Abstract
Background
Mammalian lung development consists of a series of precisely choreographed events that drive the progression from simple lung buds to the elaborately branched organ that fulfills the vital function of gas exchange. Strict transcriptional control is essential for lung development. Among the large number of transcription factors encoded in the mouse genome, only a small portion of them are known to be expressed and function in the developing lung. Thus a systematic investigation of transcription factors expressed in the lung is warranted.
Results
To enrich for genes that may be responsible for regional growth and patterning, we performed a screen using RNA in situ hybridization to identify genes that show restricted expression patterns in the embryonic lung. We focused on the pseudoglandular stage during which the lung undergoes branching morphogenesis, a cardinal event of lung development. Using a genome-scale probe set that represents over 90% of the transcription factors encoded in the mouse genome, we identified sixty-two transcription factor genes with localized expression in the epithelium, mesenchyme or both. Many of these genes have not been previously implicated in lung development.
Conclusions
Our findings provide new starting points for the elucidation of the transcriptional circuitry that controls lung development.
Keywords: lung, mouse, transcription factors, expression patterns, branching
Introduction
The mammalian lung is essential for survival starting at birth. It is a prominent branching organ, with an endoderm-derived epithelium supported by a mesoderm-derived mesenchyme. In the mature lung, the epithelial tubules assume the shape of a tree-like structure, where the proximal portion forms the conducting airways and the distal portion forms the gas-exchanging alveoli. The mesenchyme consists primarily of the vasculature, smooth muscles, and tracheal/bronchial cartilage that forms intimate contacts with the epithelial tree and are also critical for respiration (Cardoso and Whitsett, 2008; Shi et al. 2009; Warburton et al. 2010).
In mice, lung development and maturation can be divided into five stages: embryonic bud initiation (Embryonic Day, or E9.0–E11.5), pseudoglandular (E11.5–E16.5), canalicular (E16.5–E17.5), saccular (E17.5–P5), and alveolar (P5–P28) stages (Maeda et al. 2007). The first sign of respiratory specification is marked by the expression of a transcription factor encoding gene, Nkx2-1 in a small group of ventral foregut endoderm cells at E9.0 (Lu et al. 2004). Signals from the surrounding cardiac and prospective lung mesenchyme are critical for this specification (Domyan and Sun 2011). The first morphologically distinct respiratory structure, the primary lung buds, are observed at E9.5. From E9.5–11.5, these buds elongate and undergo secondary branching to establish the basis of one left lung lobe and four right lung lobes. The bulk of branching morphogenesis occurs during the pseudoglandular stage, and follows a strict program (Metzger et al. 2008). Towards the end of this stage and continuing into the canalicular stage, the conducting airway epithelium differentiates into specialized cell types, including Clara and ciliated cells (Rawlins et al. 2006). Drastic changes occur in the saccular stage as the lung transitions from being fluid filled to air breathing. The distal epithelium undergoes saccular branching and subdivides into primary alveolar sacs that allow for gas exchange. Meanwhile, the vascular network surrounding the alveolar sacs remodels to facilitate oxygen uptake. Within the distal epithelium, type II pneumocytes mature and start to produce surfactants, a protein and lipid mixture that lowers surface tension within the lung and allows lung expansion at birth (Whitsett and Weaver 2002). The expression of channel proteins also increases at birth, leading to rapid clearing of the fluid from the lung. While the lung can carry out gas-exchange at the end of saccular stage, it only contains approximately 5% of the final alveolar surface area. During alveologenesis, thousands of secondary septae rise from the walls of the primary alveolar sac into the lumen; each serving as a new surface for gas-exchange (Mund et al. 2008).
Transcription factors have been shown to play critical roles in lung development (Warburton et al. 2000; Whitsett and Matsuzaki 2006; Maeda et al. 2007). They control diverse processes, including epithelial branching, epithelial and mesenchymal cell differentiation, surfactant and channel gene expression, and septae formation. Thus studies of transcription factor function in lung development have been a fruitful direction of research in the field. There are over 1,400 transcription factors encoded in the mouse genome. To date only a small faction of these are known to be expressed and play a role in the lung. A systematic investigation of transcription factor expression in the lung is necessary, as it will help to elucidate the genetic control of lung development.
We performed an RNA in situ hybridization screen to identify transcription factor genes expressed within the branching mouse lung. There are several previously published expression screens in the branching lung (Liu and Hogan 2002, Lu et al. 2004, Lu et al. 2005, Metzger et al. 2007). Some of these focus on genes differentially expressed in particular regions of the wildtype lung (Liu and Hogan 2002, Lu et al. 2004), while others focus on genes that are regulated by specific signals essential for branching, such asFGF (Lu et al. 2005, Metzger et al. 2007). In addition to these studies, there are public databases such as GenePaint (www.genepaint.org) where gene expression patterns in tissue sections are available on a genome scale (Visel et al. 2004). However, these large-scale databases are not focused on any particular tissue. Therefore, annotation of the expression pattern is not always precise. For example, in GenePaint, Sox17 is listed as not expressed in E14.5 lung, while it is clearly expressed at this stage and plays a critical role in lung development (Park et al. 2006).
To enrich the existing datasets, we utilized a mouse transcription factor probe collection that represents approximately 90% of all transcription factors encoded in the mouse genome (Gray et al. 2004). We conducted wholemount in situ hybridization using over 1,100 probes on E13.5 mouse lungs. Using a stringent standard, we identified sixty-two genes that are expressed in a subset of the cells within the lung epithelium, mesenchyme, or both. It should be noted that not all transcription factor genes are represented in the cDNA collection. Furthermore, since one probe per gene was screened, it is likely that a fraction of the probes do not produce the optimal hybridization signal with the specific transcript. Due to these limitations, our data only account for a subset of the transcription factor genes expressed in the branching lung. However, as many of the genes identified in our screen have not been previously implicated in lung development, our findings make an important contribution to existing gene expression datasets in the branching lung.
Results/Discussions
We chose to carry out our screen in E13.5 lungs for several reasons. First, a meticulous mapping of the lung epithelial ductal system during development shows that epithelial branching follows a stereotypical program, suggestive of strict genetic control including transcriptional regulation (Metzger et al. 2008). E13.5 is a stage in the middle of pseudoglandular branching, and is thus representative of the process. Second, several key signaling pathways that are essential for lung branching, including the fibroblast growth factor (FGF), sonic hedgehog (SHH) and bone morphogenetic protein (BMP) signaling pathways, are all active in the E13.5 lung. Members of these pathways are known to influence each other’s transcription through feedback loops, yet few transcription factors that mediate these regulations have been identified. Third, E13.5 is a stage when active patterning of the epithelium and mesenchyme occurs. These broad patterns then serve as blueprints for fine-scale cellular differentiation that occurs at later stages of lung development. Finally, we chose to carry out an in situ screen because we are particularly interested in genes expressed in subsets of cells in the lung as they may direct regional growth and patterning events. We determined that E13.5 is the latest, and therefore most advanced stage when distinct patterns can still be consistently discerned from wholemount in situ hybridization.
As we are interested in genes that may drive regional patterning and morphogenesis in the lung, we focused on genes that show restricted expression patterns. Overall, we identified sixty-two genes with differential expression in distinct sub-regions of the lung (Table 1, Figs. 1–4). Some of these factors have previously been shown to play a role in lung development (Maeda et al. 2007). However, the majority of these genes do not have a well-characterized association with the branching lung. We grouped these genes into four categories to present their expression patterns and discuss known or potential functions. The first three categories are distinguished based on their expression site: genes that are expressed solely in the epithelium (Category 1) or mesenchyme (Category 2), and genes that are expressed in subregions of both tissue layers (Category 3). Hox genes are presented as a separate group (Category 4) to facilitate a direct comparison of their overlapping expression patterns (Fig. 4). For each gene, expression patterns in the wholemount lung were confirmed by the patterns in vibratome sections.
Table 1.
A summary of transcription factors identified in the screen
| Gene Name | Sites of expression in lung | Function during lung development | References | Figure |
|---|---|---|---|---|
| Ash2l | Epithelium | KO: dies at lung bud initiation stage. | Stoller et al. 2010 | 1R |
| Baz1b | Mesenchyme | KO: die at birth with heart defects, no reported lung defect. | Yoshimura et al. 2009 | 2C |
| Brd7 | Epithelium and Mesenchyme | No knockout line available. | Kaeser et al. 2008 | 3I |
| Creb3l1 | Mesenchyme | KO: viable and no reported lung defect. | Murakami et al. 2009 | 2V |
| E2f1 | Epithelium and Mesenchyme | KO: no reported lung defect. Double KO of E2f1;E2f3 develops simplified alveoli. | Tsai et al. 2008 | 3F |
| Elf5 | Epithelium | OE: dilated branching tips and disrupted epithelial differentiation. KO: no reported lung defect. |
Metzger et al. 2008 and Xin Sun unpublished | 1N |
| Elk3 | Mesenchyme | KO: survive birth, but eventually die due to respiratory distress secondary to chyle built up in thorax. | Ayadi et al. 2001 | 2L |
| Ets1 | Mesenchyme | KO: viable and no reported lung defect. | Muthusamy et al. 1995 | 2M |
| Ets2 | Mesenchyme | KO: embryonic lethal at E8.5. | Yamamoto et al. 1998 | 2N |
| Etv5 | Epithelium | DN: proximalization of the lung epithelium in late branching stage. KO: no reported lung defect. |
Liu et al. 2003; Chen et al. 2005 | 1M |
| Foxa1 | Epithelium | KO: crucial for epithelial branching and differentiation. | Wan et al. 2005 | 1K |
| Foxd4 | Epithelium | No knockout line available. | Kaestner et al. 1993 | 1L |
| Foxf1a | Mesenchyme | Heterozygous KO: lung lobe fusion and altered branch pattern. | Mahlapuu et al. 2001 | 2S |
| Foxf2 | Mesenchyme | KO: dies postnatally with no reported lung defect. | Wang et al. 2003 | 2T |
| Foxh1 | Epithelium and Mesenchyme | KO: left-right asymmetry lung defect. | Yamamato et al. 2003; Hoodless et al. 2001 | 3E |
| Foxm1 | Epithelium and Mesenchyme | KO: defects in vascular and epithelial cell differentiation and inhibit arterial smooth muscle formation in lung. | Kim et al 2005; Kalin et al. 2008 | 3D |
| Gata6 | Epithelium | KO: lung branching and epithelial differentiation defects. | Keijzer et al 2001; Yang et al. 2002 | 1G |
| Gli2 | Mesenchyme | KO: reduced lung branching. | Motoyama et al. 1998 | 2A |
| Grhl2 | Epithelium | Point mutant: reduced lung size and lung collapse at birth. KO: dies at beginning of lung development. |
Pyrgaki et al. 2011 | 1W |
| Heyl | Mesenchyme | KO: viable and no reported lung defect. | Fischer et al. 2007 | 2R |
| Hhex | Mesenchyme | KO: dies at E14.5, no reported lung defect. | Hallaq et al. 2004 | 2I |
| Hif1a | Epithelium and Mesenchyme | CKO in epithelium: lethal at birth due to low surfactant gene expression. | Saini et al 2008 | 3G |
| Hif3a | Mesenchyme | KO: impaired alveologenesis with an increase of the vasculature lining the alveolar walls. | Yamashita et al. 2008 | 2P |
| Hnf1b | Epithelium | KO: embryonic lethal at post implantation stage. | Coffinier et al. 2002 | 1E |
| Id2 | Epithelium | KO: dies postnatally, no reported lung defect. | Yokota et al. 1999 | 1U |
| Id3 | Mesenchyme | KO: viable and no reported lung defect. | Pan et al. 1999 | 2Q |
| Irf6 | Epithelium | Unknown. KO: no reported lung defect. | Ingraham et al. 2006 | 1V |
| Irx3 | Epithelium | KD: abnormal branching. | Van Tuyl et al. 2006 | 1A |
| Kat2b | Epithelium | KO: no reported lung defect. | Yamauchi et al. 2000 | 1Q |
| Lef1 | Mesenchyme | KO: dies postnatally, no reported lung defect. | van Genderen et al. 1994 | 2U |
| Lhx6 | Mesenchyme | KO: dies around 2 weeks postnatally, no reported lung defect. | Liodis et al. 2007 | 2H |
| Lmo1 | Epithelium | KO: viable and no reported lung abnormalities. | Tse et al. 2004 | 1H |
| .Meis1 | Mesenchyme | KD: dies at E14.5 and exhibit lung hypoplasia. | Hisa et al. 2004 | 2F |
| Mnx1 | Epithelium | KO: postnatal lethal due to failure to inflate lungs. | Arber et al. 1999 | 1F |
| Nab1 | Epithelium | KO: viable and no reported lung defect. | Le et al. 2005 | 1T |
| Nkx1-2 | Epithelium | No knockout line available. | Tamplin et al. 2008 | 1D |
| Nkx2-1 | Epithelium | KO or KD: failed trachea specification and lung branching arrest. | Minoo et al. 1995; Minoo et al. 1999 | 1B |
| Nkx2-9 | Epithelium | KO: tracheobronchial epithelium hyperplasia in adult lung. | Tian et al. 2006 | 1C |
| Nr2f1 | Mesenchyme | KO: postnatal lethal with no reported lung defect. | Qiu et al. 1997 | 2D |
| Nr2f2 | Mesenchyme | KO: dies at E10.0. CKO: lung hypoplasia. | Pereira et al 1999; You et al. 2005 | 2E |
| Pitx2 | Epithelium and Mesenchyme | KO: left-right asymmetry lung defect. | Lu et al. 1999; Liu et al. 2001 | 3A |
| Pou2f1 | Epithelium and Mesenchyme | KO: prenatal lethal with erythropoiesis defect, no reported lung defect. | Wang et al. 2004 | 3C |
| Pou3f1 | Mesenchyme | KO: dies at birth due to respiratory distress possibly secondary to neuronal defects. | Bermingham et al. 1996 | 2G |
| Smarca5 | Epithelium and Mesenchyme | KO: lethal at pre-implantation stage. | Stopka et al. 2003 | 3H |
| Snai1 | Mesenchyme | KO: lethal at E8.5. | Carver et al. 2001 | 2B |
| Sox18 | Mesenchyme | KO: variable prenatal lethality, cardiovascular defects, no reported lung defect. | Dunn et al. 1995 | 2K |
| Sox2 | Epithelium | CKO: disruption of cell differentiation. OE: altered branching morphogenesis. |
Gontan et al. 2008; Tompkins et al. 2009; Que et al. 2009 | 1O |
| Sox7 | Mesenchyme | No knockout line available. | Cermenati et al 2008 | 2J |
| Sox9 | Epithelium | CKO: no reported lung defect. | Perl et al. 2005 | 1P |
| Tbx3 | Mesenchyme | KO: dies before birth, no reported lung defect. | Davenport et al. 2003 | 2W |
| Tcea1 | Epithelium and Mesenchyme | KO: dies before E16.5 and no reported lung defect. | Ito et al. 2006 | 3J |
| Tcea2 | Epithelium | No knockout line available. | Reines et al. 1996 | 1J |
| Tcf21 | Mesenchyme | KO: branching defects with proximalization of the lung epithelium. | Quaggin et al. 1999 | 2O |
| Tfap2c | Epithelium | KO: dies at E7–9. | Werling et al. 2002 | 1S |
| Zfhx3 | Epithelium and Mesenchyme | Gene-trap mutant: no reported lung defect. | Stryke et al. 2003 | 3B |
| Zfp746 | Epithelium | No knockout line available. | Shin et al 2011 | 1I |
Abbreviations:
KO Knock Out
KD Knock Down
CKO Conditional Knock Out
DN Dominant Negative
OE Over Expression
Hox gene expression patterns are presented separately in Figure 4.
