Abstract
Many polymers, such as PMMA, are very susceptible to swelling or dissolution by organic solvents. Growing covalently attached polymer brushes from these surfaces by ATRP is challenging because of the typical requirement of organic solvent for initiator immobilization. We report an unprecedented, aqueous-based route to graft poly(N-isopropylacrylamide), PNIPAAm, from poly(methyl methacrylate), PMMA, surfaces by atom-transfer radical polymerization (ATRP), wherein the underlying PMMA is unaffected. Successful attachment of the ATRP initiator, N-hydroxysuccinimidyl-2-bromo-2-methylpropionate, on amine-bearing PMMA surfaces was confirmed by XPS. From this surface-immobilized initiator, thermoresponsive PNIPAAm brushes were grown by aqueous ATRP to yield optically transparent PNIPAAm-grafted PMMA surfaces. This procedure is valuable, as it can be applied for the aqueous-based covalent attachment of ATRP initiator on any amine-functionalized surface, with subsequent polymerization of a variety of monomers.
INTRODUCTION
Grafting of unique polymers with useful characteristics on an existing polymer substrate can impart new surface properties, such as wettability, adhesion, nonspecific adsorption, bio-compatibility, and stimuli-responsive behavior.1–3 Often times the specific surface properties for a given application require the use of tethered polymer brushes on these polymer surfaces.1,2 For example, nonspecific adsorption of proteins and other biomolecules, typical of most polymer substrates,4 can be decreased/prevented by surface grafting of poly(ethylene glycol) and its copolymers.5,6 Similarly, grafting of responsive polymers on other polymer surfaces has the potential to yield interfaces with characteristics that are dependent on environmental stimuli, allowing for programmed desorption of target analytes7,8 and control over fluid flow,9 to name a few.
Poly(methyl methacrylate), PMMA, is widely used in micro-and nanofabrication of microfluidic devices and other applications because of its good thermal and mechanical properties, optical transparency, low fluorescence background, nontoxicity, commercial availability, and low cost.7,10–12 In order to make polymeric micro/nanofluidic devices with desired performance characteristics, it is often required that their surfaces be modified with different functional groups that yield specific interfacial properties.13 We are interested in fabricating stimuli-responsive polymer surfaces on PMMA substrates.7 Poly(N-isopropylacrylamide), PNIPAAm, is the most-often selected material for this application,14,15 as it exhibits a lower critical solution temperature (LCST) in aqueous solution at 32 °C at which the polymer changes from a hydrophilic, expanded state to a collapsed hydrophobic state. Importantly, grafting PNIPAAm onto a substrate surface can lead to its surface hydrophobicity being thermally responsive in a reversible fashion.16 Such surfaces have promising applications in separation science,17–19 switchable membranes,9,20–23 and the attachment–detachment of cells,24–26 bacteria,27,28 and proteins.29,30
The main challenge in the development of surface modification routes for polymer substrates, such as PMMA, is discovering and optimizing aqueous-based chemistries that offer the diversity of functionalities possible with “traditional” nonaqueous paths. Specifically for PMMA, it has poor resistance to many organic solvents, resulting in its being readily swelled and dissolved in these solvents; this is particularly problematic for modification of microfluidic devices whose feature sizes and topography must not be significantly altered by the modification process.
Polymer substrate surfaces can be coated with a desired polymer by either physical adsorption or covalent grafting methods.31 Physisorption will not result in a permanent surface modification, as it is not stable toward heat and solvent influence.32–34 The covalent grafting approach typically results in a stable polymer layer that can be prepared by two distinct paths, the first of which is “grafting to” where a preformed polymer with a pendant linker group is directly attached to a complementary reactive group on the polymer substrate. The other path called “grafting from” is based on formation or growth of polymer chains from polymerization initiators attached to the substrate surface, and it is the most versatile technique because it typically results in thick polymer brushes. Such a surface-initiated polymer film formation can be achieved through use of several polymerization techniques, such as ionic, ring-opening, radical, and reversible addition–fragmentation chain transfer polymerization as well as the atom-transfer radical polymerization (ATRP) method.35
Among these, ATRP36–38 has been considered as one of the most effective surface-initiated graft polymerizations due to its tolerance to a variety of reaction conditions and monomers.3,6,39–41 However, for achievement of successful surface-initiated ATRP that yields modified surfaces with the desired qualities, the following crucial steps must occur: attachment of an appropriate ATRP initiator on a given substrate surface and then surface-initiated ATRP of monomer with an efficient catalyst. As reviewed by Fristrup et al.,3 attachment of an ATRP initiator is normally done by immersing hydroxyl- or amine-functionalized substrates in a solution of an acid halide-functionalized initiator in an organic solvent, such as tetrahydrofuran (THF). Efficient covalent attachment of acid halide initiator to substrate hydroxyl and amine surface groups is readily obtained with “traditional” nonpolymeric substrates (gold, silicon wafer, and silica) due to their inert behavior with respect to such organic solvents and derivatization reagents.
Although some synthetic polymer substrates can be modified with initiator under these rather aggressive conditions, namely cross-linked polystyrene, isotactic polypropylene, ethylene–acrylic acid copolymers, poly(vinylidene fluoride), poly(ether ether ketone), and some natural polymeric materials,42–44 such a route is not universally applicable, particularly for polymer substrates that are sensitive to organic solvents and reagents. As a result, a number of investigations have studied the feasibility of routes that minimize or do not involve organic solvents for initiator attachment, including those based on vapor-phase attachment,45 microcontact printing,46 coating24,47 or layer-by-layer deposition44,48,49 of initiator on the surface. These are attractive possibilities for solvent-sensitive substrates, but they are substrate specific or require additional efforts of preparation and purification of macroinitiators.
