Abstract
CosR is an essential response regulator in Campylobacter jejuni, a major food-borne pathogen causing enteritis worldwide. A transcriptomic analysis performed in this study discovered 93 genes whose transcriptional levels were changed >2-fold due to the repression of CosR expression by antisense peptide nucleic acid. The identified CosR-regulated genes are involved in various cellular functions, such as energy production, protein synthesis and folding, flagellum biogenesis, and lipid metabolism. Interestingly, 17 of the 93 CosR-regulated genes (18.3%) are predicted essential genes, indicating that CosR may participate in the regulation of vital biological processes in C. jejuni. In particular, CosR knockdown increased the transcriptional levels of cmeA, cmeB, and cmeC genes, whose protein product (CmeABC) is an important determinant conferring multidrug resistance in Campylobacter. Negative regulation of cmeABC by CosR was verified by quantitative real-time PCR (qRT-PCR) and PcmeABC::lacZ assay. The results of electrophoretic mobility shift assays (EMSAs) and DNase I footprinting assays demonstrated that CosR directly binds to the cmeABC promoter. Another notable finding is that CosR regulates the transcription of katA, the sole catalase gene in C. jejuni. Further characterization with qRT-PCR, the catalase enzyme assay, EMSA, and DNase I footprinting assays successfully demonstrated that CosR affects the katA transcription and the catalase activity by direct interactions with the katA promoter. The findings in this study clearly demonstrated that CosR regulates resistance mechanisms in C. jejuni by controlling the expression of genes involved in oxidative stress defense and extrusion of toxic compounds out of the cell.
INTRODUCTION
Campylobacter jejuni is a leading bacterial cause of human gastroenteritis worldwide. The symptoms of campylobacteriosis include severe abdominal cramp, watery or bloody diarrhea, and in some rare cases Guillain-Barré syndrome as a postinfection complication (21). Although C. jejuni infection is usually self-limiting and doesn't require antibiotic treatment, antibiotics are warranted for severe cases of campylobacteriosis (30). However, the increasing prevalence of Campylobacter resistant to medically important antibiotics threatens public health. Among multiple mechanisms of antibiotic resistance, drug efflux pumps are considered the major cause of multidrug resistance in pathogenic bacteria (23). In Campylobacter, a few multidrug efflux pumps have been identified (1, 17, 27), and CmeABC is considered the most important multidrug efflux pump in this pathogenic bacterium, giving resistance to different classes of antibiotics and bile salts (27).
Aerobiosis inevitably produces reactive oxygen species (ROS), such as the superoxide anion (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (HO•) as by-products, and ROS can damage intracellular materials (16). Various defense and regulatory mechanisms exist to protect the cell from oxidative stress. C. jejuni contains only a single type of catalase (KatA) and superoxide dismutase (SodB) (2), whereas Escherichia coli harbors two types of catalase (KatG and KatE) and three kinds of superoxide dismutase (SodA, SodB, and SodC) (6). In enterobacteria, such as E. coli and Salmonella, oxidative stress response is regulated by the SoxRS and OxyR transcription factors, which sense superoxide and peroxide stresses, respectively (6, 16). Exposure to redox-cycling drugs activates SoxR by the oxidation of [2Fe-2S] centers, and the activated SoxR stimulates the expression of SoxS, which then upregulates oxidative stress defense genes (13, 24, 46). OxyR controls the expression of about 40 genes mostly involved in peroxide stress resistance (16). Neither the oxyR nor the soxRS homologue has been found in the genome sequence of C. jejuni (34). Notably, C. jejuni is the first Gram-negative bacterium which has been reported to harbor PerR, a non-OxyR-dependent regulatory system to control peroxide stress genes (42). The C. jejuni PerR regulates the peroxide stress genes including katA (catalase) and ahpC (alkyl hydroperoxide reductase); thus, inactivation of PerR renders C. jejuni hyper-resistant to hydrogen peroxide due to derepression of peroxide stress genes (42).
A recent proteomics study done by Garénaux et al. demonstrated that the protein level of CosR (Cj0355c) is significantly reduced by exposure to paraquat, a superoxide-generating drug, suggesting that CosR may be associated with the regulation of oxidative stress response in C. jejuni (9). CosR is an OmpR-type response regulator and essential for the viability of C. jejuni (9, 35). CosR homologues are found mostly in epsilonproteobacteria, such as Campylobacter, Helicobacter, and Wolinella (15), and the CosR homologue in Helicobacter pylori (HP1043) is also essential for bacterial viability (4). This suggests a common critical role played by CosR and its homologues in sustaining bacterial viability. In our previous study, we overcame the lethality problem resulting from a knockout mutation of the essential gene cosR by using antisense peptide nucleic acid (PNA), which was designed to specifically inhibit CosR, and demonstrated that CosR regulates the expression of oxidative stress proteins, such as SodB, Dps, and AhpC (15).
To obtain a better understanding of the CosR regulon at the transcriptomic level, in the present study, we performed an extensive transcriptomic analysis with a DNA microarray and report that CosR regulates expression of key determinants of stress resistance in C. jejuni, including the CmeABC multidrug efflux pump and the KatA catalase.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
C. jejuni NCTC 11168 was used in the present study. A CosR-overexpression strain was constructed by integrating cosR and a chloramphenicol cassette into the chromosome according to the method described in our previous study (15). C. jejuni strains were grown at 42°C on Mueller-Hinton (MH) media (Difco) in a microaerobic condition generated by Anoxomat (Mart Microbiology BV, Lichtenvoorde, Netherlands). Kanamycin (50 μg ml−1) or chloramphenicol (10 μg ml−1) was occasionally added to culture media where required. Broth cultures were microaerobically grown with shaking at 180 rpm.
Preparation and treatment of CosR-specific PNA (CosR-PNA).
PNAs used in this study were commercially synthesized by Panagene (Daejeon, Korea). The design and use of CosR-PNA was reported in our previous study (15). Based on the genome sequence of C. jejuni NCTC 11168 (34), briefly, a 16-mer PNA (CATTTGTTCTATCCTT) was designed to reverse complementarily bind to the leader sequence spanning the ribosomal binding site and the start codon of cosR, and conjugated with the permeabilizing oligopeptide (KFFKFFKFFK) (10). The optical density of bacterial culture was adjusted to ∼0.07 at 600 nm (∼107 CFU ml−1), and CosR-PNA (1.5 μM) was added to the bacterial cultures. C. jejuni was grown for 8 h in the culture conditions described above.
Transcriptomic analysis. (i) Preparation of total RNA for a DNA microarray.
C. jejuni cells were grown at 42°C in MH broth to the mid-exponential phase for ∼8 h with shaking, and the culture media were supplemented with 1.5 μM CosR-PNA for CosR-knockdown. The total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions from three independent bacterial cultures. After DNase treatment with a Turbo DNA-free kit (Ambion), the total RNA was purified using acid-phenol solution. The integrity of purified total RNA was measured with a Bioanalyzer 2100 RNA Nano kit (Agilent Technologies, USA).
(ii) cDNA synthesis and microarray hybridization.
