Abstract
According to pulsed-field gel electrophoresis (PFGE) typing, 4,12:a:− Salmonella enterica isolates from harbor porpoises are highly diverse. However, porpoise isolates belong to only two multilocus sequence types within the eBurst group 18 (eBG18) genetic cluster, which also includes S. enterica serovars Bispebjerg and Abortusequi. Isolates of other, serologically similar serovars belong to unrelated eBGs. These assignments to eBGs were supported by eBG-specific sequences of the flagellar gene fliC.
TEXT
Isolates of 4,12:a:− Salmonella enterica subspecies enterica are frequently found in the pulmonary tract and other tissues of healthy and diseased harbor porpoises (Phocoena phocoena) from northern European coasts (4, 5, 8, 17). This monophasic antigenic formula corresponds to S. enterica serovar Fulica (6), which was first isolated from poultry in the United States in 1957 and only rarely thereafter, except for the harbor porpoise isolates (see Table S1 in the supplemental material). Twenty-seven percent of harbor porpoises in the United Kingdom are infected with 4,12:a:− strains (17), which are most commonly isolated from lung tissue (5, 17). It is possible that 4,12:a:− Salmonella isolates are transmitted by nematodes (3, 5), which are major porpoise parasites (7, 8, 13).
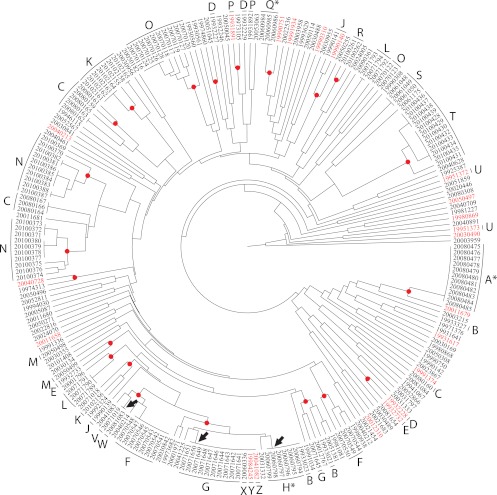
Little is known about the population structure of 4,12:a:− Salmonella from harbor porpoises (2, 10). Pulsed-field gel electrophoresis (PFGE) XbaI fingerprinting of 230 4,12:a:− isolates from harbor porpoises revealed 157 discrete fingerprint profiles (with a range of 10 to 20 fragments), of which 87% (136 profiles) were found only once (Fig. 1; see Table S2 in the supplemental material; for methods, see the supplemental material). Multiple isolates with uniform profiles were identified in three animals, and 3/157 profiles grouped strains from multiple animals. The latter profiles spanned periods of isolation of 1, 9, and 13 years. However, 15/20 porpoises with multiple isolates yielded two or more profiles. Furthermore, repeating PFGE typing of multiple single colonies from slopes, which were inoculated from a single colony and stored at room temperature for several years, revealed multiple PFGE profiles that had probably accumulated during laboratory storage (see Fig. S1 in the supplemental material).
Fig 1.
Relationships of PFGE profiles for 230 S. enterica isolates from harbor porpoises according to an unweighted-pair group method using average linkages (UPGMA) dendrogram. The animal source is indicated for selected isolates by capital letters outside the dendrogram which designate the porpoise from which strains were isolated. Letters are followed by an asterisk for porpoises from which all isolated strains possessed identical PFGE profiles. Arrows indicate identical PFGE profiles in multiple porpoises. The strain designations of isolates that were subjected to MLST analysis are highlighted in red, whereas red dots indicate nodes connecting PFGE profiles that differed by fewer than four band differences.
Our PFGE observations raise the question of whether 4,12:a:− isolates from harbor porpoises are phylogenetically related or comprise multiple lineages. Previous multilocus sequence typing (MLST) analyses of >4,000 S. enterica subspecies enterica isolates have shown that numerous serovars were polyphyletic, comprising multiple unrelated sequence types (STs) or clusters of related STs (eBurst groups [eBGs]) (1, 12). MLST of 21 representatives of the PFGE diversity showed that all isolates belong to two STs (ST416, 20 isolates; ST417, 1 isolate) within eBG18 (Fig. 2; see Table S3 in the supplemental material). ST416 and ST417 differ by one single nucleotide polymorphism (SNP) in one of the seven MLST loci that were sequenced. Thus, 4,12:a:− Salmonella isolates from porpoises are genetically monomorphic according to MLST, similar to isolates of S. enterica serovar Typhi (9, 11). Comparisons with the MLST website revealed that eBG18 is genetically unrelated to other STs or eBGs, differing by at least five loci.
