Abstract
Replication-competent (oncolytic) adenoviruses (OAV) can be adapted as vectors for the delivery of therapeutic genes, with the aim of extending the antitumor effect beyond direct cytolysis. Transgene expression using these vectors is usually intense but short-lived, and repeated administrations are hampered by the rapid appearance of neutralizing antibodies (NAbs). We have studied the performance of monocytes as cell carriers to improve transgene expression in cancer models established in athymic mice and immunocompetent Syrian hamsters. Human and hamster monocytic cell lines (MonoMac6 and HM-1, respectively) were loaded with replication-competent adenovirus-expressing luciferase. Intravenous administration of these cells caused a modest increase in transgene expression in tumor xenografts, but this effect was virtually lost in hamsters. In contrast, intratumoral administration of HM-1 cells allowed repeated cycles of expression and achieved partial protection from NAbs in preimmunized hamsters bearing pancreatic tumors. To explore the therapeutic potential of this approach, HM-1 cells were loaded with a hypoxia-inducible OAV expressing the immunostimulatory cytokine interleukin-12 (IL-12). Three cycles of treatment achieved a significant antitumor effect in the hamster model, and transgene expression was detected following each administration, in contrast with the rapid neutralization of the free virus. We propose monocytes as carriers for multiple intratumoral administrations of armed OAVs.
Bunuales and colleagues examine the therapeutic potential of monocytes as carrier cells of a replication-competent oncolytic adenovirus encoding IL-12 (OAV-12). Administration of monocytes carrying OAV-12 achieved significant antitumor effects in a hamster model, and transgene expression was detectable after each administration, in contrast with the rapid neutralization of free OAV-12.
Introduction
Oncolytic viruses (OV) are natural or modified viruses with the ability to preferentially replicate in and destroy cancer cells, in comparison with the surrounding normal cells. The number of different types of viruses proposed for the treatment of cancer is continuously expanding, in search of agents with the optimal balance between potency and specificity. However, the experience accumulated with early versions of these agents indicates that the immune system and physical barriers in the tumor microenvironment are important obstacles for the spread and amplification of OVs, especially in the clinical setting (Eager and Nemunaitis, 2011). To overcome these limitations, OVs have been adapted as vectors for the expression of therapeutic genes, with the aim of increasing their oncolytic effect (pro-apoptotic or suicide genes), or to gain a systemic antitumor effect (cytokines, tumor antigens, etc.). In fact, some of the most promising results in recent clinical trials involve the use of OVs expressing the immunostimulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) (Lei et al., 2009; Senzer et al., 2009; Breitbach et al., 2011). This approach may alleviate the need for efficient biodistribution of the virus in the tumor, but the appearance of neutralizing antibodies (NAb) remains a serious obstacle to maintain the function of the virus in repeated administrations. The influence of the immune system on OVs is especially relevant in the case of highly immunogenic agents such as oncolytic adenoviruses (OAV), which, on the other hand, are efficient gene therapy vectors (Alemany and Cascallo, 2009).
The tumor tropism of certain cell types has stimulated their use as carriers for OVs, with the double purpose of achieving tumor targeting upon systemic administration and shielding the virus from NAbs. These cells include different kinds of stem cells such as mesenchymal (Dwyer and Kerin, 2010), adipose (Josiah et al., 2010) or neural stem cells (Ahmed et al., 2011), as well as lymphocytes (Thorne et al., 2010), monocytes/macrophages (Muthana et al., 2011) or tumor cells (Raykov and Rommelaere, 2008). In general terms, cells derived from the hematopoietic system are more suited to escape from anatomical filters such as lungs and liver, whereas epithelial cells are more efficient in OAV amplification and release. Monocytes are an attractive option because they accumulate in the hypoxic areas of tumors, and they can be loaded with viruses designed to be activated in response to hypoxia-inducible pathways (Muthana et al., 2011). The use of autologous cells ensures their compatibility with the recipient, but increases the cost and complicates the logistics of the treatment. In contrast, approaches based on cell lines are easier to standardize and could be suitable if long-term expression of the transgene is not required (Liu et al., 2010).
The concept of tumor homing has been extensively demonstrated in preclinical studies, although unbiased quantification of the percentage of carrier cells that reach the tumor upon systemic administration is seldom reported. In addition, the relevance of animal models is an important issue in the case of OAV, since mice are not permissive for human adenovirus. Specificity of OAV replication, and hence transgene expression, are usually overestimated in human tumor xenografts established in athymic mice, and the defects in the immune system of the host complicates the evaluation of the protective role of carrier cells. In the present work, we have used the Syrian hamster as an immunocompetent, permissive model (Thomas et al., 2006; Bortolanza et al., 2007).
The aim of this study, rather than searching for an ideal cell candidate, was to evaluate the properties of a representative cell line, with special focus on the analysis of tumor transduction. Therefore, we used materials relatively simple and easy to standardize, such as monocytic/macrophage cell lines and a replication-competent adenovirus expressing luciferase without tumor specificity. This allowed us to reach several conclusions that can be applied to different therapeutic approaches. In contrast with xenografts, tumor homing of virus-loaded monocytes was virtually undetectable following systemic administration in hamsters. However, we provide evidence that these cells can be used to protect the virus from NAbs and preserve the efficacy of repeated intratumoral administrations. When these cells were loaded with a hypoxia-inducible OAV expressing the immunostimulatory cytokine interleukin-12 (IL-12), we demonstrated improved transgene expression and antitumor effect compared with the administration of the free virus.
Materials and Methods
Cells
The human hepatocellular carcinoma (HuH-7; JCRB Genebank, Japan) and Syrian hamster pancreatic cancer (HaP-T1) tumor cell lines (German Collection of Microorganisms and Cell Cultures DSMZ ACC 222) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The Syrian hamster macrophage cell line HM-1 (JCRB Genebank, Japan) was maintained in Roswell Park Memorial Institute medium (RPMI) supplemented with 10% FBS. The human monocyte/macrophage cell lines Mono-Mac-6 (here designated MM6) and acute monocytic leukemia (THP-1) were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, ACC 124) and the American Tissue Culture Collection (ATTC, TIB-202), respectively. These cells were maintained in RPMI supplemented with 10% FBS plus 2 mM L-glutamine, nonessential amino acids, and 1 mM sodium pyruvate. MM6 were supplemented with 10 μg/ml human insulin. Human monocytes were isolated from buffy coats using CD11c microbeads (Miltenyi Biotech) and the autoMACS Pro separator according to manufacturer instructions. All culture media were supplemented with 100 U/ml penicillin, 100μg/ml streptomycin, and 2 mM L-glutamine. All cells were maintained at 37°C with 5% CO2 in a humidified incubator.
