Abstract
Purpose
To characterize the importance of cellular Fas-associated death domain (FADD)-like interleukin 1β-converting enzyme (FLICE) inhibitory protein (c-FLIP), a key regulator of caspase 8 (FLICE)-promoted apoptosis, in modulating the response of prostate cancer (CaP) cells to androgen receptor (AR)-targeted therapy.
Experimental Design
c-FLIP expression was characterized by immunohistochemical analysis of prostatectomy tissue. The functional importance of c-FLIP to survival and modulating response to bicalutamide was studied by molecular and pharmacological interventions.
Results
c-FLIP expression was increased in high-grade prostatic intra-epithelial neoplasia (HGPIN) and CaP tissue relative to normal prostate epithelium (P<0.001). Maximal c-FLIP expression was detected in castrate-resistant CaP (CRPC) (P<0.001). In vitro, silencing of c-FLIP induced spontaneous apoptosis and increased 22Rv1 and LNCaP cell sensitivity to bicalutamide, determined by flow cytometry, PARP cleavage and caspase activity assays. The histone deacetylase inhibitors (HDACi), droxinostat and SAHA, also down-regulated c-FLIP expression, induced caspase-8 and caspase-3/7 mediated apoptosis and increased apoptosis in bicalutamide-treated cells. Conversely, the elevated expression of c-FLIP detected in the CRPC cell line VCaP underpinned their insensitivity to bicalutamide and SAHA in vitro. However, knockdown of c-FLIP induced spontaneous apoptosis in VCaP cells, indicating its relevance to cell survival and therapeutic resistance.
Conclusion
c-FLIP reduces the efficacy of AR-targeted therapy and maintains the viability of CaP cells. A combination of HDACi with androgen-deprivation therapy (ADT) may be effective in early-stage disease, using c-FLIP expression as a predictive biomarker of sensitivity. Direct targeting of c-FLIP however may be relevant to enhance the response of existing and novel therapeutics in CRPC.
Keywords: Prostate Cancer, c-FLIP, bicalutamide, castrate-resistance
INTRODUCTION
Androgen-deprivation therapy (ADT) is initially effective in the treatment of localized prostate cancer (CaP) (1) and was developed on the basis that prostate tumour outgrowth is stimulated by androgens acting via the androgen receptor (AR) (2). Bicalutamide (Casodex®) is currently one of the principal anti-androgenic drugs used in CaP therapy, interrupting androgen signalling by antagonizing ligand-induced activation of the AR (3). The onset of resistance to AR-targeted therapies is a major clinical problem resulting in patient relapse and the emergence of an incurable castrate-resistant condition. Bicalutamide does not provide effective biochemical control in all patients, and the effectiveness of the drug is often transient. Knowledge of the mechanisms underpinning bicalutamide resistance and the transition to castrate-resistant CaP (CRPC) may identify more effective combinatorial treatments to increase the proportion of patients that respond, and the duration of response to bicalutamide or alternate ADT strategies.
Elevated expression of anti-apoptotic proteins has been proposed to represent one mechanism of resistance to anti-cancer drug strategies (4), including ADT. Anti-apoptotic c-FLIP (Cellular Fas-associated death domain (FADD)-like interleukin 1β-converting enzyme (FLICE) inhibitory protein), is an endogenous inhibitor of caspase-8 (FLICE) that inhibits the activation of the extrinsic apoptosis pathway mediated by death receptors such as Fas, DR4 (TRAIL-R1) and DR5 (TRAIL-R2) (5-8). Over-expression of c-FLIP has been characterized in the cancer cells of prostate biopsy tissue and has been shown to promote the androgen-independent growth of tumours in nude mice (9). We and others have also determined that transcription of the gene encoding c-FLIP is regulated in part by the AR (9-11), although NF-κB activity can also potentiate the transcription and expression of the gene in LNCaP and PC3 CaP cell lines (11). Thus, increased expression of c-FLIP, induced as a result of failure to repress androgen signaling or alternatively promoted by compensatory cytokine/growth factor-induced signaling, may represent an important feature during the transition to CRPC.
The objective of this study was to characterize the expression of c-FLIP in prostatectomy tissue and determine whether expression of c-FLIP modulates the sensitivity of androgen-dependent CaP cells to bicalutamide. In addition to direct targeting of c-FLIP using siRNA, we also demonstrate that two histone deacetylase inhibitors (HDACi), droxinostat and SAHA (Vorinostat®) can repress c-FLIP expression in androgen-dependent cells and that these agents can potentiate the apoptosis induced by bicalutamide in non-castrate models. Conversely, we show that SAHA is ineffective in cell-based models of castrate-resistant disease, suggesting that combinations of HDACi with bicalutamide are applicable during salvage therapies but may be ineffective in rescuing castrate-resistant disease. Moreover, we show that c-FLIP plays an important role in the viability of castrate-resistant prostate cancer cells.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals were supplied by Sigma unless otherwise stated. 4-(4-chloro-2-methylphenoxy)-N-hydroxybutanamide (droxinostat) was purchased from Chembridge (San Diego, CA, USA). Bicalutamide was provided under a MTA agreement with AstraZeneca (Alderley Park, UK). Suberoylanilide hydroxamic acid (SAHA;Vorinostat®) was purchased from Selleck (Texas, USA). All drugs were reconstituted in 100% DMSO vehicle, prior to further dilution for use in experiments; appropriate controls to account for effects of DMSO in biological assays were conducted in parallel.
Cell culture
Authenticated LNCaP and 22Rv1 cells were obtained from American Type Culture Collection (ATCC) and cultured for a maximum of 4 months, as previously described (12). Human VCaP cells (European Collection of Cell Cultures (ECACC), Salisbury, UK) were maintained in DMEM supplemented with 10% v/v FCS. All cells were maintained in a humidified chamber at 37°C in 5% CO2. LN-Abl cells were provided by Prof Bill Watson (University College Dublin, Ireland).
Western blotting
Protein lysates were prepared as previously described (11, 12), subjected to SDS-PAGE and transferred onto nitrocellulose membranes for 2h. Membranes were blocked for 1h at 25°C in 5% (w/v) Marvel/PBS/3% (v/v) Tween-20 (PBS-T), then incubated overnight at 4°C with either monoclonal antibodies to c-FLIP (NF6; 1:1000 dilution; Enzo Life Sciences, Pennsylvania, USA), poly (ADP-ribose) polymerase (PARP; 1:2000; eBiosciences, Hatfield, UK), androgen receptor (AR; 1:400; Millipore, California, USA) or BCL-2 (1:1000; Cell Signaling, Herts., UK) reconstituted in 5% (w/v) Marvel/PBS-T. Membranes were washed 3×10 min in PBS-T then probed with the respective horseradish peroxidise-conjugated secondary antibodies (Amersham Life Sciences, Buckinghamshire, UK) for 1h at 25°C. Following 3×10 min washes in PBS-T, bands were detected using enhanced chemiluminescence (ECL+ reagents, Amersham). Equal protein loading was assessed by reprobing membranes with an antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:3000; AbD Serotec, Oxford, UK).
siRNA transfections
Cells (density of 1×106 per P90 in FCS-supplemented growth media) were grown to 50% confluency, replenished in serum-free Optimem 1, then transfected for 4h with a siRNA oligonucleotide pool targeting both c-FLIP splice variants (FT) (Dharmacon, Lafayette, USA) or oligonucleotides specifically targeting the c-FLIPL (FL) or c-FLIPS (FS) isoforms (13), using oligofectamine according to manufacturer’s instructions. Cells were replenished in 30% FCS-enriched medium, harvested after 24h or treated with bicalutamide for a further 48h. A non-targeting oligonucleotide pool (NT; Dharmacon) was used at the same concentration as a transfection control.
