Abstract
Within the paradigm of clinical infectious disease research, Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa represent the four most clinically relevant, and hence most extensively studied bacteria. Current culture-based methods for identifying these organisms are slow and cumbersome, and there is increasing need for more rapid and accurate molecular detection methods. Using bioinformatic tools, 962,279 bacterial 16S rRNA gene sequences were aligned, and regions of homology were selected to generate a set of real-time PCR primers that target 93.6% of all bacterial 16S rRNA sequences published to date. A set of four species-specific real-time PCR primer pairs were also designed, capable of detecting less than 100 genome copies of A. baumannii, E. coli, K. pneumoniae, and P. aeruginosa. All primers were tested for specificity in vitro against 50 species of Gram-positive and –negative bacteria. Additionally, the species-specific primers were tested against a panel of 200 clinical isolates of each species, randomly selected from a large repository of clinical isolates from diverse areas and sources. A comparison of culture and real-time PCR demonstrated 100% concordance. The primers were incorporated into a rapid assay capable of positive identification from plate or broth cultures in less than 90 minutes. Furthermore, our data demonstrate that current targets, such as the uidA gene in E.coli, are not suitable as species-specific genes due to sequence variation. The assay described herein is rapid, cost-effective and accurate, and can be easily incorporated into any research laboratory capable of real-time PCR.
Introduction
In clinical microbiology laboratories, traditional culture based techniques remain the primary methodology used for identifying bacterial isolates. These methods are time-consuming, can involve multiple biochemical tests, and can be expensive, particularly for fastidious organisms. In recent years, the introduction of automated identification instruments has resulted in greater reliability, but discrepancies between the various platforms have been reported [1]–[3]. Furthermore, many research laboratories lack the funds necessary to invest in these instruments, and often rely on labor-intensive biochemical characterization or 16S rRNA sequencing to confirm species type.
Despite significant advances in molecular biology, molecular methods for species identification have not achieved widespread use, with the possible exception of the Biodefense community [4]. Factors such as allelic variation within target genes, cross-reaction with other species [5] and the lack of experienced molecular technicians have all contributed to their relative scarcity. However, despite these limitations, molecular methods can provide significant advantages over phenotypic-based methods, including rapid turnaround time, scalability, and high sensitivity [5]. Furthermore, using appropriate bioinformatic tools and careful primer design, many of the limitations outlined above can be mitigated considerably.
Even prior to recent global calls to help combat the spread of carbapenem-resistant Enterobacteriaceae (CRE), particularly E.coli and K. pneumoniae [6]–[8], research on CRE, multi-drug resistant A. baumannii and P. aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA) has been prolific, as befits their status as the most important nosocomial pathogens. A number of conventional PCR and real-time PCR assays have been developed targeting these organisms [9]–[13], but a comprehensive panel that can target all five clinically important species is lacking.
We recently published a highly sensitive and specific real-time PCR assay for the detection of MRSA [14]. This report adds an additional four real-time PCR primers, designed using a combination of in silico and in vitro methodologies that are highly specific to the four most common nosocomial pathogens. This primer set will allow researchers to rapidly identify these pathogens directly from culture in less than 90 minutes with a high degree of accuracy and sensitivity. We also describe a novel bacterial 16S rRNA real-time PCR primer designed from the alignment of 962,279 bacterial 16S rRNA gene sequences, which will amplify a product from the 16S rRNA gene in 93.6% of all bacterial 16S rRNA genes published to date. The combination of 16S rRNA gene detection with species-specific primers can greatly reduce the effort required in screening large populations of unknown organisms, with samples negative for 16S rRNA being excluded from further testing.
Materials and Methods
Primer Design
Primer characteristics are presented in Table 1. Unless noted otherwise, all primers were designed using Primer Express version 2.0 (Applied Biosystems, Carlsbad, CA). All primers were designed to have an optimal annealing temperature of 56°C, and real-time PCR reactions were performed in 20 µl volumes with 200 nM primers. Where appropriate, alignment of individual gene sequences was performed using MegAlign version 10.0.1 (DNAStar Inc, Madison, WI).
