Summary
In this review, we discuss how working memory prefrontal cortical (PFC) circuits are modulated differently than plastic changes in sensory/motor and subcortical circuits. Working memory arises from recurrent excitation within layer III PFC pyramidal cell NMDA circuits, which are afflicted in aging and schizophrenia. Neuromodulators rapidly and flexibly alter the efficacy of these synaptic connections, while leaving the synaptic architecture unchanged, a process called Dynamic Network Connectivity (DNC). Increases in calcium-cAMP signaling open ion channels in long, thin spines, gating network connections. Inhibition of calcium-cAMP signaling, e.g. by noradrenergic α2A-adrenoceptor stimulation on spines, strengthens synaptic efficacy and increases network firing, while optimal levels of dopamine D1 receptor stimulation sculpt network inputs to refine mental representation. Generalized increases in calcium-cAMP signaling during fatigue or stress disengage dlPFC recurrent circuits, reduce firing and impair top-down cognition. Impaired DNC regulation contributes to age-related cognitive decline, while genetic insults to DNC proteins are commonly linked to schizophrenia.
The highly evolved neuronal networks of the dorsolateral prefrontal cortex (dlPFC) subserve working memory, our “mental sketch pad”, by representing information in the absence of sensory stimulation. The importance of the dlPFC to working memory was first discovered by Jacobsen (Jacobsen, 1936), who wrote that in monkeys with dlPFC lesions “It is as if ‘out of sight, out of mind’ were literally applicable”. The representational properties of the dlPFC arise from extensive neural connections that have greatly expanded in human evolution (Fig. 1A,C). These circuits engage in an ever-changing, intricate pattern of network activation that underlies the contents of thought, and provides top-down regulation of attention, action and emotion (Fuster, 2009). Multiple neuromodulatory arousal systems project to the dlPFC, and we are now learning that neuromodulation plays an essential role in shaping the contents of our “mental sketch pad”, thus coordinating arousal state with cognitive state (Arnsten et al., 2010). The critical modulatory role of the catecholamines to dlPFC function was first discovered by Brososki et al. as early as 1979, when they showed that depletion of catecholamines from the dlPFC was as detrimental as ablating the dlPFC itself (Brozoski et al., 1979). More recent physiological research has shown that neuromodulators can rapidly alter the strength of dlPFC network firing on a timescale of seconds, through powerful influences on the open states of ion channels residing near network synapses, a process called Dynamic Network Connectivity (DNC) (Arnsten et al., 2010). This work has shown that the highly evolved circuits of dlPFC are often modulated in a fundamentally different manner than sensory/motor or subcortical circuits, providing great flexibility in the pattern and strength of network connections. These neuromodulatory processes allow moment-by-moment changes in synaptic strength without alterations in underlying architecture, and can bring circuits “on-line” or “off-line” based on arousal state, thus coordinating the neural systems in control of behavior, thought and emotion. However, this extraordinary flexibility also confers great vulnerability, and errors in this process likely contribute to cognitive deficits in disorders such as schizophrenia. The following review provides an overview of this emerging field, and describes how genetic and environmental insults to DNC contribute to cognitive deficits in mental illness and in advancing age. Understanding and respecting these actions will be key for the development of effective treatments for higher cognitive disorders in humans.
Figure 1.
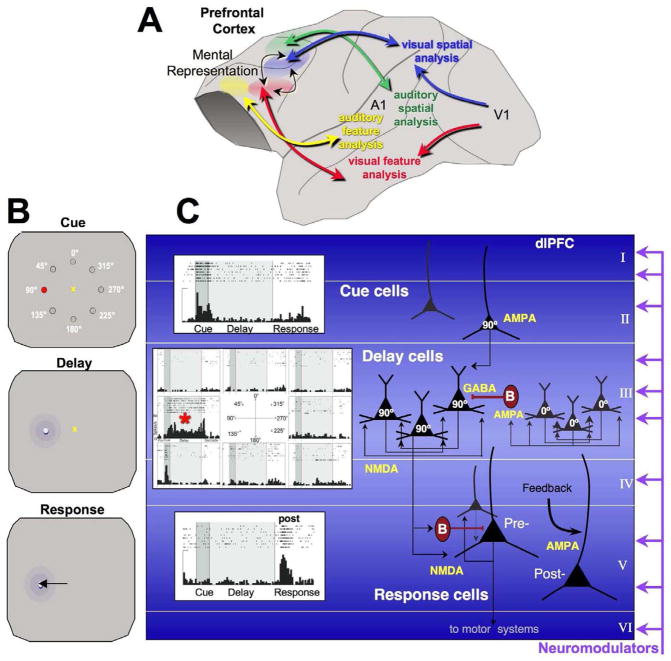
A. The reciprocal, parallel, visual and auditory cortical networks connecting with dlPFC. More ventral PFC regions connect with limbic areas subserving emotion (not shown). This figure is based on the work of Goldman-Rakic, as summarized in Arnsten, 2003 (Arnsten, 2003). B. The oculomotor delayed response (ODR) test of spatial working memory. For each trial, the monkey fixates on a central point, initiating the brief presentation (0.5s) of a cue in 1 of 8 locations. A delay period ensues (2.5–8s) in which the monkey must remember the spatial location until the fixation point extinguishes, and the monkey must make a saccade to the correct location to receive juice reward. The cued position randomly changes over hundred of trials, thus generating high levels of proactive interference. C. Cue, Delay and Response cells recorded from area 46 of the monkey dlPFC as a monkey performs the ODR task, and the corresponding microcircuitry thought to underlie physiological responses. (Goldman-Rakic, 1995). Cue cells fire during the presentation of the cue and stop firing during the Delay period. Delay cells often fire to the cue (and/or to the saccadic response), but are noted for their ability to maintain persistent firing across the delay period. Delay cells are usually spatially tuned, firing across the delay period for the neuron’s preferred direction, but decreasing firing for all other nonpreferred directions (the preferred direction for this Delay cell is indicated by a red asterisk). The microcircuits underlying Delay cell firing reside in deep layer III (and possibly in layer V as well) and are described in detail in the text. In contrast to Delay cells, Response cells are often inhibited during the delay period and instead fire leading up to, during, and/or after the motor response, initiating action and/or providing feedback. These neurons are thought to reside in layer V.
The Mental Sketch Pad: The neural basis of representation
The microcircuitry and physiology of spatial working memory
Patricia Goldman-Rakic discovered the neurobiological basis of mental representation through intensive studies of the spatial working memory system in rhesus monkeys (Goldman-Rakic, 1995). Early in her career she defined the subregion of dlPFC surrounding the principal sulcus most needed for visuospatial working memory, and then showed that this region had reciprocal connections with the parietal association cortex specialized for analyzing visuospatial position (Fig. 1A), participating in a distributed visuo-spatial network with the parietal association cortices (Selemon and Goldman-Rakic, 1988). She showed that nearby dlPFC regions received visual feature, auditory feature or auditory spatial inputs, thus extending parallel sensory processing into the dlPFC (Goldman-Rakic, 1987). Interestingly, dlPFC networks are already observed in utero and in very early life, and thus do not require experience to establish connections (Schwartz and Goldman-Rakic, 1991). The physiology of spatial working memory has been studied extensively in monkeys, using tasks such as the one shown in Figure 1B, in which the subject briefly views a spatial cue, and must remember the position over a delay period of several seconds. At the end of the delay period, the monkey can make a hand or eye movement (i.e. a saccade) to the remembered location, and if correct, receive a reward. The position of the cue randomly changes on every trial, creating extensive proactive interference. Neuronal recordings from the visuo-spatial subregion of dlPFC in monkeys performing a spatial working memory task have found a variety of neurons with task-related firing patterns (Fig. 1C). Some neurons have simple sensory or motor properties, e.g. there are Cue cells similar to those in sensory cortex that fire just to the spatial cue, and Response cells that fire in anticipation of or during the motor response (peri- and post-response neurons, respectively. Some peri- and post-saccadic neurons are thought to convey feedback from the eye movement system that an eye movement has taken place, so-called corollary discharge (Wang et al., 2004). Importantly, a large proportion of neurons in the principal sulcal dlPFC show spatially tuned, persistent firing across the delay period when the spatial position must be held in working memory (Funahashi et al., 1989). This contrasts with sensory cortices, where stimulus-evoked neuronal firing ceases once the sensory stimulation has stopped, e.g. (Maier et al., 2008). Thus, dlPFC neurons can represent visual space in the absence of sensory stimulation, the foundation of abstract thought (Funahashi et al., 1989). Goldman-Rakic appreciated that this basic representational operation is the building block of more complex dlPFC operations such as behavioral inhibition and cognitive control (Goldman-Rakic, 1996). Although spatial working memory a relatively “simple” system for revealing how brain represents information in the absence of sensory stimulation, more recent physiological studies show PFC neurons are able to represent e.g. ensuing reward or punishment (Seo and Lee, 2009), and changing rules and goals (Wallis et al., 2001). Thus, understanding the neurobiology of working memory provides an important beginning for understanding more advanced PFC cognitive operations as well.
