Abstract
The response of the actin cytoskeleton to nodulation (Nod) factors secreted by Rhizobium etli has been studied in living root hairs of bean (Phaseolus vulgaris) that were microinjected with fluorescein isothiocyanate-phalloidin. In untreated control cells or cells treated with the inactive chitin oligomer, the actin cytoskeleton was organized into long bundles that were oriented parallel to the long axis of the root hair and extended into the apical zone. Upon exposure to R. etli Nod factors, the filamentous actin became fragmented, as indicated by the appearance of prominent masses of diffuse fluorescence in the apical region of the root hair. These changes in the actin cytoskeleton were rapid, observed as soon as 5 to 10 min after application of the Nod factors. It was interesting that the filamentous actin partially recovered in the continued presence of the Nod factor: by 1 h, long bundles had reformed. However, these cells still contained a significant amount of diffuse fluorescence in the apical zone and in the nuclear area, presumably indicating the presence of short actin filaments. These results indicate that Nod factors alter the organization of actin microfilaments in root hair cells, and this could be a prelude for the formation of infection threads.
Bacterial species of the genera Rhizobium, Bradyrhizobium, and Azorhizobium are gram-negative soil bacteria that infect the roots of leguminous plants, establishing a nitrogen-fixing symbiosis (Brewin, 1991; Hirsch, 1992; Mylona et al., 1995). The interaction of rhizobia and legumes begins with the production and recognition of signal molecules by their respective eukaryotic and prokaryotic symbiotic partners (Fisher and Long, 1992). Early events prior to nodule formation involve the attachment of bacteria to the plant root hairs, root hair curling and deformation, and penetration of the bacteria as they invade the plant root hair cell by a newly formed infection thread. Simultaneously, cortical cells are mitotically activated, giving rise to the nodule primordium. Infection threads grow toward the primordium, and the bacteria are then released into the cytoplasm of the host cells, surrounded by a plant-derived peribacteroid membrane. The nodule primordium then develops into a mature nodule, and the bacteria differentiate into their endosymbiotic form that is capable of nitrogen fixation (for review, see Mylona et al., 1995).
In the first part of the signal exchange, the plant roots secrete flavonoids that lead to the activation of a set of rhizobial genes (the nod genes), which are essential for infection, nodulation, and the control of host specificity. These genes are responsible for the synthesis of LCOs, which are Nod factors that signal back to the plant (for review, see Schultze et al., 1994; Mylona et al., 1995; Dénarié et al., 1996). These Nod metabolites alone can trigger several plant responses implicated in nodule morphogenesis, including alterations in root hair morphology (Lerouge et al., 1990; Spaink et al., 1991; Price et al., 1992; Sanjuan et al., 1992; Schultze et al., 1992; Mergaert et al., 1993; Heidstra et al., 1994), changes in plant gene expression (Horvath et al., 1993; Journet et al., 1994; Cook et al., 1995), cortical cell dedifferentiation and mitosis (Spaink et al., 1991; Truchet et al., 1991; Relic et al., 1993), depolarization of root hair cell membrane potential (Ehrhardt et al., 1992; Felle et al., 1995; Kurkdjian, 1995), and, in some cases, the formation of mature structures resembling authentic nodules (Truchet et al., 1991; Mergaert et al., 1993; Stokkermans and Peters, 1994; Cárdenas et al., 1995). The root hair cells respond to Nod factors with morphological changes such as nuclear migration to the base of the cell and cytoplasmic bridges formed by cortical cells; these become the path of tip growth of the infection thread (Van Brussel et al., 1992).
The cytoskeleton is thought to be important for the initiation, development, and maintenance of the root nodules, as well as for root hair cell growth and polarity (Brewin, 1991; Pérez et al., 1994; Vidali et al., 1995). In the early stages of root nodule morphogenesis, there is evidence that the actin cytoskeleton participates in the formation of preinfection threads and induction of cortical cell divisions (Bakhuizen, 1988). Ridge (1992) showed that the actin becomes fragmented in curled root hairs of Vicia hirsuta infected with Rhizobium spp. Examining alfalfa root hairs, Allen et al. (1994) found that in curled hairs there were some actin foci close to the tip when exposed to the Nod factors; disorganization of the streaming patterns was also observed.