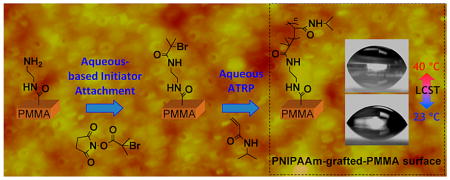
In this paper, we successfully demonstrate a simple aqueous-based route for the covalent immobilization of an ATRP initiator on PMMA surfaces and subsequent surface-initiated aqueous ATRP formation of PNIPAAm. The initiator immobilization was achieved by reacting the activated ester of a valuable ATRP initiator (N-hydroxysuccinimidyl-2-bromo-2-methylpropionate) with amine-functionalized PMMA surfaces50 in aqueous media. From the covalently attached initiator surfaces, atom-transfer polymerization in water was carried out to grow PNIPAAm brushes. This aqueous-based route to grafting polymers from surfaces can be adaptable to a variety of substrates and water-soluble ATRP monomers and is green in nature.51,52
EXPERIMENTAL SECTION
Materials
Copper(I) chloride (99.9%), 2-bromo-2-methylpropionic acid (BMP, 98%), N-hydroxysuccinimide (NHS, 98%), N,N′-dicyclohexylcarbodiimide (99%), tris[2-aminoethyl]amine (97%), ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride, and anhydrous dichloromethane were purchased from Sigma-Aldrich and used without further purification. N-Isopropylacrylamide (NIPAAm, 97%) obtained from Sigma-Aldrich was purified by recrystallization from hexane before use. Tris[2-(dimethylamino)ethyl]amine, Me6TREN, was prepared according to a literature procedure.53,54 All other chemicals were reagent grade and used without further purification. Buffers were prepared using reagent-grade chemicals and deionized water (18 MΩ cm). Poly(methyl methacrylate), PMMA, sheets were obtained from Goodfellow. The 250 μm thick PMMA sheets were cut in to small pieces (1 cm × 3 cm “slides”) and then were cleaned by immersion in 2-propanol for 10 min. Then they were washed with deionized water and dried in a stream of nitrogen.
Amine Functionalization of PMMA Surfaces
The clean PMMA samples were UV modified using a low-pressure mercury lamp for 15 min to introduce carboxylic acid functional groups on the surface.7,55 After UV modification, the slides were washed with deionized water and dried in a stream of nitrogen. The carboxylic acid groups thus formed on the surface were elaborated by reaction with ethylenediamine (5 × 10−3 M) in a solution of 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC, 0.2 M) and N-hydroxysuccinimide (NHS, 0.05 M) in deionized water for 5 h; these conditions are those found to be optimal from a previous report.7 The samples were cleaned with deionized water and dried under a stream of nitrogen.
Preparation of the ATRP Initiator, N-Hydroxysuccinimidyl-2-bromo-2-methylpropionate (NHS-BMP)
NHS-BMP was prepared56,57 by first dissolving 3.72 g of NHS to a solution of 2-bromo-2-methylpropionic acid (4.5 g) in 15 mL of dry dichloromethane (DCM). Under an argon atmosphere, this solution was cooled to 0 °C, and a solution of 7.06 g of 1,3-dicyclohexylcarbodimide in 10 mL of DCM was added dropwise with constant stirring and stirred overnight at room temperature. The solid formed was filtered, concentrated, and purified by flash chromatography on silica with a 3:1 hexanes:ethyl acetate to yield a white solid. GC-MS: (EI) m/z 264. 1H NMR (CDCl3) δ = 2.07 (s, 6H, CH3), 2.87 (s, 4H, CH2).
Initiator Immobilization
To the 120 mL of 0.01 M phosphate buffer, pH 7.3, containing 0.1 M KCl, 26 mg of NHS-BMP initiator dissolved in 12 mL of dimethyl sulfoxide, DMSO,58 was added. It was then immediately transferred to the scintillation vials containing amine-functionalized PMMA slides. The containers were closed, and the reaction was allowed to occur overnight at room temperature. Then, the slides were washed sequentially with buffer and deionized water, followed by drying in a stream of nitrogen. We also observed that a very high concentration of initiator in DMSO can cause damage to the PMMA surfaces.
Polymer Grafting
Surface grafting was carried out by first placing the initiator attached PMMA slides in small glass vials sealed with a rubber septum. The vials were evacuated and refilled with Ar three times. NIPAAm (11.3 g, 1 × 10−1 mol) and CuCl (98 mg, 1 × 10−3 mol) were weighed into a separate round-bottomed flask and sparged with Ar gas. 100 mL of degassed, deionized water was added to this flask through the septum to dissolve the contents. Afterward, the flask was placed and then kept in an ice bath, and then 254 μL (1 × 10−3 mol) of Me6TREN was injected into this flask, and the solution was degassed with Ar for an additional 30 min. This solution was then injected into different vials containing initiator–PMMA slides under an Ar atmosphere. After the addition, the vials were kept inside a refrigerator, and polymerization was carried out for 24 h. The PMMA slides were then removed from the solution, rinsed thoroughly with copious amounts of cold deionized water, and dried under a stream of nitrogen.
Instrumentation
Fourier-transform infrared spectroscopy (FTIR) was carried out using a Bruker Tensor 27 FT-IR instrument with Pike Miracle single-bounce attenuated total reflectance (ATR) cell equipped with a ZnSe single crystal. The thin PMMA sheets used were placed directly on the small crystal spot, and gentle pressure was applied to ensure good physical contact. Background spectra were measured with a bare ATR crystal. 256 scans were collected between 4000 and 600 cm−1 for both background and sample spectra. Measurements were made at a spectral resolution of 4 cm−1. 1H NMR spectra were recorded on a Bruker DPX-250 using CDCl3 as the solvent. The sessile drop contact angles of water on the surfaces were measured using a VCA 2000 contact angle system (VCA, Billerica, MA). The surface composition of the PMMA and modified surfaces were obtained with a Kratos analytical Axis 165 X-ray photoelectron spectrometer with Al Kα X-ray radiation of 1486.6 eV and takeoff angle of 90°. Peak locations were corrected based on the C 1s signal at 285 eV. Survey spectra and high-resolution spectra of individual elemental regions were recorded with pass energies of 80 and 40 eV, respectively. The morphology of the surfaces was imaged using a Nanoscope III Multimode scanning force microscope (Digital Instruments, Santa Barbara, CA) in air at ambient temperature. The operating mode was intermittent contact mode (Tapping mode). A microcantilever was used as a scanning probe with approximate resonant frequency of 325 kHz and a force constant of 40 N m−1 (MikroMasch NSC 15/50).
