Abstract
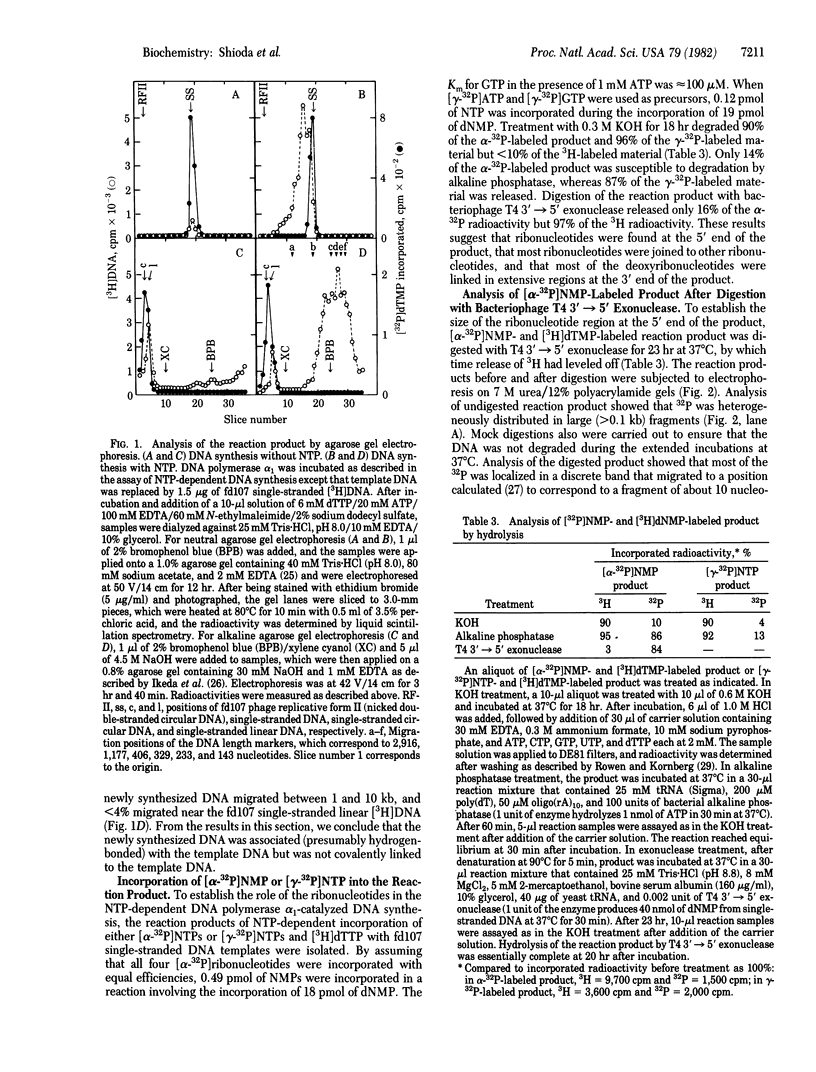
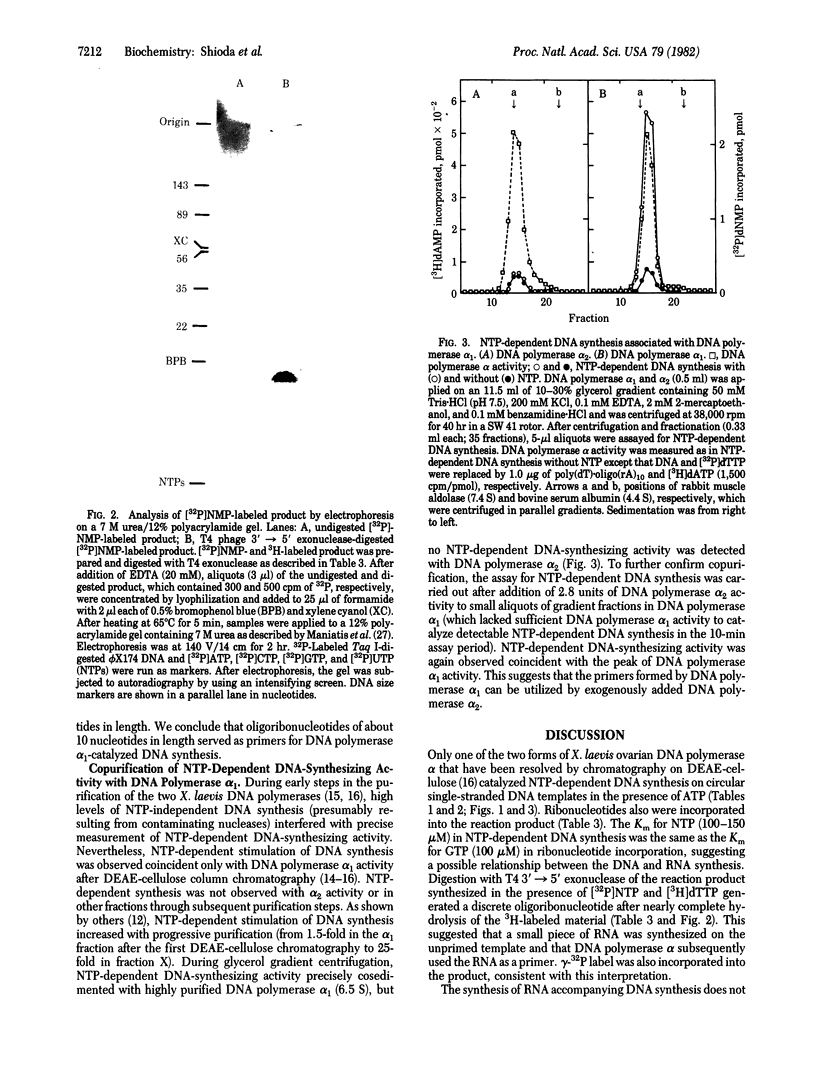
One of the two forms of DNA polymerase alpha from ovaries of the frog Xenopus laevis catalyzed ribonucleoside triphosphate-dependent DNA synthesis on single-stranded circular fd phage DNA templates. DNA synthesis was dependent on ATP and added template. CTP, GTP, and UTP stimulated DNA synthesis but were not required and could not substitute for ATP. DNA synthesis was not inhibited by alpha-amanitin. Neither poly(dT) nor double-stranded DNA served as template. Analysis of [32P]-dTMP-labeled product by neutral and alkaline agarose gel electrophoresis showed that 0.1- to 1-kilobase DNA fragments (average size of approximately equal to 0.25 kilobase) were synthesized. The fragments were not covalently linked to the template. Either [alpha-32P]NMP, [gamma-32P]ATP, or [gamma-32P]GTP were incorporated also into the product. Analysis of the product after hydrolysis by KOH, alkaline phosphatase, or bacteriophage T4 3' leads to 5' exonuclease showed the presence of a small oligoribonucleotide primer at the 5' end of the newly synthesized DNA. NTP-dependent DNA-synthesizing activity copurified on six columns and cosedimented during glycerol gradient centrifugation with one form of DNA polymerase alpha activity but not with the other form. These results suggest that DNA primase activity is associated with one of the two forms of X. laevis DNA polymerase alpha.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bayne M. L., Dumas L. B. Isolation of circular viral and complementary strand DNA from bacteriophage f1 duplex replicative-form DNA. Anal Biochem. 1978 Dec;91(2):432–440. doi: 10.1016/0003-2697(78)90528-6. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R., Reeder R. H. DNA synthesis in a multi-enzyme system from Xenopus laevis eggs. Cell. 1978 Feb;13(2):307–318. doi: 10.1016/0092-8674(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Primase initiates Okazaki pieces during polyoma DNA synthesis. Nature. 1978 Mar 9;272(5649):184–185. doi: 10.1038/272184a0. [DOI] [PubMed] [Google Scholar]
- Fox A. M., Breaux C. B., Benbow R. M. Intracellular localization of DNA polymerase activities within large oocytes of the frog, Xenopus laevis. Dev Biol. 1980 Nov;80(1):79–95. doi: 10.1016/0012-1606(80)90500-x. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Pirkl E., Zentgraf H., Schaller H. Conversion of bacteriophage fd into an efficient single-stranded DNA vector system. Mol Gen Genet. 1980 Jan;177(2):231–242. doi: 10.1007/BF00267434. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Longiaru M., Horwitz M. S., Hurwitz J. Elongation of primed DNA templates by eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5827–5831. doi: 10.1073/pnas.77.10.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Benbow R. M. A low molecular weight DNA polymerase from ovaries of the frog Xenopus laevis. DNA polymerase-beta (ovarian). J Biol Chem. 1978 Apr 25;253(8):2640–2649. [PubMed] [Google Scholar]
