Abstract
To characterize the depression of metabolism in anhydrobiotes, the redox state of cytochromes and energy metabolism were studied during dehydration of soaked cowpea (Vigna unguiculata) cotyledons and pollens of Typha latifolia and Impatiens glandulifera. Between water contents (WC) of 1.0 and 0.6 g H2O/g dry weight (g/g), viscosity as measured by electron spin resonance spectroscopy increased from 0.15 to 0.27 poise. This initial water loss was accompanied by a 50% decrease in respiration rates, whereas the adenylate energy charge remained constant at 0.8, and cytochrome c oxidase (COX) remained fully oxidized. From WC of 0.6 to 0.2 g/g, viscosity increased exponentially. The adenylate energy charge declined to 0.4 in seeds and 0.2 in pollen, whereas COX became progressively reduced. At WC of less than 0.2 g/g, COX remained fully reduced, whereas respiration ceased. When dried under N2, COX remained 63% reduced in cotyledons until WC was 0.7 g/g and was fully reduced at 0.2 g/g. During drying under pure O2, the pattern of COX reduction was similar to that of air-dried tissues, although the maximum reduction was 70% in dried tissues. Thus, at WC of less than 0.6 g/g, the reduction of COX probably originates from a decreased O2 availability as a result of the increased viscosity and impeded diffusion. We suggest that viscosity is a valuable parameter to characterize the relation between desiccation and decrease in metabolism. The implications for desiccation tolerance are discussed.
Tolerance of desiccation requires tissues to cope with physical, biochemical, and structural stresses associated with the loss of water. Two mechanisms of protection against the deleterious effects of water loss have received major attention: (a) the synthesis of the so-called late-embryogenesis-abundant proteins (Close, 1996; Kermode, 1997) and (b) the accumulation of di- and oligosaccharides (Leprince et al., 1990; Horbowicz and Obendorf, 1994; Vertucci and Farrant, 1995). In model systems these sugars protect the structural integrity of dehydrated membranes and proteins (Crowe et al., 1992; Hoekstra et al., 1997). They also participate in the formation of a glassy state (Burke, 1986; Green and Angell, 1989; Leopold et al., 1994). In glasses, diffusion and chemical reactions are severely slowed because the viscosity is assumed to be 1012-fold higher than in a liquid. However, there are numerous examples of pollen and seeds in which there is no apparent correlation between the expression of desiccation tolerance and the nature and concentration of protectants (Hoekstra et al., 1994, 1997; Vertucci and Farrant, 1995). To our knowledge there is no convincing evidence yet that the in vitro protective mechanisms of sugars also function in situ (Hoekstra et al., 1997). Glasses are formed in both dried, desiccation-sensitive and desiccation-tolerant seeds and pollen and appear to have identical properties (Sun et al., 1994; Buitink et al., 1996).
An alternative hypothesis for desiccation tolerance proposes that anhydrobiotes are capable of evading damage from oxidative and peroxidative reactions during drying (Leprince et al., 1994; Vertucci and Farrant, 1995). Indeed, loss of viability in dehydrating embryonic tissues and pollen has been associated with various symptoms of free radical-induced injury (Senaratna et al., 1987; Hendry et al., 1992; Van Bilsen and Hoekstra, 1993; Leprince et al., 1994). Such damage probably results from the formation of ROS during the water loss and subsequent dry storage. Under normal physiological conditions, tight metabolic coupling in mitochondria, together with antioxidant defense mechanisms, maintains the concentrations of ROS at nonharmful levels (Halliwell and Gutteridge, 1984; Cadenas, 1989; Skulachev, 1996). During dehydration of desiccation-tolerant tissues, a tight control of ROS production and metabolism is expected. However, this control may be lost in desiccation-sensitive tissues during dehydration, resulting in an overproduction of ROS (Leprince et al., 1994), as occurs in other oxidative stress conditions (McKersie, 1991; Foyer et al., 1994; Inzé and Van Montagu, 1995). We speculated that a coordinated down-regulation of metabolism must occur during drying to achieve desiccation tolerance (Leprince et al., 1994, 1995). Before investigating such a hypothesis, we should characterize the relations between the loss of water and the mechanisms that control the generation of ROS in tissues that retain physiological and cellular integrity during drying. Surprisingly, the significance of the changes in the physical properties of water during drying for metabolism has drawn little attention. This is in marked contrast to the wealth of studies of the transition in metabolism during imbibition before germination (Bewley and Black, 1994) and the relationship between the hydration layers of cellular and molecular structures and oxidative processes (for review, see Leopold and Vertucci, 1989).
Under physiological conditions, the generation of ROS greatly depends on the availability of molecular O2 and on the redox states of the electron-transfer components (Cadenas, 1989; Skulachev, 1996). In mitochondria, such components must be reduced to build up a membrane potential and produce ATP. However, this reduction must be regulated, since it also favors the leakage of electrons from the mitochondrial electron-transport chain to molecular O2 and the generation of ROS (Halliwell and Gutteridge, 1984; Cadenas, 1989; Skulachev, 1996). A parallel between desiccation sensitivity and oxidative stress can be assumed because they are both associated with high metabolic rates and are characterized by similar symptoms of injury (Leprince et al., 1994, 1995; Vertucci and Farrant, 1995). Based on this assumption, we assessed noninvasively the redox states of respiratory chain components and energy metabolism during drying. These redox states can be estimated in situ by spectrophotometry at low temperature, as demonstrated by the pioneering work of Wilson and Bonner (1971) on intact peanut cotyledons during imbibition.
As a result of the water loss during drying, diffusion of O2 to mitochondria may be impeded. This may have a beneficial effect, as there is less of a chance to form ROS (Skulachev, 1996). However, it also may induce anoxia during drying. It is interesting that survival of invertebrates in the dry state has been associated with tolerance to anoxia (Hofmann and Hand, 1994; Hand and Hardewig, 1996). In these animal systems, anoxia induces a coordinated suppression of the catabolic and anabolic biosynthetic pathways of energy metabolism (Hand and Hardewig, 1996). These observations prompted us to examine further the interaction between O2 diffusion as a function of WC and redox states of Cyts. Because of the physiological significance of glasses in seeds and pollen (Leopold et al., 1994; Leprince and Walters-Vertucci, 1995), we further assessed the relation between loss of water and increase in viscosity during drying. For this purpose, ESR spectroscopy was used to study the rotational motion of nitroxide spin-probes that were inserted into the cytoplasm. From the rotational diffusion coefficient, the viscosity of the cytoplasmic surrounding was calculated during drying (Freed and Fraenkel, 1963; Hemminga et al., 1993). From the relation between viscosity and the O2 diffusion coefficient, the diffusion rates of O2 to mitochondria during drying were estimated.
MATERIALS AND METHODS
Plant Material, Hydration, and Drying Treatments
Seeds of cowpea (Vigna unguiculata L. Walp.), germination > 90%, were purchased locally and allowed to soak overnight at 15°C in wet paper towels. Before drying or incubation with chemicals, the cotyledons were excised and blotted dry on filter paper. The cotyledons were placed in a sealed chamber flushed with dry air, N2, or 100% O2 for intervals up to 24 h at 22°C or at 4°C in a cold room (RH about 3%). Pollen grains of Typha latifolia L. and Impatiens glandulifera Royle were harvested in Wageningen, The Netherlands. Fresh, mature I. glandulifera pollen was used immediately (>92% germination), whereas mature T. latifolia pollen was first dried and stored at −20°C (>95% germination). To obtain hydrated T. latifolia pollen, the material was rehydrated overnight at 4°C in 100% RH. Rehydrated pollen was then uniformly spread on a Petri dish and dried back at 20°C and 55% to 65% RH while samples were taken for analysis. WC were assessed gravimetrically by comparing the sample weights before and after drying for 38 h at 96°C and are expressed on a dry- weight basis.
