Abstract
Various factors play an essential role in patterning the digestive tract. During development, Sox2 and Cdx2 are exclusively expressed in the anterior and the posterior parts of the primitive gut, respectively. However, it is unclear whether these transcription factors influence each other in determining specification of the naïve gut endoderm. We therefore investigated whether Sox2 redirects the fate of the prospective intestinal part of the primitive gut. Ectopic expression of Sox2 in the posterior region of the primitive gut caused anteriorization of the gut toward a gastric-like phenotype. Sox2 activated the foregut transcriptional program, in spite of sustained co-expression of endogenous Cdx2. However, binding of Cdx2 to its genomic targets and thus its transcriptional activity was strongly reduced. Recent findings indicate that endodermal Cdx2 is required to initiate the intestinal program and to suppress anterior cell fate. Our findings suggest that reduced Cdx2 expression by itself is not sufficient to cause anteriorization, but that Sox2 expression is also required. Moreover, it indicates that the balance between Sox2 and Cdx2 function is essential for proper specification of the primitive gut and that Sox2 may overrule the initial patterning of the primitive gut, emphasizing the plasticity of the primitive gut.
Keywords: gut development, Cdx2, Sox2
Introduction
The vertebrate digestive tract develops through a complex mechanism of patterning, expansion and differentiation of the primitive gut, which itself is formed after gastrulation as a result of folding of the definitive endodermal layer (Wells and Melton, 1999, 2000; Zorn and Wells, 2009). One of the earliest steps in patterning of the primitive gut is the regionalization into the anterior and the posterior domain, which correlates with the expression of two transcription factors, Sox2 and Cdx2, respectively (Sherwood et al., 2009). During development these domains become more subdivided and by E9.5, the foregut, midgut, and hindgut regions can be discriminated. The foregut eventually gives rise to the esophagus, stomach, and proximal part of the duodenum; the midgut develops into the caudal part of the duodenum, jejunum, ileum, caecum, and proximal part of the colon; and the hindgut forms the caudal part of the colon and rectum. Once regionalization has occurred, the gastro-intestinal tract becomes specified and cells differentiate into the characteristic types found in the various regions.
Sox2 is a member of a highly conserved family of transcription factors founded by the sex-determining gene Sry. Although individual Sox proteins share common DNA-binding properties, their specificity is the result of their expression pattern and their ability to associate with different partners (Kondoh and Kamachi, 2010; Kormish et al., 2010). Sox proteins are recognized as key players in the regulation of embryonic development and determination of cell fate and we recently described two novel partners for Sox2 (Gontan et al., 2009; Engelen et al., 2011). Sox2 plays an important role during early vertebrate embryogenesis and is required later in development in the brain, neural tube, germ cells, and in the foregut and its derivatives (Ishii et al., 1998; Avilion et al., 2003; Taranova et al., 2006; Gontan et al., 2008; Favaro et al., 2009; Que et al., 2009).
Expression of Sox2 starts at the 4–8 cell stage of embryonic development and Sox2 gene inactivation results in early peri-implantation lethality (Avilion et al., 2003). Modulating expression in mice showed the importance of Sox2 in the differentiation of the esophageal epithelium and morphogenesis of the esophagus and trachea (Que et al., 2007, 2009; Gontan et al., 2008). Several congenital malformations in humans, for instance anophthalmia, microphthalmia, and the anophthalmia–esophageal–genital syndrome (Williamson et al., 2006), have been found to be associated with heterozygote mutations in SOX2. In addition, Sox2 is one of the key players in the induction of pluripotent stem cells from somatic cells (Jaenisch and Young, 2008), confirming the importance of Sox2 in development and differentiation processes.
Cdx2 is a homeobox transcription factor involved in the establishment of the anterior–posterior polarity of the gut endoderm and is expressed from E8.5 in the posterior part of the gut (Beck et al., 1995). Later in development, Cdx2 becomes restricted to the intestinal epithelium, with a sharp boundary, marking the transition from stomach to duodenum (James and Kazenwadel, 1991; James et al., 1994; Chawengsaksophak et al., 1997; Sherwood et al., 2009).
Conditional ablation of Cdx2 from the developing gut endoderm results in severe malformation of the intestinal tract and anteriorization of the posterior gut, evidenced by changes in the expression pattern of various transcriptional regulators (Gao et al., 2009). Interestingly, quantitative RNA expression analysis of Cdx2 mutant intestinal samples demonstrated ectopic activation of Sox2 expression in the posterior region. The aberrant expression of Sox2 after Cdx2 ablation coincided with an early anteriorization event of the gut. The apparent reciprocal expression pattern of Sox2 and Cdx2 suggests that these factors are able to influence each other. However, it remains unclear whether Sox2 or Cdx2 has a dominant role in determining the fate of the developing gut endoderm. In addition, aberrant SOX2 expression may underlie the formation of gastric metaplasia associated with intestinal inflammation (Shaoul et al., 2000), and the formation of various human congenital anomalies of the gut, such as Meckel's diverticulum in which ectopic gastric tissue can be present in the small intestine (Lambert et al., 2000). Furthermore, our group and others have observed aberrant expression of SOX2 in human colorectal cancers, suggesting a role for SOX2 in colorectal tumor initiation or progression (unpublished data; Park et al., 2008).
Therefore, we ectopically expressed Sox2 in epithelial cells of the prospective intestinal part of the primitive gut to investigate whether Sox2 is able to change the differentiation of the developing gastro-intestinal tract. Our data show that expression of Sox2 in the embryonic mouse intestinal epithelium leads to a fluid-filled, translucent swollen intestine with aberrant villi. The proliferation of epithelial cells in the Sox2 expressing intestines are no longer confined to the crypts, but rather distributed randomly throughout the intestinal epithelium. Moreover, Sox2 induced the expression of esophageal and stomach specific markers, resulting in an anteriorization of the intestinal epithelium. Although the expression level of Cdx2 is unaffected by ectopic Sox2 expression, we further show that induction of Cdx2 target gene expression is strongly reduced, because Sox2 leads to a reduced binding of Cdx2 to its target genes.
