Abstract
Context
Though multivitamins aim to prevent vitamin and mineral deficiency, there is a perception that multivitamins may prevent cardiovascular disease (CVD). Observational studies examining regular multivitamin use have been inconsistently associated with CVD, with no long-term clinical trials of multivitamin use.
Objective
To determine whether long-term multivitamin supplementation decreases the risk of major cardiovascular events among men.
Design
The Physicians' Health Study II is a randomized, double-blind, placebo-controlled trial of a common daily multivitamin, that began in 1997 with continued treatment and follow-up through June 1, 2011.
Setting and Participants
A total of 14,641 male U.S. physicians initially aged ≥50 years (mean [± SD] age; 64.3 [± 9.2] years), including 754 men with a history of CVD at randomization, were enrolled.
Intervention
Daily multivitamin, as Centrum Silver.
Main Outcome Measures
The primary cardiovascular outcome was a composite endpoint of major cardiovascular events, including nonfatal myocardial infarction (MI), nonfatal stroke, and fatal CVD. Secondary outcomes included MI and stroke individually.
Results
During a median (interquartile range) follow-up of 11.2 (10.7 to 13.3) years, there were 1,732 confirmed major cardiovascular events. Compared with placebo, there was no significant effect of a daily multivitamin on major cardiovascular events (active and placebo multivitamin groups, 11.0 and 10.8 events per 1,000 person-years; hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.91–1.10; P=0.91). Further, a daily multivitamin had no effect on total MI (active and placebo multivitamin groups, 3.9 and 4.2 events per 1,000 person-years; HR, 0.93; 95% CI, 0.80–1.09; P=0.39), total stroke (active and placebo multivitamin groups, 4.1 and 3.9 events per 1,000 person-years; HR, 1.06; 95% CI, 0.91–1.23; P=0.48), or cardiovascular mortality (active and placebo multivitamin groups, 5.0 and 5.1 events per 1,000 person-years; HR, 0.95; 95% CI, 0.83–1.09; P=0.47). A daily multivitamin was also not significantly associated with total mortality (HR, 0.94; 95% CI, 0.88–1.02; P=0.13). The effect of a daily multivitamin on major cardiovascular events did not differ between men with or without a baseline history of CVD (P, interaction = 0.62).
Conclusions
A daily multivitamin did not reduce major cardiovascular events, MI, stroke, and CVD mortality after more than a decade of treatment and follow-up.
Keywords: multivitamin, cardiovascular disease, myocardial infarction, stroke, randomized clinical trial, men
INTRODUCTION
Despite uncertainty regarding its long-term health benefits, many US adults take vitamin supplements1 to prevent chronic diseases2 or for general health and well-being.3 Since multivitamins are the most common supplement taken by US adults4,5 there are broad public health implications regarding their everyday use. Individuals who feel they are deriving benefits from supplements may be less likely to engage in other preventive health behaviors, and chronic daily supplement use poses a financial burden with annual vitamin supplement sales in the billons of US dollars.6 A daily multivitamin, with its combination of essential vitamins and minerals that meet minimum RDA levels, may replicate broader, healthier dietary and food patterns identified in epidemiologic studies for CVD prevention.7,8 Observational studies of multivitamin use and cardiovascular incidence and mortality are limited and inconsistent.9–16
Randomized clinical trials have tested the effect of individual vitamins and minerals - including beta-carotene,17–19 vitamin E,19–22 vitamin C,19,22 selenium,23 and B vitamins,24,25 among others - with the vast majority showing no effect on CVD endpoints. Only a few large-scale trials have tested smaller combinations of vitamins or minerals, typically selected from those already tested individually and equivocally,26–28 for which there has been a lack of effect. There have been no large-scale trials of a multivitamin in CVD prevention. Accordingly, an NIH conference panel29 and the 2010 Dietary Guidelines concluded that there is no evidence to support a daily multivitamin in disease prevention, including CVD.
The Physicians' Health Study II (PHS II) is the only large-scale trial testing the effects of long-term use of a common multivitamin on the risk of major cardiovascular events and cancer among 14,641 male physicians. In this paper, we present the findings for multivitamin use on major cardiovascular events. Results for cancer, eye disease, and cognitive decline are being published separately.
METHODS
Study Design
The Physicians' Health Study II (PHS II) was a randomized, double-blind, placebo-controlled, 2×2×2×2 factorial trial evaluating the balance of risks and benefits of a multivitamin (Centrum Silver or its placebo daily; Pfizer (formerly Wyeth, American Home Products, and Lederle)), vitamin E, vitamin C, and beta-carotene in the prevention of CVD, cancer, eye disease, and cognitive decline among 14,641 male physicians initially aged ≥50 years.31 The beta-carotene component ended as scheduled in March 2003, and the vitamin E and C components ended as scheduled in 2007 with a lack of effect reported for CVD22 and cancer.32
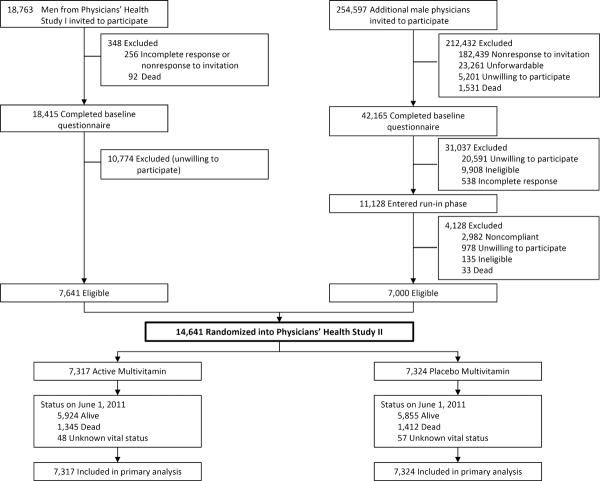
As detailed previously,22,31,32 PHS II recruitment, enrollment, and randomization occurred in two phases (Figure 1). In Phase 1, starting in July 1997, we invited 18,763 living participants from PHS I, a randomized trial of low-dose aspirin33 and beta-carotene17 among 22,071 male physicians, to participate in PHS II. Men were ineligible if they reported a history of cirrhosis, active liver disease, were taking anticoagulants, or reported a serious illness that might preclude participation. Men also must have been willing to forego current use of multivitamins or individual supplements containing more than 100% of the RDA of vitamin E, vitamin C, beta-carotene, or vitamin A. Men with a history of myocardial infarction (MI), stroke, or cancer remained eligible. We randomized 7,641 (41%) willing and eligible PHS I participants into PHS II.
Figure 1.
Flow diagram of participants from screening to completion of the multivitamin component of the Physicians' Health Study (PHS) II.*
* Those classified as “unforwardable” were not able to be contacted by mail.
Phase 2 began in July 1999 with invitational letters and baseline questionnaires sent to 254,597 additional US male physicians aged ≥50 years identified from a list from the American Medical Association that excluded PHS I participants. By July 2001, 42,165 (16.6%) men completed the baseline PHS II questionnaire, of whom 11,128 (26.4%) were willing and eligible based upon the same eligibility criteria as PHS I participants. Of 11,128 physicians who entered a run-in phase, 7,000 (63%) were compliant with their pills and were randomized into PHS II.