The synthesis of target cDNA probes and hybridization were performed as described previously (47). RNA samples (30 μg) were reverse transcribed using random primers and Superscript II kit (Invitrogen, USA) according to the manufacturer's instructions. After purification, labeling reactions were performed with a Bioprime labeling kit (Invitrogen) in a volume of 50 μl with a modified deoxynucleoside triphosphate pool containing 120 μM (each) dATP, dGTP, and dCTP; 60 μM dTTP; and 60 μM Cy5-dUTP (for CosR knockdown) or Cy3-dUTP (for the wild type). Labeled targets were subsequently purified by using a Qiaquick PCR cleanup kit (Qiagen). Labeled cDNAs were mixed together and hybridized to an assembled C. jejuni subsp. jejuni NCTC 11168 custom 3× 15K microarray slide with 97% coverage of the genome (MYcroarray, Ann Arbor, MI) at 50°C for 16 h using a hybridization oven (Agilent). The hybridized microarrays were washed according to the MYcroarray washing protocol. Finally, microarrays were spin dried and stored in the dark until scanned.
(iii) Image acquisition and analysis.
The hybridization images were analyzed by GenePix Pro 6.0 (Axon Instruments, CA). The average fluorescence intensity for each spot was calculated, and the local background was subtracted. All data normalization and selection of fold-changed genes were performed using GeneSpring 7.3.1 (Agilent). Intensity-dependent normalization (Lowess) was performed, where the ratio was reduced to the residual of the Lowess fit of the intensity-versus-ratio curve. The averages of normalized ratios were calculated by dividing the average of normalized signal channel intensity by the average of normalized control channel intensity. The entire set of microarray data was deposited to Gene Expression Omnibus (GEO) with the accession numbers GSE40201 and GPL15955.
Quantitative real-time PCR (qRT-PCR).
Total RNA was isolated with the RNeasy minikit (Qiagen) from C. jejuni cultures incubated as mentioned above in transcriptional analysis. After the removal of residual DNA contamination from the total RNA solution using the Turbo DNA-free kit (Ambion), cDNA was synthesized using the Omniscript RT kit (Qiagen) and random hexamers (Invitrogen). Quantification of cDNA was carried out using IQ SYBR green PCR Supermix (Bio-Rad), and real-time amplification of PCR product was analyzed using an iCycler optical module (Bio-Rad). The amplification program consisted of one cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, 50°C for 30 s, and 72°C for 30 s. The mRNA levels of each gene were normalized to the expression level of Cjr01 encoding 16S rRNA. The relative amount of cDNA was calculated according to the 2−ΔΔCT method (29). The sequences of primers for the expression of katA, cmeABC, and Cjr01 are presented in Table 1.
Table 1.
Primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| Amplification and EMSA | |
| cmeA_PF_F(BamH) | AAATGTTTTTCTAAATGGATCCAATAGCTCC |
| cmeA_PF_R(Xba) | AGTAAAAGCACAACATCTAGAGCTAAAATAG |
| katA_PF_F(Xba) | TAAAACAGCTCTAGAAGGAGTGATTTC |
| katA_PF_R(Xba) | TGAATTTTGGTTATCATCTAGAATGTTTCC |
| katA_ESIC_F | CGCAGGCGCAAAAGGACCTTTAC |
| katA_ESIC_R | TCAGGGAATTTATAAGCATCGCGGATG |
| cmeA_ES_F | ATGAAAAAAAATGCAGAAAAACTTGCTGTTC |
| cmeA_ES_R | TATTTTTGGTGCTTCTTCTTTGCTGC |
| cmeA_ESIC_F | CTGTAACAACCATGAGTGCTAAATCTGAAG |
| cmeA_ESIC_R | TAGACTAGCTTTTGAATTGTTAAATGTAGCAAG |
| qRT-PCR | |
| katA_F | GCTTGAAAAACTTGCTCATC |
| katA_R | TTTGGTATAAGCACTTAAGTC |
| cmeABC_F | GCTTTAGGTGTTGTGCTTTT |
| cmeABC_R | ATGGTTGTTACAGGTTGAGG |
| Cjr01_F | TCGAACGATGAAGCTTTTAG |
| Cjr01_R | TTGTCCTCTTGTGTAGGG |
Underlining indicates the enzyme recognition sites.
Measurement of catalase activity.
The catalase activity was measured with a method based on the development of colorimetric precipitation as described previously (44). After separation of protein samples on an 8% polyacrylamide gel under native conditions, the gel was washed in three changes of distilled water for a total of about 45 min. The gel was then transferred and immersed in 0.003% H2O2 solution on a rotating turntable for 10 min at room temperature in the dark. The H2O2 solution was removed, and the gel was briefly rinsed with distilled water and placed in the negative staining solution containing 2% ferric chloride and 2% potassium ferric cyanide with gentle rocking over a light until the gel turned uniformly greenish blue due to the precipitation of ferric chloride and potassium ferric cyanide mediated by H2O2.
PcmeABC::lacZ fusion assay.
The promoter and partial coding region of cmeABC were amplified with the primers cmeA_PF_F(BamHI) and cmeA_PF_R(XbaI) (Table 1) and cloned into pMW10 (45), which contains the promoterless lacZ gene. The plasmid was mobilized to C. jejuni by conjugation, and β-galactosidase assays were performed as described previously (45).
EMSA.
Electrophoretic mobility shift assay (EMSA) was performed as described previously (15). rCosR was produced in E. coli BL21(DE3) with pET15b::cosR and purified under the native conditions using Ni2+ affinity chromatography (15). The DNA fragments containing the promoter region of katA and cmeA were amplified by PCR with the primer pairs katA_PF_F(Xba)/katA_PF_R(Xba) and cmeA_ES_F/cmeA_ES_R, respectively (Table 1). The PCR products were purified from an agarose gel using a gel extraction kit (Qiagen) and labeled with [γ-32P]ATP (GE Healthcare). After the elimination of the unincorporated radioisotope using a MicroSpinTMG-25 column (GE Healthcare), the 0.2 nM 32P-labeled DNA probe was incubated with the purified rCosR protein at different concentrations (0, 0.8, 1.6, 2.4, and 3.2 nM) at 37°C for 15 min in 10 μl of the gel-shift assay buffer [20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 5 mM dithiothreitol, 0.2% Tween 20, 30 mM KCl, 0.1 μg of poly(dI-dC)]. For the competitive EMSA, unlabeled target DNA probes and internal coding regions of each gene were prepared by gel extraction after PCR amplification with the specific primer pairs listed in Table 1. The reaction mixtures were resolved in a 6% polyacrylamide gel, and the radiolabeled DNA fragments were visualized using the BAS2500 system (Fuji Film).
DNase I footprinting assay.
DNase I footprinting assays were performed as described elsewhere (8). Briefly, DNA fragments containing the katA and cmeABC promoter region were amplified by PCR using a 32P-labeled primer [katA_PF_R(Xba) and cmeA_PF_R(Xba)] and an unlabeled primer [katA_PF_F(Xba) and cmeA_ES_F], respectively (Table 1), and were purified from the agarose gel with a gel extraction kit (Qiagen). Binding reaction of rCosR to the katA and cmeABC promoters was performed at 37°C for 10 min in 40 μl of the gel-shift assay buffer (see above) containing 10 mM MgCl2. The reaction mixture was treated either with or without 0.1 or 0.5 U of DNase I (TaKaRa), and the reactions were stopped by the addition of 200 μl of ice-cold stop solution (0.4 M sodium acetate, 2.5 mM EDTA). After the purification with phenol extraction and ethanol precipitation, the digested DNA fragments were separated by electrophoresis in 6% polyacrylamide–8 M urea gels alongside sequencing ladders that were generated with the same 32P-labeled primer used to amplify DNA fragments for DNase I digestion.
RESULTS
Effects of CosR knockdown on transcriptomic profiles.
