Abstract
Monoclonal 6-19 IgG3 anti-IgG2a rheumatoid factor derived from lupus-prone MRL-Faslpr mice can induce GN and cryoglobulinemia, but the features that confer nephritogenic potential are not completely understood. Asparagine-linked oligosaccharide chains of 6-19 IgG3 mAb are poorly galactosylated and hardly sialylated, possibly contributing to the pathogenic potential of 6-19 IgG3 rheumatoid factors. Here, we used the 6-19 model of cryoglobulin-associated GN to define the relative contributions of galactosylation and sialylation, in relation to cryoglobulin activity, to the nephritogenic potential of IgG3 antibodies. We generated one highly sialylated and two distinct more galactosylated 6-19 IgG3 rheumatoid factor variants. Although the mere extent of galactosylation had no effect on either the cryogenic and nephritogenic activities of 6-19 IgG3 rheumatoid factor, terminal sialylation attenuated the nephritogenic potential of 6-19 IgG3 by limiting its cryoglobulin activity. These data suggest a protective role of IgG sialylation against the development of cryoglobulin-mediated GN, highlighting the anti-inflammatory activity of sialylated IgG antibodies.
Cryoglobulins are a heterogeneous group of Igs that precipitate at temperatures <37°C, with resolution upon warming.1,2 Most cryoglobulins are either monoclonal Ig or Ig complexes in which one component, usually IgM, has rheumatoid factor (RF) activity.1,2 Monoclonal cryoglobulins are mainly associated with various lymphoproliferative disorders, whereas mixed cryoglobulins are often found in sera of patients with autoimmune diseases, such as SLE and rheumatoid arthritis, or with chronic infectious diseases. The presence of cryoglobulins can result in a wide range of vascular, renal, and neurologic complications, likely depending on their concentration, their temperature-dependent solubility behavior, and the nature and type of proteins involved.2
Antibodies of the IgG3 subclass in humans and mice have the unique physicochemical property that allows them to self-associate via Fc-Fc interactions and to display cryoglobulin activity, independently of their specificities.3–8 Nucleotide sequence analysis of the variable regions of cryogenic and noncryogenic IgG3 mAbs, in combination with the assessment of mutant antibodies, showed that cryoglobulin activity of IgG3 was associated with the presence of more positively charged amino-acid residues at positions 6 and 23 of the heavy-chain variable domain.9,10 Moreover, structural analysis of asparagine-linked biantennary complex-type oligosaccharide chains (N-glycans) attached to the CH2 domain of different IgG3 mAbs revealed an inverse correlation between the extent of terminal sialylation and cryogenic activity.11 Thus, the cryoglobulin activity of IgG3 is likely to be determined by the presence of positively charged amino-acid residues in the variable region and of negatively charged sialic-acid residues in N-glycans attached to the Fc region. Notably, several studies reported a marked reduction of sialic-acid content in cryoglobulins from human patients.2,12–15
With regard to cryoglobulin-associated pathology, we previously established that implantation of 6-19 hybridoma secreting IgG3 anti-IgG2a RF mAb derived from lupus-prone MRL-Faslpr mice induces lupus-like GN in association with the development of cryoglobulinemia.16,17 Studies with a panel of anti-IgG2a RF mAbs, including Ig class-switch variants of 6-19 RF, demonstrated that the nephritogenicity of anti-IgG2a RF mAb is dependent on the IgG3 subclass.18–20 Moreover, the development of identical glomerular lesions in Ig-deficient mice lacking the corresponding IgG2a autoantigens indicated that the direct glomerular localization of IgG3 RF mAb, without the involvement of IgG3-IgG2a immune complex formation, is responsible for the development of such lesions.21,22 However, because IgG3 complexes self-associating due to Fc-Fc interaction could potentially be sufficient to provoke glomerular injuries, it has been unclear whether cryogenic activity is essential for the nephritogenic potential of IgG3.
The structural analysis of N-glycans revealed that 6-19 IgG3 RF mAb is poorly galactosylated and hence hardly sialylated,10 because the terminal sialylation is dependent on the presence of galactose residues. The same pattern of IgG glycosylation has been observed in MRL-Faslpr mice,23 in which IgG3 cryoglobulin formation correlates with the development of the disease,24 and in patients with rheumatoid arthritis, in parallel with the progression of the disease.25,26 Although the pathogenic significance of this association remains to be determined, it has been reported that agalactosylated and asialylated IgG could be more pathogenic due to the activation of complement by the lectin pathway and an enhanced interaction of IgG with activating IgG Fc receptors (FcγR).27–29 Moreover, hyposialylation could specifically enhance the nephritogenic potential of IgG3 mAbs by promoting their cryogenic activity.11
Using the 6-19 IgG3 RF model of cryoglobulinemia, we aimed to define the contribution of galactosylation or sialylation of N-glycans, in relation to cryoglobulin activity, to the nephritogenic potential of IgG3 antibodies. To this end, we generated two more galactosylated 6-19 IgG3 RF variants, either established from mice expressing a transgene encoding the 6-19 IgG3 heavy chain or obtained by overexpressing β-galactoside α2,6-sialyltransferase I (ST6GalI), as well as a highly sialylated variant carrying phenylalanine instead of alanine at position 243 (F243A) in the CH2 domain. The analysis of these three different 6-19 IgG3 RF variants revealed that the nephritogenic activity of 6-19 IgG3 RF mAb was dependent on the extent of terminal sialylation that negatively regulated the IgG3 cryoglobulin activity, but independent of the extent of galactosylation per se.