A total of 14,641 men were randomized into PHS II in blocks of 16 and stratified by age, prior diagnosis of cancer, prior diagnosis of CVD, and, for 7,641 PHS I participants, their original beta-carotene treatment assignment. There were 754 (5.1%) men with a history of MI or stroke before randomization. All participants provided written informed consent, and the Institutional Review Board at Brigham and Women's Hospital approved the research protocol.
Study Treatment, Follow-up, and Compliance
Every six months for the first year, then annually thereafter PHS II participants were sent monthly calendar packs containing a multivitamin or placebo. Annual mailed questionnaires asked about compliance, adverse events, endpoints, and risk factors. Blinded treatment and follow-up continued through June 1, 2011, the scheduled end of the PHS II multivitamin component. Data analyses include follow-up and validation of reported endpoints through August 2012. Morbidity and mortality follow-up in PHS II were extremely high, at 98.2% and 99.9%, respectively. In addition, morbidity and mortality follow-up as a percentage of person-time each exceeded 99.9%. Adherence with the multivitamin component was defined from participant self-reports as taking at least two-thirds of the pills, which has been shown to be very reliable in physicians.34
Confirmation of Cardiovascular Disease Endpoints
For the multivitamin component, the primary endpoint was major cardiovascular events (including nonfatal MI, nonfatal stroke, and cardiovascular mortality). Pre-specified secondary endpoints included in this report include total MI and total stroke. Other endpoints considered in these analyses included fatal and nonfatal MI and stroke, cardiovascular death, ischemic and hemorrhagic stroke, and total mortality.
For each of the above endpoints reported by participants, we requested permission from the participant to examine all relevant medical records. Upon receipt of consent, medical records were requested and reviewed by an Endpoints Committee of physicians blinded to randomized treatment assignment. We were unable to obtain adequate medical records for <5% of the endpoints. The diagnosis of MI was confirmed by evidence of symptoms in the presence of either diagnostic elevations of cardiac enzymes or diagnostic changes on electrocardiograms. For fatal events, the diagnosis of MI was also accepted based upon autopsy findings.33 We confirmed diagnoses of stroke defined as a typical neurologic deficit of sudden or rapid onset and vascular origin, lasting >24 hours. Stroke was classified according to National Survey of Stroke criteria into ischemic, hemorrhagic and unknown subtype,35 with high interobserver agreement.36 Participant deaths were usually reported by family members or postal authorities. Following a report of a participant death, death certificates and/or autopsy reports were obtained. Total mortality was confirmed by the Endpoints Committee or by death certificate. CVD mortality was additionally documented by convincing evidence of a cardiovascular mechanism from all available sources. For men with unknown vital status, we used web and National Death Index searches to identify deaths. Only confirmed endpoints of MI, stroke, and CVD death were included in this analysis. We also collected data on participant self-reports of congestive heart failure, angina pectoris, and revascularization (including coronary artery bypass graft and percutaneous coronary intervention) for inclusion in our analyses.
Statistical Analyses
All primary analyses were based upon the intention-to-treat principle, in which all 14,641 randomized PHS II participants were classified according to their randomized multivitamin treatment assignment and followed until the occurrence of major cardiovascular events, death, loss to follow-up, or the end of the multivitamin component of PHS II on June 1, 2011. We used SAS version 9.2 (SAS Institute Inc.) and S-Plus (Insightful Corp.), with statistical significance set at P<0.05 using 2-sided tests. The PHS II was estimated to have 80% power to detect a 12% reduction in the primary endpoint of major cardiovascular events.
We initially compared baseline characteristics by multivitamin treatment assignment to ensure randomization equally distributed baseline characteristics by active versus placebo groups. As done in previous PHS II trial analyses,22,32 Cox proportional hazards models estimated the HRs and 95% confidence intervals (CIs) comparing event rates in the multivitamin and placebo groups. For each pre-specified endpoint, we stratified on the presence of CVD at randomization and adjusted for PHS II study design variables, including age (in years), PHS cohort (original PHS I participant, new PHS II participant), and randomized vitamin E, vitamin C, and beta-carotene assignments. For analyses of total major cardiovascular events, all new events were included, regardless of whether the participant had a baseline history of CVD. Analyses of individual cardiovascular endpoints did not censor men upon occurrence of another cardiovascular endpoint. For analyses of total and cardiovascular mortality, we included all 14,641 PHS II participants, and for total mortality we additionally stratified by history of cancer at randomization.
We tested the proportional hazards assumptions by including an interaction term for multivitamin treatment with the logarithm of time, and this assumption was not violated for major cardiovascular events, total MI, and total stroke (each P>0.05). Cumulative incidence curves compared the overall effect of the multivitamin component on major cardiovascular events, total MI, and total stroke using a crude log-rank test. We investigated the effect of compliance with the multivitamin intervention on our primary results through sensitivity analyses using censoring and stratification.
We then conducted additional exploratory analyses on the effect of the multivitamin intervention on major cardiovascular events, total MI, and total stroke after excluding the first two or five years of follow-up to explore a possible early or late benefit associated with long-term multivitamin use. We also conducted subgroup analyses stratified by major risk factors, parental history of MI <60 years, and selected coronary biomarkers and dietary factors available in a subgroup of PHS II participants. Finally, we evaluated the effect of a daily multivitamin within the pre-specified subgroups of 754 men with and 13,887 men without a baseline history of CVD. Effect modification was assessed by using interaction terms between subgroup indicators and randomized multivitamin treatment assignment.
RESULTS
We randomized 14,641 men into PHS II with a mean (±SD) age of 64.3 (±9.2) years. Factors measured at baseline were similar between the multivitamin and placebo groups (Table 1). Among coronary risk factors, there was a low proportion of current smokers (3.6%) and a relatively high proportion of men who exercised ≥1 time/wk (59.9%), which was countered with 42.0%, 35.4% and 6.2% of men reporting a history of hypertension, high cholesterol, and diabetes, respectively. Baseline aspirin use was high (76.3%) in this population of physicians, in part reflective of their previous participation and results of the PHS I trial testing aspirin and CVD.33 There were 754 (5.1%) and 1,312 (9.0%) men with a baseline history of CVD and cancer, respectively.
Table 1.
