Abstract
Previous studies suggest that binge eating sugar leads to behavioral and neurochemical changes similar to those seen with drug addiction, including signs of opiate-like withdrawal. Studies are emerging that show multiple neurochemical and behavioral indices of addiction when animals overeat a fat-rich diet. The goal of the present study was to utilize liquid and solid diets high in sugar and fat content to determine whether opiate-like withdrawal is seen after binge consumption of these diets in Sprague Dawley rats. Control groups were given ad libitum access to the sweet-fat food or standard chow. All rats were then given a battery of tests to measure signs of opiate-like withdrawal, which included somatic signs of distress, elevated plus-maze anxiety, and locomotor hypoactivity. Neither naloxone-precipitated (3 mg/kg) nor deprivation-induced withdrawal was observed in rats that were maintained on a nutritionally complete pelleted sweet-fat diet, a sweet, high-fat diet supplemented with standard rodent chow, or a liquid sweet-fat food. Further, body weight reduction to 85%, which is known to potentiate the reinforcing effects of substance of abuse, did not affect naloxone-precipitated signs of opiate-like withdrawal. Thus, unlike previous findings reported regarding rats with binge access to a sucrose solution, rats that binge eat sweet-fat combinations do not show signs of opiate-like withdrawal under the conditions tested. These data support the idea that excessive consumption of different nutrients can induce behaviors associated with addiction in different ways, and that the behaviors that could characterize “food addiction” may be subtyped based on the nutritional composition of the food consumed.
Keywords: binge eating, food addiction, high-fat diet, withdrawal
Introduction
Neural systems that motivate and reinforce food seeking and intake also underlie behaviors associated with drug abuse [1–5]. Based on this neurological overlap, it has been suggested that the consumption of certain foods might also result in addiction-like behaviors [6–8]. Previous studies from our laboratory and others suggest that limited access to sugar leads to behavioral changes and alterations in dopamine (DA) and opioid systems that are similar, albeit smaller in magnitude, to those seen during drug addiction [8].
Of these addiction-like behaviors associated with binge consumption of sugar, evidence of opiate-like withdrawal is of particular interest. Using our laboratory animal model of binge eating sugar, we have found that when administered the opioid antagonist naloxone, rats show somatic signs of withdrawal, including teeth chattering, forepaw tremors, and head-shakes, as well as anxiety on the elevated plus-maze. Further, these behaviors are coupled with a decrease in the release of DA in the nucleus accumbens and an increase in the release of acetylcholine [9], a neurochemical imbalance that has been seen during withdrawal from several drugs of abuse [10, 11]. Behavioral and neurochemical signs of opiate-like withdrawal have also been observed without the use of naloxone (i.e., spontaneously) following a fast in rats with a history of binge eating sugar [12]. Others have noted that rats with a history of limited access to sugar have a decrease in body temperature when the sugar has been removed for 24 h [13] and can show signs of aggressive behavior [14], both which are also accepted indications of withdrawal. Further, a high-sugar diet has been shown to elicit signs of anxiety and hyperphagia that appears to be mediated by brain corticotrophin-releasing hormone systems [15].
Other studies have assessed aspects of addiction that might arise in response to other palatable foods, such as those rich in fat or sweet-fat combinations. Naloxone has been reported to produce opiate-like withdrawal signs in rats fed a cafeteria-style diet, which contained a variety of fat- and sugar-rich foods [16]. More recently, it has been shown that rodents exposed to diets rich in fat will engage in many different behaviors suggestive of addiction [11, 17–19], but the occurrence of opiate-like withdrawal has not been systematically studied within the context of overeating of fat, and not within the context of limited-access schedules.
Given that both fat and sugar affect opioid systems [3], that these macronutrients are sometimes consumed in excess and may have a role in obesity associated with overeating [20, 21] and possibly food addiction [22, 23], the goal of the present study was to determine if opiate-like withdrawal is seen in rats maintained on schedules of limited access to diets rich in sugar and fat that resulted in binge eating. In many ways, this design is akin to the human eating condition, as the binge episodes in some individuals often include combinations of these macronutrients [20, 21, 24, 25]. Further, the present study examines the effect that binge eating of fat-sugar combinations can have on the expression of withdrawal when rats are at both normal and reduced body weights, since it is known that a low body weight can potentiate the effects of drugs of abuse [26]. Further, rats with a low body weight release more DA than normal weight controls when binge eating sugar [27], which may suggest an enhanced rewarding effect at a low body weight that might affect the severity of withdrawal.
Materials and Methods
General Methods
Male Sprague-Dawley rats were obtained from Taconic Farms (Germantown, NY) and housed individually in the Princeton University vivarium on a reversed 12-h light: 12-h dark cycle. The room was maintained at 20° ± 1° C, and the animals had ad libitum access to water at all times and access to standard laboratory chow, LabDiet #5001 (PMI Nutrition International, Brentwood, MO; 3.02 kcal/g) as described below. All procedures were approved by the Princeton University Institutional Animal Care and Use Committee. The diets and procedures are summarized in Table 1.
Table 1.
Summary of groups and testing procedures for Experiments 1–4.
| Experiment | Groups | Withdrawal Testing Procedures |
|---|---|---|
| 1 |
Palatable food used: Nutritionally-complete chow (45% fat, 20% protein, 32% carbohydrate) 25 Days of Access:
|
Naloxone administration (3 mg/kg, s.c.)
|
| 2 |
Palatable food used: mixture of sugar and fat (35.7% fat, 64.3% sucrose) 27 Days of Access:
|
Deprivation for 36 h
|
| 3 |
Palatable foods used: (1) mixture of sugar and fat (35% fat, 10% sucrose), (2) Vanilla Ensure, or (3) 10%sucrose 28 Days of Access:
|
Naloxone administration (3 mg/kg, s.c.)
|
| 4 |
Palatable food used: As in Experiment 1 21 Days of Access:
|
Naloxone administration (3 mg/kg, s.c.)