However, both Ridge (1992) and Allen et al. (1994) examined only curled root hairs, which left unanswered the question of the response of the actin cytoskeleton to Nod factors during the initial minutes of exposure, well before root hair deformation. Determination of the earliest responses during the interaction is important because it is during this stage that the Nod signals secreted by Rhizobium spp. can establish the infection process. It is well known that during this stage the Nod factors induce membrane depolarization, ion mobilization, and cytoplasmic alkalinization (Ehrhardt et al., 1992; Felle et al., 1995). These processes could allow the bacteria to initiate a controlled manipulation of the host cytoskeleton and begin the events of infection thread formation.
By microinjecting FITC-phalloidin and examining the cells in the confocal microscope, we were able to visualize the actin cytoskeleton in living root hairs in the presence and absence of Rhizobium etli Nod factors. We found that these Nod factors induce processes that substantially modify the arrangement of actin microfilaments in bean (Phaseolus vulgaris) root hair cells as soon as 5 min after application of Nod factors. These changes are characterized by a dramatic breakdown of the actin bundles during the first minutes and recovery thereafter. Nod factors thus appear to induce rapid changes in the actin cytoskeleton, and these events may be a necessary prelude to the formation of infection threads.
MATERIALS AND METHODS
Plant Growth
Seeds of bean (Phaseolus vulgaris L. bv Negro Jamapa) were surface sterilized, germinated, and grown as previously described (Cárdenas et al., 1995).
Mounting Living Root Hairs
Two-day-old seedlings were placed in liquid medium containing 2 mm CaCl2 and 2.5 mm Mes, pH 6.2. After 8 h root hairs were usually well adapted to the medium. Intact seedlings containing the growing root hairs were mounted in chambers constructed on glass coverslips, forming a well, and were then visualized under the microscope (Diaphot 300, Nikon) with a ×40 water immersion lens with a numerical aperture of 0.75 (Zeiss). No mounting substance was needed and the well was filled with approximately 0.5 mL of the same medium that was replaced every 15 min to maintain the same calcium concentration during the microinjection.
Injection of FITC-Phalloidin
Microneedles were pulled in a vertical pipette puller (model 700D, David Kopf Instruments, Tujunga, CA) from filamented capillaries. FITC-phalloidin (Molecular Probes, Eugene, OR) was prepared as a 20-μm solution in 10% (v/v) methanol. Before use, the FITC-phalloidin was sonicated and centrifuged at 20,000g to remove insoluble particles. The microneedle was back-filled with 1 μL of the dye and microinjected into root hairs by hydraulic pressure. Microinjections were made with the help of a second micromanipulator that held a blunt needle to support the hair cells during impalement. Based on calculations from other plant cell systems, we estimated that there was at least a 100-fold dilution upon injection. Thus, the final phalloidin concentration was at most 200 nm.
Root hairs were treated with either the Rhizobium etli Nod factors or penta-N-acetylchitopentaose (a control factor; see below) prior to each microinjection. The living root hairs were selected before they were loaded with FITC-phalloidin by observing the normal cytoplasmic streaming (average 0.4 μm/s) and tip growth, which we have determined to be 0.4 μm/min. Microinjections were carried out anywhere in the root hair except its tip dome. Microinjected cells were scanned under the confocal microscope (MRC-600, Bio-Rad) and photographed within the 1st min after the dye was loaded to record the response of the actin cytoskeleton immediately.
Incubation of Root Hairs with Nod Factors
R. etli Nod factors were purified by HPLC as previously described (Cárdenas et al., 1995), resuspended in 1% (w/v) Chaps (the nondenaturing, zwitterionic detergent [3-{3-cholamidopropyl}-dimethylammonio]-1-propane-sulfonate) and diluted to 0.01% with the Nod factor at a final concentration of 10−8 m (the optimal concentration to induce root hair deformation). In contrast to alfalfa root hairs, which induce nodule-like structures when provided with 10−10 m Nod factor (Ehrhardt et al., 1996), bean root hairs require concentrations that are 10−8 m or higher (Martinez et al., 1993, 1995; Cárdenas et al., 1995) for an equivalent response.
Before application, the Nod factors were mixed with 0.5 mL of 2 mm CaCl2 and 2.5 mm Mes, pH 6.2, and were then added gently to the growing root hairs to replace the Nod-factor-free medium. As a negative control we used 10−7 m penta-N-acetylchitopentaose (Seikagaku America, St. Petersburg, FL) dissolved in Chaps and under the same conditions as the Nod factors. Even at a 10-fold higher concentration than the Nod factors, the control chitin oligomer failed to induce an actin or cytoplasmic response.