Baseline characteristics according to multivitamin treatment assignment in 14,641 men from the Physicians' Health Study (PHS) II.
| Men, No. (%)a |
|||
|---|---|---|---|
|
Multivitamin
b
|
|||
| Self-reported baseline characteristics | Active (n=7,317) | Placebo (n=7,324) | |
| Age (mean ± SD, years) | 64.2 ± 9.1 | 164.3 ± 9.2 | |
|
| |||
| Age (No. (%)) | |||
| 50–59 years | 2,944 (40.2) | 2,947 (40.2) | |
| 60–69 years | 2,348 (32.1) | 2,348 (32.1) | |
| ≥70 years | 2,025 (27.7) | 2,029 (27.7) | |
|
| |||
| Body mass index (mean ± SD, kg/m2) | 26.0 ± 3.6 | 26.0 ± 3.7 | |
|
| |||
| Cigarette smoking (No. (%)) | |||
| Never | 4,145 (56.7) | 4,107 (56.1) | |
| Former | 2,908 (39.8) | 2,944 (40.2) | |
| Current | 255 (3.5) | 269 (3.7) | |
|
| |||
| Exercise ≥1 time/wk (No. (%)) | |||
| No | 2,699 (37.8) | 2,806 (39.3) | |
| Yes | 4,444 (62.2) | 4,328 (60.7) | |
|
| |||
| Alcohol consumption (No. (%)) | |||
| Rarely/never | 1,391 (19.2) | 1,339 (18.4) | |
| ≥ drink/month | 5,874 (80.9) | 5,942 (81.6) | |
|
| |||
| Current aspirin use (No. (%)) | |||
| No | 1,625 (22.5) | 1,636 (22.7) | |
| Yes | 5,602 (77.5) | 5,565 (77.3) | |
|
| |||
| History of hypertension (No. (%))c | |||
| No | 4,229 (58.2) | 4,177 (57.3) | |
| Yes | 3,039 (41.8) | 3,117 (42.7) | |
|
| |||
| History of high cholesterol (No. (%))d | |||
| No | 4,534 (64.0) | 4,432 (62.7) | |
| Yes | 2,549 (36.0) | 2,641 (37.3) | |
|
| |||
| History of diabetes (No. (%)) | |||
| No | 6,838 (93.5) | 6,883 (94.1) | |
| Yes | 472 (6.5) | 433 (5.9) | |
|
| |||
| Parental history of MI <60 years (No. (%))e | |||
| No | 5,941 (90.0) | 5,928 (89.4) | |
| Yes | 661 (10.0) | 701 (10.6) | |
|
| |||
| Self-reported history of CVD (No.(%))f | |||
| No | 6,941 (94.9) | 6,946 (94.8) | |
| Yes | 376 (5.1) | 378 (5.2) | |
|
| |||
| Plasma total cholesterol (mean ± SD, mg/dL)g | 203.5 (35.5) | 203.7 (36.0) | |
|
| |||
| Plasma HDL cholesterol (mean ± SD, mg/dL)g | 44.3 (14.4) | 44.0 (14.7) | |
|
| |||
| Fruit and vegetable intake (median (25th–75th percentile), servings/d)h | 4.26 (2.95–5.75) | 4.19 (2.94–5.77) | |
|
| |||
| Whole grain intake (median (25th–75th percentile), servings/d)h | 1.13 (0.49–2.00) | 1.07 (0.49–1.99) | |
|
| |||
| Red meat intake (median (25th–75th percentile), servings/d)h | 0.63 (0.29–1.05) | 0.57 (0.29–1.00) | |
Abbreviations: CVD, cardiovascular disease; MI, myocardial infarction; SD, standard deviation
Unless otherwise indicated. The numbers do not always sum to group totals due to missing information for some variables.
P>0.05 for all comparisons between active and placebo multivitamin group.
History of hypertension was defined as self-reported systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or past/current treatment for hypertension.
History of high cholesterol was defined as self-reported total cholesterol ≥240 mg/dL or past/current treatment for high cholesterol.
Excludes 1,410 men with missing information on parental history of MI <60 years.
History of CVD included nonfatal myocardial infarction or nonfatal stroke.
Among 8,609 and 8,615 men with available biomarker data for plasma total cholesterol and HDL cholesterol, respectively.
Among 13,310, 13,280, and 13,268 men with available dietary data on fruit and vegetable intake, whole grain intake, and red meat intake, respectively.
Mean follow-up of PHS II participants was 11.2 years (median [interquartile range], 11.2 [10.7–13.3] years; maximum, 13.8 years), totaling 164,320 person-years. For active multivitamin and its placebo, adherence at 4 years was 76.8% and 77.1%, respectively (P=0.71); at 8 years, 72.3% and 70.7% (P=0.15); and at the end of follow-up, 67.5% and 67.1% (P=0.70). There were small differences between the active and placebo groups comparing the avoidance of individual non-trial multivitamin use (<30 days/year) at 4 years (86.7% and 85.4%, respectively; P=0.03) and 8 years (78.5% and 75.8%, P=0.01) follow-up, but not by the end of multivitamin follow-up (81.0% and 80.3%; P=0.35). During multivitamin treatment, we confirmed that 1,732 men had major cardiovascular events, including 652 cases (first events) of MI, 643 cases of stroke (527 and 94 cases of ischemic and hemorrhagic stroke, respectively), and 829 had cardiovascular death, with some men experiencing multiple events. A total of 2,757 (18.8%) men died during follow-up.
Multivitamin use and major cardiovascular events
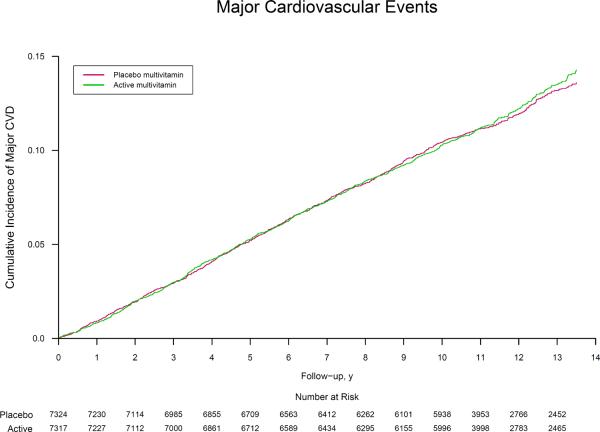
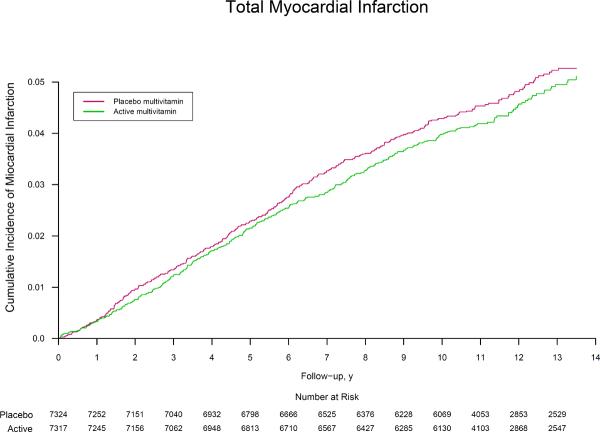
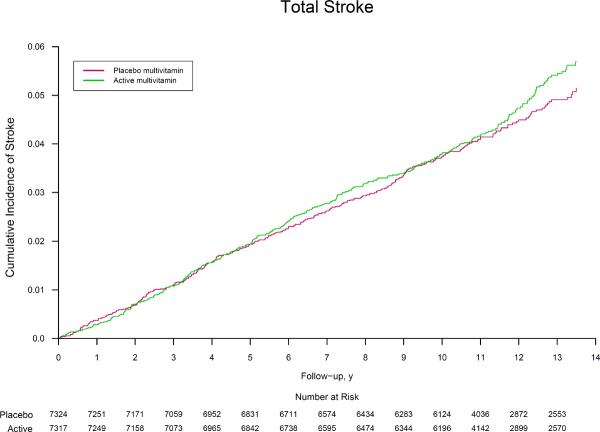
The rates of major cardiovascular events were 11.0 and 10.8 per 1,000 person-years in the active and placebo multivitamin groups, respectively. Men taking an active daily multivitamin experienced no benefit on the primary endpoint of major cardiovascular events (HR, 1.01; 95% CI, 0.91–1.10; P=0.91) (Table 2), with similar cumulative incidence curves (crude log-rank P=0.69) (Figure 2). There was a similar lack of significant benefit on the secondary endpoints of total MI (HR, 0.93; 95% CI, 0.80–1.09; P=0.39) and total stroke (HR, 1.06; 95% CI, 0.91–1.23; P=0.48) compared with men taking the placebo multivitamin. This lack of effect is illustrated in the corresponding cumulative incidence curves (both crude log-rank P>0.05) (Figure 2). In secondary analyses, there were fewer deaths among multivitamins users, but significance was marginal especially given the number of analyses conducted. (HR, 0.61; 95% CI, 0.38–1.00; P=0.048). Among stroke subtypes, a daily multivitamin had no effect on either ischemic stroke (HR, 1.10; 95% CI, 0.92–1.30; P=0.29) or hemorrhagic stroke (HR, 1.08; 95% CI, 0.72–1.63; P=0.69). We found no significant effect of a daily multivitamin on rates of congestive heart failure (HR, 0.95; 95% CI, 0.83–1.09; P=0.47), angina (HR, 1.00; 95% CI, 0.91–1.09; P=0.96), and coronary revascularization (HR, 1.03; 95% CI, 0.94–1.13; P=0.50). Daily multivitamin was not significantly associated with cardiovascular mortality (HR, 0.95; 95% CI, 0.83–1.09; P=0.47). There were fewer deaths among multivitamin users (HR, 0.94; 95% CI, 0.88–1.02; P=0.13), but this was not statistically significant.
