Abstract
The frequencies of alleles of killer cell immunoglobulin like receptor genes, KIR3DL3 and KIR3DL2, and the carrier frequency of KIR2DL4 alleles have been determined from a population of African Americans (n=100) by DNA sequencing of the coding regions. Fifty alleles of KIR3DL3 were observed with the most frequent, KIR3DL3*00901 (13%). KIR3DL2 was also diverse, 32 alleles with KIR3DL2*00103 the most frequent (17%). For KIR2DL4, of the 18 alleles observed, one allele, KIR2DL4*00103 was found in 64 of the 100 individuals. Thirty six novel alleles encoding a total of 28 unique receptors are described. Pairwise comparisons among all of the alleles at each locus suggest a predominance of synonymous substitutions. The variation at all three framework loci fits a neutral model of evolution.
Keywords: Natural killer cells, killer cell immunoglobulin-like genes (KIR), allelic polymorphism, population study
Introduction
Natural killer (NK) cells utilize a number of stimulatory and inhibitory receptors to identify danger and to prevent destruction of normal cells. The inhibitory killer-immunoglobulin-like receptors (iKIR) function early in NK maturation to ready the NK cell for response (Jonsson and Yokoyama 2009). Later, in mature NK cells, iKIR bind HLA ligands to prevent destruction of normal cells; loss of HLA expression during infection or malignancy leads to removal of the inhibitory signal allowing the NK cell to become activated (Lanier 2005). The functions of the stimulatory KIR are less well characterized.
The KIR gene family, located on chromosome 19 in the leukocyte receptor gene complex, encodes the 15 inhibitory and stimulatory receptors (Kelley et al. 2005). The KIR genes lie in two adjacent clusters separated by approximately 14 kb (Martin et al. 2000;Wilson et al. 2000). The number of genes in each cluster vary so, for example, some versions of chromosome 19 may carry only seven functional KIR loci while others may carry 12. Three functional genes and one pseudogene mark the boundaries of the two gene clusters: KIR3DL3 and KIR3DP1 (pseudogene) flank the centromeric gene cluster and KIR2DL4 and KIR3DL2 flank the telomeric cluster. These four genes are termed framework genes since the majority of KIR haplotypes include these loci. Several less frequent haplotypes have been described that either lack KIR2DL4 (Traherne et al. 2010) (Norman et al. 2002;Ordonez et al. 2008) or carry two copies of KIR2DL4 due to unequal recombination within the KIR gene complex (Martin et al. 2003).
The receptors encoded by the three functional framework genes appear to have very different properties. Two of the framework KIR are inhibitory receptors with three extracellular domains. The expression of KIR3DL3 mRNA is low and limited to a subset of NK cells; surface expression of the protein has not been detected (Trundley et al. 2006). KIR3DL3’s ligand is unknown and it lacks a functional exon to encode the extracellular membrane proximal stem region of the KIR receptor. Although little is known about the function of KIR3DL3, its extracellular domains are highly conserved between humans and apes suggesting the presence of functional constraints on this protein (Jones et al. 2006). Expressed on a subset of NK cells, KIR3DL2 appears to recognize HLA-A3 and –A11 heterodimers (Hansasuta et al. 2004) and HLA-B27 homodimers (Kollnberger et al. 2007). It also binds microbial CpG oligonucleotides for uptake into endosomes (Sivori et al. 2010). Found on NK cells in the maternal decidua, KIR2DL4 appears to recognize and internalize soluble HLA-G into endosomes and so may play a role in the vascular remodeling that takes place during pregnancy (Ponte et al. 1999;Rajagopalan et al. 2006). Variation in the transmembrane-encoding exon of this two domain KIR can alter cell surface expression including production of a secreted product (Goodridge et al. 2007). With the potential to be either inhibitory or activating (Faure and Long 2002;Kikuchi-Maki et al. 2003;Rajagopalan et al. 2001), KIR2DL4 has been conserved during primate evolution (Rajalingam et al. 2004).
The framework genes are polymorphic. The goal of this study was to examine the diversity in the functional KIR framework genes in an African American population.
Materials and Methods
Human studies were approved by the Georgetown University Institutional Review Board and conform to standards laid down in the 1964 Declaration of Helsinki. Genomic DNA was isolated from Epstein Barr Virus-transformed B-cell lines from 100 unique and unrelated African Americans from the National Institute of General Medical Sciences (NIGMS) Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/nigms/) using a QIAamp® DNA Blood Mini Kit (Qiagen, Valencia, CA).
Three overlapping PCR amplicons were generated from each framework gene covering the complete coding sequence (Supplementary Table 1, Supplementary Figure 1). KIR amplicons were purified using Agencourt AmPure magnetic beads (Beckman Coulter Genomics; http://www.beckmangenomics.com). Sequencing was performed using Applied Biosystems’ BigDye Terminator Ready Reaction mix (Applied Biosystems, Foster City, CA). Sequencing primers were positioned to obtain the sequence of both strands of each amplicon (Supplementary Table 2). Sequencing products, purified using Agencourt CleanSEQ (Beckman Coulter Genomics), were detected using an Applied Biosystems 3730XL DNA analyzer. Sample files were analyzed using Sequencher (Genecodes Corp., Ann Arbor, MI) and Assign SBT 3.2.7 (Conexio Genomics, Applecross, Western Australia) software. Sequences were compared to known KIR sequences obtained from the IPD-KIR database version 2.1.0 to determine allelic assignments. Any novel allele found that was not in that database was isolated and sequenced as described below. In this report, the numbering of nucleotides and codons is based on IPD-KIR unless noted.
Novel alleles were isolated for DNA sequencing using allele specific amplification, by cloning, or by using HaploPrep kits (Qiagen, Valencia, CA). HaploPrep™ separation reagents used included KIR2DL4-158G, KIR2DL4-712A, KIR2DL4-1062T, KIR3DL2-18T, KIR3DL2-328A, KIR3DL2-1197C, KIR3DL2-1197T, KIR3DL3-161A, KIR3DL3-1018A, and KIR3DL3-1018T. Assembly of sequences from the three amplicons covering a novel allele required either the use of an allele-specific haplotype fragment as a PCR template or an overlap of polymorphic regions carried by two adjacent cloned amplicons in order to ensure that all amplicon sequences derived from the same allele. Allele designations for novel and confirmatory KIR alleles were assigned by the WHO Nomenclature Committee for Factors of the HLA System (Marsh et al. 2003).
To characterize the deletion in cell GM17183, DNA fragments were isolated with probe 2DS5-483C (HaploPrep). Loci carried on the KIR2DS5-containing DNA fragment were identified by DNA sequencing.