Table 1. Primer characteristics.
| Name1 | Gene | Target species | Sequence (5′ to 3′) | Eff (%)2 | Length3 |
| U16SRT-F | 16S | variable | ACTCCTACGGGAGGCAGCAGT | >96.4% | 180 |
| U16SRT-R | TATTACCGCGGCTGCTGGC | ||||
| secERT-F | A.baumannii | GTTGTGGCTTTAGGTTTATTATACG | |||
| secERT-R | secE | A. nosocomialis | AAGTTACTCGACGCAATTCG | 99.4 | 94 |
| secERT-Probe | A. pitii | ACCCATCAAGGTAAAGGCTTCGTTCG | |||
| yccTRT-F | Escherichia coli | GCATCGTGACCACCTTGA | |||
| yccTRT-R | yccT | Shigella spp. | CAGCGTGGTGGCAAAA | 98.1 | 59 |
| yccTRT-Probe | TGCATTATGTTTGCCGGTATCCG | ||||
| gltART-F | gltA | K. pneumoniae | ACGGCCGAATATGACGAATTC | 97.1 | 68 |
| gltART-R | AGAGTGATCTGCTCATGAA | ||||
| ecfXRT-F | ecfX | P.aeruginosa | AGCGTTCGTCCTGCACAAGT | 93.8 | 81 |
| ecfXRT-R | TCCACCATGCTCAGGGAGAT |
Probe sequences were generated for the secE and yccT real-time PCR primers, but no in vitro testing was performed. The probe sequences are provided here for the benefit of researchers wishing to perform multiplex real-time PCR reactions using these primers. All primers have an optimal annealing temperature of 56°C.
Primer efficiency was calculated from the slope and intercept of the trendline produced following amplification of serial dilutions of genomic DNA from the ATCC strains of each species, as described previously [14].
Amplicon size in base pairs.
16S Real-time PCR Primers
16S rRNA real-time PCR primers were designed manually from a consensus sequence based on an alignment of 962,279 bacterial 16S rRNA gene sequences obtained from the Ribosomal Database Project release 10 [15]. To derive the consensus sequence, nucleotide frequencies were determined at each position in the alignment using a custom script in Perl ver 5.8.8 running on the Red Hat Linux operating system; positions where the majority of sequences had a gap were excluded. Regions of the consensus sequence where the majority nucleotide was present in >90% of the sequences were used for primer design. A synopsis of each nucleotide frequency is presented as supplemental material (Table S1).
E. coli and A. baumannii – Specific Primers
To identify genes specific to E. coli and A. baumannii, annotated complete genome sequences for 38 E. coli strains, and 8 completed and 52 draft A. baumannii genomes were selected (Table S2 and S3). In addition, the two draft genomes of A. nosocomialis (RUH 2624 and NCTC 8102) and the four draft genomes of A. pitii (SH024, D499, DSM 21653, and DSM 9306) were used to ensure that all Acinetobacter-specific primers would also amplify products from these two species. For each protein in a strain, BLASTP [16] was used to find the best match to a protein family in the HOGENOM release 5 database; orthologous proteins from different strains show the best match to the same HOGENOM protein family [17]. Genes that are not single copy (that is, two or more proteins from the same strain are members of the same HOGENOM protein family) were excluded from further analysis. In addition genes for which the nucleotide length of any ortholog varies from the mean gene length by more than 5% were excluded in order to restrict the analysis to those genes that were highly conserved. For the genes that met these criteria and had the fewest variable positions, BLASTN analysis was performed against the NCBI non-redundant nucleotide database using a gene sequence randomly selected from the final species-specific gene targets.
Four genes showed no significant match to non-Escherichia species: HBG518163 (acid chaperone protein, hdeA), HBG636731 (predicted secreted protein, function unknown, ynfB), HBG473849 (conserved protein, function unknown, yccT), and HBG758393 (function unknown, DUF1418 family, ybjC). Nineteen genes showed no significant match to non-Acinetobacter species (Table S4), and the top three candidates, based on total gene length and number of mismatches between all genomes; HBG594899 (rpiN, 50S ribosomal protein L14), HBG701403 (secE, preprotein translocase, SecE subunit), and HBG752450 (scpB, segregation and condensation protein B) were selected for further analysis.
Starting with a multiple sequence alignment, a custom Perl script was used to identify positions within each gene that are invariant among all strains. With E. coli, two of the four genes (hdeA and yccA) contained regions >100 bp that were suitable for primer design. All three genes from the Acinetobacter analysis contained extensively conserved regions suitable for primer design.