Table 2.
Association between randomized multivitamin assignment and the risk of major cardiovascular events and mortality in the Physicians' Health Study II.a
| Outcome | Multivitamin | P | ||
|---|---|---|---|---|
| Active (n=7,317) | Placebo (n=7,324) | Hazard Ratiob (95% CI) | ||
| Major cardiovascular eventsc | 876d | 856 | 1.01 (0.91–1.10) | 0.9 |
| Total myocardial infarctione | 317 | 335 | 0.93 (0.80–1.09) | 0.39 |
| Myocardial infarction death | 27 | 43 | 061 (0.38–1.00) | 0.048 |
| Total strokee | 332 | 311 | 1.06 (0.91–1.23) | 0.48 |
| Stroke death | 89 | 76 | 1.16 (0.85–1.58) | 0.34 |
| Ischemic strokef | 277 | 250 | 1.10 (0.92–1.30) | 0.29 |
| Hemorrhagic strokef | 49 | 45 | 1.08 (0.72–1.63) | 0.69 |
| Cardiovascular death | 408 | 421 | 0.95 (0.83–1.09) | 0.47 |
| Total mortalityg | 1,345 | 1,412 | 0.94 (0.88–1.02) | 0.13 |
Abbreviation: CI, confidence interval
Mean follow-up of 11.2 years for all 14,641 men through June 1, 2011.
Adjusted for age, PHS cohort (original PHS I participant, new PHS participant), randomized beta-carotene assignment, randomized vitamin E assignment, and randomized vitamin C assignment and stratified on baseline CVD.
Defined as a composite endpoint consisting of the first of any of the following individual events: nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. The individual events do not sum to the total because each individual analysis assesses the first event that occurs during follow-up. Therefore, a participant who (e.g.) has a myocardial infarction then dies of cardiovascular disease would be counted for both individual events, but only once for the primary endpoint of major cardiovascular events.
Number of events.
Includes both nonfatal and fatal events.
Stroke type was unknown for 6 men in the active multivitamin group and 16 men in the placebo multivitamin group.
Additionally stratified on baseline cancer.
Figure 2.
Cumulative incidence rates of (part A) major cardiovascular events, (part B) total myocardial infarction, and (part C) total stroke by randomized multivitamin assignment in the Physicians' Health Study II.
* The reduction in the number at risk from 10 to 11 years reflects the two phases of PHS II recruitment; PHS I physicians initially enrolled in Phase 1 starting in 1997 were followed longer on average (mean of 13 years) than new physicians recruited in Phase 2 starting in 1999 (mean of 10 years). Crude log-rank P=0.69 for major cardiovascular events, P=0.44 for total myocardial infarction, and P=0.44 for total stroke.
In secondary analyses, exclusion of the first two or five years of follow-up did not alter the results for major cardiovascular events, total MI, or total stroke. Analyses adjusting for compliance either during follow-up or averaged over the whole trial, or adjusting for drop-ins, did not materially change the effect of the multivitamin on risk of major cardiovascular events.
Modifiers of the effect between multivitamin use and major cardiovascular events
In subgroup analyses, we examined whether baseline clinical, lifestyle, familial, biochemical, and dietary risk factors for CVD, along with the other randomized PHS II interventions, modified the effect of a daily multivitamin on major cardiovascular events (Table 3). There was a suggestion of a differential effect across age groups (P, interaction = 0.041), with possible differences among men aged 50–59 years (HR, 1.27; 95% CI, 0.99–1.63; P=0.06) and men aged ≥70 years (HR, 0.91; 95% CI, 0.81–1.03; P=0.14). We found no other evidence of effect modification by baseline risk factors on major cardiovascular events (all P, interaction > 0.05). There were also no multiplicative or subadditive interactions of the multivitamin component with randomized vitamin C, vitamin E, or beta-carotene treatment in PHS II (all P, interaction > 0.05).
Table 3.