PyPop (Python for Population genetics, version 0.7.0 http://www.pypop.org) was used to carry out Hardy-Weinberg testing and Ewens-Watterson homozygosity analyses (Lancaster et al. 2003;Lancaster et al. 2007). Allele frequencies were determined by gene counting and assumed that each individual carried two copies of each locus. Allele frequencies at each locus were evaluated for deviations from Hardy-Weinberg equilibrium proportions using the exact test of Guo and Thompson (Guo and Thompson 1992). Chi-square tests were investigated for a pooled set of all heterozygotes and a pooled set of all homozygotes (Meyer et al. 2006). The Ewens-Watterson test of homozygosity was applied to each locus (Ewens 1972;Slatkin 1996), using Slatkin’s Monte-Carlo implementation of the exact test (Slatkin 1994;Slatkin 1996). The normalized deviate of F (Fnd, the difference between the observed and expected values of F, divided by the square root of the variance of the expected F) was also calculated for each locus (Salamon et al. 1999). To test the neutral theory null model, Tajima’s D and the mismatch distribution using a constant population size were calculated using DnaSP v5 (Tajima 1989) (Librado and Rozas 2009;Rogers and Harpending 1992;Slatkin and Hudson 1991). SNAP (http://www.hiv.lanl.gov) was used to estimate the ratio of synonymous to nonsynonymous substitutions (dS/dN) at a KIR locus (Korber 2000;Nei and Gojobori 1986). Alleles included were those reported in IPD-KIR database 2.3.0 and additional alleles identified in our frequency study. Phylogenetic trees were created using the neighbor –joining method (Saitou and Nei 1987) with MEGA version 4 software (Tamura et al. 2007).
Results
KIR3DL3
DNA sequencing of KIR3DL3 alleles from 100 random African Americans identified 50 alleles, 38 previously reported and 12 novel. Tests were performed to measure the goodness of fit of KIR3DL3 genotypes to Hardy-Weinberg proportions. This test measures the degree to which observed genotype frequencies differ from those expected based on the allele frequencies for that population, assuming that the population is suitably large and experiences random mating. No overall deviation from Hardy-Weinberg proportions were noted (p-value 0.2951).
Table 1 lists the KIR3DL3 allele frequencies. Five alleles were found at frequencies ≥5%: KIR3DL3*00301 (8.5%), *00402 (5%), *005 (8%), *00901 (13%) and *1406 (6.5%). Only three individuals were apparent homozygotes; all three carried alleles present at frequencies >5%. Two of the common alleles found in African Americans were observed at frequencies over 5% in European Americans—KIR3DL3*003 (22%) and *00901 (12%)(Hou et al. 2007). The third common allele in European Americans, KIR3DL3*00101 (14%), was found at a frequency of 2.5% in African Americans and could derive from admixture. Previous studies estimate that the African American population exhibits approximately 13% admixture with the European population (Tishkoff et al. 2009).
Table 1.
Frequencies of KIR framework genesa
| KIR3DL3* | Frequency % (n) | KIR3DL2* | Frequency % (n) | KIR2DL4* | Frequency % (n) |
|---|---|---|---|---|---|
| 00101 | 2.5 (5) | 00101d | 10 (20) | 00102d | 8 (8) |
| 00103 | 0.5 (1) | 00103d | 17 (34) | 00103d | 64 (64) |
| 00104 b | 1 (2) | 00201d | 7 (14) | 00202 | 1 (1) |
| 00201 | 1 (2) | 00301 | 3.5 (7) | 00501 | 21 (21) |
| 00202 | 1 (2) | 00302 | 3 (6) | 00602 | 3 (3) |
| 00205 | 2 (4) | 00501 | 1 (2) | 0080101/03d | 23 (23) |
| 00206 | 2.5 (5) | 00502b | 0.5 (1) | 0080102 | 1 (1) |
| 00207 | 2 (4) | 006 | 5.5 (11) | 00802d | 17 (17) |
| 00208 | 1 (2) | 00701d | 5.5 (11) | 011 | 11 (11) |
| 00301d | 8.5 (17) | 008 | 3.5 (7) | 01201 | 5 (5) |
| 00402d | 5 (10) | 00901 | 1.5 (3) | 01202 b | 1 (1) |
| 005 | 8 (16) | 00902 | 2.5 (5) | 013 b | 1 (1) |
| 00601 | 1 (2) | 010 | 7 (14) | 017 b | 1 (1) |
| 007 | 1 (2) | 01301 | 13 (26) | 018b | 1 (1) |
| 00801 | 2.5 (5) | 01302b | 0.5 (1) | 019b | 1 (1) |
| 00901d | 13 (26) | 01303b | 0.5 (1) | 020b | 1 (1) |
| 00902 | 1 (2) | 016 | 0.5 (1) | 021 b | 1 (1) |
| 00905 b | 0.5 (1) | 019 | 2 (4) | 022 b | 5 (5) |
| 01001 | 3.5 (7) | 023 | 2 (4) | Negative f | 1 (1) |
| 01002 | 0.5 (1) | 025 c | 0.5 (1) | ||
| 01101 | 3 (6) | 028c | 1 (2) | ||
| 01102 | 1 (2) | 029c | 3 (6) | ||
| 012 | 1.5 (3) | 032c | 0.5 (1) | ||
| 01302 | 1.5 (3) | 033c | 0.5 (1) | ||
| 01402d | 4.5 (9) | 040c | 1.5 (3) | ||
| 01404 | 1 (2) | 041c | 1 (2) | ||
| 01406 | 6.5 (13) | 057b | 1 (2) | ||
| 01408b | 1 (2) | 058b | 0.5 (1) | ||
| 01502 | 1 (2) | 059b | 0.5 (1) | ||
| 017 | 0.5 (1) | 060b | 0.5 (1) | ||
| 01802b | 0.5 (1) | 061b | 0.5 (1) | ||
| 020 | 0.5 (1) | 062b | 0.5 (1) | ||
| 02101 | 0.5 (1) | 3dl1/3dl2 fusion genese | 2.5 (5) | ||
| 022 | 0.5 (1) | ||||
| 02501 | 0.5 (1) | ||||
| 02502 | 1 (2) | ||||
| 02701 | 1 (2) | ||||
| 028 | 0.5 (1) | ||||
| 032 | 2.5 (5) | ||||
| 034 | 0.5 (1) | ||||
| 035 | 4.5 (9) | ||||
| 036 | 0.5 (1) | ||||
| 041b | 0.5 (1) | ||||
| 049b | 2 (4) | ||||
| 050b | 1 (2) | ||||
| 051b | 0.5 (1) | ||||
| 052b | 1 (2) | ||||
| 053b | 1.5 (3) | ||||
| 054b | 0.5 (1) | ||||
| 055b | 0.5 (1) |
Allele frequencies are calculated for KIR3DL3 and KIR3DL2. It was assumed that each individual carried two copies of these genes. Because not all haplotypes carry KIR2DL4, the carrier frequency is reported for this locus.
Novel allele described in this study and designated as such by the WHO Committee for Factors of the HLA System.
Alleles with sequences not reported in IPD-KIR database 2.3.0. Our sequence submissions were assigned as confirmatory by the WHO Committee for Factors of the HLA System.
Intron variation not distinguished
Five individuals carry a chimeric gene resulting from the fusion of KIR3DL1 and KIR3DL2 (Jiang et al. 2010;Norman et al. 2009). These alleles include KIR3DL1*059 (three individuals), KIR3DL1*064 (one individual), and KIR3DL1*065 (one individual).
One individual does not carry KIR2DL4 alleles.