Results
Induction of Glomerular Lesions by a More Galactosylated 6-19 Variant, X10C, Established from 6–19 Heavy-Chain Transgenic Mice
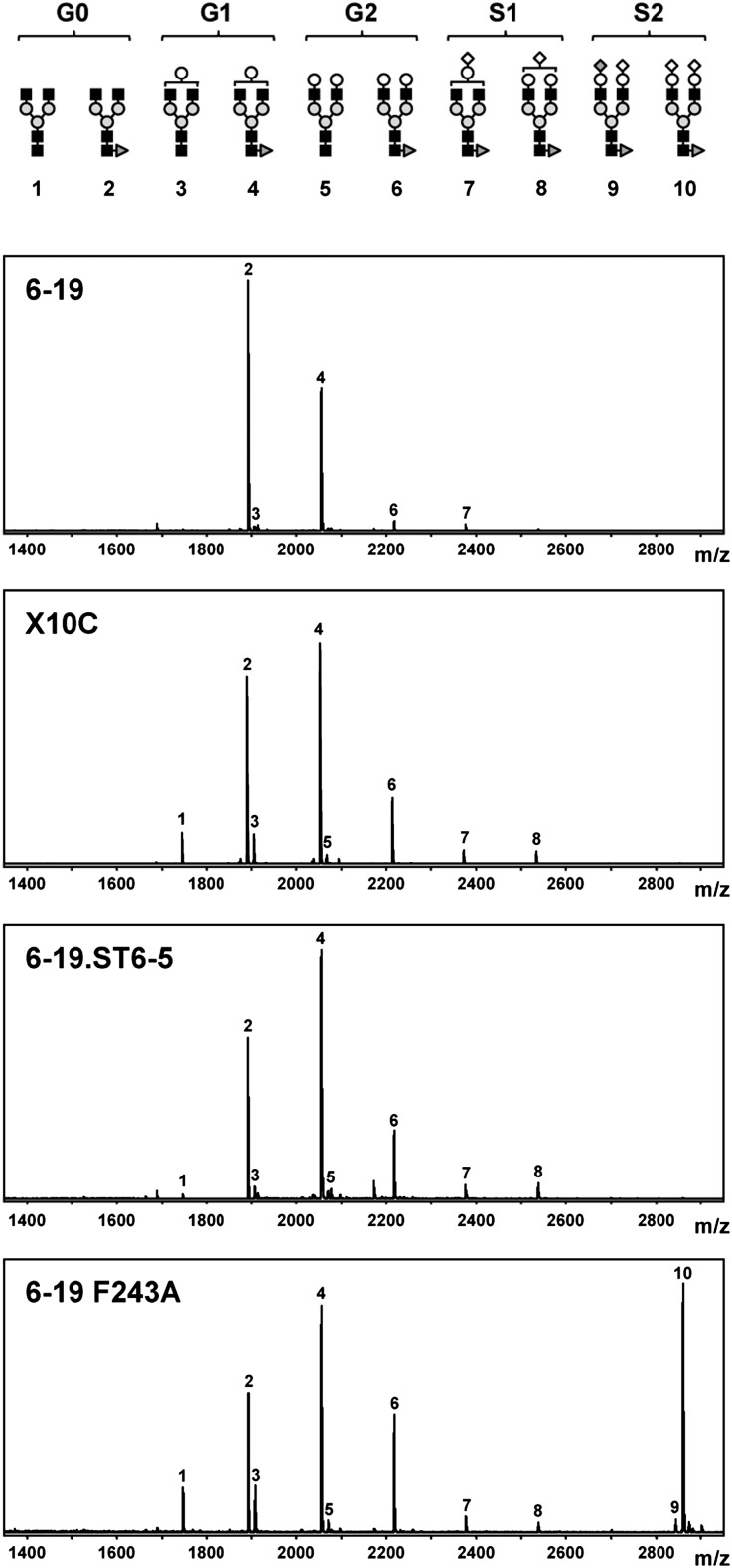
Structures of N-glycans of IgG are highly heterogeneous in terms of galactosylation and sialylation.23,25 Most of them are fucosylated and nonsialylated, ending with either two N-acetylglucosamine residues (G0; peaks 1 and 2 in Figure 1), one N-acetylglucosamine and one galactose (G1; peaks 3 and 4), or two galactoses (G2; peaks 5 and 6), whereas a minor fraction of galactosylated glycoforms bear one (S1; peaks 7 and 8) or two (S2; peaks 9 and 10) terminal sialic acids (Figure 1). It can be speculated that conformational changes in the Fc region of IgG3 cryoglobulins, resulting from limited galactosylation of 6-19 IgG3 RF mAb, could promote their glomerular deposition and subsequent inflammation. We therefore attempted to generate a 6-19 variant with amino-acid sequences of the heavy and light chains identical to the parental mAb, but different in galactosylation patterns because of their synthesis by alternative hybridoma cells. To this end, a panel of IgG3 anti-IgG2a RF mAb expressing the 6-19 idiotype were established from 6-19 heavy-chain transgenic mice, which spontaneously produce 6-19–like IgG3 anti-IgG2a RF, resulting from the combination of the transgenic 6-19 heavy chains with endogenous light chains.10 Among them, we selected the clone, X10C, which secreted the highest concentration of IgG3 anti-IgG2a (>20 μg/ml in culture supernatants), because a high-secretor hybridoma is necessary to assess the nephritogenicity of 6-19 RF mAb in vivo. Nucleotide sequence analysis confirmed that the amino-acid sequences of heavy and light chains of X10C mAb are identical to those of 6-19 IgG3 RF mAb.21
Figure 1.
Increased galactosylation of N-glycans of X10C and 6-19.ST6-5 variants and increased sialylation of the 6-19 F243A mutant as compared with the wild-type 6-19 mAb. Structures of different nonsialylated (G0, G1, and G2) and sialylated (S1 and S2) glycoforms are depicted in the upper panel, with the numbers corresponding to the respective peaks on MALDI-TOF MS of N-glycans liberated from 6-19, X10C, 6-19.ST6-5, and 6-19 F243A mAbs. Peaks 1 and 2 correspond to G0 glycoforms without or with a fucose, peaks 3 and 4 to G1 glycoforms without or with a fucose, peaks 5 and 6 to G2 glycoforms without or with a fucose, peaks 7 and 8 to S1 glycoforms with a fucose, and peaks 9 and 10 to S2 glycoforms with a fucose. Closed square, N-acetylglucosamine; gray circle, mannose; open circle, galactose; open diamond, N-glycolylneuraminic acid; dark gray diamond, N-acetylneuraminic acid; dark gray triangle, fucose. m/z, mass/charge ratio.
To estimate the content of different nonsialylated (G0, G1, and G2) and sialylated (S1 and S2) glycoforms of X10C mAb compared with 6-19 mAb, N-glycans liberated from both mAbs were subjected to matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). In contrast to hypogalactosylated 6-19 mAb, X10C mAb was more galactosylated (Table 1 and Figure 1). When the content of galactose residues in non-sialylated glycoforms was expressed as [(% of G1) + 2 × (% of G2)], X10C mAb had approximately two-fold higher value than 6-19 mAb (74.7 versus 39.2). Moreover, X10C mAb still displayed a significant cryoglobulin activity at a concentration of 2 mg/ml. Indeed, 23.0% of X10C mAb was cryoprecipitated after 24-hour incubation at 4°C, although the extent of cryoprecipitation was somehow lower than that observed with 6-19 mAb (Figure 2).
Table 1.
Structural analysis of N-glycans purified from 6–19 IgG3 RF and its variants
| mAb | Nonsialylated Glycoforms | Sialylated Glycoforms | |||
|---|---|---|---|---|---|
| G0 | G1 | G2 | S1 | S2 | |
| 6-19 | 58.8 | 34.2 | 2.5 | 2.4 | 0.1 |
| X10C | 33.1 | 48.7 | 13.0 | 4.7 | 0.1 |
| 6-19.ST6-5 | 24.5 | 51.8 | 12.5 | 8.6 | 0.1 |
| 6-19 F243A | 18.9 | 30.5 | 13.5 | 3.0 | 32.7 |
| Human IgG | 29.8 | 41.2 | 16.0 | 6.7 | 0.3 |
Results are expressed as relative abundance (%) of nonsialylated (G0, G1, and G2) and sialylated (S1 and S2) glycoforms among total oligosaccharides. Human polyclonal IgG was included for comparison. Some of the carbohydrate moieties were unable to be assigned to the glycoforms defined in Figure 1. However, these are nonsialylated and represent very minor fractions: 2.0% for 6-19, 0.4% for X10C, 2.5% for 6-19.ST6-5, 1.4% for 6-19 F243A, and 6.0% for polyclonal human IgG.
Figure 2.
Cryoglobulin activity was conserved in more galactosylated X10C and 6-19.ST6-5 variants, but lost in the highly sialylated 6-19 F243A mutant. The amounts of cryoprecipitates were estimated by measuring OD280 of supernatants, and results are expressed as percentages of cryoprecipitation (means of duplicates).
