Abstract
Here, we review the role of top-down attention in both the acquisition and the expression of perceptual learning, as well as the role of learning in more efficiently guiding attentional modulations. Although attention often mediates learning at the outset of training, many of the characteristic behavioral and neural changes associated with learning can be observed even when stimuli are task irrelevant and ignored. However, depending on task demands, attention can override the effects of perceptual learning, suggesting that even if top-down factors are not strictly necessary to observe learning, they play a critical role in determining how learning-related changes in behavior and neural activity are ultimately expressed. In turn, training may also act to optimize the effectiveness of top-down attentional control by improving the efficiency of sensory gain modulations, regulating intrinsic noise, and altering the read-out of sensory information.
Keywords: attention, perceptual learning, gain modulation, read-out, decision-making
1. Introduction
Top-down attention is a mechanism that enables the flexible modulation of neural activity so that behaviorally relevant stimuli can be processed more efficiently than competing distracters. In contrast, perceptual learning, or an improvement in sensitivity after repeated exposure to a stimulus (Gibson, 1963; 1969), typically induces a long-lasting and stable change that increases the efficiency of sensory processing for a highly specific stimulus.
Despite differences in their time-scale and flexibility, attention and perceptual learning (PL) are supported by similar neural mechanisms and appear to be intricately linked. For instance, both top-down attention and PL operate by increasing the signal-to-noise ratio (SNR) of sensory signals (Bao et al., 2010; Desimone, 1998; Desimone & Duncan, 1995; Furmanski, Schluppeck, & Engel, 2004; Martinez-Trujillo & Treue, 2004; McAdams & Maunsell, 1999; Moran & Desimone, 1985; Reynolds, Pasternak, & Desimone, 2000; Schoups et al., 2001; Zohary et al., 1994), by modulating intrinsic neural variability (Adab & Vogels, 2011; Cohen & Kohn, 2011; Cohen & Maunsell, 2009; 2011; Gu et al., 2011; Mitchell, Sundberg, & Reynolds, 2007; 2009), and by optimizing the read-out of sensory information by decision mechanisms (Dosher & Lu, 1998; 1999; 2009; Law & Gold, 2008; 2009; Palmer & Moore, 2009; Palmer, Verghese, & Pavel, 2000; Pestilli et al., 2011; Yu, Klein, & Levi, 2004). In addition, attention and PL are known to be at least partially co-dependent: attention often acts as a modulator that enables the acquisition of learning-induced improvements in behavioral performance (Ahissar & Hochstein, 1993; Fahle, 2004; 2009), but does not always appear to be necessary for the expression of learning-related changes in neural activity after training (Adab & Vogels, 2011; Bao et al., 2010; Furmanski, Schluppeck, & Engel, 2004; Hua et al., 2010).
However, despite these links, many learning-induced changes in behavioral performance and in neural activity cannot be explained solely by top-down attentional factors (Beste et al., 2011; Frankó, Seitz, & Vogels, 2010; McMahon & Leopold, 2012; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003; Watanabe, Náñez, & Sasaki, 2001; Yao & Dan, 2001; see Seitz and Wantanbe, 2009 and Sasaki, Náñez, & Watanabe, 2010 for reviews). Furthermore, task demands and the characteristics of the trained stimulus set can influence both the generality of learning and the extent to which training can improve the efficiency of attentional control. The existing data present a complex picture that is by no means resolved; however, it now seems likely that are multiple routes by which learning can occur, and that these routes might operate in parallel during everyday perceptual experience to regulate the impact of potentially relevant stimuli on perception and decision-making.
2. Attention can Modulate Learning
Traditional studies of PL have focused on training-related improvements in the discrimination of basic visual features in a specific retinal location (Ahissar & Hochstein, 1993; Andersen et al., 2010; Fahle, 1997; 2004; Schoups, Vogels, & Orban, 1995; Shiu & Pashler, 1992; Yund & Efron, 1996). In these studies, attention is usually focused on the trained stimulus, and often interpreted as necessary in order to observe robust improvements in performance (Ahissar & Hochstein, 1993; Fahle, 1997; 2004). For example, when subjects attend to only one of two vernier stimuli during training, discrimination accuracy only improves for the attended stimulus but not the unattended stimulus (Fahle, 2004). Similarly, accuracy on an orientation discrimination task improves more quickly when subjects attend to the location of the grating (Figure 1; Mukai et al., 2011), and changes in firing rates of sensory neurons that optimally discriminate a trained stimulus can rely on attention during training, and not only on repeated stimulus exposure (Figure 2; e.g., Schoups et al., 2001; Yang & Maunsell, 2004).
Figure 1.
From Mukai et al., 2011 (their figure 5a). Regression slope analyses for accuracy in three attentional cue conditions: exogenous attention, endogenous attention with an arrow cue, and endogenous attention with a color cue, for attended (blue), divided-attended (green), and unattended (red) locations. Slopes of the learning curves for attended locations were significantly greater than divided-attended locations and unattended locations. Slopes of the learning curves for divided-attended locations were also significantly greater than those for unattended locations. (Reprinted with permission: Mukai, I., Bahadur, K., Kesavabhotla, K., & Ungerleider, L.G. (2011). Exogenous and endogenous attention during perceptual learning differentially affect post-training target thresholds. Journal of Vision. 11, 1-15. © ARVO).
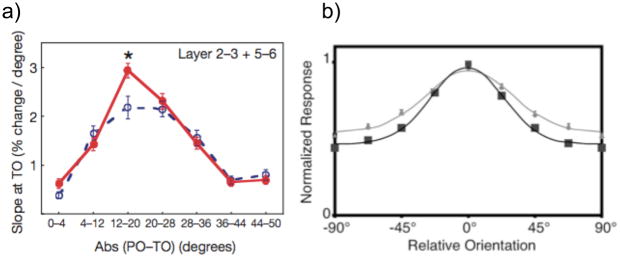
Figure 2.
a) From Schoups et al., 2001 (their figure 3a). Slope of the tuning curve for neurons tuned to orientations 0-50 degrees offset from the trained orientation. The solid red line represents trained neurons and the dashed blue line represents untrained neurons. There is a significant increase in the slope of the tuning curve for trained neurons tuned 12-20 degrees away from the trained orientation, indicating that these neurons were the most informative for accurately discriminating the trained stimulus. (Reprinted with permission from Macmillan Publishers Ltd: Nature, Schoups, A., Vogels, R., Qian, N., & Orban, G. (2001). Practising orientation identification improves orientation coding in V1 neurons. Nature. 412, 549-553., Copyright 2001). b) From Yang & Maunsell, 2004 (their figure 6). Normalized population tuning curve for trained (black curve) and untrained (gray curve) populations, where 0 degrees represents the preferred orientation of a given neuron. There is a significant decrease in bandwidth and increase in amplitude for the trained populations compared to untrained populations, indicating a gain enhancement of those populations that optimally discriminate the trained stimulus. (Reproduced with permission of Society for Neuroscience: Yang, T. & Maunsell, J.H.R. (2004). The effect of perceptual learning on neuronal responses in monkey visual area V4. The Journal of Neuroscience. 24, 1617-1626., Copyright 2004.)