In Situ Determination of Redox States of Cyts
Pollen (300 mg) was loaded directly into a spectroscope cuvette with a low-temperature attachment. To avoid changes in WC that may occur between sampling and loading, all manipulations were carried out in a glove box at 3%, 55%, or 100% RH, depending on the initial WC of the sample. After loading, the cuvette was sealed with tape and transferred into a Dewar flask filled with liquid N2.
Eight to 16 cowpea cotyledons were frozen in liquid N2 and ground into a homogeneous powder using a mortar and pestle. Frozen powder was then loaded into the spectroscope cuvette that was precooled in liquid N2. To reduce condensation of water at the surface of the frozen material, grinding and loading were performed in a glove box at 3% RH. For drying experiments under anoxia and hyperoxia, the glove box was also purged with pure N2 and O2, respectively. At the time the cuvette was filled, an additional aliquot of pollen or powdered frozen cotyledons was always secured in a small tube for WC determination.
In vivo spectra of Cyts were recorded in the visible region at liquid N2 temperature using a 1- or 3-mm-pathlength cuvette on an Aminco DW2a or DW2000 spectrophotometer (SLM Instruments, Urbana, IL) in double-beam mode, interfaced to a personal computer. A slit width of 3 mm and a scan rate of 30 nm/min were used. Instrumental noise was reduced by averaging 5 to 10 successive scans. Spectra of powdered cotyledons and pollen were routinely recorded against insoluble PVP (Polyclar AT) powder as a reference, which is assumed to have light-scattering properties similar to the biological material (Chance, 1954). The effects of various reducing conditions on the spectra were determined to ascertain that the light-scattering effects of our material did not produce artifacts and to determine the maximum absorbance values for reduced Cyts as a calibration for 100% reduction. Chemically reduced spectra were obtained from hydrated cotyledons following incubation for 4 h with 1 mm KCN or 1 h in 5% (w/v) sodium dithionite prior to drying. Difference spectra were obtained either mathematically, using the spectra obtained in the conditions described above, or by scanning the reduced samples against an aerated hydrated sample in the reference cuvette. The proportion of reduced Cyts was determined as the difference between absorbances at the peaks corresponding to Cyts b + c and a − aa3 and those of the baseline. The baseline was taken as the tangent line connecting points on the absorption scan from about 540 to 565 nm (Cyts b + c) and 590 to 608 nm (Cyt a − aa3). Actual concentrations of Cyts could not be calculated because of: (a) overlapping absorption peaks of Cyts from microsomal and mitochondrial origin (Fig. 1); (b) the unknown geometrical structure of the powdered material, which makes it impossible to determine accurately the light pathlength; and (c) the dependence of molar extinction coefficient of reduced Cyts on the osmotic potential or water activity of the solvent (Kornblatt and Hoa, 1990).
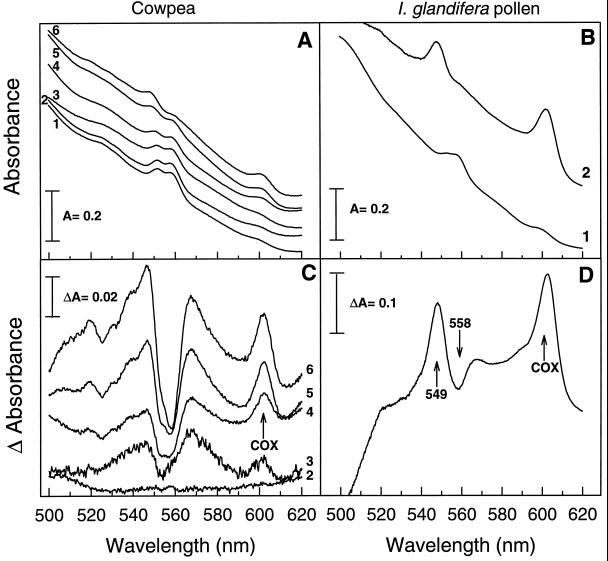
Figure 1.
Absorbance spectra at various WC of powdered cowpea cotyledons (A, Scan 1-6, WC is 1.22, 0.90, 0.52, 0.39, 0.29, and 0.15 g/g, respectively) and intact I. glandulifera pollen (B, WC is 1.2 and 0.09 g/g in scan 1 and 2, respectively). Spectra were recorded at 77 K using PVP in the reference cuvette. C (cotyledons) and D (pollen) show the effect of drying on the redox state of Cyts in more detail using difference spectra. In C, scan 1 (hydrated control) was subtracted from each individual scan (2–6). In D, scan 1 was subtracted from scan 2. Peaks and the trough (D) are indicated by upward and downward arrows, respectively.
Respiration Measurements
O2 uptake and CO2 release were measured in cotyledons of cowpea and T. latifolia pollen using a gas chromatograph with two columns in series, essentially as described by Hoekstra and Bruinsma (1980). The first column (Porapack R, 80–100 mesh, Chrompack International BV, Middelburg, The Netherlands) was operated at 65°C, and the second one (Mol Sieve 5A, 50–90 mesh) was operated at 25°C as a loop after the catharometric detector. The carrier gas was He. For calibration, pure CO2 and air were used, assuming a gas composition at atmospheric pressure of 20.95% O2, 0.93% Ar, and 78.09% N2. Three to five cotyledons that were dried to different extents were sealed in 7-mL sterile vessels and incubated at 25°C. Rates of gas exchange were calculated by linear regression analysis of three measurements taken every 20 min to 4 h, depending on respiration rate. After respiration measurement, the sample WC was approximately 5% less than before measurement.
Adenylate Extraction and AEC Measurements
At intervals during drying, samples of 250 mg of cotyledons and 50 mg of pollen were homogenized in 10% TCA and then the TCA was removed by diethyl ether (Al-Ani et al., 1985). ATP content was determined using the luciferin-luciferase system (catalog no. 1699695, Boehringer Mannheim). Analyses of ADP and AMP were performed after enzymatic conversion to ATP (Swedes et al., 1975).
Assessment of Viscosity and O2 Diffusion Coefficient Using ESR of Nitroxide Spin-Probes
Fully hydrated cotyledons of cowpea were cut into 3-mm3 pieces and incubated for 15 min at room temperature in an aerated solution of 2 mm spin-probe (4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy or CP, Sigma). Subsequently, potassium ferricyanide was added to the solution to a final concentration of 150 mm and incubation was prolonged for 30 min. Pieces of cotyledons were briefly washed in distilled water and allowed to dry at ambient conditions (22°C, 50%–70% RH) before the ESR measurements. For T. latifolia pollen, loading of the spin-probes, incubation, and washing procedures were performed in liquid germination medium according to the method of Buitink et al. (1998). Ferricyanide anions broaden the triplet ESR signal of spin-probes via spin-spin interactions (Golovina et al., 1997). Because ferricyanide cannot pass through intact membranes, the contribution of the extracellular spin-probe to the total ESR signal of our material is invisible. ESR spectra were recorded at 5°C and 25°C on an ESR X-band spectrometer (model ESP 300, Bruker Analytik, Reinstetten, Germany). Modulation amplitude was 1 G and microwave power was 6 mW. Other parameters were set as necessary to obtain the optimal signal-to-noise ratio.
To assess the cytoplasmic viscosity, τR values for anisotropic tumbling in liquid solution were derived from ESR spectra according to the method of Freed and Fraenkel (1963):
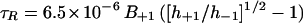 |
1 |
where h+1 and h−1 are the amplitudes of the low- and high-field lines of the spectra, respectively (Fig. 5, scan 1), B+1 is the line width of the low-field line (in tesla). Viscosity values were then determined using the following modified Stokes-Einstein relation (Keith and Snipes, 1974; Hemminga et al., 1993):
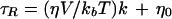 |
2 |
where η is the viscosity of the solvent, V is the volume of the rotating molecule, kb is the Boltzmann's constant, and T is the absolute temperature. η0 is the zero viscosity rotational correlation time, which is negligible (J. Buitink and M.A. Hemminga, personal communication). k is a dimensionless interaction parameter taking into account the fact that nonspherical molecules displace the solvent molecules as they rotate (Hemminga et al., 1993). O2-diffusion coefficients were calculated from the experimental correlation between viscosity of aqueous solutions and temperature for a binary mixture (Reid et al., 1987):
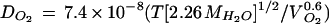 |
3 |
where T is the absolute temperature, MH2O is the molecular weight of water, and VO2 is the molar volume of O2.