Transcriptomic profile changes under the CosR-knockdown condition were analyzed with DNA microarrays. Because of the cell death problem resulting from a knockout mutation in the essential gene cosR, microarray analysis was performed with RNA samples collected under the CosR-knockdown condition where the protein level of CosR was significantly reduced by gene knockdown with CosR-PNA as described in our previous study (15). The results of microarray analysis revealed that 480 genes were regulated either positively or negatively by CosR knockdown >1.5-fold with statistical significance (P < 0.05) (see Tables S1 and S2 in the supplemental material), and among these genes >2-fold changes were observed in 93 genes involved in various cellular processes, such as energy production, transcription, protein synthesis, motility, secondary metabolite biosynthesis, and stress defense (Tables 2 and 3). Interestingly, CosR knockdown affected the transcription of 17 genes which were reported to be essential in C. jejuni (31, 38), suggesting that CosR is associated with regulation of vital cellular processes sustaining C. jejuni's viability (Tables 2 and 3; essential genes are indicated in boldface and by underlining). qRT-PCR also confirmed that the effect of CosR knockdown on the expression of the essential genes (see Fig. S1 in the supplemental material). Also, CosR knockdown affected the expression of several genes associated with flagellar biogenesis (Table 3); CosR knockdown upregulated flgD (encoding flagellum basal body rod modification protein), flgE (flagellum hook protein), flgL (flagellum hook-associated protein), and fliK (putative flagellum hook-length control protein). Based on the genome sequence of C. jejuni (34), the flgD, flgE, and flgK genes are encoded in a single polycistronic operon (data not shown). The transcriptional level changes in flgL and fliK were confirmed by qRT-PCR (see Fig. S2A in the supplemental material). CosR knockdown increased bacterial motility compared to the wild type, whereas CosR overexpression reduced motility (see Fig. S2B in the supplemental material).
Table 2.
Genes downregulated by CosR knockdowna
| Functional category | Gene | Locus tag | Description | Fold changeb | P |
|---|---|---|---|---|---|
| Translation, ribosomal structure, and biogenesis | tgt | Cj1010 | Queuine tRNA-ribosyltransferase | –2.114 | 0.01636 |
| valS | Cj0775c | Valyl-tRNA synthetase | –2.732 | 0.017 | |
| Transcription | greA | Cj0287c | Transcription elongation factor GreA | –2.008 | 0.0053 |
| DNA replication, recombination, and repair | ruvB | Cj1362 | Holliday junction DNA helicase RuvB | –2.222 | 0.00859 |
| Cell division and chromosome partitioning | mrp | Cj1606c | Putative ATP/GTP-binding protein | –4.975 | 0.015 |
| Posttranslational modification, protein turnover, and chaperones | groES | Cj1220 | Cochaperonin GroES | –13.138 | 0.00456 |
| groEL | Cj1221 | Chaperonin GroEL | –7.583 | 0.00168 | |
| Cj0954c | Cj0954c | Putative DnaJ-like protein | –2.921 | 0.00341 | |
| hypB | Cj0623 | Hydrogenase isoenzyme formation protein | –2.433 | 0.015 | |
| hypC | Cj0624 | Hydrogenase isoenzyme formation protein | –2.475 | 0.017 | |
| hypD | Cj0625 | Hydrogenase isoenzyme formation protein | –2.618 | 0.004 | |
| hypE | Cj0626 | Hydrogenase isoenzyme formation protein | –2.439 | 0.009 | |
| trxB | Cj0146c | Thioredoxin reductase | –2.398 | 0.002 | |
| Inorganic ion transport and metabolism | Cj1613c | Cj1613c | Putative pyridoxamine 5′-phosphate oxidase | –2.410 | 0.01488 |
| Cj1658 | Cj1658 | Putative iron permease | –2.066 | 0.02772 | |
| p19 | Cj1659 | Periplasmic protein p19 | –2.747 | 0.025 | |
| Energy production and conversion | Cj0037c | Cj0037c | Putative cytochrome c | –2.790 | 0.01208 |
| Cj0203 | Cj0203 | Putative citrate transporter | –3.525 | 0.00698 | |
| Cj1153 | Cj1153 | Putative periplasmic cytochrome c | –2.053 | 0.01988 | |
| napA | Cj0780 | Nitrate reductase catalytic subunit | –2.179 | 0.009 | |
| napB | Cj0783 | Periplasmic nitrate reductase small subunit | –2.262 | 0.01099 | |
| napG | Cj0781 | Quinol dehydrogenase periplasmic component | –2.326 | 0.024 | |
| napH | Cj0782 | Quinol dehydrogenase membrane component | –2.188 | 0.009 | |
| sucC | Cj0533 | Succinyl-CoA synthetase subunit beta | –2.146 | 0.027 | |
| sucD | Cj0534 | Succinyl-CoA synthetase alpha chain | –2.146 | 0.034 | |
| Amino acid transport and metabolism | dapD | Cj1605c | Putative 2,3,4,5-tetrahydropyridine-2-carboxylate N-succinyltransferase | –3.155 | 0.01173 |
| ilvH | Cj0575 | Acetolactate synthase 3 regulatory subunit | –2.070 | 0.007 | |
| ilvI | Cj0574 | Acetolactate synthase 3 catalytic subunit | –2.110 | 0.009 | |
| Nucleotide transport and metabolism | Cj0594c | Cj0594c | Putative DNA/RNA nonspecific endonuclease | –2.410 | 0.02564 |
| Cj0898 | Cj0898 | Putative histidine triad (HIT) family protein | –2.163 | 0.00825 | |
| purH | Cj0953c | Bifunctional phosphoribosylaminoimidazolecarboxamide formyltransferase/IMP cyclohydrolase | –2.392 | 0.01 | |
| purL | Cj0955c | Phosphoribosylformylglycinamidine synthase II | –2.793 | 0.003 | |
| Coenzyme metabolism | Cj0436 | Cj0436 | Putative pyridoxamine 5′-phosphate oxidase | –2.456 | 0.01122 |
| Lipid metabolism | acpP | Cj0441 | Acyl carrier protein | –7.463 | 0.00875 |
| fabF | Cj0442 | 3-Oxoacyl-(acyl carrier protein) synthase II | –6.526 | 0.00383 | |
| accA | Cj0443 | Acetyl-CoA carboxylase carboxyltransferase subunit alpha | –4.065 | 0.00599 | |
| Secondary metabolites biosynthesis, transport, and catabolism | fabG | Cj0435 | 3-Ketoacyl-(acyl-carrier-protein) reductase | –2.567 | 0.01624 |
| General function prediction only | amaA | Cj1363 | Acid membrane antigen A | –2.156 | 0.00711 |
| Cj0773c | Cj0773c | Putative binding-protein dependent transport system permease | –3.072 | 0.00907 | |
| Cj0774c | Cj0774c | ABC transporter ATP-binding protein | –2.990 | 0.00898 | |
| Function unknown | Cj0573 | Cj0573 | Putative GatB/Yqey family protein | –2.134 | 0.01623 |
| Cj0593c | Cj0593c | Putative integral membrane protein | –2.079 | 0.00487 | |
| Cj0776c | Cj0776c | Putative periplasmic protein | –2.632 | 0.015 | |
| Cj0880c | Cj0880c | Hypothetical protein | –2.070 | 0.02268 | |
| Cj1725 | Cj1725 | Putative periplasmic protein | –2.252 | 0.01401 | |
| ctsR | Cj1475c | Hypothetical protein | –2.008 | 0.01739 | |
| glpT | Cj0292c | Pseudogene | –2.012 | 0.00746 |
Table 3.