Additionally, top-down modulations have been shown to lead to learning even without the presence of a sensory signal (Dupuis-Roy & Gosselin, 2007; Tartaglia et al., 2009). For example, Tartaglia et al. (2009) demonstrated that when subjects were asked to repeatedly imagine performing a line bisection task in the absence of any visual input, their performance on a real line bisection task improved. This observation strongly suggests that top-down attentional influences are at least sufficient to observe PL. Similarly, Dupuis-Roy and Gosselin (2007) used a “no-signal” task in which subjects chose which of two noise patches matched a target texture. Although there was no actual signal inserted into either noise patch, the experimenters defined the patch that happened (by chance) to correlate more with the target stimulus as the target. Despite the absence of strong bottom-up signals, subjects improved on this task with training, suggesting an influence of top-down feedback on the tuning of lower-level populations in a Hebbian-like fashion. Thus, these studies suggest that merely exerting top-down attentional control, even in the near or complete absence of a bottom-up signal, can induce learning-related modifications in behavior.
Learning-related gain modulations can also be context-dependent, such that responses to a trained stimulus can be enhanced or suppressed based on whether or not the subject is performing a task with, and thus attending to, a given stimulus (Crist, Li, & Gilbert, 2001; Gilbert et al., 2000). Crist et al. (2001) had monkeys perform a task at fixation while previously trained stimuli were presented in flanking spatial positions. Cells that responded to the trained stimuli were suppressed, presumably because the trained stimuli were actively competing with the task-relevant stimuli being presented at fixation. However, responses in the same cells were enhanced in a second condition where the monkeys directed attention to the trained stimuli. These context-dependent effects indicate that the expression of learning-induced neural modulations can sometimes depend upon interactions between the bottom-up inputs and feedback connections from higher-level top-down control areas, which facilitate processing of a trained stimulus only when it is task-relevant (Gilbert et al., 2000). Furthermore, given that responses to trained stimuli were suppressed when they were presented as distracters in this paradigm, the context in which learning is evaluated also impacts the extent to which attention modulates the expression of learning.
Finally, the observation that learning can influence neural activity and connectivity across multiple levels of visual cortex and parietal and frontal areas (Gilbert, Sigman, & Crist, 2001; Kourtzi, 2010; Kourtzi & DiCarlo, 2006; Li, Piëch, & Gilbert, 2004) suggests that top-down attention plays a key role in coordinating task-related information from these areas during decision-making (Kourtzi, 2010; Serences & Yantis, 2006). This is particularly true in cases where learning is task-dependent (i.e., Crist, Li, & Gilbert, 2001; Li, Piëch, & Gilbert, 2004), as attention must be recruited to coordinate changes across sensory areas in a way that is sensitive to current task goals. Training with more complex objects may also require attentional coordination in order to efficiently integrate contour and feature information into a size and position invariant representation of a whole object (Kourtzi & DiCarlo, 2006). Additionally, even though feedback is not strictly required to observe learning (Ball & Sekuler, 1987; Fahle, Edelman, & Poggio, 1995; Karni & Sagi, 1991; Liu, Lu, & Dosher, 2012; McKee & Westheimer, 1978; Shiu & Pashler, 1982), it can play a facilitory role (Fahle, 2004; 2009; Gilbert, Sigman, & Crist, 2001; Herzog & Fahle, 1997; Law & Gold, 2009; Roelfsema, van Ooyen, & Watanabe, 2010; Shibata et al., 2009; Sasaki, Náñez, & Watanabe, 2010; Seitz et al., 2006), suggesting that top-down influences can be recruited throughout training to dynamically guide learning-induced changes in sensory processing or decision-making.
3. Learning Without Attention
In contrast to studies suggesting that PL depends on attentional processing, many studies have found that PL can be induced when a trained stimulus is ignored or even unseen (Beste et al., 2011; Frankó, Seitz, & Vogels, 2010; McMahon & Leopold, 2012; Sasaki, Náñez, & Watanabe, 2010; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003; Watanabe, Náñez, & Sasaki, 2001; Watanabe et al., 2002; Yao & Dan, 2001). Several of these studies induced changes in perception by manipulating the timing of stimulus presentations to exploit spike-timing dependent synaptic plasticity (Beste et al., 2011; McMahon & Leopold, 2012; Yao & Dan, 2001; see Seitz and Dinse, 2007 for a review). For example, Beste et al (2011) induced long-term potentiation (LTP) by having subjects passively view high frequency luminance changes. Following exposure to the high frequency stimulus, subjects were better at detecting luminance changes, demonstrating that improvements in a luminance change-detection task could occur even in the absence of a task during training. Moreover, low frequency luminance changes evoked long-term depression-like (LTD) effects and performance was subsequently impaired. Similarly, when cats were repeatedly presented with a target grating that was always preceded by a task-irrelevant grating that was offset by 15°, the tuning curves of neurons that preferred the target were shifted in the direction of the distracter grating (Yao & Dan, 2001). This effect was dependent on the timing of the presentation of the preceding grating, such that changes were only induced in tuning when the two stimuli were presented within a 40 ms time window. This indicates that these changes depend on the relative timing of pre- and post-synaptic firing, such that pre-synaptic activity immediately preceding post-synaptic activity leads to synaptic potentiation. The authors also performed an analogous study in human subjects and found parallel timing-dependent changes in perceptual report following repeated exposure.
There is also evidence that PL can be observed for subthreshold stimuli, so long as they are temporally yoked to a task-relevant stimulus or to the delivery of a reward (Leclercq & Seitz, 2012a; 2012b; Sasaki, Náñez, & Watanabe, 2010; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003; Watanabe, Náñez, & Sasaki, 2001; Watanabe et al., 2002). In one study, subjects were deprived of food and water before they passively viewed a sub-threshold grating. They then received a water droplet reward that was linked to the presentation of the sub-threshold grating rendered in a particular orientation. After training, subjects were better at detecting the orientation that was paired with the reward than orientations that were not paired with a reward, suggesting that the activation of subcortical reward pathways can automatically gate learning, even in the absence of overt attentional demands (Frankó, Seitz, & Vogels, 2010; Roelfsema, van Ooyen, Watanabe, 2010; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003). However, other work indicates that this task-irrelevant perceptual learning (TIPL) only occurs when there is no direct competition between target and distracter stimuli, presumably because attention will suppress concurrently presented irrelevant stimuli if they actively interfere with target processing (Choi, Seitz, & Watanabe, 2009; Gál et al., 2009; Tsushima, Seitz, & Watanabe, 2008). Thus, it appears that TIPL may be a form of learning that acts as a complement to traditional attention-modulated learning, facilitating the processing of stimuli that are temporally associated with a behaviorally relevant target or the delivery of a reward (Seitz & Watanabe, 2005; 2009). However, even though TIPL does not depend on attention in many cases, it is still subject to attentional inhibition if the irrelevant item interferes with target processing (see Seitz and Wantanbe, 2009).
These interactions between TIPL and attention are further supported by more recent studies using fast TIPL, where subjects express learning effects for task irrelevant items within a single session (Leclercq & Seitz, 2012a; 2012b). In this paradigm, the subjects task was to identify rare targets embedded within a rapid serial visual presentation (RSVP) stream of distracters while they simultaneously viewed a series of images that they would need to recall at the end of each trial. Leclercq and Seitz found that subjects were better at remembering images that were presented at the same time as or immediately following the target, indicating that enhanced top-down attention due to target detection also led to better processing of the task-irrelevant image. In a second condition where the distracter stimuli were removed from the RSVP stream, the sudden onset of the rare targets exogenously captured attention. Under these conditions, memory for the task-irrelevant images suffered when they were presented at the same time as the target, presumably because the exogenous capture of attention by the target lead to a concurrent suppression of competing stimuli. Thus, these studies provide converging evidence that attentional factors can play a dominant regulatory role in learning by enhancing relevant signals and suppressing signals associated with competing distracters.