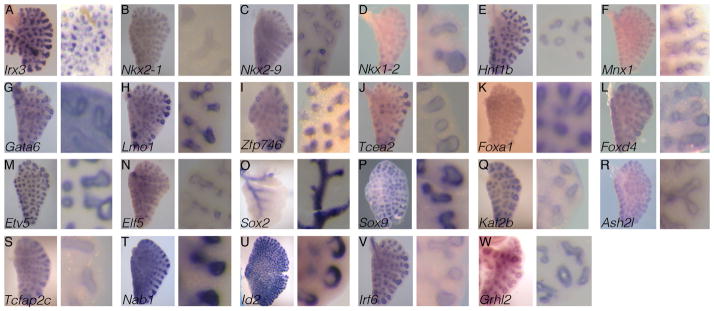
Figure 1. Patterns of transcription factor genes with primarily epithelial expression in the developing lung.
(A–W) Twenty-three genes were identified by RNA in situ hybridization as having epithelium-specific expression patterns in the E13.5 lung. For each gene, the left panel is a wholemount in situ hybridization of a left lung lobe, and the right panel is an enlarged vibratome section of a right lung lobe. The tissue in each panel is oriented so that the upper left corner and the lower right corner represent the proximal and distal regions of the lung, respectively.
Figure 4. Patterns of Hox transcription within the developing lung.
(A–L) Six Hox genes were identified by RNA in situ hybridization to be expressed within the E11.5 (A,C,E,G,I,K) and either E12.5 (J,L) or E13.5 (B,D,F,H) lung and trachea. Each gene is a wholemount in situ hybridization of both the trachea and lung.
Category 1-genes expressed in the lung epithelium
Twenty-three transcription factor genes were identified as having epithelial specific expression patterns in the lung (Fig. 1A–W). We found more than one member in each of the following families containing specific domains: homeobox, zinc finger, forkhead box (FOX), SRY box (SOX), and E-twenty six (ETS).
Homeobox containing: Irx3, Nkx2-1, Nkx2-9, Nkx1-2, Hnf1b, and Mnx1
Homeobox containing transcription factors are essential for patterning throughout the developing embryo. In the E13.5 lung, we identified five genes belonging to this family that are expressed solely in the epithelium (Fig. 1A–F). Of these, only Irx3 and Nkx2-1 have been previously shown to play roles in embryonic lung development.
The Iroquois homeobox (Irx) transcription factor family consists of six members that have extensive overlaps in expression in the developing mouse embryo (Bosse et al. 1997; Christoffels et al. 2000; Cohen et al. 2000; Houweling et al. 2001). At E13.5, Irx3 expression is detected at a high level in the distal portion of the lung epithelium (Fig. 1A). Three other Irx transcription factors (Irx1,2,5) are also expressed in the lung epithelium in a similar pattern as Irx3 (Becker et al. 2001; Van Tuyl et al. 2006; Diez-Roux et al. 2011). No lung abnormalities have been reported in any of the existing Irx single gene mutant mice (Lebel et al. 2003). However, knockdown of all Irx transcription factors using antisense oligonucleotides in lung explants causes branching defects (Van Tuyl et al. 2006), suggesting that Irx factors may act redundantly to regulate lung branching.
Three Nkx transcription factors were identified in our screen, Nkx2-1, Nkx2-9, and Nkx1-2 (Fig. 1B–D). Nkx2-1 (also termed TTF-1) is one of the earliest genes expressed in the nascent respiratory lineage (Lazzaro et al. 1991). Knockdown or knockout of Nkx2-1 leads to early lung branching arrest (Minoo et al. 1995; Minoo et al. 1999). However, lung buds form in each case, suggesting that Nkx2-1 is dispensable for lung budding, but essential for secondary branching morphogenesis. Furthermore, Nkx2-1 mutants lack the trachea, suggesting that this gene is essential for trachea specification (Minoo et al. 1999, Que et al. 2007). Nkx2-9 knockout mice form functional lungs and survive to adulthood, but show tracheobronchial epithelial hyperplasia (Tian et al. 2006). Further analysis of the Nkx2-9 mutants demonstrates that Nkx2-9 maintains the cellular architecture of the upper airway by restricting cell proliferation in the epithelium. Little is known regarding the role of Nkx1-2 in development, including in the lung. No knockout mouse line has been reported. In the Foxa2 mutant mouse gastrula, Nkx1-2 expression is downregulated (Tamplin et al. 2008). Since Foxa2 is critical for lung epithelial differentiation and alveologenesis (Wan et al. 2005), these results raise the possibility that Nkx1-2 may function downstream of Foxa2 in the lung.
The roles of either Hnf1b or Mnx1 in lung development have not been closely interrogated. Within the branching lung, Hnf1b shows higher expression in the distal region of the lung epithelium, while Mnx1 expression appears present in both the proximal and distal regions (Fig. 1E,F). Homozygous null mutants of Hnf1b (also known as vHnf1 and Tcf2) are lethal at the early post-implantation stage due to defects in extra-embryonic endoderm differentiation (Barbacci et al. 1999; Coffinier et al 1999). Using a conditional allele, it has been shown that inactivation of Hnf1b in the bile duct led to a change from cuboidal to pseudostratified squamous epithelium (Coffinier et al. 2002). Conditional inactivation of Hnf1b within the kidney results in a polycystic kidney phenotype, suggesting that Hnf1b is essential in controlling planar cell polarity (Fischer et al. 2006; Verdeguer et al. 2010). A recent study has demonstrated the importance of planar cell polarity in lung development (Yates et al. 2010), raising the possibility that Hnf1b may play a role in this process in lung. Mnx1 (also known as Hb9) knockout mice die at birth due to an inability to inflate the lung (Arber et al. 1999). However, it remains unresolved whether this is the result of a lung-specific defect or a neuronal defect in diaphragm innervation. In the epithelium of other tissues, such as the pancreas, Mnx1 is known to regulate cell differentiation, raising the possibility that Mnx1 may regulate a similar process within the lung (Harrison et al. 1999; Li et al. 2001).
Zinc finger containing: Gata6, Lmo1, Zfp746, and Tcea2
Four zinc finger containing transcription factors were found to be expressed within the E13.5 lung epithelium: Gata6, Lmo1, Zfp746, and Tcea2 (Figure 1G–J). Of these, only Gata6 has been shown to be essential for lung development (Keijzer et al. 2001; Yang et al. 2002; Liu et al. 2002; Zhang et al. 2008). Lung epithelium-specific inactivation of Gata6 leads to defects in branching morphogenesis and cell differentiation.
Homozygous Lmo1 null mutants are viable and show no obvious defects including in the lung (Tse et al. 2004). This lack of a phenotype within the lung may reflect redundancy with other Lmo transcription factors, as several other Lmo members are also expressed in the lung, including Lmo4 and Lmo7 (Sum et al. 2005; Ott et al. 2008). In neural tissues such as the hindbrain, LMO factors regulate proximal-distal patterning (Matis et al. 2007). This raises the possibility that LMO factors may function collectively to control lung epithelium patterning.
Zfp746 (also termed PARIS) has not been shown to be expressed or function within the developing lung. A recent study using overexpression and knockdown techniques show that Zfp746 promotes neural degeneration in Parkinson’s disease models (Shin et al. 2011). No Zfp746 mutant mouse has been reported.
Tcea2 is expressed within the distal region of the lung epithelium and is one of two transcriptional elongation factors identified within this screen (together with Tcea1, see below). Existing evidence suggests that Tcea2 promotes transcription by preventing the arrest of RNA polymerase (Reines et al. 1996). It remains unclear how this molecular role impacts tissue development, as no Tcea2 mutant mice have been reported. It is interesting to note that Tcea2 is not expressed in the adult lung (Umehara et al. 1997), suggesting that it may act specifically in cells and tissues that are undergoing active proliferation and expansion.
FOX domain containing: Foxa1, Foxd4
A number of FOX domain transcription factors are expressed in the developing lung (Maeda et al. 2007). Our screen highlights two that are detected within the lung epithelium at E13.5, Foxa1 and Foxd4 (Fig. 1K,L). Foxa1, along with the closely related Foxa2, has been shown to be critical for branching and epithelial differentiation in the lung (Wan et al. 2004a; Wan et al. 2004b; Wan et al. 2005). Inactivation of Foxa1 and Foxa2 in the mouse lung epithelium leads to fewer, and more dilated epithelial branches than the control. Furthermore, markers of differentiated cell types in both the proximal airway (CC10 or SCGB1A1, and FOXJ1) and distal alveoli (SFTPC and SFTPB) are no longer expressed in the absence of these FOX factors, suggesting that Foxa1;Foxa2 are required broadly in the lung epithelium for cell differentiation. On the protein level, it has been shown that FOXA1 interacts with NKX2-1 to regulate the expression of Sftpc in the lung epithelium (Minoo et al. 2007). Moreover, examination of major signaling pathways essential for lung development suggests that Foxa1 and Foxa2 act to control the expression of Shh in the lung epithelium (Wan et al. 2005).
While Foxd4 has been shown to be expressed at a low level in the adult lung (Kaestner et al. 1993), its expression has not been reported in the embryonic lung (Fig. 1L). In yeast, Foxd4 controls the cell cycle by regulating the G2/M transition (Kumar 2000). Whether it plays a similar role in mice has not been tested, and no knockout mouse has been reported. Outside of the lung, in the gastrula stage embryo, the expression of Foxd4 is positively regulated by FOXA2 (Tamplin et al. 2008). Thus it is possible that Foxd4 may function downstream of Foxa2 in the lung.
