Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that enhances glucose-stimulated insulin secretion and exerts direct and indirect actions on the cardiovascular system. GLP-1 and its related incretin hormone, glucose-dependent insulinotropic polypeptide (GIP), are rapidly inactivated by the enzyme dipeptidyl peptidase 4 (DPP-4), a key determinant of incretin bioactivity. Two classes of medications that enhance incretin action, GLP-1R agonists and DPP-4 inhibitors, are used for the treatment of type 2 diabetes mellitus (T2DM). We review herein the cardiovascular biology of GLP-1R agonists and DPP-4 inhibitors, including direct and indirect effects on cardiomyocytes, blood vessels, adipocytes, the control of blood pressure and postprandial lipoprotein secretion. Both GLP-1R activation and DPP-4 inhibition exert multiple cardioprotective actions in preclinical models of cardiovascular dysfunction, and short term studies in human subjects appear to demonstrate modest yet beneficial actions on cardiac function in subjects with ischemic heart disease. Incretin-based agents control body weight, improve glycemic control with a low risk of hypoglycemia, decrease blood pressure, inhibit the secretion of intestinal chylomicrons, and reduce inflammation in preclinical studies. Nevertheless, there is limited information on the cardiovascular actions of these agents in patients with diabetes and established cardiovascular disease. Hence, a more complete understanding of the cardiovascular risk:benefit ratio of incretin-based therapies will require completion of long term cardiovascular outcome studies currently underway in patients with T2DM.
Key terms: diabetes, glucagon-like peptides, heart, atherosclerosis, GLP-1, dipeptidyl peptidase-4
I. INTRODUCTION
Glucagon-like peptide-1 (GLP-1) is a peptide hormone that stimulates insulin and inhibits glucagon secretion in a glucose-dependent manner. GLP-1 also inhibits gastric emptying and reduces appetite, actions which contribute to improved glycemic control (Fig. 1). Several GLP-1 receptor (GLP-1R) agonists, and inhibitors of dipeptidyl peptidase-4 (DPP-4), the enzyme responsible for GLP-1 inactivation, have been developed as therapeutic agents for the treatment of type 2 diabetes mellitus (T2DM). As cardiovascular complications represent the primary source of morbidity and mortality in diabetic subjects (1–3), we now review the potential mechanisms and current understanding of the cardiovascular consequences of augmenting GLP-1 action for the treatment of T2DM.
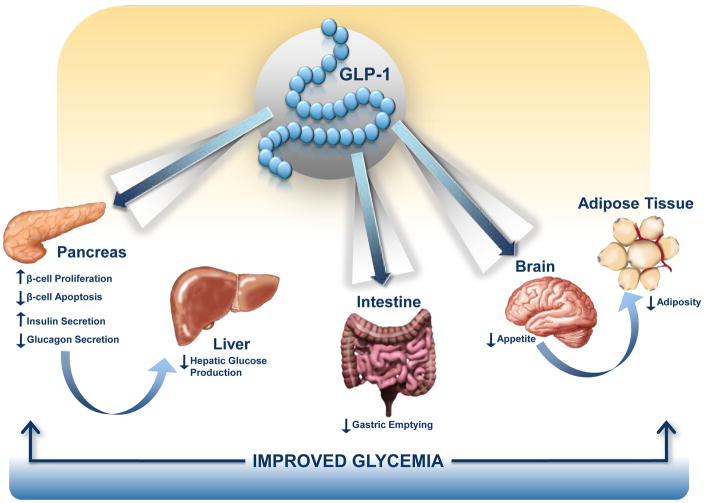
Figure 1. GLP-1 targets multiple organs to improve glucose control in T2DM.
GLP-1 acts directly and indirectly on several peripheral tissues that contribute to lowering of blood glucose levels. These include potent effects on the pancreatic β-cell to stimulate insulin secretion, inhibition of α-cell glucagon secretion that reduces hepatic glucose production, a decrease in gastric motility, and a reduction in appetite that contributes to weight loss, reduced levels of adipocytokines and decreased inflammation.
II. THE INCRETIN AXIS
Enteral glucose administration markedly potentiates insulin secretion to a much greater extent than the same level of plasma glucose achieved via parenteral glucose administration, a concept known as the incretin effect. The elucidation of the structure and function of glucose-dependent insulinotropic polypeptide (GIP) in the 1970s (4–6) established GIP as the first incretin hormone. More than a decade later, cloning of the proglucagon cDNAs enabled identification of the GLP-1 sequence and subsequent characterization of the insulinotropic biological activity of GLP-1 (7-10). Together, GIP and GLP-1 are widely viewed as the predominant gut-derived hormones that promote glucose-dependent insulin secretion following meal ingestion.
A. Glucagon-like peptide-1
Although initial studies of GLP-1 assessed the biological activity of GLP-1(1-37) and GLP-1(1-36)amide, the realization that the first 6 amino acids of GLP-1 could be cleaved to generate GLP-1(7-37) or GLP-1(7-36)amide accelerated the discovery of multiple metabolic actions of GLP-1 in vivo. GLP-1 is synthesized in and secreted from enteroendocrine L cells distributed throughout the small and large intestine, however the majority of intestinal GLP-1 content has been localized to the distal small bowel and colon. GLP-1 is also produced in the central nervous system (CNS), predominantly in the brainstem, and subsequently transported to a large number of regions within the CNS. GLP-1 is secreted from the gut at low basal levels even in the fasted state, and plasma levels of GLP-1 increase 2–3-fold following nutrient ingestion. The biology of GLP-1 synthesis and secretion and the multiple metabolic and extrapancreatic actions of GLP-1 and GIP have been extensively reviewed (11–15).
B. The GLP-1 Receptor
A single G protein coupled receptor (GPCR), with considerable amino acid homology to related class B family GPCRs (16), transduces the majority of GLP-1 actions in vivo, and represents the only specific high affinity GLP-1 receptor (GLP-1R) identified to date (17). The GLP-1R was originally identified in islet β-cells but is widely expressed in extrapancreatic tissues, including the lung, kidney, CNS, enteric and peripheral nervous system, lymphocytes, blood vessels, and heart (17, 18). Multiple actions of GLP-1 and structurally-related GLP-1R agonists have been reported in cells and tissues that do not express the classical GLP-1R, hence there continues to be analysis of mechanisms and pathways capable of transducing actions of GLP-1 independent of the known GLP-1R (19).
C. GLP-1-mediated regulation of insulin and glucagon secretion
GLP-1 directly potentiates insulin secretion in a glucose-dependent manner, minimizing the risk of hypoglycemia in diabetic subjects treated with GLP-1R agonists. GLP-1 also induces glucose competence in previously unresponsive β-cells (20), and rapidly improves β-cell glucose sensitivity thereby restoring insulin secretion towards normal in human patients with T2DM (21). GLP-1 also inhibits glucagon secretion in a glucose-dependent manner (22). Since the majority of α-cells do not express the GLP-1R, and GLP-1 inhibits glucagon secretion even in C-peptide negative subjects with type 1 diabetes, the glucagonostatic effects of GLP-1 are likely indirect, mediated through somatostatin-dependent mechanisms (23).
D. Glucose-dependent insulinotropic polypeptide
GIP is a 42 amino acid peptide synthesized in and secreted from enteroendocrine K cells localized to the proximal small bowel. Like GLP-1, GIP is secreted at low basal levels in the fasted state, and plasma levels of GIP increase within minutes of nutrient ingestion. Although the major action of GIP is the glucose-dependent stimulation of insulin secretion, GIP also promotes lipid uptake and expansion of adipocyte mass, and exerts a number of extrapancreatic actions in the brain, bone and adrenal gland, delineated predominantly in preclinical studies (24).
E. The GIP Receptor
The GIP receptor (GIPR) is a member of the class B family of GPCRs, and GIPR activation leads to cyclic AMP (cAMP) generation and insulin secretion from islet β-cells. The GIPR is widely expressed in extrapancreatic tissues, including the gastrointestinal tract, adipose tissue, heart, pituitary, adrenal cortex, and multiple regions of the central nervous system (25). Disruption or attenuation of GIP action is associated with diminished weight gain, resistance to diet-induced obesity, and improved insulin sensitivity in preclinical studies (24, 26), whereas genetic variation within the human Gipr gene is linked to control of postprandial glucose and body weight (27, 28).
F. Dipeptidyl peptidase-4 and incretin degradation
DPP-4, originally described as a lymphocyte cell surface protein, cluster of differentiation 26 (CD26), is a membrane-spanning exopeptidase that cleaves dipeptides from the N-terminus of proteins or peptides, immediately following a position 2 proline or alanine, although DPP-4 can also cleave peptides containing other position 2 residues (29, 30). DPP-4 exists in two molecular forms which both exhibit proteolytic activity; a membrane spanning protein with a short intracellular tail, and a circulating protein devoid of the membrane-spanning and intracellular regions. The biology of DPP-4 is highly complex, as both the membrane-spanning protein and the soluble circulating form exert multiple biological activities independent of the catalytic activity of the enzyme (31). The observation that DPP-4 cleaves both GLP-1 and GIP at the N-terminus (32–34), followed by demonstrations that chemical inhibition or genetic inactivation of DPP-4 increases the circulating levels of intact GLP-1 and GIP (35, 36), firmly established DPP-4 as a major regulator of incretin degradation.
III. ROLE OF THE GLP-1R IN THE CARDIOVASCULAR SYSTEM
Elucidation of GLP-1 actions in the cardiovascular system has important implications for the treatment of subjects with T2DM. Studies in animals and humans have explored the biological actions of GLP-1 on the myocardium, the vasculature, as well as on cardiovascular risk factors that will be discussed in the following section.
A. GLP-1R expression and signal transduction in the myocardium
Glp1r mRNA transcripts have been detected by reverse transcription polymerase chain reaction (RT-PCR) in the rat and mouse heart (18, 37), and in the human heart using RNAse protection assays (38). Immunohistochemistry detected GLP-1R protein in mouse cardiomyocytes and endocardium, whereas Western blotting revealed GLP-1R protein expression in all chambers of the mouse heart (37). Furthermore, immunoreactive GLP-1R protein has been detected in sarcolemmal membranes from canine myocardium by Western blotting (39).
1) GLP-1R signaling in primary cultured cardiomyocytes and cardiomyocyte cell lines
Studies in cardiomyocytes have delineated signaling pathways transduced downstream of the cardiac GLP-1R (Fig. 2). Direct treatment of adult rat cardiomyocytes with native GLP-1 (10 nM) for 20 minutes increased cAMP levels, consistent with actions of GLP-1 in pancreatic β cells (40). Surprisingly, the GLP-1-induced increase in intracellular cAMP was not coupled to an increase in intracellular Ca2+ and subsequent cardiomyocyte contractility as would be expected for a cAMP-generating agent in the heart (40). Whole-cell patch clamping of adult canine ventricular myocytes demonstrated a protein kinase A (PKA)-dependent increase in L-type Ca2+ channel current and action potential duration in response to 5 nM GLP-1 that peaked 15 min following treatment (41). In cultured neonatal mouse cardiomyocytes, treatment with exendin-4 (3 nM) for 20 min increased Akt and extracellular-regulated-kinase (ERK) phosphorylation (42), both well-characterized regulators of cardiomyocyte growth and glucose metabolism (43, 44).
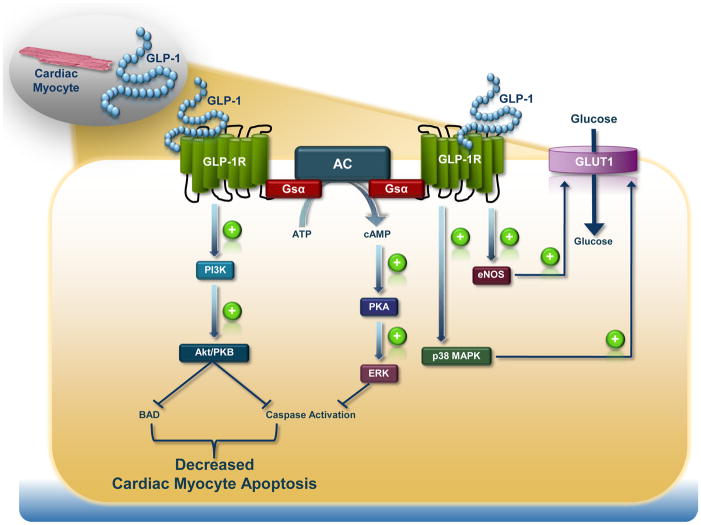
Figure 2. GLP-1R–Dependent Intracellular Signal Transduction Pathways in the Cardiomyocyte.
The signalling pathways engaged downstream of the cardiomyocyte GLP-1R lead to a reduction in apoptosis and increase in glucose uptake independent of the classical insulin-dependent pathway.
GLP-1R activation in primary cultures of neonatal mouse cardiomyocytes is anti-apoptotic, as pretreatment of cells with the GLP-1R agonist liraglutide for 1 hr (10–1000 nM) reduced caspase-3 cleavage induced by tumor necrosis factor α (TNFα, 100 ng/mL for 24 hrs) (45). Furthermore, native GLP-1 (10 nM) reduced apoptosis in neonatal rat cardiomyocytes following 16 hrs of hypoxia (92% N2/5% CO2/3% O2)/4 hr reoxygenation (95% CO2/5% O2) (46). These effects were prevented by coincubation with the phosphatidylinositol-3 kinase (PI3K) inhibitor, LY294002, or the ERK inhibitor, UO126.
GLP-1 (200 nM) also directly activated PI3K/Akt and ERK in HL-1 cardiomyocytes, reducing the extent of apoptosis induced by staurosporine, palmitic acid, or ceramide; these cytoprotective effects were abolished by co-incubation with UO126 or the PI3K inhibitor, wortmannin (47). However, whether the GLP-1R is expressed in HL-1 cells remains uncertain.
2) GLP-1R activation in isolated non-ischemic hearts and in conscious animals
The paradoxical observation that GLP-1 decreased contractility in primary cultured adult rat cardiomyocytes, despite increasing cAMP levels (40) has also been observed in isolated rat hearts, where addition of GLP-1 (0.5 nM) to the perfusate reduced left ventricular developed pressure (LVDP) (48). In contrast, 0.3 nM GLP-1 increased LVDP by ~20% at 20 min post-treatment during Langendorff aerobic perfusion of isolated mouse hearts (37). GLP-1 increased myocardial glucose uptake during aerobic perfusion (37, 48), independent of insulin-stimulated Akt phosphorylation and glucose transporter 4 (GLUT4) translocation, in association with increased p38 mitogen activated protein kinase (MAPK) activity, enhanced nitric oxide (NO) production, and increased GLUT1 protein levels at the sarcolemmal membrane (Fig. 2) (48).