Naloxone administration (3 mg/kg, s.c.)
|
Exp. 1: Naloxone-precipitated and spontaneous opiate-like withdrawal testing in rats fed a nutritionally complete, fat-and-sugar-rich diet
Rats (315–325 g) were divided into four weight-matched groups (n=10/group) and assigned to one of the following feeding conditions for 25 days: (a) 2-h daily access to sweet-fat chow (Research Diets, New Brunswick, NJ, #12451; 45% fat, 20% protein, 35% carbohydrate, 4.7 kcal/g) starting 6 h after the onset of the dark cycle, with standard rodent chow available only for the other 22 h per day; (b) 2-h access to the sweet-fat chow on Mondays, Wednesdays, and Fridays (MWF) with ad libitum access to standard rodent chow during remainder of time; (c) ad libitum sweet-fat chow; and (d) ad libitum standard chow (LabDiet #5001, PMI Nutrition International, Richmond, IN; 10% fat, 20% protein, 70% carbohydrate, 3.02 kcal/g). Food was replaced twice weekly. Food intake was measured daily (prior to and after the 2-h access period, or the equivalent time for the ad libitum-fed groups). Body weights were also measured during these times on days 1–7 and days 18–24 of access.
1a. Naloxone precipitated withdrawal testing
On days 26 and 27 rats were randomly assigned to be tested for signs of withdrawal. These tests were spread over 2 days to ensure that the tests were conducted as close to the beginning of the normal 2-h access period as possible for each rat. To test for somatic signs of opiate-like withdrawal, rats were administered the opioid antagonist naloxone (Sigma, St. Louis; 3 mg/kg, s.c.). Injections were administered 6 h after the onset of the dark cycle, when palatable food access would normally begin. Rats in the 2-h MWF group normally had a 46-h period of palatable chow deprivation between their access periods during the week (although they had standard chow available during this time), and were also deprived of the palatable food over the weekend. Thus, to standardize the 46-h deprivation period, we ensured that all rats tested had 46 h of sweet-fat chow deprivation. Ten minutes after the injection, rats were placed in a plastic cage lined with Bed-o-Cobs (The Anderson Co., Maumee, OH), and somatic signs of withdrawal were recorded for 5 min by an observer blind to experimental conditions. Instances of paw-biting, defensive burrowing, wet-dog shakes, teeth chattering, head shakes, forepaw tremor, cage crossing, and grooming were recorded for each rat, and the total instances of these behaviors were summed to produce an overall withdrawal index score, using a method modified from other reports [28, 29].
1b. Spontaneous withdrawal testing
To determine if withdrawal behavior could be observed by simply removing the palatable diet (i.e., without naloxone), rats were next given access to only standard rodent chow for 3 weeks. Then, the rats were returned to their previous feeding schedules for 14 days. During subsequent periods of sweet-fat chow deprivation, all animals were maintained on standard rodent chow for 46 h. At the end of 46 h, when the experimental group would normally receive access to sweet-fat chow, they were instead tested for somatic signs of withdrawal.
Exp. 2: Naloxone-precipitated and spontaneous withdrawal testing in rats fed standard rodent chow with a nutritionally incomplete food rich in sugar and fat
This experiment used an added assessment of opiate-like withdrawal, the elevated plus maze, to determine both somatic and anxiety-like responses to withdrawal from palatable food. Rats (350–400 g) were divided into three weight-matched groups (n=8/group) and maintained on ad libitum chow and water supplemented with the following for 28 days: (a) 12-h access to a high-sugar, high-fat mixture (4.48 kcal/g; 35.7% fat, 64.3% sucrose; butter, powdered sugar, prepared in our laboratory); (b) ad libitum access to the same sugar and fat mixture (c) ad libitum chow. Food was replaced twice weekly, at which times animals were weighed.
2a. Spontaneous withdrawal testing
On day 28, all rats were placed on a diet of ad libitum standard rodent chow. 24 h and 36 h later all rats were tested for somatic signs of opiate-like withdrawal in order to determine an overall withdrawal index, as described in Exp. 1a. Then, to test for anxiety, animals were then individually placed for 5 min in an elevated plus-maze [30]. The apparatus had four arms, each 10 cm wide by 50 cm long, and was elevated 60 cm above the floor. Two opposite arms were enclosed with high opaque walls, while the other two arms had no protective walls. The experiment was conducted under red light to minimize disruption to the rats’ circadian cycle. The rats were placed in the center of the maze with head orientation alternated toward an open or closed arm. Each plus-maze trial was videotaped and later scored for the amount of time spent with head, shoulders and forepaws on the open arm, closed arm or center section of the maze by an observer blind to the diet condition.
2b. Naloxone-precipitated withdrawal testing
Following testing in Exp. 2a, all rats were returned to their assigned diets for 21 days and then were administered naloxone (Sigma, St. Louis; 3 mg/kg, s.c.). Ten minutes after injection, rats were observed for somatic signs of withdrawal and elevated plus-maze anxiety (as described in Exp. 2a).
Exp. 3: Naloxone-precipitated opiate-like withdrawal testing in rats maintained on standard rodent chow and with a liquid food rich in sugar and fat
The diets tested in Exp. 1 and 2 were solid; we next tested a liquid diet in order to control for the effects of texture, since signs of opiate-like withdrawal in our animal model of binge eating of sugar involved the use of a sucrose solution [8, 9], and there are known differences in the effects that solid and liquid diets can have on ingestive behavior [31, 32]. Rats (300–375 g) were divided into four weight-matched groups (n=8/group) and maintained for 28 days on ad libitum chow supplemented with: (a) 12-h access to an emulsion of oil, sugar and water (3.4 Kcal/mL, 35% fat, 10% sugar; Mazola® Corn Oil, sucrose, tap water and 0.6% Emplex, Caravan Ingredients, Lenexa, KS, prepared in our laboratory), and chow; (b) 12-h access to Vanilla Ensure (1.06 Kcal/mL, 30% fat, and 30% sugar, Abbott Laboratories, Abbott Park, IL) and chow; (c) 12-h access to a 10% (w/v) sucrose solution (0.4 Kcal/mL) and chow, or (d) ad libitum chow. To prepare the emulsion, water was heated to 75–80°C and added to other ingredients. The emulsion was mixed on a high speed for 5 min and then cooled in an ice bath until it reached 20°C. All diets (except for standard chow) were liquid and were presented in a graduated drinking tube. Food was replaced daily and animals were weighed weekly.