RESULTS AND DISCUSSION
Attachment of ATRP Initiator
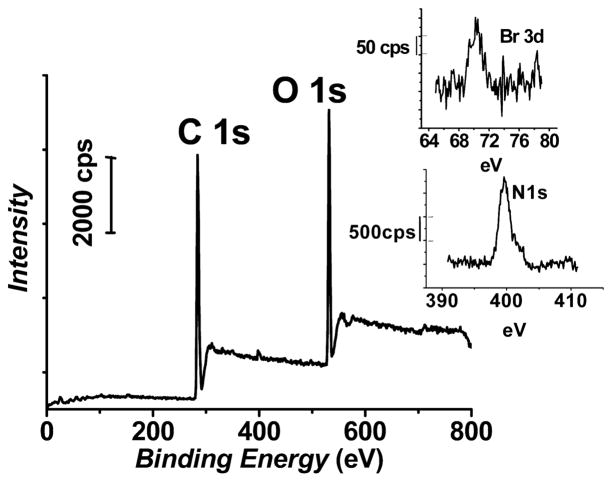
Successful attachment of the N-hydroxysuccinimidyl-2-bromo-2-methylpropionate (NHS-BMP) initiator on the amine-terminated PMMA surfaces7 using the conditions outlined in Scheme 1 was confirmed upon inspection of their X-ray photoelectron spectra (Figure 1). The peak corresponding to the Br 3d transition of the BMP initiator was observed at 70 eV in the high-resolution spectrum (top inset of Figure 1). In addition, the peak centered at 400 eV is due to the N 1s transition of the amide groups that connect the PMMA surface to the initiator, an observation similar to that we have found for attachment of other materials on PMMA via amide bonds.7,55 Control experiments with PMMA exposed to solutions of the NHS-BMP did not lead to observation of Br signals in their X-ray photoelectron spectra, indicating that the outcomes in Figure 1 are due to covalently attached BMP initiator.
Scheme 1.
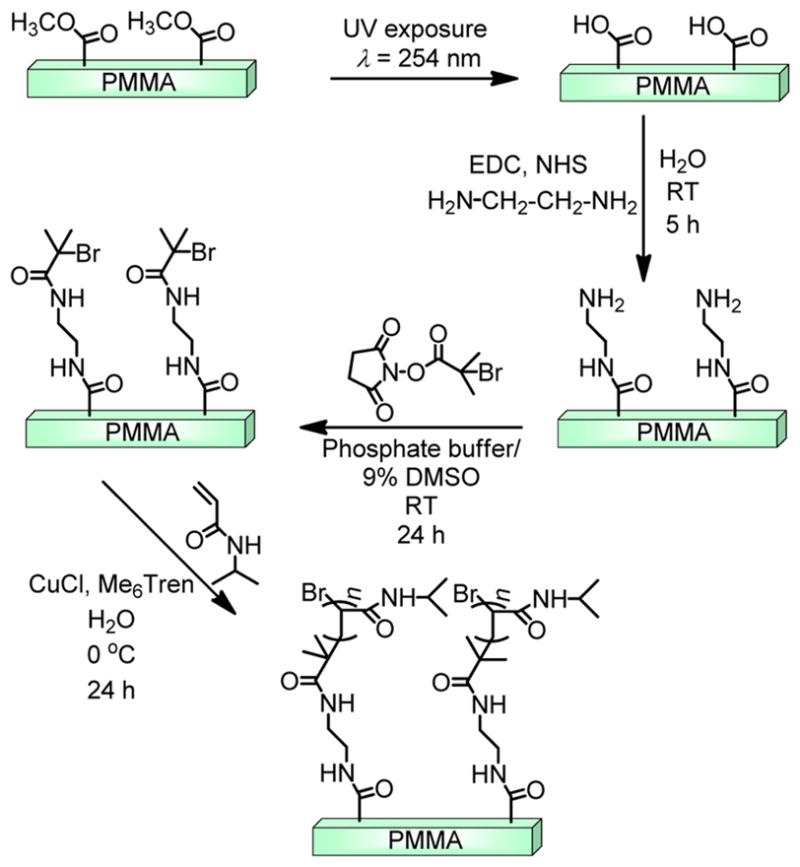
Reaction Path Used for Surface Grafting of PNIPAAm from PMMA by Aqueous-Based ATRP
Figure 1.
Representative X-ray photoelectron spectrum of BMP initiator-modified PMMA surface. Shown in the insets are the high-resolution regions for N 1s and Br 3d transitions.
Grafting of PNIPAAm from BMP–PMMA Surfaces by ATRP
PNIPAAm was grown from the initiator-terminated surface by immersing the latter in an Ar-degassed aqueous solutions of NIPAAm monomer containing the ATRP catalyst system (CuCl and Me6TREN) at 0 °C for 24 h.59–61 As we show in sections that follow, successful grafting of PNIPAAm from BMP initiator-PMMA surfaces was confirmed by spectroscopic, microscopic, and wettability measurements.