Flow cytometry
Flow cytometry was performed on siRNA- or drug treated cells using previously described protocols (11, 14). Following treatment, detached and attached cells were collected, centrifuged 2000×g for 5 min, washed twice in PBS containing 1% FCS and fixed in 100% ethanol. Cell pellets were resuspended in 500μl PI/RNaseA solution, incubated at 37°C for 30 min and analysed on a FACSCalibur machine (Becton Dickinson, New Jersey, USA). Ten thousand events were captured using forward and sidescatter detectors. Gating of the sub-G0/G1 fraction of the cell cycle calculated the percentage of cells staining positive for PI and undergoing cell death. In some experiments, apoptosis was calculated as the sum of the FITC-Annexin V positive/propidium iodide negative (early apoptosis) and FITC-Annexin V positive/propidium iodide positive (late apoptosis) cell populations (BD Biosciences, Oxford, UK). To assess mitochondrial membrane potential, tetramethyl-rhodamine ethyl ester perchlorate (TMRE) was added to drug-treated cells at a final concentration of 25nM and incubated for 15 min at 37°C. Cells were collected, pelleted by centrifugation at 2,000 r.p.m at 4°C for 5 min and resuspended in 300μl PBS. TMRE fluorescence was analysed immediately by flow cytometry using the FL2 filter. Geometric means were calculated from the FL2 fluorescence signal, normalized against background fluorescence in non-TMRE-treated cells, and expressed as percentage TMRE staining compared to vehicle control.
Quantitative Real-time PCR analysis
Samples were prepared as previously described (11, 12). The primer sequences used were as follows: 18S: Forward, 5′-CATTCGTATTGCGCCGCTA-3′; Reverse, 5′-CGACGGTATCTGATCGTC-3′; AR: Forward, 5′-CGGAAGCTGAAGAAACTTGG-3′; Reverse, 5′-CGTGTCCAGCACACACTACA-3′; c-FLIPL: Forward, 5′-CCTAGGAATCTGCCTGATAATCGA-3′; Reverse, 5′-TGCGATATACCATGCATACTGAGA-3′; c-FLIPS: Forward, 5′-GCAGCAATCCAAAAGAGTCTCA-3′; Reverse, 5′-ATTTCCAAGAATTTTCAGATCAGG-3′; PSA: Forward, 5′-TGAGCCTCCTGAAGAATCGA-3′; Reverse, 5′-TTGCGCACACACGTCATT-3′; BCL-2: Forward, 5′-GGATGCCTTTGTGGAACTG-3′; Reverse, 5′-AGAGACAGCCAGGAGAAATCA-3′. Real-time PCR was carried out in a 96-well plate using a LC480 light cycler instrument (Roche Diagnostics, Mannheim, Germany). The threshold cycle (CP) was calculated for each reaction and expression levels determined using the relative standard curve method, normalised against 18S expression.
Transfection and luciferase assay
Luciferase reporter assays to assess AR and NF-κB transcriptional activity were performed as previously described (11, 12). Transfected cells were incubated for 24h prior to drug treatment; SAHA was administered for 24h prior to the addition of bicalutamide for a further 48h. Samples were analysed using a Dual-Luciferase Reporter assay kit (Promega, Madison, USA) according to manufacturer’s instructions. Transcriptional activity was calculated by adjusting for transfection efficiency using Renilla, followed by normalisation to sample matched pGL3 readouts.
Immunohistochemistry
Expression of c-FLIP was analyzed in fifty prostate specimens obtained from the Pathology Services of the Polytechnic University of the Marche Region-United Hospitals, Ancona, Italy. The clinical characteristics associated with these specimens are defined in Supplementary Table S1. Forty radical prostatectomies were included, taken from men with clinically detected CaP. Twenty of these prostatectomies were obtained from patients who had not received any hormone treatment and HGPIN was present in all samples. A further 20 prostatectomies were studied in which patients had been under total androgen ablation for three months before surgery; HGPIN was detected in 18 of these specimens. Tissue from 10 transurethral resections of the prostate (TURP) was also included where patients had locally recurrent hormone-independent CaP, defined as a minimum of two consecutive rises in PSA, obtained at least 2 weeks apart, in the presence of castrate levels of testosterone. All tissue samples were obtained from the peripheral zone of the prostate to avoid the influence of zonal distribution upon the determination of c-FLIP expression. In addition, the cancers were all stage pT2a. In the group of 20 specimens with untreated PCa, we had a balance of Gleason score 3+3=6 (10 cases) and 4+4=8 (10 cases). In the group of 20 specimens with treated PCa, the Gleason score as determined in the initial prostate biopsy, was 3+3=6. For each case, the microscope slide with the greatest amount of cancer, HGPIN and normal tissue of the peripheral zone was selected. Five-micron thick sections were cut from archival, formalin-fixed, paraffin embedded specimens, and mounted on silane-coated slides.
c-FLIP expression was performed by standard indirect biotin–avidin immunohistochemical (IHC) analysis. Antigen retrieval was carried out using EDTA buffer in a pressure steamer at 100°C for 90 min. Slides were stained on an automated immunostainer (DakoCytomation, Denmark), using a polyclonal anti-c-FLIP antibody (Enzo Life Sciences, Pennsylvania, USA; 1:50 dilution). Bound antibodies were detected with the Dako Envision™ System. As a negative control, primary antibody was omitted and replaced with PBS. In addition, non-specific rabbit antibody was used, resulting in clean negative results in all cases tested (not shown). Slides were counter-stained with a light haematoxylin.
At least 1,000 cells were counted in contiguous 400X microscopic fields, counting separately for normal epithelium, HGPIN, and CaP. Each slide was assessed independently by two pathologists (FB and RMa). Discrepancies were resolved by a concurrent re-examination by both investigators using a double-headed microscope. c-FLIP expression was evaluated in a semi-quantitative manner whereby the levels of expression are represented as the percentage of positive cells and the intensity of the staining, as follows: [Hscore = 1 × (% weak) + 2 × (% moderate) + 3 × (% intense) generating a ranking between 0 and 300] [15].