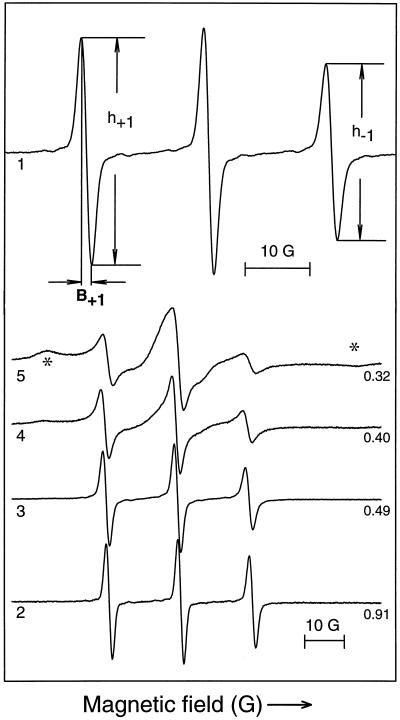
Figure 5.
ESR spectra of CP introduced into hydrated (WC is 1.2 g/g) cowpea cotyledons (scan 1) and dried to various extents (scans 2–5). In scan 1, the spectral parameters used to calculate τR are shown. Water contents (expressed on a dry-weight basis) are indicated next to each spectrum. The asterisk indicates spectral features characteristic of a rigid-solid-state-like spectrum.
Determination of Tg
Three milligrams of cotyledons dried to different extents were hermetically sealed into aluminum differential scanning calorimetry pans. Glass transitions were determined using Pyris-1 differential scanning calorimetry (Perkin-Elmer) using the settings described by Leprince and Walters-Vertucci (1995). The Tg was determined by the onset of the temperature range at which changes in specific heat, characteristic of a glass transition, occurred (Leprince and Walters-Vertucci, 1995).
Experimental Design and Statistical Treatment
All drying experiments were performed twice or three times and the resulting data were pooled in the graphics. Furthermore, the value for each measurement represents an average of 3 to 16 cotyledon pairs or 12 × 106 pollen grains. As an aid to the eye, the data were fitted using the software Tablecurve 2D (Jandel Software, San Rafael, CA), with third-order polynomial regression or asymmetric transition functions (i.e. y = a/(1 + x/b)c; y = a + b/(1 + (x/c)d); y = a + b/x + clnx/x2) giving adjusted r2 varying between 0.908 and 0.988.
RESULTS
In Situ Spectrophotometric Determination of Redox States of Cyts
The effects of decreasing WC on the absorbance spectra of powdered cowpea cotyledons and intact I. glandulifera pollen are shown in Figure 1, A and B, respectively. The peak at 599 to 603 nm that is attributable to the heme a−a3 complex of COX (Chance, 1954; Lance and Bonner, 1968; Ikuma, 1972) increased 2- and 10-fold upon drying of cowpea cotyledons and I. glandulifera pollen, respectively. The identity of this complex was confirmed by its sensitivity to cyanide (see next paragraph; Fig. 2A) and Ar (data not shown). To avoid possible light-scattering artifacts when assessing concentrations of Cyts, we studied the effects of drying on COX using difference spectra. Difference spectra were obtained by subtracting the absorbance spectrum of a hydrated sample from the spectra of samples having various WC (Fig. 1, C and D). They are referred to as air-dried minus hydrated difference spectra. For both cowpea cotyledons and I. glandulifera pollen, air-dried minus hydrated difference spectra showed an increase in absorbance at 599 to 603 nm during drying, indicating an increase in the proportion of reduced COX. Results similar to those in Figure 1, C and D, were obtained when the dried material was directly scanned against the hydrated material in the reference cuvette instead of scanning against PVP followed by spectra subtraction.
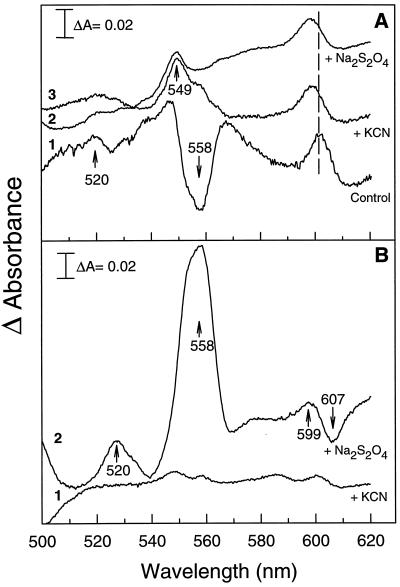
Figure 2.
A, The effect of chemical reduction on Cyt redox state of hydrated, powdered cotyledons. Difference spectra were obtained by subtracting the spectra of nontreated samples from spectra of samples obtained after chemical reduction with 1 mm KCN (scan 2) and 5% sodium dithionite (scan 3). The dried minus hydrated difference spectrum (scan 1) is shown as a control. B, The effect of drying plus chemical reduction on Cyt redox state of powdered cotyledons. Incubation with KCN or sodium dithionite were performed before drying. Difference spectra were obtained after subtraction of an untreated, dried sample from a dried KCN-treated sample (scan 1) and from a dried sodium-dithionite-treated sample (scan 2) of similar WC. Peaks and troughs are indicated by upward and downward arrows, respectively.
In addition to the presence of Cyt a−a3, cowpea cotyledons contained additional Cyt components. The presence of these components was confirmed by the presence of peaks or troughs in the various difference spectra shown in Figure 2A. Scan 1 exhibits a peak at 520 nm of unknown origin and one trough between 558 and 560 nm that indicates the presence of b-type Cyts. In scans 2 and 3 (Fig. 2A) a peak is observed at 549 nm that is attributable to a c-type Cyt. In light of previous studies of microsomal membranes isolated from legume seedlings (Hendry et al., 1981; Lüthje et al., 1997), it is most likely that b- and c-type Cyts observed in cowpea cotyledons are from plasma membrane oxidases. In I. glandulifera pollen, the difference spectra (Fig. 1D) exhibits a peak at 549 nm and a trough at 558 nm, which may correspond to mitochondrial Cyt c and b, respectively. In T. latifolia pollen, we found features similar to I. glandulifera pollen, although the maximum absorbance of Cyt c and COX was 10-fold lower (data not shown).
To further ascertain that the changes in absorbance observed in the air-dried minus hydrated difference spectra were the result of actual changes in redox states of the Cyts, we examined chemically reduced spectra that were obtained after incubating hydrated cotyledons with KCN or sodium dithionite. Spectra of untreated, hydrated cotyledons were then subtracted from these chemically reduced spectra to obtain reduced minus air-oxidized difference spectra (Fig. 2A, scans 2 and 3). The extent of COX reduction (599–603 nm peak) with the chemicals in the hydrated state (scans 2 and 3) was identical to that of the untreated, dried minus hydrated difference spectrum (control, scan 1). However, compared with this control spectrum, the COX peaks in the chemically reduced difference spectra were at lower wavelengths. To assess whether the water loss or the extent of reduction could shift the peak to a higher wavelength, difference spectra were obtained by subtracting a spectrum of hydrated cotyledons from spectra of cotyledons that were dried in the presence of either KCN or sodium dithionite.
In the presence of KCN, the spectrum (data not shown) was remarkably similar to the air-dried minus hydrated difference spectrum (Fig. 2, scan 1). Indeed, no peak was discernible in the difference spectrum between cotyledons dried in the presence and absence of KCN (Fig. 2B, scan 1). The difference spectrum between cotyledons dried in the presence and absence of sodium dithionite showed a peak at 599 nm and a trough at 607 nm (Fig. 2B, scan 2), originating from the fact that the COX peak in the presence of sodium dithionite is not sensitive to water loss. The difference between the effects of KCN and sodium dithionite may stem from differences in the mode of reduction of COX (i.e. via blocking electron transport to O2 or direct reduction, respectively). From these observations we conclude that the absorbance peak in the 599–603 nm region is associated with reduced COX. Furthermore, the desiccation-induced increase of this peak (Fig. 1) indicates that COX becomes increasingly reduced in both cotyledons and pollen during drying.
