Abstract
Corneal avascularity is necessary for the preservation of optimal vision. The cornea maintains a dynamic balance between pro- and antiangiogenic factors that allows it to remain avascular under normal homeostatic conditions; however, corneal avascularity can be compromised by pathologic conditions that negate the cornea’s “angiogenic privilege.” The clinical relevance of corneal neovascularization has long been recognized, but management of this condition has been hindered by a lack of safe and effective therapeutic modalities. Herein, the etiology, epidemiology, pathogenesis, and treatment of corneal neovascularization are reviewed. Additionally, the authors’ recent findings regarding the clinical utility of topical ranibizumab (Lucentis®) and bevacizumab (Avastin®) in the treatment of corneal neovascularization are summarized. These findings clearly indicate that ranibizumab and bevacizumab are safe and effective treatments for corneal neovascularization when appropriate precautions are observed. Although direct comparisons are not conclusive, the results suggest that ranibizumab may be modestly superior to bevacizumab in terms of both onset of action and degree of efficacy. In order to justify the increased cost of ranibizumab, it will be necessary to demonstrate meaningful treatment superiority in a prospective, randomized, head-to-head comparison study.
Keywords: angiogenesis, bevacizumab, cornea, corneal angiogenic privilege, hemangiogenesis, lymphangiogenesis, neovascularization, ranibizumab, vascular endothelial growth factor, VEGF
I. INTRODUCTION
Corneal transparency and optimal vision require an avascular cornea.1 The cornea possesses redundant antiangiogenic mechanisms that actively maintain corneal avascularity, collectively accounting for corneal angiogenic privilege.2 Although the human cornea is avascular under normal homeostatic conditions, corneal angiogenic privilege is not absolute. Corneal neovascularization (NV) is a sight-threatening condition that can develop in response to inflammation, hypoxia, trauma, or limbal stem cell deficiency.1 A variety of therapeutic modalities have been employed in the treatment of corneal NV with variable, and often limited, clinical success.3
Vascular endothelial growth factors (VEGFs) regulate the development and maintenance of blood and lymphatic vessels.4 VEGF neutralizing agents have proven invaluable in the treatment of pathologic conditions such as neovascular age-related macular degeneration and diabetic retinopathy; furthermore, recent findings suggest that VEGF inhibition may be an effective therapeutic modality for corneal NV.5-7 Because systemic anti-VEGF exposure is associated with severe and potentially life-threatening adverse events, it is prudent to pursue the route of administration that minimizes systemic exposure.8 Herein, we present a brief review of corneal NV; additionally, we summarize our recent findings regarding the clinical utility of topical ranibizumab (Lucentis®; Genentech, Inc.; San Francisco, CA) and bevacizumab (Avastin®; Genentech, Inc.) in the treatment of corneal NV.
II. BACKGROUND
A. Etiology and Epidemiology
According to the World Health Organization (WHO), approximately 285 million people are visually impaired worldwide; of these, approximately 39 million are blind.9 Corneal disease is second only to cataract as the leading cause of nonrefractive visual impairment worldwide.10 Despite aggressive international prevention efforts, corneal disease remains the most common cause of blindness in some developing countries.11 Corneal NV is a potential sequela of numerous conditions, including infection, injury, surgery, autoimmune disease, limbal stem cell deficiency, neoplasm, dystrophy, and contact lens use.2 Over a decade ago, it was estimated that there are up to 1.4 million cases of corneal NV in the USA alone.12 The clinically evident pattern of vessel invasion (eg, vascular pannus, superficial stromal NV, or deep stromal NV) is often indicative of the etiology of corneal NV; for example, deep stromal NV generally develops in response to interstitial keratitis (eg, herpetic stromal keratitis) or significant ocular trauma (Figure 1).2,12 Corneal NV ultimately alters visual acuity by inducing stromal edema, cellular infiltration, lipid deposition, hemorrhage, and scarring.13
Figure 1.
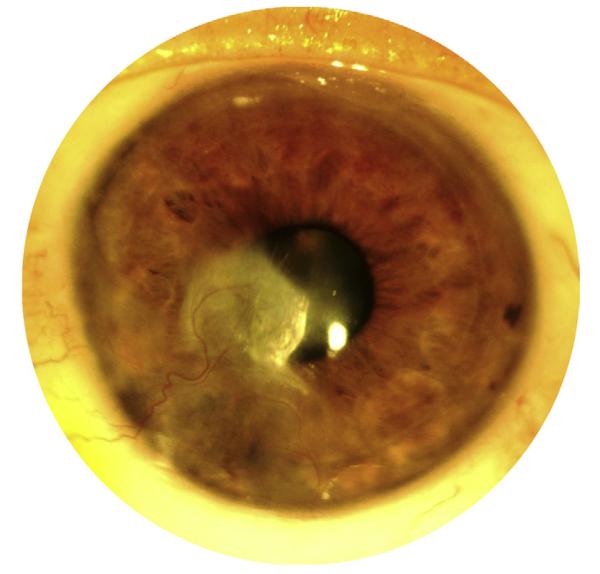
Clinical appearance of corneal neovasculariztion (NV). Superficial stromal NV, deep stromal NV, and corneal scarring secondary to recurrent herpes simplex virus (HSV) keratitis.
Corneal NV is a potential complication of numerous bacterial, parasitic, and viral infections. Trachoma is the world’s leading infectious cause of blindness.14 The WHO estimates that there are 146 million cases of Chlamydia trachomatis infection worldwide, and 5.9 million people are blind or at immediate risk of blindness from trachomatous trichiasis.14 Recurrent episodes of trachoma can damage the eyelid, resulting in eyelash-induced corneal abrasions, ulcerations, NV, and scarring.15 Onchocerciasis, commonly referred to as river blindness, is the second most common infectious cause of blindness worldwide.16 The causative filarial nematode, Onchocerca volvulus, infects an estimated 37 million people, and 270,000 cases of blindness have been attributed to onchocerciasis.17,18 Adult worms produce microfilariae that can migrate to the cornea and induce intense inflammation, NV, and opacification upon death of the worm.19 Herpes simplex virus (HSV) is the most common infectious cause of blindness in the developed world. Extrapolating from the 2010 census, approximately 64,000 episodes of HSV keratitis occur annually in the USA alone.20,21 Following the primary viral infection, HSV remains dormant in neural ganglia pending episodic reactivation.22 Recurrent episodes of HSV keratitis can cause NV, opacification, and scarring.
Ocular trauma accounts for approximately 19 million cases of unilateral visual impairment and 1.6 million cases of bilateral blindness worldwide.23 Corneal wound healing is generally an avascular process; however, corneal NV can develop in response to severe corneal injuries.2 Chemical burns in particular are known to induce a vigorous inflammatory response that promotes corneal NV. Furthermore, chemical burns are capable of damaging the corneal limbus, thereby leading to limbal stem cell deficiency.24 There are numerous potential causes of limbal stem cell deficiency, including inherited (eg, aniridia) and acquired (eg, trauma) etiologies.24,25 Disruption of the corneal limbus leads to corneal damage and provides an avenue for the extension of conjunctival epithelium and blood vessels into the cornea.
Corneal transplantation is the most common form of solid tissue transplantation.26 Nearly 40,000 corneal transplantations are performed annually in the USA alone.27 Surgery-induced corneal injury (eg, refractive surgery) generally provokes an avascular healing process; however, corneal transplantation can involve suture-induced inflammation and alloimmune responses that promote corneal NV.2,28 In addition to being a potential cause and consequence of corneal transplantation, corneal NV is a known risk factor for graft rejection.29 Corneal transplantation performed in graft beds devoid of inflammation and vasculature, referred to as low-risk transplantation, enjoys a rate of graft acceptance approaching 90%.30 However, corneal transplantation performed in previously sensitized, inflamed, or vascularized graft beds, referred to as high-risk transplantation, has a much lower rate of graft acceptance.31 The risk of corneal graft rejection has been found to correlate with the number of corneal quadrants that exhibit NV.32 Corneal NV provides the immune system with increased afferent and efferent access to graft alloantigens, thereby increasing the risk of allogeneic immune rejection.33 Nearly 20% of corneal buttons excised during corneal transplantation exhibit histologic evidence of NV.34
Approximately 38 million people in the USA and up to 125 million people worldwide wear contact lenses.35 Contact lens use is associated with a variety of inflammatory complications, including corneal NV.36 It has been estimated that 11-23% of contact lens users develop corneal NV.37 Presumably, contact lenses promote corneal NV by inducing inflammation and hypoxia.38 Risk factors for corneal NV include prolonged contact lens use, low oxygen permeability-contact lenses, and contact lens contamination. In extreme cases, contact lens use can cause deep stromal NV, hemorrhage, and opacification.39,40
B. Pathogenesis
1. Corneal Vessel Formation
Vasculogenesis refers to the de novo formation of blood vessels by endothelial precursor cells (angioblasts) or endothelial progenitor cells.41 Although vasculogenesis primarily occurs during embryologic development, endothelial progenitor cells are capable of giving rise to vascular endothelial cells during the postnatal period.42-44 Angiogenesis refers to the sprouting or splitting (intussusception) of new vessels from pre-existing vessels.4 Vasculogenesis and angiogenesis are physiologic processes that occur during normal development and tissue repair; however, these processes can also contribute to pathologic conditions, such as cancer and eye disease.41 A morphometric analysis of experimental corneal NV described the sprouting and extension of new vessels from pre-existing vessels at the corneoscleral limbal vascular plexus.45 Vascular endothelial cells in newly developed corneal vessels arise from previously established vessels at the limbal vascular plexus.46 Interestingly, a majority of the pericytes found in newly formed corneal vessels arise from bone marrow-derived precursor cells rather than the limbal vascular plexus.46
2. Corneal Angiogenic Privilege
Avascularity is a unique characteristic possessed by select tissues, such as the cornea and cartilage.1 Corneal avascularity is maintained despite intermittent exposure to potentially proangiogenic inflammatory stimuli (eg, ocular foreign body) and hypoxic conditions (eg, eyelid closure).37 Furthermore, the cornea is capable of remaining avascular in the face of significant injury (eg, refractive surgery), and corneal wound healing is generally an avascular process.2,37 A dynamic balance exists between the positive and negative regulators of angiogenesis that serves to maintain corneal avascularity (Table 1).47 In spite of this balance, pathologic conditions can override the cornea’s innate antiangiogenic defense mechanisms, thereby compromising the cornea’s avascular status.1,2 The “angiogenic switch,” a concept initially postulated to describe the induction of tumor angiogenesis, is relevant in cases of corneal angiogenesis, where it can be used to describe the transition from corneal avascularity to NV that occurs when proangiogenic factors overwhelm the cornea’s angiogenic privilege.48
Table 1.