Naloxone-precipitated withdrawal testing
After 28 days on the assigned diets, rats were administered naloxone (3 mg/kg, s.c.). Ten minutes after the injection, rats were placed on the elevated plus-maze as described in Exp. 2a. Immediately following a 5-min elevated plus-maze test, rats were placed in a computerized, open-field activity chamber under red light (MED Associates, Georgia, VT, 30.5 cm high acrylic sidewalls and 16 infrared photocells on each of the three axes). The entire field was 43.2 cm × 43.2 cm. Each rat was initially placed in the center of the open field and given a 10 min acclimation period before testing began [33, 34]. Then, locomotor activity, defined as infrared beam breaks, was monitored for 20 min.
Exp. 4: Naloxone-precipitated opiate-like withdrawal testing in rats at reduced body weight
In order to test whether withdrawal signs could be elicited at a reduced body weight, weight-matched rats (283–345 g) were maintained for 21 days on: (a) 2-h daily access to sweet-fat chow (Research Diets, New Brunswick, NJ, #12451, as used in Exp. 1) starting 6 h after the onset of the dark cycle, with standard rodent chow available only for the other 22 h per day (n=10), or (b) ad libitum standard chow with 2 h of access to the sweet-fat chow only two days (day 2 and day 22 or 23; Acute Sweet-Fat group, n=9). Food intake was measured daily at 6 h and 8 h after the onset of the dark cycle; food was replaced twice weekly.
4a. Normal body weight withdrawal testing
On day 22, 6 h into the dark period, all rats were administered naloxone (3 mg/kg, s.c.). Ten minutes after the injection, rats were observed for somatic signs of withdrawal and elevated plus-maze anxiety as described in Exp. 2a.
4b. Reduced body weight withdrawal testing
The 2-h Daily Sweet-Fat rats were reduced to 85% body weight over a 7-day period by reducing daily standard chow availability to either a half-pellet (3 g) or one pellet (5 g) and sweet-fat chow to a half-pellet (2 g) or one pellet (3.5 g). The amount of food provided was adjusted for each rat depending on rate of weight loss. The Acute Sweet-Fat group was also reduced to 85% body weight over a 7-day period by decreasing daily standard chow availability to 1–2 pellets. Rats in this group were given 2-h access to sweet-fat chow for a third time on day 30 or 31. Withdrawal tests (somatic signs and plus-maze) were conducted again on day 29 as described in Exp. 1a and 2a.
4c. Normal body weight locomotor activity testing
After testing at reduced body weight, all rats were given ad libitum access to standard chow for one month to allow them to return to a normal body weight for their age. Then, all animals were returned to their experimental diets for 14 days. Rats in the Acute Sweet-Fat group were given access to the sweet-fat chow again on the fourteenth day of access to the resumed testing diets in order to determine if behavior was due to binge eating or mere exposure to the diet. Then, 6 h after the onset of the dark cycle, naloxone (3 mg/kg, s.c.) was administered. Ten minutes after the injection, rats were placed in a computerized, open-field activity chamber under red light, as described in Exp. 3a. Each rat was initially placed in the center of the locomotor chamber, and activity counts were measured for 10 min.
4d. Reduced body weight locomotor activity testing
Following Exp. 4c, body weights of all rats were again reduced to 85% as described above over the course of 7 days. Locomotor activity testing was then conducted as described in Exp. 3c.
Statistical Analysis
Data were analyzed using one-way and two-way analyses of variance (ANOVA) with post-hoc Newman Keuls or Tukey tests when appropriate, or Student’s t-tests. For the elevated plus maze data, open arm activity was considered as the total time that each rat spent in the open arms of the maze [35]. Locomotor data were analyzed first with one-way ANOVA for each measure of locomotion and then with two-way ANOVA to compare within-group locomotor measures at normal and reduced body weight, as well as between-group measures. Error presented in this manuscript is standard error of the mean.
Results
Exp. 1: Naloxone-precipitated or spontaneous somatic signs of anxiety were not observed in rats given a nutritionally complete sweet-fat chow
Intake and body weight data
The intake data for these rats have been previously reported [36]. To briefly summarize those findings, compared with the control groups, rats with 2-h daily access and 2-h MWF access to the nutritionally complete sweet-fat food consumed excessively large amounts of the palatable chow in the 2 h of access. The body weight of these animals increased due to large meals and then decreased between binges as a result of self-restricted intake of standard chow following binges. However, despite these fluctuations in body weight the group with access to sweet-fat chow every day gained significantly more weight than the control group with standard chow available ad libitum. Further, when analyzing weight gain over the duration of the study, there was a difference among groups (F(3,39)=7.74, p<0.001), with those animals with 2-h daily access to the sweet-fat chow gaining more weight than the standard chow-fed controls (108.6 ± 6.2 g vs. 75.4 ± 3.8 g, respectively; p<0.001) and the sweet-fat-food-fed controls (88.3 ± 4.9 g; p<0.05). In addition, the rats with 2-h MWF access to the sweet-fat food gained more weight than chow-fed controls (95.0 ± 4.6 g vs. 75.4 ± 3.8 g, respectively; p<0.05).
Withdrawal tests
When given naloxone, there were no differences in withdrawal index scores for somatic behavior noted between the groups (F(3, 36) = 2.71, p = n.s.). These behaviors included forepaw tremors, cage crossing, paw biting and defensive burrowing (p = n.s. for each; see Fig. 1). Wet dog shakes were not observed in any group.