Additional information about the redox state of b-type Cyts in cotyledons can be derived by comparing the absorbance spectra in Figure 1A and the difference spectra in Figure 2. The absorbance spectra of hydrated material (Fig. 1A, scan 1) showed a double peak at approximately 558 nm. When spectra from hydrated cotyledons that were reduced with sodium dithionite were subtracted from spectra of untreated, hydrated cotyledons, no peak was observed at 558 nm in the difference spectra (Fig. 2A, scan 3). It follows that sodium dithionite had no effect on the redox states of Cyt b. Thus, we conclude that microsomal Cyts were mostly reduced in the hydrated, untreated material. After drying, their redox state changed, as demonstrated by the trough at 558 nm in the dried minus hydrated spectrum (Fig. 2A, control, scan 1). When spectra from dried cotyledons that were reduced by sodium dithionite prior to drying were subtracted from spectra of untreated, dried material, the peak reappeared at 558 nm in the difference spectra (Fig. 2B, scan 2), indicating that sodium dithionite kept the Cyt b reduced during drying. From these observations we conclude that the microsomal Cyts become more oxidized during drying of untreated material. Such oxidation can be monitored by comparing scans 1 through 6 in Figure 1, A and C. The absence of peaks in the difference spectrum of the dried material in the presence and absence of KCN suggests that cyanide does not lead to reduction of microsomal Cyts.
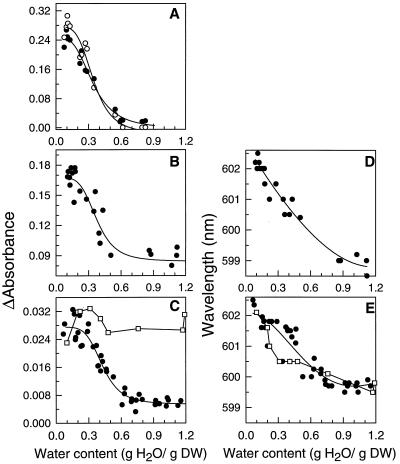
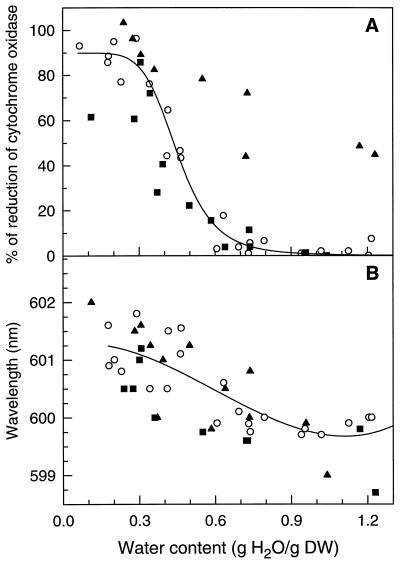
To study the response of the Cyt redox states to water loss, the absorbance of mitochondrial Cyts in cotyledons and pollen was plotted as a function of WC obtained after various times of drying (Fig. 3). In hydrated pollen of I. glandulifera (Fig. 3A) and T. latifolia (Fig. 3B) and in hydrated cotyledons (Fig. 3C), the A599 was low. Because the a − a3 complex is mostly oxidized in steady-state physiological conditions (Chance, 1954; Kariman et al., 1983; Leštan et al., 1993), we assumed that the absorbance values in the hydrated state correspond to near 100% oxidized levels. During drying, the A599–603 did not increase substantially until a WC of 0.6 g/g was reached. Thereafter, it increased markedly until 0.2 to 0.3 g/g. Below these WC values the absorbance reached a constant value that was equivalent to those obtained after reduction with sodium dithionite or KCN. In cotyledons, KCN kept COX reduced regardless of WC (Fig. 3C). The A599–603 values obtained in dried cotyledons and pollen were similar to those measured in dry, quiescent material (data not shown), indicating that the increase in absorbance was not due to synthesis of Cyts during drying. The progressive red shift in the maximum absorbance from 599 to 603 nm during drying is particularly noteworthy (Fig. 3, D and E). Treating cotyledons with KCN prior to drying did not interfere with the red shift in cowpea cotyledons (Fig. 3E). Such shifts have been previously observed in the Soret maximum of isolated beef heart COX exposed to decreasing water activity (Kornblatt and Hoa, 1990). However, the reasons why the chromophores are perturbed in our material during drying remain to be elucidated. In I. glandulifera pollen, the effects of reducing WC on absorbance of Cyt c was also recorded (Fig. 3A). The plot of the proportion of reduced mitochondrial Cyt c versus WC was similar to that of COX, indicating that the redox changes are synchronous during drying.
Figure 3.
A to C, The effects of drying on the absorbance of the α-band of Cyt c (○) and COX (•) in I. glandulifera pollen (A), T. latifolia pollen (B), and cowpea cotyledons (C). For cowpea cotyledons the effect of 1 mm KCN is also shown (□). D to E, The effect of drying on the absorbance maximum of the α-peak of COX for T. latifolia pollen (D) and cowpea cotyledons (E) in the absence (•) and presence (□) of 1 mm KCN. DW, Dry weight.
Effects of O2 Supply on COX Reduction during Drying
At least two factors may contribute to the increased reduction of mitochondrial Cyts: (a) the lack of O2, as suggested by studies of mammalian (Kariman et al., 1983), fungal (Leštan et al., 1993), and plant tissues (Lance and Bonner, 1968), and (b) viscosity that changes the solvent properties around COX (Escamilla et al., 1989; Kornblatt and Hoa, 1990). However, the interpretation of the COX redox behavior in our material during drying is further complicated because O2 availability is also related to viscosity. To test the effects of O2 availability, hydrated cotyledons of cowpea were dried for different intervals in a sealed chamber under a stream of 100% N2 or 100% O2. Under anoxia, only a 60% reduction in COX was observed in the hydrated state (Fig. 4A). Thus, traces of O2 may have penetrated the tissues and partially oxidized the COX before having been fixed in liquid N2. Beginning at a WC of 0.7 g/g, the extent of reduction increased to 100% at 0.3 g/g. Thus, O2 availability could be considered to be a factor controlling the onset of COX reduction. Drying cotyledons in the presence of 100% O2 did not affect the pattern of reduction of COX (Fig. 4A). It was estimated that approximately 70% ± 5.8% reduction was achieved in the dry state, based on the average (±se) of the data points corresponding to the four lowest WC values. The composition of the atmosphere during drying did not influence the shift in the position of the absorbance maximum (Fig. 4B). However, the resulting 70% COX reduction, despite the high concentration of O2 present during drying, suggests that there is a barrier limiting O2 availability to COX. Therefore, we investigated the relation between viscosity and the COX redox state during drying.
Figure 4.
The effects of O2 concentration on the reduction (A) and absorbance maximum (B) of COX during drying of cowpea cotyledons. Treatments were 100% N2 (▴), air (○), and 100% O2 (▪). The curves represent fits (r2 = 0.915) to the control (air) experiments. DW, Dry weight.