Studies in conscious dogs demonstrate that a 48 hour continuous infusion of recombinant GLP-1 (1.5 pmol/kg/min) increased myocardial glucose uptake during a hyperinsulinemic-euglycemic clamp (49). This effect was inhibited by pretreatment with either SB 203580 (1 mg/kg i.v.) or nitro-L-arginine (30 mg/kg i.v.), suggesting essential roles for p38 MAPK and endothelial nitric oxide synthase (eNOS) as downstream targets of GLP-1 action (39).
3) Cardiovascular phenotype of Glp1r−/− mice
In vivo hemodynamic measurements with Millar catheters in hearts from anaesthetized whole body Glp1r−/− mice demonstrate normal basal LV function, but reduced contractile function in response to intraperitoneal (i.p.) insulin (3 U/kg) or i.v. adrenaline administration (1 μg/kg) (50). Histological analysis of the 5 month old Glp1r−/− mouse heart on a CD1 genetic background revealed thicker ventricular walls, suggesting that endogenous GLP-1R activity may influence structural development of the heart. Interestingly, hearts from Glp1r−/− mice exhibited increased baseline LVDP (~25% increase) throughout the course of a 40 min Langendorff aerobic perfusion (37), findings consistent with prior observations of GLP-1 reducing contractility and LVDP in adult rat cardiomyocytes (40) and Langendorff aerobically perfused isolated rat hearts (48), respectively.
B. GLP-1R in the vasculature
An immunoreactive GLP-1R protein has been detected in human coronary artery endothelial cells (HCAECs) and human umbilical vein endothelial cells (HUVECs) (51, 52). GLP-1R protein expression has also been detected in mouse coronary endothelial and smooth muscle cells via immunohistochemistry (37).
1) GLP-1R activation in endothelial cells
A 5 hr incubation with the GLP-1R agonist liraglutide (0.1 – 100 μg/mL) increased eNOS phosphorylation and NO production via a 5′AMP-activated protein kinase (AMPK)-dependent pathway in HUVEC cultures (53). Similarly, a 48 hr incubation with either GLP-1 (100 nM) or exendin-4 (10 nM) increased Akt and eNOS phosphorylation, and subsequent NO production in HCAECs (54). GLP-1R activation also increased proliferation of HCAECs as determined via [3H]thymidine incorporation into DNA; stimulation of cell proliferation was abrogated by co-incubation with either Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) or the Akt inhibitor IV (54). In contrast, a 1 hr treatment of HUVECs with 10 nM GLP-1 had no effect on Akt phosphorylation, but increased PKA activity and cAMP response element binding protein (CREB) phosphorylation (55). Hence, the endothelial cell is a direct target for GLP-1 action (Fig. 3).
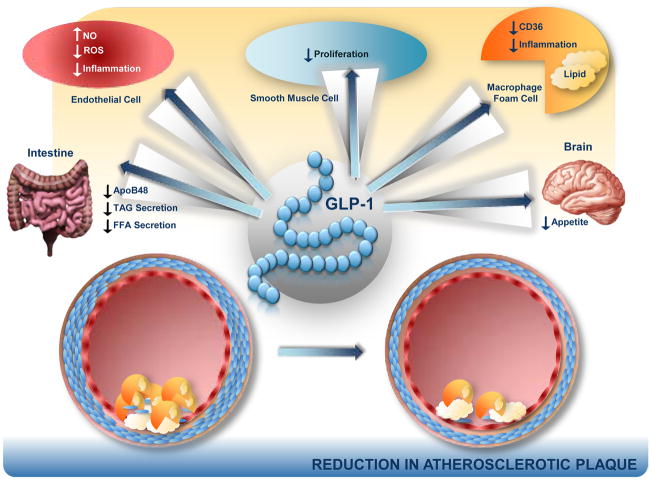
Figure 3. Anti-Atherosclerotic potential of GLP-1 action.
The direct actions of GLP-1 on blood vessels, macrophages, and on the regulation of plasma lipid profiles may impact the development and/or progression of atherosclerotic plaques.
Liraglutide (100 nM) prevented the increase in plasminogen activator inhibitor type-1 and vascular cell adhesion molecule-1 (VCAM-1) mRNA and protein expression in response to TNFα (10 ng/mL for 5 or 16 hrs) or hyperglycemia (10 mM for 48 hrs) in the C11 STH human umbilical vein endothelial cell line (56). GLP-1 (0.03 and 0.3 nM for 4 hrs) reduced reactive oxygen species and VCAM-1 mRNA expression in HUVECs following exposure to advanced glycation end products (100 μg/mL glycated bovine serum albumin) (52). Utilizing an in vitro model of vascular aging, treatment of HUVECs for 30 min with GLP-1 or exendin-4 10 min prior to a 1 hr incubation with 30 μM H2O2 reduced the number of senescent cells, in an exendin(9-39)-sensitive manner (55). These effects were also dependent on PKA activity as they were abolished by pretreatment with 1 μM H89. Hence, GLP-1R activation exerts cytoprotective actions in endothelial cells.
2) GLP-1 and endothelial function in humans
Studies in non-diabetic normotensive volunteers demonstrated that GLP-1 (1.2 pmol/kg/min) infusion enhanced acetylcholine (2–8 μg/100mL)-induced forearm blood flow measured via venous occlusion plethysmography, changes abolished by co-administration of the sulfonylurea glyburide but not glimepiride. In contrast, GLP-1 had no effect on blood flow induced via sodium nitroprusside (0.5 – 2 μg/100mL) (57). Similarly, fasted subjects with T2DM and stable coronary artery disease (n=12) exhibited improved endothelial function in response to GLP-1 infusion (2 pmol/kg/min), as indicated by an increase in flow-mediated vasodilation of the brachial artery, independent of changes in systolic and diastolic blood pressure (SBP and DBP) during a hyperinsulinemic clamp (51). A study of 16 subjects with T2DM and 12 non-diabetic controls demonstrated that infusion of 0.4 pmol/kg/min GLP-1 (plasma concentration of ~180 pg/mL) during a 2 hr hyperglycemic clamp improved flow-mediated vasodilation in both groups. However, the GLP-1-mediated improvements in blood flow were considerably attenuated after a 2 month period of improved glycemic control (58). Whether the effects of native GLP-1 on blood flow are mimicked by degradation resistant GLP-1R agonists remains unclear; GLP-1 but not exenatide produced vasodilation and increased cGMP release from isolated preconstricted blood vessels ex vivo (37) and exenatide had no effect on endothelial function in rat conduit arteries ex vivo after infusion with intralipid in vivo, whereas native GLP-1 and GLP-1(9-36) both exerted vasodilatory actions in control experiments (59).
3) GLP-1-mediated control of heart rate and blood pressure in animals
GLP-1R-dependent control of heart rate (HR) and BP is complex, and species-specific. Synthetic human GLP-1 (0.1–1000 ng) administered into the jugular vein of male rats acutely increased SBP and DBP, and HR; BP and HR returned to basal levels by 25 min post-GLP-1 administration (60). These effects were independent of catecholamines and adrenergic receptors, as pretreatment with either propranolol (1 mg/kg i.v.) or phentolamine (0.1 mg/kg i.v.) did not prevent the increases in HR and BP (60). GLP-1 also increased HR and BP in streptozotocin-induced diabetic rats, though plasma insulin levels were not measured (60). The increased BP and HR observed after administration of GLP-1 or exendin-4 in rats was dependent on GLP-1R signaling, and abolished by i.v. infusion of the antagonist exendin(9-39) (61). The GLP-1-stimulated increase in HR and BP in rodents involves dual pathways originating from both the CNS and periphery, with the neural pathway requiring intact vagus nerve transmission, as intracerebroventricular (i.c.v.) GLP-1 (100 ng) failed to increase HR and BP in vagotomized rats (62). Support for a role of the CNS in the GLP-1R-dependent control of HR and BP derives from telemetry studies of freely moving rats, whereby GLP-1R agonist–mediated increases in HR and BP were coupled to activation of autonomic control centers in the CNS (63). Administration of i.c.v. exendin-4 induced Fos-like immunoreactivity in neurons innervating sympathetic preganglionic neurons in the paraventricular hypothalamus, the arcuate nucleus, and the lateral hypothalamic area in the rat brain (63). Furthermore, double-labeling immunohistochemistry following i.c.v. exendin-4 detected induction of Fos-like and tyrosine hydroxylase (TH) immunoreactivity in catecholaminergic neurons in the nucleus of the solitary tract and locus coeruleus (63); i.c.v. exendin-4 also activated tyrosine hydroxylase (TH) transcription in adrenal medullary catecholamine neurons (63). Continuous infusion of the selective β2-adrenoceptor antagonist, ICI 118551 or the non-selective β-adrenoceptor antagonist, propranolol abolished the effects of exendin-4 (250 ng/kg i.v.) on HR in rats (64). Furthermore, exendin-4 (250 ng/kg i.v.) was unable to increase HR in adrenalectomized rats (25). Taken together, considerable data suggests that GLP-1 engages the rodent sympathetic nervous system to modify HR and BP (63–65). Nevertheless, other investigators have reported that the vasoconstrictor properties of exendin-4 were independent of the autonomic nervous system, as the increase in mean arterial pressure following exendin-4 administration persisted despite continuous infusion with propranolol or the α-adrenoceptor antagonist, phentolamine (24). Furthermore, i.v. GLP-1 (20 nmol) acutely increased HR over a 1 hr period in adult male rats that had previously undergone either adrenalectomy or vagotomy (66).
GLP-1R activation in neurons that innervate cardiac vagal neurons in the nucleus ambiguous, resulted in diminished heart rate variability and reduced parasympathetic modulation of the heart (67). GLP-1 may also increase BP in rodents through the vasopressin system, as an intra-arterially injected vasopressin receptor antagonist, B-mercapto (10 μg/kg), abolished the rise in BP following 100 ng i.c.v. GLP-1 (68). In contrast to findings of increased BP in mice and rats following acute GLP-1R activation, GLP-1 (30–60 μmol/kg body weight/min) transiently increased HR yet had no effect on aortic BP in conscious calves (69). Hence, the mechanisms underlying the acute effects of GLP-1R agonists to increase HR and BP are complex, may involve both the sympathetic and parasympathetic nervous system, and appear species-specific.
4) GLP-1-mediated control of heart rate and blood pressure in humans
Studies of GLP-1 effects on BP in humans have yielded results quite different from those obtained acutely in rodents. Infusion of GLP-1 (2.4 pmol/kg/min for 10 min followed by 1.2 pmol/kg/min) for 65 min in 55 healthy human subjects (13 men and 42 women, mean age 31) had no effect on SBP or DBP, plasma norepinephrine levels, or HR variability calculated at both low and high frequency, indices of cardiac sympathetic and parasympathetic activity, respectively (70). In contrast, acute subcutaneous injection of GLP-1 (80 nmol/mL) into the anterior abdominal wall transiently increased HR (64 ± 2 vs. 54 ± 2 b.p.m. at 40 min post-injection) and BP (83 ± 5 vs. 77 ± 4 mmHg at 20 min post injection) in 10 healthy human subjects, however values returned to near baseline by ~50 – 60 min post-injection (71).
C. GLP-1 action and dyslipidemia
1) Studies of GLP-1 action on lipoprotein synthesis and secretion
Rats infused with GLP-1 (20 pmol/kg/min) via the jugular vein for 6 hrs exhibited a reduction in triacylglycerol (TAG) absorption, decreased intestinal lymph flow and reduced intestinal apolipoprotein B-48 (ApoB-48) production. (72). Furthermore, in studies of fructose-fed hamsters, the DPP-4 inhibitor sitagliptin (5 mg/kg via oral gavage for 2 or 3 weeks) decreased fasting plasma TAG, predominantly in the very low-density lipoprotein (VLDL) fraction, whereas both exendin-4 (24 nmol/kg i.p.) and sitagliptin (10 mg/kg via oral gavage) acutely decreased postprandial TAG and ApoB-48 levels in male C57BL/6J mice administered olive oil and Triton WR1339 following a 5 hr fast (73). Exendin-4 also reduced TAG and apoB48 secretion when administered 1hr after the oral fat load, suggesting the effect on post-prandial lipid metabolism was not related to delayed gastric empting (85). Moreover, exendin-4 (0.1 nM) directly decreased ApoB-48 secretion in primary cultured hamster enterocytes, as assessed by reduced levels of 35S-labelled ApoB-48 in the media over a 90 min time course. Conversely, Glp1r−/− mice exhibited enhanced appearance of plasma TAG following olive oil administration. These findings support a direct essential role for GLP-1 in the control of chylomicron secretion (Fig. 3) independent of changes in gastric emptying (73).
2) GLP-1 action, dyslipidemia and the liver
Chronic administration of the DPP-4 inhibitor vildagliptin (1 mM in drinking water) for 8 wks reduced fasting plasma TAG and cholesterol levels in WT mice fed a high fat diet for 14 wks (45% kcal from lard), but failed to lower plasma lipid levels in mice lacking both the Glp1r and Gipr (74). Vildagliptin also reduced hepatic expression of long chain acyl CoA synthetase mRNA in WT but not in Glp1r−/−:Gipr−/− mice, suggesting that DPP-4 inhibition may reduce plasma TAG levels via prevention of VLDL assembly (74). Ob/ob mice treated for 60 days with exendin-4 (10 μg/kg every 24 hrs for the first 14 days, followed by either 10 or 20 μg/kg every 12 hrs for the remaining 46 days) exhibited a marked reduction in hepatic lipid content as assessed by oil red O staining, associated with reduced steroyl CoA desaturase and sterol response element binding protein-1c mRNA expression, and increased peroxisome proliferator activated receptor α (PPARα) mRNA expression (75). Furthermore, daily liraglutide (200 μg/kg i.p.) treatment for 4 wks in mice fed a high fat and high-fructose corn syrup diet for 8 wks reversed hepatic steatosis assessed by oil red O staining (76). This effect was associated with increased mRNA expression of acyl CoA oxidase and increased protein expression of long chain acyl CoA dehydrogenase, suggesting an elevation in peroxisomal and mitochondrial fatty acid β-oxidation, respectively (76). In primary cultured human hepatocytes, 24 hr treatment with 10 nM exendin-4 in the presence of either 0.8 mM oleate, elaidate, or palmitate reduced lipid stores assessed by oil red O staining, as well as caspase-3 cleavage and DNA condensation, resulting in improved cell survival (77). Evidence for reduced ER stress and increased macroautophagy was also observed, as exendin-4 increased protein expression of glucose regulated protein 78, reduced expression of C/EBP homologous protein, and increased autophagic vacuole formation.