Figure 1.

Exp. 1: Instances of somatic signs of naloxone-precipitated withdrawal (mean ± SEM). There were no statistically significant differences among groups on the behaviors measured. Somatic signs with close to no instances (Wet dog shakes, head shakes, paw biting and grooming) for all groups are not depicted.
Instances of somatic signs of withdrawal following deprivation from the sweet-fat chow are depicted in Fig. 2. There was no significance among groups between the total withdrawal index score (F(3, 36) = 2.04, p = n.s.). Pair-wise comparisons revealed no differences between groups for forepaw tremors, paw biting or defensive burrowing (p = n.s. for all). Significance was seen among groups in instances of cage crossing (F(3, 36) = 4.66, p < 0.05). Post hoc Tukey tests revealed that 2-h daily access rats showed significantly fewer instances of cage crossing than ad libitum Chow rats (p < 0.01) or ad libitum Sweet-Fat rats (p < 0.05). Again, wet dog shakes were not observed in any group.
Figure 2.
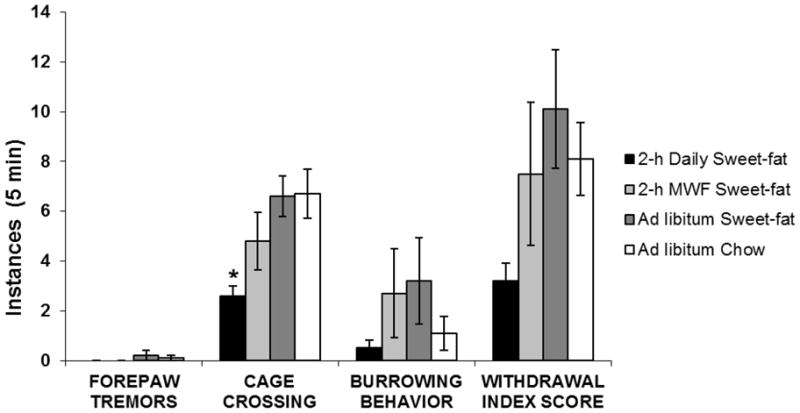
Exp. 1: Somatic signs of spontaneous withdrawal (mean ± SEM). 2-h Daily Sweet-fat rats showed significantly fewer instances of cage crossing than Ad libitum Chow rats or Ad libitum Sweet-fat rats, *p < 0.05. Somatic signs of withdrawal with close to no instances (Wet dog shakes, head shakes, paw biting and grooming) for all groups are not depicted.
Exp. 2: Naloxone-precipitated, spontaneous somatic signs, or spontaneous signs of anxiety in the elevated plus maze were not observed in rats fed a sweet-fat supplement to their standard chow
Intake and body weight data
Animals that were in the 12-h Sweet-Fat + Chow group consumed more of the sweet-fat food during the first hour of daily access compared to those animals maintained on the ad libitum Sweet-Fat + Chow diet (F(2, 21)=13.16, p < 0.001, day 28 of diet access, 5.6 vs. 1.1 g, respectively). On day 28 of diet access, the 12-h Sweet-Fat group consumed 3.5 ± 0.9 g of chow, the ad libitum Sweet-Fat group consumed 0.68 ± 0.7 g of chow, and the ad libitum Chow group consumed 2.3 ± 1.5 g of chow in the first hour. Despite the differences in intake of the sweet-fat supplement and chow, on day 28 there was no statistically significant difference among groups in the total number of calories consumed over a 24-h period (F(2, 22)= 0.62; p = n.s.; 12-h Sweet-Fat: 82.8 ± 2.6 Kcal, ad libitum Sweet-Fat: 77.3 ± 7.8 Kcal, ad libitum Chow: 83.2 ± 6.8 Kcal). On day 28, body weights of the rats were not significantly different among groups (F(2, 23)=1.87, p = n.s.). Further, analysis of weight gained over the duration of the study yielded no significant differences among groups (F(2, 21)=1.31, p = n.s.).
Withdrawal tests
There were no statistically significant differences in withdrawal index scores noted among the groups 24 h (F(2, 23)= 0.24, p = n.s.; 12-h Sweet-Fat group = 11.5 ± 2.6, ad libitum Sweet-Fat group = 13.6 ± 2.6; ad libitum Chow group= 13.4 ± 1.8) and 36 h (F(2, 23)= 0.17, p = n.s.; 12-h Sweet-Fat group = 11.8 ± 2.6, ad libitum Sweet-Fat group = 12.1±1.4; ad libitum Chow group = 10.5 ± 2.0) after the animals had been deprived of the palatable diet. The index score includes behaviors of grooming, wet dog shakes, cage crossing, forepaw tremors, paw biting and defensive burrowing (for each pair-wise comparison, p = n.s.). No instances of headshakes were observed at the 24 h and 36 h time points.
In terms of the elevated plus maze, after 24 h of deprivation there was a statistically significant difference among groups in terms of time spent on the open arm (F(2, 23)=3.77, p<0.05; 3.1 ± 1.4 s, 20.0 ± 6.0 s and 15.4 ± 4.7 s, ad libitum Sweet-Fat, 12-h Sweet-Fat and ad libitum Chow respectively), with the rats that had been maintained on ad libitum Sweet-Fat spending less time on the open arm than the 12-h Sweet-Fat group or the ad libitum Chow group (p<0.05). At 36 h of deprivation, no effects were seen in time spent on the open arm of the plus maze (F(2, 23)=0.22, p= n.s.; 26.3 ± 7.6 s, 30.0 ± 10.0 s and 23.4 ± 7.2 s, ad libitum Sweet-Fat, 12-h Sweet-Fat and ad libitum Chow respectively).