Changes in Cytoplasmic Viscosity and the O2-Diffusion Coefficient during Drying
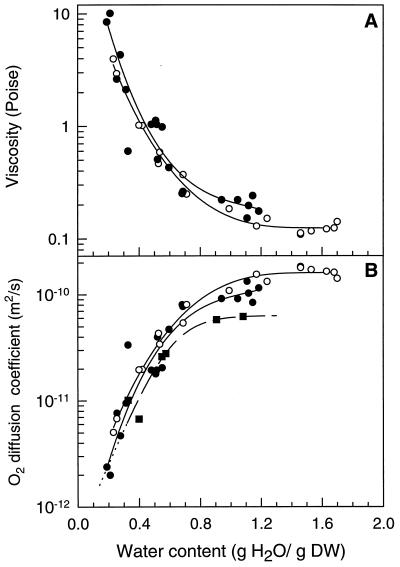
To estimate cytoplasmic viscosity, we studied the effects of drying on the rotational motion of CP that was introduced into the cytoplasm of cowpea cotyledon cells (Fig. 5) and T. latifolia pollen. With decreasing WC, both the amplitudes of and the distance between the left (low-field) and right (high-field) peaks decreased. At WC of less than 0.32 g/g (Fig. 5, scan 5), the high-field peak became progressively distorted and two additional components appeared at the extremes of the spectra. These components are characteristic of a solid-state-like spectrum in which the spin-probe is almost immobilized. The theory that predicts τR is only valid for liquid-state spectra that are representative of an (an)isotropic tumbling of the spin-probe (for theoretical details, see Hemminga et al., 1993). Thus, spectra of CP introduced in samples with WC of less than 0.25 g/g cannot be used to calculate τR. Therefore, the corresponding viscosities are unknown. Before drying, the cytoplasmic viscosities of both T. latifolia and cowpea cotyledons were approximately 0.15 poise (Fig. 6A), similar to values previously reported (Keith and Snipes, 1974). No significant changes were observed until the cotyledons and the pollen reached a WC of 0.7 and 0.9 g/g, respectively. Below these values the viscosity increased exponentially with further drying. A viscosity of 3 poise in pollen and 10 poise in cotyledons was reached at approximately 0.3 g/g (Fig. 6A). At this WC, the Tg is approximately −70°C (data not shown). As Tg progressively approaches a value close to room temperature with further drying, viscosities are expected to increase considerably. Similar results were obtained when 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy was used instead of CP.
Figure 6.
A, The effects of drying on cytoplasmic viscosity in cowpea cotyledons (•) and T. latifolia pollen (○). B, The effects of drying on calculated O2-diffusion coefficients in cowpea cotyledons dried at 22°C (•) and 5°C (▪) and in T. latifolia pollen (○) dried at room temperature. DW, Dry weight.
The O2-diffusion coefficient is inversely proportional to the viscosity of the medium (Nobel, 1983; Reid et al., 1987). It must be pointed out that the experimental correlation used to calculate O2-diffusion coefficients is valid only for binary systems and becomes less accurate with increasing viscosities (Reid et al., 1987). Therefore, in this study, the relation between O2-diffusion coefficients versus WC is regarded as qualitative (Fig. 6B). At room temperature the O2-diffusion coefficients for both T. latifolia and cowpea cotyledons prior to drying were approximately 9.5 × 10−9 m2 s−1. During drying of both materials, their respective O2-diffusion coefficients decreased exponentially below 0.8 g/g. The O2-diffusion coefficient measured at 5°C in hydrated cotyledons of cowpea was significantly lower than that at 25°C. However, this difference rapidly disappeared during the loss of water. These observations suggest that O2 diffusion through the tissues is progressively impeded upon the loss of water and increase in viscosity.
Relation among Viscosity, Temperature, and Redox State of COX
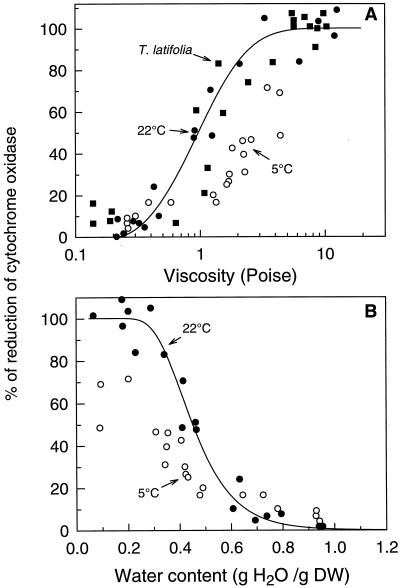
As mentioned earlier in this paper, an increased viscosity may directly or indirectly contribute to increased COX reduction. On the one hand, an increase in viscosity reduces the O2 availability and the environment around COX may experience anoxia. On the other hand, an increase in viscosity could impede the conformational changes of COX that are necessary for the intramolecular electron transfer to O2 and proton pumping (Einarsdottir et al., 1995; Wittung and Malmström, 1996). When the extent of COX reduction in cowpea cotyledons and T. latifolia pollen were plotted against viscosity, a similar relation appeared for both materials despite their differences in cellular composition and structure (Fig. 7A). The viscosity corresponding to the onset of COX reduction was approximately 0.4 poise for both pollen and cotyledons. Since temperature also affects viscosity, measurements of COX reduction were carried out in cowpea cotyledons during drying at 22°C and 5°C. When dried at 5°C, the onset of reduction shifted toward higher viscosity values (Fig. 7A). At 1.2 poise, the extent of COX reduction was 18% ± 2% (n = 2) and 56% ± 7% (n = 3) at 5°C and 22°C, respectively. In the driest state at 5°C, 63% ± 7% of maximal reduction was achieved, as estimated by the average (±se) of the data points corresponding to the three lowest WC values. This indicates that viscosity cannot be the sole factor involved in the reduction of COX.
Figure 7.
The response of COX reduction to viscosity and temperature during drying of cowpea cotyledons (•, 22°C; ○, 5°C) and in T. latifolia pollen (▪, room temperature). Reduction levels are plotted as a function of viscosity (A) or as a function of sample WC (B). Viscosity values were taken from the equation fitting the viscosity versus WC plot of Figure 6A. The relation between viscosity and COX reduction in T. latifolia pollen is also shown. The curves represent fits (r2 = 0.915) to the control (22°C) experiments. DW, Dry weight.
In vitro studies have demonstrated that the transfer of electrons from the Cyt a to the Cyt a3 − CuB center of COX requires water movements in and out of the enzyme (Kornblatt and Hoa, 1990; Kornblatt and Kornblatt, 1992). Such water movements can be inhibited by decreasing water activity or osmotic potential, which results in the reduction of the Cyt a (Escamilla et al., 1989; Kornblatt and Hoa, 1990). To test whether a decreased water activity in the cotyledons caused the reduction of Cyt a, we compared the A599–603 from material dried at 22°C and 5°C (Fig. 7B). From water sorption isotherms (data not shown), it can be derived that, for the same water activity, the WC of tissues equilibrated at 5°C is higher than that of tissues equilibrated at 22°C. The plot of the levels of reduced COX versus WC shows that the WC corresponding to the onset of reduction of COX is lower in tissues dried at 5°C than at 22°C (Fig. 7B). If water activity were responsible for the increase in reduction of COX, the WC corresponding to the onset of reduction would have shifted to higher WC, which is in contrast to what is observed in Figure 7B. Instead, it is likely that the reduced temperature during drying and the consequent reduction in metabolism maintained a concentration of dissolved O2 high enough to maintain a significant proportion of COX in the oxidized state during drying.
Response of Respiration and Energy Metabolism to Drying in Cowpea Cotyledons
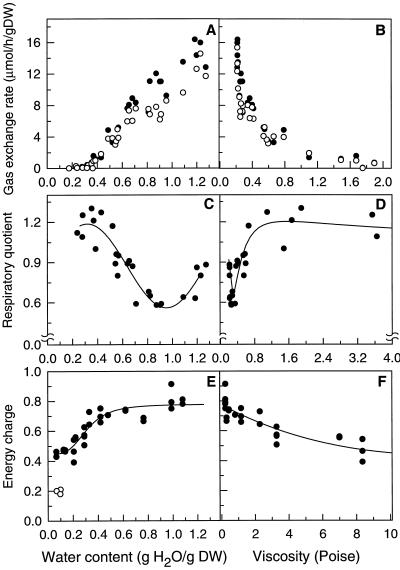
To examine the relationship between energy metabolism and changes in viscosity, O2 uptake and CO2 release rates were measured in the gas phase and plotted as a function of WC and viscosity during drying (Fig. 8, A and B). In addition, we determined the AEC as an estimate of the metabolic activity (Pradet and Raymond, 1983). The plots of gas-exchange rates versus WC show that respiration declined progressively as water was lost (Fig. 8A). At WC of less than 0.27 g/g, gas exchange was not detectable. The decline in respiration is more pronounced when plotted as a function of viscosity rather than WC (Fig. 8B). At viscosities greater than 0.27 poise, gas-exchange rates decreased exponentially.