Summary of pro- and antiangiogenic factors involved in corneal NV
| Proangiogenic Factors | Antiangiogenic Factors |
|---|---|
| Vascular endothelial growth factors • VEGF-A, VEGF-C, etc. |
Vascular endothelial growth factor receptors • sVEGFR1, mVEGFR3, etc. |
|
| |
| Fibroblast growth factors • FGF1, FGF2 (bFGF), etc. |
Pigment epithelium-derived factor |
|
| |
| Platelet-derived growth factors • PDGF-AA, PDGF-AB, etc. |
Collagen derivatives • Endostatin, canstatin, etc. |
|
| |
| Angiopoietins • Ang-1, (±) Ang-2 |
Angiopoietins • Ang-2 |
|
| |
| Angiogenin | Angiostatin |
|
| |
| Matrix metalloproteinases • MT-1MMP, MMP-2, etc. |
Matrix metalloproteinases • Epithelial MT1-MMP, MMP- 7, etc. |
|
| |
| Integrins • αVβ3, α1β1, etc. |
Tissue inhibitors of matrix metalloproteinases • TIMP-1, TIMP-2, etc. |
|
| |
| Cytokines/chemokines • IL-1, IL-8/CXCL8, etc. |
Cytokines/chemokines • IL-1RA, IP-10/CXCL10, etc. |
|
| |
| Hepatocyte growth factor/scatter factor |
Thrombospondins and CD36 • TSP-1, TSP-2, etc. |
|
| |
| Insulin-like growth factor | Death signaling pathways • Fas/FasL, CD80/PD-L1 |
|
| |
| Renin-angiotensin system |
Decorin, small leucine-rich proteoglycan |
|
| |
| Placental growth factor | Placental growth factor |
|
| |
| Leptin | Prolactin |
|
| |
| Thrombin | Anti-thrombin |
|
| |
| Platelet activating factor | Plasminogen activator inhibitor |
3. Promoters of Corneal Angiogenesis
a. Vascular Endothelial Growth Factors
VEGF is one of the most important factors implicated in the pathogenesis of corneal NV. There are multiple members of the human VEGF family, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF).49 VEGFs interact with the receptor tyrosine kinases VEGF receptor (VEGFR)-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4).49,50 VEGF-A is considered the most important member of the VEGF family, particularly with regard to pathologic hemangiogenesis. Alternative mRNA splicing allows for the production of pro- and antiangiogenic isoforms of VEGF-A, of which VEGF-A165 is the dominant proangiogenic isoform.51 Inflammation and hypoxia induce the production of VEGF-A by a variety of cell types, including blood vessel-associated pericytes and smooth muscle cells, and inflammation-associated macrophages and T cells.52-54 The binding of VEGF-A to VEGFR-2 promotes hemangiogenesis by stimulating vascular endothelial cell migration, proliferation, and survival, as well as vessel dilation and permeability.55-57 The binding of VEGF-C (or –D) to VEGFR-2 or -3 promotes lymphangiogenesis in a similar fashion.58,59 Furthermore, VEGFs serve as chemoattractants for inflammatory cells (eg, macrophages) that produce additional proangiogenic factors.60,61 The relevance of VEGF in corneal NV is well established, and VEGF inhibition is currently being investigated as a treatment for corneal NV.62-65
b. Fibroblast Growth Factors
Fibroblast growth factors (FGFs) regulate a variety of processes including angiogenesis and wound healing. There are 18 members of the mammalian FGF family that bind to the FGF receptors FGFR1, FGFR2, FGFR3, and FGFR4.66,67 FGF1 and FGF2, members of the FGF1 subfamily, are potent stimulators of angiogenesis; however, neither FGF1 nor FGF2 is required for normal growth, development, viability, or fertility.68,69 FGF2, also known as basic FGF (bFGF) or FGF-β, promotes vascular endothelial cell migration, proliferation, and differentiation, and inhibits cellular apoptosis.70,71 Abrogation of the FGF system leads to the loss of vascular integrity, suggesting that FGF-mediated signaling regulates vessel permeability.72 FGF2 is synthesized by corneal epithelial cells and passively released in response to epithelial cell injury.2,73 Once released, FGF2 binds to heparan sulfate polysaccharides expressed on membranes such as Descemet membrane, Bowman membrane, and vascular basement membrane.74,75 The intrastromal implantation of an FGF2 containing micropellet serves as an important model of experimental angiogenesis.76
c. Platelet-Derived Growth Factors
Platelet-derived growth factors (PDGFs) mediate adiverse array of biological processes, including angiogenesis and tissue remodeling.77 PDGF and VEGF are structurally and functionally related, as evidenced by their evolutionarily conserved PDGF/VEGF homology domain.78,79 Humans express four PDGF chains (PDGF-A, -B, -C, and -D), that dimerize and interact with receptor tyrosine kinase complexes of PDGFR-α or PDGFR–β.80 PDGF ligands and receptors are found throughout the human body, and many PDGFs are required for normal growth and development.77 PDGF-B and PDGFR-β are preferentially expressed by blood vessel-associated cells, and a deficiency in either PDGF-B or PDGFR-β results in fatal cardiovascular and hematological abnormalities.81,82 Nascent vascular endothelial cells secrete PDGF-B that binds to pericyte and smooth muscle cell-associated PDGFR-β, thereby promoting their migration, proliferation, and survival.83,84 PDGF-A and -B are detectable in corneal epithelial cells, stromal fibroblasts, and endothelial cells, and PDGF-BB can be isolated from tears.85,86 PDGFR-α and PDGFR–β are expressed by corneal epithelial cells, stromal fibroblasts, and endothelial cells.85,87 Inhibition of PDGFR–β leads to vessel-associated pericyte loss and decreased corneal vessel density.88
d. Angiopoietins
Angiopoietin (Ang) growth factors regulate angiogenesis, vascular extravasation, and inflammation.89 The human Ang family consists of Ang-1, -2, -4, and the tyrosine kinase receptors Tie1 and Tie2.90 Ang-1 deficiency, Ang-2 overexpression, or Tie2 deficiency all result in early embryonic death secondary to vascular abnormalities suggestive of abnormal blood vessel maturation and stabilization.91,92 Ang-1 promotes vessel assembly, maturation, and stability by facilitating vascular endothelial cell/mural cell (eg, pericyte and smooth muscle cell) interactions.93 Ang-2 antagonizes the activity of Ang-1, thereby inducing vessel destabilization and facilitating vascular sprouting and branching.89 The exogenous administration of either Ang-1 or -2 alone does not induce corneal NV; however, Ang-1 and -2 profoundly affect the development of VEGF-mediated corneal NV.94 Ang-1 promotes vessel maturation as evidenced by increased vessel density and perfusion, whereas Ang-2 promotes vessel destabilization as evidenced by the sprouting of new corneal vessels. Interestingly, Ang-2 induces the regression of blood vessels in the absence of VEGF, suggesting that Ang-2-induced destabilization alone serves an antiangiogenic function.95
e. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases involved in extracellular matrix remodeling. MMPs often promote angiogenesis by degrading the extracellular matrix and activating proangiogenic molecules.96 Proangiogenic factors upregulate the production of MMPs, such as MMP-1 (interstitial collagenase-1), MMP-2 (gelatinase A), MMP-9 (gelatinase B), and MT1-MMP (MMP-14), by vascular endothelial cells.97-100 MMP-2 and MMP-9 are type IV collagenases that degrade the extracellular matrix and liberate proangiogenic molecules.101-105 MT1-MMP degrades extracellular matrix type I collagen, thereby facilitating vascular endothelial cell invasion, migration, and tubule formation.106-108 MMP-2, MMP-9, and MT1-MMP are expressed by the cornea, and each of these MMPs is intimately involved in the promotion of corneal NV.107,109-112 Conversely, MT1-MMP expressed by corneal epithelial cells and keratocytes inhibits angiogenesis, suggesting that MT1-MMP’s location defines its function.113,114 Antiangiogenic MMP functions will be discussed later in this manuscript.
f. Inflammatory Mediators
Inflammation is a characteristic shared by all etiologies of corneal NV. Inflammatory cells are rich sources of VEGF molecules that promote hemangiogenesis and lymphangiogenesis; blood and lymphatic vessels in turn provide the inflamed eye with inflammatory cells that amplify the angiogenic cascade.33,60,61 Cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF), and transforming growth factor (TGF)-β not only activate inflammatory cells, but also upregulate the production of VEGF.115-118 Chemokines and their receptors are involved in the recruitment of inflammatory cells, and some chemokines (eg, IL-8/CXCL8) can directly stimulate angiogenesis.119 Promoters of angiogenesis and inflammation often regulate one another; for example, IL-1, IL-6, TNF, and IL-8/CXCL8 enhance MMP expression.120 Integrins (eg, α1β1, also known as very late antigen-1) facilitate the migration of not only inflammatory cells, but also vascular endothelial cells.121,122 The importance of these proinflammatory factors in angiogenesis is well-established, and their significance in corneal NV is becoming increasingly apparent.121,123,124
4. Inhibitors of Corneal Angiogenesis
a. VEGF Receptors
Corneal angiogenic privilege relies on a variety of antiangiogenic mechanisms, none more important than the endogenously occurring inhibitors of VEGF. There are several VEGF receptors (VEGFRs) expressed by the cornea that serve as “decoy” receptors for proangiogenic VEGF molecules. Soluble VEGFR-1 (sVEGFR-1/sFLT-1) inhibits hemangiogenesis by sequestering proangiogenic VEGF-A molecules.125,126 Soluble VEGFR-1 also forms inactive heterodimers with membrane-bound VEGFR-1 and VEGFR-2, further suppressing VEGF-mediated angiogenesis.127,128 The cornea expresses sVEGFR-2 that binds and sequesters prolymphangiogenic VEGF-C, thereby conserving corneal alymphaticity.129 The corneal epithelium displays membrane-bound VEGFR-3 that binds and sequesters VEGF-C and -D.130 This directly suppresses lymphangiogenesis by inhibiting VEGF-C and-D mediated signaling, and indirectly suppresses hemangiogenesis by inhibiting the recruitment of VEGF secreting macrophages.131 Soluble VEGFR-1 and sVEGFR-2 are essential for maintaining corneal avascularity under normal homeostatic conditions; epithelial expressed VEGFR-3 may be the primary factor responsible for inhibiting inflammation-mediated, pathologic corneal NV.