Viruses
The Ad-CMV-Luc virus (Vector Biolabs) is a first-generation, replication-deficient adenovirus-expressing luciferase under the control of the cytomegalovirus (CMV) promoter. The same promoter controls the expression of the green fluorescent protein (GFP) in the first-generation adenovirus-expressing GFP (Ad-GFP) virus, as previously described (Narvaiza et al., 2006). The Ad-WTLuc virus (Bortolanza et al., 2009a) is based on wild-type adenovirus type 5 (Ad5) with insertion of the luciferase gene into the E3 region (substitution of the E3-6.7 K/gp19K genes). This location determines that luciferase expression is under the control of endogenous viral promoters and is dependent on viral replication (Hawkins et al., 2001). Ad-IL12G is an OAV derived from the previously described Ad-DHscIL12 virus (Bortolanza et al., 2009b). Both versions contain the murine single-chain IL-12 as a transgene in the E3 locus and a partial deletion of the E1A gene (comprising bp 922 to 947) to obtain preferential replication in cancer cells (Fueyo et al., 2000; Heise et al., 2000).
Modifications in Ad-IL12G were designed to obtain a more compact genome. They include restoration of the wild-type E4 promoter and incorporation of a smaller promoter in the E1A region, containing 3 hypoxia-response elements (HREs) instead of the nine copies present in the previous version. No differences in hypoxia responsiveness were observed between both promoters using luciferase reporter plasmids (not shown). All viruses were amplified in 293 cells and purified by ultracentrifugation in cesium chloride (CsCl) gradient. Quantification of infectious units (iu) was done using the Adeno-X rapid titer kit (Clontech).
Infectivity assay
Cells were infected with different multiplicity of infection (MOIs) of the Ad-GFP virus, and 24 hr later the percentage of cells expressing GFP was analyzed using a FACScan flow cytometer (BD Biosciences).
In vitro viral replication assay
Cells (5×104/well) were cultured in 24-well plates overnight and then incubated for 12 hr in 300 μl of medium containing 2% FBS and the indicated amounts of viruses. After intensive washing with phosphate-buffered saline (PBS), cells were maintained in 500 μl of virus-free medium for different periods. Then, cells and supernatants were collected separately and cells were lysed by three cycles of freezing and thawing. The amount of viruses in supernatants and cell lysates was quantified by end-limiting dilution on 293 cells and expressed in total iu per well.
Tumor models and treatments
Xenografts were induced in the flank of 5–6 week-old athymic nu/nu mice (Charles River Laboratories) by subcutaneous inoculation of 107 HuH-7 cells. When tumors reached a volume of 500–700 mm3, animals were treated with viruses or virus-loaded carrier cells. For intravenous administrations, MM6 or HM-1 cells were infected ex vivo with the indicated MOIs of Ad-WTLuc for 4 hr. Virus was then intensively washed out with PBS. Viability of cells was verified by trypan blue exclusion and they were resuspended in 200 μl saline solution before tail vein injection. The same procedure was carried out for intratumoral administration, except that infection was extended for 24 hr and cells were resuspended in 50 μl saline solution before injection.
Liver metastases of pancreatic cancer were established in Syrian (Golden) hamsters (Mesocricetus auratus, HSD HAN: AURA, 5 weeks of age; Harlan) by intrahepatic injection of 3×106 HaP-T1 cells through laparotomy, as previously described (Bortolanza et al., 2007). Intravenous inoculation of viruses or virus-loaded carrier cells was performed by retro-orbital injection. Intratumoral inoculations were performed by direct injection through laparotomy for single inoculations. Repeated intratumoral inoculations were performed by ultrasound-guided percutaneous injection using a dedicated small-animal high-resolution ultrasound imaging unit (VEVO 770; Visualsonics), as previously described (Zabala et al., 2009). Ex-vivo infection of carrier cells with Ad-WTLuc was performed as described above. In the case of the Ad-IL12G virus, HM-1 cells were placed in hypoxia chambers (1% O2, 94% N2, 6% CO2; Billups Rothenberg Inc.) during infection.
Tumor volumes were calculated at necropsy using the formula V=(Dxd2)/2, where D and d are the major and minor diameters, respectively. Blood samples were obtained by retro-orbital sinus blood extraction. All procedures were carried out following protocols approved by the local ethical committee in accordance with recommendations for proper care and use of laboratory animals.
Quantification of luciferase activity in vitro
Cells (5x104/well) were cultured in 24-well plates overnight and then incubated for 12 hr in 300 μl of medium containing 2% FBS and the indicated amounts of viruses. After intensive washing with PBS, cells were maintained in 500 μl of virus-free medium. Cell lysates were collected 48 hr later, and luciferase activity was analyzed using the Luciferase Assay System (Promega).
Quantification of neutralizing antibodies
Anti-adenovirus type 5 neutralizing antibodies were determined using a modified luciferase-based virus neutralization assay, as previously described (Fontanellas et al., 2010).
In vivo bioluminescence detection
Ten minutes before luciferase detection, mice and hamsters received an intraperitoneal injection of 100 μl or 300 μl D-Luciferin Firefly (Sigma), respectively. Animals were anesthetized and placed in a dark chamber connected to a cooled charged coupled device (CCD) camera (IVIS, Xenogen). Photon emission was quantified and analyzed using Living Image Software (Caliper).
Quantification of murine IL-12 in serum
An OptEIA mouse IL-12 ELISA kit was used (BD Biosciences) according to the manufacturer's instructions.
Analysis of CCR2 and CCR5 expression
Cells (5×104/well) were left untreated or exposed to Ad-WTLuc or IFNa2b (100 U; Sicor Biotech) 4 hr before extensive washing. Sixteen hours later, cells were fixed with 0.25% paraformaldehyde and stained with the following fluorochrome-conjugated antibodies: CD192 (TG5/CCR2, Biolegend); CD195 (eBio T21/8, eBioscience), and CD14 (M5E2, BD Bioscience). Acquisition was performed in a FACSCalibur flow cytometer and was analyzed using FlowJo software (Tree Star).