Our current understanding of the dlPFC microcircuitry underlying spatial working memory, as illustrated in Figure 1C, is based on the work of Goldman-Rakic and colleagues (Goldman-Rakic, 1995). Sensory inputs from the parietal association cortex terminate in columns in the dlPFC (Selemon and Goldman-Rakic, 1988), targeting both superficial and deep layers. Recordings from monkeys doing a similar task suggest that Cue cells reside in the superficial layers (Sawaguchi et al., 1989). Importantly, the persistent firing of Delay cells appears to be generated by the recurrent excitation of glutamatergic pyramidal cell microcircuits in deep layer III (and possibly layer V as well; (Kritzer and Goldman-Rakic, 1995)). Electrophysiological and anatomical studies suggest that nearby neurons with similar spatial tuning excite each other via connections on spines to maintain firing without the need for bottom-up sensory stimulation (Goldman-Rakic, 1995; González-Burgos et al., 2000). Our recent iontophoretic studies have shown that this persistent firing is highly dependent on NMDA receptors, including those with NR2B subunits found exclusively within the synapse (Wang et al., 2011b). These physiological data are consistent with computational models predicting that persistent neuronal firing requires the slower kinetics of the NR2B receptor (Wang, 1999). The spatial tuning of Delay cells is shaped in part by lateral inhibition from GABAergic parvalbumin-containing interneurons (Goldman-Rakic, 1995). GABAergic neurons are excited by pyramidal cell microcircuits with dissimilar tuning, and this synapse appears to rely on AMPA receptors in the adult (Rotaru et al., 2011). These deep layer III microcircuits are greatly afflicted in schizophrenia, with loss of spines and neuropil, and weakening of GABAergic actions e.g. (Glantz and Lewis, 2000; Lewis and Gonzalez-Burgos, 2006; Selemon and Goldman-Rakic, 1999), likely related to profound working memory impairment and thought disorder (Perlstein et al., 2001). Deep layer III pyramidal cells are also an early target of neurofibrillary tangles and neurodegeneration in Alzheimer’s Disease (AD) (Bussière et al., 2003), and likely contribute to early signs of dlPFC dysfunction. Alterations in layer V neurons also contribute to these diseases, and these neurons likely play a variety of roles in the working memory process. In addition to their well-known projections to striatum, some layer V dlPFC neurons also engage in cortico-cortical connections, e.g. engaging in reciprocal connections with the parietal association cortex (Schwartz and Goldman-Rakic, 1984). Layer V neurons also exhibit lateral recurrent connections within the dlPFC, although to a lesser extent than deep layer III (Kritzer and Goldman-Rakic, 1995). Thus, some Delay cells may reside in layer V. It is likely that most Response cells reside in layer V, as they are selectively influenced by dopamine D2 receptors (D2Rs) (Wang et al., 2004), and D2 receptor mRNA is only observed in layer V neurons (Lidow et al., 1998). Interestingly, peri-response cells are very sensitive to NMDA but not AMPA receptor blockade, while post-saccadic response cells show reduced firing with AMPA receptor blockade (Wang et al., 2011b). This may reflect an AMPA-driven proprioceptive feedback from sensory/motor systems, e.g. from the eye movement system via the medial dorsal thalamus. Layer V pyramidal neurons are also afflicted in schizophrenia (Black et al., 2004) and in AD (Bussière et al., 2003), and may contribute to symptoms. For example, alterations in corollary discharge feedback from the PFC are thought to contribute to symptoms of hallucinations (Ford et al., 2002) and errors in feedback may also play a role in delusions (Corlett et al., 2007). Thus, this aspect of dlPFC function deserves further investigation.
Evolutionary influences on deep layer III microcircuitry
The dlPFC expands greatly over evolution, with no exact counterpart in rodents, and an enormous extension from nohuman to human primates (Elston, 2003; Elston et al., 2006; Preuss, 1995; Wise, 2008). Comparisons of dendritic complexity in human vs. animal cortices have shown that the basal dendrites of dlPFC deep layer III pyramidal cells are the ones most increased in primate evolution, with increases in both dendritic complexity and the number of spines (Elston, 2003). Layer III pyramidal cells in the dlPFC have many more spines than their counterparts in primary visual cortex (V1), e.g. an average of 16 times more spines in rhesus dlPFC, and 23 times more spines in the human dlPFC (Elston, 2000). Elston (Elston, 2003) quotes the initial observations of Ramón y Cajal, who first noted these evolutionary changes in pyramidal cells, which he termed “psychic” cells due to their likely function: “In mice the basal dendrites [of pyramidal cells] are short and have few branches, in man they [the basal dendrites] are numerous, long and highly branched……as one ascends the animal scale the psychic cell becomes larger and more complex; it’s natural to attribute this progressive morphological complexity, in part at least, to its progressive functional state”. Or, as Elston concludes: “without these specializations in the structure of pyramidal cells, and the circuits they form, human cognitive processing would not have evolved to its present state.”
Working memory vs. classic neuroplasticity
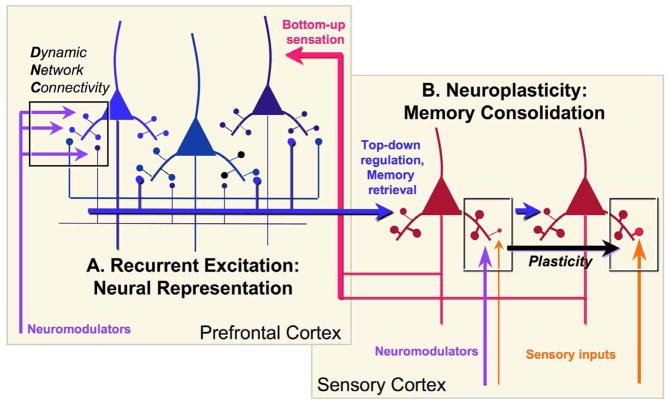
The working memory “mental sketch pad” differs from long-term memory consolidation in a number of elementary ways. Working memory is a momentary (timescale of seconds), ever-changing pattern of recurrent activation of relatively stable architectural networks (Fig. 2A), while long-term memory consolidation retains events as structural changes in synapses (Fig. 2B). Long-term plastic changes begin with relatively rapid alterations in the numbers of AMPA and NMDA receptors in the synapse (Lüscher and Malenka, 2012), leading to structural changes such as enlarging of the spine head and shortening/thickening of the spine neck (Yuste and Bonhoeffer, 2001) to create a stable, mushroom-shaped spine and enduring strengthening of a synaptic connection (Araya et al., 2006) (Fig. 2B), and/or the addition of new spines and synapses (Yuste and Bonhoeffer, 2001). Recent in vitro studies of V1 in very young mice indicate that changes in spine neck length can occur quite rapidly to increase synaptic strength, transforming long, thin ineffective spines into effective mushroom spines (R. Yuste, personal communication). These processes typically require the activation of calcium (Ca+2) and cAMP signaling, which are facilitated by neuromodulators and which lead to transcriptional events in the nucleus (Barco et al., 2003). In this way, the cortex is thought to accumulate a lifetime of experience in remote memory storage. In contrast, long, thin spines in layer III of dlPFC are the most common spine type even in very old monkeys, and thus appear to retain their long, thin shape throughout the lifespan (Dumitriu et al., 2010). This geometric shape facilitates the rapid gating of synapses via ion channel opening, likely by limiting the spine’s volume and extending the distance that the signal must travel (see below).
Figure 2.
A highly schematized diagram of the interactions between recurrent, representational circuits in PFC with the sensory association cortices to create the “mental sketch pad”, illustrating the differences between long thin spines in PFC serving the gating processes of DNC, and the thin-type spines that change to mushroom-type to store long-term changes in synaptic efficacy (e.g. traditional neuroplastic changes in sensory cortex and subcortical structures). Neuromodulators shape both processes, but in fundamentally different ways. The sensory cortices provide the PFC with “bottom-up” sensory information, while PFC networks provide “top-down” regulation over the sensory cortex to retrieve stored memories and to guide sensory processing. For example, PFC inputs may enhance the processing of a nonsalient but relevant stimulus (shown), or inhibit the processing of distractors via projections to GABAergic interneurons ((Barbas et al., 2005), not shown). Plasticity occurs in the PFC as well, but is not illustrated for the sake of clarity. Inspired by Fuster, 1997 (Fuster, 1997).
The dlPFC representational machinery interacts extensively with posterior cortices, providing top-down regulation e.g. to suppress irrelevant operations or enhance the processing and storage of a nonsalient but relevant stimulus, and to reactivate long-term memories onto the mental sketch pad as key part of memory retrieval and recall (Fuster, 1997). Although the hippocampus is not shown in Figure 2, it is also required for the reactivation of recent (~15s-2yrs) memories (amongst its many other memory functions), but not for the activation of immediate (0-~15s) or remote memories (>~2yrs) (Squire, 1992). Conversely, there is also plasticity within the dlPFC (Liu et al., 2012), but this is not shown in Figure 2 in order to highlight the differences between the elaborate networks of memory storage vs. the working memory networks that retrieve and maintain information temporarily on the mental sketch pad. In this way, representational networks can interface with plastic circuits that learn and store experience. These operations are differentially modulated by the arousal systems, further dissociating the events that shape memory storage, and those that govern mental state.
Working memory networks in dlPFC are modulated in a fundamentally different manner than those processing sensory information and consolidating long-term memories. For example, increased cAMP signaling enhances long-term consolidation in hippocampal circuits (Abel et al., 1997; Frey et al., 1993; Huang et al., 1994) but markedly weakens PFC working memory (Runyan and Dash, 2005; Taylor et al., 1999). Similarly, the physiological responses of neurons in the primary sensory cortices are excited by NE α1 adrenergic receptor (α1-AR) signaling (Mouradian et al., 1991; Wang and McCormick, 1993), while neurons in the dlPFC show marked reductions in firing in response to NE α1-AR stimulation (Birnbaum et al., 2004). The sensitivity of the PFC to its neurochemical state is perhaps best appreciated in comparison to the primary visual cortex area V1: V1 continues to process visual information in the anesthetized state (Hubel and Wiesel, 1959), while even subanesthetic doses of ketamine markedly reduce Delay cell firing in the dlPFC and preclude cognitive performance (Wang et al., 2011b). The strength of dlPFC functions vary according to our state of arousal: working memory abilities are greatly impaired during fatigue or stress (Arnsten, 2009; Thomas, 2005), and even mild pressure can impair the ability to find insightful solutions to problems (Subramaniam et al., 2009). Our data indicate that there are ionic mechanisms that can cause rapid losses of dlPFC network excitation while maintaining the architectural integrity of the immensely complex networks needed for mental representation. Thus, there can be a momentary weakness in dlPFC function (e.g. a potential stressor that takes dlPFC “off-line” and switches control of behavior to more habitual, subcortical mechanisms), quickly followed by a return to more thoughtful, top-down dlPFC regulation when safety is assured. This dissociation between arousal effects on mental state and memory consolidation allows us to make new memories even if the PFC is “off-line” during stress, e.g. high levels of catecholamines can simultaneously weaken dlPFC top-down regulation while strengthening consolidation of the stressful experience through actions in amygdala, hippocampus and sensory cortices. These dual actions in distinct brain circuits arise from differences in downstream intracellular signaling processes initiated by the modulatory arousal pathways.
