Abstract
Protein structure and function depend on a close interplay between intrinsic folding energy landscapes and the chemistry of the protein environment. Osmolytes are small-molecule compounds that can act as chemical chaperones by altering the environment in a cellular context. Despite their importance, detailed studies on the role of these chemical chaperones in modulating structure and dimensions of intrinsically disordered proteins have been limited. Here, we used single-molecule Förster resonance energy transfer to test the counteraction hypothesis of counterbalancing effects between the protecting osmolyte trimethylamine-N-oxide (TMAO) and denaturing osmolyte urea for the case of α-synuclein, a Parkinson’s disease-linked protein whose monomer exhibits significant disorder. The single-molecule experiments, which avoid complications from protein aggregation, do not exhibit clear solvent-induced cooperative protein transitions for these osmolytes, unlike results from previous studies on globular proteins. Our data demonstrate the ability of TMAO and urea to shift α-synuclein structures towards either more compact or expanded average dimensions. Strikingly, the experiments directly reveal that a 2∶1 [urea]∶[TMAO] ratio has a net neutral effect on the protein’s dimensions, a result that holds regardless of the absolute osmolyte concentrations. Our findings shed light on a surprisingly simple aspect of the interplay between urea and TMAO on α-synuclein in the context of intrinsically disordered proteins, with potential implications for the biological roles of such chemical chaperones. The results also highlight the strengths of single-molecule experiments in directly probing the chemical physics of protein structure and disorder in more chemically complex environments.
Keywords: Parkinson’s disease, protein folding, urea-TMAO counteraction, smFRET
Proteins are dynamic entities that are in constant interaction with their environment. Several components of the protein environment can affect the folding landscape (1) and function, including solvents (2), osmolytes (3), crowding agents (4), and small-molecule and macromolecular ligands (5–7). Osmolytes are naturally occurring low-molecular weight compounds that are utilized by biological systems as chemical chaperones that counteract deleterious effects of extreme physical conditions such as high osmotic and hydrostatic pressures (8, 9), dehydration (10), and high or low temperatures (11, 12). Urea, a major metabolic by-product, is known to be used as a balancing osmolyte by several marine vertebrates (8), air-breathing teleosts (13) and some amphibians (14, 15) to deal with osmotic stress. In mammalian kidneys, urea plays an important role in balancing the medullary osmotic gradient (16). However, even at physiologically relevant concentrations, urea shows a strong denaturing effect on proteins (8, 17). This apparent paradox is solved by the activity of several protecting osmolytes (e.g., methylamines and polyhydric alcohols) found in these urea-rich biological systems (8, 18, 19).
Trimethylamine N-oxide (TMAO) is the major protective methylamine found in several marine vertebrates. It is the most efficient among the protecting osmolytes (20). Yancey et al. put forth the counteraction hypothesis, which proposes that these protecting osmolytes can counteract the denaturing effects of urea (8, 20). Urea-TMAO counteraction has been studied in multiple systems both in vitro and in vivo (21, 22). TMAO has been used to reverse the effect of denaturants on proteins (23, 24), and fold mutation-destabilized (25) and partially folded or unfolded (26) proteins in vitro. Recently, Bandyopadhyay et al. showed that TMAO can fold mutant proteins in vivo to compensate the effects of mutations in Escherichia coli (27). While a few examples of intrinsically disordered proteins (IDPs) have been studied in this context (28–30), tests of the counteraction across a broad range of TMAO and urea conditions have not been reported.
Here, we investigated urea-TMAO counteraction in the context of the structural landscape of α-synuclein, a protein that is intrinsically disordered in its monomeric form (31). This protein has multiple putative biological functions and has also been linked to Parkinson’s disease (32). To study the monomeric form of α-synuclein and avoid issues with aggregation that occur at the higher protein concentrations needed for ensemble measurements (33), we turned to single-molecule experiments. Developments in optical and force spectroscopy techniques have enabled single-molecule studies on several aspects of protein dynamics, folding, and interactions (34, 35). Single-molecule techniques provide new types of information at very low sample concentrations without ensemble averaging (35–42). Using the distance dependence of single-molecule Förster/fluorescence resonance energy transfer (smFRET) to probe protein dimensions, we studied the effect on α-synuclein of urea and TMAO, both as individual cosolvents and also in varying mixing ratios. Our results provide a direct test of the urea-TMAO counteraction hypothesis for this IDP system.
Results and Discussion
smFRET Reveals a Non-Cooperative TMAO-Induced Effect on α-Synuclein Dimensions.
Previous studies on the effect of TMAO on α-synuclein and other amyloid-forming proteins have revealed that the osmolyte can induce protein oligomerization and/or aggregation (33, 43). A powerful means to study protein structural properties while avoiding the effects of protein aggregation is to perform single-molecule fluorescence experiments. The extremely low concentrations (100 pM or less) required in such experiments have been shown to minimize artifacts caused by protein aggregation (44). Therefore, smFRET experiments were utilized in this work to study osmolyte effects on monomeric α-synuclein.
