Abstract
The pulmonary vasculature is an important site of renin-angiotensin metabolism. While angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers (collectively AIABs) have a role in left ventricular (LV) disease, the impact of AIABs on right ventricular (RV) function is unknown. AIAB use was determined by medication inventory during the Multi-Ethnic Study of Atherosclerosis baseline examination. RV measures were obtained via cardiac magnetic resonance imaging. The relationship between AIAB use and RV measures was assessed using multivariable linear regression, stratified by race/ethnicity, and adjusted for multiple covariates. AIAB use was associated with lower RV mass (-0.7 g, 95% confidence interval [CI] -1.3 to -0.1, P=0.03) in African Americans (N=1012) after adjustment for multiple covariates including LV mass. Among Caucasians (N=1591), AIAB use was associated with larger RV end-diastolic volume (3.7 mL, 95% CI 0.7-6.8, P=0.02) after adjustment for LV volume. No significant associations were seen between AIAB use and other RV measures or in Hispanic or Chinese American participants. AIAB use was associated with RV morphology in a race-specific and LV-independent manner, suggesting the renin-angiotensin system may play a unique role in RV structure and function. The use of AIABs in those with RV dysfunction warrants further study.
Keywords: angiotensin-converting enzyme inhibitor, angiotensin II receptor blockers, right ventricle, epidemiology, renin-angiotensin system
The role of the renin-angiotensin system (RAS) has been well described in left ventricular (LV) structure and function, but its contribution to right ventricle (RV) performance is less well known. The pulmonary capillary bed is a major site of angiotensin I and angiotensin-converting enzyme (ACE) production.[1] Angiotensin II causes proliferation of pulmonary artery smooth muscle cells.[2] ACE2 is a counter-regulatory homolog of ACE that is located in the pulmonary endothelium and leads to vasodilatation and antiproliferation.[3,4]
Increased RAS activity has been demonstrated in various animal models of pulmonary hypertension (PH). Interventions targeting RAS prevent RV morphologic changes in the setting of pulmonary vascular disease.[5–7] For example, ACE2 augmentation prevents or reverses PH and subsequent increases in RV mass in monocrotaline animal models.[5,6] Increased pulmonary endothelial ACE activity has been documented in the explanted lungs of pulmonary arterial hypertension (PAH) patients, and ACE2 expression increases in failing RVs of cardiac transplant recipients.[8,9] Manipulation of RAS pathways (via ACE inhibition or angiotensin II receptor blockade) could impact pulmonary vascular function and/or RV remodeling. The use of ACE inhibitors or angiotensin II receptor blockers (ARBs; collectively AIABs) in the treatment of pulmonary vascular disease and RV dysfunction is not supported by the small amount of available data, however.[10–12]
We examined the relationship of AIAB use with RV structure and function assessed by cardiac magnetic resonance imaging (MRI) in a large cohort of participants without clinical cardiovascular disease. We hypothesized that AIAB use would be associated with higher RV ejection fraction (RVEF) and larger RV stroke volume (RVSV), lower RV mass, and smaller RV end-diastolic volume (RVEDV), and RV end-systolic volume (RVESV). Preliminary results from this study have been published in the abstract form.[13]
MATERIALS AND METHODS
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in Caucasians, African Americans, Hispanics, and Chinese Americans.[14] In 2000-2002, MESA recruited 6814 subjects aged 45-84 years old from six U.S. communities: Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. Exclusion criteria included clinical cardiovascular disease, weight > 300 lbs, pregnancy, or impediment to long-term participation. The presence of clinical cardiovascular disease was determined at participant screening by questionnaire. Participants were excluded if they answered “yes” to having been diagnosed by a physician with heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, and/or to having undergone any prior cardiovascular procedure. Hypertension and diabetes were not exclusion criteria. The protocols of MESA and studies described herein were approved by the Institutional Review Boards of all collaborating institutions and the National Heart Lung and Blood Institute. The MESA-Right Ventricle Study is an ancillary study that planned for the selection of 4,634 participants with interpretable cardiac MRIs at the baseline examination for measurement of RV morphology, which was completed in 4204. Participants were sampled without regard to demographics, anthropometrics, or other clinical variables.
Cardiac magnetic resonance imaging measures
The cardiac MRI protocol has been described elsewhere.[15] All imaging was performed on 1.5 T magnets with a four-element phased-array surface coil positioned anteriorly and posteriorly and electrocardiographic gating. Imaging consisted of fast gradient echo cine images with temporal resolution ≤ 50 ms.
