 688–691, 694–685, 1990 [PubMed] [Google Scholar]
- 58. Spector AC. Psychophysical evaluation of taste function in nonhuman mammals. In: Handbook of Olfaction and Gustation, edited by Doty R.L. New York: Marcel Dekker, 2003, p. 861– 879 [Google Scholar]
- 59. Spector AC. The functional organization of the peripheral gustatory system: Lessons from behavior. In: Progress in Psychobiology and Physiological Psychology, edited by Fluharty SJ, Grill HJ. New York: Academic, 2003, p. 101– 161 [Google Scholar]
- 60. Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol Regul Integr Comp Physiol 272: R1210– R1218, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110: 1096– 1109, 1996 [PubMed] [Google Scholar]
- 62. Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiol Behav 33: 127– 136, 1984 [DOI] [PubMed] [Google Scholar]
- 63. St John SJ, Garcea M, Spector AC. The time course of taste bud regeneration after glossopharyngeal or greater superficial petrosal nerve transection in rats. Chem Senses 28: 33– 43, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Steinbach S, Hundt W, Schmalfeldt B, Böhner C, Berktold S, Wolf P, Harbeck N. Effect of platinum-containing chemotherapy on olfactory, gustatory, and hearing function in ovarian cancer patients. Arch Gynecol Obstet 286: 473– 480. [DOI] [PubMed] [Google Scholar]
- 65. Stricker EM. Influence of saliva on feeding behavior in the rat. J Comp Physiol Psychol 70: 103– 112, 1970 [DOI] [PubMed] [Google Scholar]
- 66. Sánchez-Lara K, Sosa-Sánchez R, Green-Renner D, Rodríguez C, Laviano A, Motola-Kuba D, Arrieta O. Influence of taste disorders on dietary behaviors in cancer patients under chemotherapy. Nutr J 9: 15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec 227: 373– 379, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Treesukosol Y, Blonde GD, Jiang E, Gonzalez D, Smith JC, Spector AC. Necessity of the glossopharyngeal nerve in the maintenance of normal intake and ingestive bout size of corn oil by rats. Am J Physiol Regul Integr Comp Physiol 299: R1050– R1058, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vance WB. Observations on the role of salivary secretions in the regulation of food and fluid intake in the white-rat. Psychol. Monogr 79: 1– 22, 1965 [Google Scholar]
- 70. Vance WB. Water intake of partially ageusic rats. Life Sci 5: 2017– 2021, 1966 [Google Scholar]
- 71. Vigorito M, Sclafani A, Jacquin MF. Effects of gustatory deafferentation on Polycose and sucrose appetite in the rat. Neurosci Biobehav Rev 11: 201– 209, 1987 [DOI] [PubMed] [Google Scholar]
- 72. Weingarten HP, Watson SD. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav 28: 401– 407, 1982 [DOI] [PubMed] [Google Scholar]
- 73. Williamson JS, Ingrams D, Jones H. Quality of life after treatment of laryngeal carcinoma: a single centre cross-sectional study. Ann R Coll Surg Engl 93: 591– 595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Young PT. Palatability: the hedonic response to foodstuffs In: Handbook of Physiology, Section 6: Alimentary Canal, Vol 1 Control of Food and Water Intake, edited by Code CF. Washington, DC: American Physiological Society, 1967, p. 353– 366 [Google Scholar]
- 75. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255– 266, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 33 Suppl 2: S8– 13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866– R876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34: 685– 694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]