Abstract
Recent discovery of a method for derivation and culture of germline-competent rat pluripotent stem cells (PSCs) enables generation of transgenic rats or knock-out rats via genetic modification of such PSCs. This opens the way to use rats, as is routine in mice, for analyses of gene functions or physiological features. In mouse or human, one widely used technique to express a gene of interest stably and ubiquitously is to insert that gene into the Rosa26 locus via gene targeting of PSCs. Rosa26 knock-in mice conditionally expressing a reporter or a toxin gene have contributed to tracing or ablation of specific cell lineages. We successfully identified a rat orthologue of the mouse Rosa26 locus. Insertion of tdTomato, a variant of red fluorescent protein, into the Rosa26 locus of PSCs of various rat strains allows ubiquitous expression of tdTomato. Through germline transmission of one Rosa26-tdTomato knock-in embryonic stem cell line, we also obtained tdTomato knock-in rats. These expressed tdTomato ubiquitously throughout their bodies, which indicates that the rat Rosa26 locus conserves functions of its orthologues in mouse and human. The new tools described here (targeting vectors, knock-in PSCs, and rats) should be useful for a variety of research using rats.
Introduction
Use of rats for studies in behavior, pharmacology, and disease modeling has been limited because gene-targeting technology has been lacking. However, recent discovery of a culture system using small molecules specifically to inhibit spontaneous differentiation pathways of pluripotent stem cells (PSCs) [1] has permitted generation of germline-competent rat PSCs [2–4]. This stable and reproducible culture system in rat PSCs constitutes a breakthrough for generating not only transgenic rats by introducing exogenous genes into PSCs [5,6], but also knock-out rats via gene targeting [7]. Various genetically modified rats will soon be available for analyses of gene functions or physiological features like those that can now be done in mice.
To generate a genetically modified animal with stable and ubiquitous expression of a gene of interest is essential for current research. One widely used method is to insert that gene into the Rosa26 locus on mouse chromosome 6, identified by random retroviral gene-trap screening using mouse embryonic stem cells (ESCs) [8]. Rosa26 is ubiquitously expressed in embryonic as well as adult tissue, and gene targeting at this locus in ES cells is highly efficient. Insertion of a gene of interest or a loxP-flanked stop codon with a reporter or a toxin gene into Rosa26 thus has been widely used to trace specific cell lineages or, by mating with mice expressing Cre recombinase under the control of specific promoters, to ablate specific cell lineages [9,10].
As in the mouse, so in man: A human ROSA26 locus has also been identified by homology search with mouse Rosa26 sequences [11]. Insertion of sequences encoding a red fluorescent protein (RFP) into the Rosa26 locus of human ESCs allows ubiquitous expression of RFP in both undifferentiated and differentiated states [11]. Features of the Rosa26 locus may be conserved in a variety of species.
Here, as a third model, we report identification of a Rosa26 locus in the rat. Furthermore, we generated rat PSCs expressing tdTomato, a variant of RFP, by gene targeting into the Rosa26 locus, and generated a knock-in rat line via germline transmission of such PSCs.
Materials and Methods
Animals
C57BL/6NCrSlc, BDF1, and ICR mice and Wistar and DA rats were purchased from SLC Japan (Shizuoka, Japan). All experiments were performed in accordance with the animal care and use committee guidelines of the Institute of Medical Science, the University of Tokyo, and of the National Institute for Physiological Sciences.
Culture of PSCs
In this study, 3 ESC lines and 2 induced PSC (iPSC) lines were used (see Table 1). Their culture conditions were as described [12]. In brief, undifferentiated rat PSCs were maintained on mitomycin-C - treated mouse embryonic fibroblasts in an N2B27 medium [1] containing 1 μM PD0325901 (Axon, Groeningen, The Netherlands), 3 μM CHIR99021 (Axon), and 1,000 U/mL of rat leukemia inhibitory factor (Millipore, Bedford, MA).
Table 1.
Efficiency of Gene Targeting in Various Types of Rat Pluripotent Stem Cells
| Name | Strain | Cell type | Reference | Transduced cells | Drug- resistant colonies | Picked- up colonies | Targeted clones (%)a |
|---|---|---|---|---|---|---|---|
| BLK2i-1 | DA×Wistar | ESC | [18] | 5×10e6 | 3 | 2 | 1 (50) |
| 5×10e6 | 2 | 2 | 2 (100) | ||||
| 2.5×10e6 | 18 | 12 | 6 (50) | ||||
| DA3i-1 | DA | ESC | 5×10e6 | 48 | 24 | 9 (38) | |
| BN2i-4 | BN | ESC | [5] | 5×10e6 | 8 | 8 | 2 (25) |
| 5×10e6 | 13 | 9 | 2 (23) | ||||
| T1-3 | Wistar | iPSC | [4,12] | 4×10e6 | 9 | 8 | 3 (38) |
| DAT3-1 | DA | iPSC | 5×10e6 | 6 | 6 | 1 (17) | |
| Total | 107 | 71 | 26 (37) |
Judged by PCR.
ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; PCR, polymerase chain reaction.
DA rat-derived ESC (DA3i-1) and iPSC (DAT3-1) lines were newly established. DA3i-1 ESCs were derived from DA rat blastocysts as described [13]. DAT3-1 iPSC were generated from DA rat-derived tail-tip fibroblasts by introducing 3 mouse factors (Oct3/4, Klf4, and Sox2) in one retroviral vector (data not shown).
Construction of vectors and gene targeting
Homology arms were amplified from genomic DNA of DA strain rats by polymerase chain reaction (PCR) using PrimeSTAR or PrimeSTAR GXL DNA polymerase (Takara Bio, Otsu, Japan), according to the manufacturer's protocol. These arms, with an additional NheI site and an MC1-promoter driven DTA cassette amplified from an MC1-DTA vector, a kind gift from Dr. T. Yagi (Osaka University, Osaka, Japan), were inserted into pBluescript KS(+) (Stratagene, La Jolla, CA) with an infusion cloning kit (Takara Bio) (prRosa26-1 in Fig. 1A). A splice acceptor sequence amplified from a pSAβ-geo vector, a kind gift from Dr. P. Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA); tdTomato amplified from ptdTomatoN1 (Clontech, Palo Alto, CA); and IRES-Puror-pA amplified from pCAG-Cre-IRES-Puror-pA, a kind gift from Dr. J. Miyazaki (Osaka University), were inserted into the NheI site of prRosa26-1.
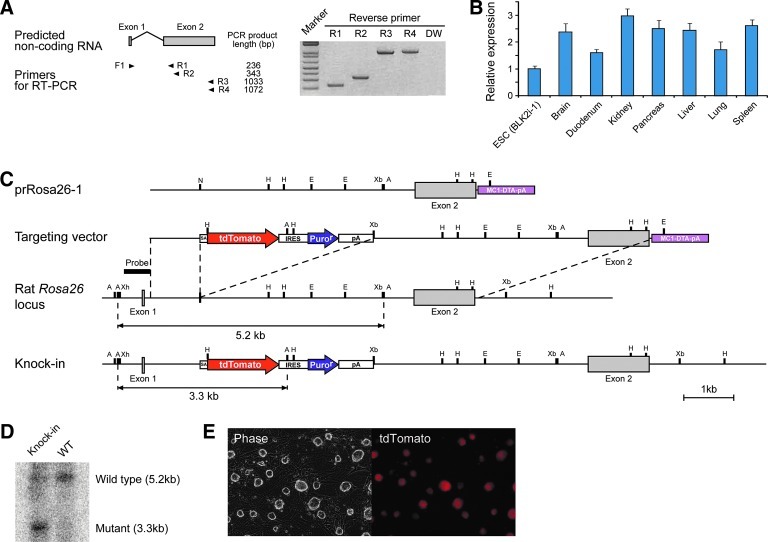
FIG. 1.
Identification of Rat Rosa26 locus and gene targeting of rat pluripotent stem cells by inserting a cassette containing tdTomato into Rosa26 locus. (A) The RT-PCR analysis of rat Rosa26 noncoding RNA in rat ESCs. The primer positions are schematically shown by arrowheads under the predicted noncoding RNA transcript. (B) Quantitative real-time PCR of Rosa26 noncoding RNA in rat ESCs and adult tissues relative to β-actin. Error bars are mean±SD (n=2). (C) Structure of targeting vectors and a schematic of knock-in genome of rat Rosa26 locus on chromosome 4. Letters on the locus indicate selected restriction enzyme sites. A, ApaI; E, EcoR1; H, HindIII; N, NheI; Xb, XbaI; Xh, XhoI. (D) Result of the Southern blot analysis using ApaI-digested genomic DNA hybridized with 5′ probe schematically shown in (A). (E) Photomicrograph of Rosa26-tdTomato knock-in rat ESCs generated from the BLK2i-1 line (BLK-RT2). These ESCs express tdTomato ubiquitously. ESCs, embryonic stem cells; RT-PCR, reverse transcription–polymerase chain reaction.