The 12 novel KIR3DL3 alleles encoding eight unique receptors are described in Table 2; six of these alleles were found in two or more individuals (Table 1). The eight receptors encoded by these novel alleles differ by a single amino acid substitution or by two substitutions from receptors encoded by a closely related allele selected based on a neighbor-joining tree (Supplemental Figure 2). In total, the amino acid variations are found throughout the protein altering all three immunoglobulin-like extracellular domains, the transmembrane region, and cytoplasmic tail. Four of the substitutions alter amino acid residues not previously polymorphic in the known alleles. Four novel alleles are synonymous variants of known alleles, altering the nucleotide sequence of the exons.
Table 2.
Description of novela alleles of KIR framework loci
| Locus | Novel Allele | Most Similar Allele |
Codons (Amino Acid) Altered | GenBank Accession No. |
Cell ID |
|---|---|---|---|---|---|
| 2DL4 | 2DL4*01202 | 2DL4*01201 | 317 GCG (A)=>GCA (A) | GU830915 | GM17179 |
| 2DL4*013 | 2DL4*00801 | 115 GCA (A) =>ACA (T), 186 CCG (P)=>GCG (A) | GU830911 | GM17199 | |
| 2DL4*017 | 2DL4*00802 | 22 GGA (G)=>AGA (R) | GU830913 | GM17132 | |
| 2DL4*018 | 2DL4*00103 | 32 CGT (R)=>GGT (G), 174 GGC (G)=>AGC (S) | GU830914 | GM17137 | |
| 2DL4*019 | 2DL4*018 | 231 TTC (F) => TTT (F), 248 AAT (N) => AT (deletion, converts 10A to 9A) | GU830916 | GM17180 | |
| 2DL4*020 | 2DL4*00802 | 137 GAA (E) =>GTA (V) | GU830917 | GM17192 | |
| 2DL4*021 | 2DL4*00103 | 158 GAC (D)=>AAC (N) | GU830918 | GM17197 | |
| 2DL4*022 | 2DL4*00103 | 317 GCG (A)=>GTG (V) | GU830912 | GM17112 | |
| 3DL2 | 3DL2*00502 | 3DL2*00501 | 122 CAA (Q)=>CAG (Q) | GU944872 | GM17134 |
| 3DL2*01302 | 3DL2*01301 | 125 TCA (S)=>TCG (S) | GU944864 | GM17109 | |
| 3DL2*01303 | 3DL2*01301 | −18 ACT (T) =>ACG (T) | GU944868 | GM17122 | |
| 3DL2*025b | 3DL2*01301 | 145 CGC (R) =>CAC (H) | GU944875 | GM17165 | |
| 3DL2*028b | 3DL2*00103 | 49 ATC (I) =>TTC (F) | GU944876 | GM17166 | |
| 3DL2*029b | 3DL2*00201 | 75 CGG (R) =>CCG (P) | GU944870 | GM17126 | |
| 3DL2*032b | 3DL2*00103 | 226 TGG (W)=>CGG (R), 231 ATC (I) =>ATG (M) | GU065733 | GM17113 | |
| 3DL2*033b | 3DL2*00103 | 219 GTG (V)=>ATG (M) | GU944866 | GM17112 | |
| 3DL2*040b | 3DL2*01301 | 87 GCA (A) =>ACA (T) | GU944863 | GM17106 | |
| 3DL2*041b | 3DL2*032 | 433 GTT (V) =>TTT (F) | GU944871 | GM17128 | |
| 3DL2*057 | 3DL2*010 | 145 CGC (R) =>CAC (H) | GU944869 | GM17124 | |
| 3DL2*058 | 3DL2*00701 | 47 GTT (V) =>ATT (I) | GU944873 | GM17137 | |
| 3DL2*059 | 3DL2*00103 | 75 CGG (R)=>CCG (P), 145 CGC (R)=>CAC (H) | GU944874 | GM17142 | |
| 3DL2*060 | 3DL2*00302 | 62 GGT (G)=>AGT (S) | GU944877 | GM17172 | |
| 3DL2*061 | 3DL2*032 | 195 GTG (V)=>ATG (M) | GU944878 | GM17193 | |
| 3DL2*062 | 3DL2*00902 | 92 CTG (L)=>GTG (V) | GU944862 | GM17199 | |
| 3DL3 | 3DL3*00104a | 3DL3*00101 | 281 CAC (H)=>CAT (H) | GU070841 | GM17150 |
| 3DL3*00905 | 3DL3*00901 | 118 ACG (T)=>ACT (T) | GU944891 | GM17156 | |
| 3DL3*01408 | 3DL3*01401 | 266 CAC (H)=>CAT (H) | GU944892 | GM17162 | |
| 3DL3*01802 | 3DL3*01801 | 2 GTA (V)=>GTG (V), 35 AAC (N)=>AAT (N), 302 CAC (H)=>CAT (H) | GU944886 | GM17117 | |
| 3DL3*041a | 3DL3*00901 | 327 GCC (A) => CCC (P), 352 GAA (E)=>GAT (D) | GU070842 | GM17181 | |
| 3DL3*049 | 3DL3*00801 | 327 GCC (A) =>CCC (P), 352 GAA (E)=>GAT (D) | GU944881 | GM17105 | |
| 3DL3*050 | 3DL3*01403 | 292 GTT (V)=>ATT (I) | GU944882 | GM17107 | |
| 3DL3*051 | 3DL3*00202 | 35 AAT (N)=>AAA (K) | GU944885 | GM17117 | |
| 3DL3*052 | 3DL3*00901 | 86 TCG (S)=>CCG (P) | GU944887 | GM17120 | |
| 3DL3*053 | 3DL3*00201 | 292 GTT (V)=>ATT (I) | GU944888 | GM17126 | |
| 3DL3*054 | 3DL3*00901 | 113 GTG (V) =>ATG (M) | GU944890 | GM17141 | |
| 3DL3*055 | 3DL3*005 | 56 CGG (R)=>CAG (Q) | GU944893 | GM17171 |
Novel alleles are defined as the first description of the allele in the IPD-KIR database. Also included are alleles whose sequences are not reported in the IPD-KIR database release 2.3.0; our submission of the sequences of these alleles was designated as confirmatory by the WHO Committee for Factors of the HLA System.
Confirmatory allele.
The relationships of the novel alleles to all other alleles at the locus are shown in Supplemental Figure 2. Since a hotspot of recombination lies just 5’ of exon 7 (Jones et al. 2006) separating the more polymorphic exons 1–5 from the more conserved 3’ exons, two neighbor-joining trees were created comparing the nucleotide sequences encoding the extracellular domains separately from the exons encoding the transmembrane and cytoplasmic regions. The relationship among alleles differs depending on whether the 5’ or 3’ exons are evaluated. For example, KIR3DL3*050 and KIR3DL3*053 carry identical nucleotide sequences for their extracellular domains but carry two different sequences for exons 7–9. Likewise, KIR3DL3*054 and KIR3DL3*055 carry identical 3’ sequences but differ in the sequences specifying their extracellular regions. Thus, it appears that, in addition to mutation, recombination is reshuffling exon blocks within the locus to yield new KIR3DL3 alleles. These reshuffling events have been previously noted by Pyo et al. for other alleles at this locus (Pyo et al. 2010).
