Abstract
Anabolic androgenic steroids (AAS), synthetic testosterone derivatives that are used for ergogenic purposes, alter neurotransmission and behaviors mediated by GABAA receptors. Some of these effects may reflect direct and rapid action of these synthetic steroids at the receptor. The ability of other natural allosteric steroid modulators to alter GABAA receptor-mediated currents is dependent upon the phosphorylation state of the receptor complex. Here we show that phosphorylation of the GABAA receptor complex immunoprecipitated by β2/β3 subunit-specific antibodies from the medial preoptic area (mPOA) of the mouse varies across the estrous cycle; with levels being significantly lower in estrus. Acute exposure to the AAS, 17α-testosterone (17α-MeT), had no effect on the amplitude or kinetics of inhibitory postsynaptic currents in the mPOA of estrous mice when phosphorylation was low, but increased the amplitude of these currents from mice in diestrus, when it was high. Inclusion of the protein kinase C (PKC) inhibitor, calphostin, in the recording pipette eliminated the ability of 17α-MeT to enhance currents from diestrous animals, suggesting that PKC-receptor phosphorylation is critical for the allosteric modulation elicited by AAS during this phase. In addition, a single injection of 17α-MeT was found to impair an mPOA-mediated behavior (nest-building) in diestrus, but not in estrus. PKC is known to target specific serine residues in the β3 subunit of the GABAA receptor. Although phosphorylation of these β3 serine residues showed a similar profile across the cycle, as did phosphoserine in mPOA lysates immunoprecipitated with β2/β3 antibody (lower in estrus than in diestrus or proestrus), the differences were not significant. These data suggest that the phosphorylation state of the receptor complex regulates both the ability of AAS to modulate receptor function in the mPOA and the expression of a simple mPOA-dependent behavior through PKC-dependent mechanism that involves the β3 subunit and other sites within the GABAA receptor complex.
Keywords: Anabolic steroid, GABAA receptor, phosphorylation, PKC, medial preoptic area, inhibitory postsynaptic current
Anabolic androgenic steroids (AAS) are synthetic derivatives of testosterone initially developed for the treatment of chronic wasting diseases but whose use is now predominantly illicit and for ergogenic purposes (for review, Basaria et al., 2010; Kanayama et al., 2010, Wood and Stanton, 2012). Secondary to their potential to build muscle mass and enhance athletic performance, AAS are known to impose significant changes on neural function and on behavior, and such changes are recapitulated in animal models (for review, Clark and Henderson, 2003; Wood 2008; Oberlander et al., 2012a,b). Many of the behavioral actions of AAS arise with long-term exposure and involve signaling mediated by classical nuclear hormone receptors. However, the AAS can also elicit rapid changes in neural function through direct allosteric modulation of ion channels, in particular modulation of the GABAA receptor, raising the possibility that even with prolonged exposure, rapid and non-genomic actions may underlie some of the behavioral actions of these synthetic steroids (for review, Henderson and Jorge, 2004; Henderson, 2007; Oberlander et al., 2012b).
Allosteric modulation of GABAA receptors by neurosteroids, naturally occurring derivatives of progesterone, testosterone and corticosterone, is an extensively studied phenomenon (for review, Vicini et al., 2004; Mitchell et al., 2008). The GABAA receptor is a target for a wide range of kinases (for review, Tasker, 2000, 2004; Brandon et al., 2002; Song and Messing, 2005), and the phosphorylation state of the receptor (and/or the receptor and its associated proteins) has complex, but significant, effects on the ability of the neurosteroids to modulate the receptor. For example, phosphorylation mediated by protein kinase C (PKC) diminishes the modulation of the GABAA receptor by neurosteroids that enhance currents (positive neurosteroids) in spinal cord neurons of young rats of undetermined sex (Vergnano et al., 2007), in pyramidal neurons of the piriform cortex of male rats (Kia et al., 2011), and in supraoptic nucleus neurons of pregnant female rats (Brussaard et al., 1997; Koksma et al., 2003). In contrast, PKC-dependent phosphorylation augments modulation by positive neurosteroids in supraoptic neurons of young, male rats (Fáncsik et al., 2000) and in dentate granule cells of young rats (of either sex) (Harney et al., 2003). Moreover, inhibition of PKC has been shown to antagonize the ability of positive neurosteroids in the ventral tegmental area to enhance lordosis; an effect likely reflecting a change in the modulation of GABAA receptors in that area (Frye and Walf, 2008). PKC binds directly to the intracellular domains of the β subunits and phosphorylates specific serine residues in these regions (serine 409 in β1, serine 410 in β2 and serines 408/409 in β3) (Brandon et al., 1999, for review, Brandon et al., 2002), suggesting that these may be key sites for PKC-dependent regulation of neurosteroid modulation of the GABAA receptor.
In regions of the forebrain and hypothalamus, the sensitivity of the GABAA receptor to neurosteroid modulation has also been shown to vary significantly with hormonal state (for review, Brussaard and Herbison, 2000; Maguire and Mody, 2009; Smith et al., 2009). Such differences in neurosteroid modulation have most often been associated with underlying changes in the expression of specific GABAA receptor subunits. For example, the insensitivity of supraoptic neurons in rats at the time of parturition to modulation by allopregnanolone was initially attributed to a switch in the relative expression of α2 to α1 subunits (Brussaard et al., 1997), although this finding was later recanted (Koksma et al., 2005). Differences in the sensitivity across the estrous cycle of mPOA neurons to the positive neurosteroids allopregnanolone and 3α-diol were suggested to correlate with the expression of the ε subunit in this region (Jorge et al., 2002). The best characterized relationship between subunit expression and neurosteroid sensitivity has been with regard to the δ subunit. In the hippocampus and other forebrain regions, it has been proposed that as neurosteroid levels rise over the estrous cycle, they upregulate the expression of δ subunits (Shen et al., 2005; Maguire and Mody, 2007), thus creating a homeostatic mechanism (Maguire and Mody, 2009) whereby these neurosteroids regulate their own impact by altering the expression of the subunit that most strongly determines the sensitivity of the GABAA receptor to their action (for review, Henderson, 2007; Mitchell et al., 2008). Such neurosteroid-induced changes in δ subunit expression have also been proposed to reciprocally regulate the sensitivity of forebrain regions to allosteric modulation by these neurosteroids with other changes in hormonal state, such as puberty (Shen et al., 2007; 2010) and pregnancy/parturition (Maguire et al., 2009). While the preponderance of studies implicate changes in subunit composition as a major mechanism underlying steroid-dependent changes in sensitivity to allosteric modulators, Koksma et al. (2003) provide evidence that steroid-dependent changes in sensitivity of GABAA receptors to neurosteroid modulation can be elicited in juvenile males or postpartum females (cycling females were not examined) by manipulating the activity of PKC, or serine/threonine phosphatases, without changes in subunit composition. Taken together with studies demonstrating the importance of phosphorylation in neurosteroid modulation (for discussion, Tasker, 2000; 2004), these data support the assertion that steroid-dependent changes in posttranslational modification of the GABAA receptor may also be an important mechanism by which the sensitivity of the receptor to allosteric modulation by neuroactive steroids varies with hormonal state.
Although both neurosteroids and the AAS share a common core ring structure, there are marked structural and functional differences between these two classes of steroid molecules. In brief, the AAS lack key residues that are present in the positive neurosteroids that are required for modulatory capacity of the neurosteroids; the AAS do not directly gate the receptor (as do the neurosteroids); the two classes of steroids exhibit different mechanisms of receptor gating; and they do not show parallel dependence on subunit composition (Henderson, 2007; Oberlander et al., 2012a,b). Thus, one cannot extrapolate from studies of the neurosteroids to know if phosphorylation of the receptor will have an impact on AAS modulation, the structural basis for any effects of that phosphorylation on AAS modulation, or its functional repercussions. Moreover, no studies have shown that the phosphorylation state of the receptor varies with the natural changes in hormonal state, such as those that occur during adolescence or in cycling females. Thus, the goals of the current study were twofold. First, to determine if there are significant differences in the extent of phosphorylation of the GABAA receptor complex between female and male mice prior to the onset of puberty and in adult female mice over the course of the estrous cycle, and second to determine if such differences alter the functional output of an identified population of neurons in response to acute exposure to the AAS, 17α-MeT.
2. Experimental Procedures
2.1 Animals
C57Bl/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) or were obtained from an in-house strain on a C57Bl/6J background (Oberlander et al., 2012b). Animals were group-housed (4/cage) with food and water ad libitum in a temperature-controlled and 12 hr light cycle facility with lights on starting at 0700 hrs. Care was taken to minimize the discomfort and the number of animals used, and all procedures were approved by the Dartmouth College Institutional Animal Care and Use Committee and conducted in accordance with guidelines from the National Institutes of Health. Estrous cycle stages in adult female mice were determined by daily vaginal lavage (Cooper, et al., 1993; Penatti, et al, 2011). Experiments were performed on adolescent male and female mice from postnatal day (PN) 38–42 and on adult females (>PN55).