The cortical arousal pathways
There are a large number of arousal pathways that project to the cortical mantle from the brainstem or ventral forebrain, e.g. norepinephrine (NE), dopamine (DA), serotonin, acetylcholine, GABA, histamine, and orexin neurons all project to the cerebral cortex, including the PFC. There is also endogenous catecholamine production in the dlPFC of some primate species, including humans (Raghanti et al., 2009). The variations in locus coeruleus (LC) NE neuronal firing across arousal states has been extensively studied by Foote and Aston-Jones: LC neurons are silent during REM sleep, show little activity in deep sleep, have robust phasic activity to relevant stimuli during alert waking, and high, tonic firing during mild stress e.g. (Foote et al., 1983; Rajkowski et al., 2004). Several systems have been studied in primates performing cognitive tasks. Recordings from the NE or DA cell bodies indicate that NE and DA would be released in dlPFC in a phasic manner in anticipation of, or in response to, salient events associated with reward or aversion (Bromberg-Martin et al., 2010; Rajkowski et al., 2004; Schultz, 1998), while recordings from basal forebrain (Richardson and DeLong, 1986) or raphe (Okada et al., 2011) neurons suggest that acetylcholine and serotonin release would occur in more direct association with rewards (aversive stimuli have not been studied). Biochemical studies of rat medial PFC show high levels of DA, NE and serotonin release following exposure to even mild, uncontrollable stress (Amat et al., 2006; Deutch and Roth, 1990; Finlay et al., 1995).
Remarkably little is known about how these modulators alter higher cortical function. Most research has focused on the PFC due to the pioneering work of Brozoski et al. showing that catecholamines are essential to the working memory functions of the dlPFC (Brozoski et al., 1979). Although their paper describes the discovery in terms of DA, the effective lesion actually depleted both DA and NE, and we now know that both modulators are critical to dlPFC function (Robbins and Arnsten, 2009). More recent work suggests regional variation in modulatory mechanisms even within the PFC, whereby orbital PFC is modulated differently than dlPFC (Robbins and Arnsten, 2009). The current review focuses on mechanisms revealed during working memory performance in the dlPFC. The reader is cautioned that molecular mechanisms likely differ by cortical region and cognitive operation, and thus the specific mechanism discussed may not apply to other PFC subregions or other association cortices. The work to date in dPFC shows that the catecholamines have an inverted-U influence on dlPFC function, whereby either too little (fatigue) or too much (stress) NE or DA impairs working memory function (Arnsten, 2010). Slice recordings have shown basic excitatory actions that are likely engaged in the PFC in the switch from sleep to waking e.g. (Gorelova and Yang, 2000; Henze et al., 2000; Seamans et al., 2001a). But there are also more intricate actions that dynamically alter mental abilities by modulating synaptic network strength.
Dynamic Network Connectivity- A unique form of neuroplasticity
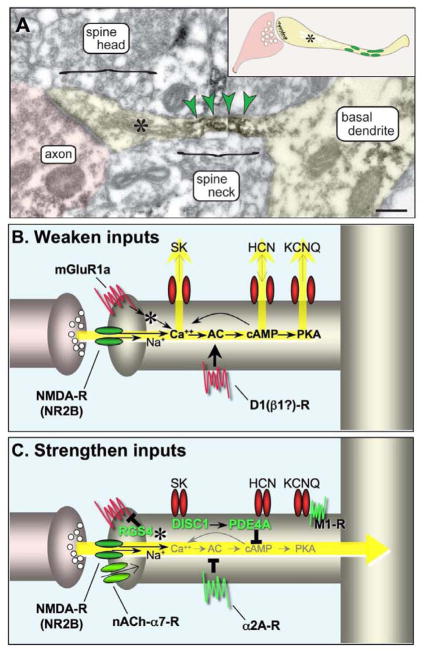
The recurrent excitatory working memory microcircuits in deep layer III of dlPFC interconnect on dendritic spines. These spines are predominately long and thin ((Dumitriu et al., 2010); Figs. 3–5), often with a narrow “bottleneck” (Paspalas et al., 2012), and they are greatly enriched in Ca+2- or cAMP-regulated ion channels and signaling proteins ((Paspalas et al., 2012); Figs. 3–5). Long, thin spines predominate even in the dlPFC of extremely old monkeys, suggesting that they are not awaiting to become mushroom spines, but rather perform an alternative function. We have proposed that their long, thin shape allows for more effective synaptic gating, whereby Ca+2- or cAMP-opening of nearby potassium (K+) channels on the spine membrane weakens the effectiveness of nearby synaptic inputs, while inhibiting Ca+2 and/or cAMP signaling closes these channels and strengthens synaptic efficacy (Fig. 3; (Arnsten et al., 2010; Wang et al., 2007). The long, thin shape facilitates gating by isolating electrical and chemical events near a specific synapse, and by increasing the effectiveness of ionic conductances on membrane potential by influencing a very small cellular volume ((Araya et al., 2006; Arnsten et al., 2010) and X.J. Wang, personal communication). For example, cAMP-related proteins and ion channels often concentrate in the very narrow “bottleneck” of the spine (e.g. Fig. 3A), where small changes in ionic conductance may be especially effective in transiently gating inputs to the parent dendrite (Paspalas et al., 2012). Thus, DNC allows rapid and flexible alterations in network strength while maintaining a stable architecture. These mechanisms have been examined physiologically through the iontophoresis of minute amounts of drug onto dlPFC Delay cells in monkeys performing the oculomotor working memory task (Fig. 1B). The data have revealed that treatments that increase Ca+2-cAMP signaling rapidly decrease dlPFC Delay cell firing, while those that inhibit Ca+2-cAMP signaling rapidly enhance task-related neuronal firing. Immunoelectron microscopy (immunoEM) has emphasized the importance of precise molecular localization and interactions, and that it is not just the amount but the exact placement of molecular events that is needed for proper modulation of cognitive circuits.
Figure 3.
Working model of the modulatory events contributing to DNC. A. DNC mechanisms engage long, thin spines with narrow neck segments that confine a minute cytosolic volume to subserve biochemical/electrical compartmentalization. DNC spines emanate from high-order dendrites, including the basal dendrites, as the one shown in layer III of monkey dlPFC (pseudocolored yellow). The spine neck is immunoreactive for PDEA (indicated by green arrowheads, and by green ovals in inset), positioned to regulate cAMP in the spine’s bottleneck. Asterisk marks the spine apparatus. Scale bar, 200 nm. B. A schematic illustration of the Ca+2-cAMP signaling events that weaken synaptic efficacy in layer III, long, thin spines. C. A schematic illustration of the Ca+2-cAMP signaling events that strengthen synaptic efficacy in layer III, long, thin spines. Please note that nicotinic α7 receptors have been documented on spines in rodent PFC (Duffy et al., 2009), but have not been studied in primate. See text for abbreviations.
Figure 5.
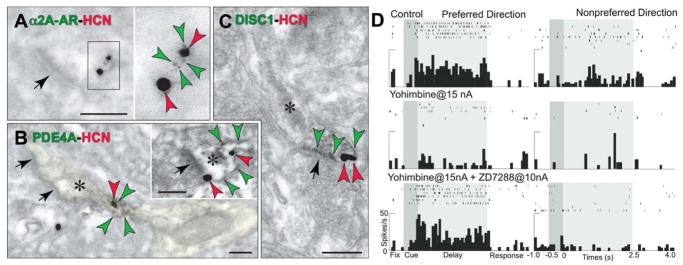
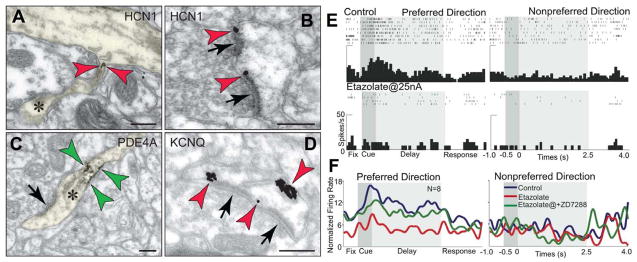
HCN channels are co-expressed with a constellation of cAMP-related molecules in layer III spines of the monkey dlPFC. A–C. Label for HCN channels (red arrowheads) on spine membranes overlaps with label for α2A-ARs (A, green arrowheads) that inhibit cAMP production, PDE4A that catabolizes cAMP (B; green arrowheads; spine is pseudocolored yellow) and DISC1 that regulates PDE4 activity (C, green arrowheads). HCN channels also co-localize with D1Rs that elevate cAMP (see Fig. 8F). Asterisks mark spine apparata; arrows point to excitatory-like synapses. Scale bars, 200 nm. Adapted from (Paspalas et al., 2012; Wang et al., 2007). D. Local increases in cAMP signaling in dlPFC caused by iontophoresis of the α2-AR antagonist, yohimbine (15 nA) markedly reduce Delay cell firing in monkeys performing a working memory task. Blockade of HCN channels with co-iontophoresis of ZD7288 (10 nA) restores normal firing patterns, demonstrating physiological interactions between HCN channels and α2A-ARs in the primate dlPFC. Adapted from (Wang et al., 2007).