Figure 1 shows the frequency distribution of nucleotide differences in the pairwise sequence comparisons for 75 KIR3DL3 alleles. The average number of pairwise differences among alleles is 6.141. The seven most divergent allele pairs differ by 13 nucleotides and include, for example, KIR3DL3*031 versus KIR3DL3*036 and KIR3DL3*00601 versus KIR3DL3*029. Calculation of the ratio of synonymous to nonsynonymous substitutions for the alleles of KIR3LD3 show values favoring synonymous substitutions (i.e., dS/dN values greater than one) (Table 3).
Figure 1.
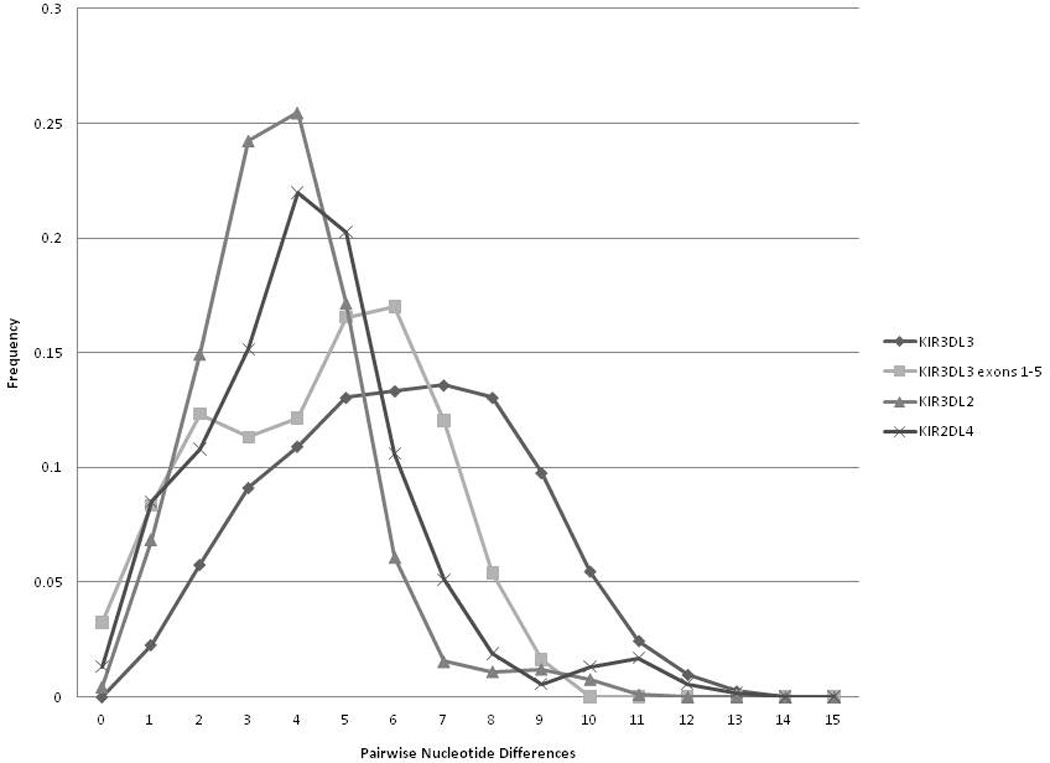
The frequency distribution of nucleotide differences in a pairwise comparison of alleles at the three framework KIR loci, KIR3DL3, KIR3DL3 exons 1–5 only, KIR2DL4, KIR3DL2. Removal of alleles KIR2DL4*003 and KIR3DL2*004 will remove the most mismatched categories from the curves for these alleles (eg removal of KIR3DL2*004 will remove the 8–11 mismatched pairs).
Table 3.
dS/dN ratiosa for the KIR framework genes
| KIR3DL3 | KIR2DL4 | KIR3DL2 | |
|---|---|---|---|
| dS/dN average of pairwise comparisons | 4.0976 | 2.8778 | 1.6483 |
The ratios are based on a pairwise comparison of all alleles at each locus. The analysis used 75 KIR3DL3, 33 KIR2DL4, and 43 KIR3DL2 alleles.
Two statistical tests were used to estimate the selection acting on KIR3DL3. In the Ewens-Watterson test of homozygosity, the observed homozygosity (F=0.0497 where F is the sum of the squares of the allele frequencies) was compared with the mean value of F expected for a population of the same size with the same number of alleles, undergoing neutral evolution (F=0.0491)(Table 4). Our data suggest that the KIR3DL3 locus is undergoing neutral evolution (p value 0.6184). [The normalized deviate of F (Fnd) was 0.0527. Fnd values significantly lower than 0 are consistent with the action of balancing selection and significantly greater than 0, with directional selection.] Secondly, Tajima’s D was derived based on the nucleotide sequences of the 200 KIR3DL3 alleles observed in the cohort (Table 5). This test is used to determine if a DNA sequence is evolving under a neutral model of evolution in a population of constant size versus being subjected to natural selection. The test provides two estimates of the population-scaled mutation rate, one based on the average nucleotide sequence variability between alleles and the second based on the total number of polymorphic sites. The neutral model of evolution predicts that these two estimates will be equal. The value calculated for KIR3DL3, −0.21130, is not statistically different from that predicted by the neutral model so the null hypothesis of neutrality can not be rejected. It should be noted, however, that the value of Tajima’s D is impacted by natural selection, admixture, and changes in population size. Since the African American population exhibits admixture and has increased in size over time (Tishkoff et al. 2009), application of this statistic to this cohort has its limitations.
Table 4.
Ewens-Waterson homozygosity test
| KIR3DL3 | KIR2DL4 | KIR3DL2 | |
|---|---|---|---|
| Fobs | 0.0497 | 0.2500 | 0.0788 |
| Fnd | 0.0527 | 1.4685 | −0.0397 |
| p-value | 0.6184a | 0.9187a | 0.5881a |
Not significant
Table 5.
Results of Tajima’s D statistical testa
| KIR3DL3 | KIR2DL4 | KIR3DL2 | |
|---|---|---|---|
| No. coding nucleotides evaluatedb/total | 1181/1233 | 1064/1134 | 1280/1368 |
| No. polymorphic sites | 31 | 16 | 23 |
| Tajima’s D | −0.21130 | −0.40250 | −1.31684 |
| p-value | NSc | NS | NS |
Based on the 200 alleles observed in the frequency study for KIR3DL3 and the 195 KIR3DL2 alleles (excluding the 5 KIR3DL1/KIR3DL2 fusion alleles). For KIR2DL4, the data included the 166 alleles described in Table 1.
Includes all of the coding sequence with the exception of a portion of the 5’ sequence where data are not available for all alleles.
Not significant
KIR2DL4
DNA sequencing of KIR2DL4 alleles identified 18 alleles, ten previously reported and eight novel. If we assumed that each individual carried two alleles at the KIR2DL4 locus, deviation from Hardy-Weinberg proportions was noted for KIR2DL4 (p-value 0.0309). Since the chi-square test detected a higher number of 2DL4*00103 homozygotes than expected (27 observed versus 21 expected with a significant p-value 0.0153), these data suggested that some individuals in the study may carry only single copies of the KIR2DL4 locus.