RESULTS
To determine the organization of actin microfilaments in P. vulgaris root hair cells, we microinjected FITC-phalloidin into the cytoplasm of individual living root hairs. FITC-phalloidin specifically stains F-actin microfilaments and permits their visualization by confocal microscopy (Zhang et al., 1993; Miller et al., 1996) (Fig. 1). Because phalloidin eventually becomes toxic to the cell, presumably through its stabilization of F-actin, all images were taken within 1 min from the time of microinjection. At this time the root hairs appeared normal: vigorous cytoplasmic streaming (approximately 0.4 μm/s) and normal cytoplasmic morphology and growth rates (approximately 0.4 μm/min) were observed.
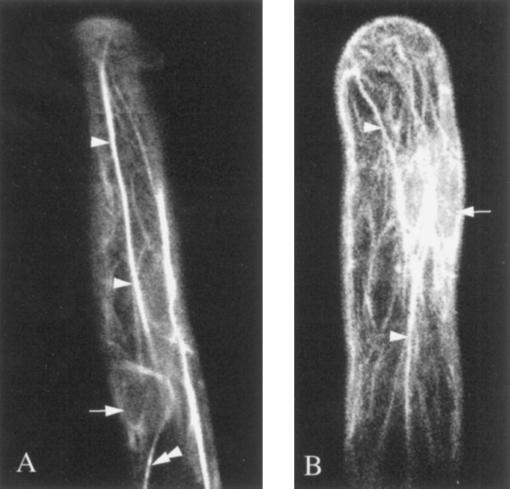
Figure 1.
Organization of actin microfilaments in the root hair cells of P. vulgaris in the absence of Nod factors (A) and in the presence of penta-N-acetylchitopentaose (B). FITC-phalloidin was microinjected into both cells and the outcome was visualized by confocal microscopy. Both A and B show large actin bundles running along the root hair (arrowheads) from the base to the tip, and microfilaments surrounding the nucleus are also observed (double arrowheads). A, Arrow indicates the nuclear area, and the double arrowhead indicates one of the large actin bundles surrounding the nucleus. B, Superimposition of 21 images acquired every 1 μm along the z axis.
With this procedure the cells were first treated with Nod factors or chitin oligomers and then with FITC-phalloidin microinjection at appropriate time intervals. Thereafter, images were acquired usually within 1 min and certainly well before cytoplasmic streaming had stopped, which generally occurred after 10 min. The efficiency of the microinjections was approximately 20%; those that were unsuccessful, e.g. the cell was injured or the FITC-phalloidin was delivered to the vacuole, were easily detected by a clearly noncytoplasmic distribution of the fluorescence (data not shown). To reduce damage, we microinjected into the shank of the hair, as shown in Figure 3D (see “n”).
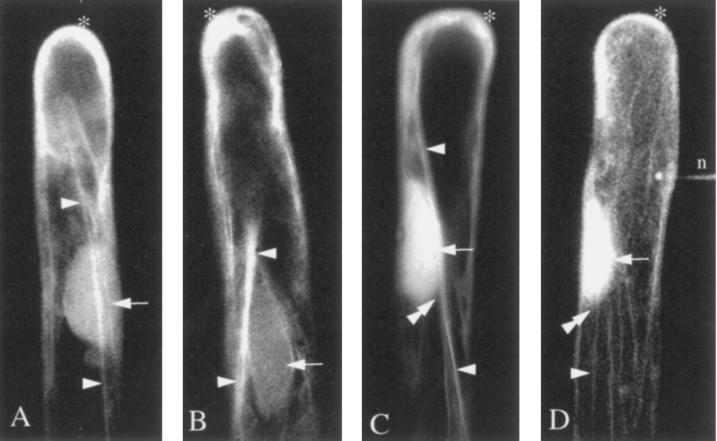
Figure 3.
Actin cytoskeleton visualized 30 to 60 min after Nod factor treatment on four independent hairs. The actin microfilaments have partially recovered to form large bundles running along the length of the hair (arrowheads). Fragmented microfilaments are still accumulated at the tip (asterisks). The bright, diffuse staining around the nucleus (arrows) suggests the presence of numerous short microfilament fragments that appear to be associated with long microfilament bundles (see C and D for detail). D, Superimposition of 21 images acquired every 1 μm. n, The site of microinjection.