Mechanisms that weaken synaptic efficacy
A variety of DNC mechanisms serve to weaken synaptic efficacy and induce a rapid (timescale of seconds) reduction in dlPFC firing (Fig. 3B). These may be synergistic, feedforward processes, e.g. Ca+2 increases cAMP generation, and cAMP facilitates intracellular Ca+2 release from the spine apparata (indicated by asterisks) that are prominent in dlPFC long thin spines near the synapse (Soulsby and Wojcikiewicz, 2005). Calcium activation of protein kinase C may exacerbate this process, e.g. by uncoupling α2A-adrenergic receptors (α2-AR) which normally serve to inhibit cAMP signaling ((Wang and Limbird, 2007); not shown in Fig. 3). Calcium can build up in spines through a number of mechanisms, e.g. through NMDA receptors (especially those with NR2B subunits) (Liu et al., 2007), and through IP3-mediated internal Ca+2 release. Internal Ca+2 release is stimulated by NE α1-AR, and by Gq coupled metabotropic glutamate receptors, (mGluR1α and/or mGluR5) which have been localized near the synapse in primate dlPFC spines (Fig. 8E; (Muly et al., 2003)). mGluR generation of IP3 increases internal Ca+2 release from the spine apparatus; cAMP-PKA signaling can increase this process through phosphorylation of IP3 receptors (Soulsby and Wojcikiewicz, 2005). Calcium opens a variety of K+ channels, e.g. SK channels, which can provide negative feedback for NMDA receptor excitation (Faber, 2010), reduce PFC cell firing (Hagenston et al., 2008), and impair working memory in rats (Brennan et al., 2008). SK channels have not yet been mapped in primate dlPFC, but are likely to reside near the spine apparatus as well as on dendrites.
Figure 8.
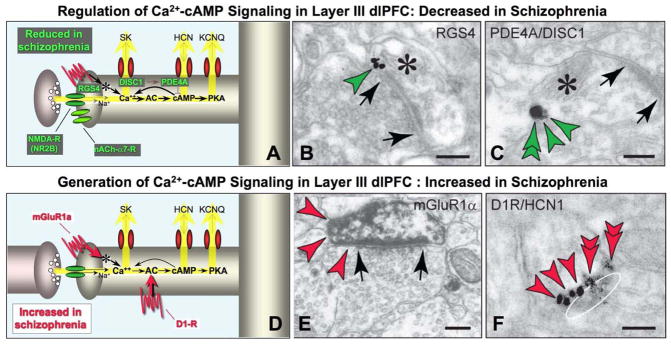
Some of the changes in DNC proteins associated with schizophrenia. A. Schematic summary showing that a number of proteins that normally serve to strengthen synaptic efficacy, e.g. by regulating Ca+2-cAMP signaling, are lost or weakened in schizophrenia. B and C. Dendritic spines in layer III of the monkey dlPFC express proteins that regulate Ca+2-cAMP signaling: B. RGS4 (green arrowheads) is found perisynaptically, positioned to regulate mGluR1α/mGluR5 signaling. C. PDE4A (green arrowhead) is found on the spine membrane with DISC1 (green double arrowheads), which likely tethers the enzyme to the correct subcellular locus. Adapted from (Paspalas et al., 2012; Paspalas et al., 2009) D and E. Proteins that generate Ca+2-cAMP signaling are localized in layer III spines in the monkey dlPFC. Stimulation of mGluR1α (D, red arrowheads) can mobilize Ca+2 from the spine’s internal stores (i.e. the spine apparatus). Adapted from (Muly et al., 2003) with permission from C. Muly. The D1Rs, which elevate cAMP signaling, are colocalized with cAMP-gated HCN channels favoring their open state. An oblique section through a synapse in F (the synaptic disk is drawn as a white oval) demonstrates perisynaptic localization for both proteins (HCN channels, red arrowheads; D1Rs, red double arrowheads). Asterisks mark spine apparata; arrows point to excitatory-like synapses. Scale bars, 100 nm.
cAMP signaling also influences the open state of a variety of ion channels: it directly increases the open state of HCN (hyperpolarization-activated cyclic nucleotide-gated) channels (Ulens and Tytgat, 2001), while cAMP-PKA signaling increases the open state of KCNQ channels ((Delmas and Brown, 2005); also known as “M” channels, as they are closed by muscarinic receptor stimulation, Fig. 3C). In hippocampus, HCN channels are concentrated on distal apical pyramidal dendrites, where they gate distal inputs e.g. (Nolan et al., 2004), and modulate excitability and plasticity e.g. (Fan et al., 2005). HCN channels are also on the distal apical dendrites of layer V dlPFC pyramidal cells (Paspalas et al., 2012; Wang et al., 2007). However, in deep layer III of dlPFC, HCN channels are enriched in long, thin spines (Paspalas et al., 2012), both in the spine neck (e.g. Fig. 4A), and next to the synapse (e.g. Fig. 4B). These are likely HCN1-HCN2 heteromers, which rapidly respond to cAMP (Chen et al., 2001; Ulens and Tytgat, 2001). A variety of cAMP-related signaling proteins can be observed in deep layer III long, thin spines near the HCN channels. The phosphodiesterase PDE4A is commonly found in the spine neck (Fig. 3A, 4C) and in the spine head (Fig. 5B, inset) near HCN channels (Fig. 5B), positioned to regulate the amount of cAMP (cAMP “hot spots”), and thus the degree of HCN channel opening. Indeed, inhibiting PDE4 regulatory activity by iontophoresis of etazolate onto dlPFC neurons induces a rapid collapse in dlPFC Delay cell firing (Figs. 4E,F). Firing can be restored by simultaneously blocking HCN channels with co-iontophoresis of ZD7288, demonstrating physiological as well as physical interactions (Fig. 4F, green trace). Similar effects have been observed at the behavioral level, where very low dose blockade of HCN channels in rat PFC can improve working memory performance (Wang et al., 2007). In some neurons, PKA phosphorylation of HCN channels can lead to sustained increases in channel opening (Vargas and Lucero, 2002); if this occurs in dlPFC, it could contribute to prolonged cognitive impairment, e.g. as occurs with fatigue and/or stress (see below). We have also documented KCNQ channels on layer III dlPFC spines (Fig. 4D). KCNQ channels are present in many other cellular compartments as well, where they influence neuronal excitability and action potential generation e.g. (Devaux et al., 2004), but may have a gating function in spines in dlPFC (e.g. KCNQ channel blockade can restore task-related firing in aged dlPFC neurons, see below). KCNQ channels are of special interest to neuromodulation, as their open state is regulated by a variety of modulatory systems, including cAMP-PKA, muscarinic and endocannabanoid/arachidonic acid signaling (Delmas and Brown, 2005). There are likely additional ionic mechanisms that contribute to rapid weakening of synaptic efficacy, but existing data already indicate a rich interplay of powerful ionic mechanisms that can rapidly disconnect dlPFC neuronal networks and reduce neuronal firing.
Figure 4.
cAMP signaling in primate dlPFC differs from traditional neuroplasticity. A and B. Dendritic spines in monkey layer III dlPFC sequester cAMP-gated HCN channels (red arrowheads), positioned to translate cAMP “hot spots” to network connectivity patterns. HCN channels are found in the spine neck (A) and in the perisynaptic membranes flanking excitatory (network) synapses (B), positioned to gate impulses to the parent dendrite. C. PDE4A, which hydrolyzes cAMP and terminates its actions, presents an identical expression pattern in the neck region (green arrowheads); please see also Figure 3A. A–C adapted from (Paspalas et al., 2012). D. KCNQ potassium channels are also expressed in dlPFC spines, and are shown here in perisynaptic and extrasynaptic membranes (red arrowheads). Asterisks mark spine apparata; arrows point to excitatory-like synapses. Scale bars, 100 nm. E. Increased cAMP signaling reduces dlPFC Delay cell firing. Iontophoresis of the PDE4 inhibitor, etazolate (25 nA), onto a dlPFC Delay cell rapidly reduces task-related firing in a monkey performing a working memory task. F. Population response of 8 Delay cells with delay-related firing under control conditions (blue trace), that is markedly reduced by iontophoresis of etazolate (red trace). Blockade of HCN channels with co-iontophoresis of ZD7288 (10 nA, green trace) restores normal firing patterns, demonstrating physiological interactions between HCN channels and cAMP signaling in the primate dlPFC. Adapted from (Wang et al., 2007).