These findings provide strong evidence that expression of Sox2 in the developing gut drives the activation of the foregut transcriptional program and leads to conversion from an intestinal into a premature gastric epithelium, despite simultaneous expression of Cdx2. This indicates that the balance between Sox2 and Cdx2 function is essential for proper specification of the primitive gut and that Sox2 can exert a dominant effect over Cdx2 to alter intestinal cell fate by redirecting the intestinal transcriptional program, emphasizing the plasticity of the primitive gut.
Results
Ectopic Sox2 expression in the caudal part of the developing gut endoderm
Sox2 expression in the developing gut is restricted to the anterior part, which forms the stomach and other foregut derivatives. The posterior part is devoid of Sox2 expression, but expresses the homeobox protein Cdx2. Expression of Sox2 and Cdx2 is thought to be mutually exclusive, but it is unclear whether one has a dominant effect on the determination of the fate of the developing gut endoderm. Therefore, we investigated the role of Sox2 in the patterning of the primitive gut and its influence on Cdx2 expression.
Conditional expression of Sox2 in the intestinal epithelium was achieved by crossing mice carrying a myc-tagged Sox2 gene under the control of a tet-inducible promoter with the Villin-rtTA transgenic mouse line, which drives expression of the reverse tetracycline transactivator (rtTA) gene in all intestinal epithelial cells (Gontan et al., 2008; Roth et al., 2009). The rtTA protein drives expression of the Sox2 transgene in the intestinal epithelium in a doxycycline-dependent manner. Induction of Sox2 in embryos was achieved by administration of doxycycline to the mother from E8.5 onward. Double transgenic pups that received doxycycline through their mother were born at Mendelian ratio, but failed to thrive. Therefore, pups were isolated at E18.5 by caesarean section in order to investigate the intestinal abnormalities.
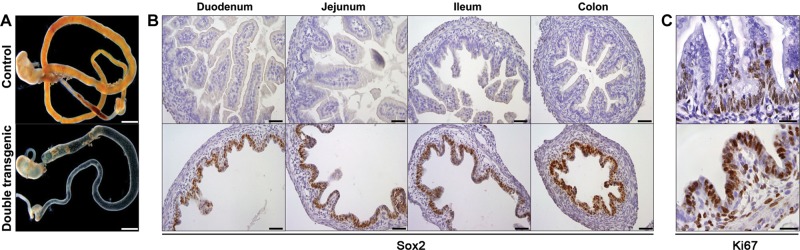
Externally, the littermates showed no significant morphological abnormalities and all the embryos were equally well developed. Intestinal tracts isolated from the pups carrying only one of the two transgenes, or non-induced myc-Sox2/Villin-rtTA double transgenic animals, appeared indistinguishable from non-transgenic control animals and showed no macroscopic or microscopic abnormalities. However, isolation of the gastro-intestinal tract of double transgenic embryos that received doxycycline immediately revealed major differences compared with single or non-transgenic animals (Figure 1A). In all the double transgenic pups, ectopic expression of Sox2 in the intestinal tract resulted in markedly abnormal intestines that were translucent and fluid-filled. Immunohistochemical analyses using an antibody against Sox2 (Figure 1B), or an antibody against the N-terminal myc-epitope present in the transgenic Sox2 protein (data not shown), revealed that nuclear Sox2 was expressed throughout the entire intestinal epithelium of the double transgenic animals. Assessment of Sox2 expression at specific time points of development, i.e. E.10.5, E12.5, E14.5, and E16.5, revealed that robust Sox2 expression could be detected only in the double transgenic intestinal epithelium from E14.5 onward (Supplementary Figure S1A), coinciding with the phase of cell specification in the embryonic gut. The level of ectopic Sox2 expression was compared with endogenous Sox2 levels in the stomach by quantitative polymerase chain reaction (qPCR) analysis, and a 6-fold increase in RNA level was found (Supplementary Figure S1B). As Sox2 expression in the E18.5 embryonic stomach is restricted to the basal cells of the forestomach, the qPCR analysis will likely overestimate transgenic Sox2 expression. Therefore, we compared expression levels by immunohistochemistry (IHC) at limiting antibody concentrations, showing that on a cell-to-cell basis transgenic Sox2 is expressed only at slightly higher levels than endogenous stomach Sox2 (Supplementary Figure S1C).
Figure 1.
Ectopic expression of Sox2 severely affects the intestinal tract. (A) Macroscopic appearances of the digestive tracts from stomach until rectum isolated at E18.5 of a non-transgenic embryo (top) and double transgenic embryo treated with doxycycline (bottom), showing that Sox2 induction leads to dilated and fluid-filled intestines. Scale bar, 2 mm. (B) IHC using an antibody against Sox2 on cross-sections of the duodenum, jejunum, ileum, and colon reveals specific nuclear staining in the epithelium of double transgenic animals throughout the intestinal tract, whereas Sox2 is absent in the control. Scale bar, 50 μm. (C) IHC using an antibody against Ki67 shows an increased number of cycling cells in the double transgenic embryos, compared with control intestine. Moreover, proliferating cells were randomly distributed throughout the intestinal epithelium of the double transgenic animals, whereas proliferation is restricted to the prospective crypt compartment at the base of villi in control intestines. Scale bar, 20 μm.