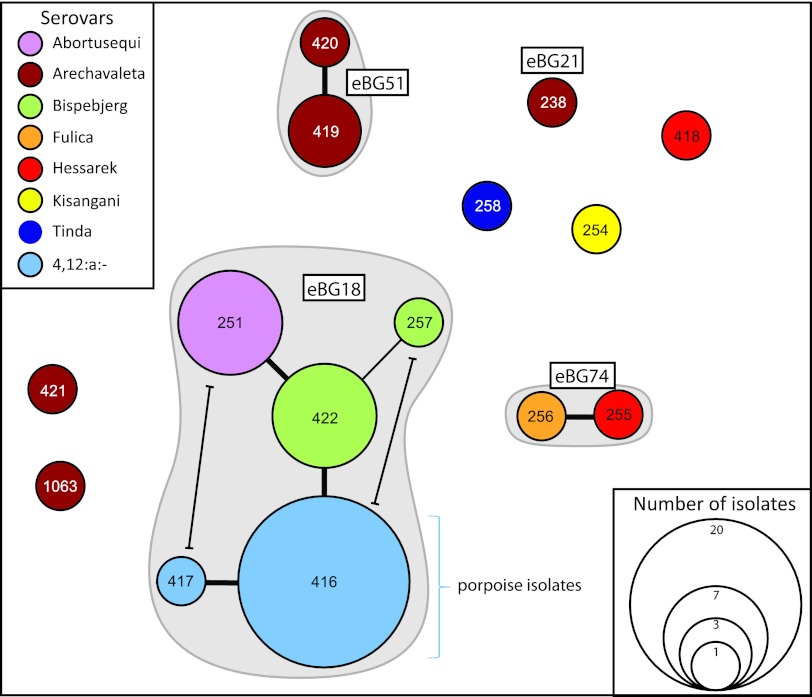
Fig 2.
Minimal spanning tree based on MLST alleles. Each ST is indicated by a circle encompassing the ST number, whose size reflects the number of isolates; eBGs are indicated by gray shading around STs, and the eBG designation is within a rectangle. STs are connected by thick black lines (six identical alleles) or thin black lines (five identical alleles). The primary connecting lines are not terminated, but two alternative connections at the level of five identical alleles are indicated by lines terminating in bars. All STs that are unconnected shared fewer than four alleles.
We also investigated the MLST patterns of 27 strains from seven other serovars with similar antigenic formulas (see Table S1 in the supplemental material). eBG18 also includes three other STs containing strains of S. enterica serovars Bispebjerg (1,4,[5],12:a:e.n.x), which has been isolated from turtles, humans, and birds, and Abortusequi (4,12:−:e,n,x), which causes disease in horses (Fig. 2; see Table S3 in the supplemental material). These serovars are found only in eBG18 according to the MLST website, and eBG18 contains no other serovars. Thus, eBG18 includes isolates with overlapping but discrete antigenic formulas.
Other serovars, including S. enterica serovar Fulica, which is antigenically identical to the porpoise isolates, were assigned to multiple, unrelated eBGs and STs (Fig. 2; see Table S3 in the supplemental material). S. enterica serovar Arechavaleta (4,[5],12:a:1,7) is polyphyletic, because seven isolates were distributed among four eBGs or STs. S. enterica serovar Hessarek (4,12,[27]:a:1,5) is also polyphyletic, with the two tested isolates belonging to two unrelated STs, one of which (ST255) is in the same eBG (eBG74) as the reference Fulica strain. Finally, single isolates of S. enterica serovars Kisangani and Tinda were assigned to two other STs. Thus, serological relatedness did not correlate with genetic relatedness for these serovars.