b. Pigment Epithelium-Derived Factor
Pigment epithelium-derived factor (PEDF) is a secreted glycoprotein that belongs to the serpin superfamily, despite lacking the ability to inhibit serine proteases.132,133 PEDF possesses potent antiangiogenic, immunomodulatory, and neurotrophic properties.134-136 PEDF exerts antiangiogenic activity by suppressing VEGF, FGF, and IL-8/CXCL8-mediated vascular endothelial cell migration and proliferation, and inducing vascular endothelial cell apoptosis.134,137,138 PEDF has been localized to the corneal epithelium and endothelium, and PEDF expression is thought to be an important component of corneal angiogenic privilege.139,140 The inhibition of PEDF promotes corneal NV, and the exogenous administration of PEDF suppresses corneal NV.141,142
c. Angiostatin
Angiostatin is a multifunctional 38 kDa protein fragment generated by the proteolytic cleavage of plasminogen.143,144 Angiostatin binds to vascular endothelial cell surface-expressed F1-F0 ATP synthase, thereby suppressing the production of ATP and inhibiting cell migration and proliferation.145,146 Angiostatin decreases cell migration and tubule formation upon binding to endothelial cell surface-expressed angiomotin.147 The binding of angiostatin to integrin αvβ3 antagonizes vascular endothelial cell survival and migration.148 Angiostatin also binds to the hepatocyte growth factor receptor (c-met), resulting in the downstream inhibition of Akt phosphorylation, thereby promoting apoptosis and cell cycle inhibition.149-151 The antiangiogenic effects of angiostatin may require the presence of IL-12 and be mediated in part by the inhibition ofinflammatory cellrecruitment.152-154 Endogenous angiostatin can be found in the corneal epithelium and tear fluid.155,156 The functional relevance of angiostatin in suppressing corneal NV has been demonstrated in models of surgical, mechanical, and alkali-induced corneal injury.156,157
d. Collagen Derivatives
Endostatin is an endogenously occurring 20 kDa fragment of collagen XVIII that inhibits angiogenesis.158 Collagen XVIII is a component of most epithelial and endothelial basement membranes, including vascular basement membrane.159-162 The proteolytic cleavage of type XVIII collagen’s C-terminal noncollagenous domain (NC1) results in the liberation of biologically active endostatin.163 Endostatin not only inhibits vascular endothelial cell migration and proliferation, but also promotes endothelial cell apoptosis and cell-cycle arrest.158 Endostatin directly binds to VEGFR-2, thereby inhibiting VEGF-A-mediated angiogenesis.164 Endostatin is not required for the normal development and function of most major organs; however, endostatin deficiency results in aberrant hyaloid vessel regression and retinal vessel development reminiscent of the abnormalities observed in Knobloch syndrome.165,166 Collagen XVIII has been immunolocalized to the corneal epithelium, epithelial basement membrane, and Descemet membrane.167 Endostatin inhibits experimental FGF- and VEGF-mediated corneal NV, implicating endostatin in corneal angiogenic privilege.168 MMP-7 and MT1-MMP-mediated cleavage of collagen-XVIII produces the antiangiogenic collagen fragments neostatin-7 and -14, respectively.169,170 Type IV collagen, a component of all basement membranes, can be cleaved to produce arrestin, canstatin, and tumstatin.171 These collagen derivatives negatively regulate angiogenesis, and their importance in corneal angiogenic privilege is becoming increasingly apparent.2,172
e. Matrix Metalloproteinases
The regulation of corneal angiogenesis is complex and multifaceted, as evidenced by the conflicting pro- and anti-angiogenic functions of many MMPs. For example, MMP-7 (matrilysin) stimulates the production of VEGF and directly promotes the proliferation of vascular endothelial cells independent of VEGF.173,174 Moreover, VEGF-mediated angiogenesis is augmented by the degradation of sVEGFR-1 by MMP-7.175 In spite of these proangiogenic functions, MMP-7 expressed by the basal epithelial layer of the cornea is thought to inhibit angiogenesis because MMP-7 deficiency dramatically increases the angiogenic response to corneal wounding.176,177 This may be a function of the MMP-7-mediated cleavage of type XVIII collagen, the precursor of antiangiogenic endostatin.167,178
f. Thrombospondins and CD36
Thrombospondins (TSPs) are multifunctional endogenous proteins known to suppress angiogenesis. TSPs inhibit vascular endothelial cell migration, proliferation, and tubule formation, and induce vascular endothelial cell apoptosis.179-182 TSP-1 has been immunolocalized to the cornea’s epithelial basement membrane, posterior Descemet membrane, and endothelium.183 TSP-1 deficiency augments the cornea’s angiogenic response to suture-induced inflammation.184 The antiangiogenic effects of TSP-1 are mediated by the ligation of TSP-1 to the transmembrane glycoprotein CD36 scavenger receptor found on vascular endothelial cells and inflammatory cells.185,186 The activation of vascular endothelial cell-expressed CD36 directly suppresses corneal angiogenesis.185 The activation of macrophage-expressed CD36 indirectly suppresses corneal hemangiogenesis and lymphangiogenesis by inhibiting the secretion of VEGF-A, -C, and -D.185,186 Genetic ablation of CD36 results in the development of age-related corneal NV, demonstrating the functional relevance of TSP and CD36 in corneal angiogenic privilege.187
g. Death Signaling Pathways
The Fas (CD95)/FasL (Fas ligand) system inhibits corneal NV by regulating the infiltration of inflammatory cells and vascular endothelial cells.188-190 Corneal epithelial- and endothelial-expressed FasL induces the apoptosis of infiltrating inflammatory cells, thereby safeguarding ocular immune privilege.188,189 The Fas/FasL system serves as a barrier against immune cells that invade the cornea in response to inflammation and secrete proangiogenic molecules. Moreover, vascular endothelial cells express Fas, and corneal-expressed FasL suppresses the proliferation of corneal NV by inducing the apoptosis of vascular cells.190 Programmed death-ligand 1 (PD-L1), a member of the B7-CD28 superfamily, binds to the receptors PD-1 and CD80 and has been shown to regulate inflammatory cell activity by inducing apoptosis.191 PD-L1 and CD80 have been identified in vascular endothelial cells, and PD-L1 is expressed by corneal epithelial cells.192 Vascular endothelial cell and corneal epithelial cell-expressed PD-L1 suppresses corneal NV through an inflammation-independent pathway.192
C. Conventional Treatment Modalities
1. Medical Therapy
Several medical therapies have been employed in the treatment of corneal NV, including corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs).193 Corticosteroids (eg, dexamethasone) are the standard medical treatment for patients with actively proliferating corneal NV, particularly in cases of corneal transplantation-associated corneal NV.194 Corticosteroids inhibit angiogenesis by suppressing inflammatory cell recruitment, proinflammatory cytokine (eg, IL-1, IL-6) expression, and arachidonic acid release.195-199 Corticosteroids may suppress the development of inflammation-induced corneal NV, but they are generally ineffective at treating stable corneal NV. Moreover, the side effects associated with long-term corticosteroid use (eg, cataract, ocular hypertension, corneal thinning, and opportunistic infections) generally make this an untenable choice. NSAIDs (eg, nepafenac) are another class of anti-inflammatory agents that have been utilized in the treatment of corneal NV.200,201 NSAIDs inhibit the production of prostaglandins that stimulate angiogenesis.201,202 Unfortunately, the variable efficacy and sometimes serious side effects associated with the use of topical NSAIDs (eg, corneal ulceration and perforation) have limited their clinical utility, particularly in the setting of patients with concomitant ocular surface disease.203
2. Laser-Induced Photocoagulation
Laser-induced photocoagulation is a procedure that can be employed in the treatment of corneal NV. To perform this procedure, a beam of light is selectively focused on the vessel of interest, resulting in heat-induced coagulation and vessel occlusion.204 Argon, Nd:YAG, and yellow lasers have all been used for this purpose with some success.205-213 Unfortunately, laser-induced tissue damage can stimulate the release of proangiogenic factors that promote the development of collateral circulation.212 The combined use of laser-induced photocoagulation and angiogenesis-specific medical treatments (eg, anti-VEGF agents) may overcome this limitation.212 Laser-induced photocoagulation is associated with a number of uncommon complications, including corneal hemorrhage, corneal thinning, iris atrophy, and necrotizing scleritis.209-211,213
3. Photodynamic Therapy
Photodynamic therapy (PDT) is another procedure utilized in the treatment of corneal NV. In this procedure, a photosensitizing agent is delivered to the tissue of interest and activated by a specific wavelength of light, resulting in the production of cytotoxic singlet oxygen.214,215 In the case of corneal NV, the photosensitizing agent is delivered to corneal vessels and activated by laser light, resulting in vascular endothelial cell damage and vessel thrombosis.216,217 Photosensitizing agents such as verteporfin, fluorescein, and dihematoporphyrin have all been used to treat corneal NV.218-221 PDT performed with verteporfin or fluorescein induces the regression of corneal NV with minimal risk.218-220 PDT performed with dihematoporphyrin also induces the regression of corneal NV, but this photosensitizing agent has been associated with deleterious local and systemic side effects.221 PDT has been shown to promote both experimental and clinical graft survival and may be an effective treatment for corneal NV following corneal transplantation.222,223 Combining PDT with angiogenesis-specific inhibitors such as bevacizumab may increase treatment efficacy and decrease the incidence of collateral vessel development.224 Although PDT performed with an appropriate photosensitizing agent appears to be a safe and effective treatment for corneal NV, the high costs associated with PDT have limited its clinical utility.
4. Fine-Needle Diathermy
Fine-needle diathermy (FND) is a surgical procedure that shows promise in the treatment of corneal NV. In this procedure, a needle is inserted parallel to or within the lumen of the vessel of interest. A diathermy probe in coagulation mode is then brought in contact with the needle, resulting in vessel cauterization. Clinical investigations have found that FND is a safe and effective procedure for occluding targeted corneal vessels.225-227 Transient corneal whitening and intra-stromal hemorrhage are the only reported complications. The procedure may need to be repeated because of vessel recanalization or collateral vessel development.225-227 FND-induced ablation of corneal vessels has successfully aided in the reversal of several cases of corneal graft rejection.225,226
5. Ocular Surface Reconstruction
Ocular surface restoration using limbal, conjunctival, or amniotic membrane transplantation can be employed as a final recourse for some patients with otherwise unresponsive corneal NV. Limbal stem cell transplantation has been successfully utilized in the treatment of limbal stem cell deficiency; unfortunately, cases of bilateral limbal stem cell deficiency may require allogeneic transplantation and be complicated by issues with long-term graft survival.228,229 Cultivated limbal epithelial transplantation is being increasingly used as an alternative to limbal transplantation.230,231 The transplantation of autologous conjunctival epithelial cells can successfully restore the ocular surface in some settings.232,233 Human amniotic membrane has long been used to restore the ocular surface because of its ability to suppress inflammation and promote wound healing.234,235 Amniotic membrane transplantation may be a safe and effective alternative to limbal transplantation; however, additional clinical trials are required to determine its place in the clinical management of corneal NV.236,237
III. TOPICAL ANTI-VEGF THERAPY
A. Corneal Penetration of Topical Bevacizumab
Bevacizumab is a humanized monoclonal antibody that binds to isoforms of VEGF-A.238 Bevacizumab was initially approved for the treatment of metastatic colorectal cancer; however, it has since been used off-label to treat a variety of ophthalmic conditions, including neovascular age-related macular degeneration, diabetic retinopathy, central retinal vein occlusion, and neovascular glaucoma.5,6,239,240 The systemic administration of bevacizumab has been associated with several severe and potentially life-threatening complications, including hypertension, impaired wound healing, gastrointestinal perforation, bleeding, arteriolar hemorrhage, and arterial thromboembolic events.241 The route of administration that provides the best combination of safety, efficacy, and practicality should be pursued; with regard to the cornea, the preferred method of administration is generally ocular surface topical instillation.