K. pneumoniae and P. aeruginosa – Specific Primers
The limited number of published genome sequences available for K. pneumoniae and P. aeruginosa precluded extensive bioinformatic analysis. Therefore, the top two candidate genes from both species (K. pneumoniae; gltA and khe; P. aeruginosa; ecfX and 23S) were selected based on conserved gene sequences from the available genomes.
Validation of Real-time PCR Primers
All primer sets were tested for sensitivity, optimal annealing temperature and primer efficiency as previously described [18], [19]. Briefly, high quality gDNA was was extracted using the GeneJET Genomic DNA Purification Kit (Fermentas Inc, Glen Burnie, MD, USA) with an additional bead-beating step to maximize lysis. DNA was quantified using both a Nanodrop 2000 (Nanodrop, Wilmington, DE, USA) and Qubit 2.0 Fluorometer (Life technologies, Grand Island, NY, USA).
Genome copies of DNA were calculated using the following formula:
Size of genome (in bp) × 650 Daltons/bp = molecular weight of Genome in g/mol.
# copies of genome in 1 ng of DNA = (1×10−9 g ÷ Mw of genome)×6.02×1023 molecules/mole (Avogadro’s number).
To get 108 copies in 2 µl = (108 ÷ # copies of genome in 1 ng)/2.
Serial dilutions of DNA from 108 to 102 copies were prepared and tested in duplicate with each primer set to calculate primer efficiency and sensitivity.
Primers were tested using two different instruments, the Roche Light Cycler 480 II (LC 480 II) with SYBR Green I Master Mix (Roche Applied Sciences, Indianapolis, IN), and the BioRad CFX96 with SsoAdvanced SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA). Each primer was tested for specificity by two methods. First, the primers were tested against genomic DNA extracted from a panel of American Type Culture Collection strains (ATCC, Manassas, VA, USA) and clinical isolates representing fifty different bacterial species, including closely related members from the same genus (Table S5). Secondly, primers were tested against 200 clinical isolates of each species, identified to the species level using three automated identification systems; the Vitek 2 (bioMerieux, Durham, NC), the BD Pheonix (Diagnostics Systems, Sparks, MD), and the Microscan Walkway (Siemens Healthcare Diagnostics Inc, Deerfield, IL), selected from a large repository of isolates (>10,000 strains) collected between 2002 to 2012 from 23 different facilities in the United States of America, Europe, Asia and the Middle East. Pulsed-field gel electrophoresis (PFGE) indicated that the selected clinical isolates represented a variety of different pulse-types (unpublished results).
High throughput Testing of Clinical Isolates
The primers were incorporated into a 96-well plate assay to allow high throughput testing of multiple clinical isolates. All bacterial isolates were cultured overnight on Blood Agar plates at 37°C, and all samples were prepared in a BioSafety hood in a certified BSL2 laboratory. Single colonies from an overnight culture of each isolate were re-suspended in 200 µl of sterile, ultra-pure water and mixed by vortexing. 10 µl of the resulting suspension (or 10 µl taken directly from an overnight broth culture) was added to 20 µl of Lyse-and-Go reagent (Thermo Scientific, Waltham, MA) in 96-well plates, and run in a thermal cycler using the manufacturer’s protocol for the isolation of total genomic DNA. Isolates were held at 80°C for 15 minutes at the end of the program to maximize lysis, and 2 µl of the resulting lysate was used directly for real-time PCR. Leftover bacterial DNA in Lyse-and-Go reagent was stored at −20°C, and no reduction in real-time PCR amplification was evidenced after 9 months (data not shown). Appropriate positive (ATCC Type strains for each species), negative (two ATCC type strains from species other than the target organism), and no template controls (ultra-pure water) were incorporated onto every plate. Cycling parameters were 95°C for 5 minutes, followed by 40 cycles of 95°C for 10 seconds and 56°C for 10 seconds. A melting curve analysis was included at the end of every program to assist in data analysis. Quantification cycle (Cq; CFX96) and crossing threshold (Ct; LC 480 II) values were calculated automatically using instrument software.
Results and Discussion
16S rRNA Real-time PCR Primers
Analysis of the alignment of 962,279 bacterial 16S rRNA gene sequences revealed considerable nucleotide variation between species (Table S1). However, two regions were identified that were suitable for primer design, where the nucleotide sequence was highly conserved in >90% of all rRNA sequences. Forward and reverse primers were manually designed, with the nucleotide sequence of the forward primer being conserved in 98.5% of 16S rRNA sequences, and the nucleotide sequence in the reverse primer being conserved in 93.8% (Table S1).