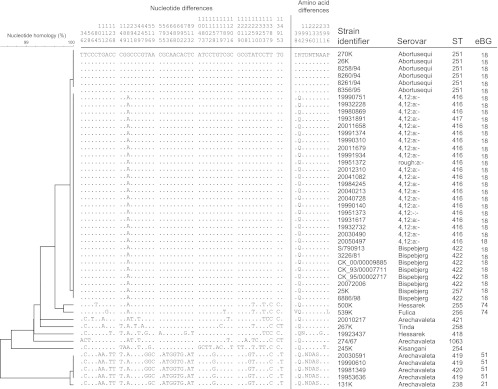
The antigenic formulas of all the serovars investigated here share similar lipopolysaccharide (LPS) epitopes and the flagellar phase 1 “a” epitope, except that S. enterica serovar Abortusequi does not express phase 1 (see Table S1 in the supplemental material). We sequenced 1,400 bp of fliC (phase 1) from all strains tested by MLST. fliC was identical in all S. enterica serovar Bispebjerg and 4,12:a:− harbor porpoise isolates (Fig. 3). Even though the FliC phase 1 antigen is not expressed in S. enterica serovar Abortusequi, fliC of 6/7 strains differed from that sequence by only one (nonsynonymous) SNP. We could not amplify fliC from the seventh strain (8259/94). fliC from all other isolates differed at multiple SNPs. Most of these SNPs were due to synonymous polymorphisms, resulting in only 10 polymorphic amino acids in the entire collection and amino acid sequences identical to that of S. enterica serovar Bispebjerg in four isolates of S. enterica serovars Hessarek, Arechavaleta, and Tinda. The ratio of nonsynonymous to synonymous rates of substitution, ω, was low (0.06) and similar to ω for MLST sequences (1), suggesting that nonsynonymous mutations are removed by purifying selection.
Fig 3.
Nucleotide and amino acid diversity within fliC of serovars containing the “a” epitope. Left: unweighted-pair group method using average linkages (UPGMA) dendrogram of relatedness. An arbitrary reference nucleotide and amino acid sequence is indicated in the first line, and dots in other lines indicate identical nucleotides or amino acids. Nucleotide positions are given at the top. Right: information on each strain regarding serovar, ST, and eBG.
The primary differences between the serovars tested here are in the phase 2 antigen, which is 1,2, 1,5, 1,7, e,n,x, e,n,z15, or not expressed (see Table S1 in the supplemental material). We sequenced 1,200 bp of fljB (phase 2) from multiple strains but could not amplify fljB from harbor porpoise isolates, with one exception (strain 20011679). The fljB amino acid sequences formed two discrete sequence clusters, encoding e,n,x or e,n,z15 epitopes (S. enterica serovars Bispebjerg, Abortusequi, and Tinda) and 1,2, 1,5 or 1,7 epitopes (S. enterica serovars Hessarek, Arechavaleta, and Kisangani) (see Fig. S2 in the supplemental material). The fljB sequence from strain 20011679 differs by three amino acids and 14 or 15 SNPs from strains of S. enterica serovars Bispebjerg and Abortusequi (e,n,x), and 14 SNPs and one amino acid from an e,n,z15 sequence from S. enterica serovar Tinda. BLASTN searches against fljB sequences encoding the e,n epitope in GenBank and unpublished sequences at the Institut Pasteur revealed that this 4,12:a:− sequence is identical to, or differs by only one SNP from, fljB of S. enterica serovars Brandenburg (4,[5],12:l,v:e,n,z15) and Goettingen (9,12:l,v:e,n,z15), both in eBG12 (see Fig. S3 in the supplemental material). Therefore, eBG12, which shares no alleles with eBG18, represents a potential source of the fljB in the exceptional 4,12:a:− isolate.
Our data show that the PFGE diversity reflects hypervariable changes that occur within individual animals and upon storage. According to MLST, 4,12:a:− Salmonella isolates from harbor porpoises form a genetically monomorphic group within eBG18 of S. enterica subspecies enterica, which also includes S. enterica serovars Bispebjerg and Abortusequi. In contrast, eBG18 isolates are unrelated to other S. enterica isolates with similar or identical serological patterns, including the reference strain for S. enterica serovar Fulica. We recommend that isolates from harbor porpoises be referred to by their ST or eBG designations rather than by their serological formulas, as the latter are not particularly informative. Our results also provide further support for the conclusion (1) that some eBGs are comprised of multiple serovars and some serovars conflate genetically unrelated bacteria. A particularly dramatic example of such conflation is S. enterica serovar Paratyphi B biovar Java (1), where distinct eBGs are differentially associated with infections of reptiles and poultry (15).