Mechanisms that strengthen synaptic efficacy
Research in nonhuman primates has also identified mechanisms that can enhance task-related neuronal firing in dlPFC either by inhibiting Ca+2-cAMP signaling in spines, or by directly depolarizing the spine compartment (Fig. 3C). For example, RGS4 (regulator of G protein signaling 4) flanks the synapse where it is positioned to inhibit perisynaptic mGluR1α/5-Gq signaling and reduce internal Ca+2 release (Fig. 8B; (Paspalas et al., 2009)). A variety of mechanisms regulate cAMP signaling in layer III dlPFC spines. As mentioned above, the phosphodiesterase PDE4A, which catabolizes cAMP, is often localized next to HCN channels in spines, and near the spine apparatus to regulate cAMP effects on internal Ca+2 release (Fig. 5B; (Paspalas et al., 2012)). (In contrast, PDE4B is in the post-synaptic density and in dendrites, ibid). It is likely that PDE4A is anchored to the correct location in the spine by the scaffolding protein, DISC1 (Disrupted in Schizophrenia), as DISC1 tethers a variety of PDE4s (Murdoch et al., 2007), and co-localizes with PDE4A in layer III spines in monkey dlPFC (Fig. 8C). DISC1 is also found next to HCN channels (Fig. 5C) and near the spine apparatus (Fig. 8C) in monkey dlPFC layer III spines (Fig. 5C), suggesting that a DISC1-PDE4A interactome is positioned to regulate both network gating and internal Ca+2 release. PDE4A may also be anchored to the spine apparatus by AKAP6 (A Kinase Anchor Protein 6, also known as AKAP100), which tethers cAMP-related proteins to endomembranes that harbor Ca+2 (Dodge-Kafka et al., 2008), such as the spine apparatus. Thus, PDE4A is positioned to reduce cAMP concentrations at several key sites in the spine, where it can decrease internal Ca+2 release and close HCN, KCNQ and possibly SK channels. KCNQ channels are also closed by cholinergic stimulation of M1 receptors within the same lipid raft as the channel itself (Oldfield et al., 2009), therefore opposing cAMP-PKA actions on these channels.
cAMP levels in the spine are also reduced by α2A-ARs, which inhibit cAMP generation. α2A-ARs co-localize with HCN channels in layer III spines near the synapse and in the spine neck (Figs. 5A; (Wang et al., 2007)). Stimulation of α2A-ARs, e.g. with the α2A-AR agonist, guanfacine, specifically increases firing for the neuron’s preferred direction, thus enhancing mental representation (Wang et al., 2007). Conversely, blockade of α2A-ARs with yohimbine causes a complete collapse of dlPFC network firing (Li et al., 1999) that can be restored by blocking HCN channels (Fig. 5D; (Wang et al., 2007)). Parallel effects are seen on cognitive behavior, where infusion of guanfacine directly into dlPFC improves working memory (Mao et al., 1999), and systemic administration of guanfacine improves a variety of PFC cognitive functions, including spatial working memory, behavioral inhibition, top-down regulation of attention, and rapid associative learning (reviewed in (Arnsten, 2010)). A recent study has shown that guanfacine improves impulse control by inhibiting responses to an immediate, small reward in order to wait over a delay for a larger reward (Kim et al., 2011). All of these tasks require behavior to be guided by mental representation. Conversely, the work of Bao-Ming Li has shown that infusion of the α2A-AR antagonist, yohimbine, into the dlPFC impairs working memory and impulse control, and induces locomotor hyperactivity in monkeys (reviewed in (Arnsten, 2010)). Thus, α2A-AR stimulation strengthens the efficacy of dlPFC microcircuit connections, enhancing mental representation and top down regulation of behavior. Based on this research in animals, guanfacine is now being used to treat a variety of PFC disorders in human patients, including Attention Deficit Hyperactivity Disorder (extended release pediatric formulation Intuniv™) (Biederman et al., 2008), Tourette’s Syndrome (Scahill et al., 2001), autism spectrum illness (McCracken et al., 2010), substance abuse (McKee, Sinha and Arnsten, in preparation), and traumatic brain injury to the frontal lobe (McAllister et al., 2011).
Recent research has revealed that acetylcholine (ACh) also plays a critical, beneficial role in dlPFC function. Depletion of ACh from the primate PFC produces a marked loss of spatial working memory function, comparable to that seen with catecholamine depletion (Croxson et al., 2011). It is likely that ACh has beneficial actions through both nicotinic and muscarinic receptors, although these receptor mechanisms are just emerging. Studies of rat medial PFC have shown that nicotinic α7 receptors are localized within the post-synaptic density in spines, likely next to NMDA receptors, as well as in their traditional locations on pre-synaptic axon terminals (Duffy et al., 2009). Our physiological data show that ionotophoresis of nicotinic α7 receptor agonists onto dlPFC neurons increases Delay cell task-related firing and rescues firing following NMDA receptor blockade, suggesting that the arousing properties of ACh may be an important “depolarizing partner” for NMDA receptors in PFC circuits (Yang, Jin, Arnsten, and Wang, unpublished). Similar results are seen with the systemic administration of nicotinic α7 receptor agonists in monkeys, which improve working memory and normalize performance following NMDA antagonists (Buccafusco and Terry, 2009; Castner et al., 2011). Thus there is converging evidence that nicotinic α7 receptors provide a vital modulatory influence in dlPFC circuits. Studies in rats indicate that acetylcholine also modulates PFC function through actions at muscarinic receptors that close KCNQ channels and increase neuronal excitability (Santini et al., 2012), and KCNQ receptors also influence neuronal excitability in the primate dlPFC during working memory (Wang et al., 2011a). Thus, cholinergic stimulation may strengthen network firing through both muscarinic and nicotinic mechanisms. Little is known about the effects of other modulators (e.g. serotonin, orexin and histamine) on the cognitive firing patterns of dlPFC neurons. This will be an important area for future work.
Inverted U dopamine D1 receptor actions sculpt network inputs
Much has been learned about DA actions in dlPFC since the first discovery of its importance by Brozoski et al. in 1979. Although the original work emphasized the beneficial effects of DA, we now know that DA stimulation of D1 receptors (D1R) has an inverted U dose-response influence on dlPFC neuronal firing and on working memory performance, with high doses decreasing firing and impairing working memory ((Arnsten et al., 1994); schematically illustrated in Figure 6). In vitro recordings from PFC slices have been ideal preparations for examining the excitatory effects of very low dose D1R stimulation, as there is no endogenous DA in the slice. PFC neurons are also hyperpolarized in the slice, without the constant excitation from neighbors that occurs in vivo. It should be noted that most of these studies are done on layer V pyramidal cells; however, as some of layer V neurons may “migrate” into layer III in the more differentiated primate PFC (Elston, 2003), these data may also be relevant to the recurrent layer III neurons. The in vitro studies have revealed excitatory effects of D1R stimulation in both rat medial PFC (Seamans et al., 2001a) and monkey dlPFC (Henze et al., 2000), e.g. by enhancing persistent sodium currents (Gorelova and Yang, 2000) and NMDA receptor actions e.g. (Seamans et al., 2001a). These data are echoed in vivo, where high doses of D1R antagonist lead to loss of dlPFC Delay cell firing and to working memory impairment (Williams and Goldman-Rakic, 1995).
Figure 6.
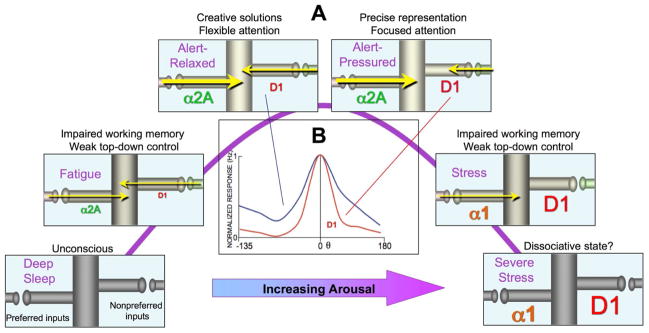
A. Hypothetical coordination of arousal state and PFC cognitive abilities based on alterations in layer III dlPFC network gating by increasing levels of catecholamines. NE stimulation of α2A-AR enhances network firing by increasing synaptic efficacy for inputs from neurons with similar preferred directions. Conversely, moderate levels of DA D1R stimulation sculpt network inputs from dissimilar neurons by decreasing synaptic efficacy. D1R sculpting actions may be helpful for some PFC cognitive operations, e.g. working memory for a precise spatial location, but may be harmful when wide network inputs are needed, e.g. creative solutions or attentional set-shifting. High levels of NE and DA release during stress disconnect all network inputs through α1-AR and high levels of D1R, respectively, switching control of behavior and thought to more primitive brain regions. α1-AR activation of PKC signaling during stress may uncouple α2A-ARs and diminish their beneficial actions (see text). B. Iontophoresis of a low dose of D1R agonist onto dlPFC Delay cells sharpens their spatial tuning (red trace), decreasing neuronal delay-related firing following cues for the neurons’ nonpreferred directions (θ≠0), but having no effect on delay-related firing following cues for the neurons’ preferred direction (θ=0). These sculpting actions are most evident in neurons with noisy firing under control conditions (blue trace). From (Vijayraghavan et al., 2007).
More moderate levels of D1R stimulation have sculpting actions on the pattern of task-related neuronal firing (Vijayraghavan et al., 2007). Iontophoresis of low doses of D1R agonists onto noisy dlPFC Delay cells can selectively decrease neuronal firing for the neurons’ nonpreferred directions while leaving firing for the neurons’ preferred direction intact (Fig. 6B; “0” θ indicates the neurons’ preferred direction; (Vijayraghavan et al., 2007)). These sculpting effects likely involve cAMP-HCN channel gating actions as illustrated in Figure 6, but may also involve facilitation of lateral inhibition from GABAergic interneurons (Kroner et al., 2007; Seamans et al., 2001b) and presynaptic inhibition of glutamate release (Gao et al., 2001). Finally, very high doses of DA D1R stimulation, as occurs during uncontrollable stressors, reduce all neuronal firing and impair working memory (Vijayraghavan et al., 2007). The deleterious effects of D1R agonists on neuronal firing and working memory performance are prevented by cAMP inhibition (Vijayraghavan et al., 2007), or HCN channel blockade (Gamo and Arnsten, in submission) but are often not reversed once the D1R agonist has taken effect. These irreversible actions may involve cAMP-PKA phosphorylation of HCN channels maintaining channels in open state (Vargas and Lucero, 2002).