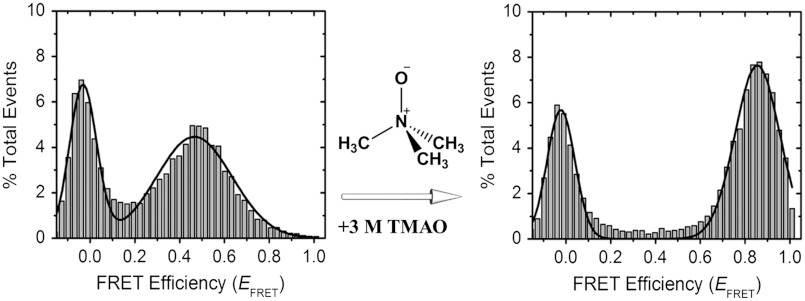
For the smFRET experiments reported here, we used a confocal detection method to monitor FRET efficiencies (EFRET) from freely diffusing individual molecules, avoiding potential perturbations from surface immobilization. α-Synuclein was labeled via cysteine chemistry at residue positions 7 and 84, a labeling scheme that we have previously shown to be nonperturbative and to report on multiple α-synuclein conformations (45–48). A combination of a small detection volume (sub-fL) and low concentrations of dual-labeled molecules (approximately 100 pM) in these experiments allowed us to detect bursts of donor and acceptor photons from individual molecules. These bursts were then analyzed to generate smFRET histograms. An smFRET histogram of α-synuclein in buffer is presented in Fig. 1, Left, showing a single FRET peak at about 0.47 EFRET (Table S1), consistent with disordered α-synuclein as reported previously (45). “Zero peaks” observed at zero EFRET originate from protein molecules having either photo-bleached or missing acceptor fluorescent probes.
Fig. 1.
Chemical chaperone-induced changes in the dimensions of α-synuclein probed by single-molecule Förster resonance energy transfer (smFRET). Addition of a high concentration of trimethylamine N-oxide (TMAO) results in increased FRET efficiency (EFRET) for α-synuclein dual-labeled at residue positions 7 and 84 with Alexa Fluor 488 (donor) and Alexa Fluor 594 (acceptor).
Using smFRET, we tested the effect of TMAO on α-synuclein structure and dimensions. Fig. 1, Right, shows a histogram of the protein in the presence of a high concentration of TMAO (3 M). The nonzero peak observed in this histogram is clearly shifted relative to that observed for the 0 M TMAO data, showing a higher EFRET of approximately 0.86, corresponding to a more compact protein dimension. This observation is consistent with the known ability of TMAO to stabilize compact folded states of proteins (25, 26, 28–30, 49) and induce folding of unfolded proteins (30, 50). To further probe the α-synuclein structural landscape in the presence of TMAO, we performed a series of smFRET experiments in varying TMAO concentrations.
In contrast with previous smFRET observations on a two-state-folding protein (51) or the SDS-induced folding of α-synuclein (45), the TMAO titration data presented single nonzero EFRET peaks that continuously shift as a function of TMAO concentration. In addition, when the peak positions were plotted as a function of TMAO concentration, within the resolution of our experiments, a clear cooperative transition was not observed (Fig. 2 A and C). Our observation is consistent with previous solution thermodynamics (52) and molecular dynamics simulation (53, 54) studies suggesting that TMAO shifts the structural ensemble towards more collapsed forms.
Fig. 2.
Contrasting effects of the denaturing osmolyte urea and the counteracting osmolyte TMAO on α-synuclein dimensions. (A) and (B) show smFRET histogram data on α-synuclein TMAO and urea isothermal titrations, color-coded on the basis of fractional populations (red to blue representing high to low populations, respectively). Projections of the 3D contour plots onto the EFRET—TMAO/EFRET—urea planes are shown at the top for each panel. (C) and (D) present mean EFRET as functions of cosolvent concentrations. EFRET values were obtained by nonlinear least-squares (NLS) fitting of individual smFRET histograms to a Gaussian function. Solid lines are shown as guides.
TMAO and Urea Have Opposing Effects on α-Synuclein Dimensions.
The effect of urea on the structural properties of α-synuclein was then studied by carrying out isothermal smFRET titrations. Similar to the TMAO data, our urea titration data showed a continuous change, but with the results instead showing gradual expansion of the protein with increasing urea concentration (Figs. 2 B and D). The observed trend is similar to the noncooperative transition previously reported for the guanidine hydrochloride-induced expansion of α-synuclein monitored using smFRET (55). This result is also consistent with the lack of a stable native structure in this disordered protein as studied previously using ensemble measurements (31). Similar results were observed for the denaturation of other IDPs (44, 55, 56). The contrasting effects of TMAO and urea on α-synuclein dimensions are consistent with unfavorable vs. favorable interactions of these two osmolytes respectively with the protein backbone (57).
A 2∶1 Ratio of [urea]∶[TMAO] Results in a Net Neutral Effect on α-Synuclein Dimensions.