Figure 8.
The effect of drying on several metabolic parameters in cowpea cotyledons. A and B, CO2 release (•) and O2 uptake (○). C and D, Respiratory quotient values (CO2 output/O2 input). E and F, AEC values during drying (•) and in dry tissues before imbibition (○). Data were plotted as a function of WC (A, C, and E) and viscosity (B, D, and F). Viscosity values were taken from the equation fitting the viscosity versus WC plot of Figure 6A. DW, Dry weight.
When the samples were dried, respiratory quotient values (CO2 release/O2 consumption) decreased from 0.9 to 0.6. It is unlikely that such a low value is a sign of breakdown of storage lipid. Since the amount of oil present in cowpea cotyledons is less than 2% of the dry weight (data not shown), storage lipid is not a likely candidate for respirable substrate during drying. Thus, the low respiratory quotient at the onset of drying may be due to the generation of ROS. At WC between 0.8 and 0.27 g/g, the respiratory quotient increased to 1.2 (Fig. 8C). It is noteworthy that a WC of 0.8 g/g corresponds to the WC at which O2 diffusion starts to decrease exponentially. The response of the respiratory quotient to drying showed a different pattern when plotted as a function of viscosity (Fig. 8D). With a 2-fold increase in viscosity, respiratory quotient values first decreased to 0.6 and then increased to 1.2 at a viscosity of 0.8 poise (Fig. 8D). They did not change with a further increase in viscosity. This suggests that during drying the tissues did not ferment, despite the reduction of the O2-diffusion coefficient. An increased breakdown of organic acids (e.g. citrate, malate) is a likely possibility for the increase in respiratory quotient values.
During the loss of water from the hydrated state to 0.5 g/g WC, the AEC remained constant at approximately 0.8 (Fig. 8E). At WC of less than 0.5 g/g, the AEC decreased to 0.4. Similar observations have been made for other plant systems in which the energy metabolism was limited by a total or partial deprivation of O2 (Pradet and Raymond, 1983; Raymond et al., 1985). In both dry seeds and pollen before imbibition, AEC was approximately 0.2 (Fig. 8E), as previously observed in seeds (Raymond et al., 1985). When plotted against viscosity, the AEC declined almost linearly (Fig. 8F).
DISCUSSION
Desiccation Induces the Reduction of Mitochondrial Cyts in Anhydrobiotes
The relations between redox status and metabolic functions of Cyts have been extensively characterized in seeds by spectrophotometry of isolated mitochondria (Lance and Bonner, 1968; Wilson and Bonner, 1971; Ikuma, 1972; Sowa et al., 1993) and isolated microsomal membranes (Hendry et al., 1981; Lüthje et al., 1997). Extraction of mitochondria from dry tissues often leads to loss of structural and functional integrity (Hoekstra and van Roekel, 1983) or, at best, gives a low yield of intact material (Attucci et al., 1991). Moreover, the necessary use of aqueous solvents during isolation from drying tissues implies that the changes in redox states of the Cyts observed in vitro may not reflect the actual in vivo redox state before the extraction. Therefore, we recorded spectra of intact or powdered material during drying. Our noninvasive analysis of the α-band of Cyts c and a−aa3 in pollen and a−aa3 in cowpea cotyledons shows clearly that drying at a WC of less than 0.6 g/g induces a steady increase in the extent of reduction of mitochondrial Cyts. Furthermore, these Cyts remained reduced at values less than 0.2 g/g. The glassy state is formed at room temperature when the WC is less than 0.12 g/g in cowpea cotyledons (data not shown) and 0.08 g/g in T. latifolia pollen (Buitink et al., 1996, 1998). Thus, both Cyt c and COX are fully reduced before a glassy state is formed during drying. In intact organisms, the only conditions known to induce total reduction in the terminal portion of the Cyts are anoxia (Lance and Bonner, 1968; Kariman et al., 1983; Leštan et al., 1993) and treatment with inhibitors that block the intramolecular transfer of electrons to the O2-binding site of the COX (Lance and Bonner, 1968). Such conditions also reduced COX in our material before drying (Figs. 1 and 2). Furthermore, our results confirm earlier suggestions that in dry pollen and seeds mitochondria are intact and the electron-transport chain is functional (Wilson and Bonner, 1971; Hoekstra and Van Roekel, 1983; Leopold and Vertucci, 1989; Attuci et al., 1991).
Impeded O2 Diffusion Is a Likely Factor Responsible for the Desiccation-Induced Reduction of Mitochondrial Cyts
Two observations point to a critical role for the O2 availability in the reduction of COX when the tissues are dried at a WC less than 0.6 g/g: (a) during drying of cowpea cotyledons and T. latifolia pollen, the exponential increase in viscosity and the corresponding decrease in the O2-diffusion coefficient (Fig. 6, A and B) coincided with the onset of the COX reduction; (b) the AEC decreased to values similar to those found in germinating seeds under anoxia (Fig. 8E; Pradet and Raymond, 1983; Al-Ani et al., 1985). Thus, we suggest that the diffusion of O2 becomes strongly impeded by the increase in solvent viscosity, which may restrict the O2 supply to the mitochondria and reduce COX. However, the observation that increasing [O2] during drying did not have an effect on the onset of COX reduction (Fig. 4A) challenges this suggestion. Two arguments are put forward to address this question. First, the decrease in diffusion rates during drying of our material could be so large that increasing the O2 gradient from outside to inside of the tissues will not significantly increase the O2 flux to the mitochondria and O2 availability to the COX. Second, previous studies (Al-Ani et al., 1985; Raymond et al., 1985) have shown that the respiratory metabolism in hydrated seeds is sustained at O2 partial pressures as low as 0.2 kPa. Furthermore, at 21 kPa, COX is saturated with substrates, judging from the low Km for O2 (Kariman et al., 1983; Leštan et al., 1993). These observations show that at 21 kPa and greater there is sufficient O2 to keep COX fully oxidized. Therefore, drying hydrated seeds or pollen at an O2 partial pressure greater than 21 kPa is not expected to influence COX activity.
One may argue that the in vivo reduction of Cyts may be attributed to mechanisms other than O2 deprivation. For instance, it has been shown that the reduction of O2 by COX is inhibited in conditions that prevent the solvent entry and expulsion between Cyt a and Cyt a3 during the COX catalytic cycle (Escamilla et al., 1989; Kornblatt and Kornblatt, 1992). Such water movements can be inhibited by decreasing the water activity of the medium (Kornblatt and Hoa, 1990). We showed that manipulating the water activity of cowpea cotyledons during drying did not affect the onset of COX reduction accordingly (Fig. 7). Therefore, the dependence of COX reduction on impeded water movements within our material is unlikely. Nevertheless, in the dry state, the low water activity may well prevent COX from being reoxidized by O2, which should still diffuse, albeit slowly, through the glassy cytoplasm. Indeed, we observed that COX in dried cowpea cotyledons remained reduced for at least 6 months of open storage.
