Abstract
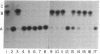
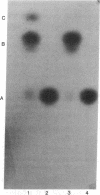
The Escherichia coli R factor-derived chloramphenicol resistance (camr) gene is functionally expressed in the yeast Saccharomyces cerevisiae. the gene was introduced by transformation into yeast cells as part of a chimeric plasmid, pYT11-LEU2, constructed in vitro. The plasmide vector consists of the E. coli plasmid pBR325 (carrying the camr gene), the yeast 2-micron DNA plasmid, and the yeast LEU2 structural gene. Yeast cells harboring pYT11-LEU2 acquire resistance to chloramphenicol and cell-free extracts prepared from such cells contain chloramphenicol acetyltransferase (acetyl-CoA: chloramphenicol 3-O-acetyltransferase, EC 2.3.1.28), the enzyme specified by the camr gene in E. coli. Resistance to chloramphenicol and the presence of chloramphenicol acetyltransferase activity segregate with the yeast marker LEU2, carried by the transforming plasmid, during both mitotic growth and meiotic division.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Lansman R. A., Clayton D. A., Cohen S. N. Studies of mouse mitochondrial DNA in Escherichia coli: structure and function of the eucaryotic-procaryotic chimeric plasmids. Cell. 1975 Oct;6(2):231–244. doi: 10.1016/0092-8674(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Markin J. S. Molecular cloning and expression in E. coli of a yeast gene coding for beta-galactosidase. Cell. 1978 Sep;15(1):123–130. doi: 10.1016/0092-8674(78)90088-0. [DOI] [PubMed] [Google Scholar]
- Gerbaud C., Fournier P., Blanc H., Aigle M., Heslot H., Guerineau M. High frequency of yeast transformation by plasmids carrying part or entire 2-micron yeast plasmid. Gene. 1979 Mar;5(3):233–253. doi: 10.1016/0378-1119(79)90080-5. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Degelmann A., Kustermann-Kuhn B., Royer H. D. Characterization of 2-mum DNA of Saccharomyces cerevisiae by restriction fragment analysis and integration in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2072–2076. doi: 10.1073/pnas.73.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Chang A. C., Houseman D., Cohen S. N. Isolation of histone genes from unfractionated sea urchin DNA by subculture cloning in E. coli. Nature. 1975 Jun 12;255(5509):533–538. doi: 10.1038/255533a0. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Bouanchaud D. H., Goldstein F. W. Mechanism of transferable resistance to chloramphenicol in Haemophilus parainfluenzae. Antimicrob Agents Chemother. 1978 Feb;13(2):326–330. doi: 10.1128/aac.13.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. The reactivity of sulfhydryl groups at the active site of an F-factor--specified variant of chloramphenicol acetyltransferase. Eur J Biochem. 1978 Feb;83(2):553–562. doi: 10.1111/j.1432-1033.1978.tb12123.x. [DOI] [PubMed] [Google Scholar]