Daily treatment of high fat diet-fed mice (6 wks, 44% kcal from fat) for 4 wks with the GLP-1R agonist CNT0736 (0.1 or 1 mg/kg i.p.), reduced fasting VLDL production in a body weight-independent manner, however exendin-4 administration (7.1 μg/kg i.p.) did not affect these parameters (78). Nevertheless, whether GLP-1 acts directly on the liver to control lipoprotein synthesis and secretion remains unclear as treatment of primary murine hepatocytes for 16 hrs with 40 nM exendin-4 had no effect on TAG or cholesterol synthesis/secretion, and Glp1r mRNA transcripts corresponding to the entire open reading frame of the GLP-1R were not detected in isolated hepatocytes (74). In contrast, a 142 bp Glp1r mRNA transcript and immunoreactive GLP-1R protein were detected in RNA from human liver biopsies and human hepatoma HepG2 cells via RT-PCR and Western blotting, respectively (79). Immunoreactive GLP-1R protein detected by Western blotting, was also identified in both isolated human and rat hepatocytes, and exenatide directly increased mRNA expression of PPARγ and PPARα in human hepatocytes (75, 79, 80). As data from multiple groups has yielded contrasting results on the presence of hepatic GLP-1R expression in rodent or human hepatocytes, the mechanism(s) through which GLP-1 acts on the liver requires further elucidation (18, 74, 81, 82).
3) GLP-1 action in adipose tissue
Whether the classical GLP-1R is expressed in the adipocyte is uncertain. GLP-1 binding sites were detected in solubilized membranes from both human and rat adipose tissue using radioligand binding assays (83, 84) whereas others have been unable to detect GLP-1R mRNA expression in adipose tissue from humans and rats (18, 38). Nevertheless, GLP-1 treatment of isolated rat adipocytes increased lipolysis (85) and exogenous GLP-1 exerted both lipolytic and lipogenic effects in human adipocytes (86). On the other hand, in situ microdialysis in 9 healthy volunteers failed to demonstrate a lipolytic action of GLP-1 (87). Hence, whether GLP-1 exerts direct actions in adipose tissue depots through the GLP-1R remains unclear.
4) GLP-1 and dyslipidemia in human subjects
GLP-1 infusion (1.2 pmol/kg/min) for 6.5 hrs in 14 healthy volunteers inhibited the postprandial increase in plasma TAG and free fatty acid (FFA) levels following a 250 kcal solid test meal (88). Short term studies in 12 subjects with T2DM demonstrated that addition of GLP-1 (subcutaneous injection of 25 nmol directly before meals) to insulin therapy for 5 days, followed by administration of GLP-1 alone for the last 2 days, decreased plasma concentrations of VLDL-TAG (1.30 ± 0.36 vs. 2.08 ± 1.11 mM), while increasing the size of LDL cholesterol particles (mean diameter of 22.9 vs. 22.3 nm) (89). A study in 50 subjects with T2DM infused for 4 hrs with GLP-1 (1.2 pmol/kg/min) following an overnight 10 hr fast revealed a reduction in plasma FFA levels (~0.25 mM decrease throughout the first 2 hrs) (90). A hyperglycemic clamp followed by a hyperinsulinemic-euglycemic clamp in 16 elderly patients with T2DM treated for 3 months with a continuous subcutaneous GLP-1 infusion (100 pmol/kg/hr) also demonstrated decreased plasma FFA levels during the hyperinsulinemic portion of the clamp, which was associated with a significant increase in plasma insulin levels (91). A double-blind crossover study in 35 patients with recent onset T2DM demonstrated that a single subcutaneous injection of exenatide (10 μg) markedly reduced postprandial levels of TAG and ApoB-48, as well as plasma remnant lipoprotein cholesterol, for up to 8 hrs in subjects fed a high-calorie (600 kcal/m2 of body surface area), fat-enriched (45%) breakfast meal following an overnight fast (92). These effects were already observed during the first 4 hrs following the breakfast meal, when no changes in plasma insulin were observed. Interpretation of studies demonstrating that acute GLP-1R activation lowers postprandial lipemia requires consideration of whether the findings are due in part to the acute effect of GLP-1R agonists to inhibit gastric emptying and gut motility, actions that are diminished with more sustained GLP-1R activation (93, 94).
DPP-4 inhibitors have also demonstrated favourable effects on postprandial dyslipidemia in T2DM. A 4 wk treatment with vildagliptin (50 mg twice daily) reduced postprandial elevations of plasma TAG, chylomicron-TAG, chylomicron-ApoB-48, and chylomicron-cholesterol for up to 8 hrs in response to a fat-rich test meal (1000 kcal, 72 g fat) following an overnight fast (95). Similarly, a double-blind cross over study in 36 subjects with T2DM treated with sitagliptin (100 mg/day taken during the morning meal) for 6 wks also demonstrated a reduction in postprandial elevations of plasma TAG, ApoB-48, and FFA levels for up to 8 hrs in response to a test meal (1003 kcal, 68.6 g fat) following a 12 hr overnight fast (96). As DPP-4 inhibitors reduce postprandial lipemia without inhibition of gastric emptying or weight loss, these findings are consistent with a role for local GLP-1 in the control of intestinal chylomicron secretion (73).
D. Direct versus indirect effects of GLP-1R agonism on cardiac function
One of the challenges in understanding the effect(s) of GLP-1 on the heart involves elucidation of the direct vs. indirect pleiotropic metabolic properties of GLP-1 (Fig. 1,2,4). The following section will briefly highlight some of the key indirect actions/effectors of GLP-1, and their potential impact on cardiac function.
Figure 4. Indirect Cardiac Actions of GLP-1.
In addition to direct actions on the cardiomyocyte, GLP-1 may also influence cardiac function indirectly through its actions on pancreatic islets to enhance glucose-dependent insulin secretion and inhibit glucagon secretion, thereby changing levels of islet hormones, glucose and fatty acids, all of which may also directly impact the heart.
1) GLP-1 and insulin
Activation of the β-cell GLP-1R during hyperglycemia usually increases plasma insulin levels, leading to increased myocardial glucose uptake, glycogen synthesis and decreased fatty acid oxidation (Fig. 4)(97). Furthermore, increased insulin receptor signaling in the heart leads to activation of the PI3K/Akt pathway, increased eNOS activity and inhibition of AMPK (Fig. 3) (98, 99). Insulin also inhibits lipolysis and reduces plasma FFA levels (100, 101) and this effect, coupled with the improvement in myocardial glucose metabolism, supports the rationale for evaluating glucose-insulin-potassium (GIK) infusions in subjects with acute myocardial infarction (AMI) (102). Hence whether the metabolic actions of GLP-1 on the heart in vivo reflect direct and/or indirect mechanisms requires careful consideration.
2) GLP-1, obesity, and adiponectin
GLP-1R activation in the hypothalamus reduces appetite and leads to weight loss (103–105). Obesity is a significant risk factor for the development of cardiovascular diseases, with every 1 kg/m2 increment in body mass index resulting in a 5% and 7% increase in the risk of heart failure in men and women, respectively (106). Thus, weight loss associated with GLP-1R agonists may contribute to potential cardioprotective effects. Furthermore, weight loss is associated with increased plasma adiponectin levels, and adiponectin protects against both AMI and cardiac hypertrophy (107, 108). Indeed, the reduction in body mass in 9-month-old spontaneously hypertensive, and heart failure-prone rats infused i.p. with 1.5 pmol/kg/min GLP-1 for 3 months is associated with improved left ventricular function, increased survival, and a ~50% increase in circulating adiponectin levels (109). Consistent with these findings, exendin-4 (2.5–5 nM) directly increased adiponectin mRNA expression in 3T3-L1 adipocytes, an effect blocked by the GLP-1R antagonist exendin (9-39) or the PKA inhibitor, H89 (110). Weight loss arising from therapy with GLP-1R agonists is also associated with reductions in dyslipidemia (111). Hence, attribution of direct versus indirect effects to the cardiovascular actions of GLP-1 may be difficult and requires careful analyses.
E. Cardiovascular Biology of GLP-1(9-36)
GLP-1(9-36) is the primary GLP-1 metabolite in vivo and circulating levels of GLP-1 (9-36) are greater than those of intact bioactive GLP-1 (34, 112). GLP-1(9-36) binds to the classical GLP-1R with low affinity and at pharmacologic doses acts as a weak competitive antagonist of the β-cell and gastrointestinal tract GLP-1R in vivo. However, i.v. administration of GLP-1(9-36) in combination with glucose has no effect on insulin secretion, glucose elimination, or insulin-independent glucose disposal in either wild-type or Glp1r−/− mice (113). In contrast, GLP-1(9-36) enhanced insulin-independent glucose clearance in anesthetized pigs (114). Similarly, infusion of GLP-1(9-36) into healthy fasted humans in conjunction with a test meal significantly reduced postprandial glycemia, independently of changes in insulin or glucagon secretion or gastric emptying (115). The magnitude of these effects were minor in comparison to those of native GLP-1. Moreover, a separate study in healthy human subjects found that GLP-1(9-36) infusion had no direct effect on glucose tolerance, insulin secretion or sensitivity, or GLP-1 action (116). Furthermore, simultaneous infusion of GLP-1(9-36) with GLP-1(7-36) did not alter the magnitude of GLP-1 (7-36)-stimulated insulin secretion (116). Intriguingly, infusion of GLP-1(9-36) had no effect on insulin sensitivity in lean subjects, but suppressed hepatic glucose production, in association with increased plasma insulin levels, in obese subjects (117). The complexity of GLP-1(9-36) action is underscored by studies demonstrating that a secondary cleavage product derived from GLP-1(9-36), GLP-1(28-36), localizes to mitochondria, and also reduces hepatic glucose production in hepatocytes (118). It is important to note that the actions of both GLP-1(9-36) and GLP-1(28-36) appear at pharmacological concentrations and are most prominent in stressed metabolic states such as insulin resistance (118, 119). Hence, further research is required to ascertain the biological relevance of GLP-1(9-36) and GLP-1(28-36) in the human endocrine and cardiovascular system.
1) GLP-1(9-36) action in cardiomyocytes
GLP-1(9-36) improved the viability of both Glp1r+/+ and Glp1r−/− mouse neonatal cardiomyocytes during reoxygenation following 48 hrs of hypoxia; preincubation with 0.3 nM GLP-1 (9-36) for 20 min before exposure to hydrogen peroxide for 7 hrs also improved cell viability (42). Unexpectedly, the actions of GLP-1(9-36) were blocked by pretreatment with the classical GLP-1R antagonist exendin (9-39). In contrast, exendin-4 (3 nM) had no effect on cell viability in hypoxic neonatal cardiac Glp1r−/− myocytes subjected to reoxygenation or in cardiomyocytes treated with hydrogen peroxide (42).
2) GLP-1(9-36) activity in the isolated heart and in conscious animals
A 48 hr continuous infusion of GLP-1(9-36) (1.5 pmol/kg/min) in conscious dogs with pacing-induced dilated cardiomyopathy significantly reduced left ventricular end diastolic pressure, and increased left ventricular contractility and myocardial glucose uptake during a hyperinsulinemic-euglycemic clamp (120). Consistent with the hypothesis that GLP-1(9-36) exerts its actions through a separate receptor, pretreatment with native GLP-1 (0.3 nM) enhanced the recovery of LVDP during reperfusion following 30 min of global ischemia in hearts from Glp1r−/− mice (37). Notably, prevention of the formation of GLP-1(9-36) with the DPP-4 inhibitor sitagliptin prevented the recovery of LVDP during reperfusion in hearts from Glp1r−/− mice. Interestingly, exendin-4 (5 nM) also modestly improved the recovery of LVDP during reperfusion following 30 min of global ischemia in Glp1r−/− mice (37), adding further support for a second receptor capable of recognizing GLP-1R agonists in the heart. Related studies demonstrated that 0.03–3 nM GLP-1(9-36) added to the perfusate for the first 15 min of a 120 min reperfusion period following a 45 min ischemic insult in isolated rat hearts resulted in a significant improvement in LVDP, though it did not reduce infarct size (121). However, follow up studies from this same group could not reproduce these findings, demonstrating that native GLP-1, but not GLP-1(9-36), was cardioprotective in isolated ischemic rat hearts ex vivo (122). Although pharmacological levels of GLP-1(9-36) are cardioprotective, results using DPP-4 inhibitors, which markedly lower levels of GLP-1(9-36), demonstrate significant cardioprotection following DPP-4 inhibition in both normal and diabetic rodent models and in short term human studies (123–125).
IV. GLP-1 AND CARDIOVASCULAR DISEASE
A. GLP-1 and hypertension
1) Animal studies
Although acute GLP-1R activation increases HR and BP in rodents, GLP-1 actions on endothelial cells, such as increased NO production (Fig. 3), would be predicted to be antihypertensive (54 ). Indeed, db/db mice chronically treated with exendin-4 (i.p. 20 nmol/kg twice daily) for 12 wks displayed a marked reduction in SBP; exendin-4 also attenuated the increase in SBP (~10 mmHg increase vs. ~25 mmHg increase) in db/db mice provided with 2% salt in their drinking water for 2 wks (126). In male C57BL/6J mice infused with angiotensin II (1 μg/kg/min for 2 wks), twice daily exendin-4 (20 nmol/kg i.p.) also reduced SBP (126). A 4 hr treatment with exendin-4 (10 nM) prevented acute angiotensin II-induced ERK phosphorylation in kidney proximal tubular cells, raising the possibility that GLP-1R signaling directly modifies the actions of exogenous angiotensin II (126). A 7-day subcutaneous infusion of exendin-4 (1 μg/kg/day) via osmotic pumps also reversed corticosterone-induced increases in SBP and DBP in rats independent of changes in body weight and caloric intake (127). Hence, although acute GLP-1R activation transiently increases BP in preclinical studies, sustained GLP-1R activation reduces BP in different animal models.
2) Studies in subjects with diabetes and/or heart disease
Infusion of GLP-1 (0.7 pmol/kg/min) for 48 hrs in 15 non-diabetic human subjects with heart failure (New York Heart Association [NYHA] class II/III) resulted in small increases in HR (67 ± 2 vs. 65 ± 2 beats/min (b.p.m)) and DBP (71 ± 2 vs. 68 ± 2 mmHg) (128). Nevertheless, the majority of clinical trials investigating the anti-diabetic actions of GLP-1R agonists have reported reductions in BP. Exenatide (synthetic exendin-4) administered twice daily to diabetic subjects for 12 weeks produced small (~ 2 b.p.m.) but non-significant changes in HR, and a trend toward a reduction in SBP, associated with a modest weight loss of 1.8 kg (129). In a 26 week head to head study of liraglutide vs. sitagliptin in subjects with T2DM, HR increased with liraglutide (3.94 b.p.m.) but not with sitagliptin, and reductions in SBP and DBP were actually greater with sitagliptin compared to liraglutide (130). A greater reduction in DBP with sitagliptin was also observed following 1 year of treatment in comparison to liraglutide (131) and BP reductions were also modestly greater with sitagliptin compared to exenatide once weekly in the Results from the Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly (DURATION-4) study (132). Nevertheless, acute administration of the high molecular weight exendin-transferrin fusion protein in subjects with T2DM produced significant increases in HR, and DBP (mean increase of 10 mm Hg) (133). Hence further analysis of the effects of structurally distinct GLP-1R agonists on HR and BP in diabetic subjects is warranted.