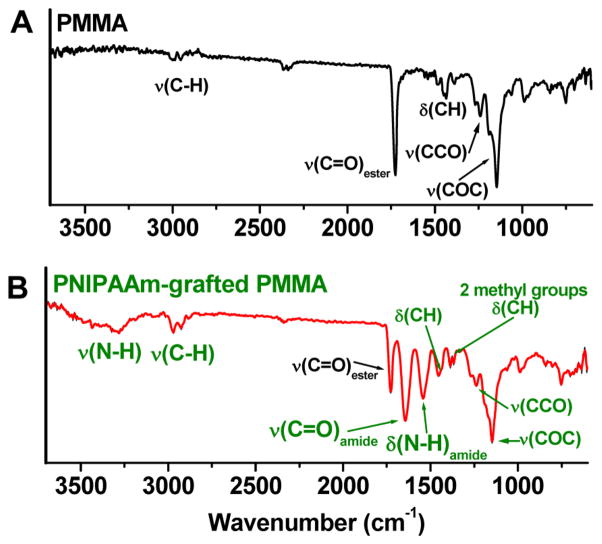
In Figure 2A,B are shown representative FTIR spectra of pristine (unmodified) PMMA and PNIPAAm-grafted PMMA substrates. Compared with the pristine PMMA surface (Figure 2A), IR transitions corresponding to PNIPAAm62 are evident in the spectrum for the grafted surface (Figure 2B). Also apparent are IR bands associated with the PMMA substrate, and they arise due to the penetration depth of the IR beam being greater than the PNIPAAm film thickness.63 For the PNIPAAm–PMMA surface, the broad band in the 3155–3595 cm−1 range and the weak intensity absorption between 3040 and 3101 cm−1 are attributed to the amide N–H stretching and amide II overtone transitions. The sharp transitions at 1645 and 1540 cm−1 are associated with amide transitions (νC=O and δN-H) of PNIPAAm. The peak at 1460 cm−1 is due to the methyl antisymmetric deformation, and those that correspond to the two methyl groups of the isopropyl functionality of the PNIPAAm are found at 1367 and 1386 cm−1. These IR transitions are virtually identical to those reported for surface plasma-polymerized PNIPAAm,62 leading us to the conclusion that formation of PNIPAAm on the initiator-terminated PMMA surfaces was successful.
Figure 2.
Fourier-transform infrared (FTIR) spectra of (A) PMMA and (B) PNIPAAm-grafted PMMA surfaces.
Representative X-ray photoelectron spectra of pristine PMMA64 and PNIPAM-grafted PMMA surfaces are shown in Figure 3. For the pristine PMMA substrate (Figure 3A), the survey spectrum possesses two peaks: one at roughly 285 eV that is associated with the carbon C 1s transition and the other at 531 eV due to the oxygen O 1s transition of PMMA. The survey spectrum of PNIPAAm-grafted PMMA (Figure 3B) has not only the C 1s and O 1s transitions but also a band near 400 eV that is attributed to the nitrogen N 1s of the amide groups of PNIPAAm; see Figure S1 for XPS of bulk, solid PNIPAAm. Compared to the survey scan of the BMP initiator-functionalized PMMA surface (Figure 1), there is a substantially larger amount of nitrogen present on the PNIPAAm–PMMA surfaces (Figure 3B). Additional support for the presence of the grafted PNIPAAm material on the PMMA surface comes from spectral fitting of the high-resolution C 1s spectra of PMMA and PMMA–PNIPAAm (Figure S2), with the presence of the characteristic C 1s signals for the amide (286.1 and 287.8 eV). As seen in Table 1, the PNIPAAm-grafted PMMA surface is composed of 78.5% carbon, 9.1% nitrogen, and 12.4% oxygen, values that are quite similar to those for the composition of PNIPAAm, which are 75% carbon, 12.5% nitrogen, and 12.5% oxygen.
Figure 3.
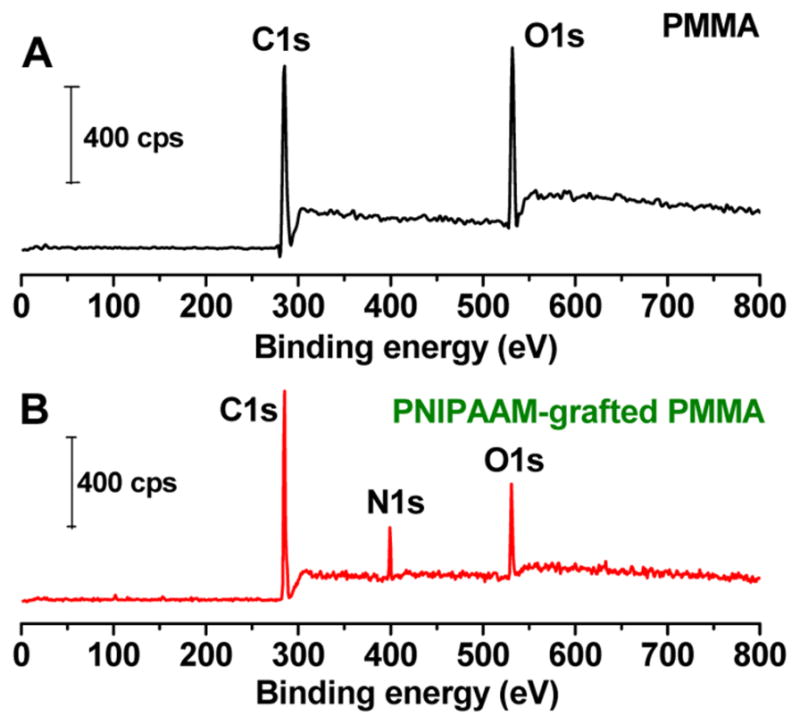
X-ray photoelectron survey spectra of (A) pristine PMMA and (B) PNIPAAm-grafted PMMA.
Table 1.
Elemental Atomic Composition of PNIPAAm/PMMA Surfaces from XPS Studies
| % C (285.5 eV) | % N (400 eV) | % O (531 eV) | |
|---|---|---|---|
| pristine PMMA | 77.1 | not observed | 22.8 |
| PNIPAAm-grafted PMMA | 78.5 | 9.1 | 12.4 |
| theor PNIPAAm composition | 75 | 12.5 | 12.5 |
The surface morphology of the PMMA surface before (initiator–PMMA, Figure 4A) and after (PNIPAAm–PMMA, Figure 4B) polymerization was examined using scanning force microscopy. The root-mean-square (rms) roughness of the initiator-modified surface was 20 nm (25 μm × 25 μm area of evaluation). This value is typical of what we have found for UV-modified PMMA (~18–27 nm),55 and it results from the substrate changes that occur during the steps needed to prepare the initiator–PMMA surface, namely washing with isopropanol, UV modification, amine functionalization, and initiator modification. From our previous work,55 the number of carboxylic acid sites, corrected for roughness from the initial UV-modification chemistry, is approximately one monolayer, pointing to a very efficient process for the 15 min UV exposure time. The roughness after PNIPAAm grafting decreased to 11 nm (25 μm × 25 μm area of evaluation). Such a decrease in surface roughness as a result of polymer grafting is characteristic of homogeneous layer polymer formation on the surface, as noted for PNIPAAm grafts on poly(propylene) and poly-(ethylene terephthalate).65,66 Importantly, the ATRP grafting-from process developed here does not cause significant damage to the underlying PMMA.