2.2. Immunoprecipitation and Western blot analyses
2.2.1. Antibodies
Primary antibodies used in this study included a polyclonal antibody directed against the β3 subunit of the GABAA receptor and a polyclonal antibody directed against phosphorylated serine 408/serine 409 of the β3 subunit (Brandon et al., 2000; Jovanovic et al., 2004), a monoclonal antibody directed against the β2/β3 subunits (AB 05-474; Millipore, Billerica, MA, USA), a rabbit polyclonal anti-phosphoserine antibody (AB1603, Millipore) and a mouse monoclonal anti-phosphoserine antibody (05-1000, Millipore). For Western blots, goat anti-rabbit secondary antibodies were obtained from either Pierce Biotechnology Inc. (Rockford, IL, USA) or BioRad (Hercules, CA, USA). The goat anti-mouse secondary was from BioRad.
2.2.2 Protein extraction and immunoprecipitation
Tissue was harvested from the mPOA of adolescent male and female mice and from adult females during proestrus, estrus and metestrus/diestrus. Tissue was lysed in 0.1 ml of lysis buffer (25mM Tris pH 7.5, 150mM NaCl, 5mM MgCl2, 1% NP-40, 5% glycerol, 0.001% TritonX-100, 1mM PMSF, 2mM NaF, and 1X Complete-mini (Roche, Indianapolis, IN, USA) protease inhibitor cocktail, and protein concentration determined using a BCA Protein Assay (Pierce). Total protein (200 μg) was immunoprecipitated (IP) with 10μg of AB 05-474 overnight at 4°C with rotation. Protein G agarose (50 μL; Pierce) was then added to the antigen-antibody complex and incubated for 2 hrs at 4°C with rotation. Subsequently, 500 μL IP buffer (25mM Tris, 150mM NaCl; pH 7.2) was added, gently mixed, centrifuged for 3 min 2,500 μg (three times, with a final wash of 50μl dH20), and the supernatant discarded. Electrophoresis loading buffer (5X: 300mM Tris, 50% glycerol, 5% SDS, 5% β-mercaptoethanol, 0.2% bromophenol blue; 50μL) was added, the sample heated for 5 min at 95°C and re-centrifuged for 3 min at 2,500 μg. Immunoprecipitates (7 μL) were separated by 7.5% SDS-PAGE and electrophoretically transferred to Immobilon-P membranes (Millipore).
2.2.3. Western blotting
Two protocols for Western blots were used. The first protocol was used for tissue from adolescent mice, as well as adult females; the second was used only for adult females. In all experiments, membranes were blocked for 1 hr at room temperature in 5% BSA/TBS, incubated in primary antibody overnight at 4°C and subsequently in secondary antibody for 1 hr at room temperature. In the first protocol, phosphoserine signal of the material immunoprecipitated by the β2/β3 antibody was detected with either the AB1603 (1:5000) or 05-1000 (1:5000) antibody in 5% BSA/TBST and a secondary anti-rabbit (Pierce; 1:5000) or anti-mouse (BioRad; 1:300,000), respectively, in 5% milk/TBST. In the second protocol, signal corresponding to phosphoserine 408/409 of the β3 subunit of the material immunoprecipitated by the β2/β3 antibody was detected with the polyclonal antibody specifically directed against these phosphoserine residues (1:5000 in 5% BSA/TBST) and with the secondary goat anti-rabbit antibody (BioRad; 1:200,000) in 1% BSA/TBST.
For both protocols, membranes were washed three times for 5min in TBST (20mm Tris, 150mm NaCl, 0.1%Tween-20, pH7.4) before incubation with the secondary antibody and again before chemiluminescent detection (SuperSignal West Femto Chemiluminescent substrate; Pierce). Signals were visualized on autoradiographic film (Kodak, Rochester, NY, USA), and densitometric measurements were obtained using a ChemiDoc™ XRS+System (BioRad). Membranes were subsequently stripped (30 min at 50°C in 70mM Tris (pH 6.8), 2% SDS, 0.1% β-mercaptoethanol), rinsed with TBS (20mm Tris, 150mm NaCl; pH 7.4), blocked (3% BSA/TBST) and re-probed with the polyclonal antibody directed against the β3 subunit (1:5000 in 1% BSA/TBST).
2.3 Physiological recordings
Coronal sections (250μm) corresponding to the rostral portion of the mPOA (0.14 mm Bregma) were prepared using an Electron Microscopy Sciences OTS-4000 vibroslicer. All recordings were made according to protocols previously described (Penatti et al., 2010; 2011) between 1300 and 1800 hr at room temperature. Briefly, slices were superfused with 95%O2/5%CO2-saturated artificial cerebrospinal fluid (aCSF; in mM: 125 NaCl, 1.2 CaCl2, 10 glucose, 4 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 1 ascorbic acid; pH 7.4) supplemented with 2mM kynurenic acid. Recordings were performed in the whole-cell configuration at a holding potential (Vhold) of −70 mV. The pipette solution consisted of (in mM): 153 CsCl, 1 MgCl2, 5 EGTA, and 10 HEPES, to which 2 MgATP was added just prior to each experiment. The identity of synaptic currents as GABAergic was confirmed in some experiments by demonstrating blockade of events by the selective GABAA receptor competitive antagonist, bicuculline (20μM). Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded for 5 min, followed by a wash-in of 1 μM 17α-MeT (Yang et al., 2002; 2005; Clark et al., 2004) or 1 μM 3α-diol (5α-androstane-3α,17β-diol; Jorge et al., 2002), a subsequent recording for 5 min, and then a 5 min washout of the AAS (and subsequent recording). To record miniature IPSCs (mIPSCs), 1 μM tetrodotoxin (TTX; Sigma) was added to the kynurenic acid-containing aCSF bath solution to block action potential-dependent GABA release. In experiments to determine the effects of inhibiting PKC, calphostin (20nM or 200nM in 0.01% DMSO; Sigma, St. Louis, MO, USA) was added to the pipette solution (Kobayashi et al., 1989; Werner et al., 2011).
All physiological data was acquired and stored using a HEKA-EPC9 amplifier (HEKA Instruments, Bellmore, NY USA). Averaged IPSCs were analyzed for peak current amplitude (Ipeak), frequency, and decay kinetics (biphasic and fitted with two time constants, τ1 and τ2) or with a single weighted time constant (τw). Recordings were only accepted for analysis if the access resistance was < 25MΩ and Ihold did not change more than 10% during the recording. In the majority of experiments, a single recording was made per slice prepared from each animal, and no one animal within a given group disproportionately contributed cells to the group averages.
2.4 RNA extraction, reverse transcription and quantitative real time PCR (qRT-PCR)
Real time PCR protocols were employed as described previously (Penatti et al., 2009). Briefly, tissue from the mPOA was dissected and stored in RNAlater® (Ambion Inc., Austin TX, USA) at −20°C. Total RNA was extracted according to the manufacturer’s protocol for RNAqueous (DNase treated with TURBO DNA-free), and the concentration of the RNA was determined by measuring the optical density at 260 nm. RNA was reverse transcribed using material and protocols included in the High Capacity RNA-to-cDNA™ kit (Ambion). Quantitative RT-PCR was performed using an AB 7500 Sequence Detection System, and all cDNAs were analyzed in triplicate with TaqMan®Gene Expression Assays (ABI). Samples with reverse transcriptase omitted were used to control for genomic DNA contamination and with template omitted to control for any reagent contamination. The specific subunit expression assays were: α1 (Mm00439046_m1), α2 (Mm00433435_m1), α5 (Mm00621092_m1), β1 (Mm00433461_m1), β2 (Mm00549788_s1), β3 (Mm00433473_m1), γ1 (Mm00439047_m1), γ2 (Mm00433489_m1), δ (Mm00433476_m1), ε (Mm00489932_m1), and 18S rRNA (Hs99999901_s1). All gene expression assays used were demonstrated to amplify with equal efficiencies. Data are expressed normalized to internal standard as 2−ΔCt values (Livak and Schmittgen, 2001; Peirson et al., 2003).
2.5 Behavioral analyses
2.5.1 Nest building
Nest building behavior, a measure of thermoregulation controlled by the mPOA, was assessed according to Deacon (2006a). Mice were placed in a clean cage overnight with a 3g nestlet (a square piece of shreddable material) following injections of either ~ 25 μL sesame oil or 17α-MeT (7.5 mg/kg in sesame oil). The next day, the built nest was assessed on a scale of 1–5, (a score of 1 was no nest building observed, and a score of 5 indicating a complete nest with walls taller than the mouse). The remaining, unshredded nestlet was also weighed as a separate measure of nest building. Each mouse was initially injected with oil and then tested. The next time that the mouse reached the same stage (either estrus or diestrus), it received an injection of 17α-MeT and was re-tested.