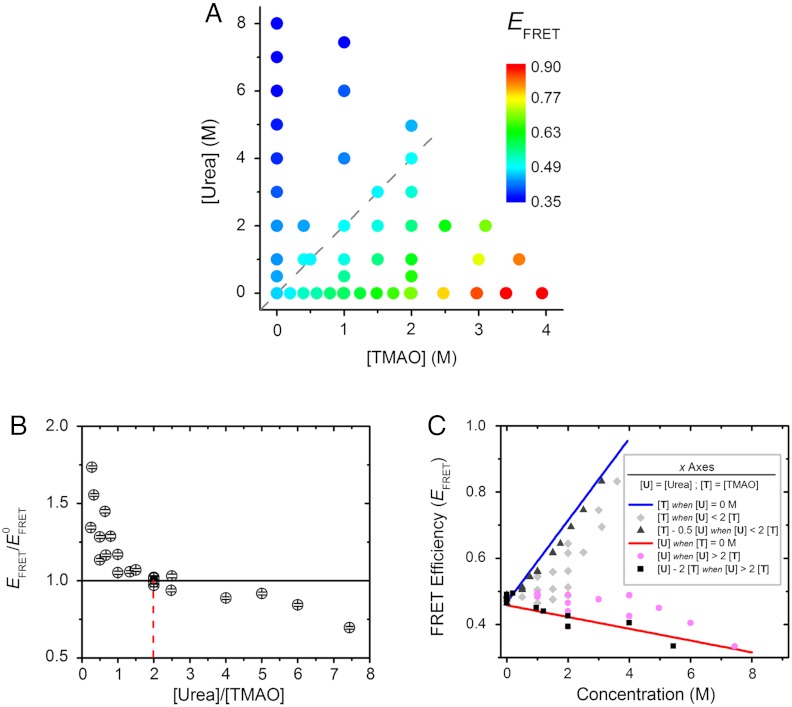
We next tested if particular [urea]∶[TMAO] ratios could balance out urea-induced α-synuclein expansion and TMAO-mediated protein contraction; i.e., effective urea-TMAO neutralization. Thus, we carried out extensive titration experiments where both the absolute and relative concentrations of individual osmolytes were varied. In total, approximately 240,000 single-molecule events were recorded and analyzed, providing high-quality statistics at single-molecule resolution (Table S1). In all conditions tested, single nonzero FRET peaks were observed, shifting as functions of individual osmolyte concentrations. The mean EFRET values of these peaks are reported color-coded in Fig. 3A. Evident from the data are the opposing trends of the effects of urea and TMAO along the osmolyte concentration axes. In addition, another trend emerges along the diagonal dashed line corresponding to a 2∶1 ratio of urea∶TMAO concentrations (cyan circles in Fig. 3A). The values of EFRET along this line is constant within error limits regardless of the absolute urea and TMAO concentrations, and is the same value corresponding to the 0 M osmolyte condition (Fig. 1, Left). Interestingly, this ratio corresponds to the 2∶1 ratiometric urea-TMAO counteraction of protein activity observed in vivo (8, 20) and in vitro (23, 58).
Fig. 3.
2∶1 Urea-TMAO counteraction ratio observed at single-molecule resolution. Changes in α-synuclein dimensions were monitored in different concentrations of urea and TMAO using smFRET. (A) Summary of the single-molecule data from approximately 240,000 events, plotted against cosolvent concentrations, color-coded on the basis of the measured EFRET values; i.e., red to blue for high to low EFRET, respectively. The gray dashed line represents a [urea]∶[TMAO] ratio of 2. (B) Normalized EFRET values (referenced to 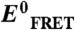 , the EFRET in 0 M cosolvent) are shown as a function of [urea]∶[TMAO] ratio when both urea and TMAO are present. An
, the EFRET in 0 M cosolvent) are shown as a function of [urea]∶[TMAO] ratio when both urea and TMAO are present. An 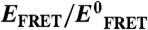 value of 1 (horizontal solid line) translates to conditions where protein dimension remains unchanged relative to 0 M osmolyte condition. The red dashed line represents the 2∶1 [urea]∶[TMAO] ratio. (C) presents the same smFRET data as shown in (A), plotting EFRET values against “residual” osmolyte concentrations, as defined in the inset; i.e., blue line: [TMAO] in the absence of urea, the same as shown in Fig. 2C; red line: [urea] in the absence of TMAO, the same as shown in Fig. 2D; gray diamonds: [TMAO] in solutions where urea is present and [urea]∶[TMAO] ratio is less than 2; black triangles: [TMAO]−0.5 [urea], in solutions where urea is present and [urea]∶[TMAO] ratio is less than 2; magenta circles: [urea] in solutions where TMAO is present and [urea]∶[TMAO] ratio is greater than 2; and, black squares: [urea]−2 [TMAO], in solutions where TMAO is present and [urea]∶[TMAO] ratio is greater than 2. See text for additional discussion.