Statistical analysis
Experimental results were compared using a two-tailed Student’s t-test analysis, comparing the statistical significance between means (GraphPad Prism, La Jolla, USA). For immunohistochemistry studies, statistical analysis was performed using SPSS software (SPSS Inc., Chicago, USA) using the Mann-Whitney test function. Results were considered significant at p<0.05 for all analyses.
RESULTS
c-FLIP expression in diseased prostate tissue correlates with sensitivity or resistance to androgen-deprivation therapy
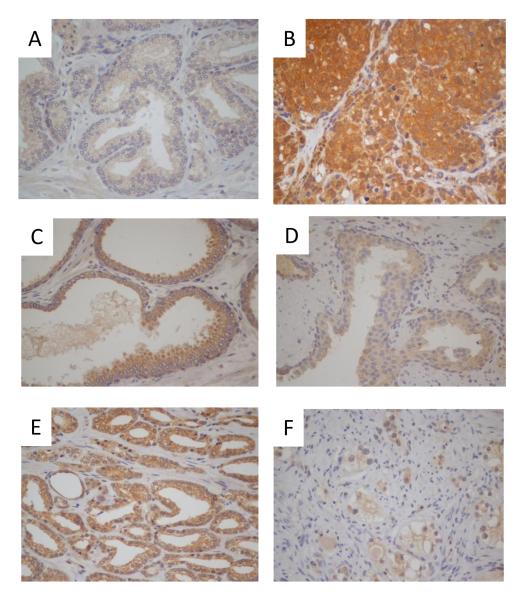
Expression of c-FLIP was evaluated by a semi-quantitative IHC-analysis of radical prostatectomy and TURP tissue (Table S1). c-FLIP expression ranged from a minimal level in histologically-normal prostate epithelium (NPE) (Figure 1A) to a maximal level in CRPC tissue (Figure 1B) (Table S2).
FIGURE 1. Characterization of c-FLIP expression in prostate tissue.
Expression of c-FLIP was determined by immunohistochemistry. Images shown depict (i) normal prostate epithelium [x10], (ii) castrate-resistant tissue [x20], (iii) prostatic-intraepithelial neoplasia [x10], (iv) untreated Gleason pattern 3 [x10], (v) untreated Gleason pattern 4 cancer [x10] and (vi) hormonally-treated Gleason pattern 4 cancer [x20]. Magnification of the image is shown in brackets.
Expression of c-FLIP in hormone-naive normal prostate epithelium, HGPIN and CaP tissue
c-FLIP was detected at low levels in NPE (Table S2; Figure 1(i)). There was no statistically significant difference in the expression of c-FLIP in the adjacent normal tissue between Gleason pattern 3+3 or Gleason pattern 4+4 cancer. Upon the transition to HGPIN, we observed a statistically significant increase in mean H-score for c-FLIP relative to expression in NPE in untreated Gleason score 3+3=6 group (p=0.008) and normal untreated Gleason score 4+4=8 group (p=0.008) (Table S2; Figure 1(iii)). However, as previously observed in normal tissue, the Gleason score of the adjacent cancer did not affect the level of c-FLIP expression detected within HGPIN. There was no observed increase in the expression of c-FLIP in cancer relative to HGPIN; the mean H-score value was 193 ± 4 in the untreated Gleason score 3+3=6 cancers and 202 ± 15 in the untreated Gleason score 4+4=8 cancers (Table S2; Figure 1(v)).
Effect of hormone treatment upon c-FLIP expression
Hormone treatment had no effect on c-FLIP expression in NPE. However, there was a statistically significant decrease in c-FLIP expression in HGPIN foci within hormonally-treated prostate tissue relative to untreated HGPIN tissue (P<0.001) (Figure 1(iv)). Similarly, c-FLIP expression was significantly lower in cancers treated for three months with hormonal-therapy (Figure 1(vi)); this decrease was also statistically significant when compared to c-FLIP expression in the untreated Gleason score 3+3=6 and hormone-treated groups (p=0.001) and between the untreated Gleason score 4+4=8 and hormone-treated groups (p=0.001). Of major significance, the expression of c-FLIP was elevated in locally-recurrent castrate-resistant tissue, with a mean H-score of 282.9 ± 12.13. This increase in the level of expression in castrate-resistant tissue was statistically significant when compared to expression in untreated Gleason score 3+3=6 cancers (p=0.001), untreated Gleason score 4+4=8 cancers and that detected in hormonally-treated cancers (p<0.001) (Figure 1(ii)).
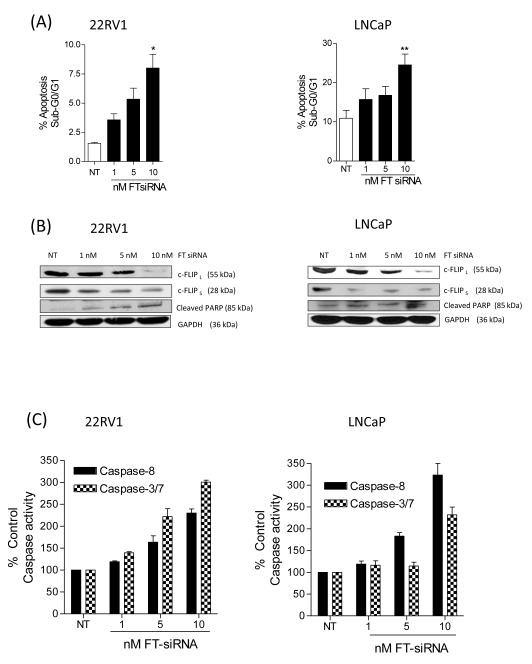
Silencing of c-FLIP induces spontaneous apoptosis in CaP cells
The significance of c-FLIP expression to CaP cell viability was studied in vitro using a previously validated c-FLIP-targeted siRNA-strategy (14). We used splice form-selective oligonucleotides (FL and FS, respectively) to target the two predominant splice variants expressed in human cells, c-FLIPL and c-FLIPS, and a non-selective oligonucleotide (FT) that targets both c-FLIP splice forms. Transfection of 22Rv1 (left panel) and LNCaP cells (right panel) with increasing concentrations of the non-selective FT-siRNA resulted in a dose-dependent increase in the apoptotic cell population (Figure 2A), compared to the effects of a non-targeting-siRNA (NT-siRNA) control. Immunoblotting confirmed the selectivity of the respective siRNAs employed and secondly, confirmed enhanced PARP cleavage, consistent with apoptosis, in cells transfected with the dual c-FLIPL/S-targeting FT siRNA (Figure 2B, left and right panels; Supplementary Figure S1). We also characterized a dose-dependent increase in caspase-8 and caspase-3/7 activity in 22Rv1 and LNCaP cells (Figure 2C, left and right panels respectively). In contrast, 22Rv1 and LNCaP cells displayed a minimal induction of apoptosis upon transfection with either FL-siRNA (c-FLIPL-targeted siRNA) or FS-siRNA (c-FLIPS-targeted siRNA) (Supplementary Figure S1), suggesting that expression of either c-FLIP splice form can maintain the viability of these CaP cell lines.