- Kowalski J., Denhardt D. T. Ribonucleotides in DNA newly synthesised in 3T6 cells in vivo. Nature. 1979 Oct 25;281(5733):704–706. doi: 10.1038/281704a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Longacre S. S., Rutter W. J. Nucleotide polymerases in the developing avian erythrocyte. J Biol Chem. 1977 Jan 10;252(1):273–283. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Morris P. W., Racine F. M. Ribonucleotidyl transferase in preparations of partially purified DNA polymerase alpha of the sea urchin. Nucleic Acids Res. 1978 Oct;5(10):3959–3973. doi: 10.1093/nar/5.10.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. Discontinuous DNA replication. Annu Rev Biochem. 1980;49:421–457. doi: 10.1146/annurev.bi.49.070180.002225. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. 3. The nucleotide sequence at the RNA-DNA junction of nascent strands. J Mol Biol. 1974 Mar 25;84(1):197–216. doi: 10.1016/0022-2836(74)90222-8. [DOI] [PubMed] [Google Scholar]
- Reichard P., Eliasson R., Söderman G. Initiator RNA in discontinuous polyoma DNA synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4901–4905. doi: 10.1073/pnas.71.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G. Multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in Xenopus laevis. Isolation and partial characterization. J Biol Chem. 1974 Jan 10;249(1):241–248. [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sato S., Hutchinson C. A., 3rd, Harris J. I. A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):542–546. doi: 10.1073/pnas.74.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. Initiator RNA of discontinuous DNA synthesis in human lymphocytes. Cell. 1977 Oct;12(2):483–489. doi: 10.1016/0092-8674(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Grafstrom R. H., Revie D., Oertel W., Goulian M. Studies on early intermediates in the synthesis of DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):263–270. doi: 10.1101/sqb.1979.043.01.032. [DOI] [PubMed] [Google Scholar]
- Wu G. J., Dawid I. B. Purification and properties of mitochondrial deoxyribonucleic acid dependent ribonucleic acid polymerase from ovaries of Xenopus laevis. Biochemistry. 1972 Sep 12;11(19):3589–3595. doi: 10.1021/bi00769a015. [DOI] [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA polymerase accompanied by a novel RNA polymerase activity: purification and partial characterization. J Biochem. 1982 Feb;91(2):607–618. doi: 10.1093/oxfordjournals.jbchem.a133732. [DOI] [PubMed] [Google Scholar]