Results from the DURATION-1 trial demonstrated that patients treated with exenatide continuously for 52 weeks had a significant reduction in SBP (134). Approximately 50% of patients with a SBP ≥ 130 mm Hg at baseline achieved a normal SBP by week 52. Consistent with these findings, 314 overweight patients receiving exenatide 10 μg twice daily for 82 weeks also experienced improvement in both SBP and DBP (135), and a retrospective analysis of 6,280 patients reported a significant reduction in both SBP and DBP with exenatide therapy (111). Similarly, combination therapy with daily liraglutide (0.6, 1.2, or 1.8 mg)/metformin (2000 mg) for 16 weeks in 928 Asian subjects with T2DM reduced SBP (>3 mm Hg decrease) in comparison to glimepiride (4 mg)/metformin (2000 mg) administration (136). A retrospective analysis of 110 patients with T2DM treated with liraglutide (0.6, 1.2, or 1.8 mg daily) for a mean duration of 7.5 months (range: 6 months – 1.1 years) demonstrated a reduction in SBP (5 ± 2 mmHg) in the first 3 months of treatment (137). In an analysis encompassing 3 randomized phase 3 trials totaling 2,665 patients, 26 weeks of treatment with once-daily liraglutide (1.2 or 1.8 mg) in combination with either metformin, glimepiride, or metformin and rosiglitazone, reduced SBP by 2.29 to 6.71 mm Hg (138). Furthermore, a study of 268 obese non-diabetic patients that completed a 20-week treatment with once-daily subcutaneous liraglutide (1.2, 1.8, 2.4 or 3.0 mg) followed by a non-blinded 2 year extension (final dose of 3.0 mg) demonstrated a mean 4.6 mm Hg decrease in SBP (139). The improvement in BP in the majority of these studies was associated with reductions in body weight. Nevertheless, the reductions in SBP with liraglutide appear rapidly, often before significant weight loss is observed (136, 138).
B. GLP-1 action in experimental models of atherosclerosis
Both direct and indirect actions of GLP-1 may contribute to the potential reduction of atherogenesis (Fig. 2). GLP-1R has been localized to mouse aortic smooth muscle and endothelial cells, as well as monocytes and macrophages, using immunocytochemistry and Western blotting (140). Continuous infusion of exendin-4 (300 pmol/kg/day or 24 nmol/kg/day) in non-diabetic C57BL/6 and ApoE−/− mice, reduced monocyte adhesion to aortic endothelial cells at 24 days, associated with a reduction in atherosclerotic lesion size after 28 days of treatment. Furthermore, treatment for 1 hr with exendin-4 (0.03–3 nM) reduced levels of mRNA transcripts for the inflammatory markers MCP-1 and TNFα in response to 1 hr lipopolysaccharide (1 μg/kg) in cultured peritoneal macrophages harvested from mice 3 days after injection of 3% thioglycolate (140). Continuous infusion of exendin-4 (24 nmol/kg/day) for 4 wks in C57BL/6 mice also reduced neointimal formation in response to endothelial denudation of the femoral artery (141). Intriguingly, a 12 hr pretreatment with 10 nM exendin-4 reduced platelet-derived growth factor (25 ng/mL)-induced bromodeoxyuridine incorporation into DNA of primary cultured mouse aortic smooth muscle cells (141). Nagashima et al. reported that continuous GLP-1 or GIP administration (4 wk osmotic mini-pump infusion of 2.2 nmol/kg/day) prevented atherosclerotic lesion development in ApoE−/− mice, which may involve reduced foam cell formation in macrophages, as peritoneal macrophages harvested from exendin-4-treated mice exhibited reduced CD36 protein expression and decreased cholesterol ester accumulation following 18 hr exposure to 10 μg/mL oxidized LDL (142). Despite these intriguing findings in animals, data on the long term effects of incretin-based therapy on atherosclerosis-associated outcomes in diabetic humans is not yet available.
C. GLP-1R activation in ischemic heart disease
1) Animal studies
Multiple pre-clinical studies have demonstrated cardioprotective effects of native GLP-1 and GLP-1R agonists in experimental models of ischemic heart disease (37, 45, 48, 143, 144). Both GLP-1 and exendin-4 improved recovery of LVDP in isolated perfused rat and mouse hearts during reperfusion following ischemia (37, 48, 121). Similarly, i.v. infusion of 4.8 pmol/kg/min GLP-1 decreased infarct size following 30 min of ischemia induced by temporary occlusion of the left anterior descending (LAD) coronary artery in rats (143), and exendin-4 (10 μg i.v. and subcutaneously 5 min before the onset of reperfusion) decreased infarct size and improved LV systolic function following 75 min of LAD coronary artery occlusion in pigs (145). In contrast, recombinant GLP-1 administered via the jugular vein at 3 pmol/kg/min 15 min prior to the onset of ischemia failed to reduce infarct size in a pig model of ischemia secondary to 60 min of left circumflex coronary artery occlusion (146). Furthermore, liraglutide (10 μg/kg) administered to pigs for 3 days prior to LAD coronary artery occlusion did not reduce infarct size or improve left ventricular function (147). The GLP-1R agonist albiglutide, injected subcutaneously for 3 days at 3 or 10 mg/kg/day, reduced infarct size assessed 24 hrs following 30 min of temporary LAD coronary artery occlusion in normoglycemic rats (148). The benefit of albiglutide was attributed to improved cardiac energetics, as in vivo 13C-nuclear magnetic resonance studies demonstrated decreased fatty acid oxidation and increased glucose oxidation rates.
A GLP-1-transferrin protein also limited infarct size following 30 min of LAD coronary artery occlusion in rabbits, whether given subcutaneously at a dose of 10 mg/kg 12 hrs before the onset of ischemia, or i.v. at the onset of ischemia (149). Furthermore, liraglutide administration (75 μg/kg i.p. twice daily) for 1 week prior to LAD occlusion improved survival and cardiac output assessed 4 wks after permanent occlusion of the LAD coronary artery in both non-diabetic and diabetic male mice (45). Interestingly, 30 nM liraglutide administered in coronary arteries at the onset of reperfusion did not protect against reperfusion injury in isolated perfused mouse hearts following 30 min of global ischemia, but liraglutide did improve recovery of LVDP during reperfusion if injected into the mouse in vivo prior to ischemia/reperfusion ex vivo. This observation suggests that liraglutide may achieve cardioprotection in part through mechanisms requiring the heart to receive its normal neural, humoral and vascular input. Furthermore, the observations that GLP-1R activation does not universally produce cardioprotection in preclinical studies, highlights the importance of future studies designed to understand the precise biological mechanisms and cellular sites of action for different GLP-1R agonists in the cardiovascular system.
2) Studies in subjects with ischemic heart disease
The observation that a 72 hr infusion of GLP-1 (1.5 pmol/kg/min) initiated ~3.5 hrs after angioplasty within ~6.5 hrs from symptom onset in patients with AMI enhanced LV ejection fraction (LVEF, 29 ± 2 vs. 39 ± 2 %) and infarct zone-related regional wall motion engendered considerable interest in the cardioprotective actions of GLP-1 in humans (150). However, this report was a single-center non-randomised pilot study in a small number of patients (n=10). Nevertheless, subsequent studies have confirmed that GLP-1 may be cardioprotective. A 1.2 pmol/kg/min i.v. infusion of GLP-1 30 min prior to dobutamine stress echocardiography and continuing for 30 min into recovery in 14 patients (4 with T2DM) with known coronary artery disease, protected against LV dysfunction and mitigated post-ischemic myocardial stunning (151). These same investigators demonstrated a reduction in LV dysfunction and myocardial stunning during dual inflation coronary balloon occlusion in 20 non-diabetic patients with single vessel disease in the LAD coronary artery, in whom GLP-1 was infused at 1.2 pmol/kg/min after completion of the first balloon occlusion (152). A larger randomized, double-blind, placebo-controlled trial investigating the effects of a 6 hr exenatide infusion initiated 15 min prior to onset of reperfusion in 172 patients undergoing primary angioplasty to treat ST-segment elevation MI also demonstrated a reduction in area at risk (153). Exenatide was infused to achieve a target plasma concentration between 0.03 – 0.3 nM (mean concentration 0.177 ± 0.069 nM), and reduced reperfusion injury in these patients as determined by an increase in myocardial salvage index and decrease in infarct size relative to the ischemic area at risk assessed by cardiac magnetic resonance at ~90 days post-infusion. However, mortality and LV contractility were not different in patients receiving exenatide (153). Although accumulating data on GLP-1 action and cardiovascular function in humans with ischemic heart disease appears promising with respect to safety and potential benefit, much larger double-blinded randomized trials are necessary to determine whether GLP-1R agonists are cardioprotective in a wide range of subjects with T2DM.
D. GLP-1R agonists in heart failure
1) Preclinical models of heart failure
Studies in animals illustrate that GLP-1R activation may produce beneficial effects on the failing heart (49, 154). In a dog model of 28 day rapid pacing-induced heart failure, a 48 hr infusion of GLP-1 (1.5 pmol/kg/min) exerted insulinomimetic properties on the heart, increasing glucose uptake during a hyperinsulinemic-euglycemic clamp (49). Furthermore, GLP-1 decreased HR and increased LV systolic function, and decreased plasma levels of norepinephrine and glucagon. In the spontaneously hypertensive and heart failure-prone rat, a 3-month i.p. infusion of 1.5 pmol/kg/min GLP-1 improved survival and preserved LV contractile function, an effect associated with reduced cardiomyocyte apoptosis (109). Similarly, liraglutide administered for 1 wk (75 μg/kg i.p. twice daily) to mice before permanent occlusion of the LAD coronary artery reduced cardiac hypertrophy, decreased LV structural remodelling, and improved cardiac output 4 wks after induction of ischemia and LV dysfunction (45). In a rat model of chronic heart failure, an 11 wk subcutaneous infusion of GLP-1 (2.5 or 25 pmol/kg/min), or the exenatide analog, AC3174 (1.7 or 5 pmol/kg/min), 2 weeks after permanent LAD coronary artery occlusion significantly enhanced LV function (increased LVEF and fractional shortening), while also reducing adverse LV remodelling (decreased LV end systolic and diastolic dimensions) and improving survival (155). Taken together, the available data supports a beneficial role for both native GLP-1 and GLP-1R agonists in preclinical models of heart failure.
2) Studies in human subjects with heart failure
Initial trials in humans demonstrated salutary effects of GLP-1 in subjects with heart failure; a 5 wk infusion of GLP-1 (2.5 pmol/kg/min) in 12 patients with New York Heart Association Class III/IV heart failure improved LVEF, oxygen consumption, and 6-minute walk test scores (154). However, this was a single-center non-randomised trial, whose results require replication. A 48 hr infusion of GLP-1 (0.7 pmol/kg/min) in 15 humans with congestive heart failure but without diabetes produced no beneficial effects on LV function, and actually resulted in minor increases in HR (2 b.p.m.) and DBP (3 mmHg) (128). Although this particular study was randomised and double-blinded, a brief duration of GLP-1 infusion may be insufficient to increase function in a decompensated failing heart.
V. DPP-4 INHIBITION AND CARDIOVASCULAR FUNCTION
This section will discuss the cardiovascular biology of DPP-4, and actions of DPP-4 inhibitors in the regulation of BP, the development of atherosclerosis, the setting of AMI, and the failing heart, illustrating differences between DPP-4 inhibitors and GLP-1R agonists where appropriate.
A. DPP-4 expression in the cardiovascular system
DPP-4 is a widely expressed enzyme, and has been localized to smooth muscle and endothelial cells in different species (156, 157). Short term exposure to high glucose induces DPP-4 activity in microvascular endothelial cells (158). Although the precise biological role(s) of DPP-4 in the cardiomyocyte, endothelial or coronary smooth muscle cell requires further study, DPP-4 is also a circulating protein and thus DPP-4 activity in the systemic and coronary circulation may influence intact levels of GLP-1 and other vasoactive DPP-4 substrates reaching the myocardium and vasculature (Fig. 5). The soluble form of DPP-4 may also interact with the mannose 6-phosphate receptor on human endothelial cells, promoting endothelial cell transmigration of T cells (159).
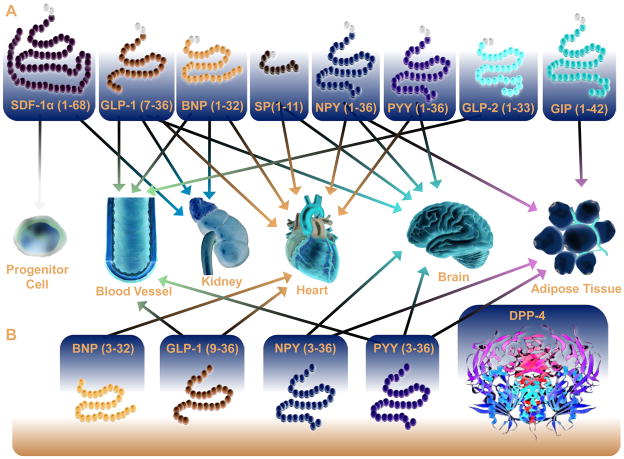
Figure 5. DPP-4 Substrates that directly or indirectly regulate cardiovascular function.
Multiple DPP-4 substrates have been identified that act on multiple peripheral tissues that influence the cardiovascular system. For a summary of these direct effects on target tissues refer to Table 1.
B. Potential Cardioactive DPP-4 substrates
Although GLP-1 is classically viewed as the primary DPP-4 substrate capable of influencing cardiovascular function, DPP-4 cleaves multiple peptides, many of which also have direct actions on the heart and blood vessels (Table 1, Fig. 5), as discussed in the following section.
Table 1.