Following naloxone, there was no statistically significant difference in withdrawal index scores of somatic behavior noted among the groups (F(2, 23) = 0.64, p = n.s.). Withdrawal index scores were 8.4 ± 2.5 for the 12-h Sweet-Fat group, 11.5 ± 2.3 for the ad libitum Sweet-Fat group and 11.4 ± 1.7 for the ad libitum Chow group. The index score includes behaviors of teeth chattering, grooming, cage crossing, forepaw tremors, paw biting and defensive burrowing (for each pair-wise comparison, p = n.s.). No instances of headshakes or wet dog shakes were observed.
Exp. 3: Naloxone-precipitated somatic signs or signs of anxiety in the elevated plus maze were not observed in rats fed a liquid high-fat, high-sucrose diet
Intake data
By the third week of diet access, there was a difference among groups in terms of their first-hour intake (Sugar-oil emulsion = 32% of kcal, Vanilla Ensure = 27% of kcal, and 10% Sucrose = 24% of kcal of total daily intake; F(2, 27) = 39.40, p < 0.001). There was also a statistically significant difference among the groups in terms of their daily consumption of standard rodent chow (F(3, 78) = 22.86, p < 0.0001), with the animals with palatable food available showing a decrease their intake of standard rodent chow by day 28 (23 ± 3 Kcal: Sugar-oil emulsion; 30 ± 4 Kcal: Vanilla Ensure; 71 ± 2 Kcal: 10% Sucrose) relative to the ad libitum Chow group (101 ± 4 Kcal). Although there was a difference seen among the groups in overall daily caloric intake (F(3, 27) = 3.50, p < 0.05), follow-up multiple comparisons indicated that there were no differences noted when each group was independently compared to the chow-consuming control group (101 ± 4 Kcal), p = n.s. in all cases (118 ± 13 Kcal: Sugar-oil emulsion; 93 ± 11 Kcal: Vanilla Ensure; 85 ± 6 Kcal: 10% Sucrose). Further, the amount of actual sucrose consumed (in grams) was consistent across groups with each group consuming 3–4.5 g of sugar/daily, even given the varying diets (F(2, 20) = 2.32, p = n.s.). At the end of 4 weeks, there were no differences in body weight among groups (F(3,31) = 0.25, p = n.s.). However, when analyzing weight gain over the duration of the study, there was a difference among groups (F(3,31) = 3.67, p<0.05), with those animals consuming the Sugar-oil emulsion gaining more weight than the Chow-fed controls (123 ± 23 g vs. 67 ± 6 g, respectively, p<0.05).
Withdrawal data
When placed on the elevated plus-maze after naloxone injections, the animals with 12-h 10% Sucrose access spent less time on the open arm of the plus-maze compared to Chow-fed controls (t(9)=2.58, p < 0.05; 52 ± 7 vs. 75 ± 3 s). No other differences were noted among the groups (12-h Sugar-oil emulsion group = 54 ± 11 s on the open arm; 12-h Vanilla Ensure group=75 ± 3 s on the open arm). Analysis of the open field maze data revealed that the 12-h 10% Sucrose group had increased locomotor activity (F(3, 29) = 3.65, p < 0.05) compared with the ad libitum Chow group (743 ± 70 and 512 ± 57 ambulatory counts, respectively). No other differences were noted among the groups in the open field maze (12-h Sugar-oil emulsion = 561 ± 71 ambulatory counts; 12-h Vanilla Ensure group = 576 ± 58 ambulatory counts).
Exp. 4: Naloxone-precipitated somatic signs or signs of anxiety in the elevated plus maze in sweet-fat-bingeing rats were not seen when reduced to 85% body weight
Intake and body weight data
Beginning the second week of sweet-fat access, rats in the 2-h Daily Sweet-Fat group consumed an excessive number of calories in the 2 h of access to sweet-fat chow (66.8% of total daily intake), which is consistent with our previous report using this model [36] and suggests binge eating behavior. The Acute Sweet-Fat group consumed 24.6 ± 12.5 kcal on day 2 and 48.1 ± 14.1 kcal on day 22 or 23 from the sweet-fat pellets. At normal body weight, repeated-measures ANOVA (with a Greenhouse-Geisser correction) showed a significant group × time interaction (F(1.63, 27.70) = 21.28, p < 0.001). Post-hoc tests revealed significantly heavier body weight for the 2-h Daily Sweet-Fat group compared to the Acute Sweet-fat group (day 8: t(1, 17) = 2.28, p < 0.05, day 12: t(1, 17) = 2.63, p < 0.05, and day 16: t(1, 17) = 2.94, p < 0.01). Further, when weight gain over the first 16 days was analyzed, the rats in the 2-h Daily Sweet-fat group were found to have gained significantly more weight than the Acute Sweet-fat group (81.0 ± 4.1 g vs. 45.3 ± 4.5 g, respectively; F(1, 18) = 33.83, p<0.001). When animals were reduced in body weight, paired samples t-tests indicated that the body weights of both groups were statistically significantly reduced (t(9) = 25.50, p <0.001 and t(8) = 19.93, p < 0.001, 2-h Sweet-Fat Chow and Acute Sweet-fat Chow, respectively).
Withdrawal data
At normal weight, the only observed difference between groups revealed that the 2-h Daily Sweet-fat rats exhibited significantly fewer instances of cage crossing compared to the Acute Sweet-fat rats (2.3 ± 0.4 vs. 4.5 ± 0.9, respectively; F(1, 16) = 5.54, p < 0.05; Fig. 3). However, no differences were seen in overall withdrawal index (2-h Daily Sweet-fat: 9.4 ± 1.2; Acute Sweet-fat: 12.5 ± 2.0; F(1, 16) = 2.00, p = n.s.). This included measures of burrowing behavior, head shakes, grooming and rearing (p = n.s. for each). No rats exhibited any instances of teeth chattering.
Figure 3.