Primers were tested against serial dilutions of genomic DNA from A. bauamnnii ATCC 19606, E. coli ATCC 35218, and P. aeruginosa ATCC 27853. The primers were reproducibly capable of detecting <100 copies of genomic DNA, and primer efficiency was 96.4%, 98.2% and 97.9% respectively, with an R2>99%. The primers successfully amplified a product from the 50 ATCC and clinical strains (Table S5), providing in vitro support for the in silico primer design method employed. No amplification was seen from no-template negative controls, or from DNA extracted from 15 clinical isolates of Candida parapsilosis and C. albicans (data not shown).
The 16S rRNA gene is a frequent target for many assays, and universal PCR primers are routinely used for species identification [20]. A number of real-time primers for this gene have also been developed, but they either contain degenerate nucleotides [21], or produce long amplicons that are not suitable for optimal real-time PCR [22]. The primer set described here overcomes both these limitations, and has proved a valuable tool in detecting bacterial contamination of environmental swabs (manuscript in preparation), as a positive control for bacterial lysis, confirming that plasmid DNA is free of contaminating genomic DNA, and determining if a colony in a mixed culture is bacterial.
Species-specific Real-time PCR Primers
Five sets of primers were tested for sensitivity and specificity for A. baumannii; three primers were generated using the genome alignment algorithm (rpiN, secE and scpB), one primer was designed in-house from an alignment of 54 ompA sequences, and the fifth primer, also targeting the ompA gene, has been published previously [11]. OmpA was included as a potential target as this gene has been used as a target for A. baumannii-specific primers in previous publications [11]. However, though ompA was identified as a potential target using our algorithm, sequence variation among A. baumannii, A. nosocomialis and A. pitii, as well as variation in the protein length, resulted in this gene being flagged as unsuitable as an Acinetobacter-specific target. All five primers did not cross-react with other bacterial species (Table S5), including A. lwoffi. Primer efficiency at 56°C, based on serial dilutions of A. baumannii AB0057 and A. baumannii ACICU, ranged from 97.2% (rpiN) to 99.4% (secE), with R2>99%, and all primers were reproducibly capable of detecting <100 genome copies of DNA. Furthermore, all five primers successfully amplified products from the 200 clinical test isolates. The primer pair targeting the secE gene consistently provided greater sensitivity than all other primers at an annealing temperature of 56°C, and was therefore selected as the optimal candidate for identifying A. baumannii. In silico analysis of the secE primer with the draft A. nosocomialis and A. pitii genomes demonstrated that these primers would also amplify a product from these two species.
Four sets of primers were tested for sensitivity and specificity for E.coli; two primers were generated using the genome alignment algorithm (hdeA and yccT), one primer was designed from an alignment of 38 uidA genes (Table S2), and a fourth primer, also targeting the uidA gene, has been described previously [7], [18]. UidA was included as this gene has been used as a species-specific target in a number of previous publications [10], [12], [23]–[25], but it did not meet the criteria demanded by the species-specific algorithm in this study. This was confirmed by both in silico and in vitro testing. An alignment of 38 E.coli genomes indicated that published uidA primer sequences [10], [12] have 3 nucleotide variations in the forward primer and 1 nucleotide variation in the reverse primer when aligned with some E.coli uidA gene sequences. When tested against the 200 clinical isolates, the primers failed to amplify any product from 8 strains (Figure 1a). A second set of uidA primers was designed, targeting a conserved region of the 38 uidA gene sequences. However, no amplification was again observed from 7 of the same 8 strains that were uidA negative from the first primer pair. One of these 7 isolates amplified a product after 28 cycles, indicative of inefficient primer annealing, most likely due to primer/target mismatches within the uidA gene.