Methods for interpretation of the LV and RV measures have been previously reported.[15,16] Briefly, RV image analysis was performed by two independent analysts on Windows workstations using QMASS software (v4.2, Medis, The Netherlands). The endocardial and epicardial borders of the RV were traced manually on short-axis cine images at end-diastole and end-systole. Papillary muscles and trabeculae were included in the RV volumes and excluded from RV mass.[17] RVEDV and RVESV were calculated using Simpson's rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at end-diastole as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of the heart (1.05 g/cm3).[15] RVSV was calculated by subtracting RVESV from RVEDV. RVEF was calculated by dividing RVSV by RVEDV. The intra-reader intraclass correlation coefficients (ICCs) from random, blinded re-reads of 229 scans for RV mass was 0.94 and for 230 scans was 0.99, 0.95, and 0.89 for RVEDV, RVESV, and RVEF, respectively. The intra-reader ICC was 0.96 for RVSV. The inter-reader ICCs from random, blinded re-reads of 240 scans for RV mass, RVEDV, RVESV, and RVEF were 0.89, 0.96, 0.94, and 0.80, respectively. The inter-reader ICC for RVSV was 0.93.
Medication use
A validated medication inventory was used to assess medication use at the baseline exam.[18] Participants were asked to bring all containers for medications (prescription and non-prescription) used during the two weeks prior to the baseline visit. Interviewers transcribed all current medication names, strengths, and dosages from participant medication bottles. Participants were queried about actual medication intake during the two weeks prior to the visit, and interviewers recorded the average number of pills taken. In circumstances where no medication was taken, the interviewer coded medication intake as “0.”
Other covariates
Race/ethnicity was self-reported during the baseline exam according to 2000 US Census criteria as race (Caucasian, African American, Chinese American) and ethnicity (Hispanic or non-Hispanic). Standard questionnaires were used to ascertain smoking status and level of education. Height was measured to the nearest 0.1 cm with the participant in stocking feet and weight was measured to the nearest pound with the participant in light clothing using a balanced scale. Resting blood pressure was measured using the Dinamap Monitor PRO 100 (Critikon, Tampa, FL) automated oscillometric device. Hypertension was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or current use of anti-hypertension medication. Fasting blood samples were drawn and sent to a central laboratory for measurement of glucose and lipids. The presence of diabetes mellitus was based on self-reported physician diagnosis or a fasting glucose value > 126 mg/dL, the latter measured by rate reflectance spectrophotometry (Johnson and Johnson Clinical Diagnostics, Inc., Rochester, N.Y.). Fasting glucose of 100-125 mg/dL was considered impaired fasting glucose. Spirometry (forced expiratory volume in 1 s [FEV1], forced vital capacity [FVC], and the FEV1/FVC ratio) was available for 2703 participants.[19]
Statistical analysis
Continuous variables were expressed as means and standard deviations. Categorical variables were expressed as percentages. As response to AIAB therapy differs by race, we decided a priori to stratify all analyses by race/ethnicity.[20] Significant (or borderline significant) interactions between AIAB use and race/ethnicity for RV volumes (P = 0.02 for RVEDV and P = 0.16 for RVESV, respectively) supported this approach.
Multivariate linear regression was used to assess the relationship of AIAB use with each RV parameter. Initial models included age, gender, height, and weight. Adjustment for height and weight avoided the assumptions made in indexing the RV measures to certain parameters of body size (e.g., body surface area), while accounting for differences in body size between participants. Models were further adjusted for hypertension and use of antihypertensive medications, systolic and diastolic blood pressure, creatinine, urine albumin, smoking (status and pack years), diabetes mellitus, impaired glucose tolerance, cholesterol, triglycerides, statin use, level of education, and respective LV parameters (e.g., the model for RVEF was adjusted for LV ejection fraction, and so forth). We assessed for collinearity of the main exposure (AIAB use) with the other blood pressure and treatment variables. Adjustment for LV parameters was performed to account for the contribution of LV abnormalities to RV changes (e.g., increased LV mass causing pulmonary venous hypertension leading to increased RV mass), to account for body size differences, and to examine RV-specific associations. RVSV was not adjusted for LV stroke volume considering the significant interdependence of these measures.
Because the RAS is active in the lung parenchyma and has been implicated in obstructive and restrictive lung diseases, we performed adjustment for lung function in the subgroup with available spirometry (N = 2,703).[6,21] Statistical significance was defined as P < 0.05. Analyses were performed using STATA 10.0 (StataCorp, College Station, Tex.).
RESULTS
MESA enrolled 6,814 participants of whom 5,098 had cardiac MRIs; 5,004 were interpretable for LV morphology (Fig. 1). Of these, 4,634 were selected for RV interpretation, 4,484 were attempted to be read, and 4,204 had RV measures completed. In addition to participants using combination AIABs with diuretics (N=117), we excluded participants missing covariate data (N=96). The final study sample consisted of 3,991 participants, of whom 1,591 (39.9%) were Caucasian, 1,012 (25.3%) were African American, 877 (22.0%) were Hispanic, and 511 (12.8%) were Chinese American. Of participants with available spirometry (N=2703), 964 (35.7%) were Caucasian, 661 (24.4%) were African American, 597 (22.1%) were Hispanic, and 481 (17.8%) were Chinese American.