Quantification of flourescence-labeled cells in tissues
MM6 cells were mock-infected or infected with Ad-WTLuc at MOI 1000 for 4 hr before extensive washing. Cells were then labeled with 2.5 μM carboxyfluorescein succinimydil ester (CFSE; Sigma) for 15 min and washed again. A total of 4×106 cells were injected intravenously or intratumorally in athymic mice-bearing subcutaneous HuH-7 xenografts. Twenty-four hours later, livers and tumors were extracted and treated with 400 U/ml collagenase D and 50 μg/ml DNAse I (Roche Diagnostics). After mechanical tissue dissociation, cells were passed through a 70 μm nylon mesh filter (BD Biosciences) and washed. To enrich liver cell suspension in leucocytes, hepatocytes were removed with Percoll gradient. Red blood cells were lysed with AcK buffer. CFSE+ cells were quantified by flow cytometry as described above.
Statistical analysis
The Mann Whitney test was applied for statistical comparisons between two groups. Analysis was performed using the GraphPad Prism program (GraphPad Software).
Results
Infectivity, transgene expression, and replication of human adenovirus in monocytic cell lines
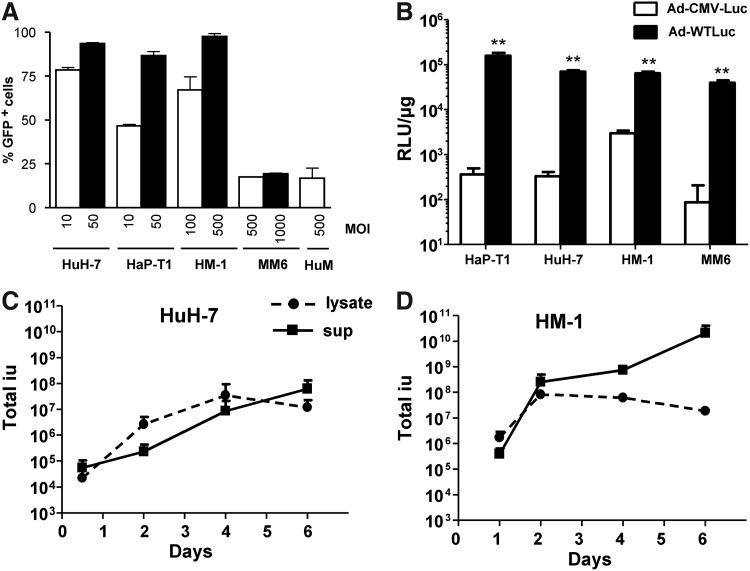
The infectivity of the different cells used in this study was evaluated using an Ad-GFP. The percentage of GFP+ cells was quantified by flow cytometry 24 h after initiation of infection. As shown in Figure 1A, epithelial-derived cancer cell lines from human (HuH-7) or hamster (HaP-T1) origin were efficiently infected with Ad-GFP at relatively low MOIs (infectivity of 50% or higher at MOI 10). In contrast, the monocytic cell line MM6 required high MOIs, and the percentage of infected cells remained below 25%. The same was observed with freshly isolated human monocytes (HuM). Interestingly, the hamster monocytic cell line HM-1 was efficiently infected at MOI 100.
FIG. 1.
Human and hamster monocytes support infection, replication, and transgene expression of adenoviral vectors. (A) Freshly isolated human monocytes (HuM); monocytic/macrophage cell lines from human (MM6) or hamster (HM-1); the human hepatocarcinoma cell line HuH-7, or the hamster pancreatic cell line HaP-T1 were infected with the first-generation adenovirus-expressing green fluorescent protein (Ad-GFP) vector at the indicated multiplicity of infection (MOIs). The percentage of GFP+ cells was determined by flow cytometry 24 h later. (B) The indicated cell lines were infected with the Ad-WTLuc or Ad-CMV-Luc vector at MOIs 2, 1, 25, and 250 for HaP-T1, HuH-7, HM-1, and MM6, respectively. Cell lysates were collected at 48 h post-infection for quantification of luciferase activity, expressed in relative light units/μg protein. (C, D) HuH-7 and HM-1 cells were infected with Ad5 at MOI 2 and 100, respectively, and viral progeny production was quantified in cell lysates and supernatants (sup) collected at the indicated days post-infection. **p<0.001.
Next, we evaluated the ability of cells to support transgene expression mediated by a replication-competent adenovirus. To this end, we compared the activity two vectors carrying the luciferase gene. Ad-CMV-Luc is an E1-deleted, replication-defective adenoviral vector, whereas Ad-WTLuc has no restrictions for replication and contains the transgene in the E3 region. Cells were infected at suboptimal MOIs (2, 1, 25, and 250 for HaP-T1, HuH-7, HM-1, and MM6, respectively) to better appreciate the influence of virus replication. We observed a dramatic increase in luciferase activity in cells infected with the replicative vector compared with the defective counterpart, even in those that were relatively refractory to adenoviral infection (Fig. 1B).
To further demonstrate replication of adenovirus in monocytic cells, we infected HuH-7 and HM-1 cells with Ad5 and determined the progression of viral progeny production over time (Fig. 1C and D, respectively). In agreement with active virus amplification and spread, we detected an initial increase in the amount of virus in cell lysates with a subsequent decline at day 6, when most cells in each well are already destroyed. In fact, release of virus in the supernatant peaked at this time in both cell lines. Note that the parallel amplification of cells and viruses explains the high titers obtained at late times.
Monocytes improve the biodistribution of adenovirus in human tumor xenografts established in athymic mice
In order to estimate the potential of MM6 cells as carriers for OAV, we analyzed the expression of key chemokine receptors involved in the trafficking of monocytes to tumor sites: CCR2, the ligand for CCL2 (monocyte chemoattractant protein-1, MCP-1) and CCR5, the ligand for CCL5 (RANTES) (Murdoch and Lewis, 2005). Using flow cytometry we found that more than 70% of MM6 were positive for the monocytic marker CD14 (not shown). Among CD14+ MM6 cells, more than 80% were positive for CCR2, which is similar to the percentage observed in primary human monocytes (Supplementary Fig. 1A; Supplementary Material available online at www.liebertonline.com/hum).
Regarding CCR5, approximately 40% of CD14+ MM6 displayed this receptor on their surface, which is actually higher than in primary monocytes. Compared with other commonly used human monocytic cell lines (THP-1), the expression of CCR2 and CCR5 was also higher in MM6, suggesting that this cell line is suitable for tumor homing. Adenoviral infection could alter the expression of these receptors, either directly or in response to the production of cytokines such as type I interferon. In order to check this possibility, we repeated the analysis in MM6 cells infected with Ad-WTLuc or treated with human interferon-alpha (IFNa). As shown in Supplementary Figure 1B, we found no reduction in the percentage of cells displaying CCR2 or CCR5 under these conditions. The intensity of expression was not affected either (not shown), suggesting that MM6 cells retain their capacity to sense chemotactic factors despite Ad5 infection.