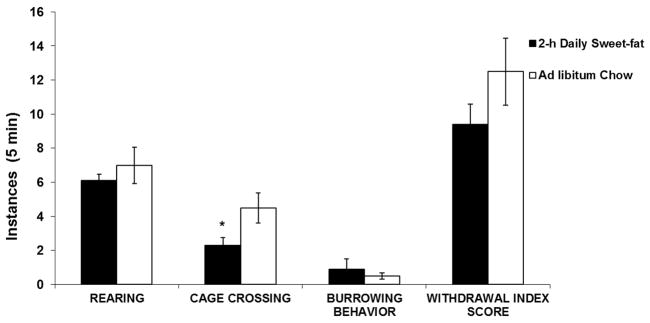
Exp. 4: Cage crossing (mean ± SEM). At normal weight, 2-h Daily Sweet-fat rats exhibited significantly fewer instances of cage crossing compared to Ad libitum Chow controls, *p < 0.05.
At reduced body weight, there were no statistically significant differences between the groups in instances of somatic signs of withdrawal, as seen by the index score (F(1, 16) = 0.49, p = n.s.). Withdrawal index scores were 13.0 ± 3.2 in the Acute Sweet-fat group vs. 10.8 ± 1.2 in the 2-h Daily Sweet-fat group. The index score includes behaviors of teeth chattering, rearing, grooming, cage crossing, forepaw tremors, paw biting and burrowing behavior. No instances of headshakes were observed.
In the elevated plus-maze testing, time spent on the open arm did not differ by group before weight reduction (2-h Daily Sweet-fat group: 22.4 ± 7.7 s; Acute Sweet-Fat Chow group: 17.4 ± 11.5 s; F(1, 16) = 0.14, p = n.s.) or after weight reduction (2-h Daily Sweet-fat group: 22.4 s ± 7.0 s; Acute Sweet-fat group: 16.5 ± 7.8 s; F(1, 16) = 0.32, p = n.s.). In the locomotor activity testing, at both normal and reduced body weight, there were no significant differences between 2-h Daily Sweet-Fat rats and Acute Sweet-Fat rats in terms of locomotor activity (Fig. 4).
Figure 4.

Exp. 4: Total ambulatory counts before and after body weight reduction (mean ± SEM). Regardless of group, all rats were more active at a reduced body weight than before weight loss, *p < 0.05.
When at a reduced body weight, regardless of group, all rats were more active (F(1, 16) = 7.13, p < 0.05, Fig. 4) and spent more time in the center (F(1, 16) = 11.83, p < 0.005; 2-h Daily Sweet-Fat: 12.0 ± 1.7 min at reduced body weight, versus 9.6 ± 1.6 min at normal weight; Acute Sweet-Fat: 12.8 ± 3.2 min at reduced body weight versus 8.8 ± 2.2 min at normal body weight) of the locomotor activity chamber compared with their behavior at a normal body weight, but there was no interaction between body weight and group.
Discussion
Based on the findings of these four experiments, rats binge eating a palatable food containing both fat and sugar did not demonstrate significant indications of naloxone-precipitated or spontaneous opiate-like withdrawal. Given previous findings that rats binge eating sugar show signs of naloxone-precipitated withdrawal [9] (which was also replicated here in Exp. 3), we expected naloxone-injected rats with limited access to a sweetened, fat-rich diet to show similar signs of withdrawal. However, the inclusion of fat in the diet may have interfered with the expression of withdrawal signs. These findings therefore suggest that opiate-like withdrawal is not observed in response to binge eating of all palatable diets, specifically those rich in fat, which is, like sugar, known to exert effects on brain opioid systems. Thus, the emergence of the opiate-like withdrawal signs seen in response to binge eating appears to be macronutrient specific.
The Addictive Properties of Diets High in Fat and Sugar
In previous reviews we have summarized the findings from our laboratory and others suggesting that limited access to sugar can result in multiple behavioral and neurochemical signs of addiction in rats, including opiate-like withdrawal [8, 37]. There have been studies using animal models suggesting that access to a fat-rich diet can also instigate some signs of addiction [16]. Corwin’s group has shown an increase in progressive-ratio responding in rats binge eating fat, suggesting enhanced motivation [38]. Bale and colleagues show that mice that are maintained on a high-fat or high-carbohydrate diet and then denied access to the food will endure an aversive stimulus (foot shock) in order to obtain the desired foods [11]. Following an acute withdrawal (deprivation) period, the mice with access to a high-fat diet show signs of anxiety as well as reduced expression of corticotropin-releasing factor in the central nucleus of the amygdala. However, McGee and colleagues have noted no signs of anxiety or enhanced motivation using a progressive ratio schedule following deprivation from limited daily access to a sweetened vegetable shortening, similar to the diet we used in Exp. 2 [17]. Collectively, these studies suggest that some specific signs of addiction can be elicited when animals are offered a fat-containing diet, but the results are complex and may be influenced by factors such as diet composition, body weight, and access schedule.
Macronutrient Specificity, Form and Availability as Factors in Palatable Food Withdrawal
The present studies incorporated a variety of high-fat diets, including some that are nutritionally complete, similar to a “meal.” Others were supplements to a standard chow diet, similar to a “snack food,” which are commonly consumed during binge episodes [25]. Diets were also varied in texture, from the pelleted diet in Exp. 1, the semi-solid diet supplement in Exp. 2 and the liquid diets in Exp. 3. The liquid diets were used because of the previously identified relationship between overconsumption of liquid diets and subsequent weight gain [31, 32]. Also, because our previous studies showing signs of opiate-like withdrawal in response to sucrose access used a sucrose solution (replicated here in Exp. 3 where animals binge eating sucrose spent less time on the open arm of the plus maze and showed signs of withdrawal induced hyperactivity in the open field maze). We tested a liquid fat to see if the form of the food could have an effect of the expression of withdrawal-associated behaviors. However, access to a fat-rich liquid did not precipitate signs of withdrawal. From this variety of diet texture and form, we conclude that regardless of type of fat-rich food or form in which it is given, the present experiments did not show clear signs of opiate-like withdrawal in animals with binge-access to fat.