value of 1 (horizontal solid line) translates to conditions where protein dimension remains unchanged relative to 0 M osmolyte condition. The red dashed line represents the 2∶1 [urea]∶[TMAO] ratio. (C) presents the same smFRET data as shown in (A), plotting EFRET values against “residual” osmolyte concentrations, as defined in the inset; i.e., blue line: [TMAO] in the absence of urea, the same as shown in Fig. 2C; red line: [urea] in the absence of TMAO, the same as shown in Fig. 2D; gray diamonds: [TMAO] in solutions where urea is present and [urea]∶[TMAO] ratio is less than 2; black triangles: [TMAO]−0.5 [urea], in solutions where urea is present and [urea]∶[TMAO] ratio is less than 2; magenta circles: [urea] in solutions where TMAO is present and [urea]∶[TMAO] ratio is greater than 2; and, black squares: [urea]−2 [TMAO], in solutions where TMAO is present and [urea]∶[TMAO] ratio is greater than 2. See text for additional discussion.
We plotted the EFRET data against the [urea]∶[TMAO] ratio for all nonzero TMAO or urea concentrations (Fig. 3B). The EFRET values were referenced to the value derived for 0 M osmolyte condition (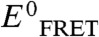 ); i.e., exactly counterbalancing conditions would result in a y-axis value of 1. Plainly observed is that the counterbalancing [urea]∶[TMAO] ratios cluster at an approximate value of 2, with all other ratios resulting in
); i.e., exactly counterbalancing conditions would result in a y-axis value of 1. Plainly observed is that the counterbalancing [urea]∶[TMAO] ratios cluster at an approximate value of 2, with all other ratios resulting in 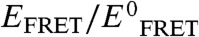 values either lower or higher than 1. Specifically, [urea]∶[TMAO] ratios less than 2 result in
values either lower or higher than 1. Specifically, [urea]∶[TMAO] ratios less than 2 result in 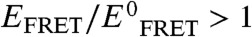 ; i.e., protein compaction, and vice versa. Additionally, Figs. 3
A and B show that although EFRET is conserved for the 2∶1 ratio, it generally varies for the other ratios.
; i.e., protein compaction, and vice versa. Additionally, Figs. 3
A and B show that although EFRET is conserved for the 2∶1 ratio, it generally varies for the other ratios.
The smFRET results described in the previous sections are consistent with a model in which urea and TMAO counterbalance each other’s effects on α-synuclein when the ratio is 2, possibly through formation of an effectively noninteracting 2∶1 complex, or via independent counterbalancing interactions of protein with urea and TMAO. Isotopic substitution neutron-scattering measurements of ternary-component urea-TMAO mixtures in water previously suggested that TMAO interacts directly with urea through preferential hydrogen bonding (59). However, recent studies show that the interaction affinity between the two osmolytes is too weak to be relevant even at high osmolyte concentrations (60, 61). Therefore, we favor a model in which the free energy of transfer of the protein in TMAO solutions is opposite of that in urea solutions, with the free energies being additive in urea-TMAO mixtures. At a 2∶1 urea-TMAO ratio, the transfer free energies counterbalance each other (23), and there is no net effect on the protein dimensions.
To further test the 2∶1 counteraction, we next calculated TMAO or urea residual concentrations for all the experimental conditions; i.e., the concentration of the osmolyte present in excess over the counterbalanced fraction of the osmolyte. For example, for a mixture of 2 M TMAO and 3 M urea, the residual concentration of TMAO is 0.5 M (see Fig. 3C for explanation). If the counterbalancing effect is additive, this transformation should collapse the EFRET data for osmolyte mixtures onto the data for individual osmolyte titrations alone. We plotted the transformed data against EFRET values for the total TMAO or urea concentrations in Fig. 3C. Comparison of the black triangles (transformed [TMAO] when [urea]∶[TMAO] < 2) with the blue line (same as shown in Fig. 2C) shows close correspondence, in contrast with the significant deviation for the grey diamonds (corresponding to the untransformed TMAO titration data). A similar trend is observed for the urea titration data. Therefore, these results support a 2∶1 counteraction regime for urea and TMAO.