Association between randomized multivitamin assignment and risk of major cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death) according to baseline characteristics and treatment assignment in the Physicians' Health Study II.a
| Group | Multivitamin | |||
|---|---|---|---|---|
| No. of Men (No. of Major Cardiovascular Events) | ||||
| Active | Placebo | Hazard Ratiob (95% CI) | P Interaction | |
| Age, years | 0.041 | |||
| 50–59 | 2,944 (136) | 2,947 (109) | 1.27 (0.99–1.63) | |
| 60–69 | 2,348 (244) | 2,348 (225) | 1.09 (0.91–1.31) | |
| ≥70 | 2,025 (496) | 2,029 (522) | 0.91 (0.81–1.03) | |
| Body mass index, kg/m2 | 0.72 | |||
| <255 | 3,039 (347) | 3,021 (355) | 0.98 (0.84–1.13) | |
| 25–29 | 3,463 (417) | 3,474 (400) | 1.01 (0.88–1.16) | |
| ≥30 | 815 (112) | 826 (100) | 1.11 (0.85–1.45) | |
| Smoking status | 0.49 | |||
| Never | 4,145 (412) | 4,107 (414) | 0.95 (0.83–1.09) | |
| Former | 2,908 (419) | 1.06 (0.92–1.21) | ||
| Current | 255 (45) | 269 (41) | 1.11 (0.72–1.70) | |
| Exercise ≥1 time/week | 0.29 | |||
| No | 2,699 (398) | 2,806 (362) | 1.07 (0.93–1.24) | |
| Yes | 4,444 (454) | 4,328 (460) | 0.97 (0.85–1.10) | |
| Alcohol consumption | 0.32 | |||
| Rarely/never | 1,391 (194) | 1,339 (189) | 0.92 (0.75–1.12) | |
| ≥1 drink/month | 5,874 (671) | 5,942 (660) | 1.02 (0.92–1.14) | |
| Current aspirin use | 0.07 | |||
| No | 1,625 (199) | 1,636 (169) | 1.20 (0.98–1.48) | |
| Yes | 5,602 (654) | 5,565 (663) | 0.96 (0.86–1.07) | |
| History of hypertensionc | 0.95 | |||
| No | 4,229 (320) | 4,477 (316) | 1.00 (0.86–1.17) | |
| Yes | 3,039 (553) | 3,117 (539) | 1.01 (0.90–1.14) | |
| History of high cholesterold | 0.65 | |||
| No | 4,534 (511) | 4,432 (472) | 1.03 (0.91–1.16) | |
| Yes | 2,549 (353) | 2,641(369) | 0.98 (0.85–1.13) | |
| History of diabetes | 0.33 | |||
| No | 6,838 (765) | 6,883 (740) | 1.02 (0.92–1.13) | |
| Yes | 472 (109) | 433 (116) | 0.89 (0.68–1.16) | |
| Parental history of MI <60 yearse | 0.17 | |||
| No | 5,941 (700) | 5,928 (660) | 1.04 (0.94–1.16) | |
| Yes | 661 (75) | 701 (94) | 0.86 (0.63–1.17) | |
| MstoryofCVDf | 0.59 | |||
| No | 6,941 (745) | 6,946 (728) | 1.02 (0.92–1.13) | |
| Yes | 376 (131) | 378 (128) | 0.96 (0.75–1.22) | |
| Total cholesterol ≥240 mg/dLg | 0.77 | |||
| No | 3,694 (457) | 3,648 (443) | 1.02 (0.89–1.16) | |
| Yes | 622 (83) | 645 (82) | 1.07 (0.79–1.46) | |
| HDL cholesterol ≥50 mg/dLg | 0.66 | |||
| No | 3,029 (397) | 3,043 (412) | 0.97 (0.84–1.11) | |
| Yes | 1,294 (145) | 1,249 (113) | 1.27 (0.99–1.62) | |
| Fruit and vegetable intakeh | 032 | |||
| <4 servings/d | 2,994 (330) | 3,075 (308) | 1.09 (0.93–1.27) | |
| 4–<7 servings/d | 2,729 (318) | 2,615 (300) | 0.99 (0.85–1.17) | |
| ≥7 servings/d | 935 (107) | 962 (120) | 0.87 (0.67–1.13) | |
| Whole grain intakeh | 0.60 | |||
| <2 servings/day | 4,952 (547) | 4,999 (543) | 0.99 (0.88–1.12) | |
| ≥2 servings/day | 1,685 (202) | 1,644 (184) | 1.05 (0.86–1.29) | |
| Red meat intakeh | 0.59 | |||
| <1 serving/day | 4,900 (511) | 4,928 (504) | 0.99 (0.88–1.12) | |
| ≥1 serving/day | 1,736 (237) | 1,704 (220) | 1.05 (0.87–1.26) | |
| Randomized to vitamin C | 0.54 | |||
| Placebo | 3,653 (424) | 3,659 (432) | 0.98 (0.85–1.11) | |
| Active | 3,664 (452) | 3,665 (424) | 1.04 (0.91–1.19) | |
| Randomized to vitamin E | 0.46 | |||
| Placebo | 3,667 (430) | 3,659 (430) | 0.97 (0.85–1.11) | |
| Active | 3,650 (446) | 3,665 (426) | 1.04 (0.91–1.19) | |
| Randomized to beta-carotene | 0.97 | |||
| Placebo | 3,632 (441) | 3,645 (427) | 1.00 (0.88–1.15) | |
| Active | 3,685 (435) | 3,679 (429) | 1.01 (0.88–1.15) | |
Abbreviation: CI, confidence interval
Mean follow-up of 11.2 years for all 14,641 men through June 1, 2011.
Adjusted for age, PHS cohort (original PHS I participant, new PHS participant), randomized beta-carotene assignment, randomized vitamin E assignment, and randomized vitamin C assignment.
History of hypertension was defined as self-reported systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or past/current treatment for hypertension.
History of high cholesterol was defined as self-reported total cholesterol ≥240 mg/dL or past/current treatment for high cholesterol.
Excludes 1,410 men with missing information on parental history of MI <60 years.
History of cardiovascular disease (CVD) included nonfatal myocardial infarction or nonfatal stroke.
Among 8,609 and 8,615 men with available biomarker data for plasma total cholesterol and HDL cholesterol, respectively.
Among 13,310, 13,280, and 13,268 men with available dietary data on fruit and vegetable intake, whole grain intake, and red meat intake, respectively.
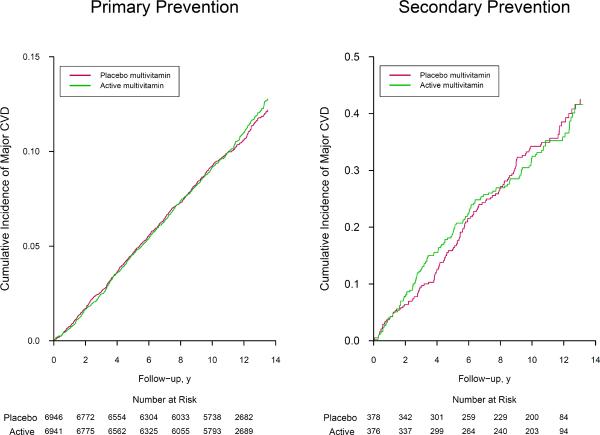
We found no significant interaction by baseline CVD history status (P, interaction = 0.62) for primary (HR, 1.02) versus secondary (HR, 0.96) prevention (Table 4). The cumulative incidence curves did not differ for primary (crude log-rank P=0.71) or secondary (crude log-rank P=0.94) prevention during up to 14 years of treatment and follow-up (Figure 3). The apparent lower rate of MI death among multivitamin users persisted (HR, 0.56; 95% CI, 0.33–0.95; P=0.032), whereas power was limited with only 9 cases of MI death among those with baseline CVD (P, interaction = 0.31). The effect of a daily multivitamin on total MI, total stroke, and other cardiovascular endpoints did not differ between those with and without baseline CVD (all P, interaction > 0.05).
Table 4.