4. Learning-Induced Neural Changes Can Persist without Attention
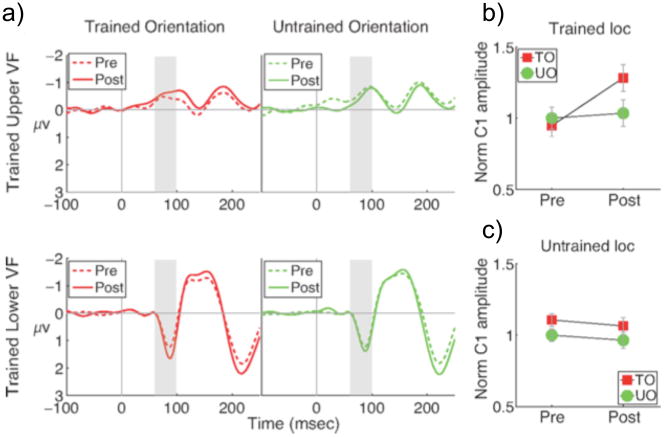
In many cases, top-down control appears to play a key role in the acquisition of learning, in part by increasing the impact of attentional control signals on sensory gain in early visual areas (Mukai et al., 2007). However, just as attention is not always required for the acquisition of learning (e.g., Beste et al., 2011; Seitz & Watanabe, 2003; Seitz, Kim, & Watanabe 2009), task-related attention is often not necessary to observe the expression of the learning-induced neural modifications after training, even for tasks where attention is recruited during training. Furmanski, Schluppeck, & Engel (2004) used functional magnetic resonance imaging (fMRI) to scan subjects before and after training on a task that required performing a discrimination on an obliquely oriented grating. Data from the pre-training scan session revealed an over-representation of cardinal compared to oblique angles in primary visual cortex. However, this oblique effect was abolished after training, such that cardinal and oblique gratings evoked a similar response profile in primary visual cortex (V1). This response increase was limited to V1 and not seen in V2 or V3, which the authors interpreted as evidence against a role for attentional factors because the influence of top-down attention tends to be larger in later visual areas (Kastner et al., 1998). Additionally, if more top-down attentional gain was applied to oblique orientations compared to cardinal orientations then behavioral performance should differ; however, no such difference was observed. Bao and colleagues (2010) subsequently used EEG to demonstrate that training a specific orientation leads to an increase in the amplitude of the temporally early C1 component of the visual evoked potential (onsetting ~50ms after the presentation of the trained stimulus). The increase in the C1 response was specific to both the location and the orientation of the trained grating, and occurred even when subjects were attending to a demanding RSVP task in the center of the screen (Figure 3; Bao et al., 2010). Thus, training-related changes in the amplitude of the C1 component could not be attributed to attentional factors because attention was focused on the center of the screen and not on the trained peripheral grating. Analogous findings have also been reported in single-unit physiology (Adab & Vogels, 2011; Raiguel et al., 2006; Schoups et al., 2001; Vogels, 2010). For example, Adab and Vogels (2011) found that training monkeys on an orientation discrimination task both increased the mean firing rate and decreased response variability of V4 neurons tuned to the trained orientation, an effect which was observed both during task performance and passive fixation.
Figure 3.
From Bao et al., 2010 (their figure 3). a) Time course of the VEPs for trained versus untrained orientation, before and after training. The gray bars mark the latency for the early segment of the C1 component. b, c) Normalized peak amplitude for the trained orientation (TO) versus untrained orientation (UO) at the trained location (b) and untrained location (c). (Reproduced with permission of Society for Neuroscience: Bao, M., Yang, L., Rios, C., He, B., & Engel, S.A. (2010). Perceptual learning increases the strength of the earliest signals in visual cortex. The Journal of Neuroscience. 30, 15080-15084., Copyright 2010.)
More dramatically, perhaps, sensory neurons in animals often show learning-induced modulations of sensory responses even under anesthesia, strongly suggesting that top-down attention is not required for the expression of all types of learning-induced neural modulations. For instance, training cats to discriminate a particular spatial frequency with one eye led to a significant improvement in neural contrast sensitivity functions recorded in V1 under anesthesia (Hua et al., 2010). These training effects were highly specific: the contrast sensitivity of V1 neurons was not only greater in trained cats compared to untrained controls, but greater for neurons receiving input from the trained eye compared to the untrained eye. Likewise, after training on a shape discrimination task, a greater proportion of neurons in the inferotemporal cortex of anesthetized monkeys responded to a set of trained shapes compared to control animals that had no prior experience with the same set of shapes (Kobatak, Wang, & Tanaka, 1998). Finally, European starlings can be trained to recognize and discriminate many different motifs and this ability can be used to index plasticity in the auditory system (see Knudsen & Gentner, 2010, for a review). Akin to visual perceptual learning, starlings improve at discriminating complex songs with practice and these behavioral improvements correspond to changes in the firing rates of neurons in the caudomedial mesopalluim (CMM) and caudolateral mesopallium (CLM), two regions of secondary auditory cortex (Gentner & Margoliash, 2003; Jeanne et al., 2011). Under anesthesia, neurons respond more strongly to trained songs compared to untrained songs, and this effect is mediated by the training regime (Figure 4; Gentner, 2007; Gentner & Margoliash, 2003). In “go-nogo” trained birds, the responses to songs associated with positive reinforcement were higher than those to songs associated with no reinforcement. The selective learning of task-relevant songs is consistent with the notion that feedback driven attentional mechanisms may play a role in modulating the acquisition of learning, even though long lasting effects of learning can be instantiated in local circuits without top-down control (Bao et al., 2010; Crist, Li, & Gilbert, 2001; Gentner, 2007; Gilbert, Sigman, & Crist, 2001).
Figure 4.
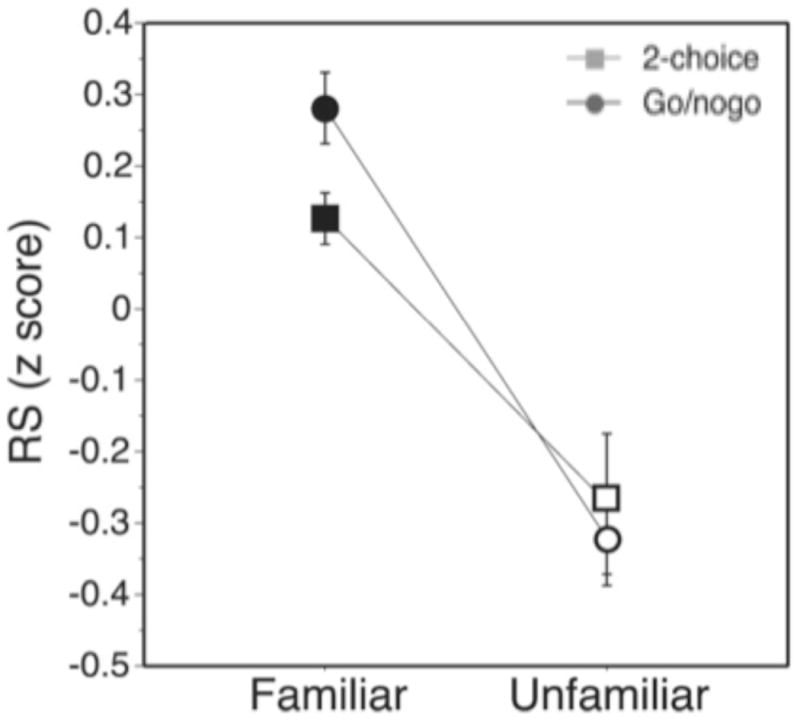
From Gentner & Margoliash, 2003 (their figure 2a). Mean normalized response strength of single units in CMM to familiar versus unfamiliar songs for the two training regimes (2AFC and go-nogo). (Reprinted by permission from Macmillan Publishers Ltd: Nature, Gentner, T.Q. & Margoliash, D. (2003). Neuronal populations and single cells representing learned auditory objects. Nature. 424, 669-674., Copyright 2003.)