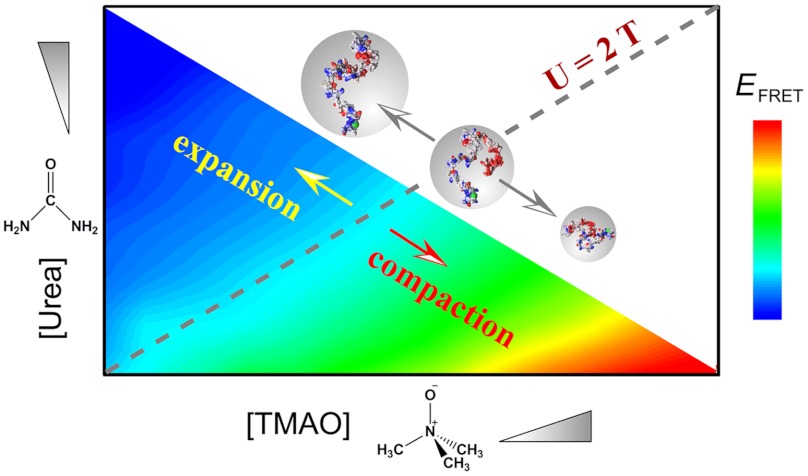
Hence overall, our data are consistent with the general model illustrated in Fig. 4. Monomeric α-synuclein is in its disordered state in the absence of both urea and TMAO (31, 45). The interaction between TMAO and the protein backbone is highly unfavorable (62). Consequently, the protein shifts to collapsed forms with increasing concentrations of the protecting osmolyte TMAO (50). On the contrary, the denaturing osmolyte urea interacts favorably with the protein backbone (62). This favorable interaction causes protein expansion with increasing urea concentrations (50). However, at a 2∶1 [urea]∶[TMAO] ratio (cyan-shaded region marked with diagonal dashed line in Fig. 4), the protein is neither expanded nor collapsed irrespective of the absolute concentrations of individual osmolytes. A residual concentration of either osmolyte over this ratio results in a change in protein dimension similar to that in the presence of the same concentration of the osmolyte alone. Our results can potentiate future theoretical treatments of the osmolyte counteraction phenomenon, perhaps in the context of linear free energy relationships in an additive and low-binding regime, as has been used extensively in biophysical and polymer physics analyses of protein folding reactions (63–66).
Fig. 4.
The urea-TMAO concentration ratio as the primary determinant for solvent-induced α-synuclein dimensional effects. The smFRET data are illustrated as a color-coded contour plot on the basis of EFRET, as a function of both osmolyte concentrations. The gray dashed line represents a [urea]∶[TMAO] ratio of 2, above which the protein dimension expands, and below which it contracts.
Conclusions
Osmolytes are members of the small-molecule chemical chaperone family, which play important roles in maintaining and regulating intracellular proteostasis (67, 68). A recent study revealed that chemical chaperone-mediated mutational buffering can prevent misfolding of mutant proteins in vivo (27). However, the mechanistic details of how osmolyte-mediated compaction/expansion of proteins effect protein folding/unfolding events both in vitro and in vivo warrant further investigation. In this paper, we showed that the Parkinson’s disease-linked α-synuclein, an intrinsically disordered protein, adopts compact/expanded structures in protecting/denaturing osmolyte solutions without clearly observed cooperative structural transitions. In addition, our study reinforces the concept of ratiometric counteraction of the protecting methylamine osmolyte TMAO and the denaturing osmolyte urea, wherein these small-molecule chaperones can effectively fine-tune protein structure and function by altering the ratio without the need for strict regulation of the absolute amounts of individual compounds in a cellular context. While some IDPs may not assume stable folded structures in the presence of a chemical chaperone such as the case here for α-synuclein, more compact/expanded and perhaps altered local structure could potentiate and modulate further binding/folding and ultimately function. In addition, our results also show that the counteraction hypothesis holds for a protein (α-synuclein) that presumably was not evolved to be tunable by the urea-TMAO system. This observation points to a more general interaction mechanism that is not protein-specific.
Finally, much theoretical and experimental work has been carried out on the mechanisms of interaction of denaturants and osmolytes on protein structure and function (3, 57, 63, 65, 69, 70). While the chemical physics of folding landscapes in these types of complex mixtures could be extremely involved, the results in our paper provide direct experimental evidence for a surprisingly simple and robust phase boundary condition between expanded and compact conformational ensembles of this important system.
Materials and Methods
All chemicals used were either analytical or reagent grade. Background fluorescence from TMAO was minimized by treatment with activated carbon and mixed-bed resin. We observed that TMAO solutions exhibit dilution-dependent pH changes. To avoid complications, individual TMAO solutions at specific concentrations were prepared by readjusting pH separately. α-Synuclein was expressed, purified, and dye-labeled as described previously (31, 45).
smFRET osmolyte titrations were carried out in αβγ buffer (0.2 M NaCl, 10 mM sodium acetate, 10 mM NaH2PO4, 10 mM glycine, pH 7.5 ± 0.1) at room temperature with dual-labeled sub-nM α-synuclein concentrations. Concentrations of urea and TMAO in stock preparations were calculated using refractive indices of respective solutions (71). Donor and acceptor fluorescence signals were recorded by simultaneous two-channel data collection with a binning time of 500 μs using a home-built confocal single-molecule setup as described previously (45, 72). The leakage of donor emission into the acceptor channel (9%) and acceptor emission due to direct excitation (5%) were taken into account. A threshold of 40 counts (the sum of signals from the two channels) was used to separate background noise from single-molecule fluorescence signals. EFRET values were calculated from the corrected donor (ID) and acceptor (IA) fluorescence intensities as
 |
The value of γ was approximated to 1 on the basis of our previous measurements (45). FRET efficiency histograms were generated and the distributions were fitted to a Gaussian function using OriginPro 8.0 (OriginLab). A more detailed description of data acquisition and analysis was described previously (45).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Robert L. Nussbaum and Nelson B. Cole for providing the plasmid construct for wildtype α-synuclein and Dr. Jörg Rösgen for helpful communications and critical review of the manuscript. This work was supported by Grant GM066833 (A.A.D.) from the National Institute of General Medical Sciences, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201802109/-/DCSupplemental.