DPP-4 cardioactive substrates and their effects on peripheral tissues that influence the cardiovascular system
| Peptide | Target Tissue | Effect | References |
|---|---|---|---|
| GLP-1 (7-36) | Heart | Increased cardiac function, glucose uptake, Decreased contractility, apoptosis | (42, 48, 49, 120) |
| Blood Vessel | Increased NO production, decreased inflammation | (37, 46, 47, 52–54, 56) | |
| Brain | Decreased appetite | (103–105) | |
| GLP-1 (9-36) | Heart | Increased cardiac function, glucose uptake, decreased apoptosis | (120),(42) (37) |
| Blood Vessel | Increased vasodilation | (37) | |
| GIP (1-42) | Adipose Tissue | Increased lipogenesis and adipogenesis | (160–163) |
| GLP-2 (1-33) | Blood Vessel | Increased blood flow, HR, and BP | (192) |
| SDF1 (1-68) | Progenitor Cell | Increased homing of progenitor cells to ischemic myocardium, increased angiogenesis | (167–171) |
| BNP (1-32) | Heart | Decreased LV remodelling | (189) |
| Blood Vessel | Increased vasodilation | (223) | |
| Kidney | Increased natriuresis | (223) | |
| BNP (3-32) | Kidney | Increased natriuresis | (223) |
| SP (1-11) | Heart | Decreased chronotropy and inotropy | (196) |
| Brain | Altered cardiac adrenergic tone | (224) | |
| NPY (1-36) | Heart | Increased [Ca2+]i current | (179, 180) |
| Brain | Increased appetite | (225) | |
| Increased adipocyte differentiation, decreased lipolysis | |||
| Adipose Tissue | (226, 227) | ||
| NPY (3-36) | Adipose Tissue | Increased lipogenesis | (183) |
| Blood Vessel | Increased angiogenesis | (182) | |
| PYY (1-36) | Blood Vessel | Increased collateral blood flow | (228, 229) |
| Adipose Tissue | Decreased lipolysis | ||
| PYY (3-36) | Blood Vessel | Increased vasoconstriction | (185) |
| Brain | Decreased appetite | (230) |
1) GIP
The GIPR has been detected by immunohistochemistry in both rat atrial and ventricular tissue (25). Although the biological function, if any, of GIP in the heart is unknown, studies in rodents have linked GIPR activation to increased adipogenesis, enhanced adipokine expression and obesity (160–163). Hence DPP-4-inhibition and the subsequent increase in plasma levels of intact bioactive GIP may have potential direct or indirect effects on the heart. The GIPR is also expressed in endothelial cells (164), and GIPR activation promotes endothelial cell proliferation in an endothelin-1-dependent manner in HUVEC cultures (165). The observation that GIP activates different signal transduction pathways in endothelial cells isolated from the hepatic artery vs. the portal vein further highlights the need for more investigation of the actions of GIP in different vascular beds (166). GIPR protein has also been detected in mouse aortic smooth muscle cells and peritoneal macrophages extracted from ApoE−/− mice (142). Moreover, administration of native GIP (25 nmol/kg/day) via continuous infusion with osmotic mini-pumps prevented atherosclerotic lesion development and macrophage infiltration in the aortic wall of ApoE−/− mice (142). As modulation of GIPR activity is being pursued for the treatment of diabetes and perhaps obesity (24), and DPP-4 inhibitors prevent the degradation of biologically active levels of intact GIP, a greater understanding of GIP action in the cardiovascular system seems prudent.
2) Stromal Cell-Derived Factor-1α (SDF-1α)
SDF-1α is a 7.97 kDa chemokine secreted from multiple cell types that promotes homing of endothelial progenitor cells to sites of cellular injury. It plays an important role in myelopoiesis and development of the embryonic heart as well as in the homing of hematopoietic stem cells and neural progenitors during embryonic development (167, 168). SDF-1 is essential for the migration of endogenous and transplanted stem cells in rodents and humans and promotes healing of injured blood vessels and myocardium (169–171). For example, SDF-1 is secreted from endothelial cells in ischemic tissue in response to activation of hypoxia-inducible factor-1, promoting migration and homing of CXCR4-positive progenitor cells to ischemic tissue (172). As SDF-1 is subject to inactivation via either DPP-4- or matrix metalloproteinase-mediated cleavage (169, 173), DPP-4 inhibitors have been used to enhance SDF-1 activity and increase stem cell number in both preclinical and clinical studies of cardiovascular injury (169, 174, 175).
3) Neuropeptide Y (NPY)
NPY is a 36 amino acid neuropeptide with potent orexigenic properties, increasing appetite and food intake. Cleavage of NPY(1-36) to NPY (3-36) by DPP-4 changes the receptor preference and biological activity of the NPY system (176). NPY receptors have been detected in cardiomyocytes and blood vessels (177, 178). Hence, inhibition of DPP-4-mediated NPY cleavage may have a number of effects on the myocardium, including modulation of ion currents (179, 180) and induction of local coronary artery vasoconstriction (181). NPY also exerts potent angiogenic actions mediated by both Y1 and Y 2 receptors, and generation of NPY(3-36) by DPP-4 enhances the angiogenic activity of NPY via the Y2 receptor (Y2R) (182). Furthermore activation of the NPY2R stimulates fat angiogenesis, macrophage infiltration, and the proliferation and differentiation of adipocytes, promoting abdominal obesity and a metabolic syndrome-like condition in preclinical studies (183). Because both NPY and NPY(3-36) appear to influence the cardiovascular system via different NPY receptor subtypes, DPP-4-mediated control of the ratio of NPY(1-36):(3-36) may have potential implications for regulation of blood flow, BP, cardiomyocyte signal transduction, adiposity, and inflammation, (184).
4) Peptide YY (PYY)
PYY is a 36 amino acid peptide released from L cells located predominantly in the ileum and colon. PYY and its DPP-4-mediated cleavage product PYY(3-36) are both agonists for NPY receptors. Preventing DPP-4-mediated PYY cleavage has been shown to enhance angiotensin II-induced vasoconstriction of isolated perfused kidneys from spontaneously hypertensive rats (185), hence the potential effects of PYY on cardiac function require further study.
5) B-type (Brain) Natriuretic Peptide (BNP)
BNP is a 32 amino acid peptide secreted from the ventricle in response to increased myocyte stretch and/or volume overload (186, 187), and is utilized as a diagnostic marker for acute heart failure. BNP is cleaved by both purified human DPP-4 and endogenous DPP-4 present in human plasma (188). BNP binds to natriuretic peptide receptors that are linked to activation of guanylyl cyclase and subsequent cGMP production, inducing arterial and venous vasodilation. Furthermore, direct intramyocardial injection of a human BNP-expressing adenovirus before permanent occlusion of the LAD coronary artery or after infusion of angiotensin II improves LV function and reduces adverse LV remodelling in rats (189). Recombinant human BNP, nesiritide, has been investigated for the management of acute decompensated heart failure, however the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial suggests that nesiritide does not improve clinical outcomes (190). Both BNP(1-32) and BNP(3-32) produce natriuretic and vasodilatory actions, hence the extent to which reduction of DPP-4 activity influences BP and LV function through modulation of BNP in diabetic subjects requires further study.
6) Glucagon-like peptide-2 (GLP-2)
GLP-2 is a 33 amino acid DPP-4-sensitive peptide (191) co-encoded together with glucagon and GLP-1 in the proglucagon gene. GLP-2 administration in humans increases mesenteric artery blood flow, which may result in a compensatory increase in HR and cardiac output (192). An immunoreactive GLP-2 receptor protein has been identified in rat heart ventricles via Western blotting, and GLP-2 treatment of Langendorff aerobic-perfused rat hearts increased contractility at 10−12 M concentrations, but decreased contractility at escalating concentrations of [10−10 – 10−7 M] GLP-2 (193). In contrast, Northern blot and RT-PCR analyses failed to detect the GLP-2R in mouse and rat heart, respectively (194), hence the mechanisms through which GLP-2 regulates cardiovascular function require additional scrutiny.
7) Other cardioactive DPP-4 substrates
DPP-4 cleaves substance P (195) and exogenous substance P administration exerts negative chronotropic and inotropic effects in Langendorff perfused guinea pig hearts (196). Substance P may also modulate adrenergic activity in the heart. Bradykinin is cleaved in part by DPP-4 and predominantly by aminopeptidase P; Whether DPP-4 inhibition significantly modulates bradykinin activity, thereby potentially contributing to the pathophysiology of angiotensin-converting enzyme inhibitor-associated angioedema, requires further study (197). As plasma levels of various DPP-4 substrates are often low and difficult to quantify, delineating the contributions of different substrates to changes observed in cardiovascular biology pursuant to DPP-4 inhibition remains challenging.
C. DPP-4 in hypertension
1) DPP-4 inhibition and blood pressure control in animals
Analysis of 20-wk-old Zucker diabetic fatty rats gavaged with sitagliptin (10 mg/kg/day) for 6 wks revealed significant reductions in SBP (~25 mmHg decrease) and DBP (~18 mmHg decrease) (198). Furthermore, 5-wk-old male spontaneously hypertensive rats treated with sitagliptin (40 mg/kg by gavage) for 8 days demonstrated significant reductions in both SBP (~26 mmHg decrease) and DBP (~16 mmHg decrease), though no effect on BP was observed in 14-wk-old rats (199). The mechanisms through which DPP-4 inhibition lowered BP in these studies remain unclear.
2) DPP-4 inhibition and blood pressure control in humans
Sitagliptin (50 or 100 mg twice daily for 5 days) reduced both SBP and DBP in 19 non-diabetic patients with mild to moderate hypertension (200). Administration of 50 mg sitagliptin every other day for 6 months in 17 hypertensive Japanese patients with T2DM resulted in a significant reduction in SBP evident after only 1 month of treatment (201). Patients treated with sitagliptin (100 mg/day) for 26 weeks in the DURATION-2 or DURATION-4 trials also demonstrated small reductions in SBP independent of changes in body weight (132, 202). Similarly, sitagliptin-treated patients experienced a small reduction in SBP and DBP over 26 wks on a background of metformin therapy (130).
D. DPP-4 in atherosclerosis
Limited information is available on the effects of DPP-4 inhibitors in the development of atherosclerosis. Treatment of high fat fed low-density lipoprotein receptor deficient mice with alogliptin (40 mg/kg/day) for 12 wks resulted in reduction of atherosclerosis (203). Furthermore, alogliptin reduced levels of plasma cholesterol, TAG, SBP (~5 mmHg decrease), adiposity, pro-inflammatory CD11b+/CD11c+ adipose tissue macrophages, atherosclerotic plaque area, plaque collagen content, and pro-inflammatory CD11b+/CD206+ macrophages in the plaque (203).
E. DPP-4 inhibition in ischemic heart disease
Studies in rats treated with valine pyrrolidide (20 mg/kg) demonstrated that DPP-4 inhibition had no impact on infarct size following LAD coronary artery occlusion in vivo or ischemia/reperfusion ex vivo (143). Similarly, acute DPP-4 inhibition with sitagliptin ex vivo conferred no benefit against ischemia/reperfusion injury in perfused Langendorff mouse hearts (123). In contrast, acute administration of sitagliptin in vivo to mice improved the recovery of LVDP in hearts subsequently subjected to ex vivo ischemia/reperfusion. Furthermore, normoglycemic Dpp4−/− mice exhibited significantly improved survival and reduced infarct size following permanent LAD occlusion in vivo. Moreover, diabetic mice treated with sitagliptin for 12 weeks exhibited improved survival following LAD ligation, and 7 days of sitagliptin administration (250 mg/kg/day) induced a cardioprotective gene expression profile in murine heart tissue (123). Pretreatment with sitagliptin for 3 or 14 days in non-diabetic mice or rats also reduced infarct size following induction of experimental ischemia through mechanisms sensitive to PKA inhibition (124). Administration of the DPP-4 inhibitor PFK275-055 (10 mg/kg/day) for 4 weeks to obese non-diabetic insulin resistant Wistar rats decreased infarct size, but interestingly, had no impact on aortic output or coronary flow (204). Taken together, the majority of preclinical studies demonstrate cardioprotection following genetic or pharmacological reduction of DPP-4 activity in young rodents.
DPP-4 inhibition likely results in cardioprotection through both GLP-1R-dependent and independent mechanisms and may protect against ischemic injury in mice by increasing angiogenesis and subsequent blood supply to the ischemic myocardium (169). Considerable evidence supports a role for SDF-1α, a potent chemoattractant of stem/progenitor cells, as a cardioactive DPP-4 substrate. Treatment of wild type mice with the DPP-4 inhibitor, diprotin A (70 μg/kg twice daily) and granulocyte colony stimulating factor (G-CSF, 100 μg/kg/day i.p.) for up to 6 days immediately after permanent LAD coronary artery occlusion improved cardiovascular outcomes at 30 days post-MI (169). These included decreased infarct size, LV wall thinning, end diastolic volume, and enhanced survival, LVEF, and neovascularization as indicated via increased CD31+ capillaries at the infarct border zone. Identical findings of improved survival, cardiac function, and ventricular remodelling were observed in G-CSF treated Dpp4−/− mice (169). Furthermore, inhibition of the SDF-1/CXCR4 axis using the antagonist AMD3100 substantially attenuated the benefits of Diprotin A/G-CSF administration in the murine LAD occlusion model (205). Follow up studies demonstrated that combined Diprotin A and G-CSF (100 μg/kg/day i.p.) also improved outcomes following LAD occlusion in mice with cardiac-specific overexpression of cyclin D2 (MHC-cycD2) (206).
Treatment of rats with a bioengineered matrix metalloproteinase and DPP-4-resistant SDF-1 led to a significant improvement in angiogenesis and ventricular function following a 3 hr ischemic insult induced via LAD coronary artery occlusion (207). The SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI) trial in humans is examining the feasibility and potential clinical utility of DPP-4 inhibition and G-CSF administration to improve myocardial function in patients pursuant to an AMI and revascularization (175).
F. DPP-4 in heart failure and cardiomyopathy
There is limited information on whether DPP-4 inhibition modifies ventricular function in the failing heart. Administration of sitagliptin, 30 mg/kg, once daily for 3 wks to normoglycemic pigs with pacing-induced heart failure, resulted in reduced HR, increased stroke volume and preservation of glomerular filtration rate (208). In contrast, administration of vildagliptin to non-diabetic rats either prior to or following LAD ligation and development of ischemic cardiomyopathy had no beneficial effects on parameters of left ventricular function or cardiac gene expression (209). Hence, whether DPP-4 inhibition directly impacts the development or progression of heart failure in animals or humans independent of its actions to reduce infarct size requires further investigation.
Studies in 6-week-old db/db mice treated with sitagliptin (16 mg/kg) for 4 wks had multiple metabolic effects on the myocardium, including reductions in AMPK and ACC phosphorylation, and decreased CD36 protein expression at the sarcolemmal membrane, suggesting that DPP-4 inhibition reduces myocardial fatty acid uptake and subsequent fatty acid metabolism (210). Sitagliptin did not improve systolic function in db/db mice, but did reduce myocardial fibrosis and improved the left ventricular relaxation constant, indicative of improved diastolic function. Furthermore, db/db mice treated with sitagliptin also demonstrated reduced myocardial p53 expression, suggestive of reduced cardiomyocyte apoptosis, though whether this reduction would prevent death of cardiomyocytes with prolonged aging and the development of heart failure in this animal model was not determined.