The distribution of actin microfilaments can be seen clearly in the cells that had received the injections. In untreated cells lacking Nod factors (Fig. 1A) or in the presence of the control factor penta-N-acetylchitopentaosa (Fig. 1B), bundles of microfilaments were observed running longitudinally from the base to the tip of the root hair cell (Fig. 1A, arrowheads) in a path that resembled the transvacuolar strands. Sometimes these bundles were quite thick, as depicted in Figure 1A (arrowheads), but more often they were thin and flexible, as indicated by their curved profiles.
Microfilaments surrounding the nucleus were also observed (Fig. 1A, arrow); these were usually thin but well defined, and in some instances they seemed to be continuous with the thicker elements (Fig. 1A, double arrowhead). Figure 1B is a sequence of 21 confocal images acquired at 1-μm intervals in the z axis and reconstructed to show the complete actin network close to the tip and the actin filaments around the nucleus (arrow). This projection image emphasizes the complexity of the actin microfilament system and reveals how it permeates all of the cytoplasmic domains of the root hair.
The Nod factors used in the present study, which had been previously isolated from R. etli strain CE3 and characterized by MS (Cárdenas et al., 1995), are N-acetylglucosamine pentasaccharides in which the nonreducing residue is N-methylated and N-acylated with cis-vaccenic acid (C18:1) or stearic acid (C18:0), and carries a carbamoyl group at C4. The reducing end is substituted at the C6 position with O-acetylfucose. Analysis of their biological activity on the host plant P. vulgaris showed that these LCOs at a concentration of 10−7 m elicited the formation of nodule primordia, which developed to the stage at which vascular bundles are formed (Cárdenas et al., 1995). In this study, the optimal concentration to induce root hair deformation was 10−8 m; a higher concentration gives the same results with regard to the root hair response, and a lower concentration results in a diminished effect.
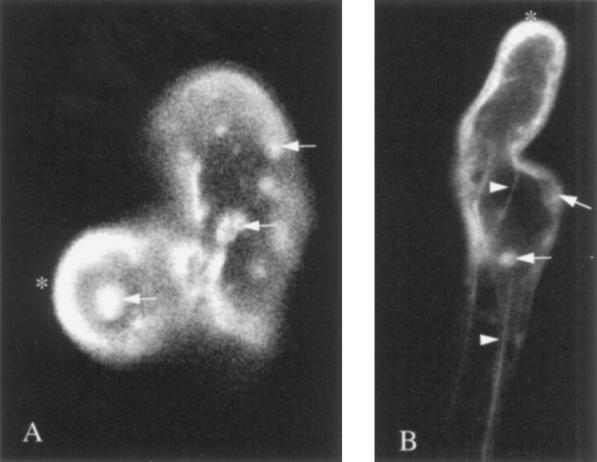
To test the effect of the R. etli Nod factors on the structure and organization of the actin microfilaments in living root hair cells of P. vulgaris, we microinjected FITC-phalloidin into root hairs that had already been exposed to these factors at a concentration of 10−8 m. During the first 5 to 10 min after the application of Nod factors, there appeared to be a rapid breakdown of the microfilament bundles, as evidenced by a reduction in their number and length, concomitantly with a marked increase in diffuse fluorescence toward the apex of the root hair (Fig. 2, asterisks). Because phalloidin does not bind actin monomers, we attribute this high level of diffuse fluorescence to the presence of very short filaments of actin. Longer bundles of microfilaments are evident farther back from the apex, but these are few in number and usually quite thin.
Figure 2.
Actin cytoskeleton stained as in Figure 1 but after 5- to 15-min treatment with Nod factors at a final concentration of 10−8 m on three independent root hairs (A, B, and C). Toward the tip of the root hair the long bundles of actin microfilaments are fragmented, which accounts for the high level of diffuse fluorescence (asterisks). Large bundles of microfilaments are also observed (arrowheads), but these are reduced in size, number, and extent compared with the controls.
After 1 h of Nod factor application, the structure of the cytoskeleton partially recovered, as evidenced by the reappearance of long actin microfilament bundles running from the base to near the tip of the root hair cell. However, the recovering cells were different from the initial controls in that they still possessed a high level of fluorescence at the tip (Fig. 3, asterisks). It was interesting that at this time the nucleus became brighter compared with controls (Fig. 3, arrows), suggesting that there had been an increase of short actin filaments around it.