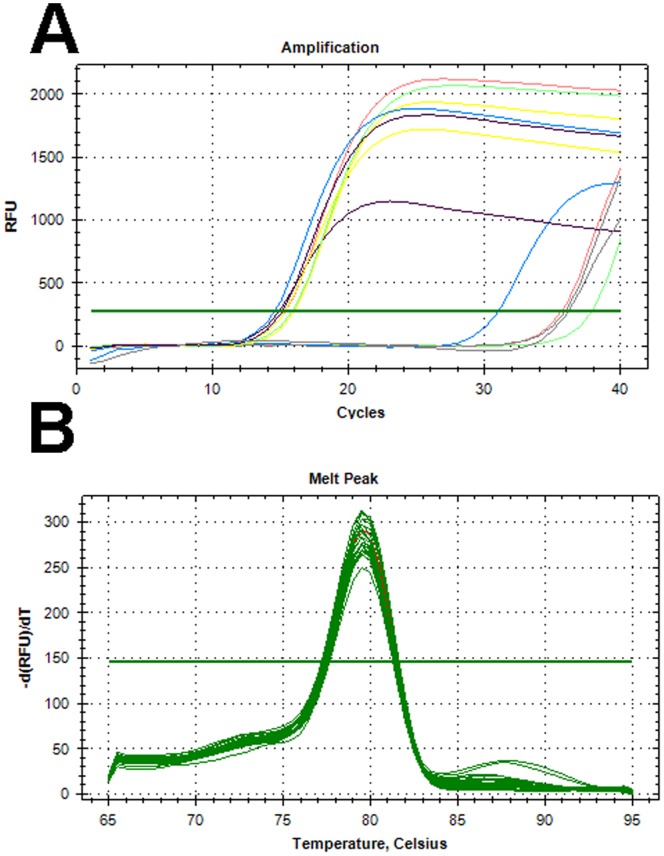
Figure 1. Amplification and melting curves for selected isolates.
(A) Amplification curves of three of the eight E. coli isolates that were uidA-negative, but yccT-positive; MRSN 1628 (Red line), MRSN 1681 (Blue Line), and MRSN 7544 (Green Line). MRSN 7851 (Yellow Line) was both uidA and yccT-positive. E.coli ATCC 35218 (Black line) was used as a positive control and K. pneumoniae ATCC 1706 (Grey Line) was used a negative control. Each amplification curve is one representative sample from quintuplicate experiments. (B) Melting curve analysis of the amplicons produced by the yccT primer pair from 30 clinical isolates of E. coli demonstrating a highly conserved sequence in all strains. Melting curve of the yccT amplicon from E. coli ATCC 35218 is shown in red. All 30 isolates represented diverse pulse-types as determined by PFGE. RFU - Relative Fluorescent Units; -d(RFU)dt – Relative change in RFU over time (in seconds).
The final two primers, targeting the yccT and hdeA genes, were identified as the most promising candidates based on the algorithm used in this study. Primer efficiency was 98.1% and 97.4% respectively, with R2>99%. However, 9 of the 200 clinical isolates failed to amplify a product with the hdeA primer, including 3 of the 8 uidA-negative isolates. In contrast, the yccT primer successfully amplified a highly conserved product from all 200 isolates (Figure 1b), and was therefore selected as the final E. coli-specific primer. As expected, due to the very high homology between E. coli and Shigella strains [26], the yccT primer set also amplified a product from Shigella flexneri ATCC 12022, but did not cross-react with any other species (Table S5).
Despite 38 sequenced genomes of E. coli, developing an E. coli-specific primer pair is challenging. We have shown that current targets, such as the uidA gene are not suitable due to considerably allelic variation, particularly among E. coli clinical isolates from diverse regions. The remarkable diversity among E. coli strains is also highlighted in the bioinformatic approach that we used, where just 2 candidate genes passed all of the criteria employed, and just a single gene, yccT, was eventually successful in vitro.
The paucity of completed genomic sequences for both K. pneumoniae and P. aeruginosa limited the application of our algorithm to just a single genome for P. aeruginosa with an additional 4 whole genome shotgun sequences, and 5 genomes for K. pneumoniae.
BLAST analysis suggested a potential cross-reaction between the K. pneumoniae khe primer set and Citrobacter fruendii, and this was confirmed in vitro. However, the gltA primer set demonstrated no cross-reactivity with other species (Table S5), a primer efficiency of 97.1%, and successfully amplified a product from all 200 clinical isolates of K. pneumoniae.
Both sets of P. aeruginosa primers demonstrated no cross-reactivity with other species, including P. flourescens, P. stutzeri and P. putida. However, when tested against a panel of 200 clinical isolates of P. aeruginosa only the ecfX primer successfully amplified a product from all strains, with 12 strains failing to generate a product with the 23S rRNA primer set. The ecfX primer set demonstrated a primer efficiency of 93.8% with an R2 value of 99.1%.