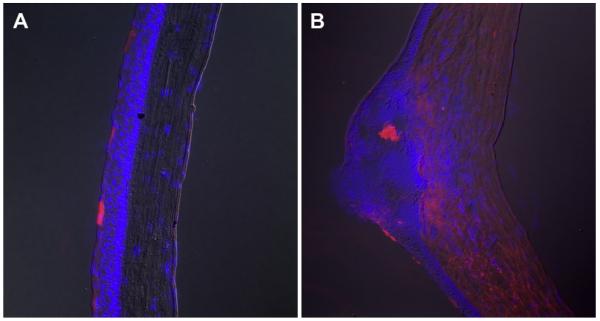
Bevacizumab is a large full-length immunoglobulin with a molecular weight of 149kD. The corneal epithelial barrier is thought to preclude the penetration of full-length immunoglobulins; nevertheless, topical bevacizumab has been successfully utilized in the treatment of corneal NV.242-248 To elucidate this incongruity, we investigated the corneal penetration of topical bevacizumab using the bFGF micropellet model of murine corneal NV.76 Topical bevacizumab 1.0% was administered to the eyes of mice with intact corneas and the eyes of mice with corneal NV. Mice were sacrificed at various time-points, and immunohistochemistry was performed to determine the extent of bevacizumab penetration. As expected, topical bevacizumab did not penetrate beyond the epithelial barrier of healthy corneas (Figure 2A); however, bevacizumab was detected in the corneal stroma of most mice with corneal NV, and staining intensity increased over time despite some variability in the extent of bevacizumab penetration (Figure 2B). These findings suggest that corneal NV diminishes the integrity of epithelial tight junctions, thereby permitting macromolecules such as bevacizumab to penetrate through the corneal epithelial barrier.
Figure 2.
Corneal penetration of topical bevacizumab. Immunohistochemical staining seven days after the initiation of topical bevacizumab 1% treatment, 3 times per day, in a normal cornea with intact epithelium (A), and a neovascular cornea (B). Immunoreactivity to bevacizumab was limited to the superficial epithelial layers of normal corneas (A), whereas immunoreactivity to bevacizumab was found in all layers of most neovascularized corneas (B) (×200).
B. The Comparison: Topical Ranibizumab (Lucentis) Vs Bevacizumab (Avastin)
1. Introduction
Topical bevacizumab is an effective treatment for corneal NV; however, there is some variability in the clinical response to topical bevacizumab treatment.244-248 Ranibizumab is a recombinant humanized monoclonal antibody fragment that binds and inhibits VEGF-A isoforms.249 Ranibizumab has a molecular weight of 48kD, making it approximately one-third the size of bevacizumab and theoretically allowing for better corneal penetration; additionally, ranibizumab has been affinity-matured to optimize its VEGF-A binding potential. These characteristics may enable ranibizumab to treat corneal NV more effectively than bevacizumab. We have completed two separate clinical studies investigating the safety and efficacy of topical bevacizumab and ranibizumab; herein, we will present a brief summary of our study results and compare treatment outcomes.250,251
2. Methods
Two prospective, open-label studies were performed investigating the safety and efficacy of topical ranibizumab and bevacizumab in the treatment of corneal NV. These studies were approved by the Institutional Review Boards of Massachusetts Eye & Ear Infirmary or Walter Reed Army Medical Center. Adults that did not meet any of the basic exclusion criteria (Table 2) and exhibited clinically stable corneal NV extending at least 2 mm beyond the corneal limbus were recruited for these studies. Patients were provided with either 1.0% ranibizumab or 1.0% bevacizumab ophthalmic solution and instructed to perform topical administration four times per day over a period of 3 weeks. Punctal plugs were placed in the superior and inferior punctae of treated eyes for the duration of the experiment in order to minimize systemic drug absorption.
Table 2.
Exclusion criteria for study enrollment
| • Age ≥ 75 years |
|---|
| • Ongoing or recent (≤3 months) episode of ocular infection |
| • Ongoing or recent (≤3 months) persistent corneal epithelial defect (≥ 14 days duration measuring ≥1 mm2) |
| • Ongoing or recent (≤3 months) contact lens use |
| • Recent (≤1 mo) change in dose or frequency of ocular steroids or NSAIDS |
| • Ocular or periocular neoplasia |
| • Recent (≤3 mo) or planned surgery |
| • Uncontrolled hypertension (systolic blood pressure ≥150 mmHg or diastolic blood pressure ≥90 mmHg) |
| • Diabetes mellitus |
| • History of a thromboembolic event (eg, myocardial infarction or cerebrovascular accident) |
| • Coagulation abnormality, including anticoagulation medications (excluding aspirin) |
Study appointments were held during weeks 1, 3, 8, and 16 of the ranibizumab study, and weeks 1, 3, 6, 12, and 24 of the bevacizumab study. Each patient visit included a detailed medical history review, blood pressure measurement, and a thorough ocular examination, including Snellen visual acuity measurement, slit-lamp biomicroscopy, and central corneal thickness measurement. Digital corneal photography was performed during all visits except week 1.
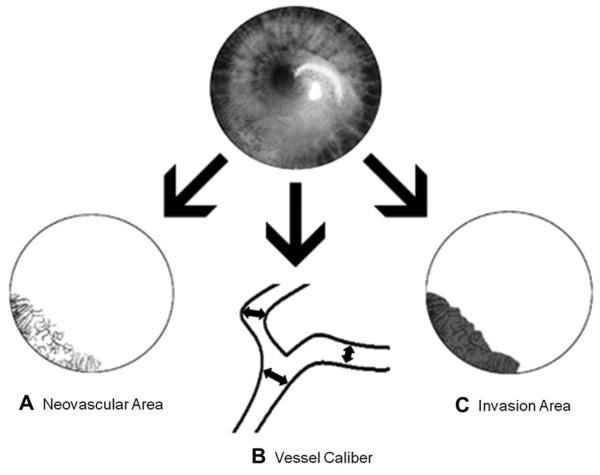
Treatment efficacy was evaluated by comparing digital slit-lamp pictures taken at baseline with pictures taken at follow-up visits. Analysis of the corneal vessels was performed using Photoshop CS2 (Adobe Systems Inc.; Berkeley, CA) and MATLAB (MathWorks, Inc.; Natick, MA). Three primary metrics of corneal NV (Figure 3) were evaluated: (A) neovascular area (NA), ie, the area of the corneal vessels; (B) vessel caliber (VC), ie, the mean corneal vessel diameter; and (C) invasion area (IA), ie, the fraction of the cornea into which vessel invasion occurred.247 Secondary measures of safety and efficacy included best-corrected visual acuity, central corneal thicknesses, and systemic blood pressure.
Figure 3.
Primary metrics of treatment efficacy. (A) Neovascular area (NA), ie, the area of the corneal vessels; (B) vessel caliber (VC), i.e., the mean corneal vessel diameter; and (C) invasion area (IA), ie, the fraction of the cornea into which vessel invasion occurs.
The paired t-test was used to compare study metrics for each cohort at follow-up visits with their baseline measures, and the unpaired t-test was used to compare study metrics between the cohorts in different studies. Data is expressed as the mean ± standard deviation (SD). P values ≤ 0.05 were considered statistically significant.
3. Results
Ten eyes from 9 patients were included in the ranibizumab study, and 20 eyes from 20 patients were included in the bevacizumab study. The average patient age was 57.3 ± 14.5 years for the ranibizumab study, and 52.5 ± 14.6 years for the bevacizumab study. The duration of corneal NV was 17.67 ± 19.18 months for the ranibizumab study and 13.69 ± 9.53 months for the bevacizumab study, excluding several cases of unknown duration.
a. Neovascular Area
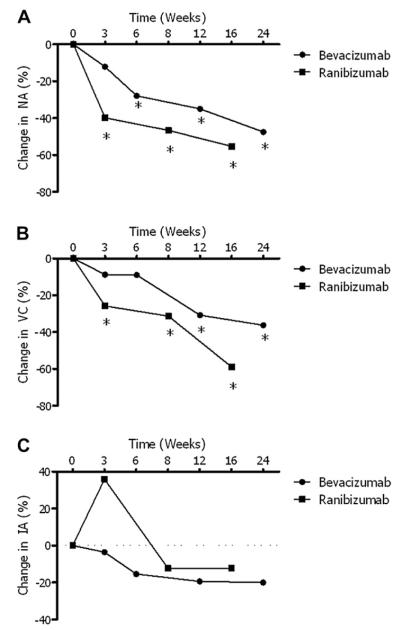
A statistically significant decrease in NA was observed from baseline to week 3 for the ranibizumab-treated group (−39.8% ± 24.1%; P < 0.001); meanwhile, a statistically significant decrease in NA was not observed until week 6 for patients treated with bevacizumab (−27.9% ± 41.2%; P = 0.007 [Figure 4A]). The average reduction in NA from baseline was 55.3% (SD, 44.4%; P < 0.001) at week 16 for the ranibizumab treated group, and 47.5% (SD, 37.5%; P < 0.001) at week 24 for patients treated with bevacizumab. Although the decrease in NA at comparable time points was consistently greater for patients treated with ranibizumab, these differences were not statistically significant.
Figure 4.