The first function evaluated in vivo in the monocytic cell lines was their ability to deliver a replication-competent adenovirus to distant tumors following intravenous administration. Tumors were established by subcutaneous inoculation of HuH-7 in athymic mice. The MM6 or HM-1 cells were infected ex vivo with the Ad-WTLuc virus for 4 hr, and then the virus was removed and 4×106 cells were administered by tail vein injection into different groups of mice. Another group received the free virus using the same route. Note that the dose of virus administered in each case cannot be directly compared. We injected 2×108 iu of the free virus, which is 2–20 times lower than the titer employed for ex-vivo infection of HM-1 and MM6 cells, respectively. However, only a small portion of these virions actually infects the cells, and the majority is washed away before inoculation. In fact, more than 90% of the infective viral particles are detected in the supernatant that is discarded after the first centrifugation of cells (data not shown).
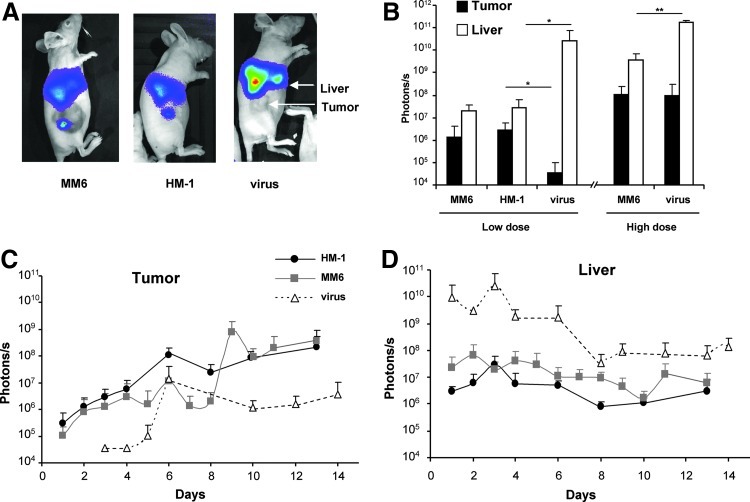
Transgene expression was analyzed in vivo by bioluminescence detection (BLI) over the next 2 weeks. At early time points (3 days after administration), we could only detect luciferase activity in the tumors of mice that received the virus incorporated in carrier cells (Fig. 2A). In contrast, transgene expression from the free virus was almost exclusively hepatic, indicating that monocytes reduced the off-target liver transduction of adenovirus. Despite this sharp difference, it became evident that tumor targeting achieved by monocytes was modest, with approximately 10% of the total BLI corresponding to the tumors and the majority still coming from the liver (Fig. 2B).
FIG. 2.
Monocytes improve adenovirus-mediated transduction of tumors following systemic administration in athymic mice. Tumors were established in athymic mice by subcutaneous inoculation of HuH-7 cells. MM6 or HM-1 cells were infected ex vivo with Ad-WTLuc at MOI 1,000 and 100, respectively. Cells were administered intravenously in two groups of mice (n=5) that received 4×106 cells (low dose) or 1.2×107 cells (high dose) (n=5). Other groups received 2×108 iu or 2×109 iu of the free virus (designed as low and high dose virus, respectively). Transgene expression in liver and tumors was quantified over time by bioluminescence detection (BLI). Representative images of mice in the low-dose groups (A) and average light emission in liver and tumor 3 days after treatment in all groups, expressed in photons/second (B). Follow-up of light emission in the tumor (C) and liver (D) of animals corresponding to the low-dose groups. In the artificial color code, the most intense light emission is represented in red and the lowest in blue. Standard deviation is represented in all cases. *p<0.05.
Similar results were obtained when treatments were administered by retro-orbital injection (not shown). We repeated the same experiment using a higher amount of carrier cells (12×106 MM6 cells/mouse). Under these circumstances, tumor transduction improves dramatically (see Fig. 2B, “high dose”), but there is a parallel increase in hepatic homing of cells. Regarding the administration of free Ad-WTLuc virus, only when the dose reaches 2×109 iu/mouse (which caused 25% mortality in athymic mice) we detected comparable luciferase expression in tumors, but liver transduction was extremely high (light emission of more than 1011 photons/sec).
In Figures 2C and 2D we represent the BLI quantification over time in the tumor and liver, respectively, corresponding the low dose of treatments. It is important to note that the absence of an efficient antiviral response in athymic mice, and the fact that only the xenografts support viral replication, lead to a misleading perception of OAV specificity in tumors at late time points. In fact, transgene expression slowly decreases in the liver but increases in the tumor due to viral amplification. Under these circumstances, even the free virus seems to accumulate in tumors one week after intravenous injection (Fig. 2C).
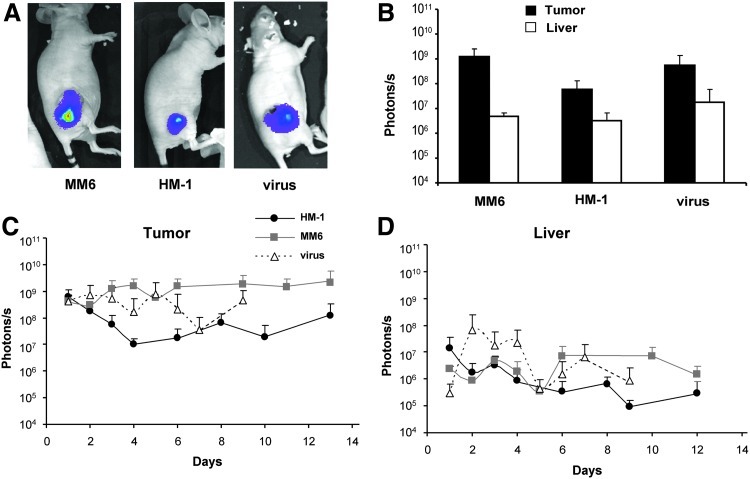
In order to estimate the proportion of cells or free virus that reaches the tumor upon systemic administration, the same doses were injected intratumorally in HuH-7 xenografts. Evaluation of transgene expression was performed as described for the intravenous administration (Fig. 3). The results indicate that virus-loaded monocytes can efficiently express the transgene in the tumor at day 3 (Fig. 3A and B). Luciferase activity was restricted to the tumor, and the BLI intensity was similar to that obtained by intratumoral administration of the free virus. If we take into account that transgene expression in tumors is 100 to 1,000 times higher if cells are administered locally versus systemically (compare Figs. 2B and 3B), we can conclude that only a small proportion (usually less than 1%) of carrier cells reach their target. Similar results were obtained when we used freshly isolated monocytes from human donors (not shown). This percentage is much lower (less than 0.01%) in the case of the free virus.