2.5.2 Marble burying test
The marble burying test (MBT) was conducted to assess defensive anxiety (Deacon, 2006b) using methods previously described (Njung’e and Handley, 1991; Deacon, 2006b). Thirty minutes following a single injection of 17α-MeT or oil, each mouse was placed in a clean cage with 5cm thickness of bedding and 18 marbles (5.0g each) placed in an array of 3 × 6 over the extent of the cage. Mice were allowed to dig/bury marbles for 30 min, before being removed. Marbles that were covered by bedding for ~2/3rd or greater of their surface were scored as 1. Marbles that were buried so that they were completely hidden within the bedding were scored as 1.5. Mice were treated and tested/re-tested as described above for nest building.
2.5.3 Elevated plus maze
The elevated plus maze used in these experiments consisted of two open arms (38L×5W cm) and two closed arms (38L×5W×15H cm) extending from a common central platform (5×5 cm) and elevated by 60 cm. The floor of the maze is dark Plexiglas, with side and end walls of closed arms made from clear Plexiglas. Tests were conducted under a dim, red light (4×60 W indirect) and the investigator was outside of the room. Animals were brought to the testing room 1 hr before assays and injections were made 30 min before testing. Tests were made for a period of 10 min and the apparatus was thoroughly cleaned with 70% ethanol between each test. Behaviors were scored by the observer in real time for arm entries and time spent in the open and closed arms and in the center compartment. Observers were blind to the treatment condition of the animals.
2.6 Statistical analysis
Values are presented as means ± standard error. To test for normality, Shapiro-Wilks or Kolmogorov-Smirnov tests were applied on the raw data. For qRT-PCR analysis, CT values calculated as described (Penatti et al., 2009, 2010, 2011) and were defined as outliers when they lay outside ± 3 standard deviations from the mean. Results were qualitatively the same whether or not outliers were included in the final analysis. Differences in the relative abundance of each mRNA between control and AAS-treated subjects were assessed using Pair-Wise Fixed Reallocation Randomization© t-test using the Excel-based Relative Expression Software Tool (REST©). Significance for all assays was determined by one-way analysis of variance (ANOVA) followed by Fisher’s Least Significant Difference Test, Student-Newman-Keuls test or a paired Student’s t-test, as appropriate. For all data, the alpha level was set at p < 0.05.
3. Results
3.1 Hormonal state-dependence of phosphoserine levels in the GABAA receptor complex
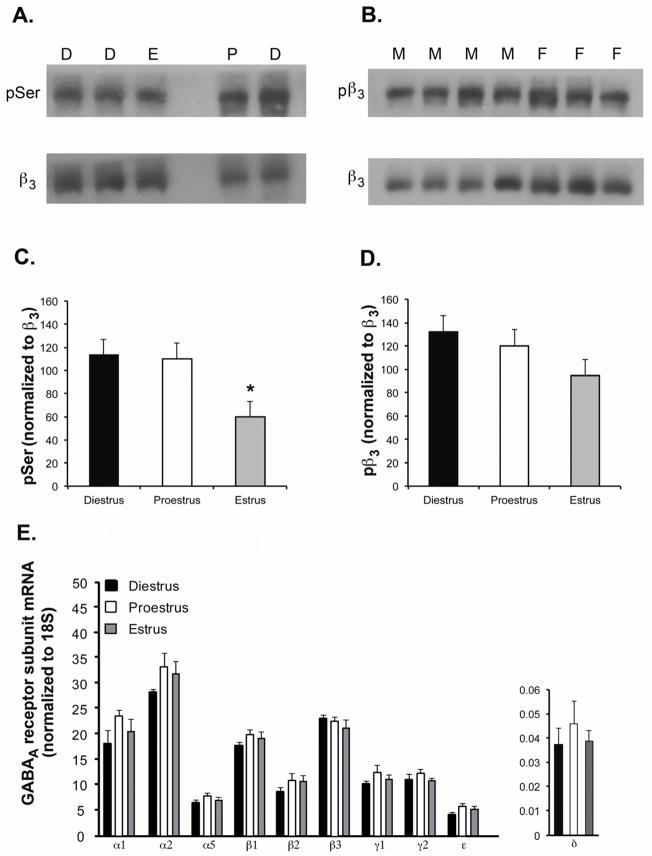
To determine if levels of serine phosphorylation of the GABAA receptor complex varied with hormonal state, tissue isolated from the mPOA of adolescent male and female mice and from adult females at different stages of the estrous cycle was immunoprecipitated with an antibody directed against the β2/β3 subunit of the receptor. The precipitate was subsequently assessed by Western blot analysis for the levels of phosphoserine, and that signal normalized to the levels of the β3 subunit. All antibodies recognized a band of the appropriate size for β3 or phosphorylated β3 (~58 kDa). No significant differences were evident in the levels of phosphoserine between adolescent male and female mice (F1,13 = 0.05, p = 0.83; n = 6 females and n = 8 males) (Figure 1B).
Figure 1. Changes in GABAA receptors across the estrous cycle.
(A) Top: representative Western blot illustrating signals corresponding to phosphoserine (pSer) in tissue harvested from individual animals in diestrus (D), proestrus (P) and estrus (E). Lysates were immunoprecipitated with a monoclonal antibody directed against the β2/β3 subunits of the GABAA receptor prior to electrophoresis and Western blotting. Bottom: Following incubation with antibody directed against pSer, the blot was stripped and re-probed with the antibody directed against β3. (B) Top: representative Western blot illustrating the signal for phosphoserine408/409 in the β3 subunit (pβ3) and the β3 subunit (β3) (Bottom) in mPOA tissue harvested from adolescent male (M) and female (F) mice. All signals corresponded to a molecular weight of 58 kDa. (C) Averaged data from female mice in diestrus (n = 7), proestrus (n = 7) and estrus (n = 9) corresponding to the signal for pSer normalized to that of β3. (D) Averaged data from female mice in diestrus (n = 9), proestrus (n = 10) and estrus (n = 11) corresponding to the signal for pβ3 normalized to that of β3. Assessment with the antibody was also made for a second group of animals (7 in diestrus, 7 in proestrus, and 9 in estrus) with a different lot of this antibody with comparable results (data not shown). (E) Levels of GABAA receptor subunit mRNAs in the mPOA. Data are presented as the 2−ΔCT values, which indicate the average levels of subunit mRNAs relative to 18S in tissue from diestrous (n = 9), proestrous (n = 10) and estrous (n = 10) mice. P values were: α1: 0.28; α2: 0.37; α5: 0.23; β1: 0.46; β2: 0.37; β3: 0.52; γ1: 0.36; γ2: 0.41; δ: 0.93; ε: 0.17.
Significant differences in the normalized signal for phosphoserine were evident in mPOA tissue isolated from adult female mice in different stages of the estrous cycle (F2,22 = 5.35, p < 0.01; Figure 1A,C), with the ratio being lower in the estrous phase than in either diestrus (p < 0.001) or proestrus (p <0.05). No significant differences from across the estrous cycle were observed in levels of any of the mRNAs encoding GABAA receptor subunits expressed at appreciable levels in the mPOA (α1, α2, α5, β1, β2, β3, γ1, γ2, and ε; Figure 1E) or in the δ subunit mRNA, which we found to be expressed at vanishingly low levels in the mPOA (Figure 1E), consistent with previous studies (Wisden et al., 1992; Pirker et al., 2000; Penatti et al., 2009). Overall, as levels of GABAA receptor mRNA and protein are in most cases believed to be comparably regulated, these data suggest that there were not significant cycle-dependent changes in the expression of subunits know to be targets for serine/threonine kinases (for review, Brandon et al., 2002; Jovanovic, 2006) that may have contributed to the observed differences in phosphoserine levels.
3.2 Acute modulation of GABAA receptor-mediated post-synaptic current by AAS is dependent on estrous cycle stage
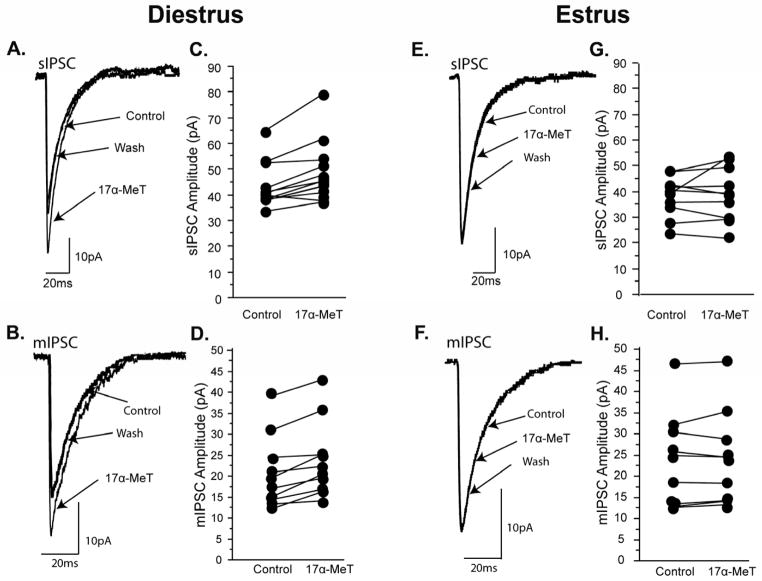
To determine if differences in the levels of serine phosphorylation of the GABAA receptor across the estrous cycle were correlated with differences in the ability of the AAS 17α-MeT (1 μM) to allosterically modulate the receptor, sIPSCs were recorded in acutely isolated slices of the mPOA at estrus, a time when serine phosphorylation was low and diestrus, a time when it was high. Estrus and diestrus were also chosen to focus on the potential importance of 17β-estradiol (E2) as a regulator of PKC in the absence of the high levels of progesterone and positive neurosteroids that are characteristic of proestrus (Freeman, 1994; Reddy, 2012). One micromolar 17α-MeT was used since serum levels of AAS have been estimated to reach micromolar concentrations in human subjects who chronically abuse these steroids (Masonis and McCarthy, 1995; Wu, 1997), and evaluation of male volunteers administered AAS in a clinical setting indicates that even moderate, short-term use can raise AAS levels in CSF to as high as 0.9 μM (Daly et al., 2001). Thus, a 1 μM concentration reflects a level believed to be present in those who illicitly self-administer these steroids, and this concentration has previously been shown to elicit significant modulation of the GABAA receptor (Yang et al., 2002; 2005; Jones et al., 2006).