Figure 4.
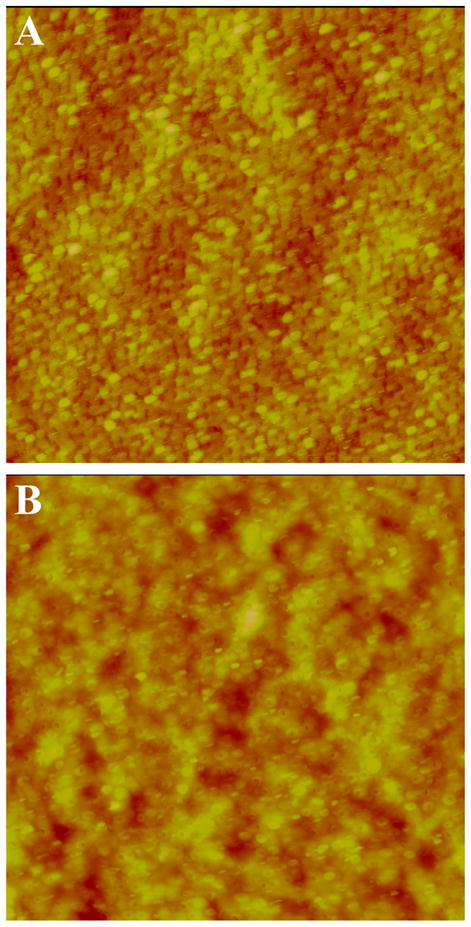
25 μm × 25 μm topographic scanning force microscopy images of (A) BMP(initiator)–PMMA and (B) PNIPAAm-grafted PMMA surfaces in air at 25 °C. Z-range is 200 nm.
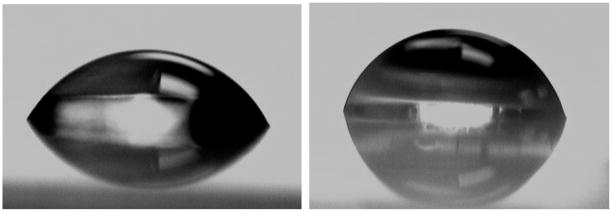
In aqueous environments, PNIPAAm surfaces are known to undergo rapid conformational changes from a coiled to expanded form in response to temperature changes near the lower critical solution temperature (LCST).15,67 As a result of this phase transition, a characteristic change in wettability occurs wherein the surface becomes more hydrophobic above the LCST near 32 °C, as found by measuring the contact angle of a sessile water droplet on thermostated surfaces. As noted in Figure 5, we found that the PNIPAAm-grafted PMMA substrates were slightly more hydrophilic with an observed contact angle of 62 ± 2° at 23 °C, whereas at temperatures of 40 °C or above, the water contact angle was 72 ± 2°. The magnitude of this change in contact angle values above and below the LCST is in agreement with previous reports for PNIPAAm on other substrates, such as gold.16,68–70 The repeated ability of the PMMA–PNIPAAM surface to change its wettability from hydrophilic to hydrophobic and vice versa (reversible nature) was tested by changing the temperature of the water several times above and below the LCST; it was found that this property is highly reversible. This property was not exhibited by PMMA or initiator–PMMA surfaces. Thus, the temperature-dependent wettability of the PNIPAAm-grafted PMMA surfaces confirmed the presence of the PNIPAAm and its ability to undergo its characteristic phase transition.
Figure 5.
Water contact angle images (sessile drop) of PNIPAAm-grafted PMMA surfaces at 23 °C (left) and 40 °C (right).
Importantly, the overall process for PNIPAAm grafting did not significantly affect the optical transparency or reflectivity of the underlying PMMA. However, a strikingly different result was observed in control experiments using surfaces possessing no initiator. Exposure of amine-terminated PMMA surfaces (the precursor to BMP initiator–PMMA) to the same polymerization medium for the same amount of time as those generated using the BMP initiator–PMMA surfaces resulted in a nonreffective, very opaque PMMA material (Figure S3, entry 6). This observed alteration of the optical properties of the underlying PMMA during the control experiment is characteristic of NIPAAm monomer-induced damage to the PMMA. The fact that the PNIPAAm-grafted PMMA surfaces do not exhibit changes in their optical properties points to the growing PNIPAAm film acting as a protective layer to the underlying PMMA substrate, similar to what we have seen during octadecyl layer formation on amine-terminated PMMA surfaces.50
CONCLUSIONS
In summary, we have demonstrated a “surface-safe”, facile, and green method for attachment of an ATRP initiator on PMMA surfaces in aqueous-based media with subsequent aqueous ATRP polymerization of NIPAAm from this surface at low temperature. Key to this route is the use of the NHS ester of the ATRP initiator and its covalent linkage to amine-modified PMMA surfaces in aqueous buffer media; this is quite suitable for PMMA substrates that are sensitive to organic solvents, and it should be readily applied to other polymers with such solvent/reagent sensitivities. The formation of poly(N-isopropylacrylamide) brushes on the initiator–PMMA surface was confirmed by XPS, FTIR, AFM, and temperature-dependent contact angle measurements. Potential applications of these responsive surfaces in nano/microfluidic devices include smart protein capture and release coatings and possibly temperature-controlled flow in embossed nanochannels.9,71 Other potential avenues include development of responsive homo- and copolymer surfaces on various polymer substrates that can subsequently be used for cell harvesting, simply by rinsing with water at a temperature lower than their LCST.