Association between randomized multivitamin assignment and the risk of major cardiovascular events and mortality among 13,887 men withouta and 754 men withb baseline cardiovascular disease (CVD) in the Physicians' Health Study II.
| Outcome | No baseline history of CVD | Baseline history of CVD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | P | No. of Events | P | P Int | |||||
| Active (n=6,941) | Placebo (n=6,946) | Adjusted HRc (95% CI) | Active (n=376) | Placebo (n=378) | Adjusted HRc (95% CI) | ||||
| Major cardiovascular eventsd | 745 | 728 | 1.02 (0.92–1.13) | 0.76 | 131 | 128 | 0.96 (0.75–1.22) | 0.73 | 0.62 |
| Total myocardial infarction e | 6,283 | 302 | 0.93 (0.79–1.09) | 0.38 | 34 | 33 | 0.99 (0.61–1.60) | 0.97 | 0.81 |
| MI Death | 22 | 39 | 0.56 (0.33–0.95) | 0.032 | 5 | 4 | 1.08 (0.29–4.07) | 0.91 | 0.31 |
| Total strokee | 281 | 265 | 1.06 (0.89–1.25) | 0.51 | 51 | 46 | 1.05 (0.70–1.56) | 0.82 | 0.94 |
| Stroke death | 64 | 60 | 1.08 (0.76–1.53) | 0.68 | 25 | 16 | 1.48 (0.79–2.78) | 0.22 | 0.40 |
| Ischemic strokef | 239 | 213 | 1.12 (0.93–1.35) | 0.23 | 38 | 37 | 0.97 (0.62–1.53) | 0.90 | 0.54 |
| Hemorrhagic strokef | 40 | 43 | 0.93 (0.60–1.43) | 074 | 9 | 2 | 4.36 (0.94–20.2) | 0.06 | 0.06 |
| Cardiovascular death | 319 | 335 | 0.96 (0.82–1.12) | 0.57 | 92 | 93 | 0.96 (0.71–1.29) | 078 | 0.95 |
| Total mortalityg | 1,166 | 1,233 | 0.95 (0.88–1.03) | 0.24 | 179 | 179 | 0.90 (0.73–1.11) | 0.34 | 0.51 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Mean follow-up of 11.3 years for 13,887 men free of baseline cardiovascular disease through June 1, 2011.
Mean follow-up of 9.3 years for 754 men with baseline cardiovascular disease through June 1, 2011.
Adjusted for age, PHS cohort (original PHS I participant, new PHS participant), and randomized treatment assignment (beta-carotene, vitamin E, and vitamin C).
Defined as a composite endpoint consisting of the first of any of the following individual events: nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. The individual events do not sum to the total because each individual analysis assesses the first event that occurs during follow-up. Therefore, a participant who (e.g.) has a myocardial infarction then dies of cardiovascular disease would be counted for both individual events, but only once for the primary endpoint of major cardiovascular events.
Includes both nonfatal and fatal events.
Stroke type was unknown for 2 men in the active multivitamin group and 9 men in the placebo multivitamin group among men with no history of CVD, and for 4 men in the active multivitamin group and 7 men in the placebo multivitamin group among men with a history of CVD.
Additionally stratified on baseline cancer.
Figure 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) of major cardiovascular events among 13,887 men with no baseline history of cardiovascular disease (part A; Primary Prevention) and 754 men with a baseline history of cardiovascular disease (part B; Secondary Prevention) in the Physicians' Health Study II.
* Crude log-rank P=0.71 for 13,887 men with no baseline history of cardiovascular disease, and crude log-rank P=0.94 for 754 men with a baseline history of cardiovascular disease.
Potential adverse effects of daily multivitamin use
Besides the primary and secondary endpoints, we assessed several potential side effects of daily multivitamin use, and found no significant effects on gastrointestinal tract symptoms (peptic ulcer, constipation, diarrhea, gastritis, and nausea), fatigue, drowsiness, skin discoloration, and migraine (all P > 0.05). Those taking the active versus placebo multivitamin use were more likely to have skin rashes (2,111 and 1,973 men in corresponding active and placebo multivitamin groups; HR, 1.08; 95% CI, 1.01–1.15; P=0.016). In addition, there were inconsistent findings for daily multivitamin use on minor bleeding, suggesting the role of chance. There was a reduction in hematuria (998 and 1,105 men in corresponding active and placebo multivitamin groups; HR, 0.89; 95% CI, 0.81–0.97; P=0.006), an increase in epistaxis (1,216 and 1,106 men in corresponding active and placebo multivitamin groups; HR, 1.11; 95% CI, 1.02–1.20; P=0.016), and no effect on easy bruising/other bleeding (1,927 and 1,902 men in corresponding active and placebo multivitamin groups; HR, 1.02; 95% CI, 0.96–1.08; P=0.59).
COMMENT
The Physicians' Health Study II represents the only large-scale, randomized, double-blind, placebo-controlled trial testing the long-term effects of a commonly available multivitamin in the prevention of chronic disease. We found that after more than a decade of daily multivitamin use among middle-aged and older men, daily multivitamin use did not reduce the primary endpoint of major cardiovascular events. Multivitamin use also did not reduce the risk of total MI, total, ischemic, or hemorrhagic stroke, cardiovascular death, or other cardiovascular endpoints including congestive heart failure, angina, or coronary revascularization. A borderline significant 39% reduction in fatal MI may have been due to chance. These findings on CVD and the decision to take a multivitamin should be put in the context of initial nutritional status and other outcomes to be considered in this trial.
The lack of an effect of a daily multivitamin on CVD appears consistent with what is known to date. Basic research indicates several mechanisms by which specific micronutrients contained in multivitamins may prevent CVD37,38 through modifications in platelet activity,39 reductions in thrombotic potential,40 and modifications in vascular reactivity.41 The consistent observation that people consuming greater amounts of fruits and vegetables tend to have lower rates of coronary heart disease (CHD)42 and stroke43 supports the idea that combinations of vitamins at moderate dose may offer CVD protection.
Observational data examining multivitamin use and CVD are sparse and inconsistent. Among 1,063,023 US adults from the Cancer Prevention Study II, men without CVD had an age-adjusted relative risk (RR) of ischemic heart disease death for multivitamin use of 0.91 (P≤0.001) attenuated upon multivariate adjustment;10 similar results were noted for women. In 80,082 Nurses' Health Study participants, multivitamin use was associated with a significant reduction in coronary heart disease (CHD) incidence (RR, 0.76; 95% CI, 0.65–0.90) after 14 years,9 a result further confirmed with additional follow-up.12 In a Swedish population-based case-control study in adults aged 45 to 70 years, the multivariate odds ratio (OR) of MI comparing regular users versus nonusers of multivitamins was 0.79 (95% CI, 0.63–0.98) among 2,053 men and 0.66 (95% CI, 0.48–0.91) among 928 women.13 In contrast, in the PHS I enrollment cohort of 83,936 initially healthy male physicians, there was no association between baseline multivitamin use and either CVD or CHD mortality.11 Among 161,808 WHI participants, of whom 41.5% took a multivitamin, there was no association between multivitamin use and the risk of CVD, MI, or stroke after a median of 8 years follow-up.14 Finally, there was also no association between multivitamin use and cardiovascular mortality in 182,099 men and women from the Multiethnic Cohort Study after a mean follow-up of 11 years.16
Several large trials of single agents or combinations of vitamins and minerals, generally at doses well above RDAs and that contained in the PHS II multivitamin, have demonstrated no effect on CVD.17,18,20,44 Primary prevention trials that have examined smaller combinations of vitamins and minerals, including the Linxian Chinese Cancer Prevention Trial26 and SU.VI.MAX,28 as well as secondary prevention trials such as the Heart Protection Study,27 found no effect on CVD. Other randomized trials have tested combinations of B vitamins with folic acid at high doses, particularly in the secondary prevention of CVD, but have not found any protective effect.45 Moreover, the Women's Health Initiative Calcium and Vitamin D trial, testing 400 IU/day vitamin D3 plus 1000 mg/day calcium, found no effect on CVD.46
Baseline nutritional status among our physician participants remains a critical consideration in the interpretation of our findings. PHS II participants likely represent, on average, a well-nourished population who already have adequate or optimum intake levels of nutrients, for which supplementation may offer no additional benefit.47 However, the requirement for PHS II participants to avoid personal use of multivitamin supplements also lowered their in-trial intake of essential vitamins and minerals. Additional studies are needed to understand how the range of baseline nutritional status among PHS II participants and other populations may modify the effect of a daily multivitamin on cardiovascular endpoints. Further, we have an arsenal of behavioral (e.g. exercise, weight loss) and pharmacological interventions (e.g. lipid-lowering therapies) that effectively lower CVD risk. This may make it difficult for vitamin supplements such as a multivitamin to meaningfully contribute toward risk reduction.