FIG. 3.
Intratumoral injection of virus-loaded monocytes achieves efficient tumor transduction. Tumors were established in athymic mice by subcutaneous inoculation of HuH-7 cells. MM6 or HM-1 cells were infected ex vivo with Ad-WTLuc at MOI 1,000 and 100, respectively, and 4×106 cells were administered intratumorally (n=5). The other group (virus) received 2×108 iu of the free virus. Transgene expression in liver and tumors was quantified over time by BLI. Representative images of mice (A) and average light emission in liver and tumor 3 days after treatment, expressed in photons/second (B). Follow-up of light emission in the tumor (C) and liver (D) of the indicated groups.
To investigate if the relatively poor tumor homing of monocytes could be a consequence of adenovirus infection, MM6 cells were mock-infected or infected with Ad-WTLuc and then labeled with CFSE before intravenous administration. The same amount of labeled cells was injected intratumorally in another set of animals. The abundance of CFSE+ mononuclear cells was analyzed 24 hr later in liver and tumor homogenates. In accordance with BLI data, the amount of cells detected in tumors following i.v. administration was only 1% compared with local injection, and this percentage was not altered by adenoviral infection (Supplementary Fig. 2).
Monocytes are not efficient carriers for systemic delivery of adenovirus in Syrian hamsters
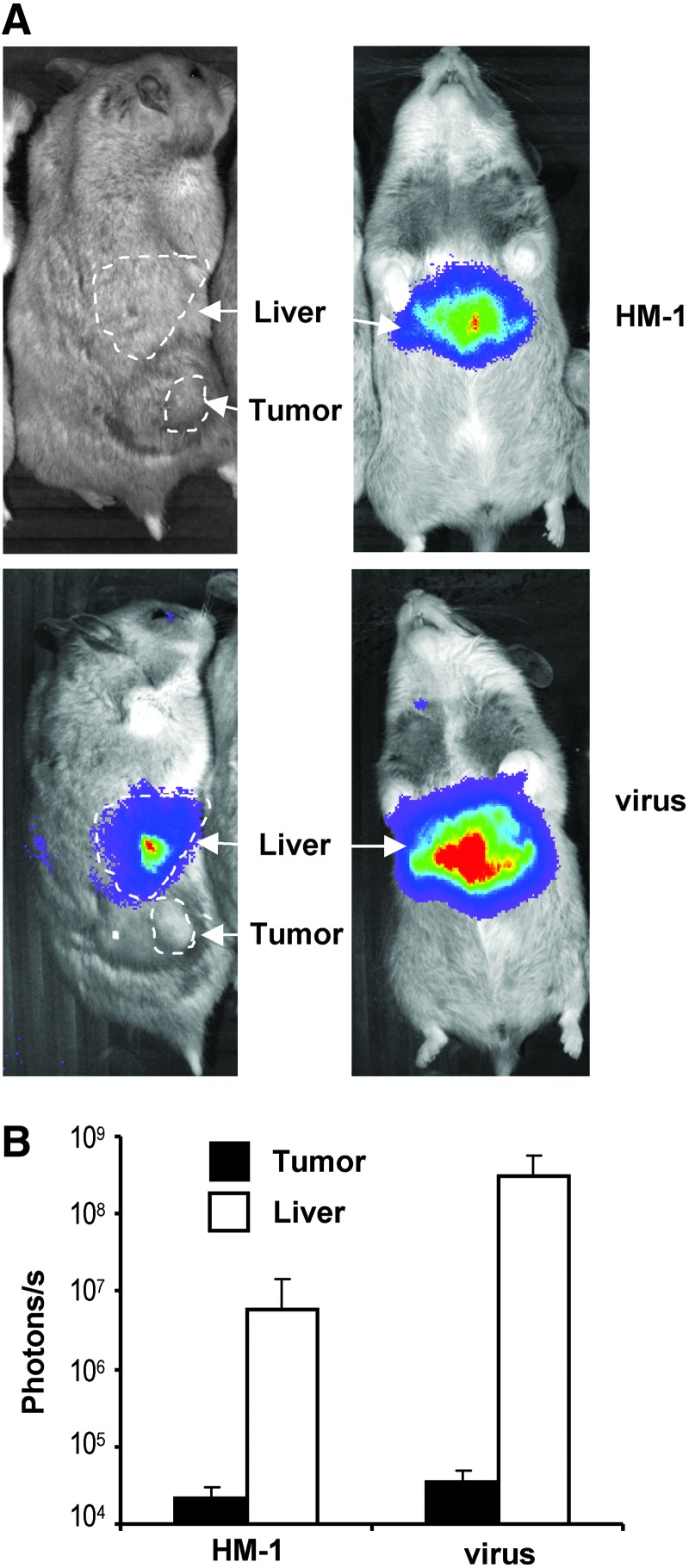
In order to evaluate the performance of carrier cells in immunocompetent animals permissive for adenovirus replication, we used a model of pancreatic cancer in Syrian hamsters. First, we established subcutaneous tumors by inoculation of HaP-T1 cells to study the tumor homing ability of cells, as previously described for athymic mice. Free Ad-WTLuc virus or virus loaded in HM-1 carrier cells were administered systemically by retro-orbital injection. In contrast with the results obtained in athymic mice, no significant tumor transduction was observed, and expression of the transgene was almost exclusively hepatic. In Figure 4, we depict an experiment performed with 5×107 cells. The effect was similar when we duplicated the number of cells, and there was no progressive amplification of luciferase expression in any organ (not shown), in line with previous description of Ad-WTLuc infection in hamsters (Bortolanza et al., 2009a).
FIG. 4.
Inefficient tumor transduction of tumors following systemic administration of virus-loaded monocytes in Syrian hamsters. Tumors were established by subcutaneous inoculation of HaP-T1 cells in Syrian hamsters. HM-1 cells were infected ex vivo with Ad-WTLuc at MOI 100, and 5×107 cells were administered intravenously (n=5). The other group (virus) received 2×109 iu of the free virus. Transgene expression in liver and tumors was quantified over time by BLI. (A) Representative images of hamsters in both groups (HM-1 top; virus bottom) 3 days after treatment. Dorso-lateral view to show the tumor (left panels) and ventral view to better appreciate the signal in liver (right panels). (B) Average light emission in both groups, expressed in photons/second.