Another mechanism that may reduce COX during drying involves the presence of electron donors in the mitochondrial matrix. As water is removed from the cell, the concentrations of various reducing substances (e.g. ascorbate) is increased. Consequently, the likelihood that these substances may act as electron donors to Cyt c and COX increases during drying. However, two sets of data do not support this hypothesis. First, we found that the extent of reduction of the ubiquinone-10 pool in cowpea cotyledons was 50% to 67% in both hydrated and dried conditions (O. Leprince and A.M. Wagner, unpublished results). This finding indicates that the electron transport between the ubiquinone pool and COX is maintained throughout drying. Furthermore, in hydrated seeds of several species, the electron transport in mitochondria occurs at a WC greater than 0.3 g/g (Leopold and Vertucci, 1989). Second, we have demonstrated that the microsomal Cyts in cowpea cotyledons are progressively oxidized during drying. This behavior is in marked contrast to that of mitochondrial Cyts, which are being reduced during drying (Figs. 1–3). If the concentration of potential electron donors to the Cyts increased during drying in all cellular compartments, the Cyts of both microsomal and mitochondrial origin would be reduced.
A Functional Role for Cytoplasmic Viscosity in Depressing Metabolism and Altering Cyt Redox States during Drying
Our study showed that viscosity can be regarded as a new, valuable parameter to characterize the effects of drying on metabolism. Characteristically, the loss of water in anhydrobiotes is associated with a decrease in respiration rates until a WC of about 0.3 g/g, below which no gas exchange can be detected (Fig. 8A; Leopold and Vertucci, 1989; Vertucci and Farrant, 1995). The precise causes that trigger the metabolic arrest during drying are not known. Electron microscopy studies have suggested that the progressive shutdown of metabolism is associated with a general dedifferentiation of organelles (Leprince et al., 1990; for review, see Vertucci and Farrant, 1995). In cowpea we observed a biphasic pattern in the relation between viscosity and respiration. At the onset of drying the initial increase in viscosity to 0.27 poise was associated with a decrease in gas-exchange rates to 50% of their initial values before drying. In contrast, the plot of respiration rates versus WC (Fig. 8A) shows a linear decline in respiration during drying. However, the initial increase in viscosity did not impede the electron transport within the mitochondrial chains, nor did it dramatically affect the AEC values. Thus, despite the loss of water, the system is still able to control the supply and demand of energy. We suggest that changes in medium viscosity and diffusion processes during drying may play a role in the control of energy supply and demand. Indeed, several studies of mitochondria showed that both the rates of electron transport and the mobility of the redox components within the lipid bilayer are more sensitive to changes in the medium bulk viscosity than in membrane microviscosity (Fato et al., 1993; Chazotte, 1994; Esmann et al., 1994).
The suggestions that there is a control of energy supply and demand during drying and that the reduction of COX during drying likely results from a desiccation-induced decrease in O2 availability are further supported by studies of metabolism depression in animal anhydrobiotes. Indeed, survival in the quiescent state of lower organisms is associated with tolerance to acute anoxia at high WC (Crowe et al., 1992; Hand and Hardewig, 1996). In Artemia cysts, the depression of metabolism (e.g. protein synthesis) is controlled by O2 limitation and acidic pH (Hofmann and Hand, 1994). In these animal systems, anoxia induces a coordinated suppression of both catabolism and anabolism (Hand and Hardewig, 1996). Whether the O2 availability directly controls the depression of metabolism in drying seeds and pollen remains to be clarified.
Implications for Desiccation Tolerance
Results of this study suggest that a regulated increase in viscosity may be regarded as a mechanism that controls the depression of metabolism during drying. An increase in viscosity may induce anoxia in dehydrating tissues before the cytoplasm enters a glassy state. It remains to be determined whether this strategy is beneficial or detrimental for desiccation tolerance. Anoxic conditions during dehydration would strongly diminish the danger of overproducing ROS. The formation of ROS is a random process that is strictly dependent on molecular collisions with O2 (Cadenas, 1989; Skulachev, 1996). Thus, entering into anoxia can be seen as a mechanism to escape from oxidative damage as suggested by Skulachev (1996). A rapid increase in viscosity during the onset of drying can be seen as a mechanism to slow metabolism and decrease O2 concentration, thereby decreasing the chances of generating ROS. It follows that the onset of drying is the critical phase for ROS formation, when the electron transport is still active and O2 is present at high concentrations. However, it can also be argued that a rapid increase in viscosity during early drying is detrimental because the induction of anoxia may increase the concentrations of metabolic end products, such as acetaldehyde and protons, to toxic levels. To clarify this ambivalence, the changes in viscosity during drying must be compared in desiccation-tolerant and -intolerant organisms. Furthermore, techniques that enable us to measure noninvasively [O2] and ROS during drying need to be developed.
ACKNOWLEDGMENTS
The authors wish to thank J. Buitink for critically reading the manuscript and Dr. A.M. Wagner (Free University of Amsterdam, The Netherlands) for the measurements of ubiquinone pools.
Abbreviations:
- AEC
adenylate energy charge
- COX
Cyt c oxidase
- CP
3-carboxy-proxyl
- ESR
electron spin resonance
- g/g
g H2O/g dry weight
- ROS
reactive oxygen species
- Tg
glass transition temperature
- τR
rotational correlation time
- WC
water content(s)
Footnotes
This work was supported by a grant from the Wageningen Agricultural University and the Laboratory of Plant Physiology to O.L.
LITERATURE CITED
- Al-Ani A, Bruzau F, Raymond P, Saint-Ges V, LeBlanc JM, Pradet A. Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiol. 1985;79:885–890. doi: 10.1104/pp.79.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attucci S, Carde JP, Raymond P, Saint-Ges V, Spiteri A, Pradet A. Oxidative phosphorylation by mitochondria extracted from dry sunflower seeds. Plant Physiol. 1991;95:390–398. doi: 10.1104/pp.95.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds, Physiology of Development and Germination. Plenum Press, New York
- Buitink J, Claessens MMAE, Hemminga MA, Hoekstra FA. Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol. 1998;118:531–541. doi: 10.1104/pp.118.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitink J, Walters-Vertucci C, Hoekstra FA, Leprince O. Calorimetric properties of dehydrating pollen. Analysis of a desiccation-tolerant and an intolerant species. Plant Physiol. 1996;111:235–242. doi: 10.1104/pp.111.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MJ (1986) The glassy state and survival of anhydrous biological systems. In AC Leopold, ed, Membranes, Metabolism and Dry Organisms. Cornell University Press, Ithaca, NY, pp 358–363
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Chance B. Spectrophotometry of intracellular respiratory pigments. Science. 1954;120:767–775. doi: 10.1126/science.120.3124.767. [DOI] [PubMed] [Google Scholar]
- Chazotte B. Comparisons of the relative effects of polyhydroxyl compounds on local versus long-range motions in the mitochondrial inner membrane. Fluorescence recovery after photobleaching, fluorescence lifetime, and fluorescence anisotropy studies. Biochim Biophys Acta. 1994;1194:315–328. doi: 10.1016/0005-2736(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Close TJ. Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant. 1996;97:795–803. [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Einarsdottir O, Georgiadis KE, Sucheta A. Intramolecular electron transfer and conformational changes in cytochrome c oxidase. Biochemistry. 1995;34:496–508. doi: 10.1021/bi00002a014. [DOI] [PubMed] [Google Scholar]