Another manipulation taken into account in this study was access period. Some of the rats were given 12-h daily access to the palatable diet, while others were given 2-h access on a daily or intermittent schedule. Both types of restricted access have been shown to precipitate binge-eating behavior [8, 39]. Binge consumption has been shown to cause changes in brain reward systems, particularly the dopamine system, in both humans [40] and in rat models, that are similar to the effects seen with some drugs of abuse [8]. Although these access schedules have been shown to lead to binge eating, which was confirmed in the present experiments, none of the access periods tested led to opioid-like signs of withdrawal in response to fat.
Interpretation of Postive Findings in the Present Experiments
While overall, the data suggest that signs of opiate-like withdrawal do not emerge when rats are offered limited access to a fat-containing palatable foods, there were some positive findings obtained in the present set of experiments that warrant discussion. In Exp. 1, after 46 h of deprivation from a sweet-fat chow, rats that previously had 2 h access showed fewer instances of cage-crossing compared with ad libitum fed controls (standard rodent chow or high-fat, sweet rodent chow). Hypoactivity has been noted during cocaine withdrawal in rodents [41]. However, no signs of opiate-like withdrawal were noted in the other tests performed on this group, such as the somatic measures of withdrawal.
In Exp. 2, after 24 h of deprivation animals with ad libitum access to the sweet-fat diet showed decreased time spent on the open arm of the elevated plus maze. This finding is interesting, as it suggest that a group with ad libitum access to the palatable food showed a change in behavior that is associated with withdrawal. However, when tested at 36 h, the effect was no longer apparent. This could be due to the fact that there is a specific window of time in which spontaneous signs of anxiety emerge, and that period had expired by the next assessment time. Or, it could suggest that repeated use of the elevated-plus maze altered the performance on the test. While some studies suggest that repeated exposure to the elevated plus maze does not affect the outcome of the test [42, 43], others do report a habituation effect [44–47]. In the present set of studies, for the most part no differences were seen between groups in anxiety, potentially indicating no differences incurred by repeated exposure. However, the positive with ad libitum-fed animals in Exp. 2 should be considered within the context of the repeated use of this test.
The Role of Body Weight in the Expression of Signs of Addiction
In the present study, we assessed the variables of binge eating and body weight, both of which have been shown to contribute to addiction-like signs. Other groups have shown that when rats are given limited access to a sweet, chocolate diet, they become obese and show anxiogenic-like behavior when denied access from the palatable food [15]. Our previous studies show withdrawal-like behavior in animals maintained on intermittent sucrose, at normal weight. Other findings have also shown that obese animals with ad libitum or limited access to a cafeteria-style diet show deficits in mesolimbic dopamine neurotransmission [18, 48], but animals with restricted access (i.e., binge access) that were not classified as obese did not show downregulated dopamine 2 receptors. This underscores the idea that the obesity itself could lead to changes in the brain reward system [18]. Conversely, studies of humans with binge eating disorder suggest that binge eating, independent of obesity, causes affect mesolimbic DA systems [40], thus highlighting the importance of studying the specific variables of overeating and obesity, both together and independently. In the present paper, we did not observe signs of opiate-like withdrawal emerge in rats that became overweight on a fat-rich food (Exp.1). Because previous studies suggest that obesity can precipitate addictive-like changes in the brain, which may or may not necessarily involve opiate-like withdrawal signs, it is possible that we might have been able to observe different (i.e., non-opiate-related) signs of withdrawal in these rats.
In Exp. 4 we assessed the effect of reduced body weight on the emergence of withdrawal signs. Rats with food-deprivation history more rapidly acquire cocaine self-administration compared to controls [49], and weight reduction has been shown to enhance the effects of drug reward [26]. Reducing rats’ body weights results in a reduction of DA levels in the NAc to 33% of baseline levels [50, 51]. We have previously found that when the body weights of rats with a history of binge eating sugar are reduced to 85%, DA release in response to sugar is further increased [27]. For these reasons, we hypothesized that reducing the rats’ body weights might enhance the expression of signs of withdrawal. However, in Exp. 4, naloxone-precipitated signs of withdrawal were not observed when rats were deprived to 85% of their normal body weight.
As an added measure of anxiety, we assessed locomotor activity. Increased locomotor activity is associated with drug withdrawal [52–54], and was found in Exp. 3 in rats binge eating sugar, but not in rats with limited access to fat. Further, in Exp. 4, there was no significant difference between rats binge eating fat and control rats in terms locomotion, either at normal or reduced body weight.
Conclusion
Rats maintained on a binge diet rich in sugar and fat did not show signs of opiate-like withdrawal when overweight, normal weight, or underweight, using both solid and liquid forms of the diets. These results contrast with previous findings from this laboratory, and others, indicating opiate-withdrawal-like behavior in rats that binge eat sugar. The present findings support the notion that signs of addiction in response to overeating palatable foods may be nutrient-specific, underscoring the importance of further investigating the differential effects that overeating of specific nutrients can have on brain reward systems.
Research Highlights.
Studies show neurochemical and behavioral indices of addiction when animals overeat a fat-rich diet.
Naloxone-precipitated withdrawal was not seen in rats binge eating a variety of high-fat, sweet diets.
Deprivation-induced withdrawal was not seen in rats binge eating a variety of high-fat, sweet diets.
Body weight reduction, which is known to potentiate the reinforcing effects of substance of abuse, did not affect naloxone-precipitated signs of opiate-like withdrawal.
Rats that binge eat sweet-fat combinations do not show signs of opiate-like withdrawal under the conditions used.