Collectively, these studies suggest that even though attention can facilitate the instantiation of PL during training, active attention is not strictly necessary for the expression of training-related changes in neural activity. However, it is important to note that even if top-down attention is not necessary to observe the effects of PL, attentional factors can still play an important role in modulating the expression of training-induced neural modulations, depending on specific task demands. Recall that in the study by Crist, Li, and Gilbert (2001), responses in neurons tuned to a trained peripheral target were enhanced when the peripheral stimulus was attended and suppressed when the monkeys performed an alternate task at fixation. This suggests that, all else being equal, top-down attentional control can override or even reverse the effects of PL on neural activity depending on task demands. Thus, trained stimuli that are task-relevant or completely task-irrelevant can evoke enhanced responses after PL, even when they are not directly attended (Adab & Vogels, 2011; Bao et al., 2010; Schoups et al., 2001; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003; 2009). In contrast, top-down attention can override the effects of PL and suppress neural activity when trained stimuli actively compete with another relevant stimulus (as in Crist, Li, & Gilbert, 2001; Li, Piëch, & Gilbert, 2004).
5. Can Training Improve the Efficiency of Attentional Modulations?
Classic PL studies focus on training a specific stimulus feature in a specific retinal location. However, this specificity can limit the applicability of learning studies to real-world cases of perceptual expertise. For example, when a radiologist examines an x-ray, she cannot expect a fracture to always be in the same location and at the same orientation; she must learn a broad strategy for detecting fine discrepancies across the entire image. Therefore, learning often requires acquiring a general strategy so that the neural modulations that support more efficient perception can be flexibly applied to a large set of potentially relevant sensory features. Allocating attention in response to dynamically changing task demands can flexibly modulate neuronal responses by enhancing the gain in early sensory populations (Martinez-Trujillo & Treue, 2004; McAdams & Maunsell, 1999; Reynolds & Chelazzi, 2004; Reynolds, Pasternak, & Desimone, 2000; Saenz, Buracas, & Boynton, 2002; Serences et al., 2009; Scolari, Byers and Serences, 2012; Serences & Yantis, 2006; Treue, 2003; Treue & Martinez-Trujillo, 1999), by improving the read-out of sensory information in later decision-making areas (Eckstein et al., 2000; Palmer & Moore, 2009; Palmer, Verghese, & Pavel, 2000; Shaw, 1984), and by mediating intrinsic neuronal noise (Cohen & Kohn, 2011; Cohen & Maunsell, 2009; 2011; Mitchell, Sundberg, & Reynolds, 2007; 2009). If the efficiency of these attentional mechanisms increases with training, then whole classes of potentially relevant stimuli could be more readily distinguished from irrelevant distracters.
Relatively few studies have examined the impact of training on the ability to flexibly deploy top-down attentional gain in the context of complex visual search tasks. Rettenbach and colleagues used a variety of visual search tasks that all recruited feature-based attention to a pre-specified target stimulus (Leonards et al., 2002; Sireteanu & Rettenbach, 1995; 2000). Even though the search displays varied in their exact composition and spatial distribution from trial-to-trial, practice led to a significant behavioral improvement, such that reaction times were eventually unaffected by increases in the size of the search array (Sireteanu & Rettenbach, 1995). The authors argued that training-related reductions in reaction time indicate a shift from inefficient to efficient search by eliminating the need to examine each object in the array serially until the target is detected (Sireteanu & Rettenbach, 2000). Training also transferred across task type, eyes, and spatial locations, which argues against a low-level locus of the training effects. Instead, the high degree of transfer suggests that subjects were learning a general attentional strategy that allowed them to more efficiently search the array for the target stimulus (Sireteanu & Rettenback, 2000). In follow-up studies, these improvements were linked to an enhanced ability to detect both brightness differences between the target and distracters, as well as a unique visual cue associated with the target (Leonards et al., 2002).
In a related line of work, Ellison and Walsh (1998) found that training on orientation, size, and color singleton search tasks transferred partially to conjunction tasks that combined these features, whereas training on a conjunction task asymmetrically transferred to several different singleton search tasks. In this case, the ability of a search strategy to transfer depends on the complexity of the training task: if subjects were able to more efficiently find a target defined by a conjunction of features, then this ability may more easily transfer to a condition in which there was only one target feature (as opposed to the other way around, see Ahissar & Hochstein, 1997; 2004; Ahissar et al., 2009). This relationship between the broadness of the trained task and the extent of transfer could account for the specificity of many traditional PL tasks, as they often employ a single stimulus feature. By extension, if subjects are trained on a more generalized task, then learning may influence the efficacy of flexible top-down attentional mechanisms and the benefits would extend beyond the specific stimulus exemplars that were used during training (Ahissar et al., 2009).
Video games have also been used as a form of generalized learning that improves perception and enhances the efficiency of attention (Bavelier et al., 2010; 2011; Clark, Fleck, & Mitroff, 2010; Dye, Green, & Bavelier, 2009; Green & Bavelier, 2003; 2012; Green, Pouget, & Bavelier, 2010; Li et al., 2009; Riesenhuber, 2004). Video game players are better at a variety of behavioral measures, including difficult flanker compatibility tasks, subitizing items in a briefly flashed display, distributing attention across wide eccentricities, overcoming the attentional blink effect, and detecting small changes in a visual array (Clark, Fleck, & Mitroff, 2010; Green & Bavelier, 2003). Non-video game players who were trained on a video game also showed post-training improvements in contrast sensitivity (Green & Bavelier, 2003; Li et al., 2009). The improvements among video game players on a wide variety of tasks suggest that training can increase the efficacy of attentional modulations in a task-general manner, enabling improvements in many different task settings. However, even though Li and colleagues (2009) found an improvement in contrast sensitivity with controlled training using video games – which is consistent with a relatively low-level change in sensory gain – it is unclear whether these improvements are tied to enhanced sensory representations, more efficient read-out of sensory responses during decision making, or more efficient distracter exclusion.