Electroporation for gene targeting was carried out as described [5]. In brief, 2.5∼5×106 rat PSCs suspended in PBS were mixed with 20 μg linearized targeting vector digested by the Sall1 restriction enzyme and were transferred to a Gene Pulser cuvette (Bio-Rad, Richmond, CA). Electroporation was carried out at 800 V, 10 μF in Gene Pulser equipment (Bio-Rad). After electroporation, PSCs were seeded onto mitomycin-C - treated puromycin-resistant mouse embryonic fibroblasts made in house, and 24 h later, 1.5 μg/mL puromycin (Invitrogen, Carlsbad, CA) was added to the culture medium.
Reverse transcription–PCR and quantitative real-time PCR analysis
cDNA synthesized using the ThermoScript™ reverse transcription (RT)–PCR System (Invitrogen, Carlsbad, CA) from extracts of BLK2i-1 rat ESCs was used for the RT-PCR analysis of Rosa26 noncoding RNA. PCR primers are shown in Supplementary Fig. S3 (Supplementary Data are available online at www.liebertpub.com/scd). One forward primer and 4 reverse primers were designed (F1 and R1-R4, shown in Fig. 1A and Supplementary Fig. S3). PCR was performed using Taq HS polymerase (Takara Bio) according to the manufacturer's protocol.
3′ and 5′ rapid amplification of cDNA ends analysis and identification of full-length noncoding RNA
3′ and 5′ rapid amplification of cDNA ends (RACE) was performed using the SMARTer™ RACE cDNA Amplification Kit (Clontech), according to the manufacturer's protocol. Synthesized cDNAs were amplified using an universal primer A mix with 3′ or 5′ RACE primers (primers shown in Supplementary Fig. S3). Amplified cDNAs were cloned into the pCR-Blunt IITOPO vector (Invitrogen), and their sequences were confirmed.
Genotyping and Southern blotting
DNA was extracted using QIAamp DNA Mini Kits (Qiagen, Germantown, MD) from picked-up PSCs. For genotyping, PCR primers for amplification of the Rosa26 knock-in locus were Fw, 5′-CAGAAAAGGCGGAGCGAGCCCAAG-3′, and Rv, 5′- GGGCCCTCACATTGCCAAAAGACGG-3′. For the Southern blot analysis, genomic DNA extracted from PSCs was digested by the ApaI restriction enzyme and hybridized with a DNA probe cloned from the upstream region of the rat Rosa26 5′-arm.
Embryo manipulation
Rat and mouse embryos were prepared using published protocols [12,14]. In brief, rat blastocysts were collected in the HER medium [15] containing 18% fetal bovine serum (Invitrogen) from the oviduct and the uterus of rats 4.5 days post coitum (dpc). These embryos were transferred into the mR1ECM medium [16] containing 80 mM NaCl and 0.01% polyvinyl alcohol (Sigma-Aldrich Co., St. Louis, MO) and were cultured for about 1 h until injection. Mouse 8-cell/morula stage embryos were collected in the M2 medium (Millipore) from the oviduct and the uterus of BDF1×C57BL/6 mice 2.5 dpc. These embryos were transferred into the KSOM-AA medium (Millipore) and were cultured for 24 h before blastocyst injection.
For micromanipulation, PSCs were trypsinized and suspended in the PSC culture medium. A piezo-driven micromanipulator (Prime Tech, Tokyo, Japan) was used to drill zona pellucida and trophectoderm under the microscope, and 10 PSCs were introduced into blastocyst cavities near the inner cell mass. After blastocyst injection, embryos underwent follow-up culture for 1–2 h. Rat blastocysts were transferred into the uteri of pseudopregnant recipient Wistar rats (3.5 dpc), and mouse blastocysts were transferred into the uteri of pseudopregnant recipient ICR mice (2.5 dpc).
Flow cytometry analysis
To analyze chimerism of chimeric rats at the adult stage, we used peripheral blood cells obtained from the retro-orbital venous plexus. Leukocytes isolated by osmotic lysis of erythrocytes were stained with APC-conjugated mouse anti-rat CD45 antibody (BD Biosciences, San Diego, CA).
To analyze chimerism of interspecific chimeras at the fetal stage, embryonic fibroblasts were stained with biotin-conjugated mouse anti-rat CD54 antibody (ICAM-1, 1A29), Alexa Fluor 647-conjugated goat anti-mouse IgG antibody, and FITC-conjugated rat anti-mouse CD54 antibody (all BD Biosciences).