FIGURE 2. Silencing of c-FLIP induces spontaneous apoptosis in CaP cells.
(A) Histograms showing a dose-dependent induction of apoptosis following FT-siRNA targeted silencing of c-FLIP for 24 h in 22Rv1 (left panel) and LNCaP cells (right panels, respectively). (B) Immunoblots illustrating the specificity of the siRNA pools in decreasing c-FLIP expression and the resultant cleavage of PARP in 22Rv1 (left panel) and LNCaP cells (right panel). Membranes were re-probed with anti-GAPDH to confirm equal loading of protein in all wells. (C) Bar graphs presenting the levels of caspase-8 and caspase-3/7 activity detected in 22Rv1 (left panel) and LNCaP cells (right panel) following transfection with increasing concentrations of the FT-oligonucleotide. All data points represent mean ± SEM, determined from four independent experiments. Statistically significant differences were obtained using a Student’s two-tailed t-test; * p<0.05; ** p<0.01.
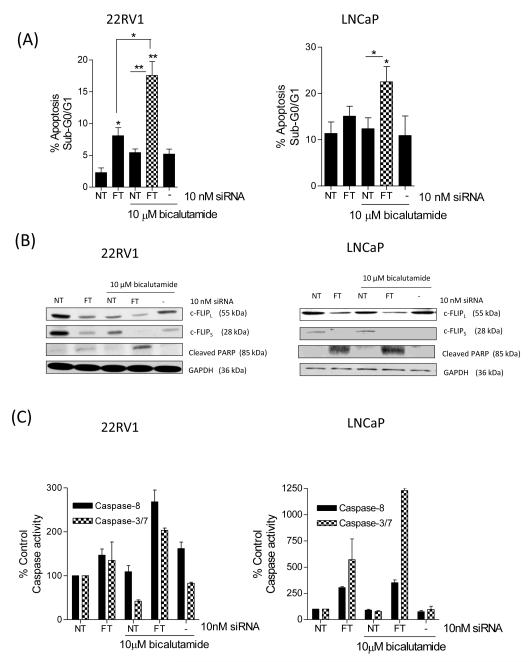
Silencing of c-FLIP potentiates the level of apoptosis in bicalutamide-treated CaP cells
We next investigated whether knockdown of c-FLIP modulated cellular sensitivity to the AR-antagonist bicalutamide. Administration of 10μM bicalutamide decreased c-FLIP expression in 22Rv1 cells but not to a level sufficient to significantly increase apoptosis (Figure 3A/B). However, transfection with FT-siRNA significantly increased apoptosis levels in bicalutamide-treated 22Rv1 cells (p<0.05, Figure 3A/B). In LNCaP cells, bicalutamide failed to induce apoptosis (Figure 3A, right panel) and had no effect on c-FLIP expression (Figure 3B, right panel). Bicalutamide-induced apoptosis was significantly increased in LNCaP cells following transfection with FT-siRNA (Figure 3B). This potentiation of apoptosis was confirmed by measurement of caspase-8 and caspase-3/7 activity. In both 22Rv1 cells (Figure 3C) and LNCaP cells (Figure 3D), the induction of caspase activation was maximal in bicalutamide-treated cells in the presence of the FT-siRNA.
FIGURE 3. Silencing of c-FLIP potentiates the level of apoptosis in bicalutamide-treated androgen-dependent CaP cells.
(A) Histograms presenting the extent of apoptosis detected in 22Rv1 (left panel) and LNCaP cells (right panel) transfected with FT-siRNA and bicalutamide. (B) Representative immunoblots confirming that c-FLIP expression is reduced in bicalutamide-treated 22Rv1 (left panel) and LNCaP cells (right panel) following transfection with the FT-siRNA-oligonucleotides and is coupled to enhanced cleavage of PARP protein. Membranes were re-probed with anti-GAPDH to confirm equal protein loading. (C) The increased apoptotic index in siRNA-transfected cells treated with bicalutamide is dependent on the activation of caspase-8 and caspase-3/7 in (left) 22Rv1 and (right) LNCaP cells. All data points presented represent the mean ± SEM values, calculated from four independent experiments. Statistically significant differences were determined using a Student’s two-tailed t-test; *, p<0.05; **, p<0.01.
HDAC inhibitors down-regulate c-FLIP expression in androgen-dependent CaP cells and potentiate bicalutamide-induced apoptosis
Droxinostat was initially identified by its capacity to potentiate apoptosis in a Fas-resistant CaP cell line due to its ability to repress c-FLIP expression (16). Droxinostat was adopted as an initial pharmacological approach to target c-FLIP expression in androgen-dependent CaP cells. Administration of droxinostat repressed c-FLIP expression and induced PARP cleavage in 22Rv1 and LNCaP cells at concentrations of 30μM and 60μM, respectively (Supplementary Figure S2A). Flow cytometry confirmed statistically significant increases in apoptosis in response to droxinostat in 22Rv1 (p<0.05) and LNCaP cells (P<0.01) at these concentrations (Supplementary Figure S2B). While bicalutamide was ineffective as a single agent, combination of bicalutamide with droxinostat further increased the level of apoptosis in 22Rv1 cells (p<0.001) and LNCaP cells (p<0.05). Maximal repression of c-FLIP was detected in both cells by combined treatment with droxinostat and bicalutamide (Supplementary Figure S2C, left and right panels).
In further experiments, we used a more clinically relevant HDACi, SAHA. SAHA also promoted a concentration-dependent decrease in c-FLIP expression that correlated with apoptosis induction, determined by PARP cleavage (Supplementary Figure S3A). Moreover, SAHA repressed c-FLIP mRNA expression consistent with inhibition of gene transcription (Supplementary Figure S3B). 22Rv1 cells were especially sensitive to SAHA-induced apoptosis (Supplementary Figure S3C). Cell viability curves determined the IC50 of SAHA as 2.2μM in 22Rv1 cells and 3.9μM in LNCaP cells, respectively (Supplementary Figure S3D).