Genes upregulated by CosR knockdowna
| Functional category | Gene | Locus tag | Description | Fold change | P |
|---|---|---|---|---|---|
| Translation, ribosomal structure and biogenesis | Cj0722c | Cj0722c | Putative DNA methylase | 2.036 | 0.00677 |
| Posttranslational modification, protein turnover, and chaperones | Cj1365c | Cj1365c | Putative secreted serine protease | 2.168 | 0.00613 |
| dsbA | Cj0872 | Putative protein disulphide isomerase | 2.404 | 0.02011 | |
| htpG | Cj0518 | Heat shock protein 90 | 2.079 | 0.01 | |
| Cell envelope biogenesis, outer membrane | dgkA | Cj0257 | Diacylglycerol kinase | 2.186 | 0.00238 |
| glmS | Cj1366c | Glucosamine–fructose-6-phosphate aminotransferase | 2.012 | 0.01338 | |
| pglF | Cj1120c | UDP-GlcNAc C4,6 dehydratase | 2.138 | 0.028 | |
| Cell motility and secretion | flgD | Cj0042 | Flagellar basal body rod modification protein | 2.159 | 0.01308 |
| flgE | Cj0043 | Flagellar hook protein | 2.156 | 0.01035 | |
| flgL | Cj0887c | Flagellar hook-associated protein FlgL | 2.228 | 0.00678 | |
| fliK | Cj0041 | Putative flagellar hook-length control protein | 2.157 | 0.00755 | |
| lspA | Cj0361 | Lipoprotein signal peptidase | 2.091 | 0.012 | |
| Inorganic ion transport and metabolism | Cj0519 | Cj0519 | Putative rhodanese-like domain protein | 2.185 | 0.00455 |
| Cj0522 | Cj0522 | Putative Na+/Pi cotransporter protein | 2.154 | 0.00264 | |
| pstS | Cj0613 | Putative periplasmic phosphate binding protein | 2.067 | 0.02 | |
| Energy production and conversion | rrc | Cj0012c | Non-haem iron protein | 2.242 | 0.002 |
| sdhB | Cj0438 | Putative succinate dehydrogenase iron-sulfur protein | 2.159 | 0.006 | |
| sdhC | Cj0439 | Putative succinate dehydrogenase subunit C | 2.068 | 0.009 | |
| Amino acid transport and metabolism | aroK | Cj0387 | Shikimate kinase | 2.827 | 0.01923 |
| putA | Cj1503c | Putative proline dehydrogenase/delta-1-pyrroline-5-carboxylate dehydrogenase | 2.028 | 0.008 | |
| putP | Cj1502c | Putative sodium/proline symporter | 2.442 | 0.009 | |
| Nucleotide transport and metabolism | pyrC | Cj0259 | Dihydroorotase | 2.075 | 0.0096 |
| Coenzyme metabolism | dfp | Cj0822 | Bifunctional phosphopantothenoylcysteine decarboxylase/phosphopantothenate synthase | 2.191 | 0.03033 |
| folD | Cj0855 | Bifunctional 5,10-methylene-tetrahydrofolate dehydrogenase/5,10-methylene-tetrahydrofolate cyclohydrolase | 2.121 | 0.01026 | |
| Secondary metabolites biosynthesis, transport, and catabolism | cmeA | Cj0367c | Periplasmic fusion protein CmeA | 2.485 | 0.01579 |
| cmeB | Cj0366c | Inner membrane efflux transporter CmeB | 2.294 | 0.01059 | |
| cmeC | Cj0365c | Outer membrane channel protein CmeC | 2.127 | 0.01653 | |
| General function prediction only | Cj0236c | Cj0236c | Putative integral membrane protein | 2.089 | 0.0283 |
| Cj0256 | Cj0256 | Putative sulfatase family protein | 2.078 | 0.00544 | |
| Cj0770c | Cj0770c | Putative NLPA family lipoprotein | 2.371 | 0.01032 | |
| Cj0771c | Cj0771c | Putative NLPA family lipoprotein | 2.408 | 0.01623 | |
| Cj0772c | Cj0772c | Putative NLPA family lipoprotein | 2.358 | 0.00361 | |
| engA | Cj0386 | GTP-binding protein EngA | 2.080 | 0.00462 | |
| Function unknown | Cj0040 | Cj0040 | Hypothetical protein | 2.306 | 0.04341 |
| Cj0069 | Cj0069 | Hypothetical protein | 2.147 | 0.00597 | |
| Cj0070c | Cj0070c | Hypothetical protein | 2.156 | 0.04309 | |
| Cj0243c | Cj0243c | Hypothetical protein | 2.362 | 0.0106 | |
| Cj0249 | Cj0249 | Hypothetical protein | 2.012 | 0.03032 | |
| Cj0391c | Cj0391c | Hypothetical protein | 2.061 | 0.01112 | |
| Cj0428 | Cj0428 | Hypothetical protein | 2.190 | 0.00859 | |
| Cj0520 | Cj0520 | Hypothetical protein | 2.168 | 0.01622 | |
| Cj0742 | Cj0742 | Pseudogene | 2.454 | 0.04945 | |
| Cj0873c | Cj0873c | Hypothetical protein | 2.070 | 0.01804 | |
| Cj1242 | Cj1242 | Hypothetical protein | 2.419 | 0.00765 | |
| Cj1501 | Cj1501 | Hypothetical protein | 2.146 | 0.00568 | |
| Cj1650 | Cj1650 | Hypothetical protein | 2.948 | 0.00732 |
Negative regulation of cmeABC by CosR.
CmeABC is a key determinant in conferring resistance to a broad range of antimicrobials and toxic compounds in Campylobacter (18, 27). The microarray results showed that the transcriptional levels of all cmeA, cmeB, and cmeC genes were increased by CosR knockdown >2-fold (Table 3). qRT-PCR and PcmeABC::lacZ fusion assay showed that the transcriptional level of cmeABC was upregulated by CosR knockdown and reduced in the CosR-overexpressing strain (Fig. 1A and B), demonstrating the negative regulation of cmeABC expression by CosR. Since the binding of bile salts to CmeR, a repressor of cmeABC, derepresses cmeABC expression due to conformational changes in CmeR (26), the PcmeABC::lacZ fusion assay carried out in the presence of cholic acid to examine whether the regulation of cmeABC by CosR would be affected by bile salts (Fig. 1B). Cholate (1 mg/ml) significantly increased the transcriptional level of cmeABC; however, the changes in the Miller units at different CosR levels were not affected by cholic acid compared to those without cholic acid (Fig. 1B). The results suggest that the regulation of cmeABC by CosR is independent of bile salts and presumably of CmeR as well, although the effect of bile salts on CosR expression is not yet known. Interaction between CosR and the cmeABC promoter was examined by performing EMSA, and the results showed that CosR bound to the cmeABC promoter (Fig. 1C). The PCR fragment from an internal coding region did not compete with the labeled probe of the cmeABC promoter; however, unlabeled cmeABC probes effectively competed with the labeled cmeABC probes (Fig. 1C). These results showed that CosR directly interacts with the cmeABC promoter and regulates cmeABC expression as a repressor.
Fig 1.
