Abstract
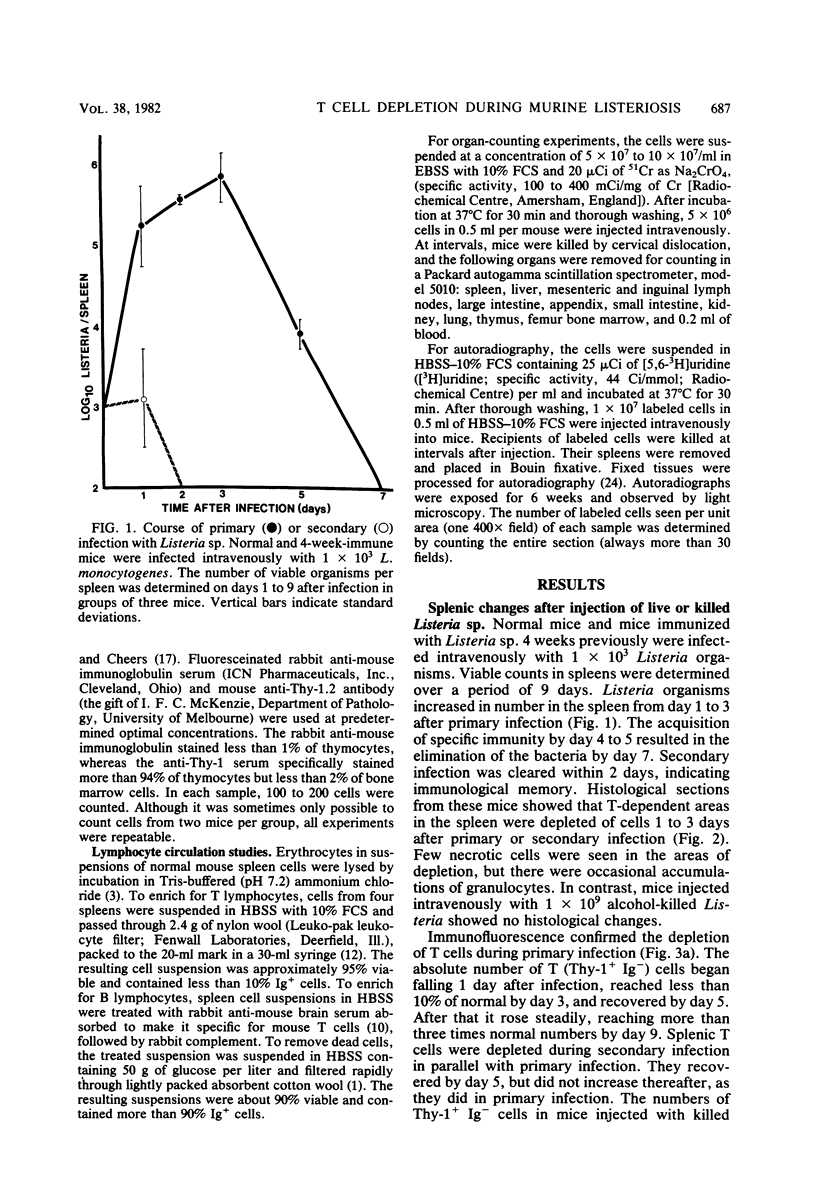
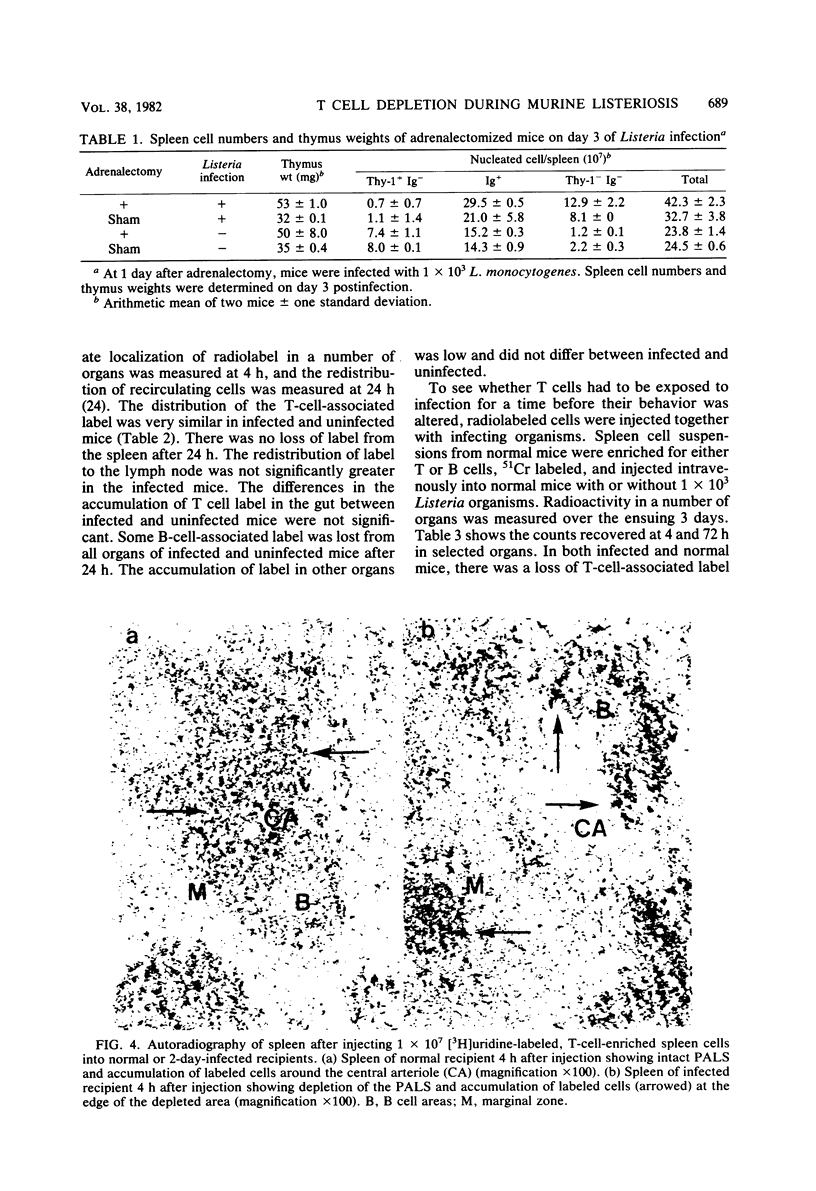
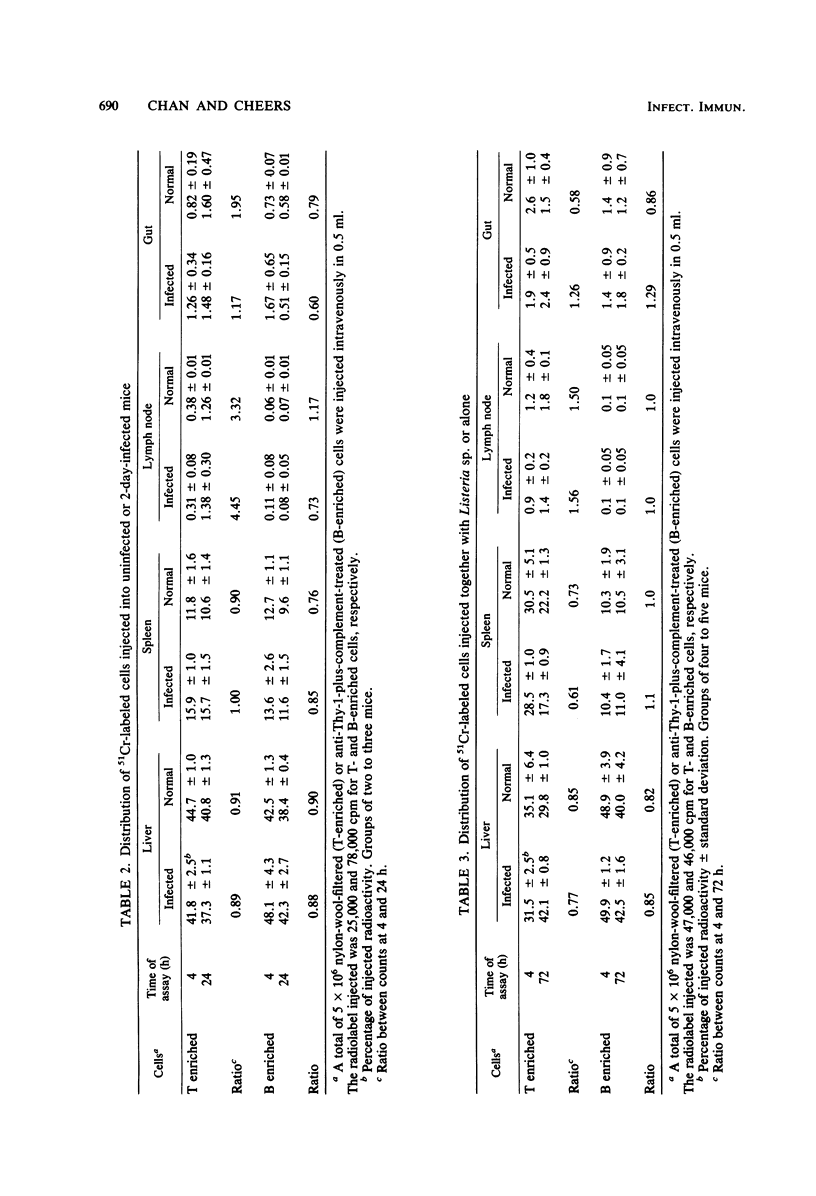
Marked changes in the splenic lymphocyte populations during murine infection with Listeria monocytogenes were observed histologically and quantitated by the immunofluorescence of Thy-1+ immunoglobulin (Ig-) (T) and Ig+ (B) cells. Cells were depleted from the T-dependent areas of the spleen, and the number of T cells in suspensions prepared from spleens of mice 1 to 3 days after primary or secondary infection were less than 1/10 of normal. High numbers of alcohol-killed Listeria sp. did not cause any depletion. Depletion was not prevented by adrenalectomy. Although injected radiolabeled T cells distributed normally between spleen, liver, lymph node, and gut in infected mice, there appeared to be a barrier to their entry into depleted T-dependent areas of the spleen. Evidence for the destruction of T cells, but not of B cells, in the infected mouse spleen was obtained.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton P. M., Kirov S. M., Donald K. J. The effects of major and minor trauma on lymphocyte kinetics in mice. Aust J Exp Biol Med Sci. 1979 Oct;57(5):479–492. doi: 10.1038/icb.1979.49. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- Chan Y. Y., Cheers C. Recovery from T cell depletion during murine listeriosis and effect on a T-dependent antibody response. Infect Immun. 1982 Nov;38(2):694–698. doi: 10.1128/iai.38.2.694-698.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F., Pavlov H., Waid C., York J. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect Immun. 1978 Mar;19(3):763–770. doi: 10.1128/iai.19.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Farr A. G., Wechter W. J., Kiely J. M., Unanue E. R. Induction of cytocidal macrophages after in vitro interactions between Listeria-immune T cells and macrophages--role of H-2. J Immunol. 1979 Jun;122(6):2405–2412. [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyöngyössy M. I., Playfair J. H. Indirect immunofluorescence of mouse thymus-derived cells using heterologous anti-brain serum. Cell Immunol. 1973 Apr;7(1):118–123. doi: 10.1016/0008-8749(73)90187-1. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns R. J., Hinrichs D. J. Heat-labile B-cell mitogen obtained from Listeria monocytogenes. Infect Immun. 1978 Dec;22(3):676–680. doi: 10.1128/iai.22.3.676-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. I. Effect of single and double-stranded polynucleotides on Brucella abortus in vivo and in vitro. Immunology. 1978 Jul;35(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Circulating T and B lymphocytes of the mouse. I. Migratory properties. Cell Immunol. 1973 Apr;7(1):10–39. doi: 10.1016/0008-8749(73)90180-9. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. II. Recirculating lymphocytes derived from antigen-activated thymus cells. Cell Immunol. 1972 Mar;3(3):385–404. doi: 10.1016/0008-8749(72)90245-6. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Immunological significance of changes in lymph nodes across the leprosy spectrum. Clin Exp Immunol. 1971 Mar;8(3):363–376. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. J., Woodruff J. F. Virus-induced alterations of lymphoid tissue. IV. The effect of Newcastle disease virus on the fate of transfused thoracic duct lymphocytes. Cell Immunol. 1974 Jan;10(1):78–85. doi: 10.1016/0008-8749(74)90153-1. [DOI] [PubMed] [Google Scholar]
- Woodruff J. J., Woodruff J. F. Virus-induced alterations of lymphoid tissues. 3. Fate of radiolabeled thoracic duct lymphocytes in rats inoculated with Newcastle disease virus. Cell Immunol. 1972 Oct;5(2):307–317. doi: 10.1016/0008-8749(72)90056-1. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]