Ectopic Sox2 expression causes alterations in proliferation and malformation of the developing intestinal tract
Cross-sections of the intestines ectopically expressing the Sox2 transgene showed a 2-fold increase in luminal space (Supplementary Figure S2). In addition, the small intestines did not form true, morphological villi. Even the epithelial folds that resembled villi were markedly reduced per equivalent surface area (Figure 1B).
One possible explanation for the dilated intestinal tract could be a disturbed balance between proliferation and apoptosis. Using Ki67 staining to mark cycling cells (Figure 1C and Supplementary Figure S3A), we observed that proliferation in the cells of the double transgenic animals was no longer confined to the intervillous, primitive crypt regions as in the controls, but distributed randomly throughout the epithelium of Sox2 expressing embryos. In addition, the total number of Ki67-positive epithelial cells increased from ∼35%–40% in controls to 85%–90% in the Sox2-expressing embryos (Supplementary Figure S3A). Staining for activated caspase 3 revealed no differences in apoptosis (data not shown).
Thus, ectopic expression of Sox2 impairs villus formation and greatly alters the appearance of normal intestinal epithelium. These changes are accompanied by an increase in the number and mislocalization of cycling cells.
Ectopic Sox2 expression in the intestine results in loss of intestinal identity
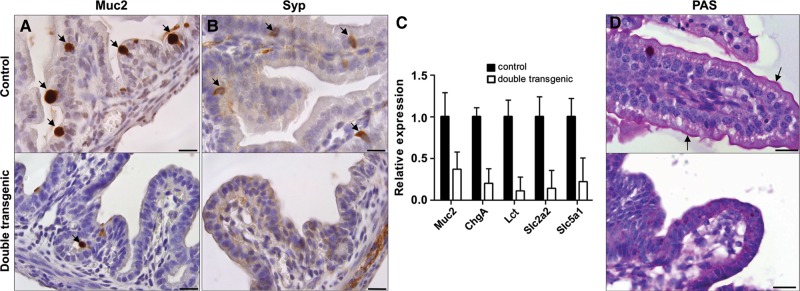
Next, we analyzed the differentiation potential of the embryonic intestinal epithelium to understand the phenotypic abnormalities observed in the Sox2 expressing intestines. Both mucin2 (Muc2) IHC and qPCR showed a dramatic loss in the number of intestinal mucin-producing goblet cells in the double transgenic animals (Figure 2A and C and Supplementary Figure S3B). Similar results were obtained for enterocytes using qPCR for Lactase (Lct) (Figure 2C). We also observed reduced numbers of synaptophysin (Syp) positive cells and reduced expression of Chromogranin A (ChgA), both markers for enteroendocrine cells though not exclusive for the intestinal epithelium (Figure 2B and C and Supplementary Figure S3C). In addition, periodic acid schiff (PAS) staining demonstrated that the intestinal brush border layer was entirely absent in the double transgenic animals, while clearly present in the controls (Figure 2D). As the brush border contains several transporters for micronutrients, lack of absorption across this border may explain both the dilatation of the small intestine and the fluid retention we observed. We tested the expression level of several transporters expressed in the intestinal epithelium by qPCR. Analysis of two members of the solute carrier family, Slc2a2 and Slc5a1, which are responsible for active and passive glucose transport, respectively (Wright et al., 2003), showed that these genes were downregulated in the double transgenic animals (Figure 2C). In addition, the expression levels of several members of the Aquaporin family, which are of major importance for water transport, were downregulated, as shown by qPCR (Supplementary Figure S4).
Figure 2.
Sox2 affects the normal differentiation of intestinal epithelium. Cross-sections of the duodenum at E18.5 of controls and double transgenic embryos, which received doxycycline. IHC using antibodies against Mucin2 (A) and synaptophysin (B) showed a reduced number of goblet cells and enteroendocrine cells, respectively, in the double transgenic animals (positive cells are indicated by arrows). Scale bar, 20 μm (A and B). (C) Analysis of the expression level of marker genes of goblet cells (Muc2), enteroendocrine cells (ChgA), and enterocytes (Lct) by qPCR showed a significant reduction of expression in the small intestine of double transgenic embryos at E18.5. Additionally, qPCR was used to determine the expression levels of two genes specific to the intestinal brush border, i.e. members of the solute carrier family (Slc), Slc2a2 and Slc5a1, which are involved in glucose transport. Both are down-regulated in double transgenic embryos. (D) PAS staining revealed the absence of the intestinal brush border in Sox2-induced animals compared with the controls (arrows indicate the positive lining of the brush border). Scale bar, 25 μm.
Overall, ectopic expression of Sox2 in the embryonic intestinal epithelium results in loss of intestinal identity which is accompanied by reduced expression of various transporters.
Sox2 expression in the intestine results in cell fate conversion
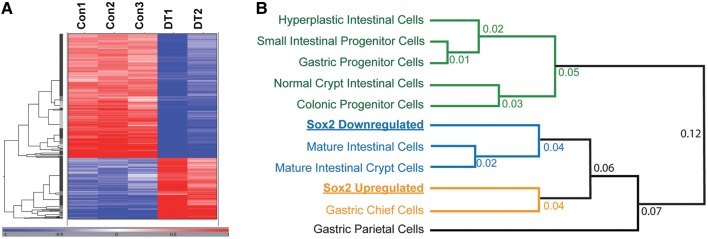
In order to better understand the underlying changes induced by expression of Sox2 in the intestinal epithelium, we performed microarray analysis using intestines of E18.5 pups of three control and two double transgenic animals, which received doxycycline from E8.5 onward. Hierarchical clustering of differentially expressed genes revealed large differences between controls and double transgenic intestines (Figure 3A). Differentially expressed genes in the Sox2-induced animals are involved in development and signal transduction, and are associated with a more immature and proliferative phenotype (Supplementary Figure S5A–C). In addition, close examination showed that the downregulated genes in the Sox2 overexpressing animals are involved in intestinal transport and lipid handling, typical processes for the absorptive function of the intestine (Supplementary Figure S5D).