All stained cells were analyzed by FACSCanto II (BD Bioscience).
Result and Discussion
Given that the transgenic rat carrying a mouse Rosa26 promoter-driven EGFP construct revealed ubiquitous expression of EGFP [17], we inferred that the promoter region of the Rosa26 locus contained a highly conserved sequence. To identify a Rosa26 locus in rat genome, we searched for similar sequences in the UCSC Genome Browser database (http://genome.ucsc.edu/) with a 5′ arm 1,088 bp sequence of the mouse Rosa26 targeting-vector [10], which corresponds to the first intron of the mouse Rosa26 transcript. We found a highly conserved region in rat chromosome 4 that contains not only a Rosa26 locus, but also genes that are neighbors to Rosa26 in mouse (Supplementary Fig. S1). In mice, transcripts from the Rosa26 locus are ubiquitously expressed noncoding RNAs [8]. To see whether the putative rat Rosa26 locus also encodes such noncoding RNAs, we confirmed the expression of Rosa26 in rat ESCs by RT-PCR. We designed primer sets to flank a region containing the first intron, with sequences based on the rat-expressed sequence tag database (Fig. 1A, Supplementary Figs. S2 and S3). Our results showed that 2 exons flanking 1 intron were amplified with each primer set (Fig. 1A and Supplementary Fig. S2). The quantitative real-time PCR analysis demonstrated that this noncoding RNA was expressed in a wide variety of adult tissues (Fig. 1B). To reveal the full-length sequence of this noncoding RNA, we performed the 3′ and 5′ RACE analysis. The sequence of at least one noncoding RNA transcribed from the rat Rosa26 locus was 1,160 bp long (Supplementary Fig. S2). It exhibited 74% homology with the sequence of one of the mouse Rosa26 noncoding RNA transcripts (NR_027009) (Supplementary Fig. S3).
To see if this region, like the Rosa26/ROSA26 locus in mouse or human, actually allowed ubiquitous expression of an inserted gene, we cloned homology arms from genomic DNA of the DA rat and constructed a targeting vector to insert a splice acceptor with the tdTomato-IRES-Puror-pA sequence (Fig. 1C). We transduced the linearized targeting vector into 3 ESC lines and 2 iPSC lines derived from various strains and tissues. Although only small numbers of colonies were observed after electroporation and drug selection, on average about 30% of picked-up clones demonstrated correct targeting judged by PCR using genomic DNA (Table 1). Gene targeting was also confirmed by Southern blotting (Fig. 1D). As expected, all positive clones ubiquitously expressed tdTomato (Fig. 1E). Survival rates of PSCs after drug selection were low compared with mouse PSCs subjected to conventional targeting. This might result from low transduction efficiency of the targeting vector or from high sensitivity to antibiotics in rat PSCs [7].
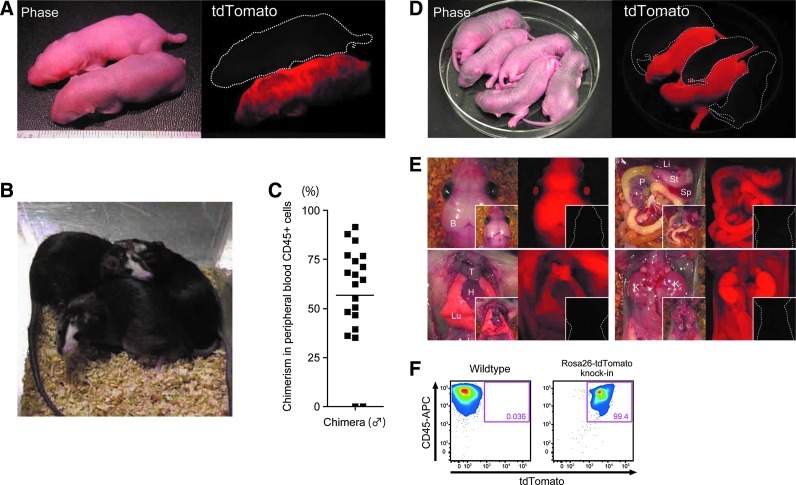
We injected rat ESCs from one of the Rosa26-tdTomato knock-in lines, BLK-RT2, into rat blastocysts. Rat ESCs of the original cell line, BLK2i-1, are germline-competent. The line was established from blastocysts of rats with black coats derived from F1 crosses of Wistar (white-coated) and DA (black-coated) rats back-crossed with Wistar rats for at least 3 generations [18]. A total of 116 injected blastocysts were transferred into uteri of pseudo-pregnant rats and at weaning 27 out of 36 pups proved chimeras. Neonatal chimeric rats expressed tdTomato throughout their bodies (Fig. 2A). They grew into adults normally and showed coat color chimerism (black, donor ESC-derived; white, host embryo Wistar strain-derived) (Fig. 2B). To estimate their chimerism, we analyzed tdTomato expression in peripheral blood of male chimeric rats by flow cytometry. Although chimerism varied individually, most chimeras showed over 50% chimerism in CD45-positive peripheral blood mononuclear cells (Fig. 2C).