The predominant GABAA receptor expressed in the mPOA is likely to contain either an α1 or an α2 subunit (Nett et al., 1999; Davis et al., 2000; McIntyre et al., 2002; Penatti et al., 2005). Expression of α2 is preferentially localized to the soma and α1 is concentrated on processes within this region (Penatti et al., 2005). In addition, pharmacological experiments indicate that α5-containing receptors make a modest, but significant, contribution to whole-cell GABA-evoked currents in mPOA neurons (Penatti et al., 2009). In the mPOA, γ1 mRNA is expressed at comparable levels to that of γ2, but substitution of a γ1 for a γ2 subunit confers no notable difference in decay kinetics or on modulation by 17α-MeT (Clark et al., 2004). Thus, currents recorded in mPOA whole-cell recordings are likely to predominantly arise from α2βxγ1/2-containing receptors. Consistent with this assumption, time constants of synaptic current decay found here were in agreement with the slower kinetics observed with native or recombinant α2 (or α5) -containing receptors (for discussion, Yang et al., 2002, 2005; Penatti et al., 2009) and with expression of high levels of α2 and moderate levels of α5 in this region (Wisden et al., 1992; Pirker et al., 2000; Jorge et al., 2002; Penatti et al., 2005, 2009; Figure 1E). Current decay kinetics were in agreement with those reported previously for neurons within the mPOA for rats and mice of both sexes (Haage and Johansson, 1999; Jorge et al., 2002; Penatti et al., 2005, 2009; Strömberg et al., 2006, 2009). Moreover, as in previous studies, there was not an observable pattern of any systematic variation in amplitude or kinetics of currents recorded in different mPOA cells.
The frequency, amplitudes, and decay kinetics of sIPSCs in the control recording conditions were not different across the stages of the estrous cycle (Table 1). Addition of 17α-MeT significantly (p < 0.01) increased the amplitude of the sIPSC in mPOA neurons of diestrous mice (Table 1 and 2A; Figure 2A,C), but was without effect (p = 0.45) on sIPSCs recorded from estrous mice (Table 1 and 2A; Figure 2E,G). The effect of 17α-MeT on sIPSC amplitudes in diestrus was reversible upon washout of the AAS. Acute application of 17α-MeT did not alter the frequency or the decay kinetics of sIPSCs in either stage. These results are consistent with the known actions of this steroid on receptor subtypes likely to predominate in the mPOA (Yang et al., 2005; Clark et al., 2004; vide supra). Recordings made in the presence of 1 μM TTX to isolate mIPSCs demonstrated comparable correlations: acute exposure to 17α-MeT significantly (p < 0.01) enhanced the amplitude of mIPSCs during diestrus (Table 1 and Figure 2B,D), but had no effect on this parameter in recordings made during estrus (p = 0.59) (Table 1 and Figure 2F,H). Moreover, as with sIPSCs, there were no significant effects of application of this AAS on the kinetics of mIPSC decay or on the frequency at either stage (Table 1). Taken together, these data suggest that AAS elicit significant effects on the postsynaptic properties of the GABAA receptor, but only during the diestrous phase when levels of phosphoserine are elevated.
Table 1.
Parameters of GABAA receptor-mediated sIPSCs and mIPSCs
| A. sIPSCs | |||||
|---|---|---|---|---|---|
| amplitude (pA) | τ1 (ms) | τ2 (ms) | τw (ms) | Frequency (Hz) | |
| Diestrus (15) | |||||
| Con | 42.6 ± 2.1 | 11.3 ± 0.8 | 42.0 ± 6.1 | 23.0 ± 3.0 | 5.0 ± 0.7 |
| AAS | 47.3 ± 2.8*** | 12.1 ± 0.9 | 45.3 ± 6.5 | 25.1 ± 2.6 | 5.3 ± 0.8 |
| ***p < 0.001 | |||||
| Estrus (12) | |||||
| Con | 38.4 ± 2.2 | 11.9 ± 0.9 | 38.5 ± 5.2 | 20.7 ± 2.0 | 5.7 ± 0.9 |
| AAS | 39.5 ± 2.7 | 12.3 ± 0.9 | 48.1 ± 6.5 | 27.9 ± 4.3 | 4.9 ± 0.5 |
| B. mIPSCs | |||||
|---|---|---|---|---|---|
| amplitude (pA) | τ1 (ms) | τ2 (ms) | τw (ms) | Frequency (Hz) | |
| Diestrus (10) | |||||
| Con | 20.8 ± 2.8 | 13.2 ± 1.3 | 48.2 ± 7.2 | 30.3 ± 2.7 | 0.6 ± 0.1 |
| AAS | 23.7 ± 2.9** | 14.8 ± 1.4 | 50.0 ± 7.3 | 28.6 ± 5.2 | 0.6 ± 0.1 |
| **p < 0.01 | |||||
| Estrus (9) | |||||
| Con | 24.4 ± 3.7 | 13.8 ± 0.8 | 46.4 ± 5.3 | 20.9 ± 1.7 | 0.7 ± 0.2 |
| AAS | 24.6 ± 3.8 | 12.5 ± 1.0 | 47.4 ± 4.7 | 22.4 ± 1.9 | 0.6 ± 0.1 |
Values are means ± sem. Numbers in parentheses indicate numbers of cells per cycle stage. Asterisk indicates values in the presence of AAS are significantly different from control.
Table 2.
Change in GABAA receptor-mediated sIPSC amplitude following acute addition of steroid
| A. Percent increase in sIPSC amplitude following acute addition of steroid | ||
|---|---|---|
| 17α-MeT | 3α-diol | |
| Diestrus | ***10.6 ± 1.7 (15) | ***14.4 ± 3.2 (7) |
| Estrus | 2.7 ± 3.5 (12) | **10.8 ± 2.6 (8) |
| ***p < 0.001; **p < 0.01 | ||
| B. Percent increase in sIPSC amplitude following acute addition of steroid in the presence of 20 or 200 nM of the PKC inhibitor, calphostin | |||
|---|---|---|---|
| 17α-MeT | 3α-diol | ||
| Diestrus | Diestrus | Estrus | |
| 20 nM | *14.9 ± 6.1 (6) | 3.5 ± 2.3 (5) | 4.2 ± 1.7 (4) |
| 200 nM | 1.4 ± 1.0 (15) | −3.4 ± 4.0 (7) | −0.2 ± 4.5 (5) |
| *p< 0.05 | |||
Measurements were made of the [amplitude (no steroid) − amplitude (steroid)) × 100] −100 to assess the percent change in sIPSC amplitude elicited in an individual cell by addition of steroid. Values represent means ± sem and numbers in parentheses indicate numbers of cells per condition. Asterisks indicate values in the presence of the steroid that were significantly different from control.
Figure 2. Allosteric modulation by AAS of GABAA receptor-mediate synaptic responses in the mPOA in diestrus and estrus.
Representative (A) sIPSCs and (B) mIPSCs from a single mPOA neuron of a diestrus mouse illustrating reversible potentiation of the peak current by acute application of 1 μM 17α-MeT. Data illustrating effect on peak currents for (C) sIPSCs (n = 15) and (D) mIPSCs (n = 10) in individual mPOA neurons from diestrus females with addition of 1 μM 17α-MeT to the bath. Representative (E) sIPSCs and (F) mIPSCs from a single mPOA neuron of an estrus female illustrating no effect of acute application of 1 μM 17α-MeT. Data illustrating effect on peak currents for (G) sIPSCs (n = 10) and (H) mIPSCs (n = 9) in individual mPOA neurons from estrous females with addition of 1 μM 17α-MeT to the bath.