Figure 3.
Transcriptome analysis reveals upregulation of gastric cell-specific transcripts by Sox2. (A) OmniViz Treescape showing the hierarchical clustering of Affymetrix probe sets that matched the selection query. Gene expression levels compared with the geometric mean are indicated in red for upregulated genes and in blue for downregulated genes. The color intensity correlates with the degree of change. Con, control; DT, double transgenic. (B) Sox2-upregulated and Sox2-downregulated gene lists were compared based on their associated GO term fractional representations with previously described GO term profiles of several other gastrointestinal cells and tissues (Doherty et al., 2008). ‘Mature intestinal crypt cells’ refers to an expression profile of genes derived from β-catenin deleted mice (Fevr et al., 2007), which causes crypt cells to mature. ‘Hyperplastic intestinal cells’ refers to PTEN-deficient intestinal cells, which become hyperplastic (He et al., 2007).
We next used the pattern of GO term enrichment to integrate our Sox2 profiles with a database of gastric and intestinal cells and tissues of varying degrees of differentiation (Figure 3B). That analysis showed that genes whose expression was induced by ectopic Sox2 most resembled the gene expression profile of gastric zymogenic chief cells, whereas genes whose expression was decreased by Sox2 most resembled genes expressed in mature intestinal cells. Thus, by GO term enrichment, Sox2 induced genes expressed by gastric cells and reduced expression of normal intestinal genes. Ectopic Sox2 had mixed effects on parietal cell genes, potentially because of overlap in the types of genes that parietal cells express and those that intestinal enterocytes express. For example, genes regulating lipid metabolism are characteristic of both enterocytes and parietal cells (Mills et al., 2001).
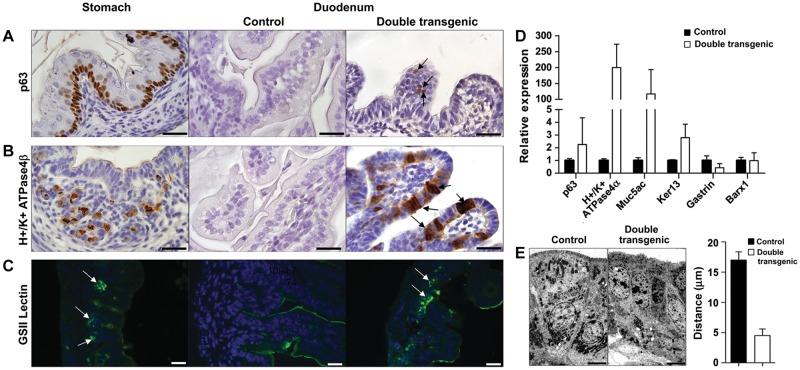
We followed up the global patterns of gene expression by confirming expression changes in individual markers with defined rostral–caudal patterns of expression in the gastro-intestinal tract. We analyzed markers identifying the presence of the esophagus and non-glandular gastric basal cells, the acid-producing glandular gastric parietal cells, and gastric mucous neck cells. This showed that unlike the controls, the double transgenic animals had focal expression of p63-positive basal cells (Figure 4A and Supplementary Figure S3D), characteristic of basal cells of the forestomach and the esophagus. Sox2-expressing intestines also showed abundant H+/K+ ATPase4β positive cells of the gastric parietal lineage (Figure 4B and Supplementary Figure S3E) and GSII lectin positive mucous neck cells (Figure 4C) within the intestinal epithelium. Mist1, a marker for the zymogenic chief cell lineage in adult stomach (Ramsey et al., 2007), did not reveal a specific staining pattern (data not shown), which is in accordance with the lack of mature chief cells in the embryonic stomach. In addition, ectopic expression of the stomach specific p63, H+/K+ ATPase4α, Mucin5ac (Muc5ac, marks gastric surface pit cells), and Keratin13 (Ker13, marks suprabasal cells) genes was detected using qPCR in the double transgenic intestine (Figure 4D). Ectopic expression of Sox2 in the intestinal epithelium did not clearly influence the underlying mesenchyme, as indicated by the unchanged low expression of the stromally expressed stomach-specific gene Barx1 (Figure 4D) (Stringer et al., 2012). Checking the expression of Gastrin revealed a reduced expression (Figure 4D), which is in line with Gastrin-producing cells being present embryonically in the intestine and pancreas, while emerging in the stomach only at birth (Larsson, 2000). Next, electron microscopy confirmed the lack of microvilli on the double transgenic intestinal epithelial cells (Figure 4E and Supplementary Figure S6). Moreover, it showed that ectopic Sox2 expression induced a more apical localization of the nuclei, in contrast to the basal orientation in controls (Figure 4E).
Figure 4.
Sox2 induces stomach-like cells in the intestinal environment. Cross-sections of the stomach and duodenum at E18.5 of controls and double transgenic embryos, which received doxycycline. IHC using markers for basal cells (p63) (A) and parietal cells (H+/K+ ATPase4β) (B) showed positive staining in the stomach. Ectopically expressed Sox2 induced the appearance of basal cells and parietal cells in duodenum of double transgenic animals (arrows), whereas control duodenum is devoid of staining. Immunofluorescence for GSII lectin (C), a marker for the mucous neck cells in the stomach, showed positive cells in the double transgenic intestine (arrows), while no expression was found in the control. Scale bar, 20 μm (A–C). (D) Analysis of the expression of marker genes for basal cells (p63), suprabasal cells (Ker13), parietal cells (H+/K+ ATPase4α), and gastric pit/surface-cell mucin (Muc5ac) by qPCR showed an increase in the small intestine of double transgenic animals. No significant change in the expression level of the stomach-specific mesenchymal marker Barx1 was detected. Expression of the Gastrin hormone was reduced in double transgenic animals. (E) Nuclei of normal intestinal epithelium are oriented toward the basal membrane, whereas the nuclei of the double transgenic intestinal epithelial cells are positioned more apically, shown by EM. Bar diagram represents the quantification of the distance of the nuclei to the apical border in control and double transgenic intestines. Scale bar, 6 μm.