Relationship of neuromodulation in dlPFC to arousal states
A primary function of neuromodulation is to coordinate cognitive abilities with arousal state, and the dlPFC is remarkably sensitive to changes in its neuromodulatory environment. Working memory functions are impaired under conditions of both fatigue and mild, uncontrollable stress (Arnsten, 2009; Arnsten et al., 2010). We have postulated that DNC mechanisms weaken recurrent connections during fatigue (inadequate NE α2A-AR stimulation) or hunger (inadequate glucose) in order to reduce neuronal firing and reserve energy stores (Arnsten et al., 2010). Recurrent firing is a very energy intensive process –PFC neurons have more mitochondria than their sensory cortex counterparts (Chandrasekaran et al., 1992)– and the weakening of synaptic connections with a build-up of Ca+2 and/or cAMP would dampen dlPFC activity to save energy. These mechanisms also serve as negative feedback on NMDA recurrent excitatory circuits to prevent seizures, and indeed, genetic insults to cAMP-PKA opening of KCNQ channels are associated with epilepsy (Schroeder et al., 1998). These protective mechanisms prevent seizures and save energy, but they likely constrain mental capability. The same feedback mechanisms appear to be actively generated during exposure to uncontrollable stress, when high levels of catecholamine release rapidly increase Ca+2 and cAMP signaling (e.g. via α1-AR and D1R stimulation) to take dlPFC “offline” and switch control of behavior to more primitive systems (Arnsten, 2009). More subtle changes in neuromodulation during nonstressed waking may serve to shape the contents of working memory, e.g. focusing network firing on events associated with reward.
The NE system has been studied most extensively both in terms of LC firing patterns during sleep, waking, and stress (see above), and in terms of its effects on dlPFC function. Varying levels of NE release engage different types of receptors, and thus can act as a neurochemical switch to alter brain state. As the NE innervation to dlPFC is quite delicate (e.g. compared to thalamus), moderate levels of phasic NE release during alert waking engage those receptors with the highest affinity for NE, the α2-AR, while high levels of NE release during stress engage the lower affinity receptors, α1-AR and β-AR (Arnsten, 2000; Li and Mei, 1994). Thus, α2A-AR stimulation strengthens network connections for the neurons’ preferred direction and increases neuronal firing to relevant stimuli (Fig. 6A), while higher levels of NE reduce dlPFC firing and impair working memory via α1-AR-Ca+2-PKC actions (Birnbaum et al., 2004), and possibly β1-AR effects (Ramos et al., 2005); these receptors are not shown in Figure 3, as immunoEM has not yet determined their subcellular location on dlPFC neurons.
Figure 6A shows a hypothetical representation of network connections for a dlPFC Delay neuron throughout the range of arousal conditions. During sleep, there is little or no NE release, and the dlPFC shows reduced levels of neuronal firing (M. Wang, unpublished) or BOLD response (Boly et al., 2008). Low levels of catecholamine receptor stimulation (α2A-AR and D1R) during the drowsy/fatigued state would weakly excite the neuron in a generalized manner. Under optimal alert conditions, more moderate levels of NE α2A-AR stimulation would strengthen synaptic connections with neuronal neighbors who shared the same spatial tuning characteristics, allowing stable mental representations. Varying levels of DA D1R stimulation would correspondingly weaken nonpreferred connections, sharpening tuning under conditions of salient events (e.g. a rewarding stimulus or pressure from a deadline). The sculpting of network inputs may be optimal for performance of a spatial working memory task in which one is trying to maintain the representation of a small location in space, but may be harmful when cognitive demands require more flexible network connections (Arnsten et al., 2009). This may explain why D1R stimulation is needed for spatial working memory, but actually impairs attentional set-shifting (Robbins and Arnsten, 2009), even though both functions depend on dlPFC. Thus, the optimal neuromodulatory environment depends on the cognitive demands: insightful solutions to problems or creative endeavors that require wide network connections would be optimal under relaxed, alert conditions with less D1R sculpting (e.g. in the shower), while more focused work may be best performed under the conditions that increase DA release (e.g. the pressure of working for a reward) (Arnsten et al., 2009). This may also explain why stimulant medications can be helpful for some schoolwork (e.g. math) but harmful to others (e.g. composing a poem or song).
The right side of the inverted U in Figure 6A shows the progressive weakening of network connections and progressive decrease in dlPFC firing with increasing stress (Arnsten, 1998, 2009). Evidence of this phenomenon has been seen in human imaging studies, where a mild uncontrollable stressor (watching a gory movie) impairs working memory and reduces the BOLD signal over the dlPFC, while disinhibiting activity in the amygdala and default mode network (Qin et al., 2009), consistent with loss of dlPFC regulation and strengthening of more primitive circuits. Data from animals indicate that that the same neurochemical pathways that take PFC off-line (D1R-cAMP and β1-AR-cAMP, α1-AR-Ca+2-PKC) serve to strengthen subcortical and sensory/motor circuits, switching the brain from a reflective to reflexive mode (Arnsten, 2009). The feed-forward nature of these signaling pathways would promote a very rapid switch to primitive circuits, i.e. “Going to Hell in a Handbasket” (Arnsten, 2009). Thus, regulatory interactors such as DISC1-PDE4A would serve a critical role to reign in feedforward Ca+2-cAMPsignaling and restore dlPFC top-down regulation of thought and behavior. Loss of this regulation and/or chronic stress exposure leads to architectural changes in PFC pyramidal cells, with loss of spines and retraction of dendrites (Cook and Wellman, 2004; Liston et al., 2006; Radley et al., 2008). The molecular basis for stress-induced atrophy has just begun to be studied. Spine loss can be prevented by inhibition of PKC signaling (Hains et al., 2009), which may disassemble the spine’s actin skeleton through phosphorylation of MARCKS (Calabrese and Halpain, 2005). Lead poisoning, which potently activates PKC signaling (Marcovac and Goldstein, 1988), may also cause PFC gray matter loss through this mechanism (Cecil et al., 2008; Hains et al., 2009). Interestingly, traumatic head injury activates stress signaling pathways in surrounding tissue, suggesting universal detrimental actions (Kobori et al., 2006). Stress-induced architectural changes in PFC neurons are reversible in young rats, but not in aged rats (Bloss et al., 2011). Thus, environmental or genetic insults that disinhibit stress signaling pathways in aging or in mental illness can readily disrupt the precise regulation needed for the integrity of PFC circuits and healthy cognitive function.
Modulatory changes with advancing age- vulnerabilities in high order circuits
PFC cognitive functions decline with advancing age, beginning in middle age, in both humans e.g. (Davis et al., 1990; Gazzaley and D’Esposito, 2007) and monkeys (Moore et al., 2006; Rapp and Amaral, 1989). Impaired dlPFC function appears to arise in part from dysregulation of DNC signaling with advancing age (Fig. 7). It is important to understand these changes, as loss of PFC function is particularly problematic in the Information Age when top-down executive abilities are essential to maintain challenging careers and to manage even basic activities such as health care and finances. Age-related vulnerabilities in the association cortices must also contribute to vulnerability to neurodegeneration, as these are the neurons that are afflicted earliest and most severely in AD (Bussière et al., 2003).
Figure 7.
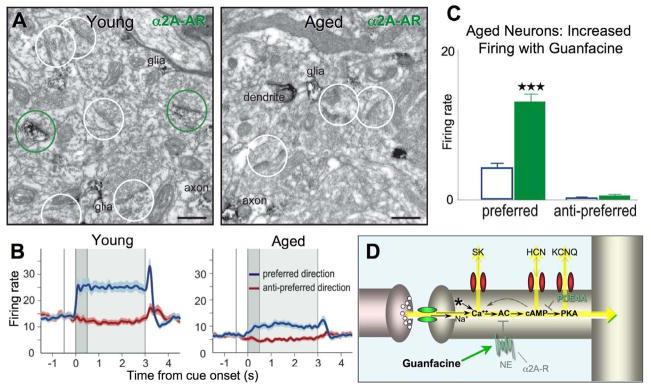
Changes in DNC regulation with advancing age weaken synaptic efficacy and contribute to reductions in the persistent dlPFC Delay cell firing underlying working memory. A. Deep layer III neuropil in the dlPFC of a young (9yo) vs. an aged (29yo) monkey. White and green circles mark unlabeled and α2A-AR-labeled dendritic spines, respectively. Please note that spine density for both categories decreases with age. The reduction in α2A-AR-labeling is consistent with autoradiographic measures of reduced α2A-AR expression in the aged monkey dlPFC (Bigham and Lidow, 1995; Moore et al., 2005). B. The persistent firing of dlPFC Delay cells during a spatial working memory task markedly declines with advancing age. Blue=firing for the neurons’ preferred direction; dark red=firing for the neurons’ nonpreferred direction. From (Wang et al., 2011a). C. The persistent firing of aged dlPFC neurons was significantly increased by iontophoresis of the α2A-AR agonist, guanfacine, which inhibits cAMP signaling. Adapted from (Wang et al., 2011a). D. A schematic representation of some of the changes in DNC signaling in layer III spines with advancing age that lead to disinhibition of Ca+2-cAMP signaling. See text for detailed description.
Neurobiological studies of aged rhesus monkeys have illuminated much of the normal aging process, as these animals do not have incipient AD, yet have well-developed association cortices. Ultrastructural studies of the dlPFC have shown large reductions in the numbers of layer III synapses with advancing age, and the loss of synapses correlates with cognitive deficits (Peters et al., 2008). Spine loss particularly afflicts the long, thin spines ((Dumitriu et al., 2010); Fig. 7A), which are the spines enriched in Ca+2-cAMP signaling proteins (Paspalas et al., 2012). Recent physiological studies have shown marked, age-related reductions in the persistent firing of Delay cells, with reductions already evident in middle age (Fig. 7B; (Wang et al., 2011a)). In contrast, the firing patterns of sensory neurons (e.g. Cue cells) remain intact with advancing age (Wang et al., 2011a). Although some of the loss of persistent neuronal firing during working memory likely arises from synapse loss in the recurrent excitatory microcircuits needed to maintain firing throughout the delay period, some of the physiological vulnerability arises from a dysregulated neurochemical environment in remaining spines (Fig. 7D). Thus, firing is restored by inhibiting cAMP signaling (e.g. with guanfacine; Fig. 7C) or blocking HCN or KCNQ channels (Wang et al., 2011a).