Although not identified by probes used in our screen, several other FOX domain transcription factors are expressed within the developing lung epithelium, including the Foxp sub family (Lu et al. 2002). Foxp1, Foxp2 and Foxp4 exhibit overlapping expression patterns within the lung epithelium and mesenchyme (Shu et al. 2001; Lu et al. 2002). Inactivation of Foxp1 and one allele of Foxp2 (Foxp1−/−;Foxp2+/−) produces a smaller lung due to a decrease in cell proliferation (Shu et al. 2007). At later stages these mice also develop a simplified alveolar network. FOXP factors act mainly as transcriptional repressors and this effect is mediated through interaction with HDAC proteins (Chokas et al 2010). In the adult lung, double heterozygous Foxp1+/− ;HDAC2+/− mice are resistant to hyperoxia-induced lung injury compared to control. These data together suggest that Foxp genes play critical roles in both lung development and homeostasis.
ETS domain containing: Etv5 and Elf5
Two genes encoding ETS domain proteins, Etv5 and Elf5, were identified as being expressed solely in the distal lung epithelium (Fig. 1M,N). Both have been shown to be positively regulated by FGF signaling, which could explain their expression within the distal epithelium, as Fgf10 is strongly expressed within the distal mesenchyme of the branching lung and signals to the adjacent epithelium (Liu et al. 2003; Metzger et al. 2007). ETV5 belongs to the three-member Pea3 subgroup of ETS domain containing proteins (ETV1 also known as ER81, ETV4 also known as PEA3, and ETV5 also known as ERM), which share homology not only in the ETS domain, but also throughout the rest of the protein. Expression of a dominant-negative form of Etv5 in the epithelium of late branching stage lung causes proximal-distal patterning defects (Liu et al. 2003). No overt lung phenotype was found in Etv5 loss-of-function mutants (Chen et al. 2005), likely due to redundancy with Etv1 and Etv4.
Overexpression of full-length form of Elf5 in the distal epithelium of the lung causes dilated branching tips, and disrupted type II cell differentiation (Metzger et al. 2008a). On the molecular level, Elf5 overexpression leads to decreased expression of Etv5 and increased expression of Spdef, another ETS family gene. This result suggests intricate feedback regulation among ETS family members. Homozygous null mutants of Elf5 die by E7.5, prior to lung initiation (Zhou et al. 2005). Lung epithelium-specific inactivation of Elf5 produces no overt lung development defects (X. Sun unpublished results), possibly due to redundancy with other ETS containing genes expressed in the lung epithelium.
SRY box containing: Sox2 and Sox9
Two genes encoding SOX domain containing transcription factors, Sox2 and Sox9, were identified as being expressed in the epithelium (Fig. 1O,P). They are known to exhibit complementary expression patterns in the epithelium, with Sox2 expression localized to the proximal epithelium and Sox9 expression localized to the distal epithelium. Conditional inactivation of Sox2 within the respiratory epithelium leads to a decrease in ciliated and Clara cells in both the trachea and bronchiole, but an increase in goblet cells in the trachea and a decrease in goblet cells in the bronchiole (Que et al. 2009; Tompkins et al. 2009). Furthermore in the adult, loss of Sox2 in the trachea disrupts its ability to repair after sulfur dioxide (SO2)-induced epithelial damage, possibly due to a reduced number of basal stem cells in the mutant epithelium (Que et al. 2009). Similarly, loss of Sox2 in the adult bronchiole disrupts the increase in goblet cell differentiation and mucus production in response to allergen challenge (Tompkins et al. 2009). Compared to these loss-of-function phenotypes, overexpression of Sox2 in the lung epithelium causes altered branching morphogenesis, proximalization of epithelial cell fate, and a slight decrease in cell proliferation (Gontan et al. 2008).
Inactivation of Sox9 in the lung epithelium does not result in any reported defects (Perl et al. 2005), suggesting that by itself, Sox9 is not required in the epithelium for lung development. However, Sox9 is also expressed in the proximal mesenchyme, specifically in the precursor and differentiated cartilages of both the trachea and main bronchi (Elluru et al. 2004). As Sox9 is a key gene essential for cartilage development (Bi et al. 2001), it likely plays a role in airway cartilage ring formation.
Miscellaneous epithelial genes: Kat2b, Ash2l, Tfap2c, Nab1, Id2, Irf6, and Grhl2
Seven of the epithelium-expressed transcription factors, Kat2b, Ash2l1, Tfap2c, Nab1, Id2, Irf6, and Grhl2, were identified in our screen as the sole member of their respective families (Fig. 1Q–W). The roles of these genes in lung development are largely unknown.
Kat2b
Kat2b (also known as Pcaf) is a bromodomain-containing acetyl transferase. Kat2b knockout mice survive postnatally, and no lung defect has been reported (Yamauchi et al. 2000). The absence of an apparent lung phenotype may be due to redundancy with a close family member Kat2a. In support of this possibility, while Kat2a is expressed at a relatively low level in the wild-type lung, its level increases dramatically in the Kat2b mutant lung (Yamauchi et al. 2000). As Kat2a global knockout mice die prior to E11.5, identification of a possible combined role of Kat2a and Kat2b in lung awaits the generation of tissue-specific double mutants. A recent study identifies Kat2b as a potential target of miR302/367, two GATA6-regulated miRNAs that control saccular branch patterning and the balance between proliferation and differentiation in the lung (Tian et al. 2011). Whether misexpression of Kat2b contributes to the defects in the miR302/367 overexpression or knockdown lungs has yet to be established.
Ash2l
Ash2l is a SPRY domain containing methyl-transferase that promotes the trimethylation of lysine 4 of histone 3 (H3K4), an epigenetic signature of actively transcribed regions (Steward et al. 2006). RNAi knockdown of Ash2l in HeLa cells causes decreased trimethylation of H3K4, and decreased transcription of target genes (Steward et al. 2006). Ash2l knockout mice die at a stage when lung buds first initiate (Stoller et al. 2010), and its role in lung development has not been studied. Despite this, Ash2l has been shown to regulate the expression of several genes implicated in lung development. For example, in muscle differentiation, Ash2l regulates the expression of MyoG (Rampalli et al. 2007). Previous studies show that MyoG is required in the lung for transition from pseudoglandular to saccular stages (Tseng et al. 2000). Ash2l has also been shown to regulate the expression of Fgfr3 in a neuroblastoma cell line (Tan et al. 2009). Fgfr3, together with Fgfr4, are required for lung alveologenesis (Weinstein et al. 1998; Srisuma et al. 2010). These data raise the possibility that Ash2l may act upstream of these factors during lung development.
Tfap2c
Tfap2c (also known as Ap-2γ) is an Ap2 family gene. The role of Tfap2c in the developing lung remains unknown. Knockout mice are lethal between E7 and E9, prior to lung budding (Werling et al. 2002). Conditional ablation of Tfap2c in the skin shows that Tfap2c is important for terminal differentiation of epidermal cells (Wang et al. 2008). This role is executed through an interaction with members of the NOTCH signaling pathway. In the lung, NOTCH signaling is essential for epithelial patterning and differentiation (Tsao et al. 2008; Guseh et al. 2009; Tsao et al. 2009; Morimoto et al. 2010). Furthermore, recent data show that CEBPα, a critical factor for the maturation of the distal epithelium, is a downstream target of TFAP2C (Martis et al. 2006; Xu et al. 2008). Thus, it is plausible that TFAP2C may interact with these genes to control lung development. It should be noted that another Tfap2 transcription factor, Tfap2 (also known as Ap-2α), is expressed within the lung epithelium (Visel et al 2004). Double knockout mice for these two TFAP2 factors die prior to the lethality of either single mutant, suggesting that they function redundantly during development, possibly also in the lung (Winger et al. 2006).
Nab1
Nab1 single mutant mice are viable, fertile, and no lung defect has been reported (Le et al. 2005). In contrast, double mutants of both Nab1 and the related Nab2 develop labored breathing. It is not clear if the respiratory defect results from the loss of Nab1 and Nab2 within the lung or in other tissues required for breathing, as histology of the double mutant lungs has not been reported (Le et al. 2005). On the molecular level, Nab1 is a transcriptional repressor of early growth response proteins 1 and 2 (EGR1 and EGR2) (Swirnoff et al. 1998; Le et al. 2005). Loss of Egr1 protects the lung from bleomycin-induced fibrosis, a mouse model for idiopathic pulmonary fibrosis (IPF) (Lee et al. 2004). Furthermore, Egr1 has been implicated in mediating cell death in smoking-induced chronic obstructive pulmonary disease (COPD) (Chen et al. 2008). Egr2 knockout mice develop increased breathing rates (Desmazieres et al. 2008). These findings suggest that Nab1 and Nab2 may control lung maturation through their ability to regulate the activity of Egr1 and Egr2.
Id2
Id2 is a member of the inhibitor of differentiation (Id) family of bHLH domain transcription factors. There are four Id transcription factor genes and all are expressed in the lung: Id2 and Id4 are expressed in the distal and proximal epithelium, respectively, while Id1 and Id3 are expressed in the mesenchyme (Fig. 1U, Fig. 2Q; Jen et al. 1996). Lineage tracing using an Id2-creERT2 has shown that Id2-expressing cells represent a multipotent progenitor population that contributes to both the proximal airway epithelial and the distal alveolar epithelial cell populations (Rawlins 2009). The expression of Id genes is regulated by BMP signaling (Miyazono and Miyazawa 2002; ten Dijke et al. 2003). Previous work has demonstrated that genetic inactivation of BMP signaling leads to trachea specification, proximal-distal lung patterning as well as lung cell differentiation defects (Bellusci 1996; Weaver et al. 1999; Eblaghie et al. 2006; Que et al. 2006; Li et al. 2008; Sun et al 2008; Domyan et al. 2011). These results suggest that the Id genes may mediate the critical roles of BMP signaling in lung. However, there are no reported lung developmental defects associated with single Id gene mutants, including Id2 mutants. These results suggest that either these Id genes function redundantly amongst themselves, or with other bHLH factors during lung development (Yokota et al. 1999).
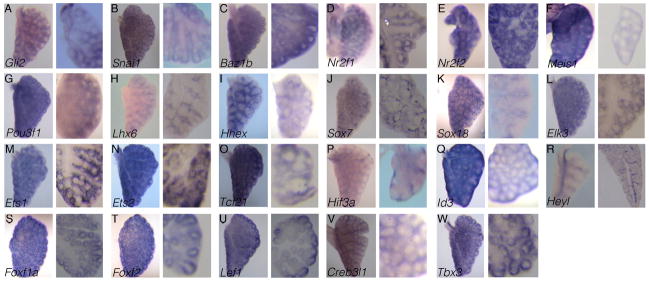
Figure 2. Patterns of transcription factor genes with primarily mesenchymal expression in the developing lung.
(A–W) Twenty-four genes were identified by RNA in situ hybridization as having mesenchyme-specific expression patterns in the E13.5 lung. For each gene, the left panel is a wholemount in situ hybridization of a left lung lobe, and the right panel is an enlarged vibratome section of a right lung lobe. The tissue in each panel is oriented so that the upper left corner and the lower right corner represent the proximal and distal regions of the lung, respectively.
Irf6
Irf6 is an interferon regulatory factor whose human homolog has been associated with Van der Woude syndrome, a craniofacial disease characterized by the formation of a cleft lip (Kondo et al. 2002). Irf6 is expressed throughout both the proximal and distal portions of the lung epithelium (Fig. 1V). Irf6 knockout mice develop skin defects as a result of increased proliferation, and decreased terminal differentiation of epidermal progenitor cells (Ingraham et al. 2006). Irf6 knockout mice also display esophageal closure and limb-tail fusion as a result of increased adhesion between epidermal layers. At the molecular level, Irf6 has been shown to act as a downstream mediator of NOTCH signaling (Restivo et al. 2011). Since NOTCH signaling is critical for lung epithelial patterning and differentiation (Tsao et al. 2008; Guseh et al. 2009; Tsao et al. 2009; Morimoto et al. 2010), it is plausible that Irf6 may also function downstream of NOTCH in the lung epithelium.
Grhl2
Grhl2 is one of three Grainyhead homologs (Grhl1-3), and all are expressed within the developing lung epithelium (Fig. 1W; Auden et al. 2006). While Grhl2 targeted knockout mutants die at the stage of development that precludes a study of their requirement in lung (Rifat et al 2010), mice carrying a Grhl2 point mutant hypomorphic allele exhibit reduced lung size and inability to inflate the lung at birth (Pyrgaki et al. 2011). In Grhl2 point mutant lungs, cell adhesion proteins including E-Cadherin, β–Catenin, and Ezrin are detected at lower levels and/or mislocalized. Regulation of cellular adhesion molecules appears to be a general function of Grhl2 during development, as a similar role was also found in the epidermis and the neural tube (Werth et al. 2010; Boglev et al. 2011). These data together suggest that Grhl2 is essential for lung development via controlling the adhesion properties of the lung epithelium.
Category 2-genes expressed in the lung mesenchyme
From our screen, twenty-three genes were identified as showing mesenchyme-specific expression patterns in the lung (Fig. 2A–W). Most of these factors have not been characterized for their role in early lung development. We found multiple transcription factors that contained the following domains: zinc finger, homeobox, SOX, ETS, bHLH, and FOX. Six of these factors, Nr2f2, Hhex, Sox7, Sox18, Elk3, and Ets1, are expressed in a pattern consistent with their general known roles in vasculature formation (Fig. 2E, I–M). Several of the rest of the factors are detected in the subepithelial mesenchyme portion of the lung (White et al 2006).