3.3 Comparison of the modulatory effects of the AAS, 17α-MeT, and the androgenic neurosteroid, 3α-diol
We had previously reported that the androgenic neurosteroid, 3α-diol, significantly and reversibly enhanced the peak current amplitudes of GABAA receptor-mediated mIPSCs in the mPOA of the female mouse at all three stages of the estrous cycle, with potentiation being greater in diestrus than in estrus (Jorge et al., 2002). Assessment of the effects of 3α-diol in the current study also demonstrated that this neurosteroid significantly potentiated the peak current of sIPSCs in the mPOA of both diestrous (p<0.001) and estrous (p < 0.01) mice (Table 2A); however while the percent increase in the peak of sIPSCs observed here was greater in diestrus than in estrus, the difference was not significant (Table 2A).
3.4 Allosteric modulation of GABAA receptors by androgenic steroids is PKC-dependent
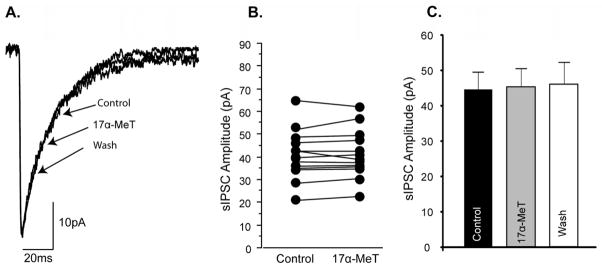
PKC consensus sequences are evident in several of the subunits that comprise the GABAA receptor (for review, Brandon et al., 2002; Jovanovic, 2006). To directly determine if the cycle-specific differences in the ability of 17α-MeT to modulate IPSC current amplitudes were due to PKC-dependent phosphorylation, recordings of sIPSCs were made from neurons of the mPOA of diestrous mice with the PKC inhibitor calphostin in the recording pipette. In the presence of 200nM calphostin, a concentration previously reported to impose ~80% inhibition of PKC activity (Kobayashi et al., 1989) acute application of 17α-MeT no longer elicited a significant enhancement of current amplitudes from mPOA neurons of diestrous mice (Figure 3 and Table 2A). In contrast, the positive modulation elicited by 17α-MeT in the presence of 20 nM calphostin, a concentration reported to impose ~ 20% inhibition (Kobayashi et al., 1989), was not significantly different from that of recordings made in the absence of calphostin (Table 2B). Thus, inhibiting the preponderance of PKC activity (estimated 80%) imposed a profile of AAS modulation in diestrus that mirrored that observed in estrus when levels of phosphoserine associated with the GABAA receptor complex are low. As noted above for recordings made in the absence of calphostin (Section 3.2), AAS application did not alter current decay or frequency of responses in the presence of calphostin at either concentration.
Figure 3. Effect of PKC inhibition on.
allosteric modulation by AAS of GABAA receptor-mediate synaptic responses in the mPOA in the diestrous mouse.
(A) Representative sIPSCs from a single mPOA neuron of a diestrous mouse illustrating the absence of an effect of acute application of 1 μM 17α-MeT when 200nM was present in the pipette solution. (B) Data illustrating effect on peak currents for sIPSCs in individual mPOA neurons from diestrous mice with addition of 1 μM 17α-MeT to the bath and 200 nM calphostin intracellularly. (C) Average peak current amplitudes for mIPSCs in mPOA neurons of diestrous mice prior to (Control), during (17α-MeT), and following (Wash) exposure to 1 μM 17α-MeT (n = 15).
We also compared the effects of calphostin (20 and 200 nM) on modulation elicited by 3α-diol: As with 17α-MeT, 200 nM calphostin abolished the potentiation elicited by this androgenic neurosteroid; in fact the responses were actually smaller than in control conditions, albeit the difference was not significant (Table 2B). For 3α-diol, significant potentiation was also abrogated by 20 nM calphostin (Table 2B), suggesting the dependence of the allosteric response may be more sensitive to the extent of PKC-mediated phosphorylation for this neurosteroid than for the synthetic AAS.
3.5 Acute AAS injection on diestrus significantly impaired an mPOA-mediated behavior
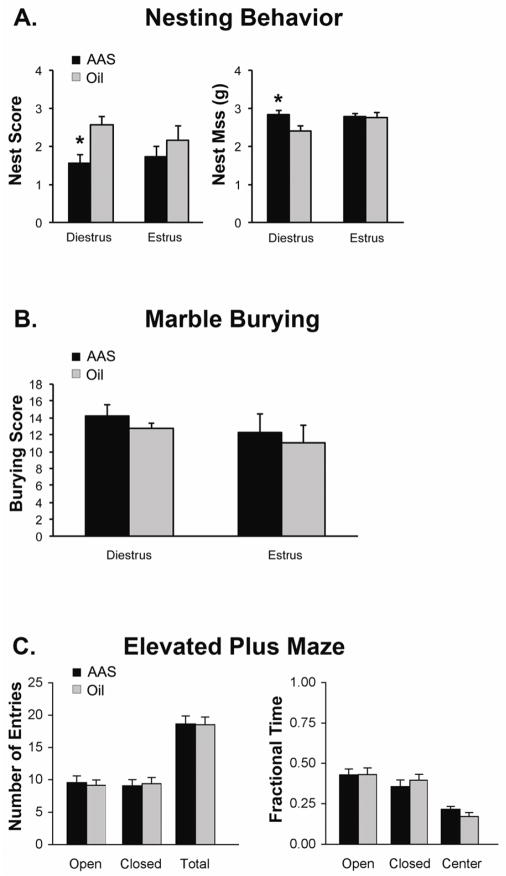
Having shown that AAS allosterically modulate neurons of the mPOA in a PKC-dependent manner, we hypothesized the AAS might affect a naturalistic mPOA-dependent behavior in a similar fashion. The mPOA is a multifunctional brain region, but key among its roles is thermoregulation, and stimulation of GABAA receptors in mPOA increases thermogenesis (Ishiwata et al., 2005; Zaretsky et al., 2006; Rusyniak et al., 2011). Nest building, in turn, is a thermoregulatory behavior dependent upon activity in the mPOA (Deacon, 2006a). In oil-injected mice, there were no differences in nest building scores between diestrous and estrous mice or the amount of nestlet mass remaining at the end of the test (Figure 4A). Following an acute injection of 17α-MeT injection, nest scores (p <0.04) were significantly lower and the amount of nest material remaining was significantly higher (p <0.04) in diestrous mice; however, AAS injection was without effect on either measure in estrous mice (Figure 4A).
Figure 4. Effects of acute systemic exposure to 17α-MeT on behaviors mediated by neural circuits that include the mPOA.
(A) Average data of nest scores and remaining nestlet material (see Section 2.5.1) for diestrous (n = 7 for each cycle stage) and estrous (n = 7 for each cycle stage) mice given a single injection of either oil or 7.5 mg/kg 17α-MeT. (B) Average marble burying scores assess during a 30 min period 30 min following an acute injection of oil or 17α-MeT in diestrus (n = 8 for each cycle stage) and estrus (n = 6 for each cycle stage) mice. (C) Number of entries into the open and closed arms, as well at total number of entries (left) and fraction of total time in the open, closed and center parts of the elevated plus maze for adult female mice (unstaged) assessed during a 5 min period 30 min following injection of oil (n = 20) or 17α-MeT (n = 20). In (A), * indicates p < 0.04.
The mPOA is part of a broader circuitry that regulates anxiety-like (for review, Oberlander and Henderson, 2012b) and locomotor (Hunt et al., 2010; Rusyniak et al., 2011) behaviors. An acute injection of 17α-MeT had no significant effect on marble burying, a measure of defensive behavior (Deacon, 2006b) (Figure 4B) or on generalized anxiety-like behavior as assessed on the elevated plus maze (Figure 4C) in either stage of the cycle.
3.6 Cycle-specific levels of serine phosphorylation of the β3 subunit
Our data indicate that levels of serine phosphorylation of the GABAA receptor complex vary with stage of the estrous cycle and that inhibition of PKC can abrogate the functional differences in AAS modulation of GABAA receptor-mediated currents in the mPOA between diestrous and estrous mice. PKC consensus sequences have been identified in the α4, γ2 and in all three β subunits of the mammalian GABAA receptor (Abramian et al., 2010; for review, Brandon et al., 2002; Jovanovic, 2006). To determine if cycle-specific differences were evident in one of these recognized sites, immunoprecipitates from adult females in diestrus, estrus and proestrus were assayed with a phospho-specific antibody directed against serines 408 and 409 in the β3 subunit (Jovanovic et al., 2004). Western blots with the pSer408/409-specific antibody were also performed for mPOA tissue isolated from adolescent mice (Figure 1B), and these experiments were run with two different preparations of this polyclonal antibody. As with assays for total phosphoserine, no differences in the levels of pSer408/409 were apparent in mPOA tissue of adolescent male versus female mice. In assays of cycling females, with either batch of the antibody, although the ratio was lower in estrus than in either diestrus or proestrus (Figure 1C), the differences were not significant.