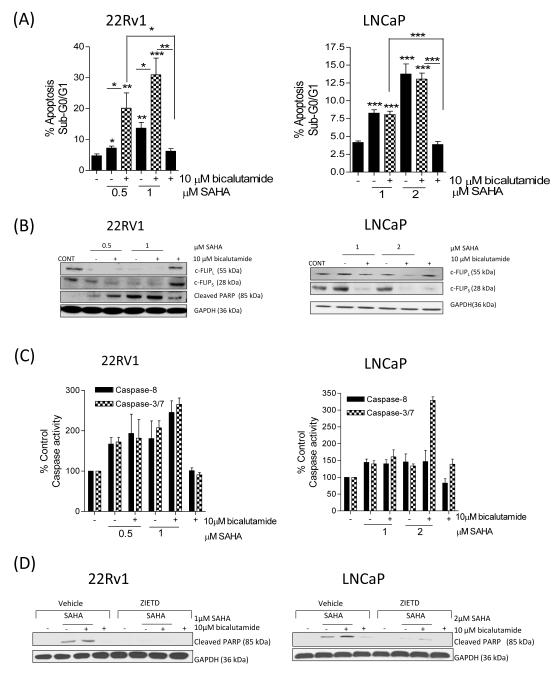
We next examined the effect of SAHA on the sensitivity of 22Rv1 and LNCaP cells to bicalutamide. In 22Rv1 cells, the apoptosis induced by 0.5μM or 1μM SAHA was significantly increased in cells co-treated with 10μM bicalutamide; this was paralleled by demonstrable knockdown of c-FLIPL and c-FLIPS expression in these cells and by an enhanced level of cleaved PARP protein (Figure 4A/B). Likewise, in LNCaP cells, SAHA promoted a significant increase in apoptosis, either in the absence or presence of bicalutamide, compared to bicalutamide alone (p<0.001) (Figure 4A). Addition of 2μM SAHA to bicalutamide (10μM)-treated cells increased apoptosis levels from 4.0±0.4% to 13.0±0.8% (p<0.001). Combination of a lower concentration of SAHA (1μM) with bicalutamide also increased apoptosis levels compared to bicalutamide alone (p<0.001). However, apoptosis levels observed in SAHA/bicalutamide-treated cells was not different from that in SAHA-treated cell populations, as determined by PI- or Annexin V-based detection (Supplementary Figure S4). Immunoblotting experiments demonstrated that the combination of SAHA with bicalutamide decreased c-FLIP isoform expression (Figure 4B).
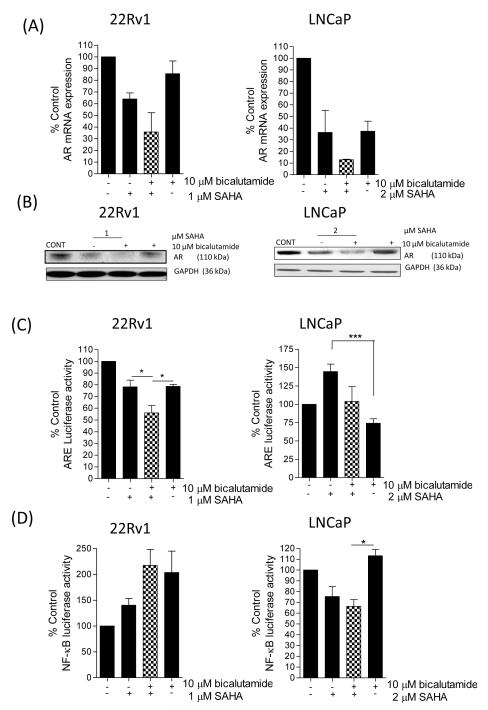
FIGURE 4. SAHA down-regulates c-FLIP expression and increases the sensitivity of CaP cells to undergo apoptosis in the presence of bicalutamide.
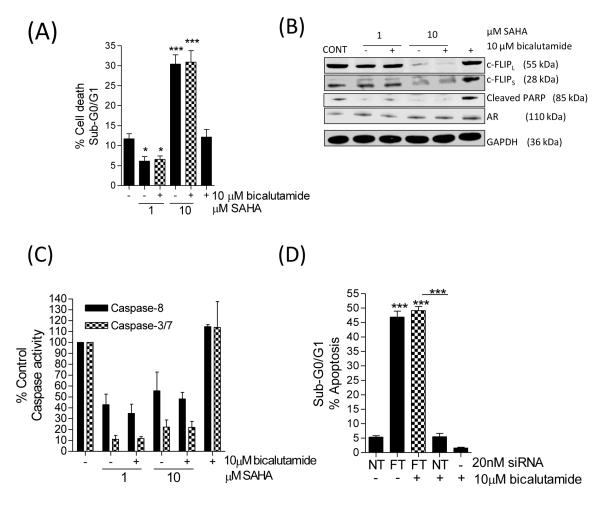
(A) Bar graph illustrating the sub-G0/G1 cell population in 22Rv1 (left panel) and LNCaP (right panel) cells in the absence and presence of 10μM bicalutamide, administered as a single agent or in combination with increasing concentrations of the HDAC inhibitor, SAHA. (B) Immunoblots demonstrating the single agent activity of bicalutamide or SAHA, or effect upon their combined administration on the expression of c-FLIP and/or the processing of PARP. Equal protein loading was confirmed by GAPDH. (C) Bar graph illustrating the effect of bicalutamide and SAHA upon the induction of caspase-8 or caspase-3/7 activity in 22Rv1 cells (left panel) and LNCaP cells (right panel). (D) Immunoblots characterizing the impact of SAHA and/or bicalutamide upon the induction of PARP cleavage, in the absence or presence of the pan-caspase inhibitor, ZIETD. ZIETD was incubated with the cells for 12 h prior to the experiment. All data points shown in (A) and (C) represent the mean ± S.E.M. value, calculated from four independent experiments. Statistically significant differences were obtained using a Student’s two-tailed t-test; *,p<0.05; **, p<0.01; ***, p<0.001).
The relevance of caspase activation in drug-induced apoptosis was addressed. In 22Rv1 cells, SAHA alone or in combination with bicalutamide (10μM) increased caspase-8 and caspase-3/7 activity relative to vehicle control or treatment with bicalutamide alone. At the highest concentration of SAHA, co-treatment induced a rise of caspase-8 and caspase-3/7 activity to 245.3±28.9% and 265.5±15.6%, respectively (Figure 4C, left panel). Similarly, in LNCaP cells, caspase activity was augmented in response to SAHA and SAHA/bicalutamide; where there was a > 3-fold increase in caspase 3/7 activity with 2μM SAHA in combination with bicalutamide (Figure 4C, right panel). To confirm the importance of caspase-8 in initiating drug-induced apoptosis, caspase-8 activity was inhibited by the addition of Z-IETD-fmk prior to the drug treatments (Figure 4D). In the absence of Z-IETD-FMK, SAHA and SAHA/bicalutamide induced PARP cleavage in 22Rv1 and LNCaP cells, however, SAHA- or SAHA/bicalutamide-induced cleavage of PARP was completely abrogated in 22Rv1 cells and attenuated in LNCaP cells following pre-treatment with Z-IETD-FMK (Figure 4D).