Supplementary Material
Acknowledgments
This work was made possible in part by financial support from the US National Science Foundation (CHE-0910845) and the US National Institutes of Health (5R21CA135585).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
X-ray photoelectron spectrum of solid PNIPAAm and high-resolution C 1s spectrum of PMMA and PNIPAAm-grafted PMMA surfaces and optical photograph of variously treated PMMA substrates. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kato K, Uchida E, Kang E-T, Uyama Y, Ikada Y. Polymer surface with graft chains. Prog Polym Sci. 2002;28(2):209–259. [Google Scholar]
- 2.Uyama Y, Kato K, Ikada Y. Surface modification of polymers by grafting. Adv Polym Sci. 1998;137:1–39. [Google Scholar]
- 3.Fristrup CJ, Jankova K, Hvilsted S. Surface-initiated atom transfer radical polymerization-a technique to develop biofunctional coatings. Soft Matter. 2009;5(23):4623–4634. [Google Scholar]
- 4.Satulovsky J, Carignano MA, Szleifer I. Kinetic and thermodynamic control of protein adsorption. Proc Natl Acad Sci U S A. 2000;97(16):9037–9041. doi: 10.1073/pnas.150236197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hucknall A, Rangarajan S, Chilkoti A. In pursuit of zero: polymer brushes that resist the adsorption of proteins. Adv Mater (Weinheim, Ger) 2009;21(23):2441–2446. [Google Scholar]
- 6.Xu FJ, Neoh KG, Kang ET. Bioactive surfaces and biomaterials via atom transfer radical polymerization. Prog Polym Sci. 2009;34(8):719–761. [Google Scholar]
- 7.McCarley RL, Vaidya B, Wei S, Smith AF, Patel AB, Feng J, Murphy MC, Soper SA. Resist-free patterning of surface architectures in polymer-based microanalytical devices. J Am Chem Soc. 2005;127(3):842–843. doi: 10.1021/ja0454135. [DOI] [PubMed] [Google Scholar]
- 8.Ernst O, Lieske A, Jager M, Lankenau A, Duschl C. Control of cell detachment in a microfluidic device using a thermo-responsive copolymer on a gold substrate. Lab Chip. 2007;7(10):1322–9. doi: 10.1039/b708619a. [DOI] [PubMed] [Google Scholar]
- 9.Lokuge I, Wang X, Bohn PW. Temperature-controlled flow switching in nanocapillary array membranes mediated by poly(N-isopropylacrylamide) polymer brushes grafted by atom transfer radical polymerization. Langmuir. 2007;23(1):305–311. doi: 10.1021/la060813m. [DOI] [PubMed] [Google Scholar]
- 10.Leonida MD, Kumar I. Microfluidics: from engineering to life sciences. Curr Nanosci. 2012;8(3):458–473. [Google Scholar]
- 11.Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC. Polymeric microelectromechanical systems. Anal Chem. 2000;72(19):643A–651A. doi: 10.1021/ac0029511. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Pumera M, Chatrathi MP, Escarpa A, Konrad R, Griebel A, Dorner W, Lowe H. Towards disposable lab-on-a-chip: poly(methylmethacrylate) microchip electrophoresis device with electrochemical detection. Electrophoresis. 2002;23(4):596–601. doi: 10.1002/1522-2683(200202)23:4<596::AID-ELPS596>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32(7):698–725. [Google Scholar]
- 14.Heskins M, Guillet JE. Solution properties of poly(N-isopropylacrylamide) J Macromol Sci, Chem. 1968;2(8):1441–55. [Google Scholar]
- 15.Schild HG. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci. 1992;17(2):163–249. [Google Scholar]
- 16.Balamurugan S, Mendez S, Balamurugan SS, O’Brien MJ, Lopez GP. Thermal response of poly(N-isopropylacrylamide) brushes probed by surface plasmon resonance. Langmuir. 2003;19(7):2545–2549. doi: 10.1021/la026787j. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi A, Okano T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog Polym Sci. 2002;27(6):1165–1193. [Google Scholar]
- 18.Kanazawa H. Thermally responsive chromatographic materials using functional polymers. J Sep Sci. 2007;30(11):1646–1656. doi: 10.1002/jssc.200700093. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Zhang H, Wirth M. Chemical modification of the surface of poly(dimethylsiloxane) by atom-transfer radical polymerization of acrylamide. Langmuir. 2002;18(25):9971–9976. [Google Scholar]
- 20.Rama Rao GV, Krug ME, Balamurugan S, Xu H, Xu Q, López GP. Synthesis and characterization of silica-poly(N-isopropylacrylamide) hybrid membranes: switchable molecular filters. Chem Mater. 2002;14(12):5075–5080. [Google Scholar]
- 21.Alem H, Duwez A-S, Lussis P, Lipnik P, Jonas AM, Demoustier-Champagne S. Microstructure and thermo-responsive behavior of poly(N-isopropylacrylamide) brushes grafted in nanopores of track-etched membranes. J Membr Sci. 2008;308(1 + 2):75–86. [Google Scholar]
- 22.Park YS, Ito Y, Imanishi Y. Permeation control through porous membranes immobilized with thermosensitive polymer. Langmuir. 1998;14(4):910–914. [Google Scholar]
- 23.Fu Q, Rama Rao GV, Basame Solomon B, Keller David J, Artyushkova K, Fulghum Julia E, Lopez Gabriel P. Reversible control of free energy and topography of nanostructured surfaces. J Am Chem Soc. 2004;126(29):8904–5. doi: 10.1021/ja047895q. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani A, Kikuchi A, Yamato M, Kanazawa H, Okano T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials. 2008;29(13):2073–2081. doi: 10.1016/j.biomaterials.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda N, Shimizu T, Yamato M, Okano T. Tissue engineering based on cell sheet technology. Adv Mater. 2007;19(20):3089–3099. [Google Scholar]