Species-specific primers targeting the ecfX gene in P. aeruginosa have been published for both conventional [27], [28] and real-time PCR assays [9], [29]. Our results support the continued use of this gene as a target for P. aeruginosa-specific primers, at least until further full genome sequences of this strain become available. In contrast, species-specific primers for K. pneumoniae are less well described, and primarily involve detecting targets that are specific to certain strains, such as the ST258 clone [30], or those with the hypermucoviscosity phenotype [31]. In addition, a number of conventional PCR assays to detect K. pneumoniae have been described [13], [32], though to our knowledge, no assay described to date uses gltA as the target gene.
Conclusion
We describe a set of real-time PCR primers, designed to have the same optimal annealing temperature, and displaying high specificity for four clinically important pathogens. The primers are well suited for high-throughput testing of isolates, with results available in less than 90 minutes from bacterial colonies or overnight broth cultures. We also demonstrate the power of bioinformatics in designing optimal primer sequences, and provide a novel 16S rRNA real-time PCR primer designed from the alignment of over 960,000 bacterial 16S rRNA sequences. The primers described herein have been an invaluable addition to our surveillance network, and have demonstrated a 100% concordance with traditional phenotypic identification systems.
Supporting Information
Nucleotide frequencies at each position from an alignment of 962,279 16S rRNA sequences.
(DOCX)
List of assembled E. coli genomes used in the design of E.coli -specific primers.
(DOCX)
Complete and draft A. baumannii genomes used the design of Acinetobacter -specific primers.
(DOCX)
The top 28 gene targets for designing Acinetobacter -specific primers.
(DOCX)
Bacterial species used for specificity testing of species-specific primers.
(DOCX)
Funding Statement
These authors have no support or funding to report.
References
- 1. Jin WY, Jang SJ, Lee MJ, Park G, Kim MJ, et al. (2011) Evaluation of VITEK 2, MicroScan, and Phoenix for identification of clinical isolates and reference strains. Diagn Microbiol Infect Dis 70: 442–447. [DOI] [PubMed] [Google Scholar]
- 2. Winstanley T, Courvalin P (2011) Expert systems in clinical microbiology. Clin Microbiol Rev 24: 515–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodford N, Eastaway AT, Ford M, Leanord A, Keane C, et al. (2010) Comparison of BD Phoenix, Vitek 2, and MicroScan automated systems for detection and inference of mechanisms responsible for carbapenem resistance in Enterobacteriaceae. J Clin Microbiol 48: 2999–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenger DA, Andreadis JD, Vora GJ, Pancrazio JJ (2002) Potential applications of DNA microarrays in biodefense-related diagnostics. Curr Opin Biotechnol 13: 208–212. [DOI] [PubMed] [Google Scholar]
- 5. Maurer JJ (2011) Rapid detection and limitations of molecular techniques. Annu Rev Food Sci Technol 2: 259–279. [DOI] [PubMed] [Google Scholar]
- 6. Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, et al. (2012) Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18: 413–431. [DOI] [PubMed] [Google Scholar]
- 7. Nordmann P, Cornaglia G (2012) Carbapenemase-producing Enterobacteriaceae: a call for action! Clin Microbiol Infect. 18: 411–412. [DOI] [PubMed] [Google Scholar]
- 8. Walsh TR (2010) Emerging carbapenemases: a global perspective. Int J Antimicrob Agents 36 Suppl 3: S8–14. [DOI] [PubMed] [Google Scholar]
- 9. Anuj SN, Whiley DM, Kidd TJ, Bell SC, Wainwright CE, et al. (2009) Identification of Pseudomonas aeruginosa by a duplex real-time polymerase chain reaction assay targeting the ecfX and the gyrB genes. Diagn Microbiol Infect Dis 63: 127–131. [DOI] [PubMed] [Google Scholar]
- 10. Frahm E, Obst U (2003) Application of the fluorogenic probe technique (TaqMan PCR) to the detection of Enterococcus spp. and Escherichia coli in water samples. J Microbiol Methods 52: 123–131. [DOI] [PubMed] [Google Scholar]
