Abstract
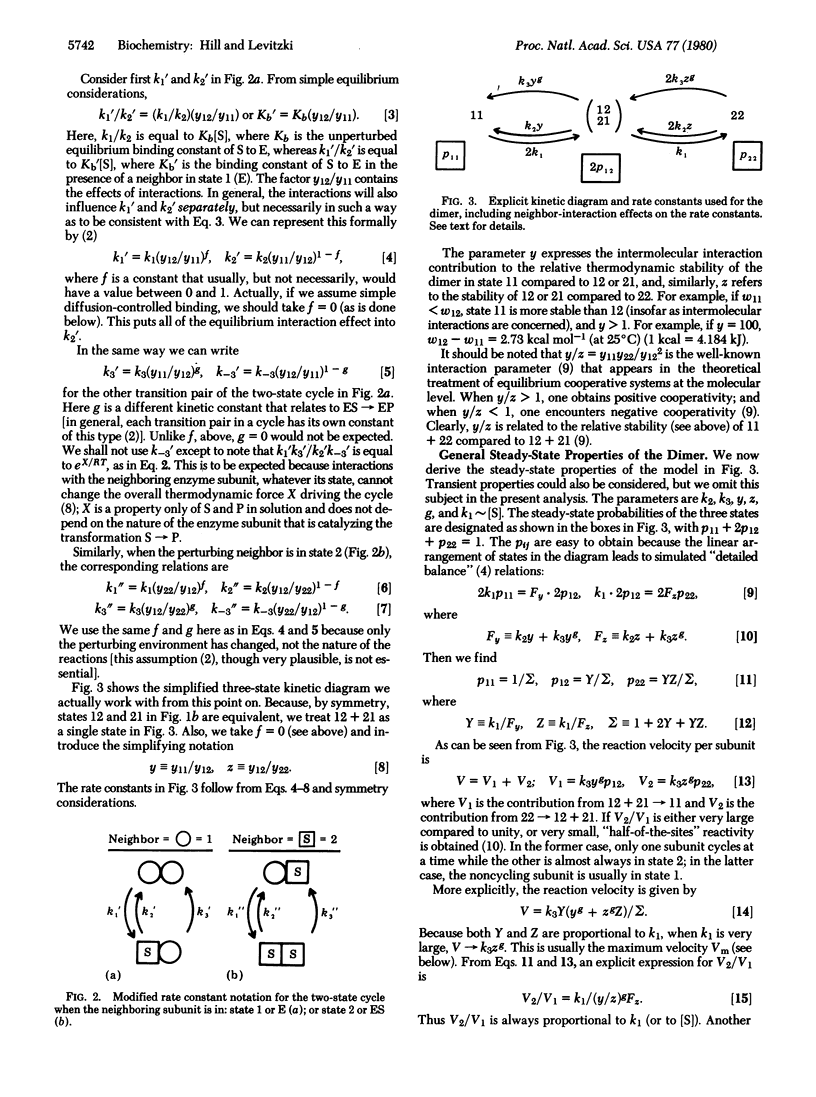
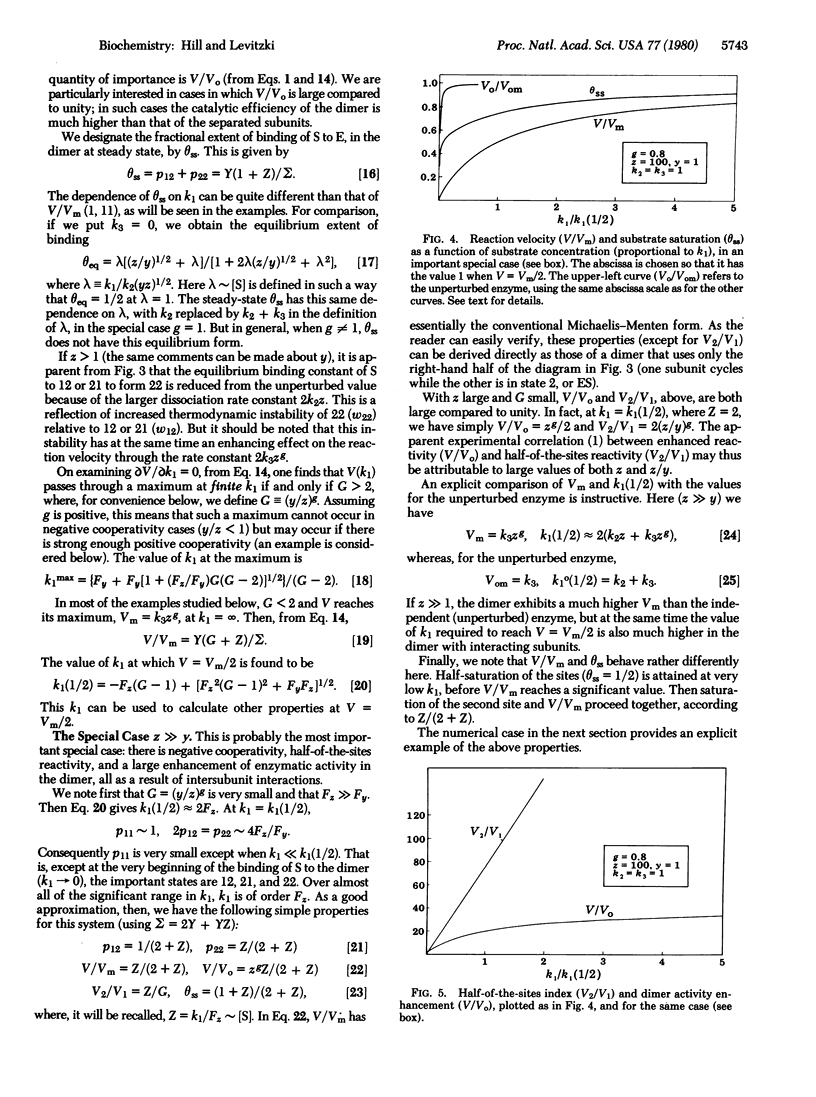
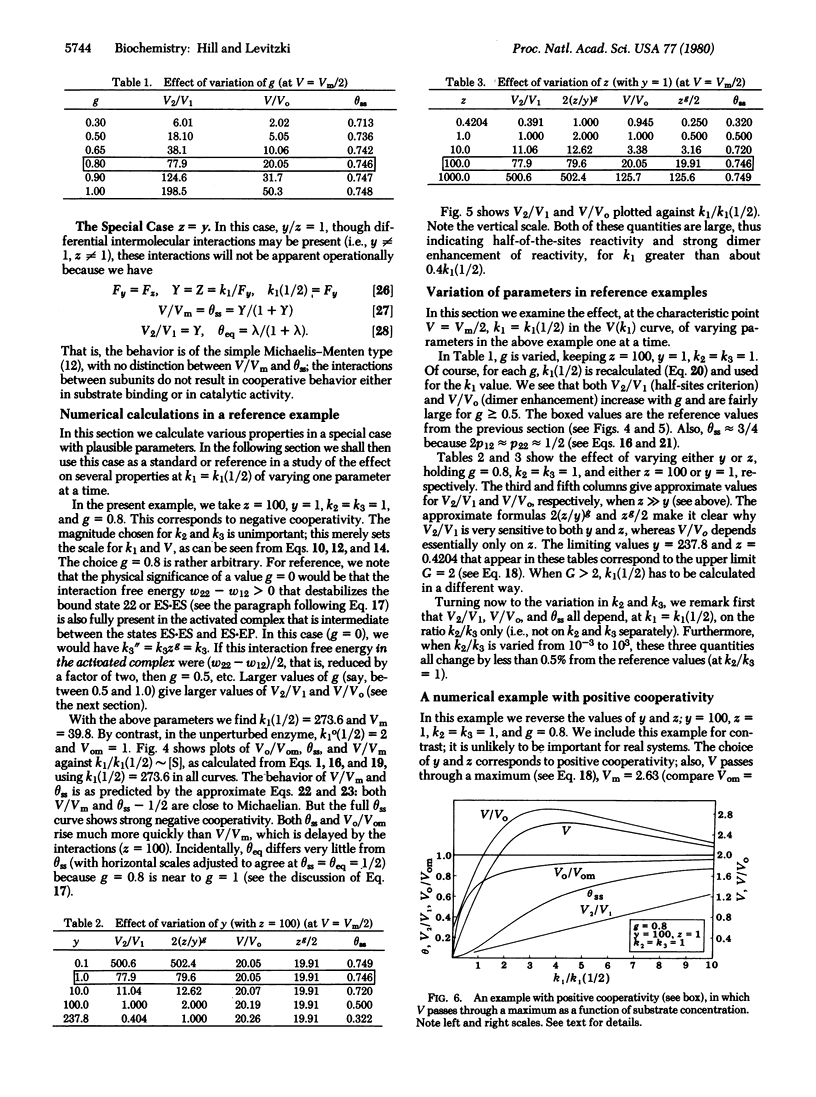
We consider an isologous enzyme dimer in which the subunits, if operating independently, would obey Michaelis-Menten kinetics. However, because of neighbor interactions, the rate constants of the kinetic cycle in either subunit depend on the state (E or ES) of the other subunit. The steady-state behavior of this dimer system, with interactions, is investigated. In what is probably the most important special case, ES x ES is destabilized considerably by the neighbor interaction compared to E x ES. This leads to half-of-the-sites reactivity (one subunit is in state ES; the other subunit cycles between E and ES), negative cooperativity, and a considerable enhancement of enzyme activity relative to the activity of independent subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hill T. L. Effect of enzyme-enzyme interactions on steady-state enzyme kinetics. IV. "Strictly steady-state" examples. J Theor Biol. 1978 Dec 21;75(4):391–416. doi: 10.1016/0022-5193(78)90352-1. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Further study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4111–4115. doi: 10.1073/pnas.74.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Steady-state phase or cooperative transitions between biochemical cycles. Proc Natl Acad Sci U S A. 1979 Feb;76(2):714–716. doi: 10.1073/pnas.76.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Unsymmetrical and concerted examples of the effect of enzyme--enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1101–1105. doi: 10.1073/pnas.75.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Stallcup W. B., Koshland D. E., Jr Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry. 1971 Aug 31;10(18):3371–3378. doi: 10.1021/bi00794a009. [DOI] [PubMed] [Google Scholar]