Figure 1.
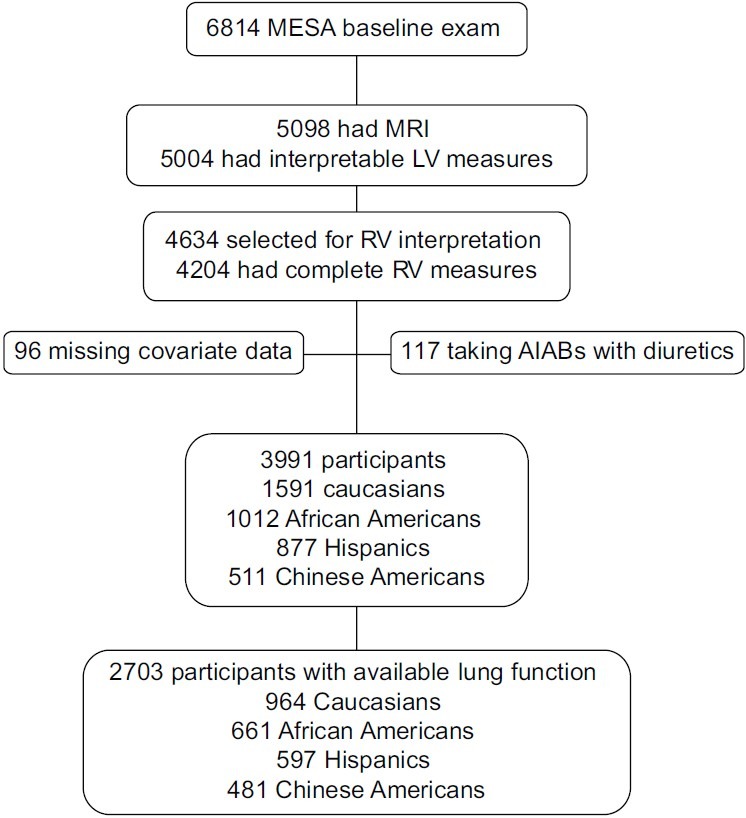
Study sample. MESA: Multi-Ethnic Study of Atherosclerosis; MRI: magnetic resonance imaging; LV: left ventricle; RV: right ventricle; AIABs: angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
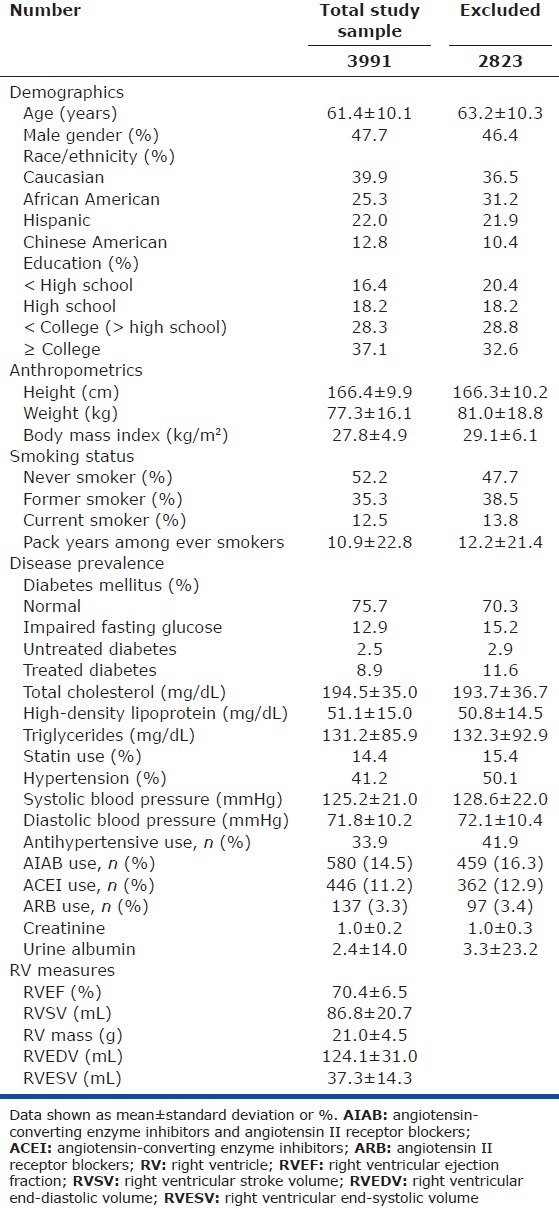
The study sample was similar to those excluded (Table 1). A total of 580 (14.5%) participants in the study sample were using AIABs, the majority of whom were using ACE inhibitors (446 of 580, 78.6%). Characteristics of the study sample stratified by race/ethnicity are shown in Table E1 (39.7KB, pdf) (Access Supplemental Table E1 (39.7KB, pdf) at www.pulmonarycirculation.org). African American participants were more likely to have hypertension and receive antihypertensive medications, including AIABs (details of African American and Caucasian participants stratified by AIAB use are shown in Table E2 (36.5KB, pdf) (Access Supplemental Table E2 (36.5KB, pdf) at www.pulmonarycirculation.org). While there were differences across race/ethnicity in socioeconomic factors (e.g., education) with Caucasians tending to have higher levels of education, within group levels of education across AIAB use were similar. AIAB users were more likely to be using statins, but this did not vary across race/ethnicity. Finally, Caucasian AIAB users were more likely to be prescribed beta-blockers (compared to non-users and African Americans), while African American AIAB users were more likely to be prescribed calcium channel blockers (compared to non-users and Caucasians).