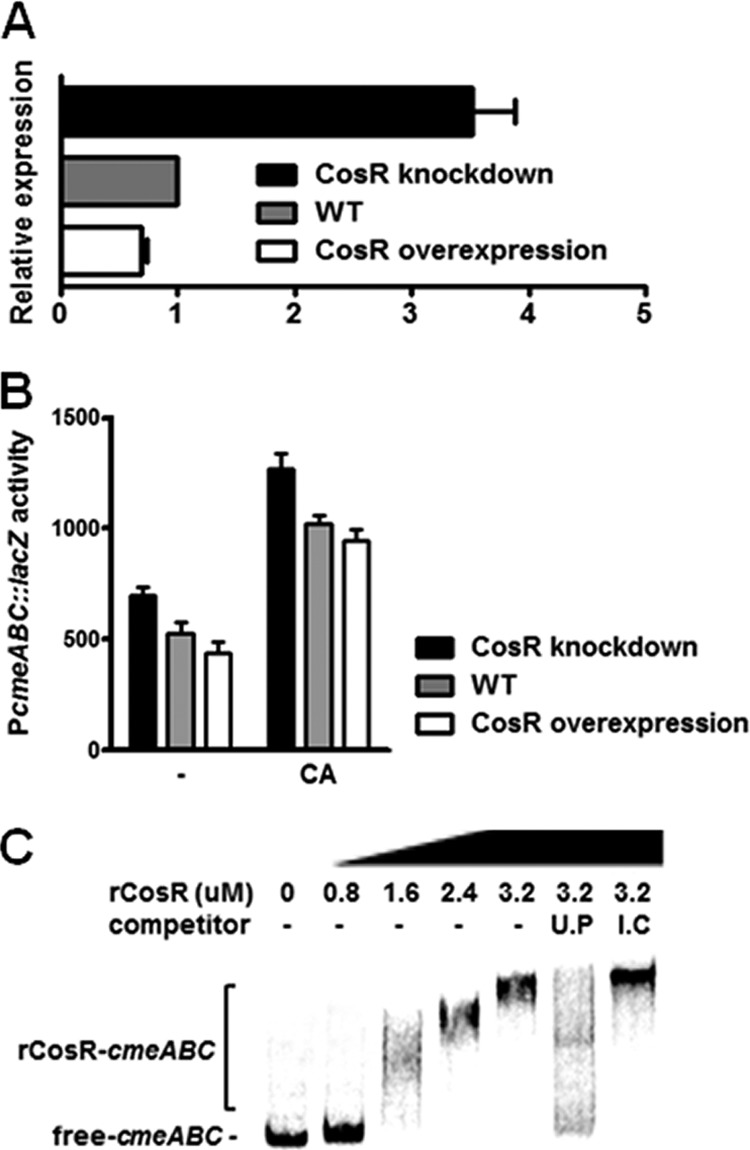
Negative regulation of cmeABC by CosR. (A) qRT-PCR analysis of cmeABC transcription. C. jejuni strains were cultured at different CosR levels at 42°C for 8 h under microaerobic conditions to extract total RNA. The CosR-knockdown condition was generated by supplementing 1.5 μM CosR-PNA to the medium. The results of three independent experiments are expressed as means and standard deviations. (B) PcmeABC::lacZ fusion assay in the absence or presence of cholate (CA). Cholate (1 mg/ml) was added to the culture medium, and C. jejuni strains were grown for 6 h prior to the assay. The “–” symbol indicates the cultures without cholate. (C) Binding of rCosR on the cmeABC promoter. Radiolabeled DNA probes of the cmeABC promoter were incubated with rCosR at different concentrations, and unlabeled DNA probes (U.P) and internal coding regions (I.C) of cmeABC were used as a competitor.
Regulation of katA by CosR.
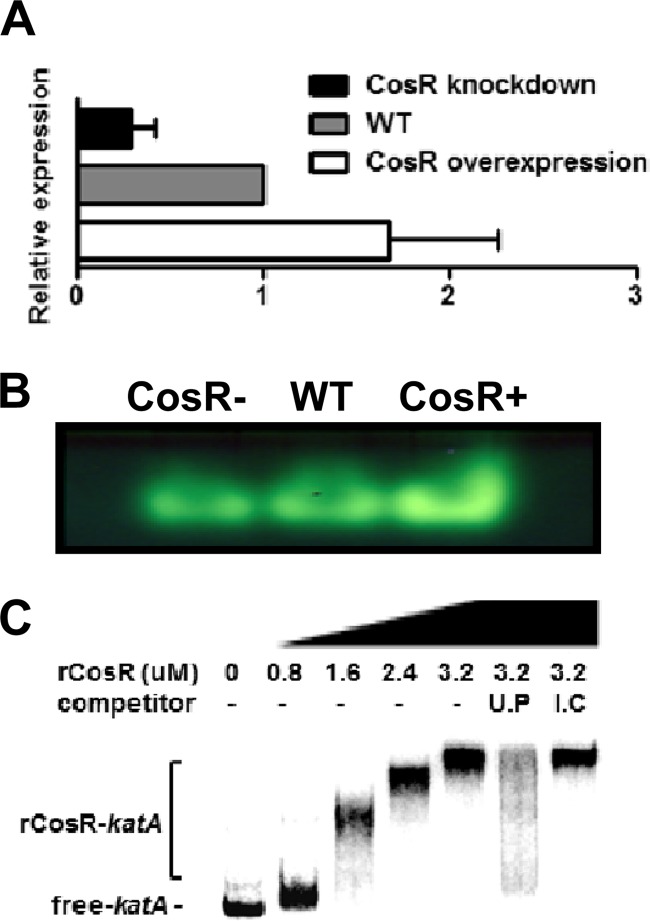
Consistent with our previous report, CosR affected the expression of oxidative stress genes. CosR-knockdown upregulated rrc (2.2-fold; Table 3), dps (1.5-fold; see Table S2 in the supplemental material), and sodB (1.7-fold; see Table S2 in the supplemental material) and downregulated katA (1.7-fold; see Table S1 in the supplemental material) and p19 (2.7-fold; Table 2). KatA is the sole catalase in C. jejuni and plays an important role in Campylobacter's resistance to H2O2 and persistence within macrophages (7). The results of qRT-PCR showed that the transcriptional level of katA was reduced by CosR knockdown and was increased by CosR overexpression compared to that of the wild type (Fig. 2A). To determine whether the transcriptional changes in katA would affect the enzyme activity, the catalase activity was measured with a catalase assay, which is based on the formation of colorimetric precipitate, ferrichloride-ferricyanide complex, via the reaction between H2O2 and ferricyanide (44). Consistent with the transcriptional changes, the catalase activity was decreased by CosR knockdown and increased in the CosR-overexpression strain (Fig. 2B). Interaction between CosR and the katA promoter was determined by EMSA, and the results showed that CosR bound to the katA promoter (Fig. 2C). These results successfully demonstrated that CosR binds to the katA promoter and positively regulates katA transcription and affects the catalase activity in C. jejuni.
Fig 2.
Positive regulation of katA by CosR. (A) qRT-PCR analysis of katA transcription. The results are expressed as the means and standard deviations of three independent experiments. (B) Catalase activity at different CosR levels. In these experiments, CosR-knockdown was achieved by adding 1.5 μM CosR-PNA to culture medium. The results show the representative of three independent experiments. The symbols “–” and “+” indicate knockdown and overexpression, respectively. (C) Binding of rCosR to the katA promoter. Radiolabeled DNA probes of the katA promoter were incubated with rCosR at different concentrations, and unlabeled DNA probes (U.P) and internal coding regions (I.C) of katA were used in the competition assay.
Determination of CosR binding sites in the katA and cmeABC promoters.