Indeed, one African American individual without KIR2DL4 (GM17183) was identified in this study. When typed for other KIR loci, this individual was found to be heterozygous at KIR2DL2/2DL3, KIR2DL5B, KIR2DS5, KIR2DS1, and KIR3DL2 loci. A number of KIR loci expected to be present based on common haplotype structures (Pyo et al. 2010) were absent (i.e., KIR2DL1, KIR3DL1/3DS1, KIR2DL5A, KIR2DS3, and KIR2DS4) suggesting a deletion likely removed several genes from the center of the complex. Both chromosomes in this individual appeared to carry similar deletions. By isolating haplotype-specific DNA fragments, we were able to show that KIR2DL5B (usually found in the centromeric segment) and KIR2DS1 (usually found in the telomeric segment) are found on the same DNA fragment as KIR2DS5 supporting our hypothesis regarding the deletion. Thirty two other individuals in our study potentially carried KIR2DL4 deletions as they exhibited single alleles at KIR2DL4. For example, eight individuals carried single alleles of KIR2DL4*00103 with single alleles at adjacent loci (KIR2DL1 and KIR3DL1/KIR3DS1) suggesting that these individuals may also carry deletions within the KIR complex and lack one copy of KIR2DL4.
Table 1 lists the frequencies of individuals carrying each KIR2DL4 allele. Five alleles were found in more than 10 individuals: KIR2DL4*00103 (64 of 100 individuals), *00501 (21/100), *0080101/03 (23/100), *00802 (17/100) and *011 (11/100). Approximately one third of individuals (32/100) exhibited only single KIR2DL4 alleles with one individual completely lacking KIR2DL4. The majority of these carried KIR2DL4*00103 as expected by its high frequency. The major difference in the frequencies from other populations is the identity of the predominant allele--KIR2DL4*00102 is the predominant allele in European Americans (37% of individuals carry this allele)(Shulse et al. 2007) and Asian populations (45–52% allele frequency)(Yawata et al. 2006;Zhu et al. 2006); it was found in 8 of 100 African Americans. The predominant allele in African Americans, KIR2DL4*00103 (64 of 100 individuals) was found in 21% of European Americans and at allele frequencies of 5–6% in Asian populations.
Eight novel KIR2DL4 alleles encoding seven unique receptors carried by single individuals are described in Table 2 and their relationships to other alleles at the locus are described in Supplemental Figure 3. The seven new receptors differ by from one to two amino acid substitutions from receptors encoded by closely related alleles. One allele, KIR2DL4*019, differs from its closely related allele, KIR2DL4*018, for the 9A / 10A variation noted to cause differences in expression (Goodridge et al. 2007). The substitutions in total alter the two immunoglobulin-like extracellular domains or the cytoplasmic tail. Four of the novel alleles, KIR2DL4*013, *017, *019, and *020 carry the 9A sequence motif (Goodridge et al. 2007) and encode a potentially secreted product. Four of the substitutions alter residues that have not been previously polymorphic in the known KIR alleles. The eighth novel allele is a synonymous variant of known allele KIR2DL4*01201.
Figure 1 shows the frequency distribution of nucleotide differences in the pairwise sequence comparisons for 33 KIR2DL4 alleles. The average number of pairwise differences among alleles is 4.258. The most divergent allele pair, KIR2DL4*003 versus KIR2DL4*0120201, differs by 14 nucleotides. KIR2DL4*003 is a part of every pairwise combination in the most mismatched categories (i.e., pairs differing for 11–14 nucleotides); other allele combinations begin to appear in the 10 nucleotide mismatch category. This suggests that either KIR2DL4*003 diverged very early on or that this allele sequence, observed only once, is an artifact. A neighbor-joining tree (Supplemental Figure 3) also shows KIR2DL4*003 as an outlier. A comparison of the ratio of synonymous to nonsynonymous substitutions (Table 3) shows a predominance of synonymous nucleotide substitutions.
Regarding selection, in the Ewens-Watterson test of homozygosity, Fnd for KIR2DL4 was 1.4685 and not significant from the value predicted by the null hypothesis of neutral evolution (p-value 0.9187) (Table 4). Tajima’s D was calculated based on the nucleotide sequences of the 166 KIR2DL4 alleles observed in the cohort (Table 5). The value, −0.4025, was not statistically different from that predicted by a neutral model of evolution.
KIR3DL2
Thirty two alleles of KIR3DL2 alleles were identified in the African American population; 16 previously reported and 16 novel. Also observed were five individuals carrying KIR3DL1/3DL2 fusion alleles which have been previously described in this study population (Jiang et al. 2010). No overall deviation from Hardy-Weinberg proportions were noted for KIR3DL2 (p-value 0.1301).
Table 1 lists the KIR3DL2 allele frequencies. Seven alleles were found at frequencies ≥5%: KIR3DL2*00101 (10%), *00103 (17%), *002 (7%), *006 (5.5%), *00701 (5.5%), *010 (7%), and *01301 (13%). Six of 100 individuals were apparent homozygotes and all carried alleles common in this population; three carried KIR3DL2*00103, one KIR3DL2*0101, one KIR3DL2*01301. KIR3DL2*029 was also observed in an apparent homozygote. European Americans shared some of the common (≥5%) alleles with African Americans: KIR3DL2*001 (23%), *002 (23%), *007 (20.1%) (Gedil et al. 2007). European Americans also exhibited common alleles KIR3DL2*005 (5.2%) and *00901 (9.7%); these alleles were present but less frequent in African Americans and could represent admixture. Common alleles in African Americans, KIR3DL2*006 and *013, were not found in European Americans. These alleles had been originally described in Black / African populations (Gardiner et al. 2001) (Artavanis-Tsakonas et al. 2003).
The 16 novel KIR3DL2 alleles encoding 13 unique receptors are described in Table 2 and their relationships to other alleles at the locus are shown in Supplemental Figure 4. Five of the novel alleles were identified in multiple individuals (Table 1). The 13 receptors differ by from one to two amino acid substitutions from receptors encoded by closely related alleles. Together, the substitutions alter all three immunoglobulin-like extracellular domains and the cytoplasmic tail. Eight of the nonsynonymous substitutions alter residues not previously polymorphic in the known KIR alleles. Three novel alleles are synonymous variants of known alleles.
Figure 1 shows the frequency distribution of nucleotide differences in the pairwise sequence comparisons for 43 KIR3DL2 alleles. The average number of pairwise differences among alleles is 3.734. The most divergent allele pairs differ by 11 nucleotides and include KIR3DL2*004 versus five alleles (KIR3DL2*008, *014, *041, *058, *061). In fact, the allele KIR3DL2*004 is a member of all the allele pairs making up the most mismatched categories (i.e., allele differing by 8–11 nucleotide differences) suggesting that either this allele diverged very early on or that this allele sequence reported only once in the IPD-KIR database is an artifact. A neighbor-joining tree (Supplemental Figure 4) also shows KIR3DL2*004 as an outlier. Table 3 shows the dS/dN ratio for KIR3DL2 is greater than one, favoring synonymous substitutions.