SAHA modulates AR and NF-kB pathways in CaP cells
We next examined how SAHA impacted upon AR and NF-κB activity, given their importance in modulating c-FLIP gene transcription. Concentrating on the AR pathway initially, we confirmed a higher level of AR expression and activity in 22Rv1 cells relative to LNCaP cells (Supplementary Figure S5A/B). SAHA decreased AR transcript levels and AR protein expression in both cell lines. In combination experiments, AR mRNA (Figure 5A) and protein levels (Figure 5B) were further down-regulated in cells co-treated with SAHA and bicalutamide. Furthermore, in 22Rv1 cells, addition of 1μM SAHA was as effective as the AR antagonist bicalutamide (10μM) in decreasing ARE-driven luciferase activity (to 77% of control). Combination of these two inhibitors had an additive effect in repressing AR activity to 54.8% of control (p<0.05 relative to individual treatments) (Figure 5C, left panel). In contrast, while bicalutamide decreased AR activity in LNCaP cells, SAHA increased ARE-driven luciferase activity to 144.7±10.1% of control. Co-treatment with SAHA and bicalutamide resulted in no overall change in AR activity relative to untreated control LNCaP cells (Figure 5C, right panel).
FIGURE 5. SAHA modulates the AR and pathway in androgen-dependent CaP cells.
(A) Bar graphs illustrating the effect of SAHA, bicalutamide or their combination upon the transcript levels encoding the AR gene in 22Rv1 cells (left panel) or LNCaP cells (right panel). (B) Immunoblots illustrating the effect of SAHA, bicalutamide or their combined administration upon the expression of the AR in 22Rv1 (left panel) or LNCaP cells (right panel). Membranes were re-probed with anti-GAPDH to ensure equal loading of protein in the wells. (C) Bar graphs showing the effect of SAHA, bicalutamide or a combined SAHA/bicalutamide administration upon AR activity in 22Rv1 (left panel) and LNCaP cells (right panel). (D) Bar graphs illustrating the effect of SAHA, bicalutamide or their combined administration upon NF-κB transcriptional activity in 22Rv1 and LNCaP cells (left and right panels, respectively). All data points shown in bar graphs represent the mean ± S.E.M. value, calculated from a minimum of 3-6 independent experiments. Immunoblots are representative of at least three experiments. Statistically significant differences between values were determined using a Student’s two-tailed t-test; *, p<0.05; ***, p<0.001).
Increased NF-κB transcriptional activity is implicated in the development of castrate-resistance (17, 18) and underpins transcription of the AR (19), c-FLIP (20, 21) and PSA (22) genes. Intrinsic levels of NF-κB activity were approximately 2-fold higher in LNCaP cells relative to 22Rv1 cells (Supplementary Figure S5C). Administration of 1μM SAHA or 10μM bicalutamide, alone or in combination, increased NF-κB activity in 22Rv1 cells (Figure 5D, left panel). In contrast, the combination of SAHA and bicalutamide reduced NF-κB activity to 65% of control levels in LNCaP cells (Figure 5D, right panel).
The cell-specific effects of SAHA and bicalutamide on AR and NF-κB activity was investigated by RT-PCR analysis of downstream genes. Consistent with the SAHA/bicalutamide combination reducing NF-κB activity in LNCaP cells, we observed a reduction in Bcl-2 mRNA transcript levels for Bcl-2 in LNCaP cells but not 22Rv1 cells (Supplementary Figure S5D). In further analysis, SAHA induced expression of the AR- and NF-κB-regulated gene PSA in either cell line, consistent with SAHA-induced AR activity in LNCaP cells and NF-κB activity in 22Rv1 cells. The combination of SAHA with bicalutamide reduced PSA gene expression back to levels detected in untreated 22Rv1 cells or to below basal levels in LNCaP cells (Supplementary Figure S5E). Finally, RT-PCR analysis confirmed that the SAHA/bicalutamide combination reduced CXCL8 gene expression in LNCaP cells consistent with the reduction of NF-κB activity. In contrast, due to drug-induced elevation of NF-κB activity in 22Rv1 cells, CXCL8 gene expression increased in this model (Supplementary Figure S5F).
Increased expression of c-FLIP correlates with cellular insensitivity to bicalutamide and SAHA
VCaP is a CRPC cell model (23). VCaP cells exhibited a significantly higher level of AR activity (~4-fold) compared to 22Rv1 cells (Supplementary Figure S6A). Immunoblots also confirmed a higher level of c-FLIPS and BCL-2 expression in VCaP cells relative to 22Rv1 and LNCaP cells (Supplementary Figure S6B). VCaP cells were more tolerant to SAHA treatment; cell viability assays determined an IC50 value of 9.2 μM (data not shown). SAHA was ineffective in inducing apoptosis (Supplementary Figure S6C), in down-regulating c-FLIP expression at concentrations < 10μM (Supplementary Figure S6D) and consistently reduced caspase-8 and caspase-3/7 activity across the concentration range (Supplementary Figure S6E). At higher concentrations, SAHA (10μM) alone or in combination with bicalutamide was shown to decrease mitochondrial TMRE-retention, indicative of mitochondrial outer membrane permeabilization (MOMP). Bicalutamide did not affect MOMP (Supplementary Figure S6F). This suggests that at higher concentrations, SAHA alone or in combination with bicalutamide, induces a mitochondrial-dependent, caspase-independent mode of cell death.
SAHA was unable to restore sensitivity to AR-directed therapy in VCaP cells; use of SAHA at a clinical achievable concentration of 1 μM with bicalutamide did not induce apoptosis (Figure 6A), or down-regulate the expression of anti-apoptotic c-FLIP protein (Figure 6B) and was incapable of potentiating caspase-8 or caspase-3/7 activity in this cell line (Figure 6C). Furthermore, the combination of SAHA with bicalutamide was unable to reduce AR or NF-κB activity, measured by PSA and Bcl-2 gene readouts (Supplementary Figure 7A/B). To investigate the role of c-FLIP over-expression in modulating the survival and therapeutic resistance of VCaP cells, cells were transfected with the FT-siRNA prior to exposure to bicalutamide. Loss of c-FLIP alone significantly increased apoptosis in these CRPC cells (Figure 6D). In addition, we detected an equivalent level of apoptosis in c-FLIP-depleted VCaP cells treated with bicalutamide. This experiment was repeated using the androgen-ablated derivative of the LNCaP cells, the LN-Abl model [24]. Consistent with prior reports, LN-Abl cells had increased AR activity relative to LNCaP cells, but did not attain the AR activity observed in VCaP cells (Supplementary Figure S7C). Correspondingly, we observed an intermediate level of c-FLIP expression in this model (Supplementary Figure S7D). While bicalutamide reduced the level of death in these cells, the knockdown of c-FLIP was shown to once again potentiate apoptosis (Supplementary Figure S7E).
FIGURE 6. Castrate-resistant VCaP cells are insensitive to bicalutamide and SAHA.
(A) Bar graph showing the percentage apoptosis detected in VCaP cells treated with 1 μM or 10 μM SAHA, in the absence or presence of 10 μM bicalutamide. (B) Immunoblot characterizing the level of c-FLIP or AR expression, or the promotion of PARP cleavage in VCaP cells following treatment with SAHA in the absence or presence of bicalutamide at the indicated concentrations. Membranes were re-probed with anti-GAPDH to ensure equal loading. (C) Bar graph illustrating the level of caspase-8 and caspase-3/7 activity in SAHA, bicalutamide or SAHA/bicalutamide-treated VCaP cells. (D) Bar graph showing the effect of a FT-siRNA to modulate the sensitivity of VCaP cells to bicalutamide. All data points shown in the figure represent the mean ± S.E.M. value, calculated from a minimum of 3 to 5 independent experiments. Statistically significant differences were determined using a Student’s two-tailed t-test; *, p<0.05; ***, p<0.001).