Collectively, our data show that Sox2 expression in the embryonic intestinal epithelium causes anteriorization of the intestine with intestinal epithelial cells adopting the morphology and gene expression profile of immature gastric cells.
Ectopic Sox2 expression in the intestine alters the functionality of Cdx2, but not its expression level
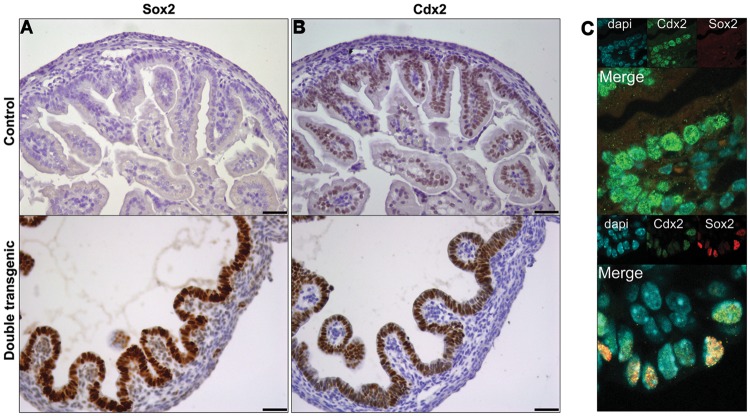
Considering all the changes caused by Sox2 expression, we examined whether Cdx2 expression was also decreased, thereby fitting the suggestion that Sox2 and Cdx2 are expressed in a mutually exclusive manner. qPCR showed that expression of the Cdx2 mRNA was not altered (data not shown). Surprisingly, IHC for Sox2 (Figure 5A) and Cdx2 (Figure 5B) revealed that both transcription factors were expressed throughout the entire intestinal epithelium of the Sox2 induced animals, whereas only Cdx2 was expressed in the control. Moreover, nuclear co-localization of Cdx2 and Sox2 was shown by immunofluorescence using confocal microscopy (Figure 5C).
Figure 5.
Sox2 and Cdx2 are co-expressed. IHC on sequential sections of E18.5 duodenum showed Sox2 expression only in double transgenic intestines (A), and Cdx2 expression throughout the epithelium in both control and double transgenic embryos (B). Confocal microscopy (C) of control and double transgenic animals showed that Cdx2 and Sox2 are co-expressed in the same intestinal epithelial cells in Sox2 expression embryos. Individual images of DAPI (blue), Cdx2 (green), and Sox2 (red) stainings are shown as inserts. Scale bar, 50 μm.
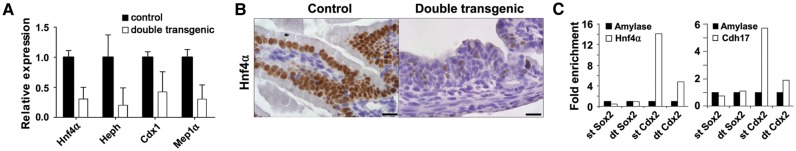
Thus, although Sox2 induced cellular changes leading to the occurrence of anterior gastro-intestinal cell types normally devoid of Cdx2, Cdx2 remained co-expressed in the Sox2 double transgenic intestines. However, the microarray analysis revealed several known Cdx2 target genes to be clearly downregulated in these Cdx2/Sox2 positive cells, including the well-established target gene Cdh17 (Hinoi et al., 2002). These reduced expression levels were confirmed by qPCR for the target genes, Hnf4α, Heph, Cdx1, and Mep1α (Figure 6A; Gao et al., 2009; Verzi et al., 2010). Using IHC we confirmed that the number of Hnf4α positive cells was strongly decreased in the double transgenic animals (Figure 6B).
Figure 6.
Sox2 affects the expression of Cdx2 target genes by inhibiting Cdx2 binding to target genes. (A) The expression levels of the Cdx2 target genes Hnf4α, Heph, Cdx1, and Mep1α are significantly downregulated in the small intestine of double transgenic animals at E18.5. (B) IHC with an antibody against Hnf4α on cross-sections of duodenum at E18.5 of control and Sox2 overexpressing animals showed a dramatic loss of staining in the double transgenic animals. Scale bar, 20 μm. (C) ChIP assay showed a dramatic loss of binding of Cdx2 to Cdh17 and Hnf4α in the double transgenic animals. Amylase served as a negative control.
These results suggest that ectopic expression of Sox2 does not directly affect the expression of Cdx2 itself, but interferes with the subsequent activation of Cdx2 target genes. Therefore, we analyzed the binding of Cdx2 to the promoter regions of two known target genes by chromatin immunoprecipitation (ChIP) using embryonic intestines from control and double transgenic animals (Figure 6C and Supplementary Figure S7). We show that ectopic expression of Sox2 in the intestine leads to a strong decrease in the binding of Cdx2 to its targets Cdh17 and Hnf4α, whereas Sox2 does not bind to these sites. Specificity of the Sox2 ChIP was confirmed by the detection of increased binding of Sox2 to its target Sox21 in the double transgenic embryos (Supplementary Figure S7). Thus, ectopic Sox2 expression in the intestinal tract does not affect the expression of Cdx2, but inhibits its function as a transcriptional regulator by interfering with the binding of Cdx2 to its gene target sites, which in turn prevents the activation of the intestinal transcriptome.