Table 1.
Characteristics of the total study sample and of those participants excluded
Characteristics of the study sample by race/ethnicity
Characteristics of the study sample (Caucasians and African Americans only), by AIAB use
Angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use
In Caucasians, AIAB use was associated with a 3.7 mL larger RVEDV (95% confidence interval [CI] 0.7-6.8 mL, P = 0.02) and possibly a 2.4 mL larger RVSV (95% CI -0.6 to 5.4 mL, P = 0.12; Table 2). The association with RVEDV was strengthened after adjustment for LV end-diastolic volume, implying an RV-specific relationship. There were no associations between AIAB use and RVEF, RV mass, or RVESV in Caucasians.
Table 2.
Associations between AIAB use and RV measures in limited and fully adjusted models, by race/ethnicity
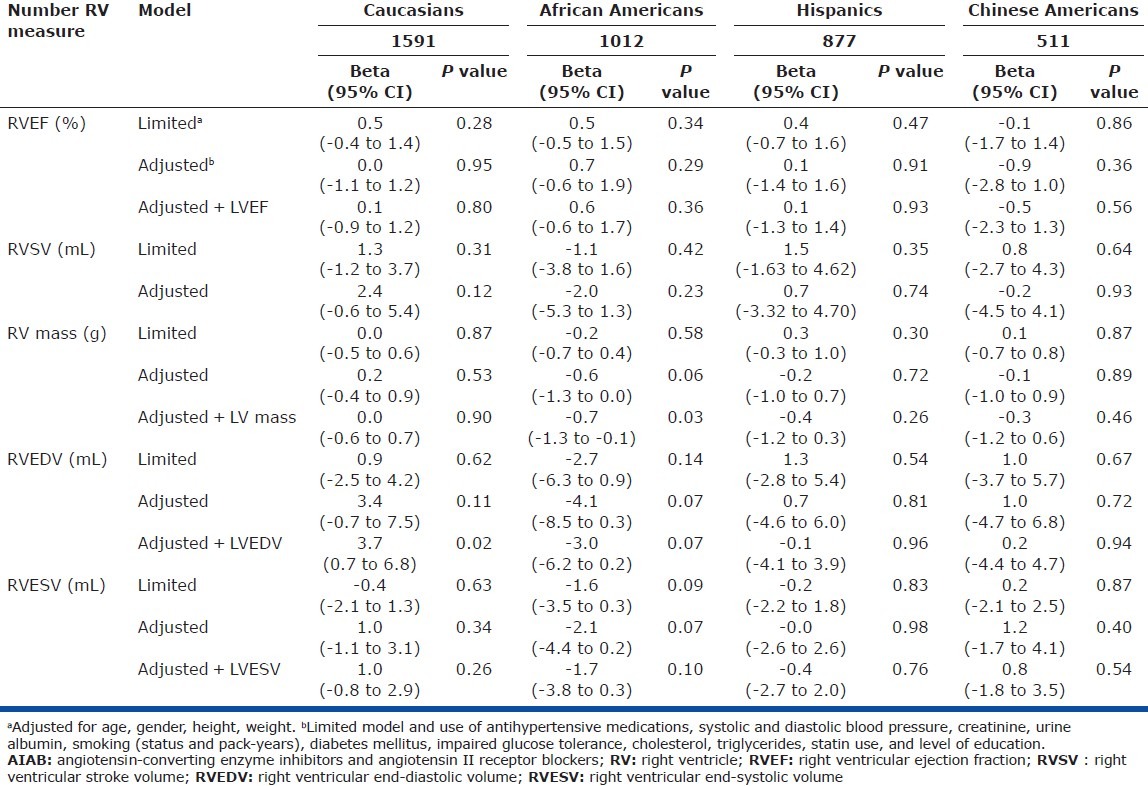
In African Americans, AIAB use was associated with a 0.7 g lower RV mass (95% CI -1.3 to -0.1 g, P = 0.03; Table 2). There was also a suggestion of smaller RVEDV and RVESV with AIAB use after adjustment for LV volumes (P = 0.07 and 0.10, respectively). There were no significant associations seen between AIAB use and RVEF or RVSV in African Americans.