Two statistical tests support a neutral model of evolution for KIR3DL2. In the Ewens-Watterson test of homozygosity, the observed homozygosity (F=0.0788) was compared with the expected value (F=0.0796) (Table 4). The normalized deviate of F (Fnd) was −0.0397. These data suggest that this locus, like the other functional framework genes, is undergoing neutral evolution (p=0.5881). A value of −1.31684 for Tajima’s D did not reject the null hypothesis of neutrality (Table 5).
Discussion
The KIR framework genes, KIR3DL3, KIR3DP1, KIR2DL4 and KIR3DL2, mark the boundaries of the two gene clusters comprising the KIR gene complex. Homologous recombination in the 14 kb region separating the two KIR gene clusters retains the outer framework genes but reshuffles the variable gene-content centromeric and telomeric clusters. Unequal crossing over creates new haplotypes by expansion and contraction of the two clusters of highly homologous KIR genes (Martin et al. 2003;Ordonez et al. 2008) (Traherne et al. 2010) but it is bounded by the centromeric (KIR3DL3) and telomeric (KIR3DL2) genes. This positioning maintains the presence of KIR3DL3 and KIR3DL2 (or 3’ exons of KIR3DL2 in the case of the KIR3DL1/KIR3DL2 chimeric gene (Norman et al. 2009)) in new haplotypes. During some unequal crossing over events, the centrally located framework genes, KIR3DP1 and KIR2DL4, may be duplicated or deleted as observed in this and other studies (Traherne et al. 2010) (Norman et al. 2002;Ordonez et al. 2008) (Martin et al. 2003).
The centromeric and telomeric framework genes are highly diverse in this African American population of 100 individuals. Fifty KIR3DL3 alleles encode 35 distinct receptors and 32 KIR3DL2 alleles encode 26 receptors. In contrast, a single allele of KIR2DL4, KIR2DL4*00103, predominates (64 of 100 individuals) although 17 other alleles are also observed encoding 14 allelic products. In general, there appears to be more alleles at each locus with lower frequencies in African Americans compared to European American or Asian populations. This diversity has also been observed for other KIR loci in this population (Hou et al. 2009;Hou et al. 2010). This increased diversity is also observed for loci other than KIR. HLA alleles and haplotypes in African Americans exhibit increased diversity when compared to other U.S. populations (Maiers et al. 2007). Studies of other polymorphic markers across the genome have also noted the high levels of genetic diversity within the African American and African populations when compared to other world-wide populations (Tishkoff et al. 2009).
Many African Americans can trace their ancestry to African slaves imported into the Americas from the west coast of Africa. While data on KIR2DL4 and KIR3DL2 alleles in African populations is limited, many of the KIR3DL3 alleles observed in this cohort of African Americans, KIR3DL3*00208, *01406, *01502, *02502, *032, *034, and *035, were originally described in a West African population from Ghana (IPD-KIR database version 2.3.0; submitted by Dr. Parham) and thus far have not been observed in populations of European origin (Hou et al. 2007). Studies of genome-wide polymorphisms have demonstrated that the African ancestry contribution to African Americans is approximately 80% (Tishkoff et al. 2009).
Comparison of the novel alleles described here to other alleles at the locus shows distinct lineages. Each lineage is marked by a consensus allele that varies subtly from other less frequent alleles in the lineage. For example, KIR2DL4*00201, KIR2DL4*0080201, KIR2DL4*0080202, KIR2DL4*017, and KIR2DL4*020 form a cluster in the neighbor-joining tree shown in Supplementary Figure 3. Since KIR2DL4*00201 was not observed in African Americans, it seems likely that KIR2DL4*00201 diverged from the more common KIR2DL4*00802 allele by the gain of a nucleotide in the exon encoding the transmembrane region producing the 9A/10A variation studied by Goodridge et al.(Goodridge et al. 2007). The newly described alleles KIR2DL4*017 and KIR2DL4*020 differ by single, apparently random, nucleotide substitutions from the KIR2DL4*00802 coding sequence. For KIR3DL2, the cluster including the common allele, KIR3DL2*01301, includes four other alleles that each differ from KIR3DL2*01301 by a single nucleotide substitution altering codons −18 (KIR3DL2*01303), 87 (KIR3DL2*040), 125 (KIR3DL2*01302), and 145 (KIR3DL2*025). The four alleles were each observed once in 100 African Americans except for KIR3DL2*040 which was observed three times. The diversification of KIR3DL3 is more complex because of the reshuffling of the two sequence blocks. But within each block, a pattern of variation similar to the other framework genes can be observed. For example, six alleles share the nucleotide sequences of exons 1–5 (KIR3DL3*00101, KIR3DL3*00102, KIR3DL3*00301, KIR3DL3*00901, KIR3DL3*00902, KIR3DL3*0041), a consensus sequence found 51 times in 100 individuals (Supplementary Figure 2a, Table 1). Another six alleles differ for a single, apparently random, substitution from that 5’ block consensus sequence (codon 86 (KIR3DL3*052), codon 111 (KIR3DL3*032), codon 113 (KIR3DL3*054), codon 118 (KIR3DL3*00905), codon 151 (KIR3DL3*00103), and codon 281 (KIR3DL3*00104). These variations from consensus were observed from one to five times in the cohort. This pattern of variation appears to support random recent mutations as a source of variation.
Synonymous nucleotide substitutions are favored over nonsynonymous substitutions within the known alleles of the three KIR genes. While the synonymous to nonsynonymous ratios can be used to estimate evolutionary pressures on genes, because our analysis was performed only within human alleles, there are limits to inferring selection from the ratios presented in this study (Kryazhimskiy and Plotkin 2008). An earlier study of KIR3DL3 diversity did evaluate dN/dS ratios in humans compared to higher primates and observed predominantly synonymous substitutions in the extracellular domains as we did. The low values for dN/dS found in that study suggested purifying selection (Jones et al. 2006).
Plots of allele mismatch distributions from the three loci show a similar pattern resembling a bell shaped curve spanning from zero to 11–13 polymorphic sites among pairwise allele combinations. The average number of differences is approximately 4–6 nucleotides with KIR3DL3 pairwise comparisons being slightly more diverse. The shape of the curves suggests that the alleles at each locus represent a continuum of diversity with relatively recent divergence. In general, the pattern of variation within the framework genes supports the hypothesis based on analysis of alu repeats and similarity in nucleotide sequences among KIR loci that these genes had a recent origin (Wilson et al. 2000).
In the mismatch distributions, KIR3DL3 exhibits a broader frequency distribution than the other two framework genes. This continuum is created, in part, by a hotspot for recombination located 5’ to exon 7 that reshuffles the sequences of 5’ (exons 1–5) and 3’ (exons 7–9) exons (Jones et al. 2006;Pyo et al. 2010). Figure 1 shows the difference in the pairwise comparison if only the sequences of KIR3DL3 exons 1–5 are compared. The peak sharpens and becomes bimodal suggesting the presence of both more recently diverged lineages and more distantly related lineages. The presence of more diverged lineages is consistent with previous studies suggesting that KIR3DL3 may be an ancient member of the KIR family (Jones et al. 2006;Rajalingam et al. 2004).