Local administration of virus-loaded monocytes allows repeated transgene expression in pancreatic tumors of Syrian hamsters
Since we could not demonstrate tumor targeting of monocytes upon systemic administration in hamsters, we studied their potential applications in local delivery. In this case, tumors were established by implantation of HaP-T1 in the liver of hamsters, to evaluate the feasibility of this approach for the treatment of internal organs. The first objective was to evaluate if monocytes can improve the efficacy of multiple administrations of OAV. In this case, ex-vivo infection of cells was extended for 24 hr, to ensure active expression of the transgene at the moment of injection. The hamster received two intratumoral administrations of free Ad-WTLuc or virus loaded in HM-1 cells, separated 13 days.
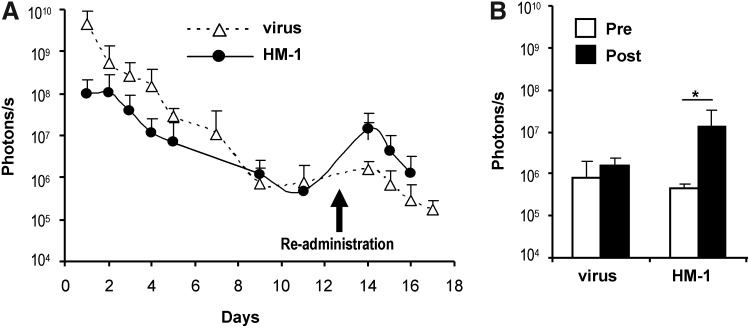
As shown in Figure 5A, the free virus was very efficient in the initial tumor transduction, but the second injection caused no significant elevation in BLI. In contrast, carrier cells achieved a second peak of transgene expression after re-administration. To better appreciate this difference, in Figure 5B we represent the quantification of light emission the day before (pre-) and the day after (post-) the second inoculation. These results suggest that carrier cells could either avoid the production of NAbs against adenovirus or protect Ad-WTLuc from them. Thus, we set up experiments to clarify this point and determine if carrier cells can allow tumor transduction in animals previously exposed to adenovirus, which is a clinically relevant scenario.
FIG. 5.
Monocytes improve the efficacy of repeated administrations of adenovirus in Syrian hamsters. Tumors were established by intrahepatic inoculation of HaP-T1 cells in Syrian hamsters. HM-1 cells were infected ex vivo with Ad-WTLuc at MOI 100, and 1×107 cells were administered intratumorally (n=5). The other group (virus) received 5×108 iu of the free virus. The same treatments were repeated 13 days later (re-administration). Transgene expression in tumors was quantified over time by BLI. (A) Progression of light emission in tumors, expressed in photons/second. (B) Comparison of transgene expression the day before (pre, white bars) and 24 hr after re-administration (post, black bars) in both groups of hamsters. *p<0.05.
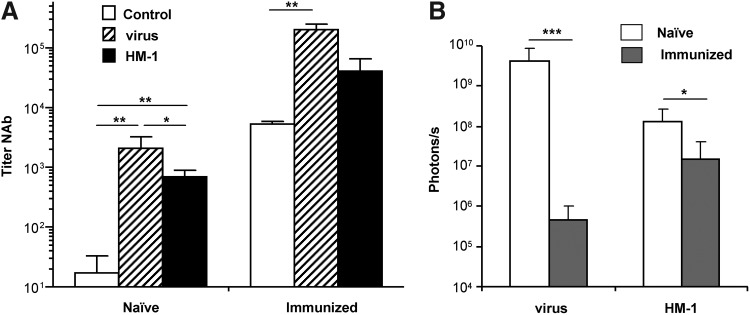
A group of hamsters received an intravenous dose of Ad5 (“immunized” animals), and one month later Ad-WTLuc or virus-loaded HM-1 cells were inoculated in the hepatic tumors, as previously described. A different set of hamsters received the same treatments, but were not pre-exposed to adenovirus (“naïve” animals). Luciferase expression was determined by BLI 24 hr after intratumoral injections, and the presence of NAbs was quantified in the serum of animals before the treatment and 2 weeks later. As shown in Figure 6A, intratumoral administration of Ad-WTLuc in naïve hamsters stimulated the production of high titers NAbs (>1,000). The use of carrier cells caused a small decrease in NAbs (average titer of approximately 700), but titers were significantly increased versus control animals, demonstrating that the virus was efficiently detected by the immune system.
FIG. 6.
Monocytes improve local tumor transduction in adenovirus-immunized hamsters. Syrian hamsters received an intravenous injection of saline solution (naïve) or 5×108 iu of Ad5 (immunized). One month later, tumors were established in both groups of animals by intrahepatic inoculation of HaP-T1 cells. A subset of naïve and immunized hamsters received an intratumoral injection of 1×107 HM-1 cells infected ex vivo with Ad-WTLuc at MOI 100 (n=7), or 5×108 iu of the free Ad-WTLuc virus. (A) Quantification of anti-adenovirus NAbs in the serum of hamsters 2 weeks after treatments. Control groups indicate the titer of naïve or immunized animals before the treatments. (B) Comparison of transgene expression (luciferase activity) in tumors of naïve or immunized hamsters 24 hr after treatment with free virus or HM-1 carrier cells. *p<0.05; **p<0.001; ***p<0.0001.
In the case of preimmunized hamsters, the same tendency was observed, with a boost of NAbs caused by the intratumoral inoculation of Ad-WTLuc and, to a lesser extent, virus-loaded monocytes. In terms of transgene expression, it became evident that immunization caused a dramatic decrease in tumor transduction of the free virus (more than 8,000-fold inhibition). In contrast, virus carried in HM-1 cells was partially protected, with an 8-fold inhibition of luciferase expression in immunized versus naïve animals (Fig. 6B).
Monocytic carrier cells increase the therapeutic effect of an OAV-expressing IL-12
Once we had demonstrated that HM-1 cells improve the function of replication-competent adenoviral vectors in preimmunized hosts, we studied if they could also increase the antitumor effect of an OAV expressing a therapeutic gene. For this purpose, we used Ad-IL12G, a hypoxia-inducible vector carrying a single-chain version of the murine immunostimulatory cytokine IL-12 (Bortolanza et al., 2009b). This virus contains a hypoxia-responsive promoter controlling the expression of the E1A region to enhance its activity in tumor tissues. In addition, a partial deletion in the E1A gene (encompassing conserved region 2) was introduced to avoid inactivation of pRB in the cells. This modification causes a relative blockade of adenoviral replication in normal quiescent cells, as previously described (Fueyo et al., 2000; Heise et al., 2000).