Summary and comparison of the primary study metrics. (A) Neovascular area (NA): the ranibizumab treated cohort experienced a significant decrease by week 3, while the bevacizumab treated cohort required 6 weeks to experience a significant decrease; (B) vessel caliber (VC): ranibizumab treated patients experienced an earlier significant reduction (3 weeks) than bevacizumab treated patients (12 weeks); (C) invasion area (IA): neither medication produced a significant decrease at any time point. (*P ≤ 0.05 as compared to baseline measures.)
b. Vessel Caliber
The decrease in VC reached statistical significance by week 3 for patients treated with ranibizumab (−25.8% ± 18.8%; P = 0.001). The decrease in VC did not reach significance until week 12 for patients treated with bevacizumab (−30.8% ± 41.7%; P = 0.006 [Figure 4B]). At the final study appointments, the average change in VC was −59.0% (SD, 34.9%; P < 0.001) for the ranibizumab group and −36.2% (SD, 44.1%; P = 0.003) for the bevacizumab group. The decrease in average VC was consistently greater for patients treated with ranibizumab at comparable time points; however, these differences were not statistically significant.
c. Invasion Area
The average change in IA was −12.3% (SD, 54.7%; P = 0.49) at week 16 for the ranibizumab-treated group, and −20.0% (SD, 42.0%; P = 0.06) at week 24 for the bevacizumab treated-group (Figure 4C). These average decreases were not statistically significant when compared to either baseline measurements or each other.
d. Additional End-points and Adverse Events
Snellen visual acuity measurements were converted to their LogMAR equivalents for analysis. The ranibizumab arm had a corrected LogMAR visual acuity of 0.68 ± 0.62 at baseline and 0.55 ± 0.37 at the final visit (week 16). The bevacizumab arm had a mean corrected LogMAR visual acuity of 0.60 ± 0.78 at baseline and 0.70 ± 0.75 at week 24. There were no statistically significant changes in visual acuity, corneal thickness, or systemic blood pressure in either study. No local (eg, corneal epitheliopathy) or systemic (eg, arteriolar hemorrhage) adverse events were observed or reported.
4. Discussion
Topical ranibizumab and bevacizumab are both effective treatments for corneal NV. Although the average decreases in NA and VC were greater for the ranibizumab-treated cohort, intergroup comparison did not reveal statistically significant differences at comparable time points. Topical ranibizumab was efficacious earlier in the course of treatment than topical bevacizumab, as measured by NA and VC. This may be due to the low molecular weight of ranibizumab allowing for more effective corneal penetration and the establishment of therapeutic concentrations earlier in the course of treatment. These findings are reminiscent of previous comparisons between ranibizumab and bevacizumab in the treatment of neovascular age-related macular degeneration. Ranibizumab was formulated in part because preliminary studies suggested that bevacizumab would not penetrate through the retina.249 Although it is not uncommon for intravitreal ranibizumab to improve measured outcomes slightly more than bevacizumab (eg, visual acuity, central retinal thickness), these differences are generally not significant.252
The results of our comparison suggest that topical ranibizumab may be superior to topical bevacizumab for the treatment of corneal NV; however, it is not possible to definitively reach this conclusion based on our study design and relatively small number of enrolled patients. Neither ranibizumab nor bevacizumab has been approved for the treatment of corneal NV; in order to justify the increased cost of ranibizumab, it will be necessary to demonstrate meaningful treatment superiority.253 Therefore, a prospective, randomized, head-to-head comparison trial will be required to make authoritative conclusions regarding the long-term efficacy of these medications.
Adverse events were neither observed nor reported for any of our patients who received topical anti-VEGF therapy. However, several clinical studies from other groups have reported local complications (eg, epithelial defects, corneal stromal thinning) associated with the application of topical bevacizumab (Table 3).245,246,248 Furthermore, ocular surface VEGF inhibition has the potential to induce neurotrophic keratopathy by blocking VEGF-mediated neural growth.254 Factors that may have contributed to the adverse events noted in previous studies include: 1) use of a bevacizumab concentration greater than 1.0%, 2) duration of treatment greater than 4 weeks, and 3) inclusion of patients with pre-existing corneal epithelial defects. Studies of the safety and efficacy of intravitreal ranibizumab and bevacizumab in the treatment of neovascular age-related macular degeneration have reported comparable rates of systemic adverse events.252,255 Topical VEGF inhibition has not been associated with any systemic adverse events in the limited number of clinical trials completed to date; however, given the risks, physicians should use discretion when selecting patients and take precautions to minimize systemic anti-VEGF exposure (eg, punctal plugs).
Table 3.
Summary of clinical trials investigating topical bevacizumab for the treatment of corneal NV
| Author | Corneal Pathology |
N | Conc. | Treatment Frequency |
Duration (Days) |
Percent with Decreased NV |
Percent with Ocular Event |
|---|---|---|---|---|---|---|---|
| DeStafeno | 4, 5 | 2 | 1.00% | 4/day | 25 | 100% | 0% |
| Bock | 1, 3 | 5 | 0.50% | 5/day | 108 | 100% | 20% |
| Kim | 1, 2, 3, 6, 9 | 10 | 1.25% | 2/day | 30-90 | 70% | 60% |
| Dastjerdi | 1, 2, 3, 6, 7, 8, 9 | 10 | 1.00% | 4/day | 21 | 100% | 0% |
| Koenig | 1, 2, 3, 6, 7, 8, 9, 10, 11 | 30 | 0.50% | 5/day | 102 | 80% | 16.7% |
Corneal pathology: 1) keratoplasty, 2) HSV keratitis, 3) chemical burn, 4) cicatricial pemphigoid, 5) traumatic rupture, 6) pterygium, 7) dry eye disease, 8) limbal stem cell deficiency, 9) Stevens-Johnson syndrome, 10) measles keratitis, 11) Salzmann’s nodular degeneration. (Conc. = Concentration).
IV. CONCLUDING COMMENTS
Although the clinical significance of corneal NV has long been recognized, management has been confounded by a lack of effective medical and surgical treatment modalities. A roundtable discussion was recently held to define unmet medical needs and formulate treatment guidelines for the management of corneal NV.256 The committee recognized the need for steroid-sparing pharmacological treatments for corneal NV and acknowledged the therapeutic potential of procedures such as FND and laser ablation. The committee’s proposed guidelines stress the importance of treating clinically relevant corneal NV, particularly in cases of infectious keratitis, keratoplasty, limbal stem cell deficiency, and chemical burns. The presence of corneal NV demonstrably increases the risk of graft rejection; therefore, pre- and post-conditioning of the host bed with anti-VEGF therapeutics is an attractive method of potentially increasing graft survival. Treatment guidelines will undoubtedly become more aggressive as the medical and surgical treatments of corneal NV become more effective.
The targeted inhibition of VEGF promises to provide clinicians with an angiogenesis-specific pharmacological approach to treating corneal NV. Based on our experience to date, topical VEGF inhibition using either ranibizumab or bevacizumab appears to be a safe, effective, and practical method for treating stable corneal NV. Clinicians wishing to integrate topical anti-VEGF in their clinical practice should take care in selecting patients and take precautions to minimize the risk of local and systemic adverse events. Novel anti-VEGF therapeutics (eg, VEGF trap, VEGF siRNA, and VEGFR tyrosine kinase inhibitors) may prove to be more effective than the currently available anti-VEGF agents.257-259 The concurrent inhibition of VEGF and other proangiogenic factors such as PDGF or Ang-2 promises to be more effective than the inhibition of VEGF alone.260,261
Combining angiogenesis-specific medical therapy with surgical procedures may overcome limitations in the efficacy of both.212,224 Novel antiangiogenic agents such as gene signal (GS)-101, a potent inhibitor of the scaffold protein insulin receptor substrate-1 (IRS-1) have shown promise in preclinical and clinical trials.262,263 The continued development of antiangiogenic agents such as these promises to provide physicians with new tools to safely and effectively treat this potentially devastating, yet often overlooked, condition.
Acknowledgments
Supported in part by NIH grants EY-12963 (RD) and EY-19098 (RD). The authors have no proprietary or commercial interests in any concept or product discussed in this article.
Abbreviations
- Ang
Angiopoietin
- CD
Cluster of differentiation
- FasL
Fas ligand
- FGF
Fibroblast growth factor
- FND
Fine-needle diathermy
- HSV
Herpes simplex virus
- IA
Invasion area
- IL
Interleukin
- MMP
Matrix metalloproteinase
- MT-MMP
Membrane-type MMP
- mVEGFR
Membrane-bound VEGF receptor
- NA
Neovascular area
- NSAID
Nonsteroidal anti-inflammatory drug
- NV
Neovascularization
- PDGF
Platelet-derived growth factor
- PD-L1
Programmed death-ligand 1
- PDT
Photodynamic therapy
- PEDF
Pigment epithelium-derived factor
- PlGF
Placental growth factor
- sVEGFR
Soluble VEGF receptor
- TGF
Transforming growth factor
- TIMP
Tissue inhibitor of metalloproteinases
- TNF
Tumor necrosis factor
- TSP
Thrombospondin
- VC
Vessel caliber
- VEGF
Vascular endothelial growth factor
- VEGFR
VEGF receptor
- WHO
World Health Organization
Footnotes
Single-copy reprint requests to Reza Dana, MD, MPH, MSc (address below).
REFERENCES
- 1.Beebe DC. Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–33. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011;30:927–38. doi: 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 7.Bock F, König Y, Dietrich T, et al. Inhibition of angiogenesis in the anterior chamber of the eye. Ophthalmologe. 2007;104:336–44. doi: 10.1007/s00347-007-1512-2. [DOI] [PubMed] [Google Scholar]
- 8.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–94. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed August 2, 2011];WHO releases the new global estimates on visual impairment. World Health Organization Web site. 2010 Available at: http://www.who.int/blindness/en.
- 10.Whitcher JP, Srinivasan M, Upadhyay MP. [79Accessed August 2, 2011];Corneal blindness: a global perspective. Bull World Health Organ. 2001 :214–21. http://www.who.int/bulletin/archives/79%283%29214.pdf. [PMC free article] [PubMed]
- 11. [Accessed August 2, 2011];Program for the prevention of blindness and deafness: data available on blindness. World Health Organization Web site. 2006 Available at: http://www.who.int/blindness/publications/global_data.pdf.
- 12.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–69. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 13.Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization. Pathogenesis and inhibition. Cornea. 1987;6:250–7. doi: 10.1097/00003226-198706040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Report of the global scientific meeting on future approaches to trachoma control; Geneva. 17–20 June 1996; World Health Organization; [Accessed August 2, 2011]. 1997. Available at: http://whqlibdoc.who.int/hq/1996/WHO_PBL_96.56.pdf. [Google Scholar]
- 15.Solomon AW, Zondervan M, Kuper H, et al. [Accessed August 2, 2011];Trachoma control: a guide for programme managers. World Health Organization. 2006 Available at: http://www.who.int/blindness/publications/tcm%20who_pbd_get_06_1.pdf.