DISCUSSION
c-FLIP is an anti-apoptotic protein which antagonizes caspase-8-mediated apoptosis. Transcription of the c-FLIP gene (cFLAR) in CaP cells has been shown by us and others to be in part under AR regulation (9-11), suggesting that this anti-apoptotic protein may constitute an important component of androgen-driven, AR-mediated survival. C-FLIP gene expression is also regulated by NF-κB (20, 21), a transcription factor whose activation is elevated in more aggressive CaPs and has been associated with the transition to castrate-resistance (17, 18, 25, 26). Therefore, we proposed that c-FLIP may be relevant to all stages of disease progression and may modulate the onset of resistance to AR-targeted therapeutics enabling the outgrowth of CRPC cells. While we have previously shown that c-FLIP confers resistance of metastatic CaP cells to docetaxel, TRAIL, and oxaliplatin (27), its relevance to AR-targeted therapeutics has not been previously investigated.
Our data shows that c-FLIP expression is increased in pre-malignant PIN foci and is retained in untreated Gleason pattern 3 and 4 cancers. Silencing c-FLIP using siRNA-oligonucleotides resulted in a spontaneous apoptosis in two CaP cell lines, 22Rv1 and LNCaP, supporting an important role of this protein in maintaining cell survival. Splice form-selective siRNA oligonucleotides further indicated that both of the predominant human isoforms of this protein have equal importance in regulating CaP cell survival. Therefore, the increased c-FLIP expression may enable cancer cells to remain viable in the face of the accumulating genetic instability present within HGPIN and locally-confined disease.
We have further defined the significance of c-FLIP as a key regulator of ADT response. Analysis of prostatectomy tissue confirmed that hormonal treatment can reduce c-FLIP expression, consistent with AR-regulated expression of this gene. In vitro, bicalutamide only partially reduced c-FLIP expression in CaP cells and therefore had a limited effect in inducing apoptosis, even when administered at concentrations up to 10μM. However, siRNA-mediated silencing of c-FLIP resulted in a marked potentiation of apoptosis in bicalutamide-treated 22Rv1 cells while underpinning the high levels of spontaneous apoptosis induced in bicalutamide-treated LNCaP cells. These results confirm that c-FLIP expression can dampen the sensitivity of CaP cells to undergo ADT-induced apoptosis.
Of major clinical relevance, we also report a very significant increase in the expression of c-FLIP in the tumours of castrate-resistant patients. This increased level of expression in tissue may relate to increased activity of its transcriptional regulators in CRPC cells, and equally, may reflect the selection of therapy-resistant cells as a consequence of high c-FLIP expression. In vitro experiments conducted on two cell-based models of CRPC clearly demonstrate that the over-expression of c-FLIP is fundamental to the viability of these cells, given the prominent induction of spontaneous apoptosis in these cells following siRNA-targeting of c-FLIP. Thus, repressing the expression of c-FLIP may be an appropriate strategy to target what may be an Achilles’ heel of CRPC.
Histone deacetylase inhibitors (HDACi) have been shown to repress c-FLIP expression in cancer cells (15, 28-31). We used droxinostat and SAHA (Vorinostat®) as representative HDACi to modulate c-FLIP expression and determine the impact on bicalutamide sensitivity in AR-expressing CaP cells. Droxinostat and SAHA both induced spontaneous apoptosis in 22Rv1 and LNCaP cells, coinciding with a potent down-regulation of both c-FLIP isoforms and moreover, potentiated or retained the maximal level of apoptosis observed in bicalutamide-treated 22Rv1 and LNCaP cells. The mode-of-action of SAHA was observed to be complex and cell-specific. SAHA-promoted down-regulation of c-FLIP expression paralleled the down-regulation of c-FLIP transcript levels in CaP cells, consistent with a predominantly transcription-mediated regulation of this gene. SAHA was subsequently shown to decrease the transcriptional activity of AR or NF-κB signaling in a cell-line specific manner, consistent with promoting decreased transcription of c-FLIP in the CaP cells. In the 22Rv1 cell line, the synergy in apoptosis induction resulting from the combination of bicalutamide with HDACi, reflects the capacity of SAHA to attenuate but not abrogate AR transcriptional activity, with the further reduction in AR transcription and survival mediated by the presence of bicalutamide. In contrast, SAHA had greater effect in attenuating NF-κB as opposed to AR activity in LNCaP cells. Therefore, in LNCaP cells, it is apparent that SAHA-induced apoptosis results predominantly from the reduction in NF-κB signaling and consequently, the addition of bicalutamide provides no further enhancement of apoptotic induction in this model. Therefore the combination of SAHA and bicalutamide is effective in attenuating the activity of two major transcription factors implicated in the progression to CRPC and sensitizes cells to undergo an increased level of apoptosis irrespective of the principal progression-driving pathway in the tumor.
HDACi have been trialled in CRPC but with limited success (32). Using VCaP as a model of CRPC, we found that HDACi were unable to down-regulate c-FLIP expression, suggesting that this class of agent may be unable to surmount the high level of AR and NF-κB signalling present in CRPC. Targeted knockdown of c-FLIP using the FT-siRNA however did induce a spontaneous apoptosis in CRPC cells, indicating that the elevated c-FLIP expression and an output of AR and NF-κB signalling in this castrate-resistant cell line may render these cells resistant to apoptosis following treatment with HDACi. Previous studies have shown that elevated BCL-2 expression in VCaP cells diminishes the sensitivity of PC3 cells to HDACi in vitro (33). However, our studies show that c-FLIP over-expression is equally important in underpinning the resistance of VCaP cells and CRPC to AR- and molecularly-targeted therapeutics. Targeting the marked over-expression of anti-apoptotic proteins in CRPC must therefore be a prime consideration for the development of more effective treatments in this currently incurable condition.