There were no significant associations between AIAB use and RV measures in Hispanic and Chinese American participants (Table 2). Analyses including participants using combination AIABs with diuretics (N = 117) did not alter the results, nor did analyses adjusting for beta-blocker and/or calcium channel blocker use (rather than adjustment for antihypertensive medications in general). Analysis of ACE inhibitor use alone showed similar results (data not shown).
Subgroup with available spirometry
We then assessed the smaller subgroup of participants with available spirometry (N = 2703). In Caucasians (N = 962), the effect estimates of AIAB use and RV morphology were similar to those seen in the total study sample and were not changed after adjustment for FEV1, FVC, and the FEV1/FVC ratio, but did not meet statistical significance likely due to smaller sample size (Table E3 (94.2KB, pdf) [Access Supplemental Table E3 (94.2KB, pdf) at www.pulmonarycirculation.org]). In African Americans in this smaller sample (N = 661), AIAB use was associated with lower RV mass even after adjustment for LV mass (-0.6 g, 95% CI -1.4 to 0.1 g, P = 0.08) and smaller RVEDV (-4.3 mL, 95% CI -8.1 to -0.5 mL, P = 0.03). These effect estimates were unchanged after adjustment for spirometry. No associations were seen between AIAB use and any RV measures in Hispanic (N = 597) or Chinese American (N = 481) participants.
Associations between AIAB use and RV measures in fully adjusted models among participants with available spirometry, by race/ethnicity
DISCUSSION
We found modest race-specific associations between AIAB use and measures of RV morphology in a large cohort of participants without clinical cardiovascular disease. In Caucasians, AIAB use was associated with larger RVEDV and possibly larger RVSV while among African Americans AIAB use was associated with lower RV mass and possibly smaller RV volumes. These findings persisted even after adjustment for respective LV measures and spirometry, implying that RAS manipulation may uniquely impact RV morphology independent of effects on the LV and pulmonary function. To our knowledge, this is the largest study of AIAB use and RV structure and function measured by cardiac MRI.
Several small studies have shown acute hemodynamic benefits of AIABs in patients with pulmonary vascular disease (particularly hypoxia-related PH), but RV effects are largely unknown.[22–25] In a single study of four patients with PAH, captopril improved radionuclide measured RVEF, but it is difficult to know whether this improvement was driven by systemic afterload reduction and concomitantly increased LV ejection fraction.[22] The impact of AIABs on RV morphology has been studied in patients with systemic RVs from congenital heart disease with mixed results.[26–28] In the largest randomized trial (N = 17), Therrien and colleagues demonstrated that ramipril had no impact on RVEF, mass, or volumes in adults with moderate systemic RV dysfunction.[27] Our study is the first to suggest that AIABs may have a role in RV modeling in the absence of clinical pulmonary or systemic vascular disease and ventricular dysfunction. While the effect sizes are modest, AIAB therapy leads to comparable effects in the LV after two years.[29] In severe PAH, long-term intravenous epoprostenol decreases RVEDV by on average 8 mL but leads to significant improvements in exercise capacity, functional status, and survival.[30,31] Similarly, it has been shown that a 10 mL change in RVSV during follow-up is a clinically relevant change for patients with pulmonary vascular disease.[32] In normal individuals, similar or smaller differences (in absolute or relative terms) may therefore have important physiologic effects.