Members of eBG18 represent three distinct patterns of host adaptation and/or restriction (16), namely, infection of harbor porpoises, multiple hosts (S. enterica serovar Bispebjerg), and horses (S. enterica serovar Abortusequi). It would be interesting to perform MLST on an additional 4,12:a:− strain from a nematode parasitizing a harbor porpoise (3), because MLST distinguishes harbor porpoise isolates from antigenically identical S. enterica serovar Fulica. Similar patterns of host diversity have been reported within eBG4, which includes S. enterica serovar Enteritidis (1), which can infect a variety of hosts, as well as the host-adapted specialists S. enterica serovar Gallinarum and S. enterica serovar Gallinarum variant Pullorum, which cause two distinct avian diseases. A genomic comparison of one genome from each of the S. enterica serovars Enteritidis and Gallinarum indicated that specialization for avian disease was accompanied by genomic decay (14). Genomic analyses of eBG18 have not yet been performed but might be expected to yield interesting insights about host specificity in Salmonella.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christian Kornschober for supplying three strains and Sylvie Issenhuth-Jeanjean for her technical assistance.
The Scottish Strandings Scheme is operated under contract to the United Kingdom Department of Environment, Food and Rural Affairs (Defra), with financial support from the Scottish Government Marine Directorate. The project was funded by the Science Foundation Ireland (05/FE1/B882).
Footnotes
Published ahead of print 5 October 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Achtman M, et al. 2012. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 8:e1002776 doi:10.1371/journal.ppat.1002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crichton PB, Henry MS, Old DC. 2000. Strain discrimination of a novel serotype of Salmonella from harbour porpoises (Phocoena phocoena) by molecular techniques. Vet. Microbiol. 76:61–69 [DOI] [PubMed] [Google Scholar]
- 3. Davison N, Barnett J, Rule B, Chappell S, Wise G. 2010. Group B Salmonella in lungworms from a harbour porpoise (Phocoena phocoena). Vet. Rec. 167:351–352 [DOI] [PubMed] [Google Scholar]
- 4. Davison NJ, et al. 2010. Prevalence of a host-adapted group B Salmonella enterica in harbour porpoises (Phocoena phocoena) from the south-west coast of England. Vet. Rec. 167:173–176 [DOI] [PubMed] [Google Scholar]
- 5. Foster G, Patterson IA, Munro DS. 1999. Monophasic group B Salmonella species infecting harbour porpoises (Phocoena phocoena) inhabiting Scottish coastal waters. Vet. Microbiol. 65:227–231 [DOI] [PubMed] [Google Scholar]
- 6. Grimont PA, Weill F-X. 2007. Antigenic formulae of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella, Paris, France [Google Scholar]
- 7. Jauniaux T, et al. 2002. Post-mortem findings and causes of death of harbour porpoises (Phocoena phocoena) stranded from 1990 to 2000 along the coastlines of Belgium and Northern France. J. Comp. Pathol. 126:243–253 [DOI] [PubMed] [Google Scholar]
- 8. Jepson PD, et al. 2000. Pulmonary pathology of harbour porpoises (Phocoena phocoena) stranded in England and Wales between 1990 and 1996. Vet. Rec. 146:721–728 [DOI] [PubMed] [Google Scholar]
- 9. Kidgell C, et al. 2002. Salmonella typhi, the causative agent of typhoid fever, is approximately 50,000 years old. Infect. Genet. Evol. 2:39–45 [DOI] [PubMed] [Google Scholar]
- 10. Old DC, Crichton PB, Taylor A, Mather H. 2001. An attempt to identify the evolutionary origin of a novel serotype of Salmonella enterica isolated from harbour porpoises. J. Med. Microbiol. 50:415–420 [DOI] [PubMed] [Google Scholar]
- 11. Roumagnac P, et al. 2006. Evolutionary history of Salmonella Typhi. Science 314:1301–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sangal V, et al. 2010. Evolution and population structure of Salmonella enterica serovar Newport. J. Bacteriol. 192:6465–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siebert U, et al. 2001. Post-mortem findings in harbour porpoises (Phocoena phocoena) from the German North and Baltic Seas. J. Comp. Pathol. 124:102–114 [DOI] [PubMed] [Google Scholar]
- 14. Thomson NR, et al. 2008. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 18:1624–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toboldt A, et al. 2012. Human infections attributable to the d-tartrate-fermenting variant of Salmonella enterica serovar Paratyphi B in Germany originate in reptiles and, on rare occasions, poultry. Appl. Environ. Microbiol. 78:7347–7357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uzzau S, et al. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valderrama Vasquez CA, Macgregor SK, Rowcliffe JM, Jepson PD. 2008. Occurrence of a monophasic strain of Salmonella group B isolated from cetaceans in England and Wales between 1990 and 2002. Environ. Microbiol. 10:2462–2468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.