- 11. McConnell MJ, Perez-Ordonez A, Perez-Romero P, Valencia R, Lepe JA, et al. (2012) Quantitative real-time PCR for detection of Acinetobacter baumannii colonization in the hospital environment. J Clin Microbiol 50: 1412–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pavlovic M, Luze A, Konrad R, Berger A, Sing A, et al. (2011) Development of a duplex real-time PCR for differentiation between E. coli and Shigella spp. J Appl Microbiol 110: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 13. Thong KL, Lai MY, Teh CS, Chua KH (2011) Simultaneous detection of methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa by multiplex PCR. Trop Biomed 28: 21–31. [PubMed] [Google Scholar]
- 14. McGann P, Kwak YI, Summers A, Cummings JF, Waterman PE, et al. (2011) Detection of qacA/B in clinical isolates of methicillin-resistant Staphylococcus aureus from a regional healthcare network in the eastern United States. Infect Control Hosp Epidemiol 32: 1116–1119. [DOI] [PubMed] [Google Scholar]
- 15. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penel S, Arigon AM, Dufayard JF, Sertier AS, Daubin V, et al. (2009) Databases of homologous gene families for comparative genomics. BMC Bioinformatics 10 Suppl 6: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bustin SA (2010) Why the need for qPCR publication guidelines?–The case for MIQE. Methods 50: 217–226. [DOI] [PubMed] [Google Scholar]
- 19. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 20. Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu CM, Aziz M, Kachur S, Hsueh PR, Huang YT, et al. (2012) BactQuant: An enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol 12: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148: 257–266. [DOI] [PubMed] [Google Scholar]
- 23. Anklam KS, Kanankege KS, Gonzales TK, Kaspar CW, Dopfer D (2012) Rapid and reliable detection of Shiga toxin-producing Escherichia coli by real-time multiplex PCR. J Food Prot 75: 643–650. [DOI] [PubMed] [Google Scholar]
- 24. Chern EC, Siefring S, Paar J, Doolittle M, Haugland RA (2011) Comparison of quantitative PCR assays for Escherichia coli targeting ribosomal RNA and single copy genes. Lett Appl Microbiol 52: 298–306. [DOI] [PubMed] [Google Scholar]
- 25. Donhauser SC, Niessner R, Seidel M (2009) Quantification of E. coli DNA on a flow-through chemiluminescence microarray readout system after PCR amplification. Anal Sci 25: 669–674. [DOI] [PubMed] [Google Scholar]
- 26. Lukjancenko O, Wassenaar TM, Ussery DW (2010) Comparison of 61 sequenced Escherichia coli genomes. Microb Ecol 60: 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hillenbrand ME, Thompson PP, Shanks RM, Kowalski RP (2011) Validation of PCR for the detection of Pseudomonas aeruginosa from corneal samples. Int J Ophthalmol 4: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lavenir R, Jocktane D, Laurent F, Nazaret S, Cournoyer B (2007) Improved reliability of Pseudomonas aeruginosa PCR detection by the use of the species-specific ecfX gene target. J Microbiol Methods 70: 20–29. [DOI] [PubMed] [Google Scholar]
- 29. Cattoir V, Gilibert A, Le Glaunec JM, Launay N, Bait-Merabet L, et al. (2010) Rapid detection of Pseudomonas aeruginosa from positive blood cultures by quantitative PCR. Ann Clin Microbiol Antimicrob 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Chavda KD, Mediavilla JR, Zhao Y, Fraimow HS, et al. (2012) Multiplex real-time PCR for detection of an epidemic KPC-producing Klebsiella pneumoniae ST258 clone. Antimicrob Agents Chemother 56: 3444–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartman LJ, Selby EB, Whitehouse CA, Coyne SR, Jaissle JG, et al. (2009) Rapid real-time PCR assays for detection of Klebsiella pneumoniae with the rmpA or magA genes associated with the hypermucoviscosity phenotype: screening of nonhuman primates. J Mol Diagn 11: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Leary J, Corcoran D, Lucey B (2009) Comparison of the EntericBio multiplex PCR system with routine culture for detection of bacterial enteric pathogens. J Clin Microbiol 47: 3449–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nucleotide frequencies at each position from an alignment of 962,279 16S rRNA sequences.
(DOCX)
List of assembled E. coli genomes used in the design of E.coli -specific primers.
(DOCX)
Complete and draft A. baumannii genomes used the design of Acinetobacter -specific primers.
(DOCX)
The top 28 gene targets for designing Acinetobacter -specific primers.
(DOCX)
Bacterial species used for specificity testing of species-specific primers.
(DOCX)