It is surprising that AIABs may have an association with lower RV mass in African Americans but not in Caucasians. African Americans have greater LV mass and more LV hypertrophy as compared to Caucasians among healthy individuals and those with systemic hypertension.[33–36] While electrocardiographic LV hypertrophy has been shown to regress over time in both whites and non-whites treated with ARB-based therapy, African Americans are more likely to have persistent echocardiographic LV remodeling/hypertrophy despite antihypertensive therapy, and these changes predict future cardiovascular events.[37,38] Moreover, African Americans with heart failure treated with ACE inhibitors have a greater risk of clinical progression, worsening LV dysfunction, and death as compared to Caucasians.[20,39,40] It has been suggested that these race-specific differences may be due to innate differences in neurohumoral activation, blood pressure response, and/or genetic variation. African Americans may have greater nitric oxide deficits and endothelial damage than Caucasians (and therefore may be less likely to respond to RAS manipulation).[40,41] In the RV, we have shown that African Americans have lower RV mass and less RV hypertrophy when compared to Caucasians after adjustment for LV mass, suggesting an RV-specific relationship. PAH therapy (such as endothelin-receptor antagonists) appears to be less efficacious in African Americans than in whites, possibly due to disparate effects on the RV.[42] Taken together, these observations suggest that AIAB therapy may (1) distinctly impact the RV as compared to the LV, and (2) that these relationships not only vary by race-ethnicity but are unlike those observed in the LV, perhaps due to differential adaptation to pressure loading and alterations in the genetic signature.[43]
Genetic variation in the ACE gene results in altered levels of cardiac ACE activity and the insertion/deletion polymorphism (ACE I/D) has been associated with LV hypertrophy, LV dysfunction, and mortality in heart failure.[44–46] While homozygotes for the deletion allele (ACE DD) are at increased risk for PAH, the ACE DD genotype appears to protect against RV dysfunction and preserve cardiac output.[47,48] Genotype frequency is known to vary by race/ethnicity, with the ACE DD genotype more commonly occurring in African Americans than the ACE I/D or ACE insertion/insertion genotypes.[49] It is possible that ACE gene interactions may explain the race/ethnicity differences seen here and that AIAB therapy may have pleiotropic effects on the pulmonary vasculature and the RV.
Whether the constellation of RV findings demonstrated (i.e., larger end-diastolic and stroke volumes in Caucasians vs. lower mass and volumes in African Americans) could be adaptive in individuals without clinical cardiovascular disease is not known. In the LV, moderate post-infarct dilatation has been proposed to be compensatory and may preserve stroke volume, yet large end-systolic volumes predict poor outcomes in high-risk patients.[29,50] In patients with systemic hypertension, lower LV stroke volume during antihypertensive treatment has been shown to predict cardiovascular risk, independent of LV morphology.[51] AIAB therapy in patients with cardiovascular disease has been shown to reduce LV mass and volumes, while exercise-induced increases in LV mass in trained athletes and in RV mass in MESA participants are believed to be adaptive.[29,37,38,52]
Our study has several limitations. No conclusions can be drawn about causality since this study was cross-sectional in nature. While we adjusted for multiple covariates, unmeasured or residual confounding is still possible, specifically confounding by indication. However, if present, we would expect such confounding effects of AIABs on both the RV and LV, so that adjustment for LV measures should eliminate the RV findings. This was not the case, as the associations between AIABs and RV morphology persisted despite LV adjustment, making confounding by indication less likely to explain our results. The duration of treatment with AIABs was not assessed. Some participants could have been treated for years with these medications, and others for only a short period of time. However, such misclassification would likely bias to the null (e.g., a participant treated for a few months before the baseline study visit would be unlikely to have any effects on RV morphology, yet would still be classified as an AIAB user). Therefore, it is possible that we have underestimated the associations between AIAB use and RV morphology. Finally, stratifying by race/ethnicity created smaller sample sizes in the Hispanic and Chinese American groups, likely limiting our power to detect associations.
We have shown that AIAB use is associated with RV mass and volumes in a race-specific and LV-independent manner in a large cohort of participants free of cardiovascular disease. The potential benefits of AIAB use in the setting of pulmonary vascular disease and in individuals with (or at risk for) RV dysfunction are unknown and warrant future study in randomized clinical trials.
ACKNOWLEDGMENTS
This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications. Significant comments have been incorporated prior to submission for publication. The authors thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Source of Support: National Institutes of Health R01-HL086719, R01-HL077612, N01-HC95159 through N01-HC95169, American Heart Association 11FTF7400032, ASPIRE Pulmonary Vascular Disease Young Investigator Research Award WS1952812.
Conflict of Interest: None declared.