- 16. [Accessed August 2, 2011];Water sanitation and health. World Health Organization Web site. 2001 Available at: http://www.who.int/water_sanitation_health/diseases/oncho/en.
- 17.African Programme for Onchocerciasis Control (APOC) APOC; Ouagadougou (Burkina Faso): 2005. [Google Scholar]; Final communiqué of the 11th session of the Joint Action Forum (JAF) of APOC; Paris, France. 6–9 December 2005. [Google Scholar]
- 18.World Health Organization . World Health Organization; Geneva: 1995. [Google Scholar]; Onchocerciasis and its control. (WHO Technical Report Series).Report of a WHO expert committee on onchocerciasis control. 1995;852:110. [PubMed] [Google Scholar]
- 19.Allen JE, Adjei O, Bain O, et al. Of mice, cattle, and humans: the immunology and treatment of river blindness. PLoS Negl Trop Dis. 2008;2:e217. doi: 10.1371/journal.pntd.0000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Bureau of the Census . U.S. Census Bureau Announces 2010 Census Population Counts – Apportionment Counts Delivered to President. US Bureau of the Census; Washington, DC: 2010. Available at: http://2010.census.gov/news/releases/operations/cb10-cn93.html. [Google Scholar]
- 21.Liesegang TJ, Melton LJ, 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–9. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 22.Liesegang TJ. Biology and molecular aspects of herpes simplex and varicella-zoster virus infections. Ophthalmology. 1992;99:781–99. doi: 10.1016/s0161-6420(92)31921-9. [DOI] [PubMed] [Google Scholar]
- 23.Negrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiol. 1998;5:143–67. doi: 10.1076/opep.5.3.143.8364. [DOI] [PubMed] [Google Scholar]
- 24.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–48. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 25.Murube J. Aniridia and the ocular surface. Ocul Surf. 2004;2:55–7. doi: 10.1016/s1542-0124(12)70144-1. [DOI] [PubMed] [Google Scholar]
- 26.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–43. [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed September 6, 2011];Cornea donation & transplantation statistics. Eye Bank Association of America Web site. Available at: http://www.restoresight.org/about-us/understanding-donation/cornea-donation-transplantation-statistics.
- 28.Dana MR, Schaumberg DA, Kowal VO, et al. Corneal neovascularization after penetrating keratoplasty. Cornea. 1995;14:604–9. [PubMed] [Google Scholar]
- 29.Volker-Dieben HJ, D’Amaro J, Kok-Van Alphen CC. Hierarchy of prognostic factors for corneal allograft survival. Aust N Z J Ophthalmol. 1987;15:11–8. doi: 10.1111/j.1442-9071.1987.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 30.Rocha G, Deschênes J, Rowsey JJ. The immunology of corneal graft rejection. Crit Rev Immunol. 1998;18:305–25. doi: 10.1615/critrevimmunol.v18.i4.20. [DOI] [PubMed] [Google Scholar]
- 31.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–403. [PubMed] [Google Scholar]
- 32.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–5.e7. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Dana MR. Angiogenesis and lymphangiogenesis-implications for corneal immunity. Semin Ophthalmol. 2006;21:19–22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 34.Cursiefen C, Kuchle M, Naumann GO. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea. 1998;17:611–3. doi: 10.1097/00003226-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Barr J. Annual Report. Contact Lens Spectrum 2005. 2004 Jan; [Google Scholar]
- 36.Suchecki JK, Donshik P, Ehlers WH. Contact lens complications. Ophthalmol Clin North Am. 2003;16:471–84. doi: 10.1016/s0896-1549(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 37.Cursiefen C, Kruse FE. Cornea and external eye disease. In: Reinhard T, Larkin DFP, editors. Essentials in ophthalmology. Springer; Germany: 2006. pp. 83–99. [Google Scholar]
- 38.Safvati A, Cole N, Hume E, Willcox M. Mediators of neovascularization and the hypoxic cornea. Curr Eye Res. 2009;34:501–14. doi: 10.1080/02713680902919557. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg RJ. Deep corneal vascularization caused by aphakic soft contact lens wear. Am J Ophthalmol. 1977;83:121–2. doi: 10.1016/0002-9394(77)90200-8. [DOI] [PubMed] [Google Scholar]
- 40.Donnenfeld ED, Ingraham H, Perry HD, et al. Contact lens-related deep stromal intracorneal hemorrhage. Ophthalmology. 1991;98:1793–6. doi: 10.1016/s0161-6420(91)32048-7. [DOI] [PubMed] [Google Scholar]
- 41.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. J Hematother Stem Cell Res. 2002;11:171–8. doi: 10.1089/152581602753658385. [DOI] [PubMed] [Google Scholar]
- 44.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–9. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 45.Burger PC, Chandler DB, Klintworth GK. Experimental corneal neovascularization: biomicroscopic, angiographic, and morphologic correlation. Cornea. 1985-1986;4:35–41. [PubMed] [Google Scholar]
- 46.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46:3502–6. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 48.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 49.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 50.Stuttfeld E, Ballmer-Hofer K. Structure and function of VEGF receptors. IUBMB Life. 2009;61:915–22. doi: 10.1002/iub.234. [DOI] [PubMed] [Google Scholar]
- 51.Harper SJ, Bates DO. VEGF-A splicing: the key to antiangiogenic therapeutics? Nat Rev Cancer. 2008;8:880–7. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCourt M, Wang JH, Sookhai S, Redmond HP. Proinflammatory mediators stimulate neutrophil-directed angiogenesis. Arch Surg. 1999;134:1325–31. doi: 10.1001/archsurg.134.12.1325. discussion 1331–2. [DOI] [PubMed] [Google Scholar]
- 53.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 54.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 56.Bates DO, Hillman NJ, Williams B, et al. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–97. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(3 Pt 2):H1054–8. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 58.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- 59.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–40. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 61.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amano S, Rohan R, Kuroki M, et al. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 63.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–22. [PubMed] [Google Scholar]
- 64.Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27:992–5. doi: 10.1097/ICO.0b013e31817786ad. [DOI] [PubMed] [Google Scholar]
- 65.Oh JY, Kim MK, Wee WR. Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009;28:1070–3. doi: 10.1097/ICO.0b013e31819839f9. [DOI] [PubMed] [Google Scholar]
- 66.Olsen SK, Garbi M, Zampieri N, et al. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J Biol Chem. 2003;278:34226–36. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- 67.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–9. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller DL, Ortega S, Bashayan O, et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–8. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nat Med. 1997;3:158–64. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]
- 71.Yanagisawa-Miwa A, Uchida Y, Nakamura F, et al. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992;257(5075):1401–3. doi: 10.1126/science.1382313. [DOI] [PubMed] [Google Scholar]
- 72.Murakami M, Nguyen LT, Zhuang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adamis AP, Meklir B, Joyce NC. In situ injury-induced release of basic-fibroblast growth factor from corneal epithelial cells. Am J Pathol. 1991;139:961–7. [PMC free article] [PubMed] [Google Scholar]
- 74.Folkman J, Klagsbrun M, Sasse J, et al. A heparin-binding angiogenic protein–basic fibroblast growth factor–is stored within basement membrane. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 75.Soubrane G, Jerdan J, Karpouzas I, et al. Binding of basic fibroblast growth factor to normal and neovascularized rabbit cornea. Invest Ophthalmol Vis Sci. 1990;31:323–33. [PubMed] [Google Scholar]
- 76.Dastjerdi MH, Sadrai Z, Saban DR, et al. Corneal penetration of topical and subconjunctival bevacizumab (Avastin) Invest Ophthalmol Vis Sci. 2011;52:8718–23. doi: 10.1167/iovs.11-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 78.McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 2003;130:3469–78. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- 79.Munier AI, Doucet D, Perrodou E, et al. PVF2, a PDGF/VEGF-like growth factor, induces hemocyte proliferation in Drosophila larvae. EMBO Rep. 2002;3:1195–200. doi: 10.1093/embo-reports/kvf242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Levéen P, Pekny M, Gebre-Medhin S, et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 82.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–96. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 83.Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 84.Hellström M, Kalén M, Lindahl P, et al. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 85.Kim WJ, Mohan RR, Mohan RR, Wilson SE. Effect of PDGF, IL-1alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci. 1999;40:1364–72. [PubMed] [Google Scholar]
- 86.Vesaluoma M, Teppo AM, Grönhagen-Riska C, Tervo T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: a potential modulator of corneal wound healing following photorefractive keratectomy. Curr Eye Res. 1997;16:825–31. doi: 10.1076/ceyr.16.8.825.8984. [DOI] [PubMed] [Google Scholar]
- 87.Hoppenreijs VP, Pels E, Vrensen GF, et al. Platelet-derived growth factor: receptor expression in corneas and effects on corneal cells. Invest Ophthalmol Vis Sci. 1993;34:637–49. [PubMed] [Google Scholar]
- 88.Dell S, Peters S, Müther P, et al. The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:1928–37. doi: 10.1167/iovs.05-1071. [DOI] [PubMed] [Google Scholar]
- 89.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 90.Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96:1904–9. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 92.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 93.Loughna S, Sato TN. Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol. 2001;20(5-6):319–25. doi: 10.1016/s0945-053x(01)00149-4. [DOI] [PubMed] [Google Scholar]
- 94.Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 95.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–10. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghajar CM, George SC, Putnam AJ. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:251–78. doi: 10.1615/critreveukargeneexpr.v18.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mignatti P, Tsuboi R, Robbins E, Rifkin DB. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989;108:671–82. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cornelius LA, Nehring LC, Roby JD, et al. Human dermal microvascular endothelial cells produce matrix metalloproteinases in response to angiogenic factors and migration. J Invest Dermatol. 1995;105:170–6. doi: 10.1111/1523-1747.ep12317080. [DOI] [PubMed] [Google Scholar]
- 99.Hanemaaijer R, Koolwijk P, Le Clercq L, et al. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Biochem J. 1993;296:803–9. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unemori EN, Bouhana KS, Werb Z. Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem. 1990;265:445–51. [PubMed] [Google Scholar]
- 101.Itoh T, Tanioka M, Yoshida H, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–51. [PubMed] [Google Scholar]
- 102.Fang J, Shing Y, Wiederschain D, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–9. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee S, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masson V, de la Ballina LR, Munaut C, et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–6. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeong JW, Cha HJ, Yu DY, et al. Induction of membrane-type matrix metalloproteinase-1 stimulates angiogenic activities of bovine aortic endothelial cells. Angiogenesis. 1999;3:167–74. doi: 10.1023/a:1009065709676. [DOI] [PubMed] [Google Scholar]
- 107.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chun TH, Sabeh F, Ota I, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–67. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–21. [PubMed] [Google Scholar]