References
- 1.Wolynes P, Onuchic J, Thirumalai D. Navigating the folding routes. Science. 1995;267:1619–1620. doi: 10.1126/science.7886447. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu N, Sharp K. Protein–solvent interactions. Chem Rev. 2006;106:1616–1623. doi: 10.1021/cr040437f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harries D, Rösgen J. A practical guide on how osmolytes modulate macromolecular properties. In: Correia JJ, Detrich HW III, editors. Methods in Cell Biology. Vol 84. San Diego: Academic; 2008. pp. 679–735. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H-X, Rivas G, Minton AP. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyson HJ, Wright PE. Coupling of folding and binding for unstructured proteins. Curr Opin Struct Biol. 2002;12:54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 6.Frankel AD, Smith CA. Induced folding in RNA-protein recognition: More than a simple molecular handshake. Cell. 1998;92:149–151. doi: 10.1016/s0092-8674(00)80908-3. [DOI] [PubMed] [Google Scholar]
- 7.Luque I, Freire E. Structural stability of binding sites: Consequences for binding affinity and allosteric effects. Proteins. 2000;Suppl 4:63–71. doi: 10.1002/1097-0134(2000)41:4+<63::aid-prot60>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Yancey P, Clark M, Hand S, Bowlus R, Somero G. Living with water stress: Evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 9.Yancey PH, Fyfe-Johnson AL, Kelly RH, Walker VP, Auñón MT. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. J Exp Zool. 2001;289:172–176. doi: 10.1002/1097-010x(20010215)289:3<172::aid-jez3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 11.Yancey PH. Water stress, osmolytes and proteins. Am Zool. 2001;41:699–709. [Google Scholar]
- 12.Kenneth BS. Organic solutes in freezing tolerance. Comp Biochem Physiol A Physiol. 1997;117:319–326. doi: 10.1016/s0300-9629(96)00270-8. [DOI] [PubMed] [Google Scholar]
- 13.Saha N, Ratha BK. Comparative study of ureogenesis in freshwater, air-breathing teleosts. J Exp Zool. 1989;252:1–8. [Google Scholar]
- 14.Gordon MS, Tucker VA. Further observations on the physiology of salinity adaptation in the crab-eating frog (Rana cancrivora) J Exp Biol. 1968;49:185–193. [Google Scholar]
- 15.Romspert AP, McClanahan LL. Osmoregulation of the terrestrial salamander, Ambystoma tigrinum, in hypersaline media. Copeia. 1981;1981:400–405. [Google Scholar]
- 16.Withers PC. Urea: Diverse functions of a “waste” product. Clin Exp Pharmacol Physiol. 1998;25:722–727. doi: 10.1111/j.1440-1681.1998.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon JA, Jencks WP. The relationship of structure to the effectiveness of denaturing agents for proteins. Biochemistry. 1963;2:47–57. doi: 10.1021/bi00901a011. [DOI] [PubMed] [Google Scholar]
- 18.Bagnasco S, Balaban R, Fales HM, Yang YM, Burg M. Predominant osmotically active organic solutes in rat and rabbit renal medullas. J Biol Chem. 1986;261:5872–5877. [PubMed] [Google Scholar]
- 19.Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 20.Yancey PH, Somero GN. Counteraction of urea destabilization of protein structure by methylamine osmoregulatory compounds of elasmobranch fishes. Biochem J. 1979;183:317–323. doi: 10.1042/bj1830317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolen DW, Rose GD. Structure and energetics of the hydrogen-bonded backbone in protein folding. Annu Rev Biochem. 2008;77:339–362. doi: 10.1146/annurev.biochem.77.061306.131357. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Rösgen J, Pettitt BM. Modeling Solvent Environments. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2010. Osmolyte influence on protein stability: Perspectives of theory and experiment; pp. 77–92. [Google Scholar]
- 23.Baskakov I, Wang A, Bolen DW. Trimethylamine-N-Oxide counteracts urea effects on rabbit muscle lactate dehydrogenase function: A test of the counteraction hypothesis. Biophys J. 1998;74:2666–2673. doi: 10.1016/S0006-3495(98)77972-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doan-Nguyen V, Loria JP. The effects of cosolutes on protein dynamics: The reversal of denaturant-induced protein fluctuations by trimethylamine N-oxide. Protein Sci. 2007;16:20–29. doi: 10.1110/ps.062393707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty K, et al. Chaperonin-catalyzed rescue of kinetically trapped states in protein folding. Cell. 2010;142:112–122. doi: 10.1016/j.cell.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Mello CC, Barrick D. Measuring the stability of partly folded proteins using TMAO. Protein Sci. 2003;12:1522–1529. doi: 10.1110/ps.0372903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay A, et al. Chemical chaperones assist intracellular folding to buffer mutational variations. Nat Chem Biol. 2012;8:238–245. doi: 10.1038/nchembio.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Y-C, Oas TG. Osmolyte-induced folding of an intrinsically disordered protein: Folding mechanism in the absence of ligand. Biochemistry. 2010;49:5086–5096. doi: 10.1021/bi100222h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskakov IV, et al. Trimethylamine N-Oxide-induced cooperative folding of an intrinsically unfolded transcription-activating fragment of human glucocorticoid receptor. J Biol Chem. 1999;274:10693–10696. doi: 10.1074/jbc.274.16.10693. [DOI] [PubMed] [Google Scholar]