- 26.da Silva RMP, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol. 2007;25(12):577–583. doi: 10.1016/j.tibtech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Ista Linnea K, Mendez S, Balamurugan Sreelatha S, Balamurugan S, Rama Rao Venkata G, Lopez Gabriel P. Smart Coatings II. Vol. 1002. American Chemical Society; Washington, DC: 2009. Smart Surfaces for the Control of Bacterial Attachment and Biofilm Accumulation; pp. 95–110. [Google Scholar]
- 28.Ista LK, Perez-Luna VH, Lopez GP. Surface-grafted, environmentally sensitive polymers for biofilm release. Appl Environ Microbiol. 1999;65(4):1603–9. doi: 10.1128/aem.65.4.1603-1609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber DL, Manginell RP, Samara MA, Kim B-I, Bunker BC. Programmed adsorption and release of proteins in a microfluidic device. Science. 2003;301(5631):352–354. doi: 10.1126/science.1080759. [DOI] [PubMed] [Google Scholar]
- 30.McCarley RL, Soper SA, Murphy MC, Wei S, Smith AF, Vaidya B, Feng J. Patterning of surface-capture architectures in polymer-based microanalytical devices. Spec Publ - R Soc Chem. 2004;297:130–132. doi: 10.1021/ja0454135. (Micro Total Analysis Systems 2004; Volume 2) [DOI] [PubMed] [Google Scholar]
- 31.Boyes SG, Granville AM, Baum M, Akgun B, Mirous BK, Brittain WJ. Polymer brushes - surface immobilized polymers. Surf Sci. 2004;570(1–2):1–12. [Google Scholar]
- 32.Bi H, Meng S, Li Y, Guo K, Chen Y, Kong J, Yang P, Zhong W, Liu B. Deposition of PEG onto PMMA microchannel surface to minimize nonspecific adsorption. Lab Chip. 2006;6(6):769–775. doi: 10.1039/b600326e. [DOI] [PubMed] [Google Scholar]
- 33.Bi H, Zhong W, Meng S, Kong J, Yang P, Liu B. Construction of a biomimetic surface on microfluidic chips for biofouling resistance. Anal Chem. 2006;78(10):3399–3405. doi: 10.1021/ac0522963. [DOI] [PubMed] [Google Scholar]
- 34.Wen X, He H, Lee LJ. Specific antibody immobilization with biotin-poly(-lysine)-g-poly(ethylene glycol) and protein A on microfluidic chips. J Immunol Methods. 2009;350(1–2):97–105. doi: 10.1016/j.jim.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Barbey R, Lavanant L, Paripovic D, SchuÌwer N, Sugnaux C, Tugulu S, Klok H-A. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev. 2009;109(11):5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 36.Percec V, Barboiu B. “Living” radical polymerization of styrene initiated by arenesulfonyl chlorides and CuI(bpy)nCl. Macromolecules. 1995;28:7970–2. [Google Scholar]
- 37.Wang J-S, Matyjaszewski K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc. 1995;117(20):5614–15. [Google Scholar]
- 38.Kato M, Kamigaito M, Sawamoto M, Higashimura T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris(triphenylphosphine)ruthenium(II)/methylaluminum bis-(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules. 1995;28(5):1721–3. [Google Scholar]
- 39.Pyun J, Kowalewski T, Matyjaszewski K. Synthesis of polymer brushes using atom transfer radical polymerization. Macromol Rapid Commun. 2003;24(18):1043–1059. [Google Scholar]
- 40.Edmondson S, Osborne Vicky L, Huck Wilhelm TS. Polymer brushes via surface-initiated polymerizations. Chem Soc Rev. 2004;33(1):14–22. doi: 10.1039/b210143m. [DOI] [PubMed] [Google Scholar]
- 41.Bontempo D, Tirelli N, Feldman K, Masci G, Crescenzi V, Hubbell JA. Atom transfer radical polymerization as a tool for surface functionalization. Adv Mater. 2002;14(17):1239–1241. [Google Scholar]
- 42.Liu P. Modification of polymeric materials via surface-initiated controlled/“living” radical polymerization. e-Polym. 2007:062. [Google Scholar]
- 43.Yameen B, Alvarez M, Azzaroni O, Jonas U, Knoll W. Tailoring of poly(ether ether ketone) surface properties via surface-initiated atom transfer radical polymerization. Langmuir. 2009;25(11):6214–6220. doi: 10.1021/la900010z. [DOI] [PubMed] [Google Scholar]
- 44.Fujie T, Park JY, Murata A, Estillore NC, Tria MCR, Takeoka S, Advincula RC. Hydrodynamic transformation of a freestanding polymer nanosheet induced by a thermoresponsive surface. ACS Appl Mater Interfaces. 2009;1(7):1404–1413. doi: 10.1021/am900111r. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X, Chen H-Y, Galvan G, Yoshida M, Lahann J. Vapor-based initiator coatings for atom transfer radical polymerization. Adv Funct Mater. 2008;18(1):27–35. [Google Scholar]
- 46.Farhan T, Huck WTS. Synthesis of patterned polymer brushes from flexible polymeric films. Eur Polym J. 2004;40(8):1599–1604. [Google Scholar]
- 47.Mauricio MR, Carvalho GM, Radovanovic E, Muniz EC, Rubira AF. Analysis of poly(N-isopropylacrylamide) grafted onto the surface of PET films by SI-ATRP technique. Mater Sci Eng, C. 2009;29(2):594–598. [Google Scholar]
- 48.Jain P, Dai J, Grajales S, Saha S, Baker GL, Bruening ML. Completely aqueous procedure for the growth of polymer brushes on polymeric substrates. Langmuir. 2007;23(23):11360–11365. doi: 10.1021/la701735q. [DOI] [PubMed] [Google Scholar]
- 49.Patrucco E, Ouasti S, Vo CD, De Leonardis P, Pollicino A, Armes SP, Scandola M, Tirelli N. Surface-initiated ATRP modification of tissue culture substrates: Poly(glycerol monomethacrylate) as an antifouling surface. Biomacromolecules. 2009;10(11):3130–3140. doi: 10.1021/bm900856r. [DOI] [PubMed] [Google Scholar]