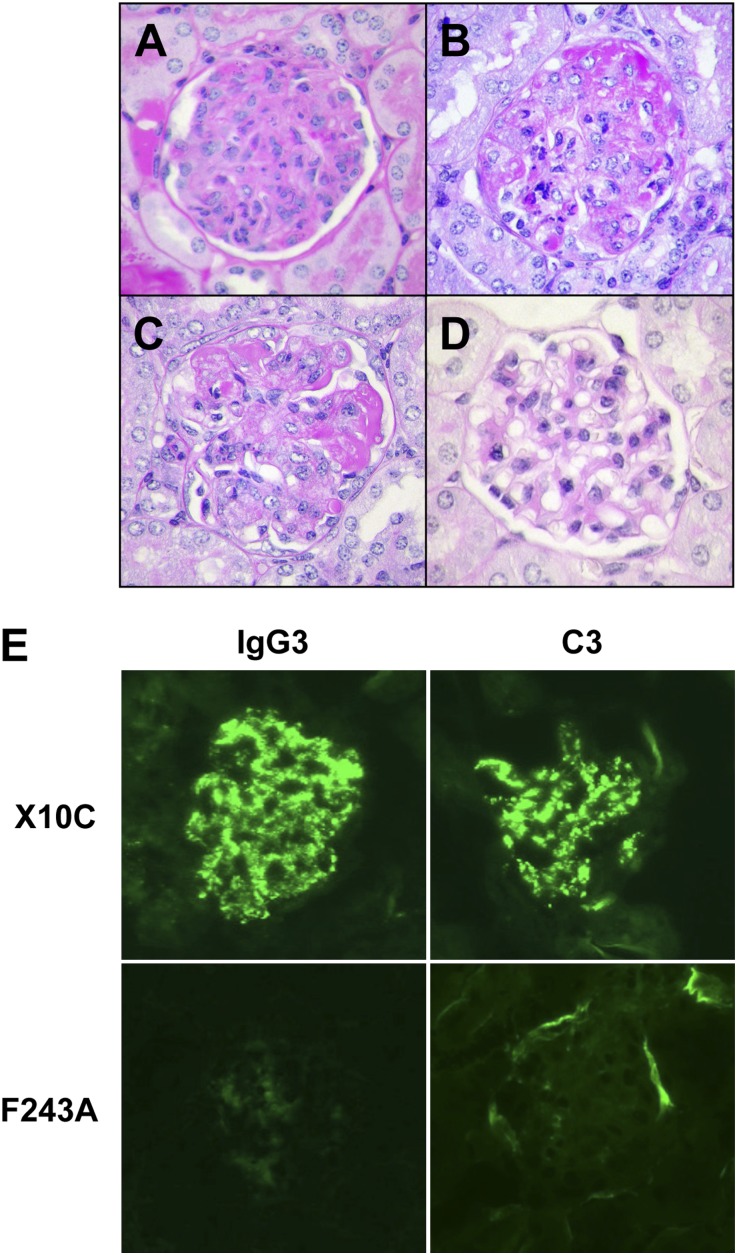
The nephritogenic potential of X10C mAb was then assessed by implantation of X10C hybridoma cells into BALB/c mice. Two weeks after intraperitoneal injection of X10C hybridoma cells, serum levels of IgG3 RF were increased to concentrations around 1–4 mg/ml and cryoglobulins were detectable in sera (Table 2). In parallel, X10C-injected mice displayed severe acute glomerular lesions essentially identical to those observed in mice implanted with 6-19 hybridoma cells.16,17 These lesions were characterized by exudation of PMN and glomerular cell proliferation in an early stage (Figure 3A and Supplemental Figure 1), followed by the marked glomerular deposition of periodic acid–Schiff (PAS)–positive materials in more advanced cases (Figure 3B and Supplemental Figure 2).
Table 2.
Serum levels of IgG3 anti-IgG2a RF and cryoglobulins and the development of glomerular lesions in BALB/c mice implanted with cells secreting one of three different 6-19 IgG3 RF variants
| mAb | IgG3 Anti-IgG2aa | Cryoglobulina | Kidneyb |
|---|---|---|---|
| X10C | 2.5±0.2 | 15.3±1.8 | 12/12 (2.9±0.2) |
| 6-19.ST6-5 | 4.9±0.4 | 28.9±4.1 | 10/10 (3.3±0.2) |
| 6-19 F243A | 2.6±0.2 | <0.1 | 0/17 (<1.0) |
Serum levels of IgG3 anti-IgG2a RF (mg/ml) and IgG3 cryoglobulins (μg/ml) in 2- to 3-month-old BALB/c mice 7–14 days after implantation of X10C, 6-19.ST6-5, or 6-19 F243A-secreting cells (mean ± SEM). The number of mice used for implantation of X10C, 6–19.ST6-5, and 6-19 F243A cells was 12, 10, and 17, respectively. Serum levels of IgG3 anti-IgG2a before injection of cells were <0.01 mg/ml.
Incidence of glomerular lesions evaluated by histologic examination. Semiquantitative values of glomerular lesions on a 0–4+ scale based on the intensity of lesions are indicated in parentheses (mean ± SEM).
Figure 3.
Induction of glomerular lesions in BALB/c mice implanted with X10C or 6-19.ST6-5 IgG3 RF-secreting cells, but not with 6-19 F243A-secreting cells. (A and B) Representative glomerular appearance of early and more advanced lesions in BALB/c mice implanted with X10C hybridoma. Note infiltrations by PMN, proliferation of mesangial cells, expansion of mesangial matrix, as well as the marked deposition of PAS-positive materials in glomeruli. (C) Representative glomerular lesions of BALB/c mice implanted with 6-19.ST6-5 hybridoma. (D) Representative appearance of glomeruli in BALB/c mice implanted with 6-19 F243A transfectoma. Note essentially normal histologic appearance of glomeruli. (E) Immunohistochemical analysis of glomerular lesions of BALB/c mice implanted with X10C or 6-19 F243A-secreting cells. Note fine granular deposits of IgG3 and C3 in the mesangium and glomerular capillary walls in mice implanted with X10C cells, and the lack of significant immune deposits in mice implanted with 6-19 F243A cells. PAS staining in A–D. Original magnification, ×400 in A–D; ×200 in E.
Induction of Glomerular Lesions by More Galactosylated 6-19 Variant Obtained through Overexpression of ST6GalI in 6-19 Hybridoma
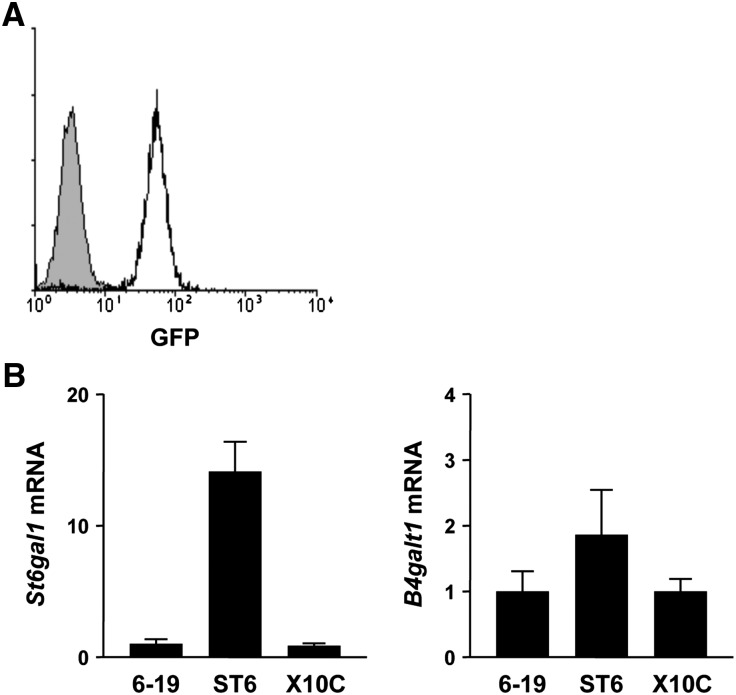
Structural analysis of N-glycans attached to the CH2 domain of different cryogenic and noncryogenic IgG3 mAbs suggested an inhibitory effect of terminal sialic acids on the cryoglobulin activity.11 Because ST6GalI is known to be one of the key enzymes involved in IgG terminal sialylation,30 we planned to generate more sialylated 6-19 variants through overexpression of ST6GalI in 6-19 hybridoma cells. After screening for the expression of green fluorescence protein (GFP) by flow cytometric analysis, clone 6-19.ST6-5 expressing the highest level of GFP was selected (Figure 4A). Quantitative PCR analysis confirmed 14–16 times increased expression of St6gal1 mRNA in this clone compared with the parental 6-19 and also X10C hybridomas (P<0.001; Figure 4B). MALDI-TOF MS analysis of N-glycans from 6-19.ST6-5 mAb showed only modest increases in the content of monosialylated S1 glycoform (Table 1 and Figure 1). In contrast, unexpectedly, 6-19.ST6-5 mAb was highly galactosylated, at levels that were indistinguishable from those of X10C mAb. However, the enhanced galactosylation of 6-19.ST6-5 mAb was not associated with an increased expression of the B4galt1 gene encoding β1,4-galatosylatransferase I that is involved in IgG galactosylation31 (Figure 4B).