- 30.Baskakov I, Bolen DW. Forcing thermodynamically unfolded proteins to fold. J Biol Chem. 1998;273:4831–4834. doi: 10.1074/jbc.273.9.4831. [DOI] [PubMed] [Google Scholar]
- 31.Ferreon ACM, Deniz AA. α-Synuclein multistate folding thermodynamics: Implications for protein misfolding and aggregation. Biochemistry. 2007;46:4499–4509. doi: 10.1021/bi602461y. [DOI] [PubMed] [Google Scholar]
- 32.Uversky VN, Eliezer D. Biophysics of Parkinson’s disease: Structure and aggregation of α-synuclein. Curr Protein Pept Sci. 2009;10:483–499. doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uversky VN, Li J, Fink AL. Trimethylamine-N-oxide-induced folding of α-synuclein. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 34.Deniz AA, Mukhopadhyay S, Lemke EA. Single-molecule biophysics: At the interface of biology, physics and chemistry. J R Soc Interface. 2008;5:15–45. doi: 10.1098/rsif.2007.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreon ACM, Deniz AA. Protein folding at single-molecule resolution. Biochim Biophys Acta. 2011;1814:1021–1029. doi: 10.1016/j.bbapap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalet X, Weiss S, Jäger M. Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem Rev. 2006;106:1785–1813. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler B, Eaton WA. Protein folding studied by single-molecule FRET. Curr Opin Struct Biol. 2008;18:16–26. doi: 10.1016/j.sbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopalan S, Huang F, Fersht AR. Single-molecule characterization of oligomerization kinetics and equilibria of the tumor suppressor p53. Nucleic Acids Res. 2011;39:2294–2303. doi: 10.1093/nar/gkq800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha T, Kozlov AG, Lohman TM. Single-molecule views of protein movement on single-stranded DNA. Annu Rev Biophy. 2012;41:295–319. doi: 10.1146/annurev-biophys-042910-155351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junker JP, Ziegler F, Rief M. Ligand-dependent equilibrium fluctuations of single calmodulin molecules. Science. 2009;323:633–637. doi: 10.1126/science.1166191. [DOI] [PubMed] [Google Scholar]
- 41.Walter NG, Huang C-Y, Manzo AJ, Sobhy MA. Do-it-yourself guide: How to use the modern single-molecule toolkit. Nat Meth. 2008;5:475–489. doi: 10.1038/nmeth.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamboy JA, Kim H, Lee KS, Ha T, Komives EA. Visualization of the nanospring dynamics of the IκBα ankyrin repeat domain in real time. Proc Natl Acad Sci USA. 2011;108:10178–10183. doi: 10.1073/pnas.1102226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borwankar T, et al. Natural osmolytes remodel the aggregation pathway of mutant huntingtin exon 1. Biochemistry. 2011;50:2048–2060. doi: 10.1021/bi1018368. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay S, Krishnan R, Lemke EA, Lindquist S, Deniz AA. A natively unfolded yeast prion monomer adopts an ensemble of collapsed and rapidly fluctuating structures. Proc Natl Acad Sci USA. 2007;104:2649–2654. doi: 10.1073/pnas.0611503104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci USA. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreon ACM, Moran CR, Ferreon JC, Deniz AA. Alteration of the α-synuclein folding landscape by a mutation related to Parkinson’s disease. Angew Chem Int Ed Engl. 2010;49:3469–3472. doi: 10.1002/anie.201000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambin Y, et al. Visualizing a one-way protein encounter complex by ultrafast single-molecule mixing. Nat Meth. 2011;8:239–241. doi: 10.1038/nmeth.