4. Discussion
Analysis of recombinant receptors has shown that the mechanisms by which the AAS elicit allosteric modulation of the GABAA receptor are distinct from those of other well-studied modulators of the receptor, including the positive neurosteroids and benzodiazepines (BZ) acting at the high affinity BZ site (Yang et al., 2002; 2005; Jones et al., 2006). Moreover, data from these heterologous expression systems has prompted intriguing, but untested hypotheses that the phosphorylation state of the receptor may impart a significant effect on the ability of the AAS to elicit this modulation (Yang et al., 2005). Despite the differences in mechanisms by which they induce modulation, studies demonstrating that phosphorylation state of the receptors alters the ability of positive neurosteroids to allosterically alter receptor function (Brussaard et al., 1997; Fáncsik et al., 2000; Koksma et al., 2003) further support the hypothesis that such post-translational changes may also have a significant impact on modulation by the AAS. The data presented here not only affirm the hypothesis that the phosphorylation state of the GABAA receptor complex regulates the ability of AAS to elicit allosteric modulation, but go on to demonstrate that such differences have important repercussions for AAS modulation of the function of GABAA receptors in native mPOA neurons and of mPOA-mediated behavior.
4.1. Gonadal steroid-dependent changes in the phosphorylation state of the GABAA receptor in the mammalian forebrain
Here we report that levels of serine phosphorylation of the GABAA receptor complex immunoprecipitated with an antibody directed against the β2/β3 subunits were significantly lower in estrus than in diestrus or proestrus. The cycle-specific differences in phosphorylation were unlikely due to changes in subunit expression since none of the major GABAA receptor subunit mRNAs showed a significant difference across the cycle. In particular, given the importance of the δ subunit in the effects of positive neurosteroids at the GABAA receptor, it is of interest to note that the mPOA expresses nearly negligible levels of δ subunit mRNA and protein (Wisden et al., 1992; Pirker et al., 2000; Penatti et al., 2009; the current study). In addition, consistent with results presented here, there is no observed difference between estrus and diestrus in the levels of δ subunit mRNA in identified gonadotropin releasing hormone neurons, which predominantly reside within the mPOA, but where δ mRNA levels are marginally higher than in the mPOA as a whole (Penatti et al., 2011). The virtual absence of δ subunit in the mPOA is unlikely to have an impact on allosteric modulation by the AAS since 17α-MeT does not elicit modulation at α2β3δ heterologous receptors (Yang et al., 2005). However, it will be of interest to determine if there are important cycle-specific differences in phosphorylation and the relationship of such differences to expression of the δ subunit in the hippocampus where expression of this subunit has a marked impact on neurosteroid effects (for review, Mitchell et al., 2008).
Assessment of the pattern of selective phosphorylation of serines 408/409 in the β3 subunit mirrors that seen with immunoprecipitation of the receptor complex, but the differences were not significant. These data suggest that while cycle-dependent changes may include the β3 subunit, they also likely include serine residues expressed in other subunits of the receptor (which are of comparable size to β3) or potentially even associated proteins of the receptor complex. Additionally, electrophysiological data (Figure 3, Table 2 and vide infra) indicate that PKC plays a significant role in this phenomenon. Thus, while we have not identified all of the residues in the receptor complex that may contribute to the cycle-dependent differences in levels of serine phosphorylation, our data are consistent with a mechanism in which the extent of PKC-dependent phosphorylation of the GABAA receptor complex in the mPOA is regulated by the changes in the endogenous steroid milieu that occur across the cycle.
The activity of PKC in the mPOA (Ansonoff and Etgen, 1998) and in other regions of the mammalian brain (Drouva et al., 1990; Joubert-Bression et al., 1990; Lachowicz and Rebas, 2002) has been shown to increase with rising concentrations of E2. Estrogen levels are higher in diestrus than in estrus (Freeman, 1994), and thus our data demonstrating augmented levels of serine phosphorylation of the GABAA receptor complex in diestrus versus estrus are consistent with an important role for E2-stimulated increases in PKC activity in this process. Clearly the interplay between estrogens and progestins and kinase activity within the hypothalamus and mPOA is multifaceted and complex: PKC influences a number of key neurophysiological targets beyond the GABAA receptor in these cells (Kelly et al., 2002), and estrogens regulate the activity of other kinases within the hypothalamus/mPOA that have the ability to phosphorylate the GABAA receptor (Gonzáles-Flores et al., 2010).
4.2 Implications of changes in phosphorylation of GABAA receptors across the estrous cycle
Previous studies have shown that the sensitivity of GABAA receptors to allosteric modulation varies across the estrous cycle, although results across studies are not consistent. Specifically, in neurons of the mouse mPOA, potentiation of peak mIPSCs is elicited by the endogenous androgenic neurosteroid, 3α-diol, at all three stages of the estrous cycle, with the potentiation being greater in diestrus than in estrus (Jorge et al., 2002). Here, we show that the potentiation of GABAA receptor-mediated mIPSCs and sIPSCs elicited by 17α-MeT is also greater in diestrus than in estrus. In contrast to the previous study, however, while the potentiation of sIPSCs by 3α-diol observed here was greater in diestrus than in estrus, the difference was not significant. This variation between the two studies may arise from differences in measurements made (mIPSCs in Jorge et al., 2002 vs. sIPSCs in the current study) or other unknown variables that may exist between studies done a decade apart.
With respect to allosteric potentiation by non-androgenic modulators, greater allopregnanolone-mediated potentiation was also observed for 35S-TBPS binding to GABAA receptors in diestrus than in estrus in multiple forebrain regions in washed tissue in which endogenous neurosteroids were presumably removed, suggesting an inherent cycle-dependent change in receptor sensitivity. Interestingly, the opposite pattern (estrus greater than diestrus) was observed in unwashed tissue, indicating that fluctuations in endogenous steroids must also be considered when assessing the overall cycle-specificity of the receptor’s response (Finn and Gee, 1993). Barbiturate-dependent potentiation of muscimol-induced depolarization of rat cuneate neurons was also found to vary over the course of the estrous cycle, but here the potentiation was highest in estrus, and lower in proestrus and diestrus. The sensitivity of these neurons to a BZ was independent of cycle stage (Westerling et al., 1991), although a significant diminution in the extent of modulation by BZs was observed in ovariectomized animals in comparison to gonadally-intact animals in estrus (Westering et al., 1993) or proestrus (Bitran et al., 1991) suggesting that the endogenous steroid milieu imposes significant effects on the extent of BZ modulation of the receptor.
While outside the scope of the current study, cyclical changes in GABAA receptor activity arising from the actions of both estrogens and progestins have been strongly implicated in the enhanced seizure activity that characterizes catamenial epilepsy. As discussed earlier (Introduction), these changes have been largely attributed to cycle-dependent differences in receptor subunit expression (for review, Reddy, 2012). However, results presented here on estrous cycle-dependent differences in GABAA receptor phosphorylation, when taken in conjunction with prior studies illustrating the impact of receptor phosphorylation on potentiation elicited by endogenous steroid modulators (Brussaard et al., 1997; Fáncsik et al., 2000; Harney et al., 2003; Koksma et al., 2003; Vergnano et al., 2007 Kia et al., 2011), suggest that such posttranslational modifications could also have an important role in changes in seizure activity that characterize catamenial epilepsy. Moreover, differences in receptor function arising from cycle-dependent changes in phosphorylation may be dramatically amplified by the known role of phosphorylation on the rapid trafficking of GABAA receptors in neuronal tissue (for review, Jacob et al., 2008; Vithlani and Moss, 2010; Vithlani et al., 2011) and its effects on pathological electrical activity (Terunuma et al., 2008).
With respect to trafficking, we note that changes in whole-cell GABA-evoked current can be elicited within minutes by compounds that alter phosphorylation due to changes in receptor trafficking (Kanematsu et al., 2006). In our study, the amplitudes of neither sIPSCs (p = 0.24) nor mIPSCs (p = 0.44) were significantly different in mPOA neurons of diestrous versus estrous mice, even following washout of 17α-MeT, suggesting that the enhanced amplitude elicited by acute 17α-MeT modulation is likely to reflect a phosphorylation-dependent difference in state transitions of the receptor rather than receptor trafficking. This conclusion would also be consistent with the interpretation of results found with ultrafast perfusion techniques (Yang et al., 2005). We also note that GABA receptors are unlikely to be saturated at mPOA synapses (for discussion, Nett et al., 1999; Jorge et al., 2002), and we did not determine if cycle-dependent differences in phosphorylation may have led to changes in receptor occupancy that, in turn, altered the modulation elicited by these androgenic steroids (although see Colquhoun 1998 for pitfalls in simple interpretations of differences in binding vs. gating).
4.3 The behavioral consequences of altered neurotransmission in the mPOA by AAS
The mPOA is a core structure at the intersection of neural circuits that regulate many critical behaviors related to homeostasis, sexual and parental behaviors, motivation, anxiety, and arousal. We chose to examine the outcomes of an acute, but systemic, exposure to 17α-MeT on thermogenesis as reflected in nest building: a naturalistic, but restrictive, behavior where the impact of influencing GABAergic activity in the mPOA might be more easily parsed than for the social behaviors whose expression involves more expansive neural circuits.