VI. CLINICAL TRIALS
A. GLP-1R agonists and cardiovascular outcomes
The majority of GLP-1R agonists are undergoing assessment in large, multi-center clinical trials of cardiovascular outcomes (Table 2, and www.clinicaltrials.gov) (211). The cardiovascular safety of the GLP-1R agonist, exenatide, is being studied in the Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL), a double-blind randomized trial investigating the time to first confirmed cardiovascular event in patients treated with once weekly exenatide (2 mg). Liraglutide is being assessed in the Liraglutide Effect and Action in Diabetes (LEADER) trial, a double-blind randomized clinical trial investigating the effects of once daily liraglutide (maximum dose up to 1.8 mg) with a primary outcomes measure of time from randomisation to first occurrence of cardiovascular death, non-fatal MI, or non-fatal stroke. Furthermore, the GLP-1R agonist lixisenatide is undergoing scrutiny in the Evaluation of Cardiovascular Outcomes in Patients with Type 2 Diabetes After Acute Coronary Syndrome During Treatment with Lixisenatide (ELIXA) trial, a double-blind randomised trial investigating whether lixisenatide can reduce cardiovascular mortality compared to placebo in type 2 diabetic patients who have recently experienced an acute coronary event. Dulaglutide is currently recruiting for the Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) trial, which will determine the effect of once weekly dulaglutide (1.5 mg) on major cardiovascular events in patients with T2DM. Patient enrolment in these trials ranges from 6,000 to 10,000.
Table 2.
GLP-1R agonist and DPP-4 inhibitor cardiovascular outcomes trials
| Drug | Study | Dose | Primary Outcome | Patient # |
|---|---|---|---|---|
| GLP-1R | ||||
| Agonists | ||||
| Exenatide | Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus | 2.0 mg injected subcutaneously once weekly | Time to 1st confirmed cardiovascular event | ~9,500 |
| Liraglutide | Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results -A Long Term Evaluation (LEADER™) | Maximum dose 1.8 mg/day injected subcutaneously | Time from randomization to 1st occurrence of non-fatal MI, non-fatal stroke, or cardiovascular death | ~8,750 |
| Lixasenatide | Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide) (ELIXA) | 20 ug in 0.2 mL once a day injection 1 hr before breakfast | Time to the 1st occurrence of non-fatal MI, non-fatal stroke, hospitalization for unstable angina, or cardiovascular death | ~6,000 |
| Dulaglutide | Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) | 1.5 mg injected subcutaneously once weekly | Time from randomization to 1st occurrence of non-fatal MI, non-fatal stroke, or cardiovascular death | ~9,600 |
| DPP-4 | ||||
| Inhibitors | ||||
| Vildagliptin | Effect of Vildagliptin on Left Ventricular Function in Patients With Type 2 Diabetes and Congestive Heart Failure | 50 mg twice daily | LV function as determined via changes in ejection fraction | ~490 |
| Sitagliptin | Sitagliptin Cardiovascular Outcome Study (0431-082 AM1) (TECOS) | 50 or 100 mg/day oral tablet | Time to 1st confirmed cardiovascular event (non-fatal MI, non-fatal stroke, hospitalization for unstable angina) | ~14,000 |
| Alogliptin | Cardiovascular Outcomes Study of Alogliptin in Subjects With Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) | 6.25 or 12.5 or 25 mg/day oral tablet | Time from randomization to the 1st occurrence of a primary major adverse cardiac event (nonfatal MI, non-fatal stroke, cardiovascular death) | ~5,400 |
| Saxagliptin | Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications (SAVOR- TIMI 53) | 2.5 or 5 mg/day oral tablet | Time to 1st confirmed cardiovascular event (non-fatal MI, non-fatal ischaemic stroke, or cardiovascular death) | ~16,500 |
| Linagliptin | CAROLINA: Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes | 5 mg/day oral tablet | Time to the 1st occurrence of non-fatal MI, non-fatal stroke, hospitalization for unstable angina, or cardiovascular death | ~6,000 |
B. DPP-4 inhibitors and cardiovascular outcomes
The majority of DPP-4 inhibitors are also being assessed in large, multi-center clinical trials for cardiovascular outcomes (Table 2, and www.clinicaltrials.gov). The cardiovascular safety of sitagliptin is being evaluated in The Sitagliptin Cardiovascular Outcome Study (TECOS), which is investigating the time to first confirmed cardiovascular event in type 2 diabetic patients being treated with once daily sitagliptin (50 or 100 mg). The consequences of vildagliptin therapy are being assessed in the Left Ventricular Function in Patients with Type 2 Diabetes and Congestive Heart Failure study, an ongoing trial evaluating vildagliptin’s effect on LVEF in subjects with T2DM and CHF having a LVEF < 40%. Saxagliptin, is under ongoing evaluation in the Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications (SAVOR-TIMI 53) study to determine whether once daily saxagliptin (2.5 or 5 mg) affects cardiovascular mortality, or non-fatal AMI or non-fatal ischemic stroke. An initial meta-analysis encompassing 8 phase II/III trials of T2DM patients treated with saxagliptin suggested a possible reduction of cardiovascular events with saxagliptin, however the event rate was extremely low, with only 40 events from a total of 4,607 enrolled subjects (212). The Cardiovascular Outcomes Study of Alogliptin in Subjects With Type 2 Diabetes and Acute Coronary Syndrome (EXAMINE) is evaluating whether once daily alogliptin (6.25, 12.5, or 25 mg) affects the time from randomisation to the occurrence of primary major adverse cardiac events (cardiovascular death, non-fatal MI and non-fatal stroke). Linagliptin is being evaluated in the Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes (CAROLINA). This study compares daily linagliptin (5 mg) versus glimepiride with regards to long-term impact on cardiovascular morbidity and mortality in patients with T2DM at elevated risk for cardiovascular disease. Patient enrolment in these trials ranges from 500 to as high as 16,500.
VII. FUTURE DIRECTIONS
Although considerable preclinical evidence supports a cardioprotective role for GLP-1R agonists and DPP-4 inhibitors, the majority of these studies are carried out in young healthy animals, without extensive atherosclerosis, diabetes or pre-existing heart disease. Whereas short term studies of GLP-1R agonists and DPP-4 inhibitors demonstrate modest improvements in cardiac function under conditions of ischemia, there is no long term data on whether these agents truly modify event rates in high risk patients. Retrospective reviews of health care claims databases suggest potential benefits of GLP-1R agonists in the reduction of cardiovascular events and cardiovascular-related hospitalization (213), however these studies are non-randomized, the events are not always verified, and firm conclusions cannot be drawn from retrospective studies. The putative reduction in cardiovascular events observed in clinical trials of DPP-4 inhibitors awaits confirmation in larger ongoing cardiovascular outcome studies (214).
A. Potential Pitfalls of Incretin-based Therapy
1) GLP-1 and pancreatitis
Case reports have linked GLP-1R agonists to the development of pancreatitis in human subjects, however, several health care database analyses of T2DM patients treated with multiple anti-diabetic agents have failed to confirm an increased rate of pancreatitis in subjects exposed to incretin-based therapy (215).
2) GLP-1 and cancer
Sustained administration of GLP-1R agonists stimulates calcitonin secretion and promotes the development of C- cell hyperplasia and medullary thyroid cancer in rats, and to a lesser extent, in mice (216). In contrast, human C-cells express a much lower level of GLP-1Rs and follow-up of thousands of subjects treated with liraglutide for diabetes or obesity for several years has not detected an increase in plasma calcitonin in exposed subjects (217). Nevertheless, as GLP-1R agonists and DPP-4 inhibitors are still relatively new agents, and the rates of many cancers are increased in subjects with T2DM, long-term follow-up is required to ascertain whether these agents modify the risk of developing specific malignancies.
3) GLP-1 and hypoglycemia
GLP-1 lowers glucose and increases plasma insulin levels in most subjects with T2DM, with low rates of treatment-associated hypoglycemia reflecting the glucose-dependent mechanism of GLP-1R signaling in the β-cell. As intensive glucose lowering reduces the number of infarction-related events, yet increases death due to cardiovascular causes (218), it will be important for future studies to carefully consider and better understand the potential risk:benefit ratio of different anti-diabetic agents that lower glucose through different mechanisms and with varying rates of treatment-associated hypoglycemia..
4) GLP-1 and heart rate
The available data suggests that 26 weeks to 1 year of therapy with GLP-1R agonists is frequently associated with a mean increase in HR of 2–4 b.p.m., while simultaneously reducing body weight and BP. As increased HR above 85 b.p.m. has been associated with increased rates of death and increased cardiovascular event rates, more information is required on the mechanisms through which GLP-1R agonists affect HR, and whether the increase in HR persists with longer durations of therapy.
5) Obesity paradox and heart failure
Although obesity is a significant risk factor for the development of cardiovascular diseases such as heart failure (106), retrospective studies suggest that obese patients with clinically significant weight loss experience a greater risk of mortality (219, 220). As GLP-1R agonists frequently decrease body weight in humans, obese patients with heart failure may represent a subset of individuals where therapy with GLP-1R agonists requires more careful scrutiny. On the other hand, a recent retrospective trial observed that the obesity paradox in heart failure did not apply in patients with diabetes (221). Furthermore, as DPP-4 inhibitors do not induce clinically significant weight loss (222), they may be associated with less risk in obese patients with heart failure. Nevertheless, more rigorous work in this area is necessary to better understand the obesity paradox in heart failure, as the majority of findings to date are derived from retrospective observational studies.
B. Novel Avenues of Research
While the majority of ongoing research in humans studies will be focused on determining the safety and long-term consequences of GLP-1R agonists and DPP-4 inhibitors on cardiovascular safety, the mechanisms and cell types mediating the potential cardioprotective effects of GLP-1R activation remain elusive. Hence, more precise elucidation of the consequences of GLP-1R activation in the cardiac myocyte or vasculature is imperative. It will also be important for future studies to measure the levels of potential DPP-4 cardioactive substrates in human subjects, as changes in these peptides may play an important role in determining cardiovascular outcomes in subjects treated with DPP-4 inhibitors. Finally, although the majority of data to date has been derived from studies in diabetic subjects, more research is required to determine whether GLP-1R activation or DPP-4 inhibition represents a useful therapeutic strategy in selected non-diabetic subjects with different types of cardiovascular disease.
C. Summary
Preclinical studies illustrate multiple cardioprotective actions of GLP-1R agonists and DPP-4 inhibitors, and short term studies of GLP-1R agonists or DPP-4 inhibitors in human subjects with heart disease have not revealed evidence for adverse effects on cardiac function. Nevertheless, the safety of incretin-based therapies in older subjects with a long duration of T2DM and established cardiovascular disease remains unknown. Although many of the actions of GLP-1R agonists and DPP-4 inhibitors on cardiovascular risk factors, blood vessels, and cardiomyocytes might be predicted to reduce cardiovascular risk, the results of multiple ongoing cardiovascular outcome studies will ultimately determine the place of incretin-based therapies in the treatment paradigm of T2DM.
Acknowledgments
Funding: JRU was supported by fellowships from the Canadian Institutes of Health Research and the Alberta Innovates Health Solutions. DJD is supported by a Canada Research Chair in Regulatory Peptides and the BBDC Novo Nordisk Chair in Incretin Biology. DJD receives research support for cardiovascular studies of gut peptides from the Heart and Stroke Foundation of Canada and Novo Nordisk Inc.
Footnotes
Disclosures: DJD has served as an advisor or consultant within the past 12 months to Amylin Pharmaceuticals, Arisaph Pharmaceuticals Inc., Diartis Pharmaceuticals., Eli Lilly Inc, Glaxo Smith Kline, Merck Research Laboratories, Novo Nordisk Inc., NPS Pharmaceuticals Inc., Takeda, and Transition Pharmaceuticals Inc. Neither DJD nor his family members hold stock directly or indirectly in any of these companies.