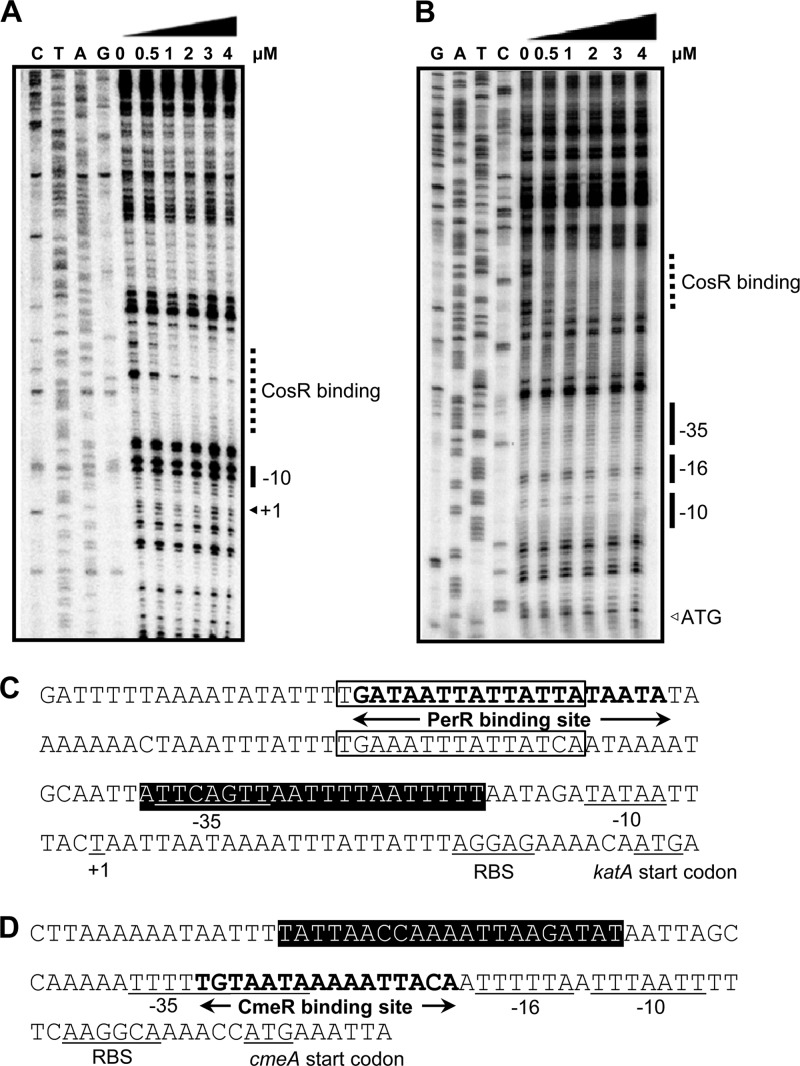
The results of microarray analysis, qRT-PCR, and EMSA demonstrated that CosR regulated katA and cmeABC by binding to their promoters (Fig. 1 and 2). DNase I footprinting assays were carried out to further determine the CosR binding sites in the katA and cmeABC promoter regions (Fig. 3). The assay found a region (from −37 to −17) overlapping with the −35 element of the katA promoter was protected from DNase I treatment (Fig. 3A and C). The CosR binding site in the katA promoter doesn't overlap with the predicted PerR binding site (−104 to −86) (43). We identified two putative holo-Fur binding sites (−51 to −65 and −91 to −105) in the katA promoter based on the consensus holo-Fur binding site (TGATAAT-T-ATTATCA) (5). Although one of the holo-Fur binding sites overlapped with the putative PerR binding site, the CosR binding site does not overlap with the holo-Fur binding sites (Fig. 3C). CosR also bound to an upstream region of the CmeR binding site in the cmeABC promoter (Fig. 3B and D) (25). Nucleotide sequences of the CosR binding sites in the katA and cmeABC promoters were aligned with the previously reported CosR binding sequences in the sodB and ahpC promoters (15), and the multiple alignment revealed a consensus CosR binding sequence (ttTaAanaAAAaTTAagaTTT; lowercase and capital letters indicate less and highly conserved residues, respectively, and “n” represents any nucleotide [Fig. 4]), which is almost identical to the consensus CosR binding sequence reported in our previous study (tttaAanAaAAaTtAtgaTTt) (15).
Fig 3.
Determination of the CosR binding sites in katA and cmeABC promoters by DNase I footprinting. The CosR binding regions in the promoter regions of katA (A) and cmeABC (B) are indicated by dotted lines; the promoters are based on previous reports (11, 25). Fur binding sites are marked with open boxes based on the consensus sequence for holo-Fur binding site (5). The CmeR binding site was reported previously by Lin et al. (25). The PerR binding region (C) in the katA promoter and the CmeR binding region (D) in cmeABC promoter are marked with boldface letters and arrows. The start codon, the transcriptional start site (+1), and the −10, −16, and −35 elements are underlined. The regions protected by rCosR from DNase I cleavage are indicated by a black background.
Fig 4.
Alignment of CosR binding sequences. Nucleotide sequences of the CosR binding sites for the katA (BkatA) and cmeABC (BcmeABC) promoters were determined from Fig. 3 and compared to the CosR binding sequences in the sodB (BsodB) and ahpC (BahpC1 and BahpC2) promoters reported in our previous study (15). Highly conserved nucleotides are shaded on black background, and identical nucleotides are indicated in capital letters. “n” means any nucleotide.
DISCUSSION
CmeABC is the major multidrug efflux pump contributing to Campylobacter's resistance to a variety of toxic compounds, antimicrobials, and bile salts (27, 28). The cmeABC mutant is highly susceptible to clinically important antibiotics and cannot colonize the chicken intestines due to elevated susceptibility to bile salts (28). CmeABC is a resistance-nodulation-cell division (RND)-type efflux pump consisting of the periplasmic protein CmeA, the inner membrane protein CmeB, and the outer membrane protein CmeC encoded in the polycistronic operon cmeABC (25, 27). The results of DNA microarray analysis in the CosR-knockdown condition demonstrated a significant change in the transcriptional levels of all three genes in the cmeABC operon (Table 3). The microarray results were further supported by qRT-PCR and PcmeABC::lacZ assays (Fig. 1A and B). CmeR has thus far been the only transcriptional regulator involved in the control of cmeABC expression (26). Although bile salts are a substrate of the CmeABC efflux pump, bile salts can also affect the CmeR regulation (26). Interaction of bile salts with the CmeR protein causes conformational changes in CmeR and results in the derepression of cmeABC expression (26). To investigate whether CosR interferes with the function of CmeR on cmeABC expression, a PcmeABC::lacZ assay was carried out in the presence of cholic acid. The results showed that cholic acid increased the transcriptional level of cmeABC (Fig. 1B), indicating that cmeABC expression was derepressed by the interaction between CmeR and bile salts; binding of bile salts to CmeR reduces the binding affinity of CmeR to the cmeABC promoter (26). However, CosR knockdown or overexpression caused similar changes in the Miller units of the PcmeABC::lacZ assay regardless of the presence or absence of cholic acid, indicating that CosR did not affect the derepression of cmeABC by bile salts; however, it is unknown whether bile salts would affect the function or expression of CosR. Also, the CosR binding site discovered by DNase I footprinting assay was located 17 bp upstream of the CmeR binding site in the cmeABC promoter (Fig. 3B and D), suggesting that binding of CosR and CmeR to the cmeABC promoter may not spatially interfere with each other.