Figure 4.
Increased levels of St6gal1 mRNA in 6-19.ST6-5 hybridoma cells as compared with wild-type 6-19 and X10C cells. (A) Expression of GFP in 6-19.ST6-5 cells was determined by flow cytometric analysis. Shaded area indicates the background obtained with parental 6-19 hybridoma cells. (B) Levels of St6gal1 and B4galt1 mRNAs in 6-19, 6-19.ST6-5, and X10C cells were quantified relative to a standard curve generated with a reference cDNA preparation and normalized using TATA-binding protein mRNA. Results (means of three different preparations of each cell type ± SEM) are expressed as fold increases relative to 6-19 hybridoma cells.
The cryoglobulin activity of 6-19.ST6-5 mAb was comparable with that of X10C mAb (Figure 2) and despite considerable alterations of the structure of N-glycans, 6-19.ST6-5 variant was still highly nephritogenic. Indeed, 7–10 days after implantation of 6-19.ST6-5 hybridoma cells, BALB/c mice developed severe glomerular lesions characterized by massive deposits of PAS-positive materials in association with infiltration of PMN (Figure 3C and Supplemental Figures 1 and 2), as was the case of mice implanted with X10C hybridoma or parental 6-19 hybridoma. The development of glomerular lesions was paralleled to marked increases in serum levels of IgG3 RF and cryoglobulins (Table 2).
Lack of Cryoglobulin and Nephritogenic Activities of a Highly Sialylated 6-19 F243A Mutant
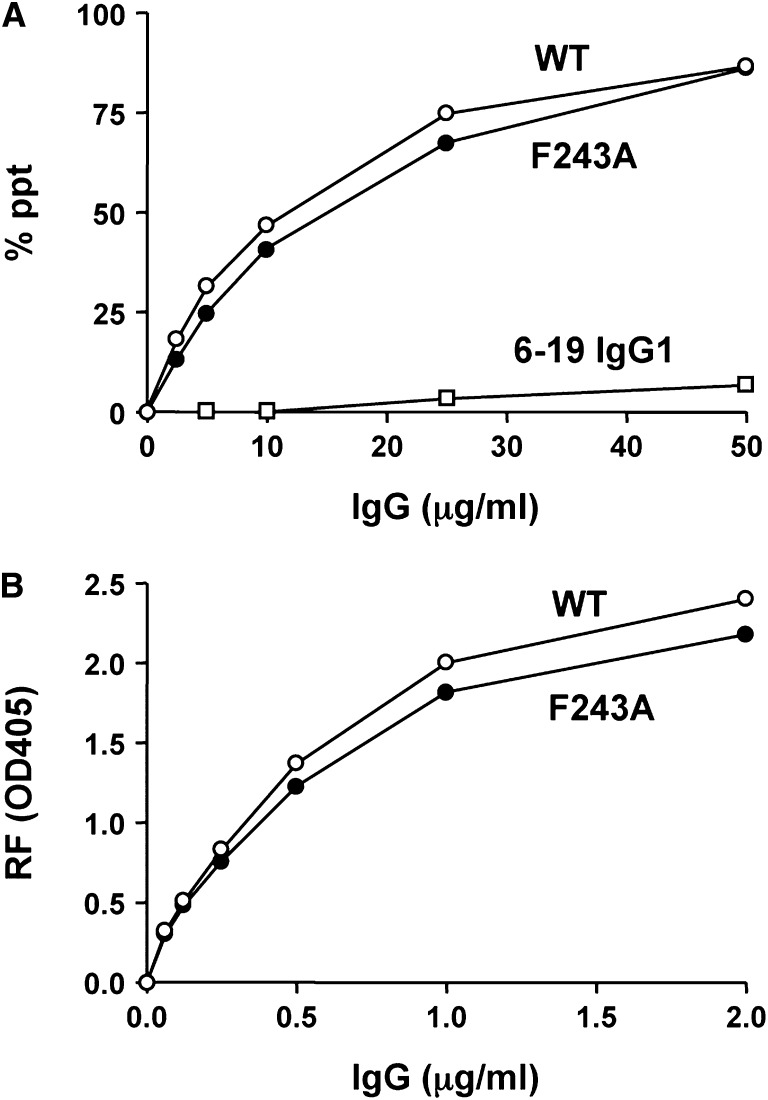
Because the generation of highly sialylated 6-19 variants through overexpression of ST6GalI was not successful, we generated a 6-19 F243A mutant with a replacement of phenylalanine by alanine at position 243. This replacement has been shown to result in a remarkable increase in the content of sialic-acid residues on human chimeric IgG3 and murine IgG2a without affecting their antigen-binding specificity.32–34 Structural analysis of N-glycans purified from the 6-19 F243A mutant confirmed a marked increase in the disialylated S2 glycoform, which accounted for approximately one-third of total oligosaccharides (Table 1 and Figure 1). When the content of sialic-acid residues was expressed as [(% of S1) + 2 × (% of S2)], the 6-19 F243A mutant had a 26-fold higher value than the parental 6-19 mAb (68.4 versus 2.6). Notably, the observed sialic-acid species in the 6-19 F243A mutant was mostly (>90%) N-glycolylneuraminic acid, rather than N-acetylneuraminic acid, as was the case with a precedent 34–3C IgG2a F243A mutant.34 As expected,11 the highly sialylated 6-19 F243A mutant failed to display cryoglobulin activity (Figure 2). This was not due to the lack of self-association, because the mutant efficiently interacted with wild-type 6-19 mAb to form IgG3-IgG3 complexes in vitro (Figure 5A). Moreover, anti-IgG2a RF activity of the 6-19 F243A mutant was comparable with that of the wild-type 6-19 mAb (Figure 5B).
Figure 5.
IgG3 self-associating and anti-IgG2a RF activities of the 6-19 F243A mutant were comparable to those of the wild-type 6-19 mAb. (A) Various amounts of 6-19 (WT, open circle), 6-19 F243A (closed circle), and 6-19 IgG1 variant (open square) were incubated with 125I-labeled 6-19 mAb and results are expressed as percentages of labeled 6-19 mAb precipitated specifically. (B) Anti-IgG2a RF activity of purified 6-19 (WT, open circle) and 6-19 F243A (closed circle) mAbs was determined by ELISA, and results are expressed as OD at 405 nm.