While the results of studies that employ complex stimuli support the notion that training increases the efficiency of attentional modulations in a fairly general manner that supports extensive transfer, recent data suggest that general improvements in task performance can also be observed even when training is carried out using a restricted stimulus set. For example, double-training paradigms reveal transfer to specific features or locations that are passively exposed while training is carried out with a separate task-relevant stimulus (Xiao et al., 2008; Zhang et al., 2010b; 2010c). For instance, the mere presence of a stimulus at the to-be-transferred location during training may enable the global transfer of feature-based learning to the exposed location (Xiao et al., 2008; see also Treue & Martinez-Trujillo, 1999; Martinez-Trujillo & Treue, 2004; Saenz et al., 2003; Serences & Boynton, 2007). Similarly, a brief pre-training exposure to the peripheral transfer location may prime this location, again enabling the transfer of learning (Zhang et al., 2010b). Lu, Liu, and Dosher (2010) further demonstrated that learning in an orientation discrimination task also transfers across external noise conditions, but only from low external noise to high external noise. Guided by the Augmented Hebbian Reweighting Model (AHRM), the authors argued that read-out mechanisms are re-tuned to more selectively pool information about relevant input signals under low noise conditions. After optimization, the re-tuned read-out mechanisms can then be utilized to enhance the stimulus signal even when viewing a stimulus that is corrupted by a high level of external noise. In addition, training with a highly discriminable motion stimulus supports transfer across directions (Liu, 1999), possibly because low difficulty tasks can be carried out based on signals in higher cortical areas, which in turn leads to a higher degree of transfer after training (see Ahissar & Hochstein, 1997; 2004; Ahissar et al., 2009). Thus, the observation of extensive transfer even with tasks that use fairly basic visual features suggests that training may increase the efficiency of top-down attentional modulations in a manner that can generalize beyond specific low-level features, echoing the general types of learning observed with more complex stimuli, such as video games.
Collectively, the existing data suggest that, just as attention plays a modulatory role in the acquisition and expression of PL, training plays a complementary role in increasing the efficiency of top-down attentional control. In line with this notion, several studies have now demonstrated a reduction in the magnitude of modulations in areas of frontoparietal cortex that are commonly thought to mediate attentional control (Mukai et al., 2007; Sigman et al., 2005), even while activation in sensory areas simultaneously increases. This suggests that active attentional control is heavily recruited before training, but that learning either improves the efficiency of top-down attentional gain or induces plasticity in local connections so that intervention by top-down attentional modulations can be minimized. In line with these general hypotheses, Sigman et al. (2005) demonstrated that the blood oxygenation level dependent (BOLD) response in posterior parietal cortex (PPC) and supplementary motor area (SMA) was significantly diminished after training on a shape discrimination task. Another study found that successful learners had strong BOLD responses in putative attention-control areas before training, including intraparietal sulcus (IPS), frontal eye fields (FEF), and supplementary eye fields (SEF). However, activation in all of these areas was reduced after training (Mukai et al., 2007). This decrease in activation was coupled with an increase in the functional connectivity between frontoparietal areas and areas of early visual cortex, which tentatively suggests that the efficacy of attentional control signals improved with training and that the associated behavioral improvements were not due solely to local plasticity in visual cortex. Thus, attentional control signals become more efficient with training and may have an equivalent impact on sensory neurons despite giving rise to a smaller metabolic trace and thus smaller BOLD responses. Since connections between putative control regions and sensory areas are known to mediate top-down attention signals (Armstrong, Fitzgerald, & Moore, 2006; Moore, 2006; Moore & Armstrong, 2003; Moore & Fallah, 2004; Noudoost et al., 2010), the reinforcement of these pathways may allow faster and more efficient gain modulations after training. Given that the capacity to attend to multiple items is limited (Huang & Pashler, 2005; Huang, Pashler, & Junge, 2004; Mullin & Egeth, 1989; Scharff, Palmer, & Moore, 2011a; 2011b), improving the efficiency of attention through PL would allow these limited attentional resources to be preferentially focused on behaviorally relevant stimuli.
6. Mechanisms of Perceptual Learning Mirror those of Attention
As we have discussed, several studies have shown that performance on complex visual search tasks can improve with training, but the mechanisms supporting these improvements are not always clear: training might improve the efficacy of attentional mechanisms by increasing the magnitude of sensory gain, by improving the read-out of sensory signals, or by more efficiently mediating internal and external sources of variability. Additionally, studies showing learning in the absence of top-down attention suggest that there may also be a separate low-level mechanism that supports learning in cases where no task-related attentional goals are in place (Beste et al., 2011; Frankó, Seitz, & Vogels, 2010; Leclercq & Seitz, 2012a; McMahon & Leopold, 2012; Sasaki, Náñez, & Watanabe, 2010; Seitz, Kim, & Watanabe, 2009; Seitz & Watanabe, 2003; Yao & Dan, 2001; Watanabe, Náñez, & Sasaki, 2001; Watanabe et al., 2002). However, in more typical settings, it may be advantageous for the mechanisms of attention to be recruited during learning in order to increase the efficiency of sensory processing and perceptual decision-making. Furthermore, parallels can be drawn between the perceptual improvements associated with learning and those observed with attention, which might guide future investigations of the two phenomena.
As with attention-driven gain enhancements, PL has been found to increase the magnitude of responses associated with the trained stimulus (Bao et al., 2010; Furmanski, Schluppeck, & Engel, 2004; Lewis et al., 2009; Op de Beeck et al., 2006; Rainer, Lee, & Logothetis, 2004; Schoups et al., 2001; Schwartz, Marquet, & Frith, 2002; Sigman et al., 2005; Yang & Maunsell, 2004; Yotsumoto et al., 2009; Yotsumoto, Watanabe, & Sasaki, 2008; Zhang et al., 2010a; Zohary et al., 1994). Although the majority of these studies used a limited set of stimuli, it is possible that learning-induced response amplification for trained stimuli corresponds to an improved ability of attention to flexibly enhance the gain of sensory neurons that respond to a wide array of task-relevant stimuli, as in the case of visual search improvements and video gaming (Sireteanu & Rettenbach, 2000; Green & Bavelier, 2003).
Training-related improvements in the efficiency of attention may also be related to an improvement in the selective read-out of sensory signals, thereby increasing the speed and accuracy of decision-making without necessarily influencing sensory gain (Palmer & Moore, 2009; Palmer, Verghese, & Pavel, 2000; Pestilli et al, 2011). Similar ideas have been advanced in the PL literature. For instance, Law and Gold (2008) found that there were no changes in the firing rates of MT neurons after training monkeys on a motion discrimination task that varied both motion direction and motion coherence (Figure 5a), but there were changes in the firing rates of the neurons in lateral intraparietal area (LIP), an area implicated in accumulating sensory evidence during decision making (Figure 5b; Law & Gold, 2008; 2009). The authors conclude that learning results from a change in read-out of the most informative sensory neurons and that these read-out changes are driven by feedback, which guides the selective enhancement of the connections between the most sensitive populations in MT and LIP in order to optimize performance (Law & Gold, 2009; 2010). This model is akin to similar proposals in the attention literature (Eckstein et al., 2000; Palmer & Moore, 2009; Palmer, Verghese, & Pavel, 2000; Pestilli et al, 2011), in which responses from the most sensitive sensory neurons are pooled and responses from uninformative sensory neurons are filtered out, leading to overall improvements in the ability to discriminate target features from distracters (Gold, Law, & Bennur, 2010; Palmer and Moore, 2009; Pestilli et al., 2011).
Figure 5.
From Law & Gold, 2008 (their figures 3a and 4a). a) Average activity of MT neurons for various motion strengths as a function of viewing time. The solid lines represent each neuron’s preferred direction and dashed lines represent each neuron’s null direction. Each panel displays the different training periods. b) Average activity of LIP neurons for various motion strengths as a function of viewing time. The solid lines represent saccades into each neuron’s receptive field and dashed lines represent saccades out of each neuron’s receptive field. (Reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience, Law, C.T. & Gold, J.I. (2008). Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neuroscience. 11, 505-513, Copyright 2008.)