Discussion
During development of the gastro-intestinal tract, Sox2 is expressed in the anterior part of the primitive gut, while the homeobox gene Cdx2 is expressed in the posterior part (Sherwood et al., 2009). Various studies have suggested that the expression of Sox2 and that of Cdx2 are mutually exclusive, but it remains elusive whether one of the two is dominant over the other. Therefore, we ectopically expressed Sox2 in the prospective intestinal part of the primitive gut to investigate whether Sox2 has a dominant role over Cdx2 in the specification of the developing endoderm. We show that ectopic expression of Sox2 in the posterior region of the primitive gut caused aberrant activation of the foregut transcriptional program, leading to anteriorization of the gut with features resembling those of an immature embryonic stomach. Sox2 induction did not alter Cdx2 expression, but interfered with its function as a transcriptional regulator, providing evidence that Sox2 can exert a dominant effect over Cdx2 on cell fate.
Ectopic expression of Sox2 in the embryonic intestinal tract leads to a translucent and dilated intestine, which had a reduced number of villus-like outfoldings per surface area. These villi were poorly developed and showed increased numbers of cycling cells that were no longer confined to the intervillous regions, but were localized randomly throughout the intestinal epithelium. The differentiation toward goblet cells, enteroendocrine cells, and enterocytes was dramatically affected. Instead, the Sox2-expressing epithelium differentiated into gastric-like cell types, such as parietal cells and basal cells, demonstrated by IHC, qPCR, and GO term analysis. GO term comparative expression profile analysis also revealed that the genes upregulated by Sox2 were closer to cells of mature gastric zymogenic chief cell differentiation than to any of a variety of normal intestinal tissue gene expression profiles. These dramatic changes were accomplished in the short time-frame that ectopic Sox2 becomes expressed, i.e. E14.5–E18.5, demonstrating the dominant influence of Sox2 in the developing gut. Apparently, Sox2 is able to convert the cell fate of already committed embryonic intestinal cells into stomach-like cells. The prominent effect of Sox2 in determining cell fate was also highlighted by our previous study, in which we have shown that ectopic expression of Sox2 in the lung also results in dramatic changes in differentiation (Gontan et al., 2008).
The fluid filled, swollen appearance of intestines of the Sox2-induced animals is most likely caused by the dramatic decreased number of enterocytes combined with the disruption of the intestinal brush border. As a consequence of the decreased absorptive area, fluid cannot be absorbed by the intestine, resulting in fluid retention. Our studies with ectopic Sox2 expression in intestines of mouse embryos complement a recent study reporting the effect of Cdx2 ablation in mouse embryos (Gao et al., 2009). Cdx2 ablation severely affects the normal intestinal development, since mutant embryos fail to form the colon and rectum properly. Furthermore, the duodenum of the Cdx2 mutant animals was distended and became translucent. Also the disturbed proliferative pattern strongly resembles our observations. In our study the total number of epithelial cells was not significantly altered (data not shown), which is in accordance with the equal number of cells in the mitotic phase of the cell cycle as revealed by phospho-Histone H3 staining (20%–25% for both genotypes, data not shown). These results indicate that although Sox2 expression strongly affects the number and position of cycling cells, it most likely also leads to an increased duration of the cell cycle, effectively resulting in an equal production of epithelial cells.
Interestingly, Gao et al. (2009) also showed ectopic activation of Sox2 in the Cdx2 mutant intestine, which implied that Sox2 may have been responsible for inducing the phenotypes that were observed. Our results strongly support this concept, as we demonstrated that Sox2 induces the differentiation toward a premature stomach phenotype, in spite of sustained Cdx2 expression. We conclude that loss of Cdx2 by itself is not sufficient to drive the activation of the foregut transcriptional program, but requires expression of Sox2 to dominantly induce anteriorization.
Inactivation of Cdx2 in adult intestines induced the expression of gastric markers, but did not lead to major morphological changes (Stringer et al., 2012). Although these authors showed that the gut stem cells in the crypts showed some plasticity to express gastric genes, the plasticity of the cells in the developing gut is much greater since Gao et al. (2009) and we showed complete induction of gastric genes and cell types. This indicates that the adult intestinal epithelial cells have partly lost their potential to change their fate upon Cdx2 ablation. In support of this, we also observed reduced plasticity when Sox2 is ectopically induced in adult animals (unpublished data).
More recently, Cdx2 was shown to also regulate epithelial cell polarity (Gao and Kaestner, 2010). Depletion of Cdx2 in the developing mouse embryo from E15.5 onward, i.e. equivalent to the time point that we induce Sox2, led to the formation of irregular packed epithelial cells containing large subapical vacuoles and a disorganized pattern of several apical and basolateral markers. In our case, staining for ZO-1 (apical), E-cadherin (basolateral), and laminin-1 (basement membrane) revealed that epithelial polarity was not clearly affected (data not shown). We also did not observe the vacuoles and irregular packing of epithelial cells. These differences in phenotype are possibly explained by the incomplete functional downregulation of Cdx2 induced by Sox2 expression.
Ectopic expression of Sox2 did not affect the expression of the intestinal specific transcription factor Cdx2. This is in apparent contrast with work published by Benahmed et al. (2008), who investigated the regulation of Cdx2 expression. Using cellular approaches with a 13 kb genomic fragment covering part of the Cdx2 locus, they showed that Sox2 had a profound inhibitory effect on Cdx2 transcriptional activity. As our study shows that the endogenous locus is not affected by Sox2, this either implies that the proposed Sox2-binding site in the 13 kb fragment is not easily accessible in the endogenous setting or that for proper Cdx2 regulation additional domains are required, e.g. the 3′ located enhancer. Previous studies have provided limited data to suggest that Cdx2 regulates Sox2 expression, but no direct binding of Cdx2 to the Sox2 promoter region was shown (Gao et al., 2009; Sherwood et al., 2009; Mutoh et al., 2011). Nishiyama et al. (2009) recently described a genome-wide analysis of Cdx2 target sites using ChIP technology and clearly demonstrated that Cdx2 did not bind to the Sox2 locus. Verzi et al. (2010) analyzed the Caco-2 intestinal carcinoma cell line for putative CDX2-binding sites and identified a ChIP peak in an intergenic region in proximity to the SOX2 locus. However, it remains unclear whether the binding site had functional relevance to the SOX2 gene.