Intraperitoneal implantation of transfectoma secreting 6-19 F243A mutant mAb into BALB/c mice induced within 2 weeks increases in serum IgG3 anti-IgG2a RF at levels comparable with those seen in mice implanted with X10C hybridoma cells (Table 2). However, these levels remained lower than those seen in mice receiving 6-19.ST6-5 hybridoma cells because of a lower mAb production rate of 6-19 F243A- and X10C-secreting cells than that of 6-19.ST6-5 hybridoma. Notably, sera of 6-19 F243A–injected mice failed to exhibit cryoglobulin activities (Table 2) and none of the mice implanted with 6-19 F243A cells developed appreciable glomerular lesions (Figure 3D and Supplemental Figure 2). Immunofluorescence analysis also showed little glomerular deposits of IgG3 and C3, which contrasted with the marked IgG3 and C3 deposits in the mesangium and along glomerular capillary walls of mice implanted with X10C hybridoma (Figure 3E).
Discussion
This study was designed to more precisely determine the contribution of galactosylation or sialylation of N-glycans to the nephritogenic potential of 6-19 IgG3 RF cryoglobulins. Our analysis of more galactosylated or sialylated 6-19 IgG3 variants demonstrated that both cryogenic and nephritogenic activities of 6-19 IgG3 RF mAb were independent of the mere extent of galactosylation but that terminal sialylation attenuated the nephritogenic potential of 6-19 IgG3 mAb by downregulating its cryoglobulin activity. These data thus demonstrate, in addition to the known anti-inflammatory property of sialylated IgG,28 a novel and previously unidentified protective role of IgG sialylation against the development of cryoglobulin-mediated GN.
N-glycans of the 6-19 IgG3 RF mAb are poorly galactosylated, and consequently hardly sialylated,35 because the terminal sialylation is dependent on the presence of galactose residues in N-glycans. The limited galactosylation of 6-19 IgG3 RF mAb is likely due to the fact that this hybridoma is derived from autoimmune-prone MRL-Faslpr mice in which the proportion of IgG lacking galactose is known to progressively increase with age.23 In contrast, it is not surprising to see that X10C mAb is highly galactosylated because this “6–19” mAb was established from 6–19 heavy-chain transgenic mice with a nonautoimmune background, in which the pattern of IgG3 galactosylation is not aberrant.10 It is, however, unexpected to see the generation of highly galactosylated IgG3 in the 6-19.ST6-5 hybridoma overexpressing ST6GalI, which is responsible for the terminal sialylation of N-glycans. At present, we cannot offer a good explanation for the observed increases in galactosylation in St6gal1-overexpressing 6-19 hybridoma.
Despite remarkable increases in the abundance of St6gal1 mRNA, the extent of terminal sialylation of N-glycans attached to the 6-19.ST6-5 variant was still limited. This contrasted with a marked increase in the content of sialic acids in 6-19 IgG3 carrying the F243A mutation. It has been speculated that phenylalanine at position 243 is one of the amino-acid residues in the CH2 domain that forms interactions with core oligosaccharides.32 Because these interactions may lead to the sequestration of the nascent oligosaccharide from glycosylation enzymes, the reduced interaction resulting from the replacement of phenylalanine by alanine at position 243 could increase the accessibility of the nascent oligosaccharide for glycosylation enzymes, thereby promoting galactosylation and sialylation.
Our studies of three 6-19 IgG3 variants differing in the content of galactose or sialic-acid residues in their N-glycans have clearly shown that the level of sialylation, but not galactosylation, determines the cryogenic activity of IgG3. Because of marked increases in the S2 glycoform carrying two sialic-acid residues, the 6-19 F243A mutant failed to display cryoglobulin activity, consistent with the notion of implication of positive charges in IgG3 cryoprecipitation.8,9,11 One may argue that a conformational change of the Fc region of IgG3 resulting from the F243A mutation could reduce its ability to display cryogenic activity. However, the hypothesis of an inhibitory role of terminal sialylation in the formation of cryoglobulins is further supported by reports of a markedly reduced content of sialic acids in cryoglobulins in several cases of human cryoglobulinemia.2,12–15 In this regard, it is worth noting that cryoglobulinemia is frequently observed in patients with liver injuries.36,37 This might be due to a lack of efficient elimination of asialylated IgG with cryogenic potential in these patients, because the liver is known to be the major site for removal of serum asialoglycoproteins due to the expression of the asialoglycoprotein receptor on hepatocytes.38,39
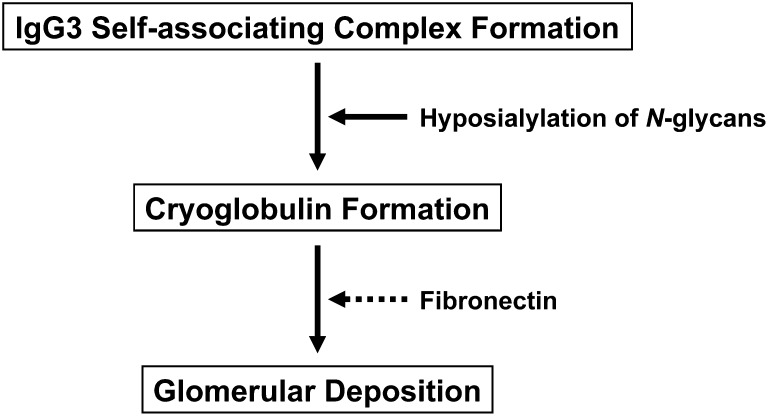
It should be stressed that the ability of the 6-19 F243A mutant to generate IgG3 self-associating complexes was not impaired. Thus, as summarized in Figure 6, self-associating IgG3 complexes resulting from Fc-Fc interaction are not sufficient for the induction of glomerular lesions and the loss of cryoglobulin activity due to increased sialylation of N-glycans is likely responsible for the lack of nephritogenic potential of the 6-19 F243A mutant. However, it is still unclear how IgG3 cryoglobulins accumulate in the glomeruli and provoke subsequent glomerular inflammation. In this regard, the constant presence of fibronectin in the cryoprecipitates from patients with cryoglobulinemia is intriguing.40–42 Although its significance remains to be determined, an attractive hypothesis would be that fibronectin present in cryoglobulins may promote glomerular localization through interaction with its receptor, such as α5β1 integrin, on endothelial cells43 and/or the extracellular matrix of the glomerular basement membrane and the mesangium. Whether the extent of terminal sialylation contributes to the interaction of cryogenic IgG3 with fibronectin is an additional issue that needs to be explored.
Figure 6.
Nephritogenic potential of IgG3 cryoglobulins. Antibodies of the IgG3 subclass in mice and humans have the unique physicochemical property to self-associate via Fc-Fc interactions. The self-associating property of the IgG3 subclass is likely to be the first step required for cryoglobulin formation but not sufficient by itself to confer cryoglobulin activity because not all monoclonal IgG3 proteins form cryoglobulins. The second step could be the electrostatic interaction between these IgG3 complexes, which would lead to the generation of larger complexes precipitating at lower temperatures. Hyposialylation of N-glycans of the Fc region could therefore enhance the cryogenic activity of IgG3 cryoglobulins and potentiate their nephritogenic activity. Glomerular localization of cryoglobulins might be mediated through their interaction with plasma fibronectin and subsequent binding to glomerular fibronectin receptors and/or extracellular matrix.