Zinc finger containing: Gli2, Snai1, Baz1b, Nr2f1, and Nr2f2
Five zinc finger containing transcription factors were identified within the mesenchyme (Fig. 2A–E). Of these, only Gli2 is known to be required for early lung development, and is detected in the subepithelial mesenchyme (Fig. 2A). Gli2, together with Gli1 and Gli3, are core components of the Hedgehog signaling pathway. Loss of Gli2 leads to reduced lung branching (Motoyama et al. 1998). Further inactivation of Gli3 in a Gli2 knockout background (generating Gli2−/−;Gli3−/−embryos) results in trachea and lung agenesis, suggestive of shared function (Motoyama et al. 1998).
Snai1 is abundantly expressed in the subepithelial mesenchyme of the lung (Fig. 2B). Its function in lung has not been directly assessed, as Snai1 knockout mutants are lethal prior to E8.5 due to aberrant conversion of epiblast cells into mesoderm cells during gastrulation (Carver et al. 2001). As exemplified by its role during gastrulation, SNAI1 is part of the core machinery that promotes epithelial to mesenchymal transitions (EMT) throughout development as well as in postnatal events such as metastasis in turmorigenesis (Vincent et al. 2009). In normal lung development, a known EMT event is the transition of mesothelium into cells in the mesenchyme (Que et al. 2008). Whether Snai1 plays a role in this event remains to be tested using conditional gene inactivation approaches. In pathological conditions of the lung, it has been shown that Snai1 promotes EMT in lung epithelium-derived cell lines (Jayachandran et al. 2009). This suggests that Snai1 may contribute to lung fibrosis in diseases such as Idiopathic Pulmonary Fibrosis (IPF) (Kim et al. 2006; Coward et al. 2010). However, a recent study throws into question the involvement of EMT in lung fibrosis (Rock et al 2011).
Baz1b has been shown to be expressed within the adult lung through Northern blot analysis (Peoples et al. 1998). Here, we localize its expression to the E13.5 lung mesenchyme (Fig. 2C). On the molecular level, BAZ1B controls transcription by interacting with a number of chromatin remodeling proteins including the SWI/SNF-type WINAC and ISWI-type WICH complexes (Oya et al. 2009). This interaction is regulated by MAPK signaling. In humans, BAZ1B deletions have been associated with the congenital disorder Williams Syndrome (WS), where patients develop supravalvular aortic stenosis along with neurological abnormalities (Williams et al. 1961; Lu et al. 1998; Riby and Porter 2010; Mohan et al. 2011). Consistent with disease symptoms, Baz1b knockout mice develop heart abnormalities, including atrial/ventricular septation defects and abnormal trabeculation (Yoshimura et al. 2009). However no lung phenotype has been reported in these mice.
Nr2f1 and Nr2f2 (also known as Coup-TfI and Coup-TfII) encode zinc finger-containing steroid/thyroid hormone nuclear receptor proteins. In the lung, they are expressed in overlapping patterns with Nr2f1 being more restricted to the subepithelial mesenchyme while Nr2f2 being more widely expressed in the mesenchyme (Fig. 2D,E). Nr2f1 has primarily been shown to control neural development, where it regulates both the patterning of neural tissues as well as the differentiation of progenitor cells into mature neurons (Qiu et al. 1997; Faedo et al. 2008). Despite neural differentiation phenotypes, Nr2f1 knockout mice survive postnatally, with no report of cyanosis or labored breathing. Nr2f2 knockout mice show abnormal angiogenesis and heart patterning, and are lethal by E10.0 (Pereira et al. 1999). Conditional knockout of Nr2f2 in the foregut mesentery using Nkx3-2-cre produces defects that mimic congenital diaphragmatic hernia (CDH), a human birth defect characterized by failed closure of the diaphragm, herniation of abdominal content into the chest cavity, and hypoplastic lungs (You et al. 2005). It remains to be determined whether this lung defect is due to a primary requirement for Nr2f2 in lung, or is secondary to the mechanical pressure exerted by the herniating tissues on the lung.
Homeobox domain containing: Meis1, Pou3f1, Lhx6 and Hhex
We identified four homeobox containing genes that are expressed within the mesenchyme of the E13.5 lung: Meis1, Pou3f1, Lhx6 and Hhex (Fig. 2F–I). While there are no reported role for the latter three factors in the developing lung, a previous study indicates that Meis1 null mutants show hypoplastic lungs (Hisa 2004).
The Meis family of transcription factors is primarily recognized as Hox cofactors in their regulation of gene expression (Moens and Selleri 2006). In mice, all three Meis genes are expressed in the developing lung mesenchyme, with Meis1 RNA being detected throughout the mesenchyme (Fig. 2F and data not shown). Multiple Hox genes are expressed in the lung mesenchyme and all are possible partners of Meis (Fig. 4). Meis1 knockout mice die around E14.5 due to hematopoietic and microvascular defects, and exhibit hypoplastic lungs (Hisa et al. 2004). However the primary role of Meis1 in lung development has not been determined.
Pou3F1 (also known as Oct6) is primarily known for its function in neuronal differentiation (Bermingham et al. 1996; Bermingham et al. 2002; Ghazvini et al. 2002). Pou3f1 mutants are unable to form myelinating Schwann cells within the embryonic peripheral nervous system. These mutants die at birth due to respiratory distress but examination of the lungs revealed no gross defect. Instead, it was proposed that the respiratory deficiency is caused by a possible decrease in number and function of phrenic motor neurons, which may lead to failed diaphragm function during respiration (Bermingham et al. 1996). However, given its expression in the lung, a more thorough analysis of the lung defects in these mutants and a tissue-specific inactivation of Pou3F1 in lung may be worthwhile to rule out a direct requirement for Pou3F1 in lung development.
Lhx6 belongs to a large family of LIM homeodomain containing transcription factors. Lhx6 knockout mice die shortly after two weeks of age due to unknown causes (Liodis et al. 2007). Whether these mice develop lung defects has not been shown. Functional redundancy among Lhx factors is observed during development. For example, during dentition, Lhx6;Lhx7 double mutants display molar agenesis due to failed mesenchyme differentiation (Denaxa et al. 2009). Following the detection of Lhx6 expression in lung (Fig. 2H), a search of existing databases revealed that Lhx4, Lhx5, and Lhx9 are also expressed in the lung mesenchyme (Visel et al. 2004). These results suggest that Lhx6 may function redundantly with other Lhx members to control lung mesenchyme development.
Hhex (also known as Hex) is expressed in the lung in a pattern that resembles the vascular network (Fig. 2I, Lazarus et al. 2011). Hhex knockout mice die prior to E14.5 due to abnormal development of the heart and vasculature (Keng et al. 2000; Hallaq et al. 2004). In the heart, Hhex mutants show abnormal outflow tract and valve formation as a result of persistent EMT of endocardial cells. This persistent EMT is thought to be mediated, at least in part, by increased VEGFA protein levels. In the vascular network of Hhex knockout mice, blood vessels initiate normally. However, the vascular lumens become dilated, possibly due to a decrease in vascular smooth muscle. Whether Hhex mutants development any lung defects has not been reported.
SOX domain containing: Sox7 and Sox18
From our screen, we identified two genes encoding F subgroup SOX domain containing transcription factors, Sox7 and Sox18. These genes, together with the remaining F subgroup member Sox17, are expressed in overlapping, but non-identical patterns in the vasculature (Fig. 2J,K) (Park et al. 2006; Sakamoto et al. 2007; François et al. 2010). For example, in a normal embryo, Sox18, but not Sox7 or Sox17, is expressed in the lymphatic endothelial cells. Interestingly, in the absence of Sox18, Sox7 becomes ectopically expressed in the lymphatics (Hosking et al. 2009). Consistent with this, these genes play redundant roles in endothelial cell differentiation in blood and lymphatic vessels in both mice and zebrafish (Cermenati et al. 2008; Sakamoto et al. 2007). In humans, a dominant-negative mutation in human SOX18, which likely interferes with the function of multiple SOX genes, is known to cause Hypotrichosis-Lymphedema-Telangiectasia, a congenital lymphatic disorder (Irrthum et al. 2003). The specific requirement for these genes in the lung vasculature has not been directly addressed.
ETS domain containing: Elk3, Ets1, and Ets2
We identified three ETS domain containing transcription factors that are expressed within the lung mesenchyme: Elk3, Ets1, and Ets2 (Fig. 2L–N). While their function in the lung remains unclear, all three of these genes have been implicated in endothelial development in other tissues.
Elk3 (also known as Net) knockout mice survive past birth and show no lung defects (Ayadi et al. 2001). While these mutant mice do eventually die due to respiratory distress, the cause is not believed to be intrinsic to the lung, but rather due to a build up of chyle in the thoracic body cavity from a defective lymphatic system (Zheng et al. 2003; McGrath et al. 2010). In addition to its role in lymphatic development, Elk3 has also been shown to be necessary for endothelial response to pro-angiogenic growth factors such as FGF2 during wound healing (Ayadi et al. 2001).
While Ets1 knockout mice are viable, Ets2 knockout embryos die around E8.5 due to a defect in extra-embryonic tissue development (Muthusamy et al. 1995; Yamamoto et al. 1998). Double mutant mice of Ets1 and Ets2 that utilize a null allele of Ets1, and either a partial loss of function allele or a conditional deletion of Ets2 within the epiblast, are lethal at approximately E14.5 and display severe hemorrhaging due to a drastic decrease of embryonic vasculature (Wei et al. 2009). While no lung phenotypes have been reported in these mice, it is possible that these Ets genes play redundant roles in the formation of the pulmonary vasculature.
bHLH containing: Tcf21, Hif3a, Id3, and Heyl
We identified four bHLH family transcription factors that were expressed within the lung mesenchyme: Tcf21, Hif3a, Id3 and Heyl (Fig. 2O–R). Of these, Tcf21 and Hif3a have known roles in lung development.
Previous work has shown that Tcf21 (also known as Pod1) is essential for lung branching morphogenesis (Quaggin et al. 1999). Tcf21 knockout mice develop fewer epithelial branches compared to control lungs and these mutant branches do not go on to form terminal sacs. The lack of terminal sacs is likely due to proximalization of the lung epithelium, as evidenced by the expansion of the proximal marker Scgb1a1 and reduction of the distal marker Sftpc. Additionally, Bmp4 expression is decreased within the Tcf21 mutant lung. Previous work has demonstrated that inhibition of BMP signaling results in a similar phenotype as the Tcf21 knockout (Bellusci 1996; Weaver et al. 1999; Sun et al 2008). Thus, Tcf21 may control lung branching and proximal-distal patterning through promoting Bmp4 expression.
Hif3a is a well-known hypoxia induced factor (HIF) that is expressed within the distal region of the lung mesenchyme (Fig. 2P). Hif3a knockout mutants show impaired alveologenesis with an increase in the amount of vasculature lining the alveolar walls (Yamashita et al. 2008). Whether the vasculature defect is the mechanism underlying the alveolar phenotype remains to be determined.
Id3, similar to Id2 mentioned above, is a member of the Id family of genes (Fig. 2Q). Id3 knockout mice are viable and no lung defect has been reported (Pan et al. 1999). The lack of an apparent developmental phenotype is likely due to its redundancy with Id1, which shows overlapping expression patterns with Id3 throughout the embryo, including in the mesenchyme of the lung (Jen et al. 1996). In support of this, Id1;Id3 double knock out mice die by E13.5 and develop severe vascular and neural defects (Lyden et al. 1999). It is not clear whether these mutants exhibit lung defects. A recent study within the postnatal lung shows that Id3 expression is increased in Id1 knockout lungs, further suggesting that these two genes may share redundant function (Lowery et al. 2010). Given that their expression is regulated by BMP signaling (Miyazono and Miyazawa et al. 2002; Ten Dijke et al. 2003), it is possible that together they may mediate the essential role of BMP signaling in lung mesenchyme development.
Heyl is a downstream component of the NOTCH signaling pathway (Nakagawa et al. 2000; Leimeister et al. 2000). As mentioned above, extensive work has demonstrated that the NOTCH signaling pathway regulates lung proximal-distal patterning and cell differentiation (Tsao et al. 2008; Guseh et al. 2009; Tsao et al. 2009; Morimoto et al. 2010). In the lung, Heyl expression is restricted to the vascular smooth muscle (Fig. 2R), likely overlapping with the expression of Notch3, which plays an essential role in vascular smooth muscle differentiation and maturation (Domenga et al. 2004). While knockout mice for Heyl have no reported lung phenotype, Heyl may function together with other components of the Notch signaling pathway during lung vascular smooth muscle development.