The mPOA is necessary for the expression of thermoregulatory behavior, and GABAA receptor-mediated transmission within the mPOA promotes thermogenesis under conditions of ambient cold (Ishiwata et al., 2005). Standard room temperatures are cold in comparison to the optimal temperature set points for mice in the wild and thus promote nest-building (Gaskill et al., 2010). Mice will “titrate” the amount of nestlet material used with changes in temperature (Deacon, 2006b; Gaskill et al., 2010), and lesions of this structure abolish nest-building behavior (Jacobson et al., 1980). As enhanced GABAergic transmission results in increased thermogenesis, we predicted that manipulations that augmented GABAergic transmission in the mPOA would diminish nest building. We found that acute exposure to AAS diminished nest building in diestrus, the cycle stage during which AAS augmented GABAA receptor-mediated currents in the mPOA, but was without effect on this behavior in estrus when there was no AAS-mediated potentiation of these currents. Thus these data are consistent with the hypothesis that cycle-specific differences in the phosphorylation state of the receptor regulate the ability of the AAS to augment GABAergic currents in this brain region and are reflected in a concomitant change in an mPOA-mediated behavior.
Acute exposure to 17α-MeT had no significant effect on a measure of defensive anxiety (marble burying) at either stage of the cycle, nor did acute exposure to this AAS promote a change in generalized anxiety in female mice across the cycle, as assessed on the elevated plus maze. The absence of a significant action of an acute injection of 17α-MeT on measures of anxiety is consistent with a previous study demonstrating a lack of effect in diestrous mice of an acute exposure to a mixture of three AAS on generalized anxiety-like behaviors, as measured on the elevated plus maze and the acoustic startle response (Oberlander and Henderson, 2012a). These data indicate that the effect of 17α-MeT in diestrus on nest building is unlikely to reflect a change in anxiety (see Gaskill et al., 2010) or a general change in locomotor responses.
4.4 Significance of acute AAS actions to human subjects
Humans self-administer AAS for protracted periods of time. To best mimic these conditions, the preponderance of studies designed to assess mechanistic actions of the AAS in the central nervous system (CNS) have employed animal models in which rodents have also been chronically exposed to these synthetic steroids. When given chronically, the AAS promote significant long-term effects in the CNS through many different mechanisms that involve long-term regulation of gene expression through classical nuclear hormone-mediated pathways, changes in the expression and the activity of enzymes that regulate steroid synthesis and metabolism, and secondary effects that arise from changes in peripheral steroid production (for review, Clark and Henderson, 2003; Clark et al., 2006; Oberlander et al., 2012a,b). Within this complex environment in these chronic use models, it is difficult to isolate actions that may arise from effects of allosteric modulation of GABAA receptors. Moreover, in many animal studies, AAS are given at concentrations to mimic high human dose regimes, which suppress estrous cyclicity (Blasberg et al., 1997; Clark et al., 1998, 2003; Penatti et al., 2009, 2011; Oberlander and Henderson, 2012a), thus negating the ability to discern any interactions of AAS on neural substrates across the cycle. Such effects may be evident under the conditions of more moderate AAS administration that likely characterize most female users (Elliot and Goldberg, 2000). Despite these uncertainties, our data suggest some provocative points of experimental pursuit. In particular, it will be interesting to determine if differential modulation of GABAA receptors across the cycle or with other environmental inputs that alter the phosphorylation state of the receptor contribute to changes in social behaviors, including the complex effects on libido that have been reported in females that illicitly use AAS (Franke and Berendonk, 1997), in those for whom AAS are prescribed as part of hormone-replacement therapy (Warnock et al., 2005; Elliot and Goldberg, 2000) and in transsexual genetic females (Elliot and Goldberg, 2000).
5. Conclusion
Our data demonstrate that there are significant differences in the extent of serine phosphorylation of the GABAA receptor complex across the estrous cycle in the mPOA, a key node in social behaviors and homeostatic regulation. The extent of phosphorylation correlates with the sensitivity of GABAA receptor-mediated synaptic currents in this region to allosteric modulation by the AAS, 17α-MeT, and inhibition of PKC abrogates the cycle-specific difference in this modulation. Analysis of a naturalistic behavior that is dependent upon GABAergic transmission in the mPOA illustrated that the ability of acute exposure to 17α-MeT to enhance this behavior occurred only during the stage of the estrous cycle when levels of phosphorylation and AAS modulation of GABAergic currents in the mPOA were high.
Highlights.
GABAA receptor phosphorylation was higher in diestrus/proestrus than in estrus
AAS elicited rapid enhancement of synaptic currents in diestrus, but not in estrus
Effects of AAS in diestrus were blocked by PKC inhibition
Levels of GABAA receptor subunit mRNAs did not vary across the estrous cycle
Single AAS injections impaired an mPOA-mediated behavior in diestrus, but not in estrus
Acknowledgments
This work was funded by DA014137 to LPH.
Abbreviations
- 17α-MeT
17α-methyltestosterone
- 3α-diol
5α-androstane-3α,17β-diol
- AAS
anabolic androgenic steroids
- E2
17β-estradiol
- mPOA
medial preoptic area
- mIPSC
miniature inhibitory postsynaptic current
- PN
postnatal
- PKC
protein kinase C
- sIPSC
spontaneous inhibitory postsynaptic current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Verena Tretter E, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM. Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology. 1998;139(7):3050–3056. doi: 10.1210/endo.139.7.6088. [DOI] [PubMed] [Google Scholar]
- Basaria S. Androgen abuse in athletes: Detection and consequences. J Clin Encodrinol Metab. 2010;95:1533–1543. doi: 10.1210/jc.2009-1579. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of γ-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105(5):653–662. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Langan CJ, Clark AS. The effects of 17α-methyltestosterone, methandrostenolone, and nandrolone decanoate on the rat estrous cycle. Physiol Behav. 1997;61(2):265–272. doi: 10.1016/s0031-9384(96)00409-x. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19(21):9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275(49):38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Brandon N, Jovanovic J, Moss S. Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharmacol Ther. 2002;94(1–2):113–122. doi: 10.1016/s0163-7258(02)00175-4. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19(5):1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- Clark AS, Blasberg ME, Brandling-Bennett EM. Stanozolol, oxymetholone, and testosterone cypionate effects on the rat estrous cycle. Physiol Behav. 1998;63(2):287–295. doi: 10.1016/s0031-9384(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27(5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Clark AS, Kelton MC, Whitney AC. Chronic administration of anabolic steroids disrupts pubertal onset and estrous cyclicity in rats. Biol Reprod. 2003;68(2):465–471. doi: 10.1095/biolreprod.102.008078. [DOI] [PubMed] [Google Scholar]
- Clark AS, Jones BL, Yang P, Henderson LP. Anabolic androgenic steroids and the brain: novel actions at the GABAA receptor and on GABAA receptor mediated-behaviors. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. Boca Raton: CRC Press LLC; 2004. pp. 119–141. [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and the effects of mutating receptors. Brit J Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the estrous cycle in the laboratory rodent by vaginal lavage. In: Heindel JJ, Chapin RE, editors. Methods in Toxicology. New York: Academic Press; 1993. pp. 45–55. [Google Scholar]
- Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, Rubinow DR. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–177. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- Davis AM, Penschuck S, Fritschy J-M, McCarthy MM. Developmental switch in the expression of GABAA receptor subunits α1 and α2 in the hypothalamus and limbic system of the rat. Dev Brain Res. 2000;119:127–138. doi: 10.1016/s0165-3806(99)00150-9. [DOI] [PubMed] [Google Scholar]
- Deacon RJ. Assessing nest building in mice. Nat Protoc. 2006a;1(3):1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Deacon RJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006b;1(1):122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Drouva SV, Gorenne I, LaPlante E, Rérat E, Enjalbert A, Kordon C. Estradiol modulates protein kinase C activity in the rat pituitary in vivo and in vitro. Endocrinology. 1990;126(1):536–544. doi: 10.1210/endo-126-1-536. [DOI] [PubMed] [Google Scholar]
- Elliot DL, Goldberg L. Women and anabolic steroids. In: Yesalis CE, editor. Anabolic steroids in sport and exercise. 2. Champaign, IL: Human Kinetics; 2000. pp. 225–246. [Google Scholar]
- Fáncsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20(9):3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The influence of estrus cycle on neurosteroid potency at the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1993;266:1374–1379. [PubMed] [Google Scholar]
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: A secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–1279. [PubMed] [Google Scholar]
- Freeman M. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press Ltd; 1994. pp. 613–658. [Google Scholar]
- Frye CA, Walf AA. Activity of protein kinase C is important for 3α,5α-THP’s actions at dopamine type 1-like and/or GABAA receptors in the ventral tegmental area for lordosis of rats. Brain Res Bull. 2008;77:91–97. doi: 10.1016/j.brainresbull.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7(3):e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Flores O, Beyer C, Gómora-Arrati P, García-Juárez M, Lima-Hernández FJ, Soto-Sánchez A, Etgen AM. A role for src-kinase in progestin facilitation of estrous behavior in estradiol-primed female rats. Horm Behav. 2010;58:223–229. doi: 10.1016/j.yhbeh.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Haage D, Johansson S. Neurosteroid modulation of synaptic and GABA-evoked currents in neurons from the rat medial preoptic nucleus. J Neurophysiol. 1999;82(1):143–151. doi: 10.1152/jn.1999.82.1.143. [DOI] [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45(6):873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP, Jorge JC. Steroid modulation of GABAA receptors: CNS roles in reproduction, dysfunction and drug abuse. In: Maue RA, editor. Advances in Molecular and Cell Biology. Molecular Insights into Ion Channel Biology in Health and Disease. Vol. 32. Amsterdam: Elsevier; 2004. pp. 217–249. [Google Scholar]