REFERENCES
- 1.Orfanos SE, Langleben D, Khoury J, Schlesinger RD, Dragatakis L, Roussos C, et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in humans. Circulation. 1999;99:1593–9. doi: 10.1161/01.cir.99.12.1593. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–94. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 4.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–28. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 5.Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, Francis J, Yamazato M, et al. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension. 2009;54:365–71. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Diez-Freire C, Dooies A, et al. The ace2/ang-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am J Respir Crit Care Med. 2010;182:1065–72. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada M, Harada T, Kikuzuki R, Yamawaki H, Hara Y. Effects of telmisartan on right ventricular remodeling induced by monocrotaline in rats. J Pharmacol Sci. 2009;111:193–200. doi: 10.1254/jphs.09112fp. [DOI] [PubMed] [Google Scholar]
- 8.Schuster DP, Crouch EC, Parks WC, Johnson T, Botney MD. Angiotensin converting enzyme expression in primary pulmonary hypertension. Am J Respir Crit Care Med. 1996;154:1087–91. doi: 10.1164/ajrccm.154.4.8887612. [DOI] [PubMed] [Google Scholar]
- 9.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, et al. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: Evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation. 2003;108:1707–12. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 10.Leier C, Bambach D, Nelson S, Hermiller J, Huss P, Magorien R, et al. Captopril in primary pulmonary hypertension. Circulation. 1983;67:155–61. doi: 10.1161/01.cir.67.1.155. [DOI] [PubMed] [Google Scholar]
- 11.Rich S, Martinez J, Lam W, Rosen KM. Captopril as treatment for patients with pulmonary hypertension. Problem of variability in assessing chronic drug treatment. Br Heart J. 1982;48:272–7. doi: 10.1136/hrt.48.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielinski J, Hawrylkiewicz I, Gorecka D, Gluskowski J, Koscinska M. Captopril effects on pulmonary and systemic hemodynamics in chronic cor pulmonale. Chest. 1986;90:562–5. doi: 10.1378/chest.90.4.562. [DOI] [PubMed] [Google Scholar]
- 13.Ventetuolo CE, Bagiella E, Barr RG, Bluemke DA, Bristow MR, Chahal H, et al. Angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use and right ventricular structure and function: The MESA-Right Ventricle Study. Am J Respir Crit Care Med. 2010;181:A4854. [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: Normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 16.Chahal H, Johnson W, Tandri H, Jain A, Hundley W, Barr R, et al. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2010;106:110–6. doi: 10.1016/j.amjcard.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, Pearson G, et al. Left ventricular papillary muscle mass: Relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comp Assist Tomogr. 2006;30:426–32. doi: 10.1097/00004728-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Psaty B, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medication in the elderly: methods and initial experience in the Cardiovascular Health Study: The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 19.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–27. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. New Engl J Med. 2001;344:1351–7. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- 21.Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: Their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18:3506–15. doi: 10.2174/092986711796642562. [DOI] [PubMed] [Google Scholar]
- 22.Ikram H, Maslowski AH, Nicholls MG, Espiner EA, Hull FT. Haemodynamic and hormonal effects of captopril in primary pulmonary hypertension. Br Heart J. 1982;48:541–5. doi: 10.1136/hrt.48.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boschetti E, Tantucci C, Cocchieri M, Fornari G, Grassi V, Sorbini CA. Acute effects of captopril in hypoxic pulmonary hypertension. Comparison with transient oxygen administration. Respiration. 1985;48:296–302. doi: 10.1159/000194843. [DOI] [PubMed] [Google Scholar]
- 24.Bertoli L, Lo Cicero S, Busnardo I, Rizzato G, Montanari G. Effects of captopril on hemodynamics and blood gases in chronic obstructive lung disease with pulmonary hypertension. Respiration. 1986;49:251–6. doi: 10.1159/000194887. [DOI] [PubMed] [Google Scholar]
- 25.Peacock AJ, Matthews A. Transpulmonary angiotensin II formation and pulmonary haemodynamics in stable hypoxic lung disease: The effect of captopril. Respir Med. 1992;86:21–6. doi: 10.1016/s0954-6111(06)80143-5. [DOI] [PubMed] [Google Scholar]
- 26.Lester SJ, McElhinney DB, Viloria E, Reddy GP, Ryan E, Tworetzky W, et al. Effects of losartan in patients with a systemically functioning morphologic right ventricle after atrial repair of transposition of the great arteries. Am J Cardiol. 2001;88:1314–6. doi: 10.1016/s0002-9149(01)02098-7. [DOI] [PubMed] [Google Scholar]
- 27.Therrien J, Provost Y, Harrison J, Connelly M, Kaemmerer H, Webb GD. Effect of angiotensin receptor blockade on systemic right ventricular function and size: A small, randomized, placebo-controlled study. Int J Cardiol. 2008;129:187–92. doi: 10.1016/j.ijcard.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Hechter SJ, Fredriksen PM, Liu P, Veldtman G, Merchant N, Freeman M, et al. Angiotensin-converting enzyme inhibitors in adults after the Mustard procedure. Am J Cardiol. 2001;87:660–3. doi: 10.1016/s0002-9149(00)01452-1. [DOI] [PubMed] [Google Scholar]