FIG. 2.
Generation of chimeric rats by injection of Rosa26-tdTomato knock-in rat ESCs into rat blastocysts. (A) Picture of a representative newborn chimeric rat generated by intrablastocystic injection of BLK-RT2 rat ESCs. Rat not expressing tdTomato is a nonchimeric littermate (white dashed outline). (B) Adult chimeric rats. Black coat color originates from injected ESCs; white coat color originates from host blastocysts. (C) The chimerism analysis in peripheral blood of male chimeric rats. Leukocytes isolated by osmotic lysis of erythrocytes were stained with antibody against rat CD45 and were analyzed by flow cytometry for intensity of tdTomato expression in CD45-positive cells. Square dots indicate values for individual rats. (D) Offspring obtained by mating a male chimeric rat with a wild-type female. tdTomato-positive neonates are Rosa26-tdTomato knock-in rats that resulted from germline transmission of genes contained in BLK-RT2 rat ESCs. (E) tdTomato expression in main organs of Rosa26-tdTomato knock-in rats. Insets show organs obtained from tdTomato-negative littermates (white-dashed outline). In bright-field panels, B, brain; T, thymus; H, heart; Lu, lung; Li, liver; P, pancreas; St, stomach; Sp, spleen; and K, kidney. (F) FACS patterns obtained using peripheral blood cells from wild-type rats and Rosa26-tdTomato knock-in rats. In knock-in rats, almost all CD45-positive cells express tdTomato. FACS, fluorescence activated cell sorting.
Next, we mated 3 male rats exhibiting relatively higher chimerism with wild-type females to see if BLK-RT2 rat ESCs were capable of germline transmission. Offspring of 2 chimeras expressed tdTomato ubiquitously throughout their bodies, indicating successful germline transmission (Fig. 2D). Expression of tdTomato was detected in all main organs at the neonatal stage (Fig. 2E) and in CD45-positive blood cells at the adult stage (Fig. 2F). These data provided strong evidence for functional conservation of the Rosa26/ROSA26 locus among rat, mouse, and human.
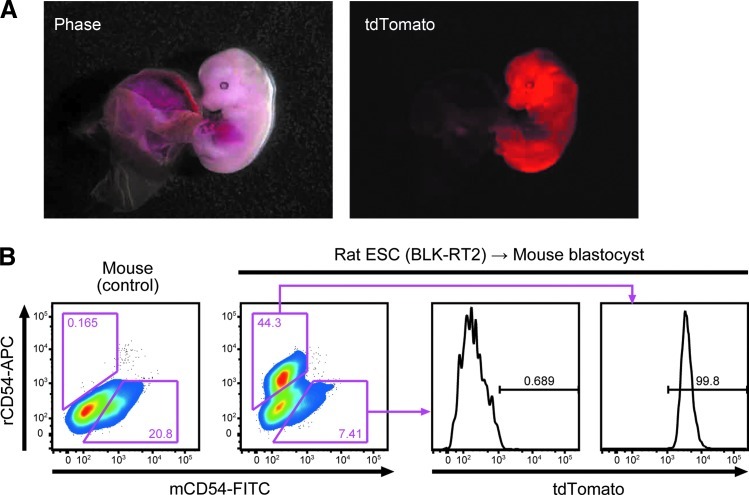
We also injected BLK-RT2 rat ESCs into mouse blastocysts. A total of 32 injected blastocysts were transferred into uteri of pseudo-pregnant mice, and 13 out of 16 fetuses proved interspecific chimeras (Fig. 3A). To distinguish host- and donor-derived cells, we cultured embryonic fibroblasts from these chimeras and stained them with a specific antibody against mouse or rat CD54. Only cells expressing rat CD54 also expressed tdTomato (Fig. 3B). Therefore, Rosa26 knock-in rat PSCs can also be used for interspecific blastocyst complementation to make rat organs in mice [12].