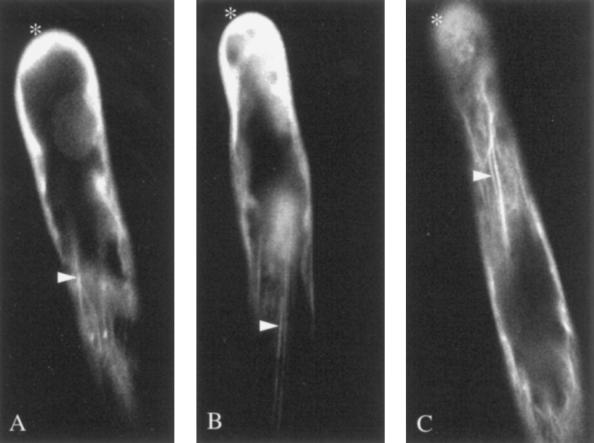
After 4 h of exposure to Nod factors, and when curling of the root hair had become evident, we noted the appearance of punctate foci of fluorescence in the apical region (Fig. 4, arrows). The elevated level of diffuse fluorescence also persisted at the tip (Fig. 4, asterisks). At this time the actin cytoskeleton was evident as long bundles running along the root hair cell (Fig. 4B, arrowheads).
Figure 4.
Two different curled root hairs after 4- to 6-h treatment (A and B) with Nod factor. The actin cytoskeleton is present as long actin bundles that run along the hair (arrowheads), some short actin microfilaments at the tip remain (asterisks), and there are punctate foci of actin evident at the tip region and scattered around the root hair (arrows).
Because the Nod factors are structurally related to chitin-oligosaccharide molecules, which have biological activity on some plant cells (Boller, 1995), it is possible that they were degraded to chitin fragments at the plant surface and that these chitin fragments may be the active molecules stimulating the actin reorganization. To rule out this possibility, phalloidin was microinjected in the presence of 10−7 m penta-N-acetylchitopentaose, an inactive analog of the active LCOs, into root hair cells (Fig. 1B). As when Nod factors were absent, no changes were found in the organization of actin microfilaments.
DISCUSSION
Our results show that there is a dramatic change in the actin cytoskeleton in response to Nod factors. These responses were detected after 5 to 10 min of exposure to Nod factors and were well characterized by a rapid breakdown of the actin bundles. The most dramatic effect was detected at the tip, which was visualized as a region of abundant fluorescence, suggesting the presence of short actin filaments. It was interesting that during the continued exposure to Nod factors, bundles of actin microfilaments reappeared in the shank of the root hair. However, a zone of short filaments remained at the tip.
It was proposed previously that the actin cytoskeleton could have a pivotal role during the establishment of the interaction between Rhizobium sp. and the legume plant. Ridge (1992), using Rhizobium sp. bacteria to elicit a response, showed a diffuse fluorescence in the deformed region of curled root hairs of Vicia; no actin bundles were evident in the treated hairs. He proposed that this area is a region where the actin could be fragmented in response to the presence of the bacteria. In a further study, Allen et al. (1994), using the purified Nod factors from Rhizobium meliloti, showed the presence of actin foci in curled root hairs of alfalfa. These actin foci were not detected by Ridge (1992), perhaps because of the different approaches used in each study, with bacteria in one (Ridge, 1992) and Nod factor in the other (Allen et al., 1994). However, these studies did not address the question of the response of actin cytoskeleton at the beginning of the infection process by Rhizobium spp. Thus, the results herein reveal for the first time, to our knowledge, that the effects of Nod factors are both dramatic in magnitude and fast, being evident within 5 to 10 min. In addition, it is important to note that the images shown in this study were obtained from living cells and do not contain artifacts arising from chemical fixation procedures.
We conclude that R. etli Nod factors induce substantial alterations to the architecture of actin microfilaments in the root hair cells of the host plant. The root hair cells that are most susceptible to Rhizobium sp.-induced deformation and infection thread development are those that are rapidly expanding (Bhuvaneswari et al., 1981). This suggests that the inward growth of infection threads may involve a reorganization of the normal processes of cell growth. It has been proposed that attached rhizobia incite a local stimulation in the rate of plant cell wall expansion (Callaham and Torrey, 1981; Ridge and Rolfe, 1985; Van Batenburg et al., 1986; Van Spronsen et al., 1994).