- Hunt JL, Zaretsky DV, Sarkar S, DiMicco JA. Dorsomedial hypothalamus mediates autonomic, neuroendocrine, and locomotor responses evoked from the medial preoptic area. Am J Physiol/Regul Integr Comp Physiol. 2010;298:R130–R140. doi: 10.1152/ajpregu.00574.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata T, Saito T, Hasegawa H, Yazawa T, Kotani Y, Otokawa M, Aihara Y. Changes of body temperature and thermoregulatory responses of freely moving rats during GABAergic pharmacological stimulation to the preoptic area and anterior hypothalamus in several ambient temperatures. Brain Res. 2005;1048:32–40. doi: 10.1016/j.brainres.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Terkel J, Gorski RA, Sawyer CH. Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res. 1980;194(2):471–478. doi: 10.1016/0006-8993(80)91226-3. [DOI] [PubMed] [Google Scholar]
- Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of mammalian ε-subunit-containing GABAA receptors. J Physiol (Lond) 2006;573:571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABAA receptors change with hormonal state in the adult mouse. J Neurobiol. 2002;50(2):137–149. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Joubert-Bression D, Brandi AM, Birman P, Peillon F. Effect of oestradiol on dopamine receptors in the rat pituitary: binding of oestradiol to pituitary membranes. Ciba Found Symp. 1990;153:156–168. doi: 10.1002/9780470513989.ch9. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24(2):522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN. Phosphorylation site-specific antibodies as research tools in studies of native GABAA receptors. In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Chpt 4. Boca Raton: CRC Press; 2006. [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Treatment of anabolic-androgenic steroid dependence: Emerging evidence and its implications. Drug Alcohol Depend. 2010;109:6–13. doi: 10.1016/j.drugalcdep.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Moss SJ, Nabekura J, Hirata M. Modulation of GABAA receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol Chem. 2006;281(31):22180–22189. doi: 10.1074/jbc.M603118200. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K+ channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–456. doi: 10.1016/s0039-128x(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SSG, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem. 2011;116:1043–1056. doi: 10.1111/j.1471-4159.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphoston C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophy Res Comm. 1989;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Koksma JJ, Fritschy JM, Mack V, Van Kersteren RE, Brussaard AB. Differential GABAA receptor clustering determines GABA synapse plasticity in rat oxytocin neurons around parturition and the onset of lactation. Mol Cell Neurosci. 2005;28:128–140. doi: 10.1016/j.mcn.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Koksma JJ, van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Lüddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J Neurosci. 2003;23:788–797. doi: 10.1523/JNEUROSCI.23-03-00788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowicz A, Rebas E. Gender differences in steroid modulation of angiotensin II-induced protein kinase C activity in anterior pituitary of the rat. Biochem Biophys Res Comm. 2002;294:95–100. doi: 10.1016/S0006-291X(02)00433-3. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29(30):9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABAA receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masonis AET, McCarthy MP. Direct effects of the anabolic/androgenic steroids, stanozolol and 17α-methyltestosterone, on benzodiazepine binding to the GABAA receptor. Neurosci Lett. 1995;189(1):35–38. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABAA receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43:634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: Molecular determinants and significance in health and disease. Neurochem Internatl. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Nett ST, Jorge-Rivera J-C, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing γ1 subunit mRNA in the preoptic area of the rat. J Neurophysiol. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38(1):63–67. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. Corticotropin releasing factor modulation of forebrain GABAergic transmission plays a pivotal role in the expression of anabolic steroid induced anxiety in the female mouse. Neuropysychopharmacology. 2012a;37(6):1483–1499. doi: 10.1038/npp.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. The Sturm und Drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012b;35(6):382–392. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Penatti CAA, Porter DM, Henderson LP. The Buzz About Anabolic Androgenic Steroids: Electrophysiological Effects in Excitable Tissues. Neuroendocrinology. 2012a;2012 doi: 10.1159/000339123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Porter DM, Penatti CA, Henderson LP. Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain. J Neuroendocrinol. 2012b;24(1):202–214. doi: 10.1111/j.1365-2826.2011.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Costine BA, Porter DM, Henderson LP. Effects of chronic exposure to an anabolic androgenic steroid mixture on α5-receptor-mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009;161:526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Davis MA, Porter DM, Henderson LP. Altered GABAA receptor-mediated synaptic transmission disrupts the firing of gonadotropin releasing hormone neurons in male mice under conditions of steroid abuse. J Neurosci. 2010;30 (19):6497–6506. doi: 10.1523/JNEUROSCI.5383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Oberlander JG, Davis MC, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology. 2011;61(4):653–664. doi: 10.1016/j.neuropharm.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Porter DM, Jones BL, Henderson LP. Sex-specific differences in the effects of chronic anabolic androgenic steroid treatment on GABAA receptor expression and function in adolescent mice. Neuroscience. 2005;135(2):533–543. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptor immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neuroendocrine aspects of catamenial epilepsy. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE, Zaretsky DV, Zaretskaia MV, DiMicco JA. The role of orexin-1 receptors in physiologic responses evoked by microinjection of PgE2 or muscimol into the medial preoptic area. Neurosci Lett. 2011;498:162–166. doi: 10.1016/j.neulet.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10(4):469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: Pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABAA receptor plasticity. Psychoneuroendocrinology. 2009;34(Supp 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell Mol Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PubMed] [Google Scholar]
- Strömberg J, Haage D, Taube M, Bäckström T, Lundgren P. Neurosteroid modulation of allopregnanolone and GABA effect on the GABA-A receptor. Neuroscience. 2006;143:73–81. doi: 10.1016/j.neuroscience.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Strömberg J, Lundgren P, Taube M, Bäckström T, Wang M, Haage D. The effect of the neuroactive steroid 5β-pregnane-3β, 20(R)-diol on the time course of GABA evoked currents is different to that of pregnenolone sulphate. Eur J Pharmacol. 2009;605:78–86. doi: 10.1016/j.ejphar.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Tasker J. Coregulation of ion channels by neurosteroids and phosphorylation. Sci STKE. 2000 Nov 21;2000(59):1. doi: 10.1126/stke.2000.59.pe1. [DOI] [PubMed] [Google Scholar]
- Tasker J. Teaching resources. Regulation of GABA receptor activity by neurosteroids and phosphorylation. Sci STKE. 2004 May 18;2004(234):tr4. doi: 10.1126/stke.2342004tr4. [DOI] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28(2):376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnano AM, Schlichter R, Poisbeau P. PKC activation sets an upper limit to the functional plasticity of GABAergic transmission induced by endogenous neurosteroids. Eur J Neurosci. 2007;26:1173–1182. doi: 10.1111/j.1460-9568.2007.05746.x. [DOI] [PubMed] [Google Scholar]
- Vicini S. THDOC and the GABAA receptor. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor. Boca Raton: CRC Press LLC; 2004. pp. 63–75. [Google Scholar]
- Vithlani M, Moss SJ. The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition. Biochem Soc Trans. 2010;37(Pt 6):1355–1358. doi: 10.1042/BST0371355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91(3):1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock JK, Swanson SG, Borel RW, Zipfel LM, Brennan JJ ESTRATEST Clinical Study Group. Combined esterified estrogens and methyltestosterone versus esterified estrogens alone in the treatment of loss of sexual interest in surgically menopausal women. Menopause. 2005;12(4):374–384. doi: 10.1097/01.GME.0000153933.50860.FD. [DOI] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. PKCγ is required for ethanol-induced increases in GABAA receptor α4 subunit expression in cultured cerebral cortical neurons. J Neurochem. 2011;116(4):554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling P, Lindgren S, Meyerson B. Functional changes in GABAA receptor stimulation during the oestrous cycle of the rat. Br J Pharmacol. 1991;103(2):1580–1584. doi: 10.1111/j.1476-5381.1991.tb09830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29(4):490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Stanton SJ. Testosterone and sport: Current perspectives. Horm Behav. 2012;61:147–155. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC. Endocrine aspects of anabolic steroid. Clin Chem. 1997;43:1289–1292. [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Mechanisms of anabolic androgenic steroid modulation of α1β3γ2L GABAA receptors. Neuropharmacology. 2002;43:619–633. doi: 10.1016/s0028-3908(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABAA receptors by anabolic androgenic steroids. Neuropharmacology. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: Comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–296. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]