Other models, such as the Perceptual Template Model (PTM) and the Augmented Hebbian Reweighting Model (AHRM), hold that learning is driven both by improved filtering of internal and external noise as well as by a selective enhancement of the most sensitive sensory inputs (Dosher & Lu, 1998; 1999; 2009; Lu, Liu, & Dosher, 2010; Lu & Dosher, 2004; Petrov, Dosher, & Lu, 2005). As with Law and Gold’s model, learning results primarily from changing the weights associated with early sensory inputs being read-out during decision-making. However, in order for learning to occur in high noise environments, sensory systems must simultaneously enhance the representation of the stimulus as well as filter out stimulus noise (Petrov, Dosher, & Lu, 2005). In these cases, PL results from an improvement in the efficiency with which top-down control selectively modulates sensory gain enhancement and in the filtering of sensory signals.
General improvements in the efficiency of attentional modulations may also involve a reduction in intrinsic neural noise and the extent to which shared noise among sensory neurons is correlated (Cohen & Kohn, 2011; Cohen & Maunsell, 2009; 2011; Dosher & Lu, 2000; Mitchell, Sundberg, & Reynolds, 2007; 2009). Reductions in variability at the single-unit level (Mitchell, Sundberg, & Reynolds, 2007) complement increases in gain by further improving the signal-to-noise ratio of cells that encode relevant stimuli. While more complex (Abbott and Dayan, 1999; Cohen and Kohn, 2011; Cohen and Maunsell, 2011), decorrelating shared variability across populations of sensory neurons is often advantageous, as removing correlated noise increases the precision of a response estimate generated via simple pooling operations (Shadlen et al., 1996; Mitchell, Sundberg, & Reynolds, 2009; Cohen & Maunsell, 2009). Although many of these empirical observations regarding the modulation of intrinsic noise have been documented in the realm of attention studies, recent PL studies have observed similar modulations. For example, Gu et al. (2011) found that training on a direction-heading task reduced noise correlations across sensory neurons in macaque dorsal medial superior temporal area (MSTd), compared to untrained control animals. Trained monkeys matched controls in terms of the time course of responses, tuning curve bandwidth and amplitude, and sensitivity to the stimulus, which led the authors to conclude that the observed noise decorrelations serve to improve the read-out of sensory inputs to decision-making mechanisms. Mathematical models of PL also indicate that internal noise suppression is a key factor in the behavioral improvements associated with learning (Bejjanki et al., 2010; Dosher & Lu, 1998; 1999; 2009; Lu et al., 2011). Thus, a reduction in neural (co)variability combined with enhanced sensory signals likely increases the amount of information carried by population responses in visual cortex, allowing for more efficient decision-making and improved behavioral performance (Bejjanki et al., 2010).
Given the broad similarities between documented behavioral and neural manifestations of attention and PL, training subjects on more general tasks may lead to correspondingly general and flexible improvements in sensory gain, read-out, and in the modulation of neural variability. In contrast, training on a highly specific stimulus may instead lead to relatively long lasting changes in local cortical circuits that, once instantiated, no longer need to be guided by top-down attentional control.
7. Conclusions
In many cases, top-down attention can mediate PL for task-relevant stimuli and can support very specific, as well as more general, learning effects that transfer widely across tasks and stimulus features (as in the case of video games, Green & Bavelier, 2003; 2012). On the other hand, PL can also occur for irrelevant stimuli that are presented outside the focus of attention by pairing an irrelevant stimulus with a reward, by pairing an irrelevant stimulus with another task-relevant stimulus, or even via specific types of bottom-up sensory input (e.g. spike-time dependent plasticity; Frankó, Seitz, & Vogels, 2010; McMahon & Leopold, 2012; Yao & Dan, 2007). This observation that the acquisition of learning can proceed without top-down attention suggests that at a very basic level, there are multiple routes that can support the instantiation of long lasting changes in the efficiency of perceptual decision making. Moreover, many changes in processing that are induced by PL do not require active attention to be expressed, as learning-related modulations can be observed when subjects are attending elsewhere and even when animals are under anesthesia (Adab & Vogels, 2011; Bao et al., 2010; Gentner & Margoliash, 2003; Mukai et al., 2007; Raiguel et al., 2006; Schoups et al., 2001). However, attention and PL are not completely independent, as changes in attentional state can override the bottom-up instantiation of PL. This is particularly apparent when trained stimuli actively compete with other stimuli that are currently task relevant (Fahle, 2004; 2009; Crist, Li, & Gilbert, 2001; Gilbert et al., 2000). Thus, even though PL can occur in the absence of top-down attention, attention may still play a critical role as a gatekeeper to determine how training-induced changes in processing are expressed based on current task demands and behavioral goals. However, developing a more complete characterization of the extent to which attention can override all forms of PL is a major avenue for future research, particularly in situations that involve more complex and ecologically relevant stimulus sets.
Highlights.
We investigate the relationship between attention and perceptual learning
Attention sometimes modulates the acquisition of learning
Some types of learning do not require attention, suggesting multiple mechanisms
Task-demands play an important role in the expression of learning
Attention can be trained and made more efficient
Acknowledgments
Supported by NIMH grant RO1-092345 to JTS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Computation. 1999;11:91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- Adab HZ, Vogels R. Practicing coarse orientation discrimination improves orientation signals in macaque cortical area v4. Current Biology. 2011;21:1661–1666. doi: 10.1016/j.cub.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proceedings of the National Academy of Sciences. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. TRENDS in Cognitive Sciences. 2004;10:457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Ni R, Bower JD, Watanabe T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. Journal of Vision. 2010;10:1–13. doi: 10.1167/10.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Research. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Bao M, Yang L, Rios C, He B, Engel SA. Perceptual learning increases the strength of the earliest signals in visual cortex. The Journal of Neuroscience. 2010;30:15080–15084. doi: 10.1523/JNEUROSCI.5703-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Han DH, Renshaw PF, Merzenich MM, Gentile DA. Brains on video games. Nature Reviews Neuroscience. 2011;12:763–768. doi: 10.1038/nrn3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi D, Li R, Dan Y, Hensch T. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. The Journal of Neuroscience. 2010;30:4964–4971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjanki VR, Beck JM, Lu ZL, Pouget A. Perceptual learning as improved probabilistic inference in early sensory areas. Nature Neuroscience. 2010;14:642–648. doi: 10.1038/nn.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Wascher E, Güntürkün O, Dinse HR. Improvement and impairment of visually guided behavior through LTP- and LTD-like exposure- based visual learning. Current Biology. 2011;21:876–882. doi: 10.1016/j.cub.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Choi H, Seitz AR, Watanabe T. When attention interrupts learning: Inhibitory effects of attention on TIPL. Vision Research. 2009;49:2586–2590. doi: 10.1016/j.visres.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Fleck MS, Mitroff SR. Enhanced change detection performance reveals improved strategy use in avid action video game players. Acta Psychologia. 2010;136:67–72. doi: 10.1016/j.actpsy.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nature Neuroscience. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nature Neuroscience. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Using neuronal populations to study the mechanisms underlying spatial and feature attention. Neuron. 2011;70:1192–1204. doi: 10.1016/j.neuron.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nature Neuroscience. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Sciences. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Noise exclusion in spatial attention. Psychological Science. 2000;11:139–146. doi: 10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Hebbian Reweighting on stable representations in perceptual learning. Learning & Perception. 2009;1:37–58. doi: 10.1556/LP.1.2009.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis-Roy N, Gosselin F. Perceptual learning without signal. Vision Research. 2007;47:349–356. doi: 10.1016/j.visres.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein MP, Thomas JP, Palmer J, Shimozaki SS. A signal detection model predicts the effects of set size on visual search accuracy for feature, conjunction, triple conjunction, and disjunction displays. Perception & Psychophysics. 2000;62:425–451. doi: 10.3758/bf03212096. [DOI] [PubMed] [Google Scholar]