In contrast to the loss of pathogenic activity by the highly sialylated 6-19 F243A IgG3 variant, this study also showed that highly galactosylated 6-19 IgG3 variants obtained by two different manners were as pathogenic as poorly galactosylated wild-type 6-19 IgG3 mAb. Notably, it has previously been claimed that galactose-less IgG could be more pathogenic based on the following findings. First, it can more efficiently activate complement in vitro through the lectin pathway, in which mannose-binding lectin can interact with N-acetylglucosamines exposed as a result of absence of galactosylation.27 Second, in a murine model of collagen-induced arthritis, in vitro treatment of anticollagen antibodies with β-galactosidase enhanced their arthritogenic activity.44 However, results obtained in mice deficient in mannose-binding lectin demonstrated a dominant role of stimulatory FcγRs in mediating the pathogenicity of galactose-less IgG antibodies in experimental models of autoimmune thrombocytopenia and arthritis.45 Notably, the interaction of IgG with stimulatory FcγRs was substantially downmodulated by the presence of terminal sialic-acid residues in its N-glycans.28,29 Thus, the enhanced pathogenicity of galactose-less IgG could rather be attributed to their inability to undergo terminal sialylation, thereby favoring the interaction with stimulatory FcγRs and the ensuing activation of immune effector cells.
It should be emphasized that the development of glomerular lesions induced by 6-19 IgG3 cryoglobulins is independent of the activation of FcγR-bearing immune effector cells and of complement.22,46,47 Thus, the lack of nephritogenic activity of the highly sialylated, noncryogenic 6-19 F243A variant was unrelated to the previously reported anti-inflammatory property of highly sialylated IgG antibodies, because the latter is owed to their reduced capacity to interact with stimulatory FcγRs.28,29 Nevertheless, all of these results are consistent with the idea that the level of IgG sialylation could be an important factor in determining the pathogenic potential of autoantibodies. This would also be in line with the increased levels of agalactosylated and thus asialylated IgG in patients with rheumatoid arthritis and in MRL-Faslpr mice, in parallel with the progression of the disease.23,25,26 Consequently, the dysregulated expression of galactosyltransferase and/or sialyltransferase involved in IgG glycosylation could be a significant factor predisposing to the development of immune complex-mediated glomerular lesions. Further elucidation of the molecular basis responsible for the regulation of IgG glycosylation could thus ultimately help to develop new therapeutic approaches for GN and autoantibody-mediated autoimmune diseases.
Concise Methods
Mice
The generation of 6-19 heavy-chain transgenic mice was previously described.10 BALB/c mice were purchased from Charles River France (L’Arbresle, France). Animal studies described in this study have been approved by the Ethics Committee for Animal Experimentation of the University of Geneva.
Plasmids
The St6gal1 plasmid was constructed using the following DNA fragments: an St6gal1 cDNA isolated by RT-PCR from 6–19 IgG3 RF hybridoma, the promoter region isolated from pSV-Vμ1,48 the Ig heavy-chain enhancer isolated from pSVE2-neo,49 and a GFP cDNA. The VDJ6-19-Cγ3 plasmid containing the complete 6-19 IgG3 heavy-chain gene was constructed as previously described.35 The VDJ6-19-Cγ3(F243A) mutant plasmid bearing a mutation at position 243 (phenylalanine to alanine) was generated by oligonucleotide-directed mutagenesis, as described.10
mAb
The 6-19 IgG3 anti-IgG2a RF hybridoma derived from MRL-Faslpr mice and its IgG1 variant were previously described.16,19 X10C IgG3 anti-IgG2a RF mAb bearing heavy and light chains identical to those of the 6-19 mAb was established from mice expressing a transgene encoding the 6-19 IgG3 heavy chain.10 The 6-19 hybridoma cells overexpressing ST6GalI were generated by transfecting the St6gal1 plasmid (containing a GFP cDNA) together with a pSVE2-neo plasmid (containing the neomycin-resistance gene). The 6-19 F243A mutant was generated by transfecting a 6-19 heavy-chain-loss mutant cells with the VDJ6-19-Cγ3(F243A) mutant plasmid, together with the pSVE2-neo plasmid. IgG mAbs were purified from culture supernatants by protein A affinity column chromatography. The purity was >95% as documented by SDS-PAGE.
RT-PCR and cDNA Sequencing
RNA from hybridomas was purified with TRIzol reagent (Invitrogen AG, Basel, Switzerland). St6gal1 cDNA was isolated from 6–19 hybridoma through amplification with the following primers: 5′-ATTCATACCAACTTGAAG-3′ and 5′-TTCAAGCAGTGGTAACC-3′. For nucleotide sequencing of the variable regions of IgG3 anti-IgG2a RF mAb, cDNA was amplified with Pfu DNA polymerase (Stratagene Cloning Systems, La Jolla, CA) using the following pairs of primers: 5′-untranslated 6-19 VH primer (5′-CACTGACTTTCACCATG-3′) and Cγ3-CH1 primer (5′-CGATAGACAGATGG-3′) for the heavy chain, and Vk6-19 leader primer (5′-GTTGCCTCCTCAAATG-3′) and Ck primer (5′-TGGATGGTGGGAAGATG-3′) for the light chain.
Analyses of Oligosaccharide Structures
Purification of oligosaccharides from different 6-19 IgG3 mAbs and from polyclonal human IgG (Sigma-Aldrich, St. Louis, MO), as a control, was performed based on chemoselective glycoblotting technique, as described.50,51 Briefly, IgG samples were reductively alkylated under the presence of detergent, and then digested with trypsin and peptide N-glycosidase F (Roche Diagnostics, Penzberg, Germany).52 The digested sample was mixed with a novel hydrazide-functionalized glycoblotting polymer51 and after washing of the unbound substances, sialic acids were methyl-esterified to render sialylated oligosaccharides chemically equivalent to neutral oligosaccharides.53 The IgG oligosaccharides were finally recovered as derivatives of aoWR (Nα-((aminooxy)acetyl)tryptophanylarginine methyl ester), an oligosaccharide labeling reagent that allows highly sensitive detection on MS.54 The recovered glycans were subjected to MALDI-TOF MS using an Ultraflex II mass spectrometer (Bruker Daltonik, Bremen, Germany) controlled by the FlexControl 2.0 software package. The utilized analytical procedure was proven to be reproducible with a coefficient of variation <15% by analysis of N-glycans prepared from normal human serum and from human IgG.51 Estimation of N-glycan structures was obtained by input of peak masses into the GlycoMod Tool (http://au.expasy.org/tools/glycomod/) or GlycoSuite Tool (https://glycosuite.proteomesystems.com/glycosuite/glycodb).
Implantation of Hybridoma or Transfectoma Cells
To study the nephritogenicity of 6-19 IgG3 anti-IgG2a RF mAb, 107 hybridoma or transfectoma cells secreting different 6-19 IgG3 RF variants were injected intraperitoneally into pristine-treated BALB/c mice. Two to three independent experiments (five to seven mice per group for each experiment) were conducted. To avoid rejection of the hybridoma or transfectoma cells, immunosuppression was achieved by a simultaneous injection of a mixture of anti-mouse CD4 (GK1.5) and anti-mouse CD8 (H-35) mAb (0.5 mg of each mAb), as previously described.46
Histopathology
Kidneys and other major organs were obtained at autopsy, processed for histologic examination, and stained with PAS. Glomerular lesions were scored on a 0–4 scale based on the intensity and extent of histologic changes, as described previously.55 Glomerular deposition of IgG3 was determined by staining frozen kidney sections with rat anti-IgG3 (H139.61) mAb, followed by FITC-labeled goat anti-rat Ig conjugates (Vector Laboratories, Inc., Burlingame, CA). C3 deposits were examined by direct staining with FITC-labeled goat anti-mouse C3 conjugates (Cappel Laboratories, West Chester, PA).