- 110.Kato T, Kure T, Chang JH, et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–90. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 111.Samolov B, Steen B, Seregard S, et al. Delayed inflammation-associated corneal neovascularization in MMP-2-deficient mice. Exp Eye Res. 2005;80:159–66. doi: 10.1016/j.exer.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 112.Mimura T, Han KY, Onguchi T, et al. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–50. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Azar DT, Casanova FH, Mimura T, et al. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008;33:954–62. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azar DT, Casanova FH, Mimura T, et al. Corneal epithelial MT1-MMP inhibits vascular endothelial cell proliferation and migration. Cornea. 2010;29:321–30. doi: 10.1097/ICO.0b013e3181b1165d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li J, Perrella MA, Tsai JC, et al. Induction of vascular endothelial growth factor gene expression by interleukin-1 beta in rat aortic smooth muscle cells. J Biol Chem. 1995;270:308–12. doi: 10.1074/jbc.270.1.308. [DOI] [PubMed] [Google Scholar]
- 116.Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 117.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–8. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 118.Pertovaara L, Kaipainen A, Mustonen T, et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–4. [PubMed] [Google Scholar]
- 119.Li A, Dubey S, Varney ML, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 120.Sun J. Matrix metalloproteinases and tissue inhibitor of metalloproteinases are essential for the inflammatory response in cancer cells. J Signal Transduct. 2010;2010:985132. doi: 10.1155/2010/985132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, Huq S, Gardner H, et al. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007;125:783–8. doi: 10.1001/archopht.125.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Senger DR, Perruzzi CA, Streit M, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakao S, Hata Y, Miura M, et al. Dexamethasone inhibits interleukin-1beta induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171:1058–65. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ebrahem Q, Minamoto A, Hoppe G, et al. Triamcinolone acetonide inhibits IL-6- and VEGF-induced angiogenesis downstream of the IL-6 and VEGF receptors. Invest Ophthalmol Vis Sci. 2006;47:4935–41. doi: 10.1167/iovs.05-1651. [DOI] [PubMed] [Google Scholar]
- 125.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ambati BK, Patterson E, Jani P, et al. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol. 2007;91:505–8. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its hetero-dimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–8. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 129.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–30. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–10. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung ES, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–92. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simonovic M, Gettins PG, Volz K. Crystal structure of human PEDF, a potent antiangiogenic and neurite growth-promoting factor. Proc Natl Acad Sci U S A. 2001;98:11131–5. doi: 10.1073/pnas.211268598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem. 2009;106:769–75. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 134.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 135.Zamiri P, Masli S, Streilein JW, Taylor AW. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci. 2006;47:3912–8. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- 136.Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv Exp Med Biol. 1997;425:223–37. [PubMed] [Google Scholar]
- 137.Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–9. [PubMed] [Google Scholar]
- 138.Mori K, Gehlbach P, Ando A, et al. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43:2428–34. [PubMed] [Google Scholar]
- 139.Karakousis PC, John SK, Behling KC, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001;7:154–63. [PubMed] [Google Scholar]
- 140.Ogata N, Wada M, Otsuji T, et al. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1168–75. [PubMed] [Google Scholar]
- 141.Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002;277:45400–7. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- 142.Jin J, Ma JX, Guan M, Yao K. Inhibition of chemical cautery-induced corneal neovascularization by topical pigment epithelium-derived factor eyedrops. Cornea. 2010;29:1055–61. doi: 10.1097/ICO.0b013e3181cc7987. [DOI] [PubMed] [Google Scholar]
- 143.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–28. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 144.Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9) J Biol Chem. 1997;272:28823–5. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- 145.Moser TL, Stack MS, Asplin I, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–6. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moser TL, Kenan DJ, Ashley TA, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–61. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Troyanovsky B, Levchenko T, Månsson G, et al. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–54. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J Biol Chem. 2001;276:39562–8. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- 149.Wajih N, Sane DC. Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood. 2003;101:1857–63. doi: 10.1182/blood-2002-02-0582. [DOI] [PubMed] [Google Scholar]
- 150.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mirza AM, Kohn AD, Roth RA, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–92. [PubMed] [Google Scholar]
- 152.Albini A, Brigati C, Ventura A, et al. Angiostatin antiangiogenesis requires IL-12: the innate immune system as a key target. J Transl Med. 2009;7:5. doi: 10.1186/1479-5876-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benelli R, Morini M, Carrozzino F, et al. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–9. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- 154.Chavakis T, Athanasopoulos A, Rhee JS, et al. Angiostatin is a novel anti-inflammatory factor by inhibiting leukocyte recruitment. Blood. 2005;105:1036–43. doi: 10.1182/blood-2004-01-0166. [DOI] [PubMed] [Google Scholar]
- 155.Sack RA, Beaton AR, Sathe S. Diurnal variations in angiostatin in human tear fluid: a possible role in prevention of corneal neovascularization. Curr Eye Res. 1999;18:186–93. doi: 10.1076/ceyr.18.3.186.5367. [DOI] [PubMed] [Google Scholar]
- 156.Gabison E, Chang JH, Hernández-Quintela E, et al. Antiangiogenic role of angiostatin during corneal wound healing. Exp Eye Res. 2004;78:579–89. doi: 10.1016/j.exer.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 157.Ambati BK, Joussen AM, Ambati J, et al. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002;120:1063–8. doi: 10.1001/archopht.120.8.1063. [DOI] [PubMed] [Google Scholar]
- 158.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 159.Muragaki Y, Timmons S, Griffith CM, et al. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc Natl Acad Sci U S A. 1995;92(19):8763–7. doi: 10.1073/pnas.92.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rehn M, Pihlajaniemi T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci U S A. 1994;91:4234–8. doi: 10.1073/pnas.91.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rehn M, Hintikka E, Pihlajaniemi T. Primary structure of the alpha 1 chain of mouse type XVIII collagen, partial structure of the corresponding gene, and comparison of the alpha 1(XVIII) chain with its homologue, the alpha 1(XV) collagen chain. J Biol Chem. 1994;269(19):13929–35. [PubMed] [Google Scholar]
- 162.Saarela J, Rehn M, Oikarinen A, et al. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611–26. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferreras M, Felbor U, Lenhard T, et al. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–51. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 164.Kim YM, Hwang S, Kim YM, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277(31):27872–9. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 165.Sertié AL, Sossi V, Camargo AA, et al. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome) Hum Mol Genet. 2000;9:2051–8. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]
- 166.Fukai N, Eklund L, Marneros AG, et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–44. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin HC, Chang JH, Jain S, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–24. [PubMed] [Google Scholar]
- 168.Yu Y, Moulton KS, Khan MK, et al. E-selectin is required for the antiangiogenic activity of endostatin. Proc Natl Acad Sci U S A. 2004;101:8005–10. doi: 10.1073/pnas.0402551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chang JH, Javier JA, Chang GY, et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579(17):3601–6. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 170.Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582(17):2515–20. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74(2-3):85–9. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Y, Yin H, Chen P, Xie L, Wang Y. Inhibitory effect of canstatin in alkali burn-induced corneal neovascularization. Ophthalmic Res. 2011;46:66–72. doi: 10.1159/000322804. [DOI] [PubMed] [Google Scholar]
- 173.Huo N, Ichikawa Y, Kamiyama M, et al. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett. 2002;177:95–100. doi: 10.1016/s0304-3835(01)00772-8. [DOI] [PubMed] [Google Scholar]
- 174.Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 175.Ito TK, Ishii G, Saito S, et al. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood. 2009;113:2363–9. doi: 10.1182/blood-2008-08-172742. [DOI] [PubMed] [Google Scholar]
- 176.Lu PC, Ye H, Maeda M, Azar DT. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–7. [PubMed] [Google Scholar]
- 177.Kure T, Chang JH, Kato T, et al. Corneal neovascularization after excimer keratectomy wounds in matrilysin-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:137–44. doi: 10.1167/iovs.01-1058. [DOI] [PubMed] [Google Scholar]
- 178.Yeh SI, Han KY, Sabri A, et al. MMP-7 knock-in corneal fibroblast cell lines secrete MMP-7 with proteolytic activity towards collagen XVIII. Curr Eye Res. 2010;35:799–805. doi: 10.3109/02713683.2010.494239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Panetti TS, Chen H, Misenheimer TM, et al. Endothelial cell mitogenesis induced by LPA: inhibition by thrombospondin-1 and thrombospondin-2. J Lab Clin Med. 1997;129:208–16. doi: 10.1016/s0022-2143(97)90141-4. [DOI] [PubMed] [Google Scholar]
- 180.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–72. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iruela-Arispe ML, Bornstein P, Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991;88:5026–30. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jiménez B, Volpert OV, Crawford SE, et al. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 183.Hiscott P, Seitz B, Schlötzer-Schrehardt U, Naumann GO. Immuno-localisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997;289:307–10. doi: 10.1007/s004410050877. [DOI] [PubMed] [Google Scholar]
- 184.Cursiefen C, Masli S, Ng TF, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–24. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 185.Mwaikambo BR, Sennlaub F, Ong H, et al. Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4356–64. doi: 10.1167/iovs.05-1656. [DOI] [PubMed] [Google Scholar]
- 186.Cursiefen C, Maruyama K, Bock F, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mwaikambo BR, Sennlaub F, Ong H, et al. Genetic ablation of CD36 induces age-related corneal neovascularization. Cornea. 2008;27:1037–41. doi: 10.1097/ICO.0b013e31817780b6. [DOI] [PubMed] [Google Scholar]
- 188.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 189.Griffith TS, Yu X, Herndon JM, et al. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 190.Stuart PM, Pan F, Plambeck S, Ferguson TA. FasL-Fas interactions regulate neovascularization in the cornea. Invest Ophthalmol Vis Sci. 2003;44:93–8. doi: 10.1167/iovs.02-0299. [DOI] [PubMed] [Google Scholar]