FOX domain containing: Foxf1a and Foxf2
Foxf1a (also known as Foxf1) and Foxf2 encode closely related FOX domain containing factors and have very similar patterns preferentially in the subepithelial mesenchyme of the lung (Fig. 2S,T). This expression is positively regulated by SHH signaling and negatively regulated by BMP signaling (Mahlapuu et al. 2001; Lim et al. 2002), both critical signals for lung development. Foxf1a has previously been shown to be essential for lung development in a haploinsufficient manner, since mice carrying one mutant allele (Foxf1a+/−) display lobe fusion, altered epithelial branching, and high incidents of postnatal lethality due to pulmonary hemorrhaging (Mahlapuu et al. 2001; Lim et al. 2002). Tissue-specific inactivation of both copies of Foxf1a in the lung has not been reported. Foxf2 knockout mice have no known lung phenotype and die postnatally due to inability to feed (Wang et al. 2003). Foxf1a and Foxf2 have been shown to act redundantly in the intestine, where they regulate extracellular matrix production through their ability to mediate WNT signaling (Ormestad et al. 2006). Whether Foxf1a and Foxf2 have redundant roles in lung development has not been reported.
Miscellaneous: Lef1, Creb3l1, and Tbx3
Three of the mesenchyme-expressed transcription factors, Lef1, Creb3l1 and Tbx3, were identified in our screen as the sole member of their respective families (Fig. 2U–W). Of these, Lef1 is the only gene implicated in lung development (Okubo et al. 2004).
Lef1
Lef1 is an HMG domain-containing transcription factor, and a downstream component of the WNT signaling pathway. Within the lung, Lef1 expression is higher within the distal mesenchyme, which corresponds to the distal expression of Wnt2 and Wnt7b within the mesenchyme and epithelium respectively (Fig. 2U and Visel et al. 2004). Both canonical (e.g. Wnt2, Wnt2b and Wnt7b) and non-canonical (e.g. Wnt5a) WNT signaling has been shown to control branching morphogenesis, patterning, and differentiation in the developing lung (Li et al. 2002; Li et al. 2005; Cohen et al. 2009; Goss et al. 2009; Goss et al. 2011). Consistent with this, expression of a β-Catenin-Lef1 fusion protein within the lung epithelium leads to constitutive activation of WNT signaling and a change of cell fate of the lung epithelium into the intestinal epithelium (Okubo et al. 2004). Lef1 knockout mice die after birth and before weaning (van Genderen et al. 1994), however no lung defect has been reported. The lack of a lung phenotype in Lef1 knockout mice may result from its overlapping expression with the highly related Tcf1, which is also expressed within the lung mesenchyme (Oosterwegel et al. 1993).
Creb3ll
Creb3l1 (also known as Oasis) is a bZIP (basic leucine zipper) domain containing transcription factor that is tethered to the endoplasmic reticulum (ER) and cleaved in response to ER stress (Murakami et al. 2006). Upon cleavage, the DNA binding domain-containing portion of the protein can be transported to the nucleus where it regulates downstream gene expression. Creb3l1 cleavage can also be regulated by signaling ligands such as BMPs (Murakami et al. 2009). Consistent with this regulation, Creb3l1 knockout mice show decreased bone formation due to reduced secretion of bone matrix proteins. Creb3l1 has also been shown to regulate extracellular matrix production within the pancreas (Vellanki et al. 2010). Creb3l1 mutant mice are viable and no lung defect has been reported (Murakami et al. 2006; Murakami et al. 2009).
Tbx3
Tbx3 is one of the four members of the T-box transcription factor family genes, Tbx2-5 that have been shown to be expressed within the lung mesenchyme (Fig. 2W, Chapman et al. 1996; Li et al. 2004). Their expression is promoted by SHH signaling, which is critical for lung epithelial branching and mesenchymal differentiation (Pepicelli et al. 1998; Li et al. 2004). However, no lung phenotype has been reported in Tbx3 knockout mice (Davenport et al. 2003), potentially due to overlapping expression with Tbx2,4,5.
Category 3-genes expressed in both the epithelium and mesenchyme
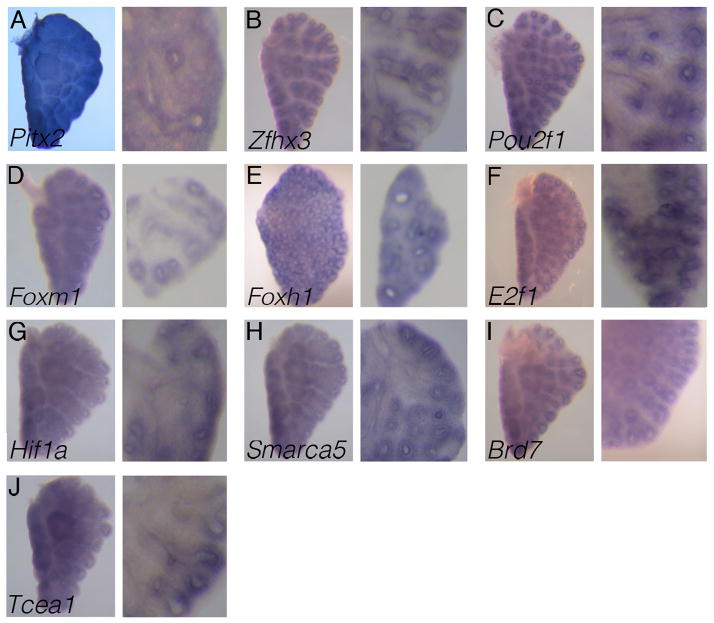
We identified ten transcription factors as being expressed in both the epithelium and the surrounding mesenchyme (Fig. 3A–J). Within this group, there are multiple members of the homeobox and FOX domain containing transcription factors.
Figure 3. Patterns of transcription factor genes with both epithelial and mesenchymal expressions in the developing lung.
(A–J) Ten transcription factor genes were identified by RNA in situ hybridization to be expressed in both the epithelium and the mesenchyme of the E13.5 lung. For each gene, the left panel is a wholemount in situ hybridization of a left lung lobe, and the right panel is an enlarged vibratome section of a right lung lobe. The tissue in each panel is oriented so that the upper left corner and the lower right corner represent the proximal and distal regions of the lung, respectively.
Homeobox domain-containing: Pitx2, Zfhx3, and Pou2f1
Of the three homeobox domain-containing transcription factors found to be expressed in both the epithelium and the mesenchyme (Fig. 3A–C), Pitx2 is the only gene known to be required for normal lung development. Pitx2 knockout mice exhibit a left-right asymmetry defect, which manifests in the transformation of the normally single-lobed left lung into the multi-lobed right lung (Lu et al. 1999; Liu et al. 2001). It remains to be determined whether this phenotype is due to a primary role of Pitx2 in the lung, or an earlier requirement in the node or lateral plate mesenchyme for overall determination of left-right asymmetry. Of note, Pitx2 is expressed in only the left lobe of the E9.5 lung, but it becomes bilaterally expressed throughout the lung epithelium and mesenchyme by E13.5 (Kitamura et al. 1999 and data not shown). Two other Pitx transcription factor genes, Pitx1 and Pitx3, are also expressed in regions of the lung mesenchyme that overlap with the Pitx2 expression domain, suggesting possible redundancy (Fig. 3A; De Langhe et al. 2008). In addition, it has been shown that the expression of both Pitx2 and Pitx3 are positively regulated by WNT signaling in the lung (De Langhe et al. 2008), raising the possibility that Pitx genes may mediate the essential functions of WNT signaling during lung development.
Zfhx3 (also known as Atbf1) shows strong expression in the epithelium and subepithelial mesenchyme of the developing lung (Fig. 3B) (Ido et al. 1996). It has been shown that Zfhx3 promotes Pdgfrsβ expression in P19 mouse embryonic carcinoma cells, protecting these cells from oxidative stress (Kim et al. 2010). Pdgfrsβ is expressed within the subepithelial mesenchyme of the lung, and a recent study suggests that signaling through PDGFRB promotes smooth muscle development in the lung (Cohen et al. 2009). Mice carrying a gene-trap allele of Zfhx3 have been produced, but no lung phenotype has been described (Stryke et al. 2003; Qi et al. 2008).
Pou2f1 (also known as Oct1) is strongly expressed in the lung epithelium, and weakly expressed in the adjacent mesenchyme (Fig. 3C). Pou2f1 knockout embryos are smaller in size, underrepresented after E13.5, and none survive after birth (Wang et al. 2004). No lung phenotype has been reported. However, in lung derived NCI-H441 cells, Pou2f1 has also been shown to directly bind to the promoter of SCGB1A1 (also known as CC10 or CCSP), a key marker for Clara cells (Sawaya et al. 1993). Furthermore, in the lens and nasal placode, Pou2f1 genetically interacts with Sox2 to promote the induction of these tissues (Donner et al. 2007). Sox2 is essential for the differentiation of airway epithelial cells (Gontan et al. 2008; Que et al. 2009; Tompkins et al. 2009). Whether similar interactions occur between Pou2f1 and Sox2 in the lung remains to be determined.
FOX domain containing: Foxm1 and Foxh1
Two FOX domain containing transcription factor genes, Foxm1 and Foxh1 are identified as being expressed in both the epithelium and mesenchyme of the lung (Fig. 3D,E). The expression level of Foxm1 in lung diminishes from E11.0–E18.5, but peaks again during the initiation of alveologenesis in the postnatal lung (Wang et al. 2010). During lung development, Foxm1 promotes blood vessel formation, epithelial cell differentiation, and inhibits arterial smooth muscle formation. Conditional inactivation of Foxm1 in the lung mesenchyme leads to an increase of smooth muscle around the proximal airways and a decrease of pulmonary microvasculature (Kim et al. 2005). Conditional inactivation of Foxm1 in the lung epithelium leads to a decrease in sacculation and a delay in the differentiation of alveolar type I cells (Kalin et al. 2008). Expression of a constitutively active form of Foxm1 within the embryonic lung epithelium results in impaired sacculation and epithelial hyperplasia in the major airways (Wang et al. 2010). These results demonstrate that precise control of Foxm1 level is crucial to multiple processes of lung development.
Similar to the loss of Pitx2, inactivation of Foxh1 in the lateral plate mesoderm leads to left-right asymmetry defects, which includes the transformation of the one-lobe left lung into the four-lobe right lung (Hoodless et al. 2001; Yamamato et al. 2003; Kofron et al. 2004; Von Both et al. 2004). And like in Pitx2 mutants, it is unclear whether this transformation of lung lobes is due to the requirement for Foxh1 in the lung, or at earlier stages in cells essential for left-right determination. In other tissues, Foxh1 has been shown to interact with several signaling pathways that are critical for lung development. For example, in the brain, Foxh1 acts downstream of TGFβ and upstream of retinoic acid signaling (Silvestri et al. 2008). However, whether similar relationships operate within the lung has not been shown.
Miscellaneous: E2f1, Hif1a, Smarca5, Brd7, and Tcea1
Of the five single family member transcription factors expressed in both the epithelium and mesenchyme (Fig. 3F–J), E2f1 and Hif1a have been previously shown to play a role in lung development.
E2f1
E2f1 is one of eight E2f transcription factor genes present in the mouse genome. E2F transcription factors play very diverse roles including the control of cell proliferation, cell death, and cell differentiation (Wang et al. 1998; Fajas et al. 2002; Sharma et al. 2006; Chong et al. 2009). In the lung, E2f1 is more strongly expressed within the distal epithelium and mesenchyme (Fig. 3F). In multiple settings especially that of cancer, E2F transcription factors interact closely with retinoblastoma (RB) and p53 proteins (Udayakumar et al. 2010). Recent studies have identified Rb as an important regulator of cell proliferation and differentiation in lung development and repair, raising the possibility that E2f1 may play a role in these processes through its association with RB (Wikenheiser-Brokamp 2004; Mason-Richie et al. 2008;Simpson et al. 2009). While E2f1 single knockout mice show no lung phenotype, E2f1;E2f3 double knockout mice have simplified alveoli (Tsai et al. 2008). The precise role of these E2f factors in lung development has not been determined.
Hif1a
Like Hif3a described above, Hif1a is a member of the HIF group. It is known to promote vascular growth in response to low oxygen levels (Tian et al. 1997; Semenza et al. 2001a; Semenza et al. 2001b). Knockout mice are lethal by E11.0, due to defective vascular development and cardiac patterning (Iyer et al. 1998; Compernolle et al. 2003). As for its role in the lung, an early report shows that conditional inactivation of Hif1a in the lung epithelium results in lethality shortly after birth due to loss of surfactant production and increased septal wall thickness (Saini et al. 2008). However, this result is challenged by a more recent study, which shows that a similar lung epithelium-specific inactivation of Hif1a permits survival of the mutant mice (Shannon and Young 2011). While the absolute requirement for Hif1a in the lung for perinatal survival awaits further clarification, the importance of a close regulation of Hif1a level in lung is further demonstrated by the findings that overexpression of Hif1a in lung epithelium leads to defects in epithelial branching, epithelium differentiation and vasculature maturation, which resulted in lethality at birth (Bridges et al. 2012).