- 29.Cowan B, Young A, Anderson C, Doughty R, Krittayaphong R, Lonn E, et al. Left ventricular mass and volume with telmisartan, ramipril, or combination in patients with previous atherosclerotic events or with diabetes mellitus (from the ONgoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial [ONTARGET]) Am J Cardiol. 2009;104:1484–9. doi: 10.1016/j.amjcard.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Roeleveld RJ, Vonk-Noordegraaf A, Marcus JT, Bronzwaer JG, Marques KM, Postmus PE, et al. Effects of epoprostenol on right ventricular hypertrophy and dilatation in pulmonary hypertension. Chest. 2004;125:572–9. doi: 10.1378/chest.125.2.572. [DOI] [PubMed] [Google Scholar]
- 31.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. The Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 32.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139:1003–9. doi: 10.1378/chest.10-1066. [DOI] [PubMed] [Google Scholar]
- 33.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women: The CARDIA Study. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 34.Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African-American and white young adults: The CARDIA study. J Am Coll Cardiol. 2003;41:955–60. doi: 10.1016/s0735-1097(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 35.Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, et al. Differences in left ventricular structure between black and white hypertensive adults. Hypertension. 2004;43:1182–8. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez CJ, Diez-Roux AV, Moran A, Jin Z, Kronmal RA, Lima J, et al. Left ventricular mass and ventricular remodeling among hispanic subgroups compared with non-hispanic blacks and whites: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:234–42. doi: 10.1016/j.jacc.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Trial. Circulation. 2003;108:684–90. doi: 10.1161/01.CIR.0000083724.28630.C3. [DOI] [PubMed] [Google Scholar]
- 38.Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlöf B, Devereux RB. Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the LIFE study) Eur J Echocardiogr. 2008;9:809–15. doi: 10.1093/ejechocard/jen155. [DOI] [PubMed] [Google Scholar]
- 39.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. New Engl J Med. 1999;340:609–16. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 40.Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: Analysis of the vasodilator-heart failure trials. J Card Fail. 1999;5:178–87. doi: 10.1016/s1071-9164(99)90001-5. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Ferdinand K, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. New Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 42.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–6. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urashima T, Zhao M, Wagner R, Fajardo G, Farahani S, Quertermous T, et al. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol. 2008;295:H1351–68. doi: 10.1152/ajpheart.91526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, Riegger GA, et al. Angiotensin-converting enzyme in the human heart: Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–8. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 45.Pinto YM, van Gilst WH, Herre Kingma J, Schunkert H. Deletion-type allele of the angiotensin-converting enzyme gene is associated with progressive ventricular dilation after anterior myocardial infarction. J Am Coll Cardiol. 1995;25:1622–6. doi: 10.1016/0735-1097(95)00090-q. [DOI] [PubMed] [Google Scholar]
- 46.Andersson B, Sylven C. The DD genotype of the angiotensin-converting enzyme gene is associated with increased mortality in idiopathic heart failure. J Am Coll Cardiol. 1996;28:162–7. doi: 10.1016/0735-1097(96)00098-8. [DOI] [PubMed] [Google Scholar]
- 47.Abraham WT, Raynolds MV, Gottschall B, Badesch DB, Wynne KM, Groves BM, et al. Importance of angiotensin-converting enzyme in pulmonary hypertension. Cardiology. 1995;86:9–15. doi: 10.1159/000176939. [DOI] [PubMed] [Google Scholar]
- 48.Abraham WT, Raynolds MV, Badesch DB, Wynne KM, Groves BM, Roden RL, et al. Angiotensin-converting enzyme DD genotype in patients with primary pulmonary hypertension: increased frequency and association with preserved haemodynamics. J Renin Angiotensin Aldosterone Syst. 2003;4:27–30. doi: 10.3317/jraas.2003.003. [DOI] [PubMed] [Google Scholar]
- 49.Arnett DK, Davis BR, Ford CE, Boerwinkle E, Leiendecker-Foster C, Miller MB, et al. Pharmacogenetic association of the angiotensin-converting enzyme insertion/deletion polymorphism on blood pressure and cardiovascular risk in relation to antihypertensive treatment: The Genetics of Hypertension-Associated Treatment (GenHAT) Study. Circulation. 2005;111:3374–83. doi: 10.1161/CIRCULATIONAHA.104.504639. [DOI] [PubMed] [Google Scholar]
- 50.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–63. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 51.Lønnebakken MT, Gerdts E, Boman K, Wachtell K, Dahlöf B, Devereux RB. In-treatment stroke volume predicts cardiovascular risk in hypertension. J Hypertens. 2011;29:1508–14. doi: 10.1097/HJH.0b013e32834921fb. [DOI] [PubMed] [Google Scholar]
- 52.Aaron C, Tandri H, Barr R, Johnson C, Bagiella E, Chahal H, et al. Physical activity and right ventricular structure and function: The MESA-Right Ventricle Study. Am J Respir Crit Care Med. 2011;183:396–404. doi: 10.1164/rccm.201003-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the study sample by race/ethnicity
Characteristics of the study sample (Caucasians and African Americans only), by AIAB use
Associations between AIAB use and RV measures in fully adjusted models among participants with available spirometry, by race/ethnicity