- Escamilla E, Ayala G, De Gomez-Puyou MT, De Gomez-Puyou A, Millan L, Darszon A. Catalytic activity of cytochrome oxidase and cytochrome c in apolar solvents containing phospholipids and low amounts of water. Arch Biochem Biophys. 1989;272:332–343. doi: 10.1016/0003-9861(89)90227-0. [DOI] [PubMed] [Google Scholar]
- Esmann M, Hideg K, Marsh D. Influence of poly(ethylene glycol) and aqueous viscosity on the rotational diffusion of membranous Na,K-ATPase. Biochemistry. 1994;33:3693–3697. doi: 10.1021/bi00178a028. [DOI] [PubMed] [Google Scholar]
- Fato R, Cavazzoni M, Castlluccio C, Parenti Castelli G, Palmer G, Degli Esposti M, Lenaz G. Steady-state kinetics of ubiquinol-cytochrome c reductase in bovine heart submitochondrial particles: diffusional effects. Biochem J. 1993;290:225–236. doi: 10.1042/bj2900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Freed JH, Fraenkel GK. Theory of linewidths in electron spin resonance spectra. J Chem Phys. 1963;39:326–348. [Google Scholar]
- Golovina EA, Tikhonov AN, Hoekstra FA. An electron paramagnetic resonance spin probe study of membrane permeability changes with seed aging. Plant Physiol. 1997;114:383–389. doi: 10.1104/pp.114.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Angell CA. Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly. J Phys Chem. 1989;93:2880–2882. [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand SC, Hardewig I. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol. 1996;58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- Hemminga MA, Roozen MJGW, Walstra P. Molecular motions and the glassy state. In: Blanshard JMV, Lillford PJ, editors. The Glassy State in Foods. Nottingham. Nottingham, UK: University Press; 1993. pp. 157–171. [Google Scholar]
- Hendry GAF, Finch-Savage WE, Thorpe PC, Atherton NM, Buckland SM, Nilsson KA, Seel WE. Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytol. 1992;122:273–279. doi: 10.1111/j.1469-8137.1992.tb04231.x. [DOI] [PubMed] [Google Scholar]
- Hendry GAF, Houghton JD, Jones OT. The cytochromes in microsomal fractions of germinating mung beans. Biochem J. 1981;194:743–751. doi: 10.1042/bj1940743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Bruinsma J. Control of respiration of binucleate and trinucleate pollen under humid conditions. Physiol Plant. 1980;48:71–77. [Google Scholar]
- Hoekstra FA, Haigh AM, Tetteroo FAA, Van Roekel T. Changes in soluble sugars in relation to desiccation tolerance in cauliflower seeds. Seed Sci Res. 1994;4:143–147. [Google Scholar]
- Hoekstra FA, van Roekel T. Isolation-inflicted injury to mitochondria from fresh pollen gradually overcome by an active strengthening during germination. Plant Physiol. 1983;73:995–1001. doi: 10.1104/pp.73.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Wolkers WF, Buitink J, Golovina EA, Crowe JH, Crowe LM. Membrane stabilization in the dry state. Comp Biochem Physiol A Comp Physiol. 1997;117:335–341. [Google Scholar]
- Hofmann GE, Hand SC. Global arrest of translation during invertebrate quiescence. Proc Natl Acad Sci USA. 1994;91:8492–8496. doi: 10.1073/pnas.91.18.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols—review and survey. Seed Sci Res. 1994;4:385–405. [Google Scholar]
- Ikuma H. Electron transport in plant respiration. Annu Rev Plant Physiol. 1972;23:419–436. [Google Scholar]
- Inzé D, Van Montagu M. Oxidative stress in plants. Curr Opin Biotechnol. 1995;6:153–158. [Google Scholar]
- Kariman K, Hempel FG, Jobsis FF. In vivo comparison of cytochrome aa3 redox state and tissue PO2 in transient anoxia. J Appl Physiol Respir Environ Exercise Physiol. 1983;55:1057–1063. doi: 10.1152/jappl.1983.55.4.1057. [DOI] [PubMed] [Google Scholar]
- Keith AD, Snipes W. Viscosity of cellular protoplasm. Science. 1974;183:666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- Kermode AR. Approaches to elucidate the basis of desiccation-tolerance in seeds. Seed Sci Res. 1997;7:75–95. [Google Scholar]
- Kornblatt JA, Hoa GHB. A nontraditional role for water in the cytochrome c oxidase reaction. Biochemistry. 1990;29:9370–9376. doi: 10.1021/bi00492a010. [DOI] [PubMed] [Google Scholar]
- Kornblatt JA, Kornblatt MJ. Cytochrome c oxidase: the presumptive channel holds at least four water molecules. Biochim Biophys Acta. 1992;1099:182–184. [Google Scholar]
- Lance C, Bonner WD., Jr The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968;43:736–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold AC, Sun WQ, Bernal-Lugo I. The glassy state in seeds: analysis and function. Seed Sci Res. 1994;4:267–274. [Google Scholar]
- Leopold AC, Vertucci CW (1989) Moisture as a regulator of physiological reaction in seeds. In PC Stanwood, MB McDonald, eds, Seed Moisture, Special Publication no. 14. Crop Science Society of America, Madison, WI, pp 51–67
- Leprince O, Atherton NM, Deltour R, Hendry GAF. The involvement of respiration in free radical processes during loss of desiccation tolerance in germinating Zea mays L. An electron paramagnetic resonance study. Plant Physiol. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince O, Bronchart R, Deltour R. Changes in starch and soluble sugars in relation to the acquisition of desiccation tolerance during maturation of Brassica campestris seed. Plant Cell Environ. 1990;13:539–546. [Google Scholar]
- Leprince O, Vertucci CW, Hendry GAF, Atherton NM. The expression of desiccation-induced damage in orthodox seeds is a function of oxygen and temperature. Physiol Plant. 1995;94:233–240. [Google Scholar]
- Leprince O, Walters-Vertucci C. A calorimetric study of the glass transition behaviors in axes of bean seeds with relevance to storage stability. Plant Physiol. 1995;109:1471–1481. doi: 10.1104/pp.109.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Les̆tan D, Podgornik H, Perdih A. Analysis of fungal pellets by UV-visible spectrum diffuse reflectance spectroscopy. Appl Environ Microbiol. 1993;59:4253–4260. doi: 10.1128/aem.59.12.4253-4260.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthje S, Döring O, Heuer S, Lüthen H, Böttger M. Oxidoreductases in plant plasma membranes. Biochim Biophys Acta. 1997;1331:81–102. doi: 10.1016/s0304-4157(96)00016-0. [DOI] [PubMed] [Google Scholar]
- McKersie BD (1991) The role of oxygen free radicals in mediating freezing and desiccation stress in plants. In EJ Pell, K Steffen, eds, Active Oxygen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 107–118
- Nobel PS. Biophysical Plant Physiology and Ecology. San Francisco, CA: WH Freeman; 1983. [Google Scholar]
- Pradet A, Raymond P. Adenine nucleotide ratios and adenylate energy charge in energy metabolism. Annu Rev Plant Physiol. 1983;34:199–224. [Google Scholar]
- Raymond P, Al-Ani A, Pradet A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol. 1985;79:879–884. doi: 10.1104/pp.79.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RC, Prausnitz JM, Poling BE. The Properties of Gases and Liquids, Ed 4. New York: McGraw-Hill; 1987. [Google Scholar]
- Senaratna T, McKersie BD, Borochov A. Desiccation and free radical mediated changes in plant membranes. J Exp Bot. 1987;38:2005–2014. [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- Sowa S, Roos EE, Caughey WS. Effector molecules to probe cytochrome c oxidase activity in germinating Phaseolus vulgaris L. seeds. J Plant Physiol. 1993;141:647–653. [Google Scholar]
- Sun WQ, Irving TC, Leopold AC. The role of sugar, vitrification and membrane phase transition in seed desiccation tolerance. Physiol Plant. 1994;90:621–628. [Google Scholar]
- Swedes JS, Sedo RJ, Atkinson DE. Relation of growth and protein synthesis to the adenylate energy charge in an adenine-requiring mutant of Escherichia coli. J Biol Chem. 1975;250:6930–6938. [PubMed] [Google Scholar]
- Van Bilsen DGJL, Hoekstra FA. Decreased membrane integrity in aging Typha latifolia L. pollen. Accumulation of lysolipids and free fatty acids. Plant Physiol. 1993;101:675–682. doi: 10.1104/pp.101.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Farrant JM (1995) Acquisition and loss of desiccation tolerance. In J Kigel, G Galili, eds, Seed Development and Germination. Marcel Dekker, New York, pp 237–271
- Wilson SB, Bonner WD. Studies of electron transport in dry and imbibed peanut embryos. Plant Physiol. 1971;48:340–344. doi: 10.1104/pp.48.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittung P, Malmstrom BG. Redox-linked conformational changes in cytochrome c oxidase. FEBS Lett. 1996;388:47–49. doi: 10.1016/0014-5793(96)00513-3. [DOI] [PubMed] [Google Scholar]