- Ellison A, Walsh V. Perceptual learning in visual search: some evidence of specificities. Vision Research. 1998;38:333–345. doi: 10.1016/s0042-6989(97)00195-8. [DOI] [PubMed] [Google Scholar]
- Fahle M. Specificity of learning curvature, orientation, and vernier discriminations. Vision Research. 1997;37:1885–1895. doi: 10.1016/s0042-6989(96)00308-2. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: A case for early selection. Journal of Vision. 2004;4:879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning and sensomotor flexibility: Cortical plasticity under attentional control? Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2009;364:313–319. doi: 10.1098/rstb.2008.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M, Edelman S, Poggio T. Fast perceptual learning in hyperacuity. Vision Research. 1995;35:3003–3013. doi: 10.1016/0042-6989(95)00044-z. [DOI] [PubMed] [Google Scholar]
- Frankó E, Seitz AR, Vogels R. Dissociable neural effects of long-term stimulus-reward pairing in macaque visual cortex. Journal of Cognitive Neuroscience. 2010;22:1425–1439. doi: 10.1162/jocn.2009.21288. [DOI] [PubMed] [Google Scholar]
- Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Current Biology. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Gál V, Kozák LR, Kóbor I, Bankó EM, Serences JT, Vidnyánszky Z. Learning to filter out visual distractors. European Journal of Neuroscience. 2009;29:1723–1731. doi: 10.1111/j.1460-9568.2009.06724.x. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ. Mechanisms of temporal auditory pattern recognition in songbirds. Language Learning and Development. 2007;3:157–178. [Google Scholar]
- Gibson EJ. Perceptual learning. Annual Review of Psychology. 1963;14:29–56. doi: 10.1146/annurev.ps.14.020163.000333. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Principles of Perceptual Learning and Development. Appelton-Century-Croft; New York: 1969. [Google Scholar]
- Gilbert CD, Ito M, Kapadia M, Westheimer G. Interactions between attention, context and learning in primary visual cortex. Vision Research. 2000;40:1217–1226. doi: 10.1016/s0042-6989(99)00234-5. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Gold JI, Law CT, Connolly P, Bennur S. Relationships between the threshold and slope of psychometric and neurometric functions during perceptual learning: Implications for neuronal pooling. Journal of Neurophysiology. 2010;103:140–154. doi: 10.1152/jn.00744.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Learning, attentional control, and action video games. Current Biology. 2012;22:R197–206. doi: 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Pouget A, Bavelier D. Improved probabilistic inference as a general learning mechanism with action video games. Current Biology. 2010;20:1573–1579. doi: 10.1016/j.cub.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Liu S, Fetsch CR, Yang Y, Fok S, Sunkara A, DeAngelis GC, Angelaki DE. Perceptual Learning Reduces Interneuronal Correlations in Macaque Visual Cortex. Neuron. 2011;71:750–761. doi: 10.1016/j.neuron.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog MH, Fahle M. The role of feedback in learning a vernier discrimination task. Vision Research. 1997;37:2133–2141. doi: 10.1016/s0042-6989(97)00043-6. [DOI] [PubMed] [Google Scholar]
- Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. Perceptual learning improves contrast sensitivity of V1 neurons in cats. Current Biology. 2010;20:887–894. doi: 10.1016/j.cub.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Pashler H. Attention capacity and task difficulty in visual search. Cognition. 2005;94:B101–B111. doi: 10.1016/j.cognition.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Huang L, Pashler H, Junge JA. Are there capacity limitations in symmetry perception? Psychonomic Bulletin Review. 2004;11:862–869. doi: 10.3758/bf03196713. [DOI] [PubMed] [Google Scholar]
- Jeanne JM, Thompson JV, Sharpee TO, Gentner TQ. Emergence of learned categorical representations within an auditory forebrain circuit. The Journal of Neuroscience. 2011;31:2595–2606. doi: 10.1523/JNEUROSCI.3930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Knudsen DP, Gentner TQ. Mechanisms of song perception in oscine birds. Brain and Language. 2010;115:59–68. doi: 10.1016/j.bandl.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatak E, Wang G, Tanaka K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. Journal of Neurophysiology. 1998;80:324–330. doi: 10.1152/jn.1998.80.1.324. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z. Visual learning for perceptual and categorical decisions in the human brain. Vision Research. 2010;50:433–440. doi: 10.1016/j.visres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, DiCarlo JJ. Learning and neural plasticity in visual object recognition. Current Opinion in Neurobiology. 2006;16:152–158. doi: 10.1016/j.conb.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Law CT, Gold JI. Neural correlates of perceptual learning in a sensory- motor, but not a sensory, cortical area. Nature Neuroscience. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nature Neuroscience. 2009;12:655–663. doi: 10.1038/nn.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Shared mechanisms of perceptual learning and decision making. Topics in Cognitive Science. 2010;2:226–238. doi: 10.1111/j.1756-8765.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards U, Rettenbach R, Nase G, Sireteanu R. Perceptual learning of highly demanding visual search tasks. Vision Research. 2002;42:2193–2204. doi: 10.1016/s0042-6989(02)00134-7. [DOI] [PubMed] [Google Scholar]
- Leclercq V, Seitz AR. Fast task-irrelevant perceptual learning is disrupted by sudden onset of central task elements. Vision Research. 2012a;61:70–76. doi: 10.1016/j.visres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Leclercq V, Seitz AR. The impact of orienting attention in fast task-irrelevant perceptual learning. Attention, Perception, & Psychophysics. 2012b;74:648–660. doi: 10.3758/s13414-012-0270-7. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Polat U, Makous W, Bavelier D. Enhancing the contrast sensitivity function through action video game training. Nature Neuroscience. 2009;12:549–551. doi: 10.1038/nn.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nature Neuroscience. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Perceptual learning in motion discrimination that generalizes across motion directions. Proceedings of the National Academy of Sciences. 1999;96:14085–7. doi: 10.1073/pnas.96.24.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu ZL, Dosher BA. Mixed training at high and low accuracy levels leads to perceptual learning without feedback. Vision Research. 2012;61:15–24. doi: 10.1016/j.visres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Perceptual learning retunes the perceptual template in foveal orientation identification. Journal of Vision. 2004;4:44–56. doi: 10.1167/4.1.5. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Hua T, Huang CB, Zhou Y, Dosher BA. Visual Perceptual Learning. Neurobiology of Learning and Memory. 2011;95:145–151. doi: 10.1016/j.nlm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Liu J, Dosher BA. Modeling mechanisms of perceptual learning with augmented Hebbian re-weighting. Vision Research. 2010;50:375–390. doi: 10.1016/j.visres.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. The Journal of Neuroscience. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Westheimer G. Improvement in vernier acuity with practice. Perception & Psychophysics. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- McMahon DBT, Leopold DA. Stimulus timing-dependent plasticity in high-level vision. Current Biology. 2012;22:332–337. doi: 10.1016/j.cub.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: Finding sources. Current Opinion in Neurobiology. 2006;16:159–65. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. Journal of Neurophysiology. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mukai I, Bahadur K, Kesavabhotla K, Ungerleider LG. Exogenous and endogenous attention during perceptual learning differentially affect post-training target thresholds. Journal of Vision. 2011;11:1–15. doi: 10.1167/11.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai I, Kim D, Fukunaga M, Japee S, Marrett S, Ungerleider LG. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. The Journal of Neuroscience. 2007;27:11401–11411. doi: 10.1523/JNEUROSCI.3002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin PA, Egeth HE. Capacity limitations in visual word processing. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:111–123. doi: 10.1037//0096-1523.15.1.111. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Current Opinion in Neurobiology. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck HP, Baker CI, DiCarlo JJ, Kanwisher NG. Discrimination training alters object representations in human extrastriate cortex. The Journal of Neuroscience. 2006;26:13025–13036. doi: 10.1523/JNEUROSCI.2481-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Moore CM. Using a filtering task to measure the spatial extent of selective attention. Vision Research. 2009;49:1045–1064. doi: 10.1016/j.visres.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Verghese P, Pavel M. The psychophysics of visual search. Vision Research. 2000;40:1227–1268. doi: 10.1016/s0042-6989(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Carrasco M, Heeger DJ, Gardner JL. Attentional Enhancement via Selection and Pooling of Early Sensory Responses in Human Visual Cortex. Neuron. 2011;72:832–846. doi: 10.1016/j.neuron.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov AA, Dosher BA, Lu ZL. The dynamics of perceptual learning: an incremental reweighting model. Psychological Review. 2005;112:715–743. doi: 10.1037/0033-295X.112.4.715. [DOI] [PubMed] [Google Scholar]
- Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. The Journal of Neuroscience. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Lee H, Logothetis NK. The effect of learning on the function of monkey extrastriate visual cortex. PLoS Biology. 2004;2:275–283. doi: 10.1371/journal.pbio.0020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annual Reviews of Neuroscience. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Riesenhuber M. An action video game modifies visual processing. TRENDS in Neurosciences. 2004;27:72–74. doi: 10.1016/j.tins.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, van Ooyen A, Watanabe T. Perceptual learning rules based on reinforcers and attention. TRENDS in Cognitive Sciences. 2010;14:64–71. doi: 10.1016/j.tics.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nature Neuroscience. 2002;5:631–332. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Náñez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nature Reviews Neuroscience. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff A, Palmer J, Moore CM. Extending the simultaneous-sequential paradigm to measure perceptual capacity for features and words. Journal of Experimental Psychology: Human Perception & Performance. 2011a;37:813–833. doi: 10.1037/a0021440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff A, Palmer J, Moore CM. Evidence of fixed capacity in visual object categorization. Psychonomic Bulletin Review. 2011b;18:713–721. doi: 10.3758/s13423-011-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 1995;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Marquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proceedings of the National Academy of Sciences. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari M, Byers A, Serences JT. Optimal deployment of attentional gain during fine discriminations. The Journal of Neuroscience. 2012;32:7723–7733. doi: 10.1523/JNEUROSCI.5558-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR. A common framework for perceptual learning. Current Opinion in Neurobiology. 2007;17:148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Náñez JE, Holloway S, Tsushima Y, Watanabe T. Two cases requiring external reinforcement in perceptual learning. Journal of Vision. 2006;6:966–973. doi: 10.1167/6.9.9. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. Is subliminal learning really passive. Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. A unified model for perceptual learning. TRENDS in Cognitive Sciences. 2005;9:329–334. doi: 10.1016/j.tics.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. The phenomenon of task-irrelevant perceptual learning. Vision Research. 2009;49:2604–2610. doi: 10.1016/j.visres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Boynton GM. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron. 2007;55:301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Serences JT, Saproo S, Scolari M, Ho T, Muftuler LT. Estimating the influence of attention on population codes in human visual cortex using voxel- based tuning functions. NeuroImage. 2009;44:223–231. doi: 10.1016/j.neuroimage.2008.07.043. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. TRENDS in Cognitive Sciences. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. Journal of Neuroscience. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ML. Division of attention among spatial locations: A fundamental difference between detection of letters and detection of luminance increments. In: Bouma H, Bouwhais DG, editors. Attention & Performance X. Erlbaum; Hillsdale, NJ: 1984. pp. 109–121. [Google Scholar]
- Shibata K, Yamagishi N, Ishii S, Kawato M. Boosting perceptual learning by fake feedback. Vision Research. 2009;49:2574–2585. doi: 10.1016/j.visres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Perception & Psychophysics. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Sigman M, Pan H, Yang Y, Stern E, Silbersweig D, Gilbert CD. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireteanu R, Rettenbach R. Perceptual learning in visual search: Fast, enduring, but non-specific. Vision Research. 1995;35:2037–2043. doi: 10.1016/0042-6989(94)00295-w. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Rettenbach R. Perceptual learning in visual search generalizes over tasks, locations, and eyes. Vision Research. 2000;40:2925–2949. doi: 10.1016/s0042-6989(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Tartaglia EM, Bamert L, Mast FW, Herzog MH. Human perceptual learning by mental imagery. Current Biology. 2009;19:2081–2085. doi: 10.1016/j.cub.2009.10.060. [DOI] [PubMed] [Google Scholar]
- Treue S. Visual attention: the where, what, how and why of saliency. Current Opinion in Neurobiology. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Treue S, Martinez-Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Tsushima Y, Seitz AR, Watanabe T. Task-irrelevant learning occurs only when the irrelevant feature is weak. Current Biology. 2008;18:R516–R517. doi: 10.1016/j.cub.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels R. Mechanisms of visual perceptual learning in macaque visual cortex. Topics in Cognitive Science. 2010;2:239–250. doi: 10.1111/j.1756-8765.2009.01051.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Náñez JE, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature Neuroscience. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Náñez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Current Biology. 2008;18:1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell JHR. The effect of perceptual learning on neuronal responses in monkey visual area V4. The Journal of Neuroscience. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–323. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Yotsumoto Y, Sasaki Y, Chan P, Vasios CE, Bonmassar G, Ito N, Náñez JE, Sr, Shimojo S, Watanabe T. Location-specific cortical activation changes during sleep after training for perceptual learning. Current Biology. 2009;19:1278–1282. doi: 10.1016/j.cub.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Klein SA, Levi DM. Perceptual learning in contrast discrimination and the (minimal) role of context. Journal of Vision. 2004;4:169–182. doi: 10.1167/4.3.4. [DOI] [PubMed] [Google Scholar]
- Yund EW, Efron R. Guided search: The effects of learning. Brain & Cognition. 1996;31:369–386. doi: 10.1006/brcg.1996.0051. [DOI] [PubMed] [Google Scholar]
- Zhang J, Meeson A, Welchman A, Kourtzi Z. Learning alters the tuning of functional magnetic resonance imaging patterns for visual forms. The Journal of Neuroscience. 2010a;30:14127–14133. doi: 10.1523/JNEUROSCI.2204-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Xiao LQ, Klein SA, Levi DM, Yu C. Decoupling location specificity from perceptual learning of orientation discrimination. Vision Research. 2010b;50:368–374. doi: 10.1016/j.visres.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Zhang GL, Xiao LQ, Klein SA, Levi DM, Yu C. Rule-based learning explains visual perceptual learning and its specificity and transfer. The Journal of Neuroscience. 2010c;37:12323–12328. doi: 10.1523/JNEUROSCI.0704-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Celebrini S, Britten KH, Newsome WT. Neuronal plasticity that underlies improvement in perceptual performance. Science. 1994;263:1289–1292. doi: 10.1126/science.8122114. [DOI] [PubMed] [Google Scholar]