ELISA
IgG3 anti-IgG2a RF activities in sera and culture supernatants were determined by ELISA as described.18 Briefly, microtiter plates were coated with TNP8-BSA and subsequently incubated with Hy1.2 IgG2a anti-TNP mAb, before the addition of serum samples. The assay was developed with alkaline phosphatase-labeled H139.61 rat anti-mouse IgG3 mAb. Results are expressed micrograms per milliliter of IgG3 anti-IgG2a by referring to a standard curve obtained with purified 6-19 IgG3 anti-IgG2a RF mAb. The expression of the 6-19 idiotype was determined as described.35 Concentrations of IgG3 in cryoglobulins were determined by IgG3-specific ELISA as described.18
Cryoglobulin Activity of IgG3 mAb
We placed 0.1 ml of 2 mg/ml of purified IgG3 mAb in conical glass tubes at 4°C for 24 hours. The samples were centrifuged at 3000 rpm at 4°C for 30 minutes. The amounts of cryoprecipitates were estimated by measuring OD280 of supernatants, and results are expressed as percentages of cryoprecipitation.
IgG3 Self-Association Assay
The ability of IgG3 mAbs to form IgG3-IgG3 self-associating complexes was determined by a RIA, as previously described.19 Briefly, various amounts of mAb were incubated with 125I-labled 6-19 mAb (10 ng) in the presence of 10 μl of normal mouse serum at 4°C overnight. After 1 hour of additional incubation at 4°C with 1 ml of 7.5% polyethylene glycol 6000 (Hänseler AG, Herisau, Switzerland), IgG3-IgG3 complexes were precipitated by centrifugation at 3000 rpm at 4°C for 30 minutes. Results are expressed as percentages of labeled 6-19 mAb precipitated specifically after correction for nonspecific precipitation (<10%) obtained in the presence of normal mouse serum alone.
Quantitative Real-Time PCR
The abundance of St6gal1 and B4galt1 mRNAs was quantified by real-time PCR with the following primers: St6gal1 (5′-AACTACCATCCGCCTAGTGAAC-3′) and reverse primer (5′-CTGCGGAATGTCTGCATGATAC-3′); and B4galt1 forward primer (5′-ATCCTTCAGCGCCAGCAAC-3′) and reverse primer (5′-TCCTTCAAGGCCTCTTGAAAGC-3′). PCR was performed using the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Philadelphia, PA) and iQ SYBR green Supermix (Bio-Rad). Results were quantified using a standard curve generated with serial dilutions of a reference cDNA preparation from 6–19 IgG3 hybridoma and normalized using TATA-binding protein mRNA.
Statistical Analyses
Unpaired comparison for levels of different mRNAs was analyzed by the t test. Probability values <5% were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. Montserrat Alvarez and Mr. Guy Brighouse for their excellent technical assistance and Dr. Thomas Moll for critically reading the manuscript.
This work was supported by a grant from the Swiss National Foundation for Scientific Research and by Project for the Developing Innovation Systems of the Ministry of Education, Culture, Sports, Science and Technology, of the Japanese Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050477/-/DCSupplemental.
References
- 1.Grey HM, Kohler PF: Cryoimmunoglobulins. Semin Hematol 10: 87–112, 1973 [PubMed] [Google Scholar]
- 2.Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M: Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 57: 775–788, 1974 [DOI] [PubMed] [Google Scholar]
- 3.Capra JD, Kunkel HG: Aggregation of γG3 proteins: Relevance to the hyperviscosity syndrome. J Clin Invest 49: 610–621, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grey HM, Kohler PF, Terry WD, Franklin EC: Human monoclonal γG-cryoglobulins with anti-γ-globulin activity. J Clin Invest 47: 1875–1884, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saulk PH, Clem W: Studies on the cryoprecipitation of a human IgG3 cryoglobulin: The effects of temperature-induced comformational changes on the primary interaction. Immunochemistry 12: 29–37, 1975 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura Y, Nakamura H: Human monoclonal cryoimmunoglobulins. I. Molecular properties of IgG3 kappa (Jir protein) and the cryo-coprecipitability of its molecular fragments by papain. J Biochem 95: 255–265, 1984 [DOI] [PubMed] [Google Scholar]
- 7.Abdelmoula M, Spertini F, Shibata T, Gyotoku Y, Luzuy S, Lambert PH, Izui S: IgG3 is the major source of cryoglobulins in mice. J Immunol 143: 526–532, 1989 [PubMed] [Google Scholar]
- 8.Spertini F, Coulie PG, Van Snick J, Davidson E, Lambert PH, Izui S: Inhibition of cryoprecipitation of murine IgG3 anti-dinitrophenyl (DNP) monoclonal antibodies by anionic DNP-amino acid conjugates. Eur J Immunol 19: 273–278, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Panka DJ, Salant DJ, Jacobson BA, Minto AW, Marshak-Rothstein A: The effect of VH residues 6 and 23 on IgG3 cryoprecipitation and glomerular deposition. Eur J Immunol 25: 279–284, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Kuroki A, Kuroda Y, Kikuchi S, Lajaunias F, Fulpius T, Pastore Y, Fossati-Jimack L, Reininger L, Toda T, Nakata M, Kojima N, Mizuochi T, Izui S: Level of galactosylation determines cryoglobulin activity of murine IgG3 monoclonal rheumatoid factor. Blood 99: 2922–2928, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Kuroda Y, Kuroki A, Kikuchi S, Funase T, Nakata M, Izui S: A critical role for sialylation in cryoglobulin activity of murine IgG3 monoclonal antibodies. J Immunol 175: 1056–1061, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zinneman HH, Levi D, Seal US: On the nature of cryoglobulins. J Immunol 100: 594–603, 1968 [PubMed] [Google Scholar]
- 13.McIntosh RM, Kulvinskas C, Kaufman DB: Cryoglobulins. II. The biological and chemical properties of cryoproteins in acute post-streptococcal glomerulonephritis. Int Arch Allergy Appl Immunol 41: 700–715, 1971 [DOI] [PubMed] [Google Scholar]
- 14.Zlotnick A, Slavin S, Eliakim M: Mixed cryoglobulinemia with a monoclonal IgM component, associated with chronic liver disease. Isr J Med Sci 8: 1968–1979, 1972 [PubMed] [Google Scholar]
- 15.Hansson UB, Lindström FD: Some factors affecting precipitation and complex formation of an IgG cryoglobulin. Clin Exp Immunol 14: 427–435, 1973 [PMC free article] [PubMed] [Google Scholar]
- 16.Gyotoku Y, Abdelmoula M, Spertini F, Izui S, Lambert PH: Cryoglobulinemia induced by monoclonal immunoglobulin G rheumatoid factors derived from autoimmune MRL/MpJ-lpr/lpr mice. J Immunol 138: 3785–3792, 1987 [PubMed] [Google Scholar]
- 17.Lemoine R, Berney T, Shibata T, Fulpius T, Gyotoku Y, Shimada H, Sawada S, Izui S: Induction of “wire-loop” lesions by murine monoclonal IgG3 cryoglobulins. Kidney Int 41: 65–72, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Berney T, Fulpius T, Shibata T, Reininger L, Van Snick J, Shan H, Weigert M, Marshak-Rothstein A, Izui S: Selective pathogenicity of murine rheumatoid factors of the cryoprecipitable IgG3 subclass. Int Immunol 4: 93–99, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Fulpius T, Spertini F, Reininger L, Izui S: Immunoglobulin heavy chain constant region determines the pathogenicity and the antigen-binding activity of rheumatoid factor. Proc Natl Acad Sci U S A 90: 2345–2349, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moll S, Menoud PA, Fulpius T, Pastore Y, Takahashi S, Fossati L, Vassalli JD, Sappino AP, Schifferli JA, Izui S: Induction of plasminogen activator inhibitor type 1 in murine lupus-like glomerulonephritis. Kidney Int 48: 1459–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Reininger L, Berney T, Shibata T, Spertini F, Merino R, Izui S: Cryoglobulinemia induced by a murine IgG3 rheumatoid factor: Skin vasculitis and glomerulonephritis arise from distinct pathogenic mechanisms. Proc Natl Acad Sci U S A 87: 10038–10042, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izui S, Fulpius T, Reininger L, Pastore Y, Kobayakawa T: Role of neutrophils in murine cryoglobulinemia. Inflamm Res 47[Suppl 3]: S145–S150, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Mizuochi T, Hamako J, Nose M, Titani K: Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice. J Immunol 145: 1794–1798, 1990 [PubMed] [Google Scholar]