In summary, we have shown that c-FLIP expression is increased in pre-malignant and malignant CaP, with further increased expression detected within castrate-resistant tumors. CaP cells exploit the increased expression of c-FLIP to maintain their viability and to diminish the sensitivity of the cells to androgen ablation therapy. While HDACi can down-regulate c-FLIP expression through down-regulation of AR and NF-κB activity, and increase the sensitivity of androgen-dependent CaP cells to undergo caspase-8 and caspase-3/7 promoted apoptosis in the presence of the AR-antagonist bicalutamide, our data indicates that there are levels to which this strategy is effective. The elevated expression of c-FLIP in the castrate-resistant VCaP cells and CRPC is consistent with these cells being insensitive to HDACi in vitro and the sub-optimal performance of HDACi in trials conducted on metastatic CaP patients, respectively. Consistent with a prior publication (34), our data suggests that an earlier introduction of HDACi may increase the effectiveness of bicalutamide in treating progressive CaP, where increasing response rates or the duration of response to androgen-targeted therapies would significantly impact on patient outcomes, retarding the rate and incidence of progression to castrate-resistant disease. Moreover, the expression of c-FLIP in diagnostic tissue may assist in predicting response to androgen-deprivation therapies, where elevated expression of this protein is indicative of resistance.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
AR signaling is the principal pathway driving prostate cancer (CaP) growth. Inhibition of androgen-driven signaling is the predominant therapeutic strategy for CaP, reinforced by the recent development of two clinically effective agents, abiraterone-acetate and MDV3100. Here we show that c-FLIP, an AR-regulated anti-apoptotic protein constitutes a novel mode of resistance to AR-targeted therapeutics. Down-regulation of c-FLIP in response to siRNA-targeting or histone deacetylase inhibitors (HDACi) induced apoptosis co-incident with down-regulation of c-FLIP expression and potentiated the response to the AR-targeted drug bicalutamide in non-castrate-resistant cells. In contrast, the elevated c-FLIP expression detected in castrate-resistant cells antagonized HDACi and bicalutamide response. Accordingly, HDACi may be useful in prolonging the therapeutic response to AR-targeted drugs in non-castrate conditions. Moreover, the increase in c-FLIP expression determined in castrate-resistant disease may assist in predicting patient response to existing and emerging androgen deprivation strategies and alternatively, may itself be a rationale therapeutic target to improve treatment of CRPC.
ACKNOWLEDGEMENTS
The long-standing support of the Ulster Cancer Foundation (DW) and the provision of equipment funding from the Friends of the Cancer Centre (DW, JO’S) is also gratefully acknowledged. We also extend our thanks to Dr Alfredo Santinelli for providing statistical analysis of the marker expression in the tissue sections.
GRANT SUPPORT
This work was supported by a grant from Prostate Cancer Research Foundation (Prostate Action) (DW, DL, JO’S) and a Stipend from the Department of Employment and Learning (CMcC).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2(5):389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 3.Floyd MS, Jr, Teahan SJ, Fitzpatrick JM, Watson RW. Differential mechanisms of bicalutamide-induced apoptosis in prostate cell lines. Prostate Cancer Prostatic Dis. 2009;12(1):25–33. doi: 10.1038/pcan.2008.23. [DOI] [PubMed] [Google Scholar]
- 4.Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers. 2011;3:1639–1671. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388(6638):190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 6.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: Regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21(24):8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274(3):1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 8.Shirley S, Micheau O. Targeting c-FLIP in cancer. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, et al. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19(7):1792–802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao S, Wang H, Lee P, Melamed J, Li CX, Zhang F, et al. Androgen receptor and prostate apoptosis response factor-4 target the c-FLIP gene to determine survival and apoptosis in the prostate gland. J Mol Endocrinol. 2006;36(3):463–83. doi: 10.1677/jme.1.01991. [DOI] [PubMed] [Google Scholar]
- 11.Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7(9):2649–61. doi: 10.1158/1535-7163.MCT-08-0148. [DOI] [PubMed] [Google Scholar]
- 12.Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, et al. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis. 2008;29(6):1148–56. doi: 10.1093/carcin/bgn109. [DOI] [PubMed] [Google Scholar]
- 13.Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L, et al. c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene. 2006;25(6):838–48. doi: 10.1038/sj.onc.1209122. [DOI] [PubMed] [Google Scholar]
- 14.Wilson TR, McLaughlin KM, McEwan M, Sakai H, Rogers KM, Redmond KM, et al. c-FLIP: A key regulator of colorectal cancer cell death. Cancer Res. 2007;67(12):5754–62. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- 15.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR, et al. Identification of small molecules that sensitize resistant tumor cells to tumor necrosis factor-family death receptors. Cancer Res. 2006;66(4):2367–75. doi: 10.1158/0008-5472.CAN-05-1061. [DOI] [PubMed] [Google Scholar]
- 17.Andela VB, Gordon AH, Zotalis G, Rosier RN, Goater JJ, Lewis GD, et al. NFkappaB: A pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res. 2003;415(415 Suppl):S75–85. doi: 10.1097/01.blo.0000093048.96273.aa. [DOI] [PubMed] [Google Scholar]
- 18.Ismail HA, Lessard L, Mes-Masson AM, Saad F. Expression of NF-kappaB in prostate cancer lymph node metastases. Prostate. 2004;58(3):308–13. doi: 10.1002/pros.10335. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489–99. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21(16):5299–305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21(12):3964–73. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22(8):2862–70. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15(2):163–8. [PubMed] [Google Scholar]
- 24.Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–51. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91(4):417–20. doi: 10.1046/j.1464-410x.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 26.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, et al. Nuclear factor-kappaB/p65 (rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6(4):390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7(9):2649–61. doi: 10.1158/1535-7163.MCT-08-0148. [DOI] [PubMed] [Google Scholar]
- 28.Yerbes R, Lopez-Rivas A. Itch/AIP4-independent proteasomal degradation of cFLIP induced by the histone deacetylase inhibitor SAHA sensitizes breast tumour cells to TRAIL. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9597-x. [DOI] [PubMed] [Google Scholar]
- 29.Lucas DM, Alinari L, West DA, Davis ME, Edwards RB, Johnson AJ, et al. The novel deacetylase inhibitor AR-42 demonstrates pre-clinical activity in B-cell malignancies in vitro and in vivo. PLoS One. 2010;5(6):e10941. doi: 10.1371/journal.pone.0010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bijangi-Vishehsaraei K, Saadatzadeh MR, Huang S, Murphy MP, Safa AR. 4-(4-chloro-2-methylphenoxy)-N-hydroxybutanamide (CMH) targets mRNA of the c-FLIP variants and induces apoptosis in MCF-7 human breast cancer cells. Mol Cell Biochem. 2010;342(1-2):133–42. doi: 10.1007/s11010-010-0477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood TE, Dalili S, Simpson CD, Sukhai MA, Hurren R, Anyiwe K, et al. Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. Mol Cancer Ther. 2010;9(1):246–56. doi: 10.1158/1535-7163.MCT-09-0495. [DOI] [PubMed] [Google Scholar]
- 32.Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (national cancer institute trial 6862): Trial results and interleukin-6 analysis: A study by the Department of Defense prostate cancer clinical trial consortium and University of Chicago phase 2 consortium. Cancer. 2009;115(23):5541–9. doi: 10.1002/cncr.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W, Ngo L, Perez G, Dokmanovic M, Marks PA. Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. Proc Natl Acad Sci U S A. 2006;103(42):15540–5. doi: 10.1073/pnas.0607518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrocco DL, Tilley WD, Bianco-Miotto T, Evdokiou A, Scher HI, Rifkind RA, et al. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6(1):51–60. doi: 10.1158/1535-7163.MCT-06-0144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.