Several unique strengths of this trial include more than a decade of treatment and follow-up, high statistical power for our primary endpoint of major cardiovascular events, consistently good adherence in taking a daily multivitamin, and the inclusion of physician participants in providing high-quality reporting of health information. We are unaware of any other long-term clinical trials that have tested a multivitamin in the prevention of CVD and other chronic diseases, highlighting the importance of trials like PHS II to test the efficacy of supplements and assess potential causality across a range of clinically relevant outcomes. Finally, we selected a commonly used multivitamin formulation, Centrum Silver, when we initiated PHS II to increase the generalizability of our findings.
There are also important potential limitations to be considered. We relied upon a specific, constant formulation of a Centrum Silver (eTable 1), which is one of many multivitamin formulations. There was an observed reduction in total cancer found for the PHS II multivitamin48 suggesting, however, that the formulation used may be adequate for cancer, but not for CVD. This highlights the need to understand how essential vitamins and minerals may differentially interact and influence cardiovascular and cancer mechanisms, even at usual levels of vitamin and mineral intake. Though PHS II included more than a decade of treatment, an even longer duration of multivitamin use may be required to derive any cardiovascular benefits. Existing epidemiologic data can provide insight on this concept, while PHS II remains the only trial of its kind for which extended follow-up of CVD endpoints can provide important longer-term mechanistic perspectives.
PHS II may also have limited generalizability, since our study population was confined to middle-aged and older, predominantly Caucasian male physicians. Despite some multivitamin non-compliance and drop-ins during PHS II, compliance- and drop-in-adjusted analyses reiterated a lack of effect of multivitamin use on major cardiovascular events. As with any trial, chance may be important when multiple hypotheses are tested, thus cautious interpretation of secondary analyses is warranted. Finally, long-term multivitamin use may be more effective when initiated earlier in life to counter the initiation and progression of atherosclerosis that often begins at an earlier age.
In this randomized trial after a mean of 11.2 years of treatment and follow-up in 14,641 men, daily multivitamin supplementation did not reduce the risk of major cardiovascular events. These data do not support multivitamin use to prevent CVD, demonstrating the importance of long-term clinical trials of commonly used nutritional supplements. Whether or not to take a daily multivitamin requires consideration of an individual's nutritional status, since the main reason is to prevent vitamin and mineral deficiency, plus consideration of other potential effects, including a modest reduction in cancer48 and other important outcomes in PHS II that will be reported separately.
Supplementary Material
Acknowledgments
Supported by grants CA 097193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health (Bethesda, MD), and an investigator-initiated grant from BASF Corporation (Florham Park, NJ). Study agents and packaging were provided by BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle) (New York, NY), and study packaging was provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ).
Trial Registration: http://www.clinicaltrials.gov identifier: NCT00270647
Study concept and design: Sesso, Christen, Manson, Glynn, Buring, Gaziano.
Acquisition of data: Sesso, Christen, Bubes, Smith, MacFadyen, Schvartz, Manson, Glynn, Buring, Gaziano.
Analysis and interpretation of data: Sesso, Christen, Bubes, Manson, Glynn, Buring, Gaziano.
Drafting of the manuscript: Sesso, Gaziano.
Critical revision of the manuscript for important intellectual content: Sesso, Christen, Bubes, Smith, MacFadyen, Schvartz, Manson, Glynn, Buring, Gaziano.
Statistical analysis: Sesso, Bubes, Glynn, Gaziano.
Obtained funding: Sesso, Buring, Gaziano.
Administrative, technical, or material support: Sesso, Bubes, Smith, MacFadyen, Schvartz, Manson, Glynn, Gaziano.
Study supervision: Sesso, Bubes, Smith, MacFadyen, Glynn, Gaziano.
Relevant Financial
Dr. Sesso reported receiving investigator-initiated research funding from the National Institutes of Health, the Tomato Products Wellness Council, and Cambridge Theranostics, Ltd.
Dr. Christen reported receiving research funding support from the National Institutes of Health, Harvard University (Clinical Nutrition Research Center), and DSM Nutritional Products Inc. (formerly Roche Vitamins).
Dr. Manson reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from BASF and Cognis Corporations for the Women's Antioxidant and Folic Acid Cardiovascular Study and from Pronova BioPharma and Pharmavite for the VITamin D and OmegA-3 TriaL, and funding from the non-profit Aurora Foundation.
Dr. Glynn reported receiving investigator-initiated research funding from the National Institutes of Health, Bristol-Meyers Squibb, AstraZeneca, and Novartis, and signed a consulting agreement with Merck to give an invited talk.
Dr. Buring reported receiving investigator-initiated research funding from the National Institutes of Health, and assistance with study pills and packaging from Natural Source Vitamin E Association and Bayer Healthcare for the Women's Health Study.
Dr. Gaziano reported receiving investigator-initiated research funding from the National Institutes of Health the Veterans Administration, and the BASF Corporation to assist in the establishment of this trial cohort, assistance with study agents and packaging from BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle), and assistance with study packaging provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins).
Funding/Support: Supported by grants CA 097193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health (Bethesda, MD), and an investigator-initiated grant from BASF Corporation (Florham Park, NJ). Study agents and packaging were provided by BASF Corporation and Pfizer (formerly Wyeth, American Home Products, and Lederle) (New York, NY), and study packaging was provided by DSM Nutritional Products, Inc. (formerly Roche Vitamins) (Parsippany, NJ).
Role of the Sponsors: NIH, BASF, Pfizer, and DSM Nutritional Products Inc had no role in the study design; conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs. Sesso and Gaziano had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. Drs. Sesso, Bubes, Glynn, and Gaziano performed and are responsible for the statistical analyses for this article.
Disclosures:No other authors reported financial disclosures.