Smarca5 and Brd7
Two of the transcription factors expressed in the epithelium and mesenchyme, Smarca5 and Brd7 are known chromatin regulators (Fig. 3H,I). SMARCA5 is a SWI/SNF related protein that is highly expressed within the distal epithelium and mesenchyme, while BRD7 is a bromodomain containing protein that acts as a component of the SWI/SNF complex and is expressed more uniformly in the lung (Fig. 3H, I and Stopka et al. 2003; Kaeser et al. 2008). Smarca5 global knockout mice are lethal during the pre-implantation stage of development (Stopka et al. 2003). Smarca5+/− embryonic stem cells or spermatocytes exhibit a global decrease in H3K9Ac and H3K79Me2 levels, indicating an inactive gene expression state (Vargova et al. 2009). These results suggest that the general role of Smarca5 within the embryo is to promote an active chromatin state. No Brd7 knockout mutant has been reported, and the function of either of these genes during lung development awaits to be determined.
Tcea1
Similar to Tcea2 described above, Tcea1 is a transcriptional elongation factor that assists RNA polymerases read through transcriptional blocks (Wind et al. 2000). Tcea1 is strongly expressed in the distal tips of the branches in both the epithelium and mesenchyme (Fig. 3J). Recent work has suggested that Tcea1 boosts RNA polymerase function through regulation of histone modifications (Nagata et al. 2009). Tcea1 knockout mice die prior to E16.5, with defects in the maintenance and differentiation of hematopoietic progenitor cells (Ito 2006). No lung phenotype has been reported, possibly due to redundancy with Tcea2, which is also expressed in the lung epithelium (Fig. 1J).
Category 4-Hox genes
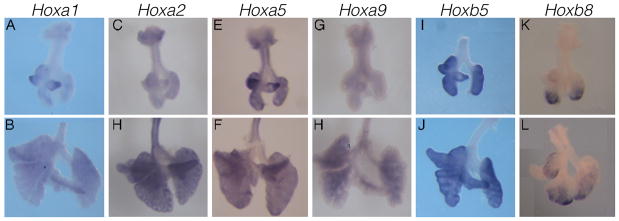
Hox genes encode an evolutionarily conserved family of transcription factors that control pattern formation in animals. Thirty-nine Hox genes are encoded in the mouse genome. Previous results obtained using multiple approaches including Northern blotting, RT-PCR/qRT-PCR and in situ hybridization suggest that as many as twenty Hox genes are expressed in the lung during development (Cardoso 1995; Mollard and Dziadek 1997; Grier et al. 2009). Despite the clear presence of expression, the function of Hox genes during lung development is largely unknown as only a single Hox mutant, Hoxa5, displays primary lung defects (Aubin et al. 1997; Kinkead et al 2004; Mandeville et al. 2006). Hoxa5 mutant mice exhibit respiratory distress and partial lethality at birth, likely due to reduced surfactant production and/or reduced and disorganized tracheal cartilage, which may lead to tracheal occlusion. Hoxa5 mutants that survive postnatally develop impaired alveologenesis. The lack of a lung phenotype in other single Hox mutants suggests that Hox factors may act redundantly. Furthermore, the range of defects observed in Hoxa5 mutants demonstrates that Hox factors regulate a variety of processes over the course of lung development. To elucidate the function of Hox in lung branching morphogenesis, we assessed the expression of fourteen Hox genes (Hoxa1, Hoxa2, Hoxa5, Hoxa9, Hoxb2, Hoxb3, Hoxb5, Hoxb6, Hoxb8, Hoxb9, Hoxc5, Hoxc6, Hoxd4 and Hoxd9) previously noted for their expression in the lung. We identified six genes, Hoxa1, Hoxa2, Hoxa5, Hoxa9, Hoxb5, and Hoxb8 that showed restricted expression patterns in E11.5, and either E12.5 or E13.5 lung and trachea (Fig. 4A–L).
While most of the Hox genes we assayed show similar expression between E11.5 and E13.5, Hoxa1 expression changes over time. At E11.5, similar to at E10.5, Hoxa1 expression is readily detected, particularly in the distal mesenchyme of both the medial and accessory buds (Fig. 4A and data not shown). However, this expression tapers off by E13.5 (Fig. 4B). Progressive loss of Hoxa1 expression is also supported by data from northern blot analysis (Mollard and Dziadek 1997). Northern blot data further indicate that other genes, Hoxa7, Hoxb3, and Hoxb4 also exhibit temporally dynamic expression (Mollard and Dziadek 1997). It should be noted that Hoxa1 mutants develop breathing abnormalities at birth (Lufkin et al. 1991). However, existing evidence has attributed the defect to a requirement for Hoxa1 in patterning the respiratory neuronal circuits in the brain (Chatonnet et al. 2002; Gray 2008).
Different Hox genes are often expressed in distinct cell lineages. For example, Hoxa2, Hoxa5, and Hoxa9 are expressed in the trachea/bronchi region, while Hoxb5 and Hoxb8 are not. At both E11.5 and E13.5, Hoxa2 and Hoxa5 are observed in the trachea and main bronchi in a pattern consistent with cartilage precursors (Fig. 4C–F). Interestingly, while no cartilage defects have been reported in Hoxa2 mutants, Hoxa5 mutants do exhibit tracheal cartilage malformations, suggesting that Hoxa5 may be the primary Hox factor that controls airway cartilage patterning (Aubin et al. 1997). Although Hoxa9 is also expressed in the trachea/bronchi region, its expression is primarily in the epithelium (Fig. 4G,H). The epithelial expression of Hoxa9 continues into the lung, where it is also detected in the lung mesenchyme with a proximal bias. In contrast to this bias, Hoxa2, Hoxa5, and Hoxb5 expression in the lung is more widespread throughout the mesenchyme at E12.5/E13.5 (Fig. 4D,F,J).
A vast majority of the genes that have been studied in the mouse lung show similar expression in the five lung lobes. Hence, it is interesting that several of the Hox genes that we have assayed exhibit biased expression in the different lobes. For example, at E11.5, both Hoxa1 and Hoxa5 show concentrated expression in the distal mesenchyme of the medial and accessory lobes (Fig. 4A,E). At both E11.5 and E12.5, HoxB8 shows concentrated expression in the distal mesenchyme of the left lobe and in the cranial and caudal lobes of the right lung, but is not detected in the right, medial, and accessory lobes (Fig. 4K,L). Whether these transcription factors coordinate the shape and size of the lung lobes remains to be determined. It is important to note that the Hox cluster was first identified as genes that provide segmental identity in Drosophila. In mouse, Hox gene expression often marks particular sub-regions within a tissue lineage such as in the anterior-posterior axial skeleton, the brain and the limb bud. Our results suggest that Hoxa1, Hoxa5 and Hoxb8 can be used as markers of early lobe compartments within the developing lung.
Conclusions
Using a probe collection that represents a majority of the transcription factors encoded in the mouse genome, we identified sixty-two transcription factors that show patterned expression in the pseudoglandular stage mouse lung. Many of the transcription factors that we identified have not been previously implicated in lung development. Furthermore, characterization of the expression pattern of some of these genes, such as Grhl2 and Tbx3, has led to consideration of other members of the gene families and their expression in lung. We envision that our findings will promote future studies by: providing additional genes to be investigated for their roles in lung development; increasing nodes of the transcriptional circuits that act either upstream or downstream of known factors; and suggesting putative markers for specialized cell populations in the lung at later stages of development and maturation.
Going beyond development, our dataset may be of value to lung disease research such as the studies of lung cancer. Recent investigations of lung cancer have revealed strong correlations between patient survival rates and misexpression of a number of the transcription factors highlighted in our screen. For example, increased nuclear localization of ID2 or increased expression of FOXM1, HIF1A, SNAI1 or HOXA9 is associated with higher mortality rates (Choi et al. 2006; Rauch et al. 2007; Gialmanidis et al. 2009; Hung et al. 2009; Rollin et al. 2009). These findings raise the possibility that while the expression of many of these factors is downregulated at the completion of development, they are reactivated in tumor cells. Thus, our dataset may suggest candidate biomarkers that can be used in early lung cancer diagnosis.
Experimental Procedures
Tissue Collection
The embryos used in these experiments were harvested from time-mated females, with noon the day of the vaginal plug counted as E0.5. Dissected lung tissues were fixed in 4% paraformaldehyde for 4 hours to overnight at 4°C. After fixation the right lungs were embedded in 4% low melt agarose and sectioned at 100um thickness using a vibratome. Intact left lung lobes and the right lung sections were dehydrated through a methanol series and stored at −20 °C in 100% methanol until RNA in situ hybridization.
RNA in situ hybridization
RNA in situ probes were generated from a published plasmid collection (Gray 2004). RNA in situ hybridization was performed following a protocol that was adapted from previously published protocols (Wall and Hogan 1995; Abler et al. 2011). Briefly, either wholemount of vibratome-sectioned tissues stored in methanol were rehydrated using PBS with 0.1% Tween (PBT). Tissues were then permeabilized using 10μg/ml proteinase K in PBT for 10 minutes. Tissues were fixed with 4% paraformaldehyde, 0.2% glutaraldehyde in PBT for 20 minutes. They were then washed and allowed to hybridize with probes at 70°C overnight. After the hybridization, tissues were washed three times, twenty minutes each in 2x SSC, 0.1% CHAPS, and then in 0.2x SSC, 0.1% CHAPS at 70°C. Tissues were then washed in KTBT and blocked for 2 hours in 2% blocking solution (Roche) and 20% heat inactivated sheep serum (Sigma) in KTBT at room temperature. The tissues were then incubated overnight at 4°C in the primary antibody, AP conjugated α-DIG, Fab fragment (Roche) at a dilution of 1:2000 using 2% blocking solution and 20% heat inactivated sheep serum in KTBT. The tissues were then washed 5 times, 1hour each in KTBT at room temperature. Following this, the tissues were washed twice, 5 minutes each with 0.1% Tween, 1mM Levamisole in ddH2O. Finally the tissues were stained in BM purple (Roche) containing 0.1% Tween and 1mM levamisole. The staining was carried out for varying amount of time depending on the probes until desired intensity, from 30 minutes to 8 hours.
Acknowledgments
Grant information: Burroughs-Wellcome career award #1002361, American Heart grant #0950041G, March of Dimes grant 6-FY10-339 and NHLBI HL113870 (to X.S.); NSFC grant 31028012 (to X.S. and G.X.); American Heart predoctoral fellowship # 11PRE5540006 (to J.C.H); American Heart predoctoral fellowship # 0610087Z (to L.Y.); and National Science Foundation predoctoral fellowship # 2011101268 (to E.A.H.). Parker B. Francis Fellowship in Pulmonary Medicine (to PAG).
We would like to thank members of the Sun lab for insightful discussions and critical readings of the manuscript. Special thanks to Amber Lashua for technical assistance. This work was supported by Burroughs-Welcome career award #1002361, American Heart grant #0950041G, March of Dimes grant 6-FY10-339 and NHLBI HL113870 (to X.S.); NSFC grant 31028012 (to X.S. and G.X.); American Heart predoctoral fellowship # 11PRE5540006 (to J.C.H); American Heart predoctoral fellowship # 0610087Z (to L.Y.) and National Science Foundation predoctoral fellowship # 2011101268 (to E.A.H).