- 24.Takahashi S, Nose M, Sasaki J, Yamamoto T, Kyogoku M: IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol 147: 515–519, 1991 [PubMed] [Google Scholar]
- 25.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, Takeuchi F, Nagano Y, Miyamoto T, Kobata A: Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316: 452–457, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Parekh R, Isenberg D, Rook G, Roitt I, Dwek R, Rademacher T: A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun 2: 101–114, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB: Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med 1: 237–243, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Kaneko Y, Nimmerjahn F, Ravetch JV: Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313: 670–673, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS: Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol 44: 1524–1534, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV: Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320: 373–376, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keusch J, Lydyard PM, Delves PJ: The effect on IgG glycosylation of altering β1, 4-galactosyltransferase-1 activity in B cells. Glycobiology 8: 1215–1220, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R: Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fcγ receptor I and influence the synthesis of its oligosaccharide chains. J Immunol 157: 4963–4969, 1996 [PubMed] [Google Scholar]
- 33.Jassal R, Jenkins N, Charlwood J, Camilleri P, Jefferis R, Lund J: Sialylation of human IgG-Fc carbohydrate by transfected rat α2,6-sialyltransferase. Biochem Biophys Res Commun 286: 243–249, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Baudino L, Shinohara Y, Nimmerjahn F, Furukawa J-I, Nakata M, Martínez-Soria E, Petry F, Ravetch JV, Nishimura S-I, Izui S: Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J Immunol 181: 6664–6669, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mizuochi T, Pastore Y, Shikata K, Kuroki A, Kikuchi S, Fulpius T, Nakata M, Fossati-Jimack L, Reininger L, Matsushita M, Fujita T, Izui S: Role of galactosylation in the renal pathogenicity of murine immunoglobulin G3 monoclonal cryoglobulins. Blood 97: 3537–3543, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Levo Y, Gorevic PD, Kassab HJ, Zucker-Franklin D, Franklin EC: Association between hepatitis B virus and essential mixed cryoglobulinemia. N Engl J Med 296: 1501–1504, 1977 [DOI] [PubMed] [Google Scholar]
- 37.Levo Y, Gorevic PD, Kassab HJ, Tobias H, Franklin EC: Liver involvement in the syndrome of mixed cryoglobulinemia. Ann Intern Med 87: 287–292, 1977 [DOI] [PubMed] [Google Scholar]
- 38.Van Lenten L, Ashwell G: The binding of desialylated glycoproteins by plasma membranes of rat liver. Development of a quantitative inhibition assay. J Biol Chem 247: 4633–4640, 1972 [PubMed] [Google Scholar]
- 39.Marshall JS, Williams S, Jones P, Hepner GW: Serum desialylated glycoproteins in patients with hepatobiliary dysfunction. J Lab Clin Med 92: 30–37, 1978 [PubMed] [Google Scholar]
- 40.Strevey J, Beaulieu AD, Ménard C, Valet JP, Latulippe L, Hébert J: The role of fibronectin in the cryoprecipitation of monoclonal cryoglobulins. Clin Exp Immunol 55: 340–346, 1984 [PMC free article] [PubMed] [Google Scholar]
- 41.Villar M, García-Bragado F, Joven J, Biosca M, Antolin M, Rodrigo MJ, Schwartz S: Cryoglobulinemia and fibronectin. Acta Haematol 74: 161–163, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Blain H, Cacoub P, Musset L, Costedoat-Chalumeau N, Silberstein C, Chosidow O, Godeau P, Frances C, Piette JC: Cryofibrinogenaemia: A study of 49 patients. Clin Exp Immunol 120: 253–260, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii H, Nakatani K, Arita N, Ito MR, Terada M, Miyazaki T, Yoshida M, Ono M, Fujiwara T, Saiga K, Ota T, Ohtani H, Lockwood M, Sasaki T, Nose M: Internalization of antibodies by endothelial cells via fibronectin implicating a novel mechanism in lupus nephritis. Kidney Int 64: 1662–1670, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Rademacher TW, Williams P, Dwek RA: Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A 91: 6123–6127, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nimmerjahn F, Anthony RM, Ravetch JV: Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A 104: 8433–8437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe N, Akikusa B, Park SY, Ohno H, Fossati L, Vecchietti G, Gessner JE, Schmidt RE, Verbeek JS, Ryffel B, Iwamoto I, Izui S, Saito T: Mast cells induce autoantibody-mediated vasculitis syndrome through tumor necrosis factor production upon triggering Fcγ receptors. Blood 94: 3855–3863, 1999 [PubMed] [Google Scholar]
- 47.Fulpius T, Lemoine R, Berney T, Pastore Y, Moll S, Izui S: Polymorphonuclear leukocytes play a key role in the generation of “wire-loop” lesions induced by a murine IgG3 rheumatoid factor. Kidney Int 49: 647–655, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Neuberger MS: Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J 2: 1373–1378, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon T, Rajewsky K: ‘Enhancer-constitutive’ vectors for the expression of recombinant antibodies. Nucleic Acids Res 16: 354, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura S-I, Niikura K, Kurogochi M, Matsushita T, Fumoto M, Hinou H, Kamitani R, Nakagawa H, Deguchi K, Miura N, Monde K, Kondo H: High-throughput protein glycomics: Combined use of chemoselective glycoblotting and MALDI-TOF/TOF mass spectrometry. Angew Chem Int Ed Engl 44: 91–96, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Furukawa J-I, Shinohara Y, Kuramoto H, Miura Y, Shimaoka H, Kurogochi M, Nakano M, Nishimura S-I: Comprehensive approach to structural and functional glycomics based on chemoselective glycoblotting and sequential tag conversion. Anal Chem 80: 1094–1101, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Kita Y, Miura Y, Furukawa J, Nakano M, Shinohara Y, Ohno M, Takimoto A, Nishimura S-I: Quantitative glycomics of human whole serum glycoproteins based on the standardized protocol for liberating N-glycans. Mol Cell Proteomics 6: 1437–1445, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Miura Y, Shinohara Y, Furukawa J, Nagahori N, Nishimura S: Rapid and simple solid-phase esterification of sialic acid residues for quantitative glycomics by mass spectrometry. Chemistry 13: 4797–4804, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Uematsu R, Furukawa J-I, Nakagawa H, Shinohara Y, Deguchi K, Monde K, Nishimura S-I: High throughput quantitative glycomics and glycoform-focused proteomics of murine dermis and epidermis. Mol Cell Proteomics 4: 1977–1989, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Kikuchi S, Pastore Y, Fossati-Jimack L, Kuroki A, Yoshida H, Fulpius T, Araki K, Takahashi S, Lemoine R, Reininger L, Izui S: A transgenic mouse model of autoimmune glomerulonephritis and necrotizing arteritis associated with cryoglobulinemia. J Immunol 169: 4644–4650, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.