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandelinder V, Ferreon ACM, Gambin Y, Deniz AA, Groisman A. High-resolution temperature–concentration diagram of α-synuclein conformation obtained from a single Förster resonance energy transfer image in a microfluidic device. Anal Chem. 2009;81:6929–6935. doi: 10.1021/ac901008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burns JN, et al. Rescue of glaucoma-causing mutant myocilin thermal stability by chemical chaperones. ACS Chem Biol. 2010;5:477–487. doi: 10.1021/cb900282e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auton M, Ferreon ACM, Bolen DW. Metrics that differentiate the origins of osmolyte effects on protein stability: A test of the surface tension proposal. J Mol Biol. 2006;361:983–992. doi: 10.1016/j.jmb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Deniz AA, et al. Single-molecule protein folding: Diffusion fluorescence resonance energy transfer studies of the denaturation of chymotrypsin inhibitor 2. Proc Natl Acad Sci USA. 2000;97:5179–5184. doi: 10.1073/pnas.090104997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu Y, Bolen CL, Bolen DW. Osmolyte-driven contraction of a random coil protein. Proc Natl Acad Sci USA. 1998;95:9268–9273. doi: 10.1073/pnas.95.16.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu CY, Lynch GC, Kokubo H, Pettitt BM. Trimethylamine N-oxide influence on the backbone of proteins: An oligoglycine model. Proteins. 2010;78:695–704. doi: 10.1002/prot.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho SS, Reddy G, Straub JE, Thirumalai D. Entropic stabilization of proteins by TMAO. J Phys Chem B. 2011;115:13401–13407. doi: 10.1021/jp207289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreon ACM, Moran CR, Gambin Y, Deniz AA. Single-molecule fluorescence studies of intrinsically disordered proteins. In: Walter NG, editor. Methods in Enzymology. Vol 472. San Diego: Academic Press; 2010. pp. 179–204. [DOI] [PubMed] [Google Scholar]
- 56.Müller-Späth S, et al. Charge interactions can dominate the dimensions of intrinsically disordered proteins. Proc Natl Acad Sci USA. 2010;107:14609–14614. doi: 10.1073/pnas.1001743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auton M, Rösgen J, Sinev M, Holthauzen LMF, Bolen DW. Osmolyte effects on protein stability and solubility: A balancing act between backbone and side-chains. Biophys Chem. 2011;159:90–99. doi: 10.1016/j.bpc.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sackett DL. Natural osmolyte trimethylamine N-oxide stimulates tubulin polymerization and reverses urea inhibition. Am J Physiol Regul Integr Comp Physiol. 1997;273:R669–R676. doi: 10.1152/ajpregu.1997.273.2.R669. [DOI] [PubMed] [Google Scholar]
- 59.Meersman F, Bowron D, Soper AK, Koch MHJ. Counteraction of urea by trimethylamine N-oxide is due to direct interaction. Biophys J. 2009;97:2559–2566. doi: 10.1016/j.bpj.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meersman F, Bowron D, Soper AK, Koch MHJ. An X-ray and neutron scattering study of the equilibrium between trimethylamine N-oxide and urea in aqueous solution. Phys Chem Chem Phys. 2011;13:13765–13771. doi: 10.1039/c1cp20842j. [DOI] [PubMed] [Google Scholar]
- 61.Rösgen J, Jackson-Atogi R. Volume exclusion and H-bonding dominate the thermodynamics and solvation of trimethylamine-N-oxide in aqueous urea. J Am Chem Soc. 2012;134:3590–3597. doi: 10.1021/ja211530n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auton M, Bolen DW. Additive transfer free energies of the peptide backbone unit that are independent of the model compound and the choice of concentration scale. Biochemistry. 2004;43:1329–1342. doi: 10.1021/bi035908r. [DOI] [PubMed] [Google Scholar]
- 63.Schellman JA. Destabilization and stabilization of proteins. Q Rev Biophys. 2005;38:351–361. doi: 10.1017/S0033583505004099. [DOI] [PubMed] [Google Scholar]
- 64.Greene RF, Pace CN. Urea and guanidine hydrochloride denaturation of ribonuclease, lysozyme, α-chymotrypsin, and β-lactoglobulin. J Biol Chem. 1974;249:5388–5393. [PubMed] [Google Scholar]
- 65.Rösgen J. Molecular basis of osmolyte effects on protein and metabolites. In: Häussinger D, Sies H, editors. Methods in Enzymology. Vol 428. San Diego: Academic Press; 2007. pp. 459–486. [DOI] [PubMed] [Google Scholar]
- 66.Haran G. How, when and why proteins collapse: The relation to folding. Curr Opin Struct Biol. 2012;22:14–20. doi: 10.1016/j.sbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Özcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- 70.Thirumalai D, O’Brien EP, Morrison G, Hyeon C. Theoretical perspectives on protein folding. Annu Rev Biophys. 2010;39:159–183. doi: 10.1146/annurev-biophys-051309-103835. [DOI] [PubMed] [Google Scholar]
- 71.Qu Y, Bolen DW. Hydrogen exchange kinetics of RNase A and the urea∶TMAO paradigm. Biochemistry. 2003;42:5837–5849. doi: 10.1021/bi0206457. [DOI] [PubMed] [Google Scholar]
- 72.Gambin Y, et al. Direct single-molecule observation of a protein living in two opposed native structures. Proc Natl Acad Sci USA. 2009;106:10153–10158. doi: 10.1073/pnas.0904461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.