Although Cdx2 was not affected at the transcriptional level, its function is severely affected as deduced from the strong downregulation of several Cdx2 target genes. We show that Sox2 interferes with the binding of Cdx2 to its genomic target sites, thereby preventing its transcriptional activity. At present, the precise molecular mechanism to explain the inhibitory effect on Cdx2 DNA binding is unclear. Possibly Sox2 competes with co-factors that are needed by Cdx2 for its binding activity (Verzi et al., 2010), or Sox2 may induce differentiation into cell types that lack the expression of factors required by Cdx2 to bind efficiently to its target sequences.
In conclusion, we show that ectopic expression of Sox2 leads to impairment of villus formation and strongly alters the appearance of the normal intestinal epithelium. We show anteriorization of the primitive gut, demonstrated by cell fate conversion from intestinal epithelial into immature stomach-like cells. This demonstrates the dominant influence of Sox2 in the developing gut, since Sox2 is able to convert the cell fate of already committed intestinal cells into stomach-like cells. Sox2 induction does not alter Cdx2 expression, but inhibits its function as transcriptional activator by interfering with its DNA-binding capacity. This supports the idea that a balance between Sox2 and Cdx2 may dictate proper patterning of the foregut endoderm and that Sox2 may exert a dominant effect on intestinal cell fate by altering the differentiation program, emphasizing the plasticity of the primitive gut.
Our work improves the understanding of the mechanisms underlying the formation of the gastrointestinal tract and provides insight into the possible origin of various human congenital anomalies of the gut, such as Meckel's diverticulum, in which ectopic gastric tissue can be present in the small intestine. In addition, it may clarify the functional consequences of the ectopic SOX2 expression that we and others observed in human colorectal cancer (unpublished data; Park et al., 2008).
Materials and methods
Transgenic animals
The myc-Sox2 and Villin-rtTA transgenic mouse lines were previously described (Gontan et al., 2008; Roth et al., 2009). Administration of doxycycline to dams from E8.5 or E14.5 onward in the drinking water (2 mg/ml doxycycline, 5% sucrose) induced expression of myc-Sox2 in intestinal epithelium of double transgenic embryos. Each experiment was performed on at least three independent litters containing double transgenic, single transgenic and wild-type pups. All double transgenic animals receiving doxycycline expressed nuclear Sox2 in the intestinal epithelium throughout the entire intestinal tract and showed described phenotype.
Histology
Embryos were obtained by caesarean section at E18.5. The gastro-intestinal tract was isolated and stretched on pieces of filter paper, followed by fixation in 4% phosphate-buffered saline (PBS)-buffered paraformaldehyde (PFA) overnight at 4°C. Intestines were cut into four to five pieces and pre-embedded in 5% bacto-agar (BD) in PBS, before being embedded in paraffin according to routine protocols.
IHC, PAS staining and electron microscopy were performed as described previously (Darbas et al., 2004; Huang et al., 2012). Antibodies are listed in Supplementary Table S1. Differentiated and proliferative cells in the epithelium were quantified by counting at least three microscopic fields relative to the total number of epithelial cells.
Microarray analysis
The intestinal tracts of three control and two double transgenic embryos, which received doxycycline from E8.5 onward, were isolated at E18.5 and individually used for transcriptome analysis using Affymetrix Mouse Genome 430 2.0 microarrays as previously described (Gontan et al., 2008). Subsequent analysis of the data were performed as described in detail in Supplementary Materials and methods.
Chromatin immunoprecipitation
Small intestines of 10 double transgenic and 10 control E18.5 embryos were pooled and mechanically disrupted. Subsequently, the material was crosslinked, sonicated, and used to immunoprecipitate either Cdx2 or Sox2 complexes. The precipitated material was de-crosslinked and the co-immunoprecipitated DNA fragments were used as template in qPCR reactions to amplify specific target sequences (primers are listed in Supplementary Table S2). Details of the protocol are provided in Supplementary Materials and methods.
Quantitative polymerase chain reaction
RNA isolation, cDNA synthesis, and subsequent qPCR analysis were performed as previously described (Rajatapiti et al., 2008). Data were analyzed using the 2−ΔΔCt-method (Livak and Schmittgen, 2001). The gene-specific primers used are listed in Supplementary Table S3.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported in part by an Erasmus MC grant (to L.R.) and R01 NIH DK079798-3, 2P30 DK052574-12 (to J.C.M.).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors are indebted to Marcel Vermeij (Department of Pathology, Erasmus MC, The Netherlands), Xiao Yu, Widia Soochit, and Ralph Stadhouders (Department of Cell Biology, Erasmus MC, The Netherlands) for their technical assistance. We thank Katharina Biermann (Department of Pathology, Erasmus MC, The Netherlands) for her helpful insights in pathology.