Acknowledgments
The research was supported by USPHS grant AA-12882 (BGH) and DK-079793 and the National Eating Disorders Foundation (NMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoebel BG. Brain neurotransmitters in food and drug reward. Am J Clin Nutr. 1985;42(5 Suppl):1133–50. doi: 10.1093/ajcn/42.5.1133. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42(18):1705–12. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13(2–3):129–33. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- 6.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Gold MS, Frost-Pineda K, Jacobs WS. Overeating, binge eating, and eating disorders as addictions. Psychiatric Annals. 2003;33(2):112–116. [Google Scholar]
- 8.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10(6):478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 10.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7(6):617–27. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61(9):1021–9. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94(3):309–15. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci. 2005;8(5–6):269–76. doi: 10.1080/10284150500485221. [DOI] [PubMed] [Google Scholar]
- 14.Galic MA, Persinger MA. Voluminous sucrose consumption in female rats: increased “nippiness” during periods of sucrose removal and possible oestrus periodicity. Psychol Rep. 2002;90(1):58–60. doi: 10.2466/pr0.2002.90.1.58. [DOI] [PubMed] [Google Scholar]
- 15.Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009;34(1):38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Magnen J. A role for opiates in food reward and food addiction. In: Capaldi PT, editor. Taste, Experience, and Feeding. American Psychological Association; Washington, D. C: 1990. pp. 241–252. [Google Scholar]
- 17.McGee HM, Amare B, Bennett AL, Duncan-Vaidya EA. Behavioral effects of withdrawal from sweetened vegetable shortening in rats. Brain Res. 2010;1350:103–11. doi: 10.1016/j.brainres.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering C, Alsio J, Hulting AL, Schioth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology (Berl) 2009;204(3):431–43. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 20.Guertin TL, Conger AJ. Mood and forbidden foods’ influence on perceptions of binge eating. Addict Behav. 1999;24(2):175–93. doi: 10.1016/s0306-4603(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 21.Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr. 1989;50(4):759–66. doi: 10.1093/ajcn/50.4.759. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal DM, Gold MS. Neurobiology of food addiction. Curr Opin Clin Nutr Metab Care. 2010;13(4):359–65. doi: 10.1097/MCO.0b013e32833ad4d4. [DOI] [PubMed] [Google Scholar]
- 23.Corsica JA, Pelchat ML. Food addiction: true or false? Curr Opin Gastroenterol. 2010;26(2):165–9. doi: 10.1097/MOG.0b013e328336528d. [DOI] [PubMed] [Google Scholar]
- 24.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav. 1990;48(6):837–40. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- 25.Allison S, Timmerman GM. Anatomy of a binge: food environment and characteristics of nonpurge binge episodes. Eat Behav. 2007;8(1):31–8. doi: 10.1016/j.eatbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91(5):459–72. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156(4):865–71. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanarek RB, D’Anci KE, Jurdak N, Mathes WF. Running and addiction: precipitated withdrawal in a rat model of activity-based anorexia. Behav Neurosci. 2009;123(4):905–12. doi: 10.1037/a0015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cicero TJ, Nock B, Meyer ER. Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav. 2002;72(3):691–7. doi: 10.1016/s0091-3057(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 30.File SE, Lippa AS, Beer B, Lippa MT. Unit 8.4 Animal tests of anxiety. In: Crawley JN, et al., editors. Current Protocols in Neuroscience. John Wiley & Sons, Inc; Indianapolis: 2004. [DOI] [PubMed] [Google Scholar]
- 31.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 32.Mattes RD. Hunger and thirst: issues in measurement and prediction of eating and drinking. Physiol Behav. 2010;100(1):22–32. doi: 10.1016/j.physbeh.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21(2):205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 34.Whimbey AE, Denenberg VH. Two independent behavioral dimensions in open-field performance. J Comp Physiol Psychol. 1967;63(3):500–4. doi: 10.1037/h0024620. [DOI] [PubMed] [Google Scholar]
- 35.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berner LA, Avena NM, Hoebel BG. Bingeing, Self-restriction, and Increased Body Weight in Rats With Limited Access to a Sweet-fat Diet. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 37.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wojnicki FH, Roberts DC, Corwin RL. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol Biochem Behav. 2006;84(2):197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82(1):123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced Striatal Dopamine Release During Food Stimulation in Binge Eating Disorder. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baldo BA, Markou A, Koob GF. Increased sensitivity to the locomotor depressant effect of a dopamine receptor antagonist during cocaine withdrawal in the rat. Psychopharmacology (Berl) 1999;141(2):135–44. doi: 10.1007/s002130050817. [DOI] [PubMed] [Google Scholar]
- 42.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 43.File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5(3):195–201. [PubMed] [Google Scholar]
- 44.Andreatini R, Bacellar LF. Animal models: trait or state measure? The test-retest reliability of the elevated plus-maze and behavioral despair. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(4):549–60. doi: 10.1016/s0278-5846(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 45.Treit D, Menard J, Royan C. Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav. 1993;44(2):463–9. doi: 10.1016/0091-3057(93)90492-c. [DOI] [PubMed] [Google Scholar]
- 46.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Espejo EF. Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav Brain Res. 1997;87(2):233–8. doi: 10.1016/s0166-4328(97)02286-9. [DOI] [PubMed] [Google Scholar]
- 48.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–9. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Specker SM, Lac ST, Carroll ME. Food deprivation history and cocaine self-administration: an animal model of binge eating. Pharmacol Biochem Behav. 1994;48(4):1025–9. doi: 10.1016/0091-3057(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 50.Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15(10):6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pothos EN, Hernandez L, Hoebel BG. Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obes Res. 1995;3(Suppl 4):525S–529S. doi: 10.1002/j.1550-8528.1995.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 52.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26(24):6450–7. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43(3):245–54. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- 54.Stinus L, Robert C, Karasinski P, Limoge A. Continuous quantitative monitoring of spontaneous opiate withdrawal: locomotor activity and sleep disorders. Pharmacol Biochem Behav. 1998;59(1):83–9. doi: 10.1016/s0091-3057(97)00319-5. [DOI] [PubMed] [Google Scholar]