Cytological examination of the apical growing tip of the uninfected root hair revealed cytoskeletal connections between the nucleus and the growing root hair tip (Lloyd et al., 1987). It is known, for instance, that the infection process uncouples the nucleus from the tip and that the uncoupled nucleus guides the infection thread toward the base of the cell (Fahraeus, 1957; Nutman, 1959). Presumably, the initiation of an inwardly growing infection thread is brought about by a reorganization of the cytoskeleton that targets cytoplasmic vesicles containing wall components to the growing point of the cell (Brewin, 1991) and regulates the nucleus-to-tip distance (Lloyd et al., 1987). In this respect, it is interesting to note that the nucleus is usually found surrounded by microfilaments, which may be necessary for the subsequent movement of the nucleus and the advancing infection thread.
Many bacteria have the capacity to enter and live within eukaryotic cells by triggering the host's signal transduction mechanism, which usually involves kinases or messengers such as calcium and inositol phosphates (Rosenshine and Finlay, 1993). These induce rearrangements of the host cytoskeleton, thereby facilitating bacteria uptake (Theriot, 1995). It is reasonable to suggest that Rhizobium spp. and other nodulating bacteria possess these same characteristics.
It has been proposed that the increases in the intracellular level of calcium triggered by Nod factors could participate in the modulation of the actin cytoskeleton (Sánchez et al., 1991; Allen et al., 1994). Specifically, it seems plausible that the appearance of the short filaments soon after the application of Nod factor is due to a calcium elevation. In support of this proposal is the observation that alfalfa root hairs exhibit oscillatory increases in intracellular calcium in response to Nod factors (Ehrhardt et al., 1996). Furthermore, in tip-growing pollen tubes, elevated levels of calcium induce fragmentation of F-actin (Kohno and Shimmen, 1987) and cause an arrest of cytoplasmic streaming (Kohno and Shimmen, 1988). Cytosolic alkalinization (Felle et al., 1996) and membrane depolarization (Ehrhardt et al., 1992; Felle et al., 1995) in root hairs exposed to Nod factor have been reported. Although their role in the infection process remains to be elucidated, it is possible that they affect the structure and distribution of actin. Note, for example, the pronounced effect that elevated pH has on the remodeling of actin in Dictyostelium sp. (Edmonds et al., 1995).
Experiments are in progress to investigate the factors that trigger the actin cytoskeleton response and to correlate them with effects on cytoplasmic streaming and other cellular processes. Although the detailed processes are not yet established, it seems likely that the dramatic reorganization of the actin cytoskeleton depicted in the present study participates in a fundamental way in controlling the apical root hair deformation and curling that precede bacterial infection and Nod.
ACKNOWLEDGMENTS
The authors are grateful to Drs. B. Barkla, G. Cassab, O. Pantoja, and M.A. Villanueva for critical reading of the manuscript. We also thank the central microscopy facility at the University of Massachusetts, Amherst, for the use of the confocal microscope.
Abbreviations:
- F-actin
filamentous actin
- FITC
fluorescein isothiocyanate
- LCO
lipochitin-oligosaccharide
- Nod
nodulation
Footnotes
This research was supported by grants from Dirección General de Asuntos del Personal Académico/Universidad Nacional Autónoma de México (DGAPA-UNAM; nos. IN202595 and IN200196) and Consejo Nacional de Ciencia y Tecnológia (CONACYT), México (no. N-9608 to C.Q.), and by grants from the National Science Foundation (no. MCB-9601087 to P.K.H.; no. BBS-8714235 to the Microscopy Facility, University of Massachusetts, Amherst). L.C. and L.V. were supported by fellowships from CONACYT and DGAPA-UNAM, respectively.