- 50.Henry AC, Tutt TJ, Galloway M, Davidson YY, McWhorter CS, Soper SA, McCarley RL. Surface modification of poly(methyl methacrylate) used in the fabrication of microanalytical devices. Anal Chem. 2000;72(21):5331–5337. doi: 10.1021/ac000685l. [DOI] [PubMed] [Google Scholar]
- 51.Tsarevsky NV, Matyjaszewski K. “Green” atom transfer radical polymerization: from process design to preparation of well-defined environmentally friendly polymeric materials. Chem Rev. 2007;107(6):2270–2299. doi: 10.1021/cr050947p. [DOI] [PubMed] [Google Scholar]
- 52.Poliakoff M, Licence P. Green chemistry. Nature. 2007;450(7171):810–812. doi: 10.1038/450810a. [DOI] [PubMed] [Google Scholar]
- 53.Ciampolini M, Nardi N. Five-coordinated high-spin complexes of bivalent cobalt, nickel, and copper with tris(2-dimethylaminoethyl)-amine. Inorg Chem. 1966;5(1):41–4. [Google Scholar]
- 54.Queffelec J, Gaynor SG, Matyjaszewski K. Optimization of atom transfer radical polymerization using Cu(I)/tris(2-(dimethylamino)ethyl)amine as a catalyst. Macromolecules. 2000;33(23):8629–8639. [Google Scholar]
- 55.Wei S, Vaidya B, Patel AB, Soper SA, McCarley RL. Photochemically patterned poly(methyl methacrylate) surfaces used in the fabrication of microanalytical devices. J Phys Chem B. 2005;109(35):16988–16996. doi: 10.1021/jp051550s. [DOI] [PubMed] [Google Scholar]
- 56.Zhang A, Barner J, Goessl I, Rabe JP, Schuleter AD. A covalent-chemistry approach to giant macromolecules and their wetting behavior on solid substrates. Angew Chem, Int Ed. 2004;43(39):5185–5188. doi: 10.1002/anie.200460390. [DOI] [PubMed] [Google Scholar]
- 57.Lecolley F, Tao L, Mantovani G, Durkin I, Lautru S, Haddleton DM. A new approach to bioconjugates for proteins and peptides (“pegylation”) utilising living radical polymerization. Chem Commun. 2004;18:2026–2027. doi: 10.1039/b407712a. [DOI] [PubMed] [Google Scholar]
- 58.Hermanson GT. Bioconjugate Techniques. Elsevier Academic Press; San Diego: 2008. [Google Scholar]
- 59.Millard P-EM, Nathalie C, Böker A, Müller AHE. Controlled/Living Radical Polymerization: Progress in ATRP. Vol. 1023. American Chemical Society; Washington, DC: 2009. Controlling the Fast ATRP of N-Isopropylacrylamide in Water; pp. 127–137. [Google Scholar]
- 60.Idota N, Kikuchi A, Kobayashi J, Akiyama Y, Sakai K, Okano T. Thermal modulated interaction of aqueous steroids using polymer-grafted capillaries. Langmuir. 2006;22(1):425–430. doi: 10.1021/la051968h. [DOI] [PubMed] [Google Scholar]
- 61.Kim DJ, Kong B, Jung YH, Kim KS, Kim W-J, Lee K-B, Kang SM, Jeon S, Choi IS. Formation of thermoresponsive surfaces by surface-initiated, aqueous atom-transfer radical polymerization of N-isopropylacrylamide: Application to cell culture. Bull Korean Chem Soc. 2004;25(11):1629–1630. [Google Scholar]
- 62.Pan YV, Wesley RA, Luginbuhl R, Denton DD, Ratner BD. Plasma polymerized N-isopropylacrylamide: synthesis and characterization of a smart thermally responsive coating. Biomacromolecules. 2001;2(1):32–36. doi: 10.1021/bm0000642. [DOI] [PubMed] [Google Scholar]
- 63.McAuley B, Cabaniss SE. Quantitative detection of aqueous arsenic and other oxoanions using attenuated total reflectance infrared spectroscopy utilizing iron oxide coated internal reflection elements to enhance the limits of detection. Anal Chim Acta. 2007;581(2):309–317. doi: 10.1016/j.aca.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 64.Briggs D. Analysis of polymer surfaces by SIMS. Part 14. aliphatic hydrocarbons revisited. Surf Interface Anal. 1990;15(12):734–8. [Google Scholar]
- 65.Curti PS, de Moura MR, Radovanovic E, Rubira AF, Muniz EC, Moliterno RA. Surface modification of polystyrene and poly(ethylene terephthalate) by grafting poly(N-isopropylacrylamide) J Mater Sci: Mater Med. 2002;13(12):1175–1180. doi: 10.1023/a:1021154424189. [DOI] [PubMed] [Google Scholar]
- 66.Bucio E, Burillo G, Adem E, Coqueret X. Temperature sensitive behavior of poly(N-isopropylacrylamide) grafted onto electron beam-irradiated poly(propylene) Macromol Mater Eng. 2005;290(8):745–752. [Google Scholar]
- 67.Wischerhoff E, Badi N, Laschewsky A, Lutz J-F. Smart Polymer Surfaces: Concepts and Applications in Biosciences, Advances in Polymer Science. Springer; Berlin: 2010. pp. 1–33. [Google Scholar]
- 68.Plunkett KN, Zhu X, Moore JS, Leckband DE. PNIPAM chain collapse depends on the molecular weight and grafting density. Langmuir. 2006;22(9):4259–4266. doi: 10.1021/la0531502. [DOI] [PubMed] [Google Scholar]
- 69.Kong B, Choi JS, Jeon S, Choi IS. The control of cell adhesion and detachment on thin films of thermoresponsive poly[(N-isopropylacrylamide)-r-((3-(methacryloylamino)propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide)] Biomaterials. 2009;30(29):5514–5522. doi: 10.1016/j.biomaterials.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 70.Cho EC, Kim YD, Cho K. Thermally responsive poly(N-isopropylacrylamide) monolayer on gold: synthesis, surface characterization, and protein interaction/adsorption studies. Polymer. 2004;45(10):3195–3204. [Google Scholar]
- 71.Huang J, Wang Y, Laradji M. Flow control by smart nanofluidic channels: a dissipative particle dynamics simulation. Macromolecules. 2006;39(16):5546–5554. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.