A variety of methods have shown age-related disinhibition of Ca+2-PKC (Brennan et al., 2009) and cAMP-PKA signaling (Ramos et al., 2003; Wang et al., 2011a) in the PFC. Depletion in neuromodulators in the aged PFC has been observed for many years (Goldman-Rakic and Brown, 1981; Wenk et al., 1989), and there is also a marked loss of excitatory inputs onto LC NEergic neurons (Downs et al., 2006). Receptor binding studies have shown a reduction in the numbers of α2A-AR (Bigham and Lidow, 1995; Moore et al., 2005), some of which may be post-synaptic on spines (Fig. 7A). Recent data indicate reduced expression and misplacement of PDE4A in the aged PFC, with specific loss from spines (Carlyle, Nairn, Simen, Arnsten, Paspalas, in preparation), which likely plays an important role in the dysregulation of both Ca+2 and cAMP signaling. The dysregulation of Ca+2-cAMP signaling in the highest order cognitive circuits would explain why high-order cognitive functions are most vulnerable to aging and neurodegeneration, and why sensory/motor neurons remain relatively intact. Dysregulation of neuromodulatory events would also explain why stress (Arnsten, 2009) or head injury (Kobori et al., 2006) magnifies age-related processes and hastens cognitive deficits (Broglio et al., 2012; Kremen et al., 2012).
Genetic insults in schizophrenia- contributions to thought disorder
A remarkable number of DNC proteins are altered in schizophrenia, which likely contribute to profound PFC dysfunction beginning in adolescence and worsening in adulthood. Patients with schizophrenia are impaired on the same working memory task used in monkey studies (Fig. 1B; (Keedy et al., 2006)), and hypofrontality of the right dlPFC during working memory strongly correlates with symptoms of thought disorder (Perlstein et al., 2001). Waves of gray matter loss from the dlPFC herald the descent into illness (Sun et al., 2009), and neuropathological studies have identified substantial atrophy in deep layer III dlPFC microcircuits, including loss of neuropil (Selemon and Goldman-Rakic, 1999), loss of pyramidal cell dendritic spines (Glantz and Lewis, 2000), and weakened GABAergic lateral inhibition (Lewis and Gonzalez-Burgos, 2006). There is also evidence of atrophy in layer V dlPFC pyramidal cells (Black et al., 2004). As pyramidal neurons drive GABAergic interneurons (Goldman-Rakic, 1995), and glutamate decarboxylase (GAD) expression is activity dependent, the weakening of GABAergic inhibition is likely secondary to pyramidal cell insults (Lewis and Gonzalez-Burgos, 2006). Decreases in cortical DA and increases in subcortical DA may also arise from primary insults to pyramidal cell circuits (Lewis and Gonzalez-Burgos, 2006), and may interact with catechol-O-methyltransferace (COMT) genotype to confer risk (Egan et al., 2001).
Insults to DNC modulation of layer III synapses may play a key role in increasing the vulnerability of these microcircuits in schizophrenia (Fig. 8). Direct or indirect insults to NMDA receptor signaling have been appreciated for some time e.g. (Beneyto and Meador-Woodruff, 2008; Kristiansen et al., 2010; Krystal et al., 2003; Ross et al., 2006). There are also longstanding links between gating deficits in schizophrenia and genetic alterations in nicotinic α7 receptors (Martin and Freedman, 2007). However, an increasing number of studies are now revealing genetic insults in schizophrenia that dysregulate Ca+2-cAMP signaling. In general, schizophrenia is associated with genetic and compensatory alterations that weaken the regulation of Ca+2-cAMP signaling (Fig. 8A), and/or strengthen the generation of Ca+2-cAMP signaling (Fig. 8D). For example, RGS4 normally serves to inhibit Gq signaling, and RGS4 is markedly reduced from the dlPFC of patients with schizophrenia (Erdely et al., 2006; Mirnics et al., 2001; Volk et al., 2010. There are also genetic links between RGS4 and schizophrenia in some families {Chowdari, 2002 #1520). RGS4 is primarily a synapse-associated protein in dlPFC neurons, including in layer III spines next to the synapse (Fig. 8B; (Paspalas et al., 2009)), the same subcellular location as mGluR-Gq linked receptors (Fig. 8E; figure from (Muly et al., 2003)). Another DNC protein directly linked to schizophrenia is the scaffolding protein, DISC1. Translocations in the disc1 gene are associated with extensive mental illness in a large Scottish pedigree (Millar et al., 2005). Animal studies have shown that loss of DISC1 interferes with the development of PFC circuits and neurite formation (reviewed in (Brandon and Sawa, 2011)). DISC1 also tethers a large range of proteins, including the PDE4s (Millar et al., 2005; Murdoch et al., 2007). ImmunoEM studies of layer III monkey dlPFC show extensive DISC1 interactions with PDE4A in spines near the spine apparatus (Fig. 8C) and near HCN channels in the spine neck and head (see above). Thus, genetic insults to DISC1-PDE4A regulation of cAMP signaling would likely dyregulate Ca+2 as well as cAMP signaling. A recent study has linked genetic insults to PDE4A with schizophrenia in a Japanese cohort (Deng et al., 2011). Although its localization in dlPFC is not yet known, mGluR3 also has been linked to schizophrenia, and this receptor normally inhibits cAMP signaling (Harrison et al., 2008; Sartorius et al., 2008). Thus, a number of mechanisms that normally serve to constrain Ca+2-cAMP signaling in layer III of dlPFC may be weaker in patients with schizophrenia.
Conversely, schizophrenia is associated with the increased expression of receptors that promote Ca+2-cAMP signaling. For example, there is increased expression of mGluR1α (Volk et al., 2010) which evoke internal Ca release (Fig. 8E; image from (Muly et al., 2003)), and increased expression of D1R even in drug naïve patients (Abi-Dargham et al., 2011), which would increase the generation of cAMP signaling (Fig. 8F). Although it is possible that the increase in D1R compensates for reduced DA levels in dlPFC, it is not known if DA is altered in PFC at this early stage of disease (see below). Genetic studies have also found insults to VIPR2 (vasoactive intestinal polypeptide receptor 2) that would increase cAMP signaling (Levinson et al., 2011; Vacic et al., 2011), and alterations in a primate-specific, cAMP-regulated potassium channel, KCNH2, (Huffaker et al., 2009); these proteins are not shown on Figure 8, as immunoEM has yet to localize their subcellular distribution in dlPFC. Thus, a variety of different genetic insults could all lead to the same phenotype of dysregulated Ca+2-cAMP signaling and weakened layer III dlPFC pyramidal cell connections. These findings would also explain why stress is such an important factor in precipitating the onset of symptoms in this illness.
Adolescence is a period of great vulnerability for the onset of serious mental illness, and it is a time of synaptic pruning and reorganization in PFC. Increased vulnerability may also arise from increased DA innervation of layer III in the primate dlPFC during adolescence, which may further drive dysregulated stress signaling pathways in dlPFC (Rosenberg and Lewis, 1995). It is possible that these actions contribute to dlPFC gray matter loss at onset of illness. In addition to weakening connections in layer III microcircuits, increased DA actions may alter the feedback (corollary discharge) from D2R-modulated layer V Response cells in dlPFC (Wang et al., 2004). D2R stimulation alters the timing and magnitude of Response cell firing, which in human subjects may contribute to symptoms of hallucinations (Ford et al., 2002) and delusions (Corlett et al., 2007). These cortical errors would be magnified by increased DA D2R signaling in caudate (Laruelle et al., 1996), weakening inhibition of inappropriate network activity by the striatal indirect pathway (Arnsten, 2011). Disruptions in PFC-striatal operations, compounded with insults to the formation of circuits during development (Brandon and Sawa, 2011), would lead to profound cognitive disorder (Arnsten, 2011).
Summary
Research on the primate dlPFC has revealed that the highly evolved microcircuits underlying representational knowledge are modulated in a unique manner, different from sensory/motor and subcortical circuits. These differences must be respected if we are to understand the neurobiology underlying higher cognitive disorders, and thus create effective treatments. We need to understand how genetic and environmental alterations in higher cognitive circuits impact their physiological integrity, and learn how to substitute for insults by identifying targets in the same subcellular compartment that can restore function. The success of guanfacine in treating PFC disorders serves as a proof of concept, showing that understanding the unique modulation of higher cortical circuits can lead to effective treatments for humans. Dynamic neuromodulatory mechanisms in PFC provide great flexibility in selecting which brain regions and networks mediate our behavior and thought, but it is a process that requires precise regulatory abilities to rapidly disengage or reengage synaptic connections. Insults to these mechanisms are common, not only in aging and mental illness, but in the everyday foibles of “our frail and feeble mind” (Albert Einstein), and in the lapse of rational behavior during stress. Thus, understanding the dependence of PFC on modulatory events will help to illuminate both the mechanisms of human weakness, and the goals for remediation.
Four bullet points.