References
- Avilion A.A., Nicolis S.K., Pevny L.H., et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F., Erler T., Russell A., et al. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev. Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Benahmed F., Gross I., Gaunt S.J., et al. Multiple regulatory regions control the complex expression pattern of the mouse Cdx2 homeobox gene. Gastroenterology. 2008;135:1238–1247. doi: 10.1053/j.gastro.2008.06.045. 1247.e1–1247.e3. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K., James R., Hammond V.E., et al. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Darbas A., Jaegle M., Walbeehm E., et al. Cell autonomy of the mouse claw paw mutation. Dev. Biol. 2004;272:470–482. doi: 10.1016/j.ydbio.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Doherty J.M., Geske M.J., Stappenbeck T.S., et al. Diverse adult stem cells share specific higher-order patterns of gene expression. Stem Cells. 2008;26:2124–2130. doi: 10.1634/stemcells.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen E., Akinci U., Bryne J.C., et al. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 2011;43:607–611. doi: 10.1038/ng.825. [DOI] [PubMed] [Google Scholar]
- Favaro R., Valotta M., Ferri A.L., et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fevr T., Robine S., Louvard D., et al. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., Kaestner K.H. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes Dev. 2010;24:1295–1305. doi: 10.1101/gad.1921510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., White P., Kaestner K.H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev. Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C., de Munck A., Vermeij M., et al. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev. Biol. 2008;317:296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Gontan C., Güttler T., Engelen E., et al. Exportin 4 mediates a novel nuclear import pathway for Sox family transcription factors. J. Cell Biol. 2009;185:27–34. doi: 10.1083/jcb.200810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.C., Yin T., Grindley J.C., et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi T., Lucas P.C., Kuick R., et al. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002;123:1565–1577. doi: 10.1053/gast.2002.36598. [DOI] [PubMed] [Google Scholar]
- Huang Y., Buscop-van Kempen M., Boerema-de Munck A., et al. Hypoxia-inducible factor 2α plays a critical role in the formation of alveoli and surfactant. Am. J. Respir. Cell Mol. Biol. 2012;46:224–232. doi: 10.1165/rcmb.2011-0024OC. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Rex M., Scotting P.J., et al. Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial–mesenchymal interactions. Dev. Dyn. 1998;213:464–475. doi: 10.1002/(SICI)1097-0177(199812)213:4<464::AID-AJA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Kazenwadel J. Homeobox gene expression in the intestinal epithelium of adult mice. J. Biol. Chem. 1991;266:3246–3251. [PubMed] [Google Scholar]
- James R., Erler T., Kazenwadel J. Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J. Biol. Chem. 1994;269:15229–15237. [PubMed] [Google Scholar]
- Kondoh H., Kamachi Y. SOX-partner code for cell specification: regulatory target selection and underlying molecular mechanisms. Int. J. Biochem. Cell Biol. 2010;42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kormish J.D., Sinner D., Zorn A.M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M.P., Heller D.S., Bethel C. Extensive gastric heterotopia of the small intestine resulting in massive gastrointestinal bleeding, bowel perforation, and death: report of a case and review of the literature. Pediatr. Dev. Pathol. 2000;3:277–280. doi: 10.1007/s100249910036. [DOI] [PubMed] [Google Scholar]
- Larsson L.I. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc. Res. Tech. 2000;48:272–281. doi: 10.1002/(SICI)1097-0029(20000301)48:5<272::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mills J.C., Syder A.J., Hong C.V., et al. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc. Natl Acad. Sci. USA. 2001;98:13687–13692. doi: 10.1073/pnas.231332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H., Sashikawa M., Sugano K. Sox2 expression is maintained while gastric phenotype is completely lost in Cdx2-induced intestinal metaplastic mucosa. Differentiation. 2011;81:92–98. doi: 10.1016/j.diff.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Xin L., Sharov A.A., et al. Uncovering early response of gene regulatory networks in ESCs by systematic induction of transcription factors. Cell Stem Cell. 2009;5:420–433. doi: 10.1016/j.stem.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.T., Gum J.R., Kakar S., et al. Aberrant expression of SOX2 upregulates MUC5AC gastric foveolar mucin in mucinous cancers of the colorectum and related lesions. Int. J. Cancer. 2008;122:1253–1260. doi: 10.1002/ijc.23225. [DOI] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J.R., et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Luo X., Schwartz R.J., et al. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajatapiti P., van der Horst I.W., de Rooij J.D., et al. Expression of hypoxia-inducible factors in normal human lung development. Pediatr. Dev. Pathol. 2008;11:193–199. doi: 10.2350/07-04-0257.1. [DOI] [PubMed] [Google Scholar]
- Ramsey V.G., Doherty J.M., Chen C.C., et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- Roth S., Franken P., van Veelen W., et al. Generation of a tightly regulated doxycycline-inducible model for studying mouse intestinal biology. Genesis. 2009;47:7–13. doi: 10.1002/dvg.20446. [DOI] [PubMed] [Google Scholar]
- Shaoul R., Marcon P., Okada Y., et al. The pathogenesis of duodenal gastric metaplasia: the role of local goblet cell transformation. Gut. 2000;46:632–638. doi: 10.1136/gut.46.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R.I., Chen T.Y., Melton D.A. Transcriptional dynamics of endodermal organ formation. Dev. Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer E.J., Duluc I., Saandi T., et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development. 2012;139:465–474. doi: 10.1242/dev.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova O.V., Magness S.T., Fagan B.M., et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi M.P., Hatzis P., Sulahian R., et al. TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc. Natl Acad. Sci. USA. 2010;107:15157–15162. doi: 10.1073/pnas.1003822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.M., Melton D.A. Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Wells J.M., Melton D.A. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Williamson K.A., Hever A.M., Rainger J., et al. Mutations in SOX2 cause anophthalmia–esophageal–genital (AEG) syndrome. Hum. Mol. Genet. 2006;15:1413–1422. doi: 10.1093/hmg/ddl064. [DOI] [PubMed] [Google Scholar]
- Wright E.M., Martin M.G., Turk E. Intestinal absorption in health and disease—sugars. Best Pract. Res. Clin. Gastroenterol. 2003;17:943–956. doi: 10.1016/s1521-6918(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Zorn A.M., Wells J.M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.