Lin et al. reported that a mutation of cmeR resulted in a >6-fold increase in the level of PcmeABC transcription and increased the MICs of ciprofloxacin (2-fold), cefotaxime (2-fold), and erythromycin (4-fold) (25). Recently, it was reported that salicylate, a nonsteroidal anti-inflammatory compound, induces cmeABC transcription by inhibiting CmeR binding to the cmeABC promoter (37). An ∼2-fold increase in the level of cmeABC transcription at 100 μg of salicylate/ml did not change MICs of antibiotics except for only a moderate change in ciprofloxacin (2-fold) but enhanced bacterial growth in the presence of antibiotics at sub-MICs (37). Similarly, neither CosR knockdown nor overexpression produced noticeable changes in the MICs of several antibiotics tested here, including ciprofloxacin, erythromycin, and tetracycline (data not shown). Based on previous reports and our findings in the present study, both CmeR and CosR function as repressors for cmeABC, and it would be interesting to investigate whether CosR and CmeR would interplay in the regulation of cmeABC under certain conditions.
It is an interesting finding that CosR is involved in the regulation of both oxidative stress and drug efflux pump. A similar example can be found in SoxRS, a well-known oxidative stress regulator in E. coli. SoxRS not only controls oxidative stress defense but also affects antibiotic resistance by upregulating the AcrAB multidrug efflux pump (22). The E. coli AcrAB is functionally coupled to the outer membrane protein TolC and significantly contributes to E. coli's resistance to a wide range of toxic compounds, including antibiotics, detergents, and dyes (32). Increased levels of AcrAB-TolC resulting from elevated soxR expression by mutations render E. coli and Salmonella resistant to antibiotics (12, 19). The removal of toxic compounds by efflux pumps may contribute to the detoxification of cells, and presumably this is why some oxidative stress regulators control the expression of oxidative stress genes and efflux pumps as well. To the best of our knowledge, CosR is the first reported transcriptional regulator that controls the expression of genes involved in oxidative stress resistance and a multidrug efflux pump in C. jejuni.
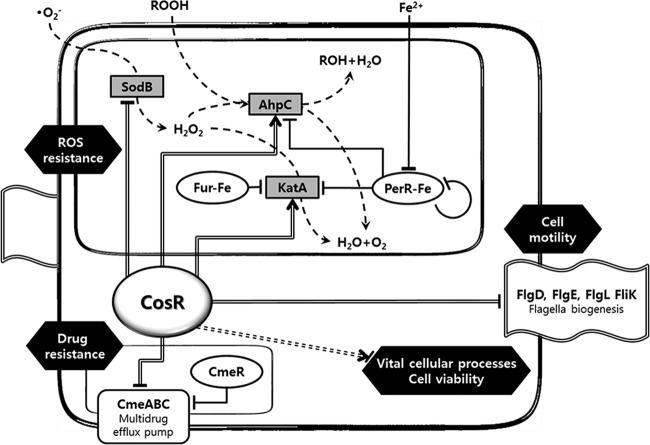
Another important finding of the present study is that CosR positively regulates katA expression at the transcriptional level and affects the catalase activity in C. jejuni (Fig. 2). As the sole catalase in Campylobacter, KatA plays an important role in oxidative stress resistance and intracellular survival in the macrophage (7). Like Gram-positive bacteria, C. jejuni uses PerR as a repressor to control peroxide resistance genes including katA and ahpC (33, 42). Iron is also a regulatory factor of katA expression; iron represses katA, ahpC, and perR as well (3, 14). Although the effect of iron on perR expression results from negative perR autoregulation (20), the iron responsiveness of katA was eliminated not by inactivation of either perR or fur but only by the double mutation of perR and fur, indicating that both PerR and Fur participate in the control of katA expression (42). In fact, it was recently shown that holo-Fur binds to the katA promoter and negatively regulates katA expression (5). In contrast to the negative regulation of katA expression by PerR and holo-Fur, CosR activated katA expression like the E. coli OxyR. The CosR binding site is separated from the predicted PerR and Fur binding sites in the katA promoter (Fig. 3C), suggesting that CosR binding to the katA promoter may not interfere with PerR and Fur binding. The regulation of katA expression involves PerR, Fur, iron, and CosR, and further studies are required to explain how these multiple regulatory factors interplay to coordinate the expression of the sole catalase gene katA. The regulatory network of CosR is summarized in Fig. 5.
Fig 5.
Schematic representation of CosR regulatory network in C. jejuni. The detoxification flow of oxidants by oxidative stress resistance enzymes (gray boxes) is drawn with dashed lines, and the transcriptional regulators (CosR, Fur, PerR, and CmeR) are boxed in ovals. The regulation by CosR is indicated with double-strand lines, and positive and negative regulations by CosR are marked with arrowheads and flat-line heads, respectively. CosR is indispensable to Campylobacter's viability, although its regulation mechanism is not known. This is indicated with a dashed double-strand line.
Although the E. coli OxyR is subject to negative autoregulation, OxyR acts mostly as an activator of the peroxide defense genes (39). The OxyR binding sites in the promoters of positively regulated genes typically overlap the −35 region and extend upstream from the promoter regions (41). This unique positioning of OxyR binding sites in the promoter region stimulates the interaction between OxyR and the α subunit of the RNA polymerase, activating gene expression (40). The region protected by CosR from DNase I treatment in the katA promoter overlaps with the −35 region of the katA promoter sequence (Fig. 3A and C). In a previous study, we showed that CosR positively regulates AhpC, and one of the CosR binding sites also overlaps the −35 region in the ahpC promoter (15). These findings suggest that CosR may interact with the RNA polymerase for activation similar to the E. coli OxyR. To elucidate the CosR regulation, in addition, further studies are required to determine whether CosR has its cognate sensor kinase or is an orphan response regulator and whether CosR is phosphorylated or not, since the phosphorylation status can affect the DNA binding affinity of a response regulator. It has been reported that HP1043, the CosR homologue in H. pylori, functions in a phosphorylation-independent manner (36); however, it remains unknown whether CosR is phosphorylated or not.
The transcriptomic analysis described here discovered 93 genes whose transcriptional levels were changed >2-fold by CosR knockdown (Tables 2 and 3). The functions of the identified genes are involved in a wide range of cellular processes, such as energy production, lipid metabolism, motility, amino acid transport, and drug efflux; however, 21.5% of the genes (20 of 93) have not been fully characterized. The DNA microarray analysis in the present study identified 53% of the genes that were reported in our previous proteomic study, such as greA, groEL, rrc, p19, and htpG (15). Consistent with our previous proteomic analysis (15), sodB, rrc, and dps were upregulated by CosR knockdown (Table 3 and see Table S2 in the supplemental material); however, ahpC was not downregulated by CosR knockdown in a DNA microarray analysis despite obvious expression changes at the protein level in our previous study (15), suggesting that posttranscriptional factors may also affect AhpC expression. Interestingly, ca. 18.3% of the CosR-regulated genes (17 of 93) are predicted essential genes in C. jejuni (31, 38). Although it is not certain whether CosR affects the expression of these essential genes directly or indirectly, CosR appears to be closely involved in the regulation of vital biological processes in C. jejuni. Given that CosR regulates genes related to resistance to oxidative stress and toxic compounds, CosR may participate in the overall stress resistance and survival of Campylobacter. Future studies will fill in the knowledge gaps regarding the environmental stimuli affecting the regulatory function of CosR and the coordination of regulatory factors of oxidative stress defense in Campylobacter.
ACKNOWLEDGMENTS
This research was supported by startup funding from the University of Alberta to B.J. Q.Z. is supported by grant R01DK063008 from the National Institute of Diabetes and Digestive and Kidney Diseases. S.H. is supported by grant 2012-0004344 from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology, and S.H. is a recipient of the graduate fellowship provided by the Ministry of Education through the Brain Korea 21 Project.