LITERATURE CITED
- Allen NS, Bennett MN, Cox DN, Shipley A, Ehrhardt DW, Long SR. Effects of Nod factors on alfalfa root hair Ca++ and H+ currents and on cytoskeletal behavior. In: Daniels MJ, Downie JA, Osbourn AE, editors. Advances in Molecular Genetics of Plant-Microbe Interactions, Vol 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 107–113. [Google Scholar]
- Bakhuizen R (1988) The plant cytoskeleton in the Rhizobium-legume symbiosis. PhD thesis. Leiden University, The Netherlands
- Bhuvaneswari TV, Bhagwat AA, Bauer WD. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981;68:1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Brewin NJ. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Callaham DA, Torrey JG. The structural basis for infection of root hairs of Trifoliumrepens by Rhizobium. Can J Bot. 1981;59:1647–1664. [Google Scholar]
- Cárdenas L, Domínguez J, Quinto C, López-Lara IM, Lugtenberg BJJ, Spaink HP, Rademaker GJ, Haverkamp J, Thomas-Oates JE. Isolation, chemical structures and biological activity of the lipo-chitin oligosaccharide nodulation signals from Rhizobium etli. Plant Mol Biol. 1995;29:453–464. doi: 10.1007/BF00020977. [DOI] [PubMed] [Google Scholar]
- Cook D, Dreyer D, Bonnet D, Howell M, Nony E, VandenBosch K. Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell. 1995;7:43–55. doi: 10.1105/tpc.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénarié J, Debellé F, Promé JC. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu Rev Biochem. 1996;65:503–535. doi: 10.1146/annurev.bi.65.070196.002443. [DOI] [PubMed] [Google Scholar]
- Edmonds BT, Murray J, Condeelis J. pH regulation of the F-actin binding properties of Dictyostelium elongation factor 1a. J Biol Chem. 1995;270:15222–15230. doi: 10.1074/jbc.270.25.15222. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Fahraeus G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957;16:374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Nod signal-induced plasma membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipochitooligosaccharide. Plant J. 1995;7:939–947. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- Fisher R, Long SR. Rhizobium-plant signal exchange. Nature. 1992;357:655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Heidstra R, Geurts R, Franssen H, Spaink HP, Van Kammen A, Bisseling T. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 1994;105:787–797. doi: 10.1104/pp.105.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. Developmental biology of legume nodulation. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Horvath B, Hedistra R, Lados M, Moerman M, Spaink HP, Promé JC, Van Kammen A, Bisseling T. Lipooligosaccharides of Rhizobium induce infection-related early nodulin gene expression in pea root hairs. Plant J. 1993;4:727–733. doi: 10.1046/j.1365-313x.1993.04040727.x. [DOI] [PubMed] [Google Scholar]
- Journet EP, Pichon M, Dedieu A, De Billy F, Truchet G, Barker DG. Rhizobium meliloti Nod factors elicit cell specific transcription of the ENOD12 gene in transgenic alfalfa. Plant J. 1994;6:241–249. doi: 10.1046/j.1365-313x.1994.6020241.x. [DOI] [PubMed] [Google Scholar]
- Kohno T, Shimmen T. Ca2+-induced fragmentation of actin filaments in pollen tubes. Protoplasma. 1987;141:177–179. [Google Scholar]
- Kohno T, Shimmen T. Mechanism of Ca2+ inhibition of cytoplasmic streaming in lily pollen tubes. J Cell Sci. 1988;91:501–509. [Google Scholar]
- Kurkdjian AC. Role of the differentiation of root epidermal cells in Nod factor from Rhizobium meliloti-induced root-hair depolarization of Medicago sativa. Plant Physiol. 1995;107:783–790. doi: 10.1104/pp.107.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénairé J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990;344:781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Lloyd CW, Pearce KJ, Rawlins DJ, Ridge RW, Shaw PJ. Endoplasmic microtubules connect the advancing nucleus to the tip of legume root hairs, but F-actin is involved in basipetal migration. Cell Motil Cytoskeleton. 1987;8:27–36. [Google Scholar]
- Martinez E, Laeremans T, Poupot R, Rogel MA, López L, Garcia F, Vanderleyden J, Prome JC, Lara F. Nod metabolites and other compounds excreted by Rhizobium spp. In: Tikhonovich IA, Provorov NA, Romanov VI, Newton WE, editors. Nitrogen Fixation: Fundamentals and Applications. Proceedings of the 10th International Congress on Nitrogen Fixation, St. Petersburg, Russia, May 28–June 3, 1995. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. [Google Scholar]
- Martinez E, Poupot R, Prome JC, Pardo MA, Segobia L, Truchet G, Denarie J. Chemical signaling of Rhizobium nodulating bean. In: Palacios R, Mora J, Newton WE, editors. New Horizons in Nitrogen Fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 171–175. [Google Scholar]
