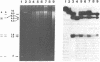
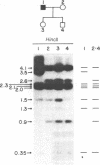
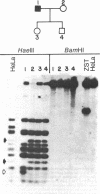
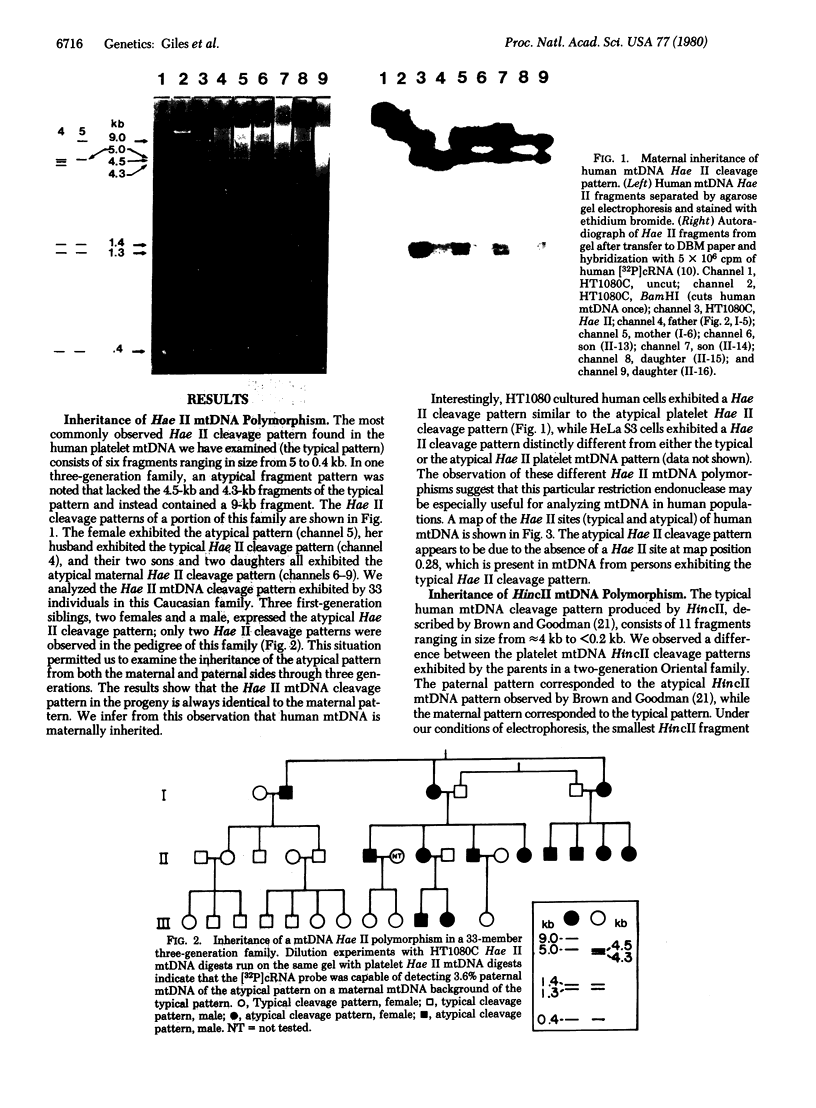
Abstract
Human mitochondrial DNA was obtained from peripheral blood platelets donated by the members of several independent families. The samples were screened for nucleotide sequence polymorphisms between individuals within these families. In each family in which we were able to detect a distinctly different restriction endonuclease cleavage pattern between the parents, the progeny exhibited the maternal cleavage pattern. Informative polymorphisms were detected for Hae II (PuGCGCPy) in a three-generation family composed of 33 members, for HincII (GTPyPuAC) in a two-generation family composed of four members, and for Hae III(GGCC) in a two-generation family composed of four members. The Hae II polymorphism was analyzed through all three generations in both the maternal and paternal lines. The results of this study demonstrate that human mitochondrial DNA is maternally inherited. The techniques described for using peripheral blood platelets as a source of human mitochondrial DNA represent a convenient way to obtain data on mitochondrial DNA variation in both individuals and populations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agam G., Bessler H., Djaldetti M. In vitro DNA and RNA synthesis by human platelets. Biochim Biophys Acta. 1976 Feb 18;425(1):41–48. doi: 10.1016/0005-2787(76)90214-8. [DOI] [PubMed] [Google Scholar]
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Avise J. C., Giblin-Davidson C., Laerm J., Patton J. C., Lansman R. A. Mitochondrial DNA clones and matriarchal phylogeny within and among geographic populations of the pocket gopher, Geomys pinetis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6694–6698. doi: 10.1073/pnas.76.12.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise J. C., Lansman R. A., Shade R. O. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. I. Population structure and evolution in the genus Peromyscus. Genetics. 1979 May;92(1):279–295. doi: 10.1093/genetics/92.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon H. G., Horak I., Dawid I. B. Propagation of both parental mitochondrial DNAs in rat-human and mouse-human hybrid cells. J Mol Biol. 1973 Dec 15;81(3):285–298. doi: 10.1016/0022-2836(73)90142-3. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Blackler A. W. Maternal and cytoplasmic inheritance of mitochondrial DNA in Xenopus. Dev Biol. 1972 Oct;29(2):152–161. doi: 10.1016/0012-1606(72)90052-8. [DOI] [PubMed] [Google Scholar]