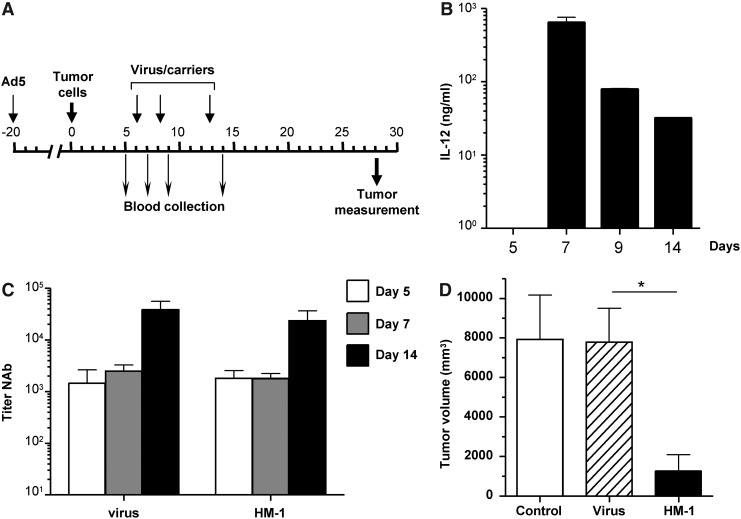
First we verified that HM-1 cells infected with Ad-IL12G produced high amounts of IL-12 under hypoxic conditions (Supplementary Fig. 3). The biological function of murine IL-12 on Syrian hamsters has been previously demonstrated (Bortolanza et al., 2009b). For in vivo evaluation of the antitumor effect, we used a stringent model consisting on preimmunized hamsters bearing HaP-T1–derived tumors in the liver. A schematic representation of the experiment is depicted in Figure 7A. Twenty days before tumor implantation, animals received an intravenous injection of Ad5. Intratumoral administration of Ad-IL12G or virus-loaded HM-1 cells was repeated three times on days 6, 8, and 13 after tumor implantation. Blood collection was performed at the indicated days for quantification of IL-12 concentration and titer of NAbs. In agreement with results obtained with the reporter gene luciferase, discrete peaks of IL-12 concentration were observed following each administration of Ad-IL12G-loaded cells (Fig. 7B). The intensity was progressively attenuated, but at least three cycles of treatment are effective in these preimmunized animals.
FIG. 7.
Monocytes improve the antitumor effect of an OAV-expressing IL-12 in preimmunized Syrian hamsters. (A) Schematic representation of the experiment. Syrian hamsters received an intravenous injection of Ad5 (5×108 iu) for immunization. Twenty days later, tumors were established by intrahepatic inoculation of HaP-T1 cells (day 0). Six days later, tumor formation was confirmed by ultrasound and animals received an intratumoral injection of saline solution (control) or 2.5×107 iu of Ad-IL12G (virus) or 1×107 HM-1 cells infected ex vivo with Ad-IL12G at MOI 100 (HM-1). Treatments were repeated at days 8 and 13, as indicated. Blood collection was carried out before initiation of treatment and after each tumor inoculation to determine concentration of IL-12 (B) and titer of anti-adenovirus NAbs (C). Hamsters were sacrificed 3 weeks after initiation of treatment and tumors were directly measured at necropsy (D). *p<0.05.
In sharp contrast, injection of the free virus caused only a marginal increase in IL-12 (less than 1 ng/ml, not represented in the graphic). This difference was not due to a reduction in NAbs, because both groups showed a strong increase in titers following the third dose of treatment (Fig. 7C). More importantly, necropsies performed 3 weeks after initiation of treatment revealed a significant reduction in tumor growth in the group of hamsters treated with carrier cells (Fig. 7D). In summary, our results suggest that monocyte/macrophage cell lines used as carriers for armed OAVs allow repeated cycles of transgene expression and increase the therapeutic effect of these agents in immunocompetent subjects.
Discussion
Treatment of disseminated tumors with OAVs that rely mainly on their direct oncolytic effect will require carrier cells with a marked tumor tropism, especially if accumulation of virus can be toxic in normal tissues such as liver or lungs. Our data indicate that monocytes do not match this requirement. The percentage of systemically administered carrier cells that reach a distant tumor is not usually reported in the literature. Here we provide a relative estimation based on the comparison of transgene expression when the same number of cells is administered intravenously or intratumorally. Even in our xenograft tumor model, barely 1% of the total number of intravenously injected monocytes reaches a subcutaneous tumor. We corroborated this data by direct quantification of CFSE-labeled cells. The use of freshly isolated human monocytes instead of monocytic/macrophage cell lines did not increase this percentage. Although this is better than the injection of free virus, and a significant antitumor effect has been recently reported using a similar approach (Muthana et al., 2011), there is clearly room for improvement. The challenge is evident in immunocompetent hosts such as Syrian hamsters. Apart from the barrier imposed by the immune system (Bortolanza et al., 2009a), differences in the anatomy of animals and the architecture of tumors may contribute to the blockade of tumor transduction. We increased 10–20 times the number of cells in hamsters versus mice to compensate for the difference in body weight. Although we cannot rule out that injection of larger numbers of virus-loaded cells could lead to the same degree of transgene expression in tumors, the ratio of tumor/liver transduction would be probably unfavorable. Ahmed et al. have recently described the use of MSCs as carriers for OAVs in another semi-permissive model (cotton rat) (Ahmed et al., 2010). Direct comparison between liver/tumor transduction was not performed in this study, but they report substantial persistence of the cells in the liver upon systemic administration.
In a different approach, OAVs expressing therapeutic genes with systemic antitumor effect may be administered locally in accessible lesions, using new interventional radiology techniques. In this case, carrier cells may play an important role in protection from NAbs and allow multiple rounds of transgene expression. Therefore, we focused on the hamster model and evaluated the ability of monocytes to improve tumor transduction in the context of local administration in hepatic tumors. An important concept in virotherapy is the efficacy of repeated administrations of OVs in solid tumors. This issue is controversial both in animal models and in clinical reports. Our data indicate that a single intratumoral injection of Ad-WTLuc causes a sharp increase in NAbs that are able to block the effect of subsequent doses. The titer of NAbs rises as early as one week after injection (not shown) and peaks approximately at three weeks. This is in agreement with the clinical experience reported with a first-generation adenovirus expressing the suicide gene thymidine kinase in hepatocellular carcinoma (Penuelas et al., 2005).