- 191.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin Y, Chauhan SK, Annan JE, et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am J Pathol. 2011;178:1922–9. doi: 10.1016/j.ajpath.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–9. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 194.Cursiefen C, Wenkel H, Martus P, et al. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2001;239:514–21. doi: 10.1007/s004170100313. [DOI] [PubMed] [Google Scholar]
- 195.Wahl SM. Corticosteroid inhibition of chemotactic lymphokine production by T and B lymphocytes. Ann N Y Acad Sci. 1975;256:375–85. doi: 10.1111/j.1749-6632.1975.tb36064.x. [DOI] [PubMed] [Google Scholar]
- 196.Claman HN. Corticosteroids and lymphoid cells. N Engl J Med. 1972;287:388–97. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- 197.Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–5. [PubMed] [Google Scholar]
- 198.Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990;20:2439–43. doi: 10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- 199.Jørgensen KA, Stoffersen E. Hydrocortisone inhibits platelet prostaglandin and endothelial prostacyclin production. Pharmacol Res Commun. 1981;13:579–86. doi: 10.1016/s0031-6989(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 200.Takahashi K, Saishin Y, Saishin Y, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003;44:409–15. doi: 10.1167/iovs.02-0346. [DOI] [PubMed] [Google Scholar]
- 201.Frucht J, Zauberman H. Topical indomethacin effect on neovascularization of the cornea and on prostaglandin E2 levels. Br J Ophthalmol. 1984;68:656–9. doi: 10.1136/bjo.68.9.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.BenEzra D. Neovasculogenic ability of prostaglandins, growth factors, and synthetic chemoattractants. Am J Ophthalmol. 1978;86:455–61. doi: 10.1016/0002-9394(78)90289-1. [DOI] [PubMed] [Google Scholar]
- 203.Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108:936–44. doi: 10.1016/s0161-6420(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 204.Halldorsson T. Alteration of optical and thermal properties of blood by Nd:YAG laser irradiation. Proceedings of the 4th congress of the international society for laser surgery; Tokyo, Japan. Nov 23–27, 1981. [Google Scholar]
- 205.Nirankari VS. Laser photocoagulation for corneal stromal vascularization. Trans Am Ophthalmol Soc. 1992;90:595–669. [PMC free article] [PubMed] [Google Scholar]
- 206.Baer JC, Foster CS. Corneal laser photocoagulation for treatment of neovascularization. Efficacy of 577 nm yellow dye laser. Ophthalmology. 1992;99:173–9. [PubMed] [Google Scholar]
- 207.Parsa CF, Temprano J, Wilson D, et al. Hemorrhage complicating YAG laser feeder vessel coagulation of cornea vascularization. Cornea. 1994;13:264–8. doi: 10.1097/00003226-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 208.Cherry PM, Garner A. Corneal neovascularization treated with argon laser. Br J Ophthalmol. 1976;60:464–72. doi: 10.1136/bjo.60.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Marsh RJ, Marshall J. Treatment of lipid keratopathy with argon laser. Br J Ophthalmol. 1982;66:127–35. doi: 10.1136/bjo.66.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Marsh RJ. Argon laser treatment of lipid keratopathy. Br J Ophthalmol. 1988;72:900–4. doi: 10.1136/bjo.72.12.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nirankari VS, Baer JC. Corneal argon laser photocoagulation for neovascularization in penetrating keratoplasty. Ophthalmology. 1986;93:1304–9. doi: 10.1016/s0161-6420(86)33581-4. [DOI] [PubMed] [Google Scholar]
- 212.Gerten G. Bevacizumab (avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea. 2008;27:1195–9. doi: 10.1097/ICO.0b013e318180e50f. [DOI] [PubMed] [Google Scholar]
- 213.Pai VH, Handary SV. Necrotizing scleritis following laser therapy for corneal vascularization. Ann Ophthalmol. 2009;41:50–1. [PubMed] [Google Scholar]
- 214.Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001;5:105–29. [Google Scholar]
- 215.Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photo-inactivation of a murine tumor. Cancer Res. 1976;36:2326–9. [PubMed] [Google Scholar]
- 216.Reed MW, Miller FN, Wieman TJ, et al. The effect of photodynamic therapy on the microcirculation. J Surg Res. 1988;45:452–9. doi: 10.1016/0022-4804(88)90195-3. [DOI] [PubMed] [Google Scholar]
- 217.Fingar VH. Vascular effects of photodynamic therapy. J Clin Laser Med Surg. 1996;14:323–8. doi: 10.1089/clm.1996.14.323. [DOI] [PubMed] [Google Scholar]
- 218.Fossarello M, Peiretti E, Zucca I, et al. Photodynamic therapy of corneal neovascularization with verteporfin. Cornea. 2003;22:485–8. doi: 10.1097/00003226-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 219.Yoon KC, You IC, Kang IS, et al. Photodynamic therapy with verteporfin for corneal neovascularization. Am J Ophthalmol. 2007;144:390–5. doi: 10.1016/j.ajo.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 220.Gordon YJ, Mann RK, Mah TS, et al. Fluorescein-potentiated argon laser therapy improves symptoms and appearance of corneal neovascularization. Cornea. 2002;21:770–3. doi: 10.1097/00003226-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 221.Sheppard JD, Jr, Epstein RJ, Lattanzio FA, Jr, et al. Argon laser photodynamic therapy of human corneal neovascularization after intravenous administration of dihematoporphyrin ether. Am J Ophthalmol. 2006;141:524–9. doi: 10.1016/j.ajo.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 222.Corrent G, Roussel TJ, Tseng SCG, et al. Promotion of graft survival by photothrombotic occlusion of corneal neovascularization. Arch Ophthalmol. 1989;107:1051–6. doi: 10.1001/archopht.1989.01070020575043. [DOI] [PubMed] [Google Scholar]
- 223.Brooks BJ, Ambati BK, Marcus DM, Ratanasit A. Photodynamic therapy for corneal neovascularisation and lipid degeneration. Br J Ophthalmol. 2004;88:840. doi: 10.1136/bjo.2003.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.You IC, Im SK, Lee SH, Yoon KC. Photodynamic therapy with verteporfin combined with subconjunctival injection of bevacizumab for corneal neovascularization. Cornea. 2011;30:30–3. doi: 10.1097/ICO.0b013e3181dc81a0. [DOI] [PubMed] [Google Scholar]
- 225.Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–53. [PubMed] [Google Scholar]
- 226.Wertheim MS, Cook SD, Knox-Cartwright NE, et al. Electrolysis-needle cauterization of corneal vessels in patients with lipid keratopathy. Cornea. 2007;26:230–1. doi: 10.1097/01.ico.0000248383.09272.ee. [DOI] [PubMed] [Google Scholar]
- 227.Thatte S. Fine needle diathermy - A choice for managing corneal vascularization. Nepal J Ophthalmol. 2011;3:23–6. doi: 10.3126/nepjoph.v3i1.4274. [DOI] [PubMed] [Google Scholar]
- 228.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–22. doi: 10.1016/s0161-6420(89)32833-8. discussion 722-3. [DOI] [PubMed] [Google Scholar]
- 229.Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–66. doi: 10.1016/s0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 230.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990–3. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 231.Pauklin M, Steuhl KP, Meller D. Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium. Ophthalmology. 2009;116:1048–56. doi: 10.1016/j.ophtha.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 232.Ono K, Yokoo S, Mimura T, et al. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol Vis. 2007;13:1138–43. [PMC free article] [PubMed] [Google Scholar]
- 233.Ang LP, Tanioka H, Kawasaki S, et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2010;51:758–64. doi: 10.1167/iovs.09-3379. [DOI] [PubMed] [Google Scholar]
- 234.Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001;12:269–81. doi: 10.1097/00055735-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 235.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–87. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 236.Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251–9. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 237.Kheirkhah A, Casas V, Raju VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008;145:787–94. doi: 10.1016/j.ajo.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 239.Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 240.Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–6. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 241.Avastin package insert. Genentech, Inc; South San Francisco, CA: 2009. [Google Scholar]
- 242.Thiel MA, Coster DJ, Standfield SD, et al. Penetration of engineered antibody fragments into the eye. Clin Exp Immunol. 2002;128:67–74. doi: 10.1046/j.1365-2249.2002.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brereton HM, Taylor SD, Farrall A, et al. Influence of format on in vitro penetration of antibody fragments through porcine cornea. Clin Exp Immunol. 2002;128:67–74. doi: 10.1136/bjo.2005.066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007;125:834–6. doi: 10.1001/archopht.125.6.834. [DOI] [PubMed] [Google Scholar]
- 245.Bock F, König Y, Kruse F, et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–4. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 246.Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–8. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 247.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–9. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Koenig Y, Bock F, Horn F, et al. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247:1375–82. doi: 10.1007/s00417-009-1099-1. [DOI] [PubMed] [Google Scholar]
- 249.Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 250.Ferrari G, Okanobo A, Dastjerdi M, et al. Topical ranibizumab as a treatment of corneal neovascularization. 2011. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- 251.Cheng SF, Ferrari G, Okanobo A, et al. Sustained efficacy of topical bevacizumab in the treatment of corneal neovascularization (NV) Invest Ophthalmol Vis Sci. 2011 ARVO:6399–D863. [Google Scholar]
- 252.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Steinbrook R. The price of sight–ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–12. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 254.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue–beyond blood vessels. Exp Neurol. 2004;187:246–53. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 255.Van der Reis MI, La Heij EC, Jong-Hesse YD, et al. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31:1449–69. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 256.Cursiefen C, Colin J, Dana R, et al. Consensus statement on indications for antiangiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol. 2012;96:3–9. doi: 10.1136/bjo.2011.204701. [DOI] [PubMed] [Google Scholar]
- 257.Heier JS, Boyer D, Nguyen QD, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology. 2011;118:1098–106. doi: 10.1016/j.ophtha.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 258.Zuo L, Fan Y, Wang F, et al. A siRNA targeting vascular endothelial growth factor-A inhibiting experimental corneal neovascularization. Curr Eye Res. 2010;35:375–84. doi: 10.3109/02713681003597230. [DOI] [PubMed] [Google Scholar]
- 259.Keskin U, Totan Y, Karadağ R, et al. Inhibitory effects of SU5416, a selective vascular endothelial growth factor receptor tyrosine kinase inhibitor, on experimental corneal neovascularization. Ophthalmic Res. 2011;47:13–8. doi: 10.1159/000324994. [DOI] [PubMed] [Google Scholar]
- 260.Chaoran Z, Zhirong L, Gezhi X. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves the antiangiogenic efficacy for advanced stage mouse corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2011;249:1493–501. doi: 10.1007/s00417-011-1709-6. [DOI] [PubMed] [Google Scholar]
- 261.Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–23. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Al-Mahmood S, Colin S, Farhat N, et al. Potent in vivo antiangiogenic effects of GS-101 (5′-TATCCGGAGGGCTCGCCATGCTGCT-3′), an antisense oligonucleotide preventing the expression of insulin receptor substrate-1. J Pharmacol Exp Ther. 2009;329:496–504. doi: 10.1124/jpet.108.147496. [DOI] [PubMed] [Google Scholar]
- 263.Cursiefen C, Bock F, Horn FK, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–7. doi: 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]