Prefrontal cortical circuits are uniquely modulated, different from neuroplasticity
Ca-cAMP signaling opens ion channels on long, thin spines to rapidly gate connections
This process, called Dynamic Network Connectivity (DNC), shapes mental state
DNC dysregulation with advanced age and in schizophrenia leads to cognitive deficits
Acknowledgments
The authors are grateful to our colleagues Y. Yang, N. Gamo, L. Jin, and A. Duque for their inspiration and hard work. This work was funded by NIH awards PO1 AG030004, RL1AA017536 and a NARSAD Distinguished Investigator Award to A. Arnsten, and by NIH MH 09335401 and an Alzheimer’s Association New Investigator Research Grant to M. Wang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban NB, Narendran R, Hwang DR, Laruelle M, Slifstein M. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2011 Jul 18; doi: 10.1177/0269881111409265. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci U S A. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. The use of alpha2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10:1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Through the looking glass: Differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Patricia Goldman-Rakic: A Remembrance. Neuron. 2003;40:465–470. [Google Scholar]
- Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;32:267–287. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Paspalas CD, Gamo NJ, YY, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cog Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine’s influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Bjorklund A, Dunnett S, Iversen L, Iversen S, editors. Dopamine Handbook. Oxford, UK: Oxford University Press; 2009. pp. 230–249. [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7:101–114. doi: 10.1517/14728222.7.1.101. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N, Group SS. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Bigham MH, Lidow MS. Adrenergic and serotonergic receptors in aged monkey cortex. Neurobiol Aging. 1995;16:91–104. doi: 10.1016/0197-4580(95)80012-g. [DOI] [PubMed] [Google Scholar]
- Birnbaum SB, Yuan P, Wang M, Vijayraghavan S, Bloom A, Davis D, Gobeske K, Sweatt D, Manji HK, Arnsten AFT. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Dolinsky B, Vu MA, Stanley M, Yeckel MF, Arnsten AF. Blockade of IP3-mediated SK channel signaling in the rat medial prefrontal cortex improves spatial working memory. Learn Mem. 2008;15:93–96. doi: 10.1101/lm.767408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Yuan P, Dickstein DL, Rocher AB, Hof PR, Manji HK, Arnsten AF. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol Aging. 2009;30:782–792. doi: 10.1016/j.neurobiolaging.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc Sport Sci Rev. 2012;40:138–144. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AVJ. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem Pharmacol. 2009;78:852–862. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Castner SA, Smagin GN, Piser TM, Wang Y, Smith JS, Christian EP, Mrzljak L, Williams GV. Immediate and sustained improvements in working memory after selective stimulation of α7 nicotinic acetylcholine receptors. Biol Psychiatry. 2011;69:12–18. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran K, Stoll J, Giordano T, Atack JR, Matocha MF, Brady DR, Rapoport SI. Differential expression of cytochrome oxidase (COX) genes in different regions of monkey brain. J Neurosci Res. 1992;32:415–423. doi: 10.1002/jnr.490320313. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MR, Shanks DR, Robbins TW, Bullmore ET, Dickinson A, Fletcher PC. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(Pt 9):2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Kyriazis DA, Baxter MG. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci. 2011;14:1510–1512. doi: 10.1038/nn.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Cohen A, Gandy M, Colombo P, Van Dusseldorp G, NS, Romano J. Lexical priming deficits as a function of age. Behav Neurosci. 1990;104:286–295. [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nature Reviews Neuroscience. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Deng X, Takaki H, Wang L, Kuroki T, Nakahara T, Hashimoto K, Ninomiya H, Arinami T, Inada T, Ujike H, et al. Positive association of phencyclidine-responsive genes, PDE4A and PLAT, with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:850–858. doi: 10.1002/ajmg.b.31233. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog in Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Bauman A, Kapiloff MS. A-kinase anchoring proteins as the basis for cAMP signaling. Handb Exp Pharmacol. 2008;186:3–14. doi: 10.1007/978-3-540-72843-6_1. [DOI] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2006 Jul 24; doi: 10.1016/j.neurobiolaging.2006.05.025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Zhou P, Milner TA, Pickel VM. Spatial and intracellular relationships between the alpha7 nicotinic acetylcholine receptor and the vesicular acetylcholine transporter in the prefrontal cortex of rat and mouse. Neuroscience. 2009;161:1091–1103. doi: 10.1016/j.neuroscience.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrande V, Kaas JH. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol. 2010;588:1281–1292. doi: 10.1113/jphysiol.2009.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–2072. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Bethesda: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil Trans R Soc London. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. mGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cerebral Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker SJ, Chen J, Nicodemus KK, Sambataro F, Yang F, Mattay V, Lipska BK, Hyde TM, Song J, Rujescu D, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15:509–518. doi: 10.1038/nm.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates. Comp Psychol Monogr. 1936;13:1–68. [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kim S, Bobeica I, Gamo NJ, Arnsten AF, Lee D. Effects of α-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology. 2011 Oct 7; doi: 10.1007/s00213-011-2520-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Lachman ME, Pruessner JC, Sliwinski MJ, Wilson RS. Mechanisms of age-related cognitive change and targets for intervention: Social interactions and stress. J Gerontol A Biol Sci Med Sci. 2012 May 8; doi: 10.1093/gerona/gls125. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian VH, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Kroner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos GR. Pathophysiologically based treatment interventions in schizophrenia. Nature Medicine. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Li B-M, Mao Z-M, Wang M, Mei Z-T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacol. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V pyramidal neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao ZM, Arnsten AFT, Li BM. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- Marcovac J, Goldstein G. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- McAllister TW, McDonald BC, Flashman LA, Ferrell RB, Tosteson TD, Yanofsky NN, Grove MR, Saykin AJ. Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: Altered working memory and BOLD response. Int J Psychophysiol. 2011;82:107–114. doi: 10.1016/j.ijpsycho.2011.06.022. [Available on 3212012/3210910/3210921] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, Aman MG, McDougle CJ, Tierney E, Shiraga S, Whelan F, Arnold LE, Posey D, Ritz L, Vitiello B, Scahill L. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity. J Child Adolesc Psychopharmacol. 2010;20:1–5. doi: 10.1089/cap.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James RS, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Mouradian RD, Seller FM, Waterhouse BD. Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence of mediation by an alpha1-receptor-linked second messenger pathway. Brain Res. 1991;546:83–95. doi: 10.1016/0006-8993(91)91162-t. [DOI] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Okada K, Nakamura K, Kobayashi Y. A neural correlate of predicted and actual reward-value information in monkey pedunculopontine tegmental and dorsal raphe nucleus during saccade tasks. Neural Plast. 2011:579840. doi: 10.1155/2011/579840. epub 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield S, Hancock J, Mason A, Hobson SA, Wynick D, Kelly E, Randall AD, Marrion NV. Receptor-mediated suppression of potassium currents requires colocalization within lipid rafts. Mol Pharmacol. 2009;76:1279–1289. doi: 10.1124/mol.109.058008. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Min Wang M, Arnsten AFT. Constellation of HCN Channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex — Potential substrate for working memory deficits in schizophrenia. Cereb Cortex. 2012 Jun 12;2012 doi: 10.1093/cercor/bhs152. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Selemon LD, Arnsten AF. Mapping the Regulator of G Protein Signaling 4 (RGS4): Presynaptic and postsynaptic substrates for neuroregulation in prefrontal cortex. Cereb Cortex. 2009;19:2145–2155. doi: 10.1093/cercor/bhn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cognit Neurosci. 1995;7:1–26. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJF, Lou J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Spocter MA, Stimpson CD, Erwin JM, Bonar CJ, Allman JM, Hof PR, Sherwood CC. Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons in the prefrontal cortex of anthropoid primates. Neuroscience. 2009;158:1551–1559. doi: 10.1016/j.neuroscience.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 2004;92:361–371. doi: 10.1152/jn.00673.2003. [DOI] [PubMed] [Google Scholar]
- Ramos B, Birnbaum SB, Lindenmayer I, Newton SS, Duman R, Arnsten AFT. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Ramos B, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AFT. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biological Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR. Nucleus basalis of Meynert neuronal activity during a delayed response task in a monkey. Brain Res. 1986;399:364–368. doi: 10.1016/0006-8993(86)91529-5. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal cortices: A tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes. Learn Mem. 2005;12:232–238. doi: 10.1101/lm.92405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius LJ, Weinberger DR, Hyde TM, Harrison PJ, Kleinman JE, Lipska BK. Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology. 2008;33:2626–2634. doi: 10.1038/sj.npp.1301669. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Depth distribution of neuronal activity related to a visual reaction time task in the monkey prefrontal cortex. J Neurophysiol. 1989;61:435–446. doi: 10.1152/jn.1989.61.2.435. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, Arnsten AFT, Cohen DJ, Leckman JF. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Amer J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Advances in Pharmacology. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: relation between intraparietal and principal sulcal cortex. J Comp Neurol. 1984;226:403–420. doi: 10.1002/cne.902260309. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol. 1991;307:144–162. doi: 10.1002/cne.903070113. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001a;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Daniel D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001b;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 2009;29:3627–3641. doi: 10.1523/JNEUROSCI.4726-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby MD, Wojcikiewicz RJ. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Biochem J. 2005:493–497. doi: 10.1042/BJ20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Kounios J, Parrish TB, Jung-Beeman M. A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci. 2009;415–32:415–432. doi: 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- Sun D, Stuart GW, Jenkinson M, Wood SJ, McGorry PD, Velakoulis D, van Erp TG, Thompson PM, Toga AW, Smith DJ, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry. 2009;14:976–986. doi: 10.1038/mp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum SG, Ubriani R, Arnsten AFT. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J Neuroscience (Online) 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ. Fatigue in the executive cortical network demonstrated in narcoleptics using functional magnetic resonance imaging--a preliminary study. Sleep Med. 2005;6:399–406. doi: 10.1016/j.sleep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;47:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Lucero MT. Modulation by PKA of the hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Membr Biol. 2002;188:115–125. doi: 10.1007/s00232-001-0178-y. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1α and Regulator of G Protein Signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167:1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AFT. Neuronal basis of age-related working memory decline. Nature. 2011a;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley AG, Nou E, et al. Alpha2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Gamo NJ, Jin LE, Mazer JA, Wang XJ, AFTA Roles of NMDA and AMPA Receptor Stimulation in Persistent Firing in Dorsolateral Prefrontal Cortical Networks During a Working Memory Task. Society for Neuroscience Abstracts 2011b [Google Scholar]
- Wang Q, Limbird LL. Regulation of α2AR trafficking and signaling by interacting proteins. Biochem Pharmacol. 2007;73:1135–1145. doi: 10.1016/j.bcp.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobio Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]