Footnotes
Published ahead of print 12 October 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Akiba M, Lin J, Barton YW, Zhang Q. 2006. Interaction of CmeABC and CmeDEF in conferring antimicrobial resistance and maintaining cell viability in Campylobacter jejuni. J. Antimicrob. Chemother. 57:52–60 [DOI] [PubMed] [Google Scholar]
- 2. Atack JM, Kelly DJ. 2009. Oxidative stress in Campylobacter jejuni: responses, resistance, and regulation. Future Microbiol. 4:677–690 [DOI] [PubMed] [Google Scholar]
- 3. Baillon ML, van Vliet AH, Ketley JM, Constantinidou C, Penn CW. 1999. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J. Bacteriol. 181:4798–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beier D, Frank R. 2000. Molecular characterization of two-component systems of Helicobacter pylori. J. Bacteriol. 182:2068–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butcher J, Sarvan S, Brunzelle JS, Couture JF, Stintzi A. 2012. Structure and regulon of Campylobacter jejuni ferric uptake regulator Fur define apo-Fur regulation. Proc. Natl. Acad. Sci. U. S. A. 109:10047–10052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang SM, Schellhorn HE. 2012. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 525:161–169 [DOI] [PubMed] [Google Scholar]
- 7. Day WA, Jr, Sajecki JL, Pitts TM, Joens LA. 2000. Role of catalase in Campylobacter jejuni intracellular survival. Infect. Immun. 68:6337–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delany I, Spohn G, Rappuoli R, Scarlato V. 2002. Growth phase-dependent regulation of target gene promoters for binding of the essential orphan response regulator HP1043 of Helicobacter pylori. J. Bacteriol. 184:4800–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garénaux A, et al. 2008. Role of the Cj1371 periplasmic protein and the Cj0355c two-component regulator in the Campylobacter jejuni NCTC 11168 response to oxidative stress caused by paraquat. Res. Microbiol. 159:718–726 [DOI] [PubMed] [Google Scholar]
- 10. Good L, Awasthi SK, Dryselius R, Larsson O, Nielsen PE. 2001. Bactericidal antisense effects of peptide-PNA conjugates. Nat. Biotechnol. 19:360–364 [DOI] [PubMed] [Google Scholar]
- 11. Grant KA, Park SF. 1995. Molecular characterization of katA from Campylobacter jejuni and generation of a catalase-deficient mutant of Campylobacter coli by interspecific allelic exchange. Microbiology 141:1369–1376 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg JT, Monach P, Chou JH, Josephy PD, Demple B. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 87:6181–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu M, Imlay JA. 2011. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 79:1136–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmes K, et al. 2005. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology 151:243–257 [DOI] [PubMed] [Google Scholar]
- 15. Hwang S, Kim M, Ryu S, Jeon B. 2011. Regulation of oxidative stress response by CosR, an essential response regulator in Campylobacter jejuni. PLoS One 6:e22300 doi:10.1371/journal.pone.0022300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeon B, Wang Y, Hao H, Barton YW, Zhang Q. 2011. Contribution of CmeG to antibiotic and oxidative stress resistance in Campylobacter jejuni. J. Antimicrob. Chemother. 66:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeon B, Zhang Q. 2009. Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter. J. Antimicrob. Chemother. 63:946–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kehrenberg C, Cloeckaert A, Klein G, Schwarz S. 2009. Decreased fluoroquinolone susceptibility in mutants of Salmonella serovars other than Typhimurium: detection of novel mutations involved in modulated expression of ramA and soxS. J. Antimicrob. Chemother. 64:1175–1180 [DOI] [PubMed] [Google Scholar]
- 20. Kim M, Hwang S, Ryu S, Jeon B. 2011. Regulation of perR expression by iron and PerR in Campylobacter jejuni. J. Bacteriol. 193:6171–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirkpatrick BD, Tribble DR. 2011. Update on human Campylobacter jejuni infections. Curr. Opin. Gastroenterol. 27:1–7 [DOI] [PubMed] [Google Scholar]
- 22. Koutsolioutsou A, Martins EA, White DG, Levy SB, Demple B. 2001. A soxRS-constitutive mutation contributing to antibiotic resistance in a clinical isolate of Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 45:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Demple B. 1994. SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J. Biol. Chem. 269:18371–18377 [PubMed] [Google Scholar]
- 25. Lin J, Akiba M, Sahin O, Zhang Q. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob. Agents Chemother. 49:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin J, et al. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 187:7417–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin J, Michel LO, Zhang Q. 2002. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob. Agents Chemother. 46:2124–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin J, Sahin O, Michel LO, Zhang Q. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 71:4250–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 30. Luangtongkum T, et al. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 4:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metris A, Reuter M, Gaskin DJ, Baranyi J, van Vliet AH. 2011. In vivo and in silico determination of essential genes of Campylobacter jejuni. BMC Genomics 12:535 doi:10.1186/1471-2164-12-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikaido H, Takatsuka Y. 2009. Mechanisms of RND multidrug efflux pumps. Biochim. Biophys. Acta 1794:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palyada K, et al. 2009. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC Genomics 10:481 doi:10.1186/1471-2164-10-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parkhill J, et al. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 35. Raphael BH, et al. 2005. The Campylobacter jejuni response regulator, CbrR, modulates sodium deoxycholate resistance and chicken colonization. J. Bacteriol. 187:3662–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schar J, Sickmann A, Beier D. 2005. Phosphorylation-independent activity of atypical response regulators of Helicobacter pylori. J. Bacteriol. 187:3100–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen Z, Pu XY, Zhang Q. 2011. Salicylate functions as an efflux pump inducer and promotes the emergence of fluoroquinolone-resistant Campylobacter jejuni mutants. Appl. Environ. Microbiol. 77:7128–7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stahl M, Stintzi A. 2011. Identification of essential genes in Campylobacter jejuni genome highlights hyper-variable plasticity regions. Funct. Integr. Genomics 11:241–257 [DOI] [PubMed] [Google Scholar]
- 39. Tao K. 1999. In vivo oxidation-reduction kinetics of OxyR, the transcriptional activator for an oxidative stress-inducible regulon in Escherichia coli. FEBS Lett. 457:90–92 [DOI] [PubMed] [Google Scholar]
- 40. Tao K, Fujita N, Ishihama A. 1993. Involvement of the RNA polymerase alpha subunit C-terminal region in co-operative interaction and transcriptional activation with OxyR protein. Mol. Microbiol. 7:859–864 [DOI] [PubMed] [Google Scholar]
- 41. Tartaglia LA, Storz G, Ames BN. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 210:709–719 [DOI] [PubMed] [Google Scholar]
- 42. van Vliet AH, Baillon ML, Penn CW, Ketley JM. 1999. Campylobacter jejuni contains two Fur homologs: characterization of iron-responsive regulation of peroxide stress defense genes by the PerR repressor. J. Bacteriol. 181:6371–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Vliet AH, Ketley JM, Park SF, Penn CW. 2002. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol. Rev. 26:173–186 [DOI] [PubMed] [Google Scholar]
- 44. Woodbury W, Spencer AK, Stahman MA. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 44:301–305 [DOI] [PubMed] [Google Scholar]
- 45. Wosten MM, Boeve M, Koot MG, van Nuenen AC, van der Zeijst BA. 1998. Identification of Campylobacter jejuni promoter sequences. J. Bacteriol. 180:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu J, Weiss B. 1992. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J. Bacteriol. 174:3915–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang SH, et al. 2003. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Int. J. Oncol. 22:741–750 [PubMed] [Google Scholar]