- Mergaert P, Van Montagu M, Promé JC, Holsters M. Three unusual modifications, a d-arabinosyl, an N-methyl, and a carbamoyl group are present on Nod factors of Azorhizobium caulinodans strain ORS571. Proc Natl Acad Sci USA. 1993;90:1551–1555. doi: 10.1073/pnas.90.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, Lancelle SA, Hepler PK. Actin microfilaments do not form a dense meshwork in Lilium longiflorum pollen tube tips. Protoplasma. 1996;195:123–132. [Google Scholar]
- Mylona P, Pawlowski K, Bisseling T. Symbiotic nitrogen fixation. Plant Cell. 1995;7:869–885. doi: 10.1105/tpc.7.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman PS. Some observations on root-hair infection by nodule bacteria. J Exp Bot. 1959;10:250–263. [Google Scholar]
- Pérez HE, Sánchez N, Vidali L, Hernández JM, Lara M, Sánchez F. Actin isoforms in non-infected roots and symbiotic nodules of Phaseolus vulgaris L. Planta. 1994;193:51–56. [Google Scholar]
- Price NPJ, Relic B, Talmont F, Lewin A, Promé D, Pueppke SG, Maillet F, Dénairé J, Promé JC, Broughton WJ. Broad-host range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol Microbiol. 1992;6:3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Relic B, Talmont F, Kopcinska J, Golinowski W, Promé JC, Broughton WJ. Biological activity of Rhizobium sp. NGR234 Nod-factors on Macroptilium atropurpureum. Mol Plant-Microbe Interact. 1993;6:764–774. doi: 10.1094/mpmi-6-764. [DOI] [PubMed] [Google Scholar]
- Ridge RW. A model of legume root hair growth and Rhizobium infection. Symbiosis. 1992;14:359–373. [Google Scholar]
- Ridge RW, Rolfe BG. Rhizobium sp. degradation of legume root hair cell wall at the site of infection thread origin. Appl Environ Microbiol. 1985;50:717–720. doi: 10.1128/aem.50.3.717-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I, Finlay BB. Exploitation of host signal transduction pathways and cytoskeletal functions by invasive bacteria. Bioassays. 1993;15:17–24. doi: 10.1002/bies.950150104. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Padilla JE, Pérez HE, Lara M. Control of nodulin genes in root-nodule development and metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:507–528. [Google Scholar]
- Sanjuan J, Carlson RW, Spaink HP, Bhat R, Barbour WM, Glushka J, Stacey G. A 2-O-methylfucose moiety is present in the lipo-oligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc Natl Acad Sci USA. 1992;89:8789–8793. doi: 10.1073/pnas.89.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Kondorosi E, Ratet P, Buiré M, Kondorosi A. Cell and molecular biology of Rhizobium-plant interactions. Int Rev Cytol. 1994;156:1–75. [Google Scholar]
- Schultze M, Quiclet-Sire B, Kondorosi E, Virelizier H, Glushka JN, Endre G, Géro SD, Kondorosi A. Rhizobium meliloti produces a family of sulphated lipo-oligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci USA. 1992;89:192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink HP, Sheeley DM, Van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991;354:125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Stokkermans TJW, Peters NK. Bradyrhizobium elkanii lipooligosaccharide signal induces complete nodule structures on Glycine soja Siebold et Zucc. Planta. 1994;193:413–420. doi: 10.1007/BF00201821. [DOI] [PubMed] [Google Scholar]
- Theriot J. The cell biology of infection by intracellular bacterial pathogen. Annu Rev Cell Dev Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F, Promé JC, Dénarié J. Sulphated lipooligosaccharide signals from Rhizobium meliloti elicit nodule organogenesis in alfalfa. Nature. 1991;351:670–673. [Google Scholar]
- Van Batenburg FHD, Jonker R, Kijne JW. Rhizobium induces marked root hair curling by redirection of tip growth: a computer simulation. Physiol Plant. 1986;66:476–480. [Google Scholar]
- Van Brussel AAN, Bakhuizen R, Van Spronsen PC, Spaink HP, Tak T, Lugtenberg BJJ, Kijne JW. Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipo-oligosaccharides of Rhizobium. Science. 1992;257:70–72. doi: 10.1126/science.257.5066.70. [DOI] [PubMed] [Google Scholar]
- Van Spronsen PC, Bakhuisen R, Van Brussel AAN, Kijne JW. Cell wall degradation during infection thread formation by the root nodule bacterium Rhizobium leguminosarum is a two-step process. Eur J Cell Biol. 1994;64:88–94. [PubMed] [Google Scholar]
- Vidali L, Pérez HE, Valdés VV, Noguez R, Zamudio F, Sánchez F. Plant Physiol. 1995;108:115–123. doi: 10.1104/pp.108.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Wadsworth P, Hepler PK. Dynamics of microfilaments are similar, but distinct from microtubules during cytokinesis in living, dividing plant cells. Cell Motil Cytoskeleton. 1993;24:151–155. [Google Scholar]