The impact of the KIR diversity on the function of these framework KIR receptors remains to be determined. For KIR3DL3, KIR2DL4, and KIR3DL2, Tajima’s D and the Ewens-Watterson test suggest that these loci are undergoing neutral evolution in which the alleles are indistinguishable with respect to one another (i.e., there is no difference in fitness creating a selective advantage or disadvantage among the alleles). In this model, variation is occurring through mutation and genetic drift is the predominant force altering allele frequencies. It should be noted, however, that there is considerable discussion in the population genetics field regarding the ability of the statistical methods used in this study and in other studies of genomic data to detect selection (for example, (Garrigan and Hedrick 2003;Nei et al. 2010). Thus, we may have to await more powerful statistical methods to identify alleles affected by selection and to predict potentially critical residues within the KIR molecules.
For the KIR loci, because we do not yet understand the ligands detected (KIR3DL3) or the biological impact of allelic variation on ligand binding (KIR2DL4, KIR3DL2) and subsequent NK activity or the role of secreted versus membrane bound receptors for KIR2DL4, it is difficult to predict how selection might be acting on these loci. Since NK cell activity is determined by the balance of signals received from a variety of inhibitory and stimulatory receptors (Bryceson and Long 2008), it may be that the impact of selection on the KIR loci will be subtle and in the context of selection acting on other immune response genes. Further studies correlating structure with function are needed to clarify the impact of allelic variation in the KIR framework gene.
Supplementary Material
Acknowledgments
This research is supported by funding from the Office of Naval Research N00014-08-1-1078. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. government. The authors would like to thank Drs. Steven Mack and Matthew Hamilton for helpful discussions.
References
- Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, Riley EM. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected. erythrocytes. J Immunol. 2003;171:5396–5405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr. Opin. Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. The sampling theory of selectively neutral alleles. Theor Pop Biol. 1972;3:87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Faure M, Long EO. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory. potential. J. Immunol. 2002;168:6208–6214. doi: 10.4049/jimmunol.168.12.6208. [DOI] [PubMed] [Google Scholar]
- Gardiner CM, Guethlein LA, Shilling HG, Pando M, Carr WH, Rajalingam R, Vilches C, Parham P. Different NK cell surface phenotypes defined by the DX9 antibody are due to KIR3DL1. gene polymorphism. J Immunol. 2001;166:2992–3001. doi: 10.4049/jimmunol.166.5.2992. [DOI] [PubMed] [Google Scholar]
- Garrigan D, Hedrick PW. Perspective: detecting adaptive molecular polymorphism:. lessons from the MHC. Evolution. 2003;57:1707–1722. doi: 10.1111/j.0014-3820.2003.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Gedil MA, Steiner NK, Hurley CK. Genomic characterization of KIR2DL4 in families and. unrelated individuals reveals extensive diversity in exon and intron sequences including a common frameshift variation occurring in several alleles. Tissue Antigens. 2005;65:402–418. doi: 10.1111/j.1399-0039.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- Gedil MA, Steiner NK, Hurley CK. KIR3DL2: diversity in a hematopoietic stem cell. transplant population. Tissue Antigens. 2007;70:228–232. doi: 10.1111/j.1399-0039.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Lathbury LJ, Steiner NK, Shulse CN, Pullikotil P, Seidah NG, Hurley CK, Christiansen FT, Witt CS. Three common alleles of KIR2DL4 (CD158d) encode. constitutively expressed, inducible and secreted receptors in NK cells. Eur J Immunol. 2007;37:199–211. doi: 10.1002/eji.200636316. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for. multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud. VM. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- Hou L, Chen M, Jiang B, Kariyawasam K, Ng J, Hurley CK. In contrast to other stimulatory. natural killer cell immunoglobulin-like receptor loci, several KIR2DS5 alleles predominate in. African Americans. Hum Immunol. 2009;70:733–737. doi: 10.1016/j.humimm.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Chen M, Jiang B, Ng J, Hurley CK. African Americans exhibit a predominant allele in. the midst of extensive KIR2DL1 allelic diversity. Tissue Antigens. 2010;76:31–34. doi: 10.1111/j.1399-0039.2010.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Chen M, Steiner NK, Belle I, Turino C, Ng J, Hurley CK. Seventeen novel alleles add. to the already extensive KIR3DL3 diversity. Tissue Antigens. 2007;70:449–454. doi: 10.1111/j.1399-0039.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Jiang B, Hou L, Chen M, Ng J, Hurley CK. The profile of KIR3DL1 and KIR3DS1 alleles in an. African American population resembles that found in African populations. Tissue Antigens. 2010;76:64–66. doi: 10.1111/j.1399-0039.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Hiby SE, Moffett A, Trowsdale J, Young NT. Nature of allelic sequence. polymorphism at the KIR3DL3 locus. Immunogenetics. 2006;58:614–627. doi: 10.1007/s00251-006-0130-5. [DOI] [PubMed] [Google Scholar]
- Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. . Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- Kelley J, Walter L, Trowsdale J. Comparative genomics of natural killer cell receptor gene. clusters. PLoS Genet. 2005;1:129–139. doi: 10.1371/journal.pgen.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Maki A, Yusa S, Catina TL, Campbell KS. KIR2DL4 is an IL-2-regulated NK cell. receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. . J Immunol. 2003;171:3415–3425. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- Kollnberger S, Chan A, Sun MY, Chen LY, Wright C, di Gleria K, McMichael A, Bowness P. Interaction of HLA-B27 homodimers with KIR3DL1 and KIR3DL2, unlike HLA-B27 heterotrimers,. is independent of the sequence of bound peptide. Eur J Immunol. 2007;37:1313–1322. doi: 10.1002/eji.200635997. [DOI] [PubMed] [Google Scholar]
- Korber B. In: HIV signature and sequence variation analysis. Rodrigo AG, Learn GH, editors. Dordrecht, Netherlands: Kluwer Academic Publishers; 2000. pp. 55–72. [Google Scholar]
- Kryazhimskiy S, Plotkin JB. The population genetics of dN/dS. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000304. e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster AK, Nelson MP, Single RM, Meyer D, Thomson G. In: PyPop: a software. framework for population genomics: analyzing large-scale multi-locus genotype data. Altman RB, Dunker K, Hunter L, Jung T, Klein T, editors. Singapore: World Scientific; 2003. pp. 514–525. [PMC free article] [PubMed] [Google Scholar]