FIG. 3.
Generation of interspecific chimeras by injection of Rosa26-tdTomato knock-in rat ESCs into mouse blastocysts. (A) Embryonic day (E) 13.5 interspecific chimera generated by injection of BLK-RT2 rat ESCs into a mouse blastocyst. (B) FACS patterns obtained using embryonic fibroblasts established from an interspecific chimera and stained with a specific antibody against mouse or rat CD54. As a control, mouse embryonic fibroblasts were also analyzed.
Via gene targeting of PSCs, we have successfully generated Rosa26 knock-in rats that express tdTomato ubiquitously. These PSCs and rats will provide useful tools for cellular or organ transplantation experiments, and the targeting vector constructed here will be useful for expressing genes of interest stably and ubiquitously in a variety of rat PSCs. These powerful tools should contribute to progress in a variety of research using rats.
Supplementary Material
Acknowledgment
We thank Dr. A. Knisely for critical reading of the article. This work was supported by grants from the Japan Science and Technology Agency (JST), KAKENHI (23700507) Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (JSPS), and the Ministry of Education, Culture, Sport, Science, and Technology (MEXT).
Author Disclosure Statement
Hiromitsu Nakauchi is a founder and shareholder of ReproCELL, Inc. There is no conflict of interest to disclose.
Reference
- 1.Ying QL. Wray J. Nichols J. Batlle-Morera L. Doble B. Woodgett J. Cohen P. Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buehr M. Meek S. Blair K. Yang J. Ure J. Silva J. McLay R. Hall J. Ying QL. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Li P. Tong C. Mehrian-Shai R. Jia L. Wu N. Yan Y. Maxson RE. Schulze EN. Song H, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamanaka S. Yamaguchi T. Kobayashi T. Kato-Itoh M. Yamazaki S. Sato H. Umino A. Wakiyama Y. Arai M, et al. Generation of germline-competent rat induced pluripotent stem cells. PLoS One. 2011;6:e22008. doi: 10.1371/journal.pone.0022008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirabayashi M. Kato M. Sanbo M. Kobayashi T. Hochi S. Nakauchi H. Rat transgenesis via embryonic stem cells electroporated with the Kusabira-orange gene. Mol Reprod Dev. 2010;77:474. doi: 10.1002/mrd.21181. [DOI] [PubMed] [Google Scholar]
- 6.Kawamata M. Ochiya T. Generation of genetically modified rats from embryonic stem cells. Proc Natl Acad Sci U S A. 2010;107:14223–14228. doi: 10.1073/pnas.1009582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong C. Li P. Wu NL. Yan Y. Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrowicz BP. Imamoto A. Fiering S. Herzenberg LA. Kerr WG. Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao X. Fujiwara Y. Chapdelaine A. Yang H. Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- 10.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 11.Irion S. Luche H. Gadue P. Fehling HJ. Kennedy M. Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477–1482. doi: 10.1038/nbt1362. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T. Yamaguchi T. Hamanaka S. Kato-Itoh M. Yamazaki Y. Ibata M. Sato H. Lee YS. Usui J, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi M. Kato M. Kobayashi T. Sanbo M. Yagi T. Hochi S. Nakauchi H. Establishment of rat embryonic stem cell lines that can participate in germline chimerae at high efficiency. Mol Reprod Dev. 2010;77:94. doi: 10.1002/mrd.21123. [DOI] [PubMed] [Google Scholar]
- 14.Nagy A. Gertsenstein M. Vintersten K. Behringer R. Manipulating the Mouse Embryo A Laboratory Manual. 3rd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2003. [Google Scholar]
- 15.Ogawa S. Sato K. Hashimoto H. In vitro culture of rabbit ova from the single cell to the blastocyst stage. Nature. 1971;233:422–424. doi: 10.1038/233422a0. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi K. Abeydeera LR. Okuda K. Niwa K. Effects of osmolarity and amino acids in a chemically defined medium on development of rat one-cell embryos. J Reprod Fertil. 1995;103:27–32. doi: 10.1530/jrf.0.1030027. [DOI] [PubMed] [Google Scholar]
- 17.Kisseberth WC. Brettingen NT. Lohse JK. Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi M. Tamura C. Sanbo M. Goto T. Kato-Itoh M. Kobayashi T. Nakauchi H. Hochi S. Ability of tetraploid rat blastocysts to support fetal development after complementation with embryonic stem cells. Mol Reprod. 2012;Dev79:402–412. doi: 10.1002/mrd.22043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.