VII. REFERENCES
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Killilea T. Long-term consequences of type 2 diabetes mellitus: economic impact on society and managed care. Am J Manag Care. 2002;8:S441–449. [PubMed] [Google Scholar]
- 3.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JC, Dryburgh JR. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971;49:867–872. doi: 10.1139/o71-122. [DOI] [PubMed] [Google Scholar]
- 5.Cataland S, Crockett SE, Brown JC, Mazzaferri EL. Gastric inhibitory polypeptide (GIP) stimulation by oral glucose in man. J Clin Endocrinol Metab. 1974;39:223–228. doi: 10.1210/jcem-39-2-223. [DOI] [PubMed] [Google Scholar]
- 6.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreymann B, Ghatei MA, Williams G, Bloom SR. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet ii. 1987:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 9.Mojsov S, Weir GC, Habener JF. Insulinotropin: Glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988;123:2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker PL. Minireview: update on incretin biology: focus on glucagon-like peptide-1. Endocrinology. 2010;151:1984–1989. doi: 10.1210/en.2010-0115. [DOI] [PubMed] [Google Scholar]
- 14.Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–516. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asmar M, Holst JJ. Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: new advances. Curr Opin Endocrinol Diabetes Obes. 2010;17:57–62. doi: 10.1097/MED.0b013e3283339051. [DOI] [PubMed] [Google Scholar]
- 16.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The Glucagon Receptor Family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 17.Thorens B, Porret A, BŸhler L, Deng S-P, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor: Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 18.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide 1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. The Journal of clinical endocrinology and metabolism. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holz GGt, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC. Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide 1(7-36)amide in patients with NIDDM. Diabetes. 1996;45:1524–1530. doi: 10.2337/diab.45.11.1524. [DOI] [PubMed] [Google Scholar]
- 22.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in Type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 23.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008 doi: 10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni RN. GIP: no longer the neglected incretin twin? Sci Transl Med. 2010;2:49ps47. doi: 10.1126/scitranslmed.3001027. [DOI] [PubMed] [Google Scholar]
- 25.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin- vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 26.Irwin N, Flatt PR. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009;52:1724–1731. doi: 10.1007/s00125-009-1422-8. [DOI] [PubMed] [Google Scholar]
- 27.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko I, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O’Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Kottgen A, Le Bacquer O, Pattou F, Taneera J, Steinthorsdottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proenca C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, G JC, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jorgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Levy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparso T, Swift AJ, Syddall H, Thorleifsson G, Tonjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvanen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JI, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy MI, Boehnke M, Barroso I, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proenca C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJ, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grassler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen AL, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jorgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, Konig IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaloy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimaki T, Lettre G, Liu J, Lokki ML, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Pare G, Parker AN, Perola M, Pichler I, Pietilainen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstrale M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo ML, Tardif JC, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tonjes A, Tuomi T, van Meurs JB, van Ommen GJ, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CI, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kahonen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Gronberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin MR, Kaprio J, Karpe F, Khaw KT, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PE, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, McCarthy MI, Hirschhorn JN, Ingelsson E, Loos RJ. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha Roy R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38) J Biol Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 30.Brandt I, Lambeir AM, Maes MB, Scharpe S, De Meester I. Peptide substrates of dipeptidyl peptidases. Adv Exp Med Biol. 2006;575:3–18. doi: 10.1007/0-387-32824-6_1. [DOI] [PubMed] [Google Scholar]
- 31.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 32.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 33.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 34.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 35.Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–1670. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- 36.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Mojsov S. Distribution of GLP-1 and PACAP receptors in human tissues. Acta Physiol Scand. 1996;157:355–357. doi: 10.1046/j.1365-201X.1996.42256000.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–452. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- 41.Xiao YF, Nikolskaya A, Jaye DA, Sigg DC. Glucagon-like peptide-1 enhances cardiac L-type Ca2+ currents via activation of the cAMP-dependent protein kinase A pathway. Cardiovasc Diabetol. 2011;10:6. doi: 10.1186/1475-2840-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ban K, Kim H, Cho J, Diamandis E, Backx PH, Drucker DJ, Husain M. GLP-1(9-36) protects cardiomyocytes and endothelial cells from ischemia-reperfusion injury via cytoprotective pathways independent of the GLP-1 receptor. Endocrinology. 2010;151:1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 43.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, Quijada P, Mason M, Konstandin MH, Malhowski A, Cheng Z, Khan M, McGregor M. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SX, Xie Y, Zhou X, Sha WW, Wang WL, Han LP, Wang JC, Yu DM. Effect of glucagon-like peptide-1 on hypoxia-reoxygenation induced injury in neonatal rat cardiomyocytes. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:72–75. [PubMed] [Google Scholar]
- 47.Ravassa S, Zudaire A, Carr RD, Diez J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. American journal of physiology Heart and circulatory physiology. 2011;300:H1361–1372. doi: 10.1152/ajpheart.00885.2010. [DOI] [PubMed] [Google Scholar]
- 48.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 49.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 50.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 51.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 52.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochemical and biophysical research communications. 2010;391:1405–1408. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 53.Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, Hayashi T. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia. 2010;53:2256–2263. doi: 10.1007/s00125-010-1831-8. [DOI] [PubMed] [Google Scholar]
- 54.Erdogdu O, Nathanson D, Sjoholm A, Nystrom T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–1414. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 56.Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201:59–66. doi: 10.1677/JOE-08-0468. [DOI] [PubMed] [Google Scholar]
- 57.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 58.Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care. 2011;34:697–702. doi: 10.2337/dc10-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nathanson D, Erdogdu O, Pernow J, Zhang Q, Nystrom T. Endothelial dysfunction induced by triglycerides is not restored by exenatide in rat conduit arteries ex vivo. Regul Pept. 2009;157:8–13. doi: 10.1016/j.regpep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36 amide) in rats. Am J Physiol. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 61.Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regulatory Peptides. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- 62.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- 65.Gardiner SM, March JE, Kemp PA, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol. 2008;154:60–71. doi: 10.1038/bjp.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26:1623–1631. doi: 10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 67.Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, Mughal MR, Mendelowitz D, Mattson MP. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovascular research. 2011;89:72–78. doi: 10.1093/cvr/cvq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isbil-Buyukcoskun N, Gulec G. Effects of centrally injected GLP-1 in various experimental models of gastric mucosal damage. Peptides. 2004;25:1179–1183. doi: 10.1016/j.peptides.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Edwards CM, Edwards AV, Bloom SR. Cardiovascular and pancreatic endocrine responses to glucagon-like peptide-1(7–36) amide in the conscious calf. Exp Physiol. 1997;82:709–716. doi: 10.1113/expphysiol.1997.sp004059. [DOI] [PubMed] [Google Scholar]
- 70.Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295:R874–880. doi: 10.1152/ajpregu.00153.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edwards CM, Todd JF, Ghatei MA, Bloom SR. Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycaemia in fasted healthy subjects. Clin Sci (Lond) 1998;95:719–724. doi: 10.1042/cs0950719. [DOI] [PubMed] [Google Scholar]
- 72.Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, D’Alessio DA, Tso P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G943–949. doi: 10.1152/ajpgi.00303.2004. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion. Diabetologia. 2010;53:552–561. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- 74.Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- 75.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mells JE, Fu PP, Sharma S, Olson DE, Cheng L, Handy JA, Saxena NK, Sorescu D, Anania FA. GLP-1 analogue, liraglutide ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a western diet. American journal of physiology Gastrointestinal and liver physiology. 2011 doi: 10.1152/ajpgi.00274.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parlevliet ET, Schroder-van der Elst JP, Corssmit EP, Picha K, O’Neil K, Stojanovic-Susulic V, Ort T, Havekes LM, Romijn JA, Pijl H. CNTO736, a novel glucagon-like peptide-1 receptor agonist, ameliorates insulin resistance and inhibits very low-density lipoprotein production in high-fat-fed mice. The Journal of pharmacology and experimental therapeutics. 2009;328:240–248. doi: 10.1124/jpet.108.144154. [DOI] [PubMed] [Google Scholar]
- 79.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 80.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. The Journal of biological chemistry. 2009;284:33509–33520. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomas E, Stanojevic V, Habener JF. GLP-1 (9-36) amide metabolite suppression of glucose production in isolated mouse hepatocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2010;42:657–662. doi: 10.1055/s-0030-1253421. [DOI] [PubMed] [Google Scholar]
- 83.Valverde I, Merida E, Delgado E, Trapote MA, Villanueva-Penacarillo ML. Presence and characterization of glucagon-like peptide-1(7-36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology. 1993;132:75–79. doi: 10.1210/endo.132.1.8380388. [DOI] [PubMed] [Google Scholar]
- 84.Merida E, Delgado E, Molina LM, Villanueva-Penacarrillo ML, Valverde I. Presence of glucagon and glucagon-like peptide-1-(7-36)amide receptors in solubilized membranes of human adipose tissue. The Journal of clinical endocrinology and metabolism. 1993;77:1654–1657. doi: 10.1210/jcem.77.6.8263154. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz-Grande C, Alarcon C, Merida E, Valverde I. Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides. 1992;13:13–16. doi: 10.1016/0196-9781(92)90134-o. [DOI] [PubMed] [Google Scholar]
- 86.Villanueva-Penacarrillo ML, Marquez L, Gonzalez N, Diaz-Miguel M, Valverde I. Effect of GLP-1 on lipid metabolism in human adipocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2001;33:73–77. doi: 10.1055/s-2001-12428. [DOI] [PubMed] [Google Scholar]
- 87.Bertin E, Arner P, Bolinder J, Hagstrom-Toft E. Action of glucagon and glucagon-like peptide-1-(7-36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J Clin Endocrinol Metab. 2001;86:1229–1234. doi: 10.1210/jcem.86.3.7330. [DOI] [PubMed] [Google Scholar]
- 88.Meier JJ, Gethmann A, Gotze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006:1–7. doi: 10.1007/s00125-005-0126-y. [DOI] [PubMed] [Google Scholar]
- 89.Juntti-Berggren L, Pigon J, Karpe F, Hamsten A, Gutniak M, Vignati L, Efendic S. The antidiabetogenic effect of GLP-1 is maintained during a 7-day treatment period and improves diabetic dyslipoproteinemia in NIDDM patients. Diabetes Care. 1996;19:1200–1206. doi: 10.2337/diacare.19.11.1200. [DOI] [PubMed] [Google Scholar]
- 90.Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3853–3860. doi: 10.1210/jcem.86.8.7743. [DOI] [PubMed] [Google Scholar]
- 91.Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D. Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care. 2003;26:2835–2841. doi: 10.2337/diacare.26.10.2835. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212:217–222. doi: 10.1016/j.atherosclerosis.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 93.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. The Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 94.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Foley JE, Taskinen MR. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–2057. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- 96.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2011;13:366–373. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 97.Brownsey RW, Boone AN, Allard MF. Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovascular research. 1997;34:3–24. doi: 10.1016/s0008-6363(97)00051-5. [DOI] [PubMed] [Google Scholar]
- 98.Ji L, Fu F, Zhang L, Liu W, Cai X, Zheng Q, Zhang H, Gao F. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. American journal of physiology Endocrinology and metabolism. 2010;298:E871–880. doi: 10.1152/ajpendo.00623.2009. [DOI] [PubMed] [Google Scholar]
- 99.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. The Journal of biological chemistry. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 100.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annual review of nutrition. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell metabolism. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Zhao YT, Weng CL, Chen ML, Li KB, Ge YG, Lin XM, Zhao WS, Chen J, Zhang L, Yin JX, Yang XC. Comparison of glucose-insulin-potassium and insulin-glucose as adjunctive therapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart. 2010;96:1622–1626. doi: 10.1136/hrt.2010.194563. [DOI] [PubMed] [Google Scholar]
- 103.Scrocchi LA, Brown TJ, MacLusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide receptor gene. Nature Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 104.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 105.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 107.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nature medicine. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nature medicine. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun. 2009;390:613–618. doi: 10.1016/j.bbrc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 111.Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759–1765. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 113.Rolin B, Deacon CF, Carr RD, Ahren B. The major glucagon-like peptide-1 metabolite, GLP-1-(9-36)-amide, does not affect glucose or insulin levels in mice. Eur J Pharmacol. 2004;494:283–288. doi: 10.1016/j.ejphar.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 114.Deacon CF, Plamboeck A, Moller S, Holst JJ. GLP-1-(9-36) amide reduces blood glucose in anesthetized pigs by a mechanism that does not involve insulin secretion. Am J Physiol Endocrinol Metab. 2002;282:E873–879. doi: 10.1152/ajpendo.00452.2001. [DOI] [PubMed] [Google Scholar]
- 115.Meier JJ, Gethmann A, Nauck MA, Goetze O, Schmitz F, Deacon CF, Gallwitz B, Schmidt WE, Holst JJ. The glucagon-like peptide 1 metabolite GLP-1 (9-36)amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am J Physiol Endocrinol Metab. 2006;290:E1118–1123. doi: 10.1152/ajpendo.00576.2005. [DOI] [PubMed] [Google Scholar]
- 116.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7-36)NH(2), GLP-1-(7-37), and GLP-1- (9-36)NH(2) on Intravenous Glucose Tolerance and Glucose-Induced Insulin Secretion in Healthy Humans. J Clin Endocrinol Metab. 2003;88:1772–1779. doi: 10.1210/jc.2002-021479. [DOI] [PubMed] [Google Scholar]
- 117.Elahi D, Egan JM, Shannon RP, Meneilly GS, Khatri A, Habener JF, Andersen DK. GLP-1 (9-36) Amide, Cleavage Product of GLP-1 (7-36) Amide, Is a Glucoregulatory Peptide. Obesity (Silver Spring) 2008;16:1501–1509. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- 118.Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regulatory peptides. 2011;167:177–184. doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Tomas E, Wood JA, Stanojevic V, Habener JF. Glucagon-like peptide-1(9-36)amide metabolite inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Diabetes, obesity & metabolism. 2011;13:26–33. doi: 10.1111/j.1463-1326.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 120.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active Metabolite of GLP-1 Mediates Myocardial Glucose Uptake and Improves Left Ventricular Performance in Conscious Dogs with Dilated Cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 121.Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 122.Ossum A, van Deurs U, Engstrom T, Jensen JS, Treiman M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1(9-36)a in an isolated rat heart. Pharmacol Res. 2009;60:411–417. doi: 10.1016/j.phrs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Sauve M, Ban K, Momen A, Zhou Y-Q, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes following myocardial infarction in mice. Diabetes. 2010;59:1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size limiting effects of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol. 2010;298:H1454–1465. doi: 10.1152/ajpheart.00867.2009. [DOI] [PubMed] [Google Scholar]