- Lancaster AK, Single RM, Solberg OD, Nelson MP, Thomson G. PyPop update - a software. pipeline for large-scale multilocus population genomics. Tissue Antigens. 2007;69:192–197. doi: 10.1111/j.1399-0039.2006.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu. Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA. polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United. States population. Hum Immunol. 2007;68:779–788. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Marsh SGE, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D, Vilches C, Carrington M, Witt C, Guethlein LA, Shilling H, Garcia CA, Hsu KC, Wain H. Killer-cell immunoglobulin-. like receptor (KIR) nomenclature report,2002. Tissue Antigens. 2003;62:79–86. doi: 10.1034/j.1399-0039.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Martin AM, Freitas EM, Witt CS, Christiansen FT. The genomic organization and evolution. of the natural killer immunoglobulin-like receptor (KIR) gene cluster. Immunogenetics. 2000;51:268–280. doi: 10.1007/s002510050620. [DOI] [PubMed] [Google Scholar]
- Martin MP, Bashirova A, Traherne J, Trowsdale J, Carrington M. Cutting edge: expansion. of the KIR locus by unequal crossing over. J Immunol. 2003;171:2192–2195. doi: 10.4049/jimmunol.171.5.2192. [DOI] [PubMed] [Google Scholar]
- Meyer D, Single R, Mack SJ, Lancaster A, Nelson MP, Erlich HA, Fernandez-Vina M, Thomson G. 13th IHWS anthropology/human genetic diversity joint report. Chapter 4. In: Hansen JA, editor. Single locus polymorphism of classical HLA genes. Seattle: International Histocompatibility Working Group Press; 2006. pp. 653–704. [Google Scholar]
- Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and. nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Nei M, Suzuki Y, Nozawa M. The neutral theory of molecular evolution in the genomic. era. Annu Rev Genomics Hum Genet. 2010;11:265–289. doi: 10.1146/annurev-genom-082908-150129. [DOI] [PubMed] [Google Scholar]
- Norman PJ, Abi-Rached L, Gendzekhadze K, Hammond JA, Moesta AK, Sharma D, Graef T, McQueen KL, Guethlein LA, Carrington CV, Chandanayingyong D, Chang YH, Crespi C, Saruhan-. Direskeneli G, Hameed K, Kamkamidze G, Koram KA, Layrisse Z, Matamoros N, Mila J, Park MH, Pitchappan RM, Ramdath DD, Shiau MY, Stephens HA, Struik S, Tyan D, Verity DH, Vaughan RW, Davis RW, Fraser PA, Riley EM, Ronaghi M, Parham P. Meiotic recombination generates. rich diversity in NK cell receptor genes, alleles, and haplotypes. Genome Res. 2009;19:757–769. doi: 10.1101/gr.085738.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman PJ, Carrington CVF, Byng M, Maxwell LD, Curran MD, Stephens HAF, Chandanayingyong D, Verity DH, Hameed K, Ramdath DD, Vaughan RW. Natural killer cell immunoglobulin-. like receptor (KIR) locus profiles in African and South Asian populations. Genes and Immunity. 2002;3:86–95. doi: 10.1038/sj.gene.6363836. [DOI] [PubMed] [Google Scholar]
- Ordonez D, Meenagh A, Gomez-Lozano N, Castano J, Middleton D, Vilches C. Duplication,. mutation and recombination of the human orphan gene KIR2DS3 contribute to the diversity of. KIR haplotypes. Genes Immun. 2008;9:431–437. doi: 10.1038/gene.2008.34. [DOI] [PubMed] [Google Scholar]
- Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, Bertone S, Moretta A, Moretta L, Mingari MC. Inhibitory receptors sensing HLA-G1 molecules in pregnancy:. decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-. G1-specific receptor. Proc. Natl Acad. Sci U.S.A. 1999;96:5674–5679. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo CW, Guethlein LA, Vu Q, Wang R, Abi-Rached L, Norman PJ, Marsh SG, Miller JS, Parham P, Geraghty DE. Different patterns of evolution in the centromeric and telomeric regions of. group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS ONE. 2010;5:e15115. doi: 10.1371/journal.pone.0015115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der MA, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS. Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-gamma production but not. cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001;167:1877–1881. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- Rajalingam R, Parham P, Abi-Rached L. Domain shuffling has been the main mechanism. forming new hominoid killer cell Ig-like receptors. J Immunol. 2004;172:356–369. doi: 10.4049/jimmunol.172.1.356. [DOI] [PubMed] [Google Scholar]
- Rogers AR, Harpending H. Population growth makes waves in the distribution of pairwise. genetic differences. Mol Biol Evol. 1992;9:552–569. doi: 10.1093/oxfordjournals.molbev.a040727. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing. phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salamon H, Klitz W, Easteal S, Gao X, Erlich HA, Fernandez-Vina M, Trachtenberg EA, McWeeney SK, Nelson MP, Thomson G. Evolution of HLA class II molecules: Allelic and amino acid. site variability across populations. Genetics. 1999;152:393–400. doi: 10.1093/genetics/152.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulse C, Steiner NK, Hurley CK. Allelic diversity in KIR2DL4 in a bone marrow transplant. population: description of three novel alleles. Tissue Antigens. 2007;70:157–159. doi: 10.1111/j.1399-0039.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- Sivori S, Falco M, Carlomagno S, Romeo E, Soldani C, Bensussan A, Viola A, Moretta L, Moretta. A. A novel KIR-associated function: evidence that CpG DNA uptake and shuttling to early. endosomes is mediated by KIR3DL2. Blood. 2010;116:1637–1647. doi: 10.1182/blood-2009-12-256586. [DOI] [PubMed] [Google Scholar]
- Slatkin M. An exact test for neutrality based on the Ewens sampling distribution. Genetical Research. 1994;64:71–74. doi: 10.1017/s0016672300032560. [DOI] [PubMed] [Google Scholar]
- Slatkin M. A correction to the exact test based on the Ewens sampling distribution. . Genetical Research. 1996;68:259–260. doi: 10.1017/s0016672300034236. [DOI] [PubMed] [Google Scholar]
- Slatkin M, Hudson RR. Pairwise comparisons of mitochondrial DNA sequences in stable. and exponentially growing populations. Genetics. 1991;129:555–562. doi: 10.1093/genetics/129.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA. polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis. (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, Ibrahim M, Juma AT, Kotze MJ, Lema G, Moore JH, Mortensen H, Nyambo TB, Omar SA, Powell K, Pretorius GS, Smith MW, Thera MA, Wambebe C, Weber JL, Williams SM. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traherne JA, Martin M, Ward R, Ohashi M, Pellett F, Gladman D, Middleton D, Carrington M, Trowsdale J. Mechanisms of copy number variation and hybrid gene formation in the KIR. immune gene complex. Hum Mol Genet. 2010;19:737–751. doi: 10.1093/hmg/ddp538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trundley AE, Hiby SE, Chang C, Sharkey AM, Santourlidis S, Uhrberg M, Trowsdale J, Moffett A. Molecular characterization of KIR3DL3. Immunogenetics. 2006;57:904–916. doi: 10.1007/s00251-005-0060-7. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Torkar M, Haude A, Milne S, Jones T, Sheer D, Beck S, Trowsdale J. Plasticity in. the organization and sequences of human KIR/ILT gene families. Proc. Natl Acad. Sci U. S. A. 2000;97:4778–4783. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR. polymorphisms in natural killer cell repertoire selection and modulation of effector function. J. Exp. Med. 2006;203:633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FM, Jiang K, Lv QF, He J, Yan LX. Investigation of killer cell immunoglobulin-like. receptor KIR2DL4 diversity by sequence-based typing in Chinese population. Tissue Antigens. 2006;67:214–221. doi: 10.1111/j.1399-0039.2006.00562.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