Apart from the problem of re-administration, pre-existing NAbs against Ad5 is a potential drawback for the efficacy of OAVs. Experimental immunization with Ad5 in the hamster model recapitulated the situation observed with a significant segment of the adult population (Sprangers et al., 2003; Thorner et al., 2006; Appaiahgari et al., 2007; Sun et al., 2011). NAbs may differ in many characteristics such as affinity and isotype, but we used a functional assay (luciferase-based virus neutralization assay) to harmonize results. The titers described here are apparently higher than those usually reported in humans, probably because we considered the 50% inhibition threshold (instead of 90%) to increase the sensitivity of the assay (Sprangers et al., 2003). Importantly, monocytes protected Ad-WTLuc from NAbs and enabled tumor transduction in preimmunized animals and in repeated administrations.
Finally, we evaluated the therapeutic potential of monocytes as carrier cells using the Ad-IL12G virus. We had determined in previous studies that the antitumor effect of this kind of OAV depends mainly on the expression of the transgene (Bortolanza et al., 2009b). The HaP-T1 tumor model used in this work is very aggressive and causes death of the animals approximately one month after hepatic cell implantation. In addition, hamsters were preimmunized with Ad5. Under these circumstances, administration of the free virus had virtually no antitumor effect, and detection of the transgene was negligible in serum. In contrast, monocytes allowed at least three productive administrations and achieved a significant reduction in the growth of pancreatic tumors.
In summary, we conclude that the most immediate application of carrier cells such as monocytes in virotherapy is the repeated local delivery of OVs expressing therapeutic transgenes with systemic antitumor effect.
Supplementary Material
Acknowledgments
We thank Itziar Echeverria, Pablo Sarobe, and Aitziber Echeverria for their help with flow cytometry, and Pilar Alzuguren for excellent technical assistance. C.B. was partially funded by the MICINN's program of Promotion of Technical Personal Support (PTA2008-1228-I). This work has been funded by grants SAF2006-04755 and SAF2009-11324 from the Spanish Department of Science; DPI2009-14115-C03-03 and the UTE project CIMA.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmed A.U. Rolle C.E. Tyler M.A., et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol. Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A.U. Tyler M.A. Thaci B., et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R. Cascallo M. Oncolytic viruses from the perspective of the immune system. Future Microbiol. 2009;4:527–536. doi: 10.2217/fmb.09.28. [DOI] [PubMed] [Google Scholar]
- Appaiahgari M.B. Pandey R.M. Vrati S. Seroprevalence of neutralizing antibodies to adenovirus type 5 among children in India: implications for recombinant adenovirus-based vaccines. Clin. Vaccine Immunol. 2007;14:1053–1055. doi: 10.1128/CVI.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolanza S. Alzuguren P. Bunuales M., et al. Human adenovirus replicates in immunocompetent models of pancreatic cancer in Syrian hamsters. Hum Gene Ther. 2007;18:681–90. doi: 10.1089/hum.2007.017. [DOI] [PubMed] [Google Scholar]
- Bortolanza S. Bunuales M. Alzuguren P., et al. Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters. Cancer Gene Ther. 2009a;16:703–712. doi: 10.1038/cgt.2009.12. [DOI] [PubMed] [Google Scholar]
- Bortolanza S. Bunuales M. Otano I., et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther. 2009b;17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach C.J. Burke J. Jonker D., et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- Dwyer R.M. Kerin M.J. Mesenchymal stem cells and cancer: tumor-specific delivery vehicles or therapeutic targets? Hum. Gene Ther. 2010;21:1506–1512. doi: 10.1089/hum.2010.135. [DOI] [PubMed] [Google Scholar]
- Eager R.M. Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- Fontanellas A. Hervas-Stubbs S. Mauleon I., et al. Intensive pharmacological immunosuppression allows for repetitive liver gene transfer with recombinant adenovirus in nonhuman primates. Mol. Ther. 2010;18:754–765. doi: 10.1038/mt.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fueyo J. Gomez-Manzano C. Alemany R., et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Hawkins L.K. Johnson L. Bauzon M., et al. Gene delivery from the E3 region of replicating human adenovirus: evaluation of the 6.7 K/gp19 K region. Gene Ther. 2001;8:1123–1131. doi: 10.1038/sj.gt.3301507. [DOI] [PubMed] [Google Scholar]
- Heise C. Hermiston T. Johnson L., et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- Josiah D. T. Zhu D. Dreher F., et al. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther. 2010;18:377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N. Shen F.B. Chang J.H., et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- Liu C. Russell S.J. Peng K.W. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C. Lewis C.E. Macrophage migration and gene expression in response to tumor hypoxia. Int. J. Cancer. 2005;117:701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- Muthana M. Giannoudis A. Scott S.D., et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71:1805–1815. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- Narvaiza I. Aparicio O. Vera M., et al. Effect of adenovirus-mediated RNA interference on endogenous microRNAs in a mouse model of multidrug resistance protein 2 gene silencing. J. Virol. 2006;80:12236–12247. doi: 10.1128/JVI.01205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas I. Mazzolini G. Boan J.F., et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128:1787–1795. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Raykov Z. Rommelaere J. Potential of tumour cells for delivering oncolytic viruses. Gene Ther. 2008;15:704–710. doi: 10.1038/gt.2008.34. [DOI] [PubMed] [Google Scholar]
- Senzer N. N. Kaufman H. L. Amatruda T., et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Sprangers M.C. Lakhai W. Koudstaal W., et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Zhang Y. Feng L., et al. Epidemiology of adenovirus type 5 neutralizing antibodies in healthy people and AIDS patients in Guangzhou, southern China. Vaccine. 2011;29:3837–3841. doi: 10.1016/j.vaccine.2011.03.042. [DOI] [PubMed] [Google Scholar]
- Thomas M.A. Spencer J.F. La Regina M.C., et al. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006;66:1270–1276. doi: 10.1158/0008-5472.CAN-05-3497. [DOI] [PubMed] [Google Scholar]
- Thorne S.H. Liang W. Sampath P., et al. Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy. Mol Ther. 2010;18:1698–1705. doi: 10.1038/mt.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner A.R. Vogels R. Kaspers J., et al. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J. Clin. Microbiol. 2006;44:3781–3783. doi: 10.1128/JCM.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala M. Alzuguren P. Benavides C., et al. Evaluation of bioluminescent imaging for noninvasive monitoring of colorectal cancer progression in the liver and its response to immunogene therapy. Mol. Cancer. 2009;8:2. doi: 10.1186/1476-4598-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.