- 125.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 Inhibition by Sitagliptin Improves the Myocardial Response to Dobutamine Stress and Mitigates Stunning in a Pilot Study of Patients with Coronary Artery Disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 126.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–49. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Laugero KD, Stonehouse AH, Guss S, Landry J, Vu C, Parkes DG. Exenatide Improves Hypertension in a Rat Model of the Metabolic Syndrome. Metab Syndr Relat Disord. 2009;7:327–334. doi: 10.1089/met.2008.0095. [DOI] [PubMed] [Google Scholar]
- 128.Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Toftegard T, Eiskjaer H, Botker HE, Wiggers H. Cardiovascular and Metabolic Effects of 48-Hour Glucagon-like Peptide 1 Infusion in Compensated Chronic Heart Failure Patients. Am J Physiol Heart Circ Physiol. 2010;298:H1096–1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 129.Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Thomsen AB, Sondergaard RE, Davies M. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 131.Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, Garber A, Thomsen AB, Hartvig H, Davies M. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. International journal of clinical practice. 2011;65:397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, Wolka AM, Boardman MK. Efficacy and Safety of Exenatide Once Weekly Versus Metformin, Pioglitazone, and Sitagliptin Used as Monotherapy in Drug-Naive Patients With Type 2 Diabetes (DURATION-4): A 26-week double-blind study. Diabetes Care. 2011 doi: 10.2337/dc11-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gustavson SM, Chen D, Somayaji V, Hudson K, Baltrukonis DJ, Singh J, Boyden TL, Calle RA. Effects of a long-acting GLP-1 mimetic (PF-04603629) on pulse rate and diastolic blood pressure in patients with type 2 diabetes mellitus. Diabetes, obesity & metabolism. 2011;13:1056–1058. doi: 10.1111/j.1463-1326.2011.01479.x. [DOI] [PubMed] [Google Scholar]
- 134.Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, Wilhelm K, Trautmann M, Shen LZ, Porter LE. DURATION-1: Exenatide Once Weekly Produces Sustained Glycemic Control and Weight Loss Over 52 Weeks. Diabetes Care. 2010;33:1255–1261. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 136.Yang W, Chen L, Ji Q, Liu X, Ma J, Tandon N, Bhattacharyya A, Kumar A, Kim KW, Yoon KH, Bech OM, Zychma M. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*) Diabetes Obes Metab. 2011;13:81–88. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 137.Varanasi A, Patel P, Makdissi A, Dhindsa S, Chaudhuri A, Dandona P. Clinical Use of Liraglutide in Type 2 Diabetes, and Its Effects on Cardiovascular Risk Factors. Endocr Pract. 2011:1–13. doi: 10.4158/EP11169.OR. [DOI] [PubMed] [Google Scholar]
- 138.Gallwitz B, Vaag A, Falahati A, Madsbad S. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. International journal of clinical practice. 2010;64:267–276. doi: 10.1111/j.1742-1241.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- 139.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. International journal of obesity. 2011 doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goto H, Nomiyama T, Mita T, Yasunari E, Azuma K, Komiya K, Arakawa M, Jin WL, Kanazawa A, Kawamori R, Fujitani Y, Hirose T, Watada H. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces intimal thickening after vascular injury. Biochemical and biophysical research communications. 2011;405:79–84. doi: 10.1016/j.bbrc.2010.12.131. [DOI] [PubMed] [Google Scholar]
- 142.Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, Miyazaki A, Hirano T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide-1 (GLP-1) can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 144.Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- 145.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 146.Kavianipour M, Ehlers MR, Malmberg K, Ronquist G, Ryden L, Wikstrom G, Gutniak M. Glucagon-like peptide-1 (7-36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003;24:569–578. doi: 10.1016/s0196-9781(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 147.Kristensen J, Mortensen UM, Schmidt M, Nielsen PH, Nielsen TT, Maeng M. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc Disord. 2009;9:31. doi: 10.1186/1471-2261-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bao W, Aravindhan K, Alsaid H, Chendrimada T, Szapacs M, Citerone DR, Harpel MR, Willette RN, Lepore JJ, Jucker BM. Albiglutide, a long lasting glucagon-like peptide-1 analog, protects the rat heart against ischemia/reperfusion injury: evidence for improving cardiac metabolic efficiency. PLoS One. 2011;6:e23570. doi: 10.1371/journal.pone.0023570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsubara M, Kanemoto S, Leshnower BG, Albone EF, Hinmon R, Plappert T, Gorman JH, 3rd, Gorman RC. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res. 2011;165:38–45. doi: 10.1016/j.jss.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 151.Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2011 doi: 10.1136/hrt.2010.219345. [DOI] [PubMed] [Google Scholar]
- 152.Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, West NE, Dutka DP. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–272. doi: 10.1161/CIRCINTERVENTIONS.110.960476. [DOI] [PubMed] [Google Scholar]
- 153.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 154.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 155.Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, Parkes DG. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palmieri FE, Ward PE. Dipeptidyl(amino)peptidase IV and post proline cleaving enzyme in cultured endothelial and smooth muscle cells. Adv Exp Med Biol. 1989;247A:305–311. doi: 10.1007/978-1-4615-9543-4_45. [DOI] [PubMed] [Google Scholar]
- 157.Matheeussen V, Baerts L, De Meyer G, De Keulenaer G, Van der Veken P, Augustyns K, Dubois V, Scharpe S, De Meester I. Expression and spatial heterogeneity of dipeptidyl peptidases in endothelial cells of conduct vessels and capillaries. Biol Chem. 2011;392:189–198. doi: 10.1515/BC.2011.002. [DOI] [PubMed] [Google Scholar]
- 158.Pala L, Pezzatini A, Dicembrini I, Ciani S, Gelmini S, Vannelli BG, Cresci B, Mannucci E, Rotella CM. Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells. Acta Diabetol. 2010:1–5. doi: 10.1007/s00592-010-0195-3. [DOI] [PubMed] [Google Scholar]
- 159.Ikushima H, Munakata Y, Iwata S, Ohnuma K, Kobayashi S, Dang NH, Morimoto C. Soluble CD26/dipeptidyl peptidase IV enhances transendothelial migration via its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cell Immunol. 2002;215:106–110. doi: 10.1016/s0008-8749(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 160.Kim SJ, Nian C, McIntosh CH. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. J Biol Chem. 2007;282:34139–34147. doi: 10.1074/jbc.M704896200. [DOI] [PubMed] [Google Scholar]
- 161.Kim SJ, Nian C, McIntosh CH. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. J Lipid Res. 2010;51:3145–3157. doi: 10.1194/jlr.M006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 163.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhong Q, Bollag RJ, Dransfield DT, Gasalla-Herraiz J, Ding KH, Min L, Isales CM. Glucose-dependent insulinotropic peptide signaling pathways in endothelial cells. Peptides. 2000;21:1427–1432. doi: 10.1016/s0196-9781(00)00287-4. [DOI] [PubMed] [Google Scholar]
- 165.Ding KH, Zhong Q, Isales CM. Glucose-dependent insulinotropic peptide stimulates thymidine incorporation in endothelial cells: role of endothelin-1. Am J Physiol Endocrinol Metab. 2003;285:E390–396. doi: 10.1152/ajpendo.00509.2002. [DOI] [PubMed] [Google Scholar]
- 166.Ding KH, Zhong Q, Xu J, Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E773–779. doi: 10.1152/ajpendo.00507.2003. [DOI] [PubMed] [Google Scholar]
- 167.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 168.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 169.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 170.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal-cell-derived factor-1alpha (SDF-1alpha) treatment. Regul Pept. 2005;125:1–8. doi: 10.1016/j.regpep.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 171.Vagima Y, Lapid K, Kollet O, Goichberg P, Alon R, Lapidot T. Pathways implicated in stem cell migration: the SDF-1/CXCR4 axis. Methods Mol Biol. 2011;750:277–289. doi: 10.1007/978-1-61779-145-1_19. [DOI] [PubMed] [Google Scholar]
- 172.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature medicine. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 173.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. The Journal of biological chemistry. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 174.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes mellitus. Possible role of stromal derived factor-1{alpha} Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Theiss HD, Brenner C, Engelmann MG, Zaruba MM, Huber B, Henschel V, Mansmann U, Wintersperger B, Reiser M, Steinbeck G, Franz WM. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial) - Rationale, design and first interim analysis. Int J Cardiol. 2010;145:282–284. doi: 10.1016/j.ijcard.2009.09.555. [DOI] [PubMed] [Google Scholar]
- 176.Mentlein R, Dahms P, Grandt D, Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 177.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 178.Pellieux C, Sauthier T, Domenighetti A, Marsh DJ, Palmiter RD, Brunner HR, Pedrazzini T. Neuropeptide Y (NPY) potentiates phenylephrine-induced mitogen-activated protein kinase activation in primary cardiomyocytes via NPY Y5 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bryant SM, Hart G. Effects of neuropeptide Y on L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 1996;118:1455–1460. doi: 10.1111/j.1476-5381.1996.tb15560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Heredia Mdel P, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, Benitah JP, Gomez AM. Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol. 2005;38:205–212. doi: 10.1016/j.yjmcc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 181.Tseng CJ, Robertson D, Light RT, Atkinson JR, Robertson RM. Neuropeptide Y is a vasoconstrictor of human coronary arteries. Am J Med Sci. 1988;296:11–16. doi: 10.1097/00000441-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 182.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- 183.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature medicine. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 184.Kos K, Baker AR, Jernas M, Harte AL, Clapham JC, O’Hare JP, Carlsson L, Kumar S, McTernan PG. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes, obesity & metabolism. 2009;11:285–292. doi: 10.1111/j.1463-1326.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 185.Jackson EK, Zhang M, Liu W, Mi Z. Inhibition of renal dipeptidyl peptidase IV enhances peptide YY1-36-induced potentiation of angiotensin II-mediated renal vasoconstriction in spontaneously hypertensive rats. The Journal of pharmacology and experimental therapeutics. 2007;323:431–437. doi: 10.1124/jpet.107.126847. [DOI] [PubMed] [Google Scholar]
- 186.Hasegawa K, Fujiwara H, Doyama K, Miyamae M, Fujiwara T, Suga S, Mukoyama M, Nakao K, Imura H, Sasayama S. Ventricular expression of brain natriuretic peptide in hypertrophic cardiomyopathy. Circulation. 1993;88:372–380. doi: 10.1161/01.cir.88.2.372. [DOI] [PubMed] [Google Scholar]
- 187.Ogawa T, Linz W, Stevenson M, Bruneau BG, Kuroski de Bold ML, Chen JH, Eid H, Scholkens BA, de Bold AJ. Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation. 1996;93:2059–2067. doi: 10.1161/01.cir.93.11.2059. [DOI] [PubMed] [Google Scholar]
- 188.Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-Peptidase IV Converts Intact B-Type Natriuretic Peptide into Its des-SerPro Form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 189.Moilanen AM, Rysa J, Mustonen E, Serpi R, Aro J, Tokola H, Leskinen H, Manninen A, Levijoki J, Vuolteenaho O, Ruskoaho H. Intramyocardial BNP gene delivery improves cardiac function through distinct context-dependent mechanisms. Circ Heart Fail. 2011;4:483–495. doi: 10.1161/CIRCHEARTFAILURE.110.958033. [DOI] [PubMed] [Google Scholar]
- 190.Pleister AP, Baliga RR, Haas GJ. Acute study of clinical effectiveness of nesiritide in decompensated heart failure: nesiritide redux. Curr Heart Fail Rep. 2011;8:226–232. doi: 10.1007/s11897-011-0066-4. [DOI] [PubMed] [Google Scholar]
- 191.Drucker DJ, Shi Q, Crivici A, Sumner-Smith M, Tavares W, Hill M, DeForest L, Cooper S, Brubaker PL. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997;15:673–677. doi: 10.1038/nbt0797-673. [DOI] [PubMed] [Google Scholar]
- 192.Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept. 2010;159:67–71. doi: 10.1016/j.regpep.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Angelone T, Filice E, Quintieri AM, Imbrogno S, Amodio N, Pasqua T, Pellegrino D, Mule F, Cerra MC. Receptor identification and physiological characterisation of glucagon-like peptide-2 in the rat heart. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 194.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 195.Wang LH, Ahmad S, Benter IF, Chow A, Mizutani S, Ward PE. Differential processing of substance P and neurokinin A by plasma dipeptidyl(amino)peptidase IV, aminopeptidase M and angiotensin converting enzyme. Peptides. 1991;12:1357–1364. doi: 10.1016/0196-9781(91)90220-j. [DOI] [PubMed] [Google Scholar]
- 196.Chiao H, Caldwell RW. Local cardiac effects of substance P: roles of acetylcholine and noradrenaline. Br J Pharmacol. 1995;114:283–288. doi: 10.1111/j.1476-5381.1995.tb13224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, Adam A, Brown NJ. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, Vala H, Pinto R, Garrido P, Sereno J, Fernandes R, Santos P, Velada I, Melo A, Nunes S, Teixeira F, Reis F. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat) Mediators Inflamm. 2010;2010:592760. doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pacheco BP, Crajoinas RO, Couto GK, Davel AP, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 200.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 201.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223:133–135. doi: 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 202.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 203.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huisamen B, Genis A, Marais E, Lochner A. Pre-treatment with a DPP-4 inhibitor is infarct sparing in hearts from obese, pre-diabetic rats. Cardiovasc Drugs Ther. 2011;25:13–20. doi: 10.1007/s10557-010-6271-7. [DOI] [PubMed] [Google Scholar]
- 205.Theiss HD, Vallaster M, Rischpler C, Krieg L, Zaruba MM, Brunner S, Vanchev Y, Fischer R, Grobner M, Huber B, Wollenweber T, Assmann G, Mueller-Hoecker J, Hacker M, Franz WM. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res. 2011;7:244–255. doi: 10.1016/j.scr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 206.Zaruba MM, Zhu W, Soonpaa MH, Reuter S, Franz WM, Field LJ. Granulocyte colony-stimulating factor treatment plus dipeptidylpeptidase-IV inhibition augments myocardial regeneration in mice expressing cyclin D2 in adult cardiomyocytes. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 208.Gomez N, Touihri K, Matheeussen V, Mendes Da Costa A, Mahmoudabady M, Mathieu M, Baerts L, Peace A, Lybaert P, Scharpe S, De Meester I, Bartunek J, Vanderheyden M, Mc Entee K. Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing-induced heart failure. Eur J Heart Fail. 2011 doi: 10.1093/eurjhf/hfr146. [DOI] [PubMed] [Google Scholar]
- 209.Yin M, Sillje HH, Meissner M, van Gilst WH, de Boer RA. Early and late effects of the DPP-4 inhibitor vildagliptin in a rat model of post-myocardial infarction heart failure. Cardiovasc Diabetol. 2011;10:85. doi: 10.1186/1475-2840-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lenski M, Kazakov A, Marx N, Bohm M, Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol. 2011;51:906–918. doi: 10.1016/j.yjmcc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 211.Fonseca VA. Ongoing clinical trials evaluating the cardiovascular safety and efficacy of therapeutic approaches to diabetes mellitus. The American journal of cardiology. 2011;108:52B–58B. doi: 10.1016/j.amjcard.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 212.Frederich R, Alexander JH, Fiedorek FT, Donovan M, Berglind N, Harris S, Chen R, Wolf R, Mahaffey KW. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgraduate medicine. 2010;122:16–27. doi: 10.3810/pgm.2010.05.2138. [DOI] [PubMed] [Google Scholar]
- 213.Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, Hussein MA. Risk of Cardiovascular Disease Events in Patients with Type 2 Diabetes Prescribed the GLP-1 Receptor Agonist Exenatide Twice Daily or Other Glucose-Lowering Therapies: A Retrospective Analysis of the LifeLinkTM Database. Diabetes Care. 2011;34:90–95. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27(Suppl 3):57–64. doi: 10.1185/03007995.2011.602964. [DOI] [PubMed] [Google Scholar]
- 215.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies--review of the scientific evidence. J Clin Endocrinol Metab. 2011;96:2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 216.Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 217.Hegedus L, Moses AC, Zdravkovic M, Thi TL, Daniels GH. GLP-1 and Calcitonin Concentration in Humans: Lack of Evidence of Calcitonin Release from Sequential Screening in over 5000 Subjects with Type 2 Diabetes or Nondiabetic Obese Subjects Treated with the Human GLP-1 Analog, Liraglutide. J Clin Endocrinol Metab. 2011;96:853–860. doi: 10.1210/jc.2010-2318. [DOI] [PubMed] [Google Scholar]
- 218.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 220.Futter JE, Cleland JG, Clark AL. Body mass indices and outcome in patients with chronic heart failure. Eur J Heart Fail. 2011;13:207–213. doi: 10.1093/eurjhf/hfq218. [DOI] [PubMed] [Google Scholar]
- 221.Adamopoulos C, Meyer P, Desai RV, Karatzidou K, Ovalle F, White M, Aban I, Love TE, Deedwania P, Anker SD, Ahmed A. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail. 2011;13:200–206. doi: 10.1093/eurjhf/hfq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 223.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 224.Wang LL, Guo Z, Han Y, Wang PF, Zhang RL, Zhao YL, Zhao FP, Zhao XY. Implication of Substance P in myocardial contractile function during ischemia in rats. Regulatory peptides. 2011;167:185–191. doi: 10.1016/j.regpep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 225.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 226.Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. Faseb J. 2008;22:2452–2464. doi: 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- 227.Kos K, Harte AL, James S, Snead DR, O’Hare JP, McTernan PG, Kumar S. Secretion of neuropeptide Y in human adipose tissue and its role in maintenance of adipose tissue mass. American journal of physiology Endocrinology and metabolism. 2007;293:E1335–1340. doi: 10.1152/ajpendo.00333.2007. [DOI] [PubMed] [Google Scholar]
- 228.Cruze CA, Su F, Limberg BJ, Deutsch AJ, Stoffolano PJ, Dai HJ, Buchanan DD, Yang HT, Terjung RL, Spruell RD, Mittelstadt SW, Rosenbaum JS. The Y2 receptor mediates increases in collateral-dependent blood flow in a model of peripheral arterial insufficiency. Peptides. 2007;28:269–280. doi: 10.1016/j.peptides.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 229.Valet P, Berlan M, Beauville M, Crampes F, Montastruc JL, Lafontan M. Neuropeptide Y and peptide YY inhibit lipolysis in human and dog fat cells through a pertussis toxin-sensitive G protein. The Journal of clinical investigation. 1990;85:291–295. doi: 10.1172/JCI114425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]