Data and Safety Monitoring Board: Voting members over the course of the PHS II trial included Lawrence Cohen, Rory Collins, Theodore Colton, I. Craig Henderson, Andrea LaCroix, Ross Prentice, and Nanette Wenger (chair); ex-officio members included Mary Francis Cotch, Jeffrey Cutler, Frederick Ferris, Jerome Fleg, Peter Greenwald, Natalie Kurinij, Howard Parnes, Marjorie Perloff, Eleanor Schron, and Alan Zonderman.
Disclaimer: Dr Gaziano, a contributing editor for JAMA, was not involved in the editorial review of or decision to publish this article.
Additional Contributions: We are deeply indebted to the 14,641 physician participants for their long-standing dedication and conscientious collaboration. We also acknowledge the long-term contributions of Charles Hennekens, MD, DrPH, Florida Atlantic University, to the Physicians' Health Study, and the exemplary contributions of the staff of the Physicians' Health Study at Brigham and Women's Hospital, under the leadership of Joanne Smith: Charlene Belanger, Eileen Bowes, Kenneth Breen, Mary Breen, Mary G. Breen, Jose Carrion, Shamikhah Curry, Colleen Evans, Ivan Fitchorov, Natalya Gomelskaya, Cindy Guo, Delia Guo, Jasmah Hanna, Beth Holman, Andrea Hrbek, Gregory Kotler, Tony Laurinaitis, Matthew Lyle, Hannah Mandel, Chandra McCarthy, Geneva McNair, Annie Murray, Leslie Power, Philomena Quinn, Harriet Samuelson, Fred Schwerin, Mickie Sheehey, Sara Tower, Martin Van Denburgh, Diana Walrond, Phyllis Johnson Wojciechowski, and Angela Zhang. Finally, we are deeply grateful for the efforts of the Physicians' Health Study Endpoints Committee, including Samuel Goldhaber, Carlos Kase, Meir Stampfer, and James Taylor, over the course of Physicians' Health Study II. Each named individual was compensated for his/her contribution as part of the grant support.
REFERENCES
- 1.Timbo BB, Ross MP, McCarthy PV, Lin CT. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J Am Diet Assoc. 2006;106(12):1966–1974. doi: 10.1016/j.jada.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Ervin RB, Wright JD, Kennedy-Stephenson J. Use of dietary supplements in the United States, 1988–94. 244. Vol. 11. National Center for Health Statistics; Washington DC: 1999. pp. i–14. Vital Health Stat. [PubMed] [Google Scholar]
- 3.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans' views on the use and regulation of dietary supplements. Arch Intern Med. 2001;161(6):805–810. doi: 10.1001/archinte.161.6.805. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;(61):1–8. [PubMed] [Google Scholar]
- 6.Muth MK, Anderson DW, Domanico JL, Smith JB, Wendling B. Economic characterization of the dietary supplement industry. Center for Food Safety and Administration, Food and Drug Administration; Washington, DC: 1999. [Google Scholar]
- 7.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshipura KJ, Hung HC, Li TY, et al. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12(1):115–121. doi: 10.1017/S1368980008002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimm EB, Willett WC, Hu FB, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279(5):359–364. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 10.Watkins ML, Erickson JD, Thun MJ, Mulinare J, Heath CW., Jr. Multivitamin use and mortality in a large prospective study. Am J Epidemiol. 2000;152(2):149–162. doi: 10.1093/aje/152.2.149. [DOI] [PubMed] [Google Scholar]
- 11.Muntwyler J, Hennekens CH, Manson JE, Buring JE, Gaziano JM. Vitamin supplement use in a low-risk population of US male physicians and subsequent cardiovascular mortality. Arch Intern Med. 2002;162(13):1472–1476. doi: 10.1001/archinte.162.13.1472. [DOI] [PubMed] [Google Scholar]
- 12.Osganian SK, Stampfer MJ, Rimm E, et al. Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol. 2003;42(2):246–252. doi: 10.1016/s0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 13.Holmquist C, Larsson S, Wolk A, de Faire U. Multivitamin supplements are inversely associated with risk of myocardial infarction in men and women--Stockholm Heart Epidemiology Program (SHEEP) J Nutr. 2003;133(8):2650–2654. doi: 10.1093/jn/133.8.2650. [DOI] [PubMed] [Google Scholar]
- 14.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch Intern Med. 2009;169(3):294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rautiainen S, Akesson A, Levitan EB, Morgenstern R, Mittleman MA, Wolk A. Multivitamin use and the risk of myocardial infarction: a population-based cohort of Swedish women. Am J Clin Nutr. 2010;92(5):1251–1256. doi: 10.3945/ajcn.2010.29371. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol. 2011;173(8):906–914. doi: 10.1093/aje/kwq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 18.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst. 1999;91(24):2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 22.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 25.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease- specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 27.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 28.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164(21):2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health State-of-the-science conference statement: multivitamin/mineral supplements and chronic disease prevention. Ann Intern Med. 2006;145(5):364–371. doi: 10.7326/0003-4819-145-5-200609050-00136. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Agriculture and US Department of Health and Human Services . Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Jun 15, 2010. [Accessed on Nov. 18, 2010]. Available at: http://www.cnpp.usda.gov/DGAs2010-DGACReport.htm. [Google Scholar]
- 31.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10(2):125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 32.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 34.Satterfield S, Greco PJ, Goldhaber SZ, et al. Biochemical markers of compliance in the Physicians' Health Study. Am J Prev Med. 1990;6(5):290–294. [PubMed] [Google Scholar]
- 35.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 Pt 2 Suppl 1):I13–44. [PubMed] [Google Scholar]
- 36.Berger K, Kase CS, Buring JE. Interobserver agreement in the classification of stroke in the Physicians' Health Study. Stroke. 1996;27(2):238–242. doi: 10.1161/01.str.27.2.238. [DOI] [PubMed] [Google Scholar]
- 37.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 38.Keaney JF, Jr., Gaziano JM, Xu A, et al. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proc Natl Acad Sci U S A. 1993;90(24):11880–11884. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mabile L, Bruckdorfer KR, Rice-Evans C. Moderate supplementation with natural alpha-tocopherol decreases platelet aggregation and low-density lipoprotein oxidation. Atherosclerosis. 1999;147(1):177–185. doi: 10.1016/s0021-9150(99)00169-0. [DOI] [PubMed] [Google Scholar]
- 40.Mehta J, Li D, Mehta JL. Vitamins C and E prolong time to arterial thrombosis in rats. J Nutr. 1999;129(1):109–112. doi: 10.1093/jn/129.1.109. [DOI] [PubMed] [Google Scholar]
- 41.Paolisso G, Tagliamonte MR, Barbieri M, et al. Chronic vitamin E administration improves brachial reactivity and increases intracellular magnesium concentration in type II diabetic patients. J Clin Endocrinol Metab. 2000;85(1):109–115. doi: 10.1210/jcem.85.1.6258. [DOI] [PubMed] [Google Scholar]
- 42.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134(12):1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 43.Joshipura KJ, Ascherio A, Manson JE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282(13):1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 44.Leppala JM, Virtamo J, Fogelholm R, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20(1):230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 45.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170(18):1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 46.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 47.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305(13):1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the Prevention of Cancer in Men: The Physicians' Health Study II Randomized Controlled Trial. JAMA. 2012 doi: 10.1001/jama.2012.14641. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.