- Francisco J. F., Brown G. G., Simpson M. V. Further studies on types A and B rat mtDNAs: cleavage maps and evidence for cytoplasmic inheritance in mammals. Plasmid. 1979 Jul;2(3):426–436. doi: 10.1016/0147-619x(79)90026-x. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Stroynowski I., Wallace D. C. Characterization of mitochondrial DNA in chloramphenicol-resistant interspecific hybrids and a cybrid. Somatic Cell Genet. 1980 Jul;6(4):543–554. doi: 10.1007/BF01539155. [DOI] [PubMed] [Google Scholar]
- Hayashi J. I., Yonekawa H., Gotoh O., Watanabe J., Tagashira Y. Strictly maternal inheritance of rat mitochondrial DNA. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1032–1038. doi: 10.1016/0006-291x(78)91499-7. [DOI] [PubMed] [Google Scholar]
- Hudgson P., Bradley W. G., Jenkison M. Familial "mitochondrial" myopathy. A myopathy associated with disordered oxidative metabolism in muscle fibres. 1. Clinical, electrophysiological and pathological findings. J Neurol Sci. 1972 Jul;16(3):343–370. doi: 10.1016/0022-510x(72)90197-9. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Jakovcic S., Casey J., Rabinowitz M. Sequence homology between mitochondrial DNAs of different eukaryotes. Biochemistry. 1975 May 20;14(10):2043–2050. doi: 10.1021/bi00681a002. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Pepe G., Bakker H., Holtrop M., Bollen J. E., Van Bruggen E. F., Cantatore P., Terpstra P., Saccone C. The restriction fragment map of rat-liver mitochondrial DNA: a reconsideration. Biochim Biophys Acta. 1977 Sep 20;478(2):128–145. doi: 10.1016/0005-2787(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., de Vos W. M., Bakker H. The heterogeneity of rat-liver mitochondrial DNA. Biochim Biophys Acta. 1978 Jun 22;519(1):269–273. doi: 10.1016/0005-2787(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M. Restriction endonuclease cleavage maps of rat and mouse mitochondrial DNAs. Nucleic Acids Res. 1977;4(5):1291–1299. doi: 10.1093/nar/4.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Reilly J. G., Thomas C. A., Jr Length polymorphisms, restriction site variation, and maternal inheritance of mitochondrial DNA of Drosophila melanogaster. Plasmid. 1980 Mar;3(2):109–115. doi: 10.1016/0147-619x(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsfold M., Park D. C., Pennington R. J. Familial "mitochondrial" myopathy. A myopathy associated with disordered oxidative metabolism in muscle fibres. 2. Biochemical findings. J Neurol Sci. 1973 Jul;19(3):261–274. doi: 10.1016/0022-510x(73)90090-7. [DOI] [PubMed] [Google Scholar]
- de Francesco L., Attardi G. Analysis of sequence homology between human and mouse mitochondrial DNA. J Mol Biol. 1980 May 5;139(1):85–93. doi: 10.1016/0022-2836(80)90117-5. [DOI] [PubMed] [Google Scholar]