References
- Abler LL, Mehta V, Keil KP, Joshi PS, Flucus CL, Hardin HA, Schmitz CT, Vezina CM. A high throughput in situ hybridization method to characterize mRNA expression patterns in the fetal mouse lower urogenital tract. J Vis Exp. 2011;(54):e2912. doi: 10.3791/2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieux M, Tremblay M, Bérard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Auden A, Caddy J, Wilanowski T, Ting SB, Cunningham JM, Jane SM. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns. 2006;6:964–970. doi: 10.1016/j.modgep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Ayadi A, Zheng H, Sobieszczuk P, Buchwalter G, Moerman P, Alitalo K, Wasylyk B. Net-targeted mutant mice develop a vascular phenotype and up-regulate egr-1. EMBO J. 2001;20:5139–5152. doi: 10.1093/emboj/20.18.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- Becker MB, Zülch A, Bosse A, Gruss P. Irx1 and Irx2 expression in early lung development. Mech Dev. 2001;106:155–158. doi: 10.1016/s0925-4773(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Scherer SS, O’Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct-6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Shumas S, Whisenhunt T, Sirkowski EE, O’Connell S, Scherer SS, Rosenfeld MG. Identification of genes that are downregulated in the absence of the POU domain transcription factor pou3f1 (Oct-6, Tst-1, SCIP) in sciatic nerve. J Neurosci. 2002;22:10217–10231. doi: 10.1523/JNEUROSCI.22-23-10217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, Hislop NR, Cangkrama M, Ting SB, Jane SM. The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol. 2011;349:512–522. doi: 10.1016/j.ydbio.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Bosse A, Zülch A, Becker MB, Torres M, Gómez-Skarmeta JL, Modolell J, Gruss P. Identification of the vertebrate Iroquois homeobox gene family with overlapping expression during early development of the nervous system. Mech Dev. 1997;69:169–181. doi: 10.1016/s0925-4773(97)00165-2. [DOI] [PubMed] [Google Scholar]
- Bridges JP, Lin S, Ikegami M, Shannon JM. Conditional hypoxia inducible factor-1α induction in embryonic pulmonary epithelium impairs maturation and augments lymphangiogenesis. Dev Biol. 2012;362:24–41. doi: 10.1016/j.ydbio.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV. Transcription factors and pattern formation in the developing lung. Am J Physiol. 1995;269:L429–442. doi: 10.1152/ajplung.1995.269.4.L429. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Whitsett JA. Resident cellular components of the lung: developmental aspects. Proc Am Thorac Soc. 2008;5:767–771. doi: 10.1513/pats.200803-026HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermenati S, Moleri S, Cimbro S, Corti P, Del Giacco L, Amodeo R, Dejana E, Koopman P, Cotelli F, Beltrame M. Sox18 and Sox7 play redundant roles in vascular development. Blood. 2008;111:2657–2666. doi: 10.1182/blood-2007-07-100412. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Thoby-Brisson M, Abadie V, Domínguez del Toro E, Champagnat J, Fortin G. Early development of respiratory rhythm generation in mouse and chick. Respir Physiol Neurobiol. 2002;131:5–13. doi: 10.1016/s1569-9048(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, Nakahira K, Pilewski JM, Lee JS, Zhang Y, Ryter SW, Choi AM. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Zheng LT, Ha E, Lim YJ, Kim YH, Wang YP, Lim Y. Comparative genomic hybridization array analysis and real-time PCR reveals genomic copy number alteration for lung adenocarcinomas. Lung. 2006;184:355–362. doi: 10.1007/s00408-006-0009-0. [DOI] [PubMed] [Google Scholar]
- Chokas AL, Trivedi CM, Lu MM, Tucker PW, Li S, Epstein JA, Morrisey EE. Foxp1/2/4-NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin-6. J Biol Chem. 2010;285:13304–13313. doi: 10.1074/jbc.M109.088468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Wenzel PL, Sáenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, Wang SH, Trikha P, Culp B, Mezache L, Winton DJ, Sansom OJ, Chen D, Bremner R, Cantalupo PG, Robinson ML, Pipas JM, Leone G. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Keijser AG, Houweling AC, Clout DE, Moorman AF. Patterning the embryonic heart: identification of five mouse Iroquois homeobox genes in the developing heart. Dev Biol. 2000;224:263–274. doi: 10.1006/dbio.2000.9801. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Thépot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- Cohen DR, Cheng CW, Cheng SH, Hui CC. Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech Dev. 2000;91:317–321. doi: 10.1016/s0925-4773(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P. Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003;60:569–579. doi: 10.1016/j.cardiores.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–388. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Sharpe PT, Pachnis V. The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev Biol. 2009;333:324–336. doi: 10.1016/j.ydbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazières A, Decker L, Vallat JM, Charnay P, Gilardi-Hebenstreit P. Disruption of Krox20-Nab interaction in the mouse leads to peripheral neuropathy with biphasic evolution. J Neurosci. 2008;28:5891–5900. doi: 10.1523/JNEUROSCI.5187-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, Lin-Marq N, Koch M, Bilio M, Cantiello I, Verde R, De Masi C, Bianchi SA, Cicchini J, Perroud E, Mehmeti S, Dagand E, Schrinner S, Nürnberger A, Schmidt K, Metz K, Zwingmann C, Brieske N, Springer C, Hernandez AM, Herzog S, Grabbe F, Sieverding C, Fischer B, Schrader K, Brockmeyer M, Dettmer S, Helbig C, Alunni V, Battaini MA, Mura C, Henrichsen CN, Garcia-Lopez R, Echevarria D, Puelles E, Garcia-Calero E, Kruse S, Uhr M, Kauck C, Feng G, Milyaev N, Ong CK, Kumar L, Lam M, Semple CA, Gyenesei A, Mundlos S, Radelof U, Lehrach H, Sarmientos P, Reymond A, Davidson DR, Dollé P, Antonarakis SE, Yaspo ML, Martinez S, Baldock RA, Eichele G, Ballabio A. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev Biol. 2007;303:784–799. doi: 10.1016/j.ydbio.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TL, Mynett-Johnson L, Wright EM, Hosking BM, Koopman PA, Muscat GE. Sequence and expression of Sox-18 encoding a new HMG-box transcription factor. Gene. 1995;161:223–225. doi: 10.1016/0378-1119(95)00280-j. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Elluru RG, Whitsett JA. Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Arch Otolaryngol Head Neck Surg. 2004;130:732–736. doi: 10.1001/archotol.130.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and beta-catenin signaling. Cereb Cortex. 2008;18:2117–2131. doi: 10.1093/cercor/bhm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Landsberg RL, Huss-Garcia Y, Sardet C, Lees JA, Auwerx J. E2Fs regulate adipocyte differentiation. Dev Cell. 2002;3:39–49. doi: 10.1016/s1534-5807(02)00190-9. [DOI] [PubMed] [Google Scholar]
- Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, Knobeloch KP, Gessler M. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ Res. 2007;100:856–863. doi: 10.1161/01.RES.0000260913.95642.3b. [DOI] [PubMed] [Google Scholar]
- Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- Francois M, Koopman P, Beltrame M. SoxF genes: Key players in the development of the cardio-vascular system. Int J Biochem Cell Biol. 2010;42:445–448. doi: 10.1016/j.biocel.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Ghazvini M, Mandemakers W, Jaegle M, Piirsoo M, Driegen S, Koutsourakis M, Smit X, Grosveld F, Meijer D. A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J. 2002;21:4612–4620. doi: 10.1093/emboj/cdf475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialmanidis IP, Bravou V, Amanetopoulou SG, Varakis J, Kourea H, Papadaki H. Overexpression of hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. 2009;66:64–74. doi: 10.1016/j.lungcan.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317:296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Grier DG, Thompson A, Lappin TR, Halliday HL. Quantification of Hox and surfactant protein-B transcription during murine lung development. Neonatology. 2009;96:50–60. doi: 10.1159/000201739. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallaq H, Pinter E, Enciso J, McGrath J, Zeiss C, Brueckner M, Madri J, Jacobs HC, Wilson CM, Vasavada H, Jiang X, Bogue CW. A null mutation of Hhex results in abnormal cardiac development, defective vasculogenesis and elevated Vegfa levels. Development. 2004;131:5197–5209. doi: 10.1242/dev.01393. [DOI] [PubMed] [Google Scholar]
- Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat Genet. 1999;23:71–75. doi: 10.1038/12674. [DOI] [PubMed] [Google Scholar]
- Hisa T, Spence SE, Rachel RA, Fujita M, Nakamura T, Ward JM, Devor-Henneman DE, Saiki Y, Kutsuna H, Tessarollo L, Jenkins NA, Copeland NG. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J. 2004;23:450–459. doi: 10.1038/sj.emboj.7600038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless PA, Pye M, Chazaud C, Labbé E, Attisano L, Rossant J, Wrana JL. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking B, François M, Wilhelm D, Orsenigo F, Caprini A, Svingen T, Tutt D, Davidson T, Browne C, Dejana E, Koopman P. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development. 2009;136:2385–2391. doi: 10.1242/dev.034827. [DOI] [PubMed] [Google Scholar]
- Houweling AC, Dildrop R, Peters T, Mummenhoff J, Moorman AF, Rüther U, Christoffels VM. Gene and cluster-specific expression of the Iroquois family members during mouse development. Mech Dev. 2001;107:169–174. doi: 10.1016/s0925-4773(01)00451-8. [DOI] [PubMed] [Google Scholar]
- Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082–1089. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- Ido A, Miura Y, Watanabe M, Sakai M, Inoue Y, Miki T, Hashimoto T, Morinaga T, Nishi S, Tamaoki T. Cloning of the cDNA encoding the mouse ATBF1 transcription factor. Gene. 1996;168:227–231. doi: 10.1016/0378-1119(95)00740-7. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, Murray JC, Schutte BC. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Arimitsu N, Takeuchi M, Kawamura N, Nagata M, Saso K, Akimitsu N, Hamamoto H, Natori S, Miyajima A, Sekimizu K. Transcription elongation factor S-II is required for definitive hematopoiesis. Mol Cell Biol. 2006;26:3194–3203. doi: 10.1128/MCB.26.8.3194-3203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran A, Königshoff M, Yu H, Rupniewska E, Hecker M, Klepetko W, Seeger W, Eickelberg O. SNAI transcription factors mediate epithelial-mesenchymal transition in lung fibrosis. Thorax. 2009;64:1053–1061. doi: 10.1136/thx.2009.121798. [DOI] [PubMed] [Google Scholar]
- Jen Y, Manova K, Benezra R. Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn. 1996;207:235–252. doi: 10.1002/(SICI)1097-0177(199611)207:3<235::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kaeser MD, Aslanian A, Dong MQ, Yates JR, Emerson BM. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. J Biol Chem. 2008;283:32254–32263. doi: 10.1074/jbc.M806061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Lee KH, Schlöndorff J, Hiemisch H, Monaghan AP, Schütz G. Six members of the mouse forkhead gene family are developmentally regulated. Proc Natl Acad Sci U S A. 1993;90:7628–7631. doi: 10.1073/pnas.90.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin TV, Wang IC, Meliton L, Zhang Y, Wert SE, Ren X, Snyder J, Bell SM, Graf L, Whitsett JA, Kalinichenko VV. Forkhead Box m1 transcription factor is required for perinatal lung function. Proc Natl Acad Sci U S A. 2008;105:19330–19335. doi: 10.1073/pnas.0806748105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development. 2001;128:503–511. doi: 10.1242/dev.128.4.503. [DOI] [PubMed] [Google Scholar]
- Keng VW, Yagi H, Ikawa M, Nagano T, Myint Z, Yamada K, Tanaka T, Sato A, Muramatsu I, Okabe M, Sato M, Noguchi T. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem Biophys Res Commun. 2000;276:1155–1161. doi: 10.1006/bbrc.2000.3548. [DOI] [PubMed] [Google Scholar]
- Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem. 2005b;280:22278–22286. doi: 10.1074/jbc.M500936200. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Kawaguchi M, Suzuki M, Jung CG, Asai K, Shibamoto Y, Lavin MF, Khanna KK, Miura Y. The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress. Dis Model Mech. 2010;3:752–762. doi: 10.1242/dmm.004689. [DOI] [PubMed] [Google Scholar]
- Kinkead R, LeBlanc M, Gulemetova R, Lalancette-Hébert M, Lemieux M, Mandeville I, Jeannotte L. Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa5 gene function. Pediatr Res. 2004;56:553–562. doi: 10.1203/01.PDR.0000139427.26083.3D. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kofron M, Puck H, Standley H, Wylie C, Old R, Whitman M, Heasman J. New roles for FoxH1 in patterning the early embryo. Development. 2004;131:5065–5078. doi: 10.1242/dev.01396. [DOI] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, McDonald-McGinn DM, Zackai EH, Lammer EJ, Aylsworth AS, Ardinger HH, Lidral AC, Pober BR, Moreno L, Arcos-Burgos M, Valencia C, Houdayer C, Bahuau M, Moretti-Ferreira D, Richieri-Costa A, Dixon MJ, Murray JC. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Reynolds DM, Shevchenko A, Goldstone SD, Dalton S. Forkhead transcription factors, Fkh1p and Fkh2p, collaborate with Mcm1p to control transcription required for M-phase. Curr Biol. 2000;10:896–906. doi: 10.1016/s0960-9822(00)00618-7. [DOI] [PubMed] [Google Scholar]
- Lazarus A, Del-Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J. Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci. 2005;8:932–940. doi: 10.1038/nn1490. [DOI] [PubMed] [Google Scholar]
- Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasingham V, Zhang X, Cohen DR, Husain M, Cheng SH, Bruneau BG, Hui CC. The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol Cell Biol. 2003;23:8216–8225. doi: 10.1128/MCB.23.22.8216-8225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister C, Schumacher N, Steidl C, Gessler M. Analysis of HeyL expression in wild-type and Notch pathway mutant mouse embryos. Mech Dev. 2000;98:175–178. doi: 10.1016/s0925-4773(00)00459-7. [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li H, Edlund H. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev Biol. 2001;240:247–253. doi: 10.1006/dbio.2001.0440. [DOI] [PubMed] [Google Scholar]
- Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–231. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Lim L, Kalinichenko VV, Whitsett JA, Costa RH. Fusion of lung lobes and vessels in mouse embryos heterozygous for the forkhead box f1 targeted allele. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1012–1022. doi: 10.1152/ajplung.00371.2001. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Liu W, Lu MF, Brown NA, Martin JF. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- Liu C, Morrisey EE, Whitsett JA. GATA-6 is required for maturation of the lung in late gestation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L468–475. doi: 10.1152/ajplung.00044.2002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hogan BL. Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns. 2002;2:229–233. doi: 10.1016/s1567-133x(02)00057-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang H, Crawford HC, Hogan BL. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev Biol. 2003;261:10–24. doi: 10.1016/s0012-1606(03)00359-2. [DOI] [PubMed] [Google Scholar]
- Lowery JW, Frump AL, Anderson L, DiCarlo GE, Jones MT, de Caestecker MP. ID family protein expression and regulation in hypoxic pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1463–1477. doi: 10.1152/ajpregu.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Lu MM, Li S, Yang H, Morrisey EE. Foxp4: a novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Mech Dev. 2002;119(Suppl 1):S197–202. doi: 10.1016/s0925-4773(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Lu X, Meng X, Morris CA, Keating MT. A novel human gene, WSTF, is deleted in Williams syndrome. Genomics. 1998;54:241–249. doi: 10.1006/geno.1998.5578. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Dierich A, LeMeur M, Mark M, Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991;66:1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Lü J, Izvolsky KI, Qian J, Cardoso WV. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. J Biol Chem. 2005;280:4834–4841. doi: 10.1074/jbc.M410714200. [DOI] [PubMed] [Google Scholar]
- Lü J, Qian J, Izvolsky KI, Cardoso WV. Global analysis of genes differentially expressed in branching and non-branching regions of the mouse embryonic lung. Dev Biol. 2004;273:418–435. doi: 10.1016/j.ydbio.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- Mandeville I, Aubin J, LeBlanc M, Lalancette-Hébert M, Janelle MF, Tremblay GM, Jeannotte L. Impact of the loss of Hoxa5 function on lung alveogenesis. Am J Pathol. 2006;169:1312–1327. doi: 10.2353/ajpath.2006.051333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBPalpha is required for lung maturation at birth. Development. 2006;133:1155–1164. doi: 10.1242/dev.02273. [DOI] [PubMed] [Google Scholar]
- Mason-Richie NA, Mistry MJ, Gettler CA, Elayyadi A, Wikenheiser-Brokamp KA. Retinoblastoma function is essential for establishing lung epithelial quiescence after injury. Cancer Res. 2008;68:4068–4076. doi: 10.1158/0008-5472.CAN-07-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis C, Oury F, Remacle S, Lampe X, Gofflot F, Picard JJ, Rijli FM, Rezsohazy R. Identification of Lmo1 as part of a Hox-dependent regulatory network for hindbrain patterning. Dev Dyn. 2007;236:2675–2684. doi: 10.1002/dvdy.21266. [DOI] [PubMed] [Google Scholar]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104:1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Metzger DE, Stahlman MT, Shannon JM. Misexpression of ELF5 disrupts lung branching and inhibits epithelial differentiation. Dev Biol. 2008a;320:149–160. doi: 10.1016/j.ydbio.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DE, Xu Y, Shannon JM. Elf5 is an epithelium-specific, fibroblast growth factor-sensitive transcription factor in the embryonic lung. Dev Dyn. 2007;236:1175–1192. doi: 10.1002/dvdy.21133. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008b;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Hamdan H, Bu D, Warburton D, Stepanik P, deLemos R. TTF-1 regulates lung epithelial morphogenesis. Dev Biol. 1995;172:694–698. doi: 10.1006/dbio.1995.8080. [DOI] [PubMed] [Google Scholar]
- Minoo P, Hu L, Xing Y, Zhu NL, Chen H, Li M, Borok Z, Li C. Physical and functional interactions between homeodomain NKX2.1 and winged helix/forkhead FOXA1 in lung epithelial cells. Mol Cell Biol. 2007;27:2155–2165. doi: 10.1128/MCB.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291:193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Mohan B, Mittal CM. Supravalvular aortic stenosis in William’s syndrome. Ann Pediatr Cardiol. 2011;4:213–214. doi: 10.4103/0974-2069.84662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard R, Dziadek M. Homeobox genes from clusters A and B demonstrate characteristics of temporal colinearity and differential restrictions in spatial expression domains in the branching mouse lung. Int J Dev Biol. 1997;41:655–666. [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama J, Heng H, Crackower MA, Takabatake T, Takeshima K, Tsui LC, Hui C. Overlapping and non-overlapping Ptch2 expression with Shh during mouse embryogenesis. Mech Dev. 1998;78:81–84. doi: 10.1016/s0925-4773(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn. 2008;237:2108–2116. doi: 10.1002/dvdy.21633. [DOI] [PubMed] [Google Scholar]
- Murakami T, Kondo S, Ogata M, Kanemoto S, Saito A, Wanaka A, Imaizumi K. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006;96:1090–1100. doi: 10.1111/j.1471-4159.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–642. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- Nagata M, Ito T, Arimitsu N, Koyama H, Sekimizu K. Transcription arrest relief by S-II/TFIIS during gene expression in erythroblast differentiation. Genes Cells. 2009;14:371–380. doi: 10.1111/j.1365-2443.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, McFadden DG, Nakagawa M, Yanagisawa H, Hu T, Srivastava D, Olson EN. Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc Natl Acad Sci U S A. 2000;97:13655–13660. doi: 10.1073/pnas.250485597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118:439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- Ott EB, van den Akker NM, Sakalis PA, Gittenberger-de Groot AC, Te Velthuis AJ, Bagowski CP. The lim domain only protein 7 is important in zebrafish heart development. Dev Dyn. 2008;237:3940–3952. doi: 10.1002/dvdy.21807. [DOI] [PubMed] [Google Scholar]
- Oya H, Yokoyama A, Yamaoka I, Fujiki R, Yonezawa M, Youn MY, Takada I, Kato S, Kitagawa H. Phosphorylation of Williams syndrome transcription factor by MAPK induces a switching between two distinct chromatin remodeling complexes. J Biol Chem. 2009;284:32472–32482. doi: 10.1074/jbc.M109.009738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19:5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006a;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006b;294:192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Peoples RJ, Cisco MJ, Kaplan P, Francke U. Identification of the WBSCR9 gene, encoding a novel transcriptional regulator, in the Williams-Beuren syndrome deletion at 7q11.23. Cytogenet Cell Genet. 1998;82:238–246. doi: 10.1159/000015110. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Ranish JA, Zhu X, Krones A, Zhang J, Aebersold R, Rose DW, Rosenfeld MG, Carrière C. Atbf1 is required for the Pit1 gene early activation. Proc Natl Acad Sci U S A. 2008;105:2481–2486. doi: 10.1073/pnas.0712196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Pereira FA, DeMayo FJ, Lydon JP, Tsai SY, Tsai MJ. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11:1925–1937. doi: 10.1101/gad.11.15.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampalli S, Li L, Mak E, Ge K, Brand M, Tapscott SJ, Dilworth FJ. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol. 2007;14:1150–1156. doi: 10.1038/nsmb1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch T, Wang Z, Zhang X, Zhong X, Wu X, Lau SK, Kernstine KH, Riggs AD, Pfeifer GP. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc Natl Acad Sci U S A. 2007;104:5527–5532. doi: 10.1073/pnas.0701059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Reines D, Conaway JW, Conaway RC. The RNA polymerase II general elongation factors. Trends Biochem Sci. 1996;21:351–355. [PMC free article] [PubMed] [Google Scholar]
- Restivo G, Nguyen BC, Dziunycz P, Ristorcelli E, Ryan RJ, Özuysal Ö, Di Piazza M, Radtke F, Dixon MJ, Hofbauer GF, Lefort K, Dotto GP. IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. EMBO J. 2011;30:4571–4585. doi: 10.1038/emboj.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riby DM, Porter MA. Williams syndrome. Adv Child Dev Behav. 2010;39:163–209. doi: 10.1016/b978-0-12-374748-8.00005-6. [DOI] [PubMed] [Google Scholar]
- Rifat Y, Parekh V, Wilanowski T, Hislop NR, Auden A, Ting SB, Cunningham JM, Jane SM. Regional neural tube closure defined by the Grainy head-like transcription factors. Dev Biol. 2010;345:237–245. doi: 10.1016/j.ydbio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin J, Bléchet C, Régina S, Tenenhaus A, Guyétant S, Gidrol X. The intracellular localization of ID2 expression has a predictive value in non small cell lung cancer. PLoS One. 2009;4:e4158. doi: 10.1371/journal.pone.0004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini Y, Harkema JR, LaPres JJ. HIF1alpha is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J Biol Chem. 2008;283:33650–33657. doi: 10.1074/jbc.M805927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Sawaya PL, Stripp BR, Whitsett JA, Luse DS. The lung-specific CC10 gene is regulated by transcription factors from the AP-1, octamer, and hepatocyte nuclear factor 3 families. Mol Cell Biol. 1993;13:3860–3871. doi: 10.1128/mcb.13.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001a;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 2001b;49:614–617. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Young LR. Deletion Of HIF-1Alpha From Mouse Lung Epithelial Cells Exacerbates Bleomycin-Induced Lung Injury. Am J Respir Crit Care Med. 2011:A5984. [Google Scholar]
- Sharma N, Timmers C, Trikha P, Saavedra HI, Obery A, Leone G. Control of the p53-p21CIP1 Axis by E2f1, E2f2, and E2f3 is essential for G1/S progression and cellular transformation. J Biol Chem. 2006;281:36124–36131. doi: 10.1074/jbc.M604152200. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Narimatsu M, von Both I, Liu Y, Tan NB, Izzi L, McCaffery P, Wrana JL, Attisano L. Genome-wide identification of Smad/Foxh1 targets reveals a role for Foxh1 in retinoic acid regulation and forebrain development. Dev Cell. 2008;14:411–423. doi: 10.1016/j.devcel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Simpson DS, Mason-Richie NA, Gettler CA, Wikenheiser-Brokamp KA. Retinoblastoma family proteins have distinct functions in pulmonary epithelial cells in vivo critical for suppressing cell growth and tumorigenesis. Cancer Res. 2009;69:8733–8741. doi: 10.1158/0008-5472.CAN-09-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ. Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med. 2010;181:838–850. doi: 10.1164/rccm.200904-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MM, Lee JS, O’Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13:852–854. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Huang L, Tan CC, Huang F, Zhou DD, Yang J, Gelb BD, Epstein JA. Ash2l interacts with Tbx1 and is required during early embryogenesis. Exp Biol Med (Maywood) 2010;235:569–576. doi: 10.1258/ebm.2010.009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. Proc Natl Acad Sci U S A. 2003;100:14097–14102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryke D, Kawamoto M, Huang CC, Johns SJ, King LA, Harper CA, Meng EC, Lee RE, Yee A, L’Italien L, Chuang PT, Young SG, Skarnes WC, Babbitt PC, Ferrin TE. BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res. 2003;31:278–281. doi: 10.1093/nar/gkg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum EY, O’Reilly LA, Jonas N, Lindeman GJ, Visvader JE. The LIM domain protein Lmo4 is highly expressed in proliferating mouse epithelial tissues. J Histochem Cytochem. 2005;53:475–486. doi: 10.1369/jhc.4A6553.2005. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, Milbrandt J. Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Mol Cell Biol. 1998;18:512–524. doi: 10.1128/mcb.18.1.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin OJ, Kinzel D, Cox BJ, Bell CE, Rossant J, Lickert H. Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genomics. 2008;9:511. doi: 10.1186/1471-2164-9-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CC, Walsh MJ, Gelb BD. Fgfr3 is a transcriptional target of Ap2delta and Ash2l-containing histone methyltransferase complexes. PLoS One. 2009;4:e8535. doi: 10.1371/journal.pone.0008535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Tian J, Mahmood R, Hnasko R, Locker J. Loss of Nkx2.8 deregulates progenitor cells in the large airways and leads to dysplasia. Cancer Res. 2006;66:10399–10407. doi: 10.1158/0008-5472.CAN-06-1564. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, Morrisey EE. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One. 2009;4:e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, Feria-Arias E, Timmers C, Opavska J, de Bruin A, Chong JL, Trikha P, Fernandez SA, Stromberg P, Rosol TJ, Leone G. Mouse development with a single E2F activator. Nature. 2008;454:1137–1141. doi: 10.1038/nature07066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse E, Smith AJ, Hunt S, Lavenir I, Forster A, Warren AJ, Grutz G, Foroni L, Carlton MB, Colledge WH, Boehm T, Rabbitts TH. Null mutation of the Lmo4 gene or a combined null mutation of the Lmo1/Lmo3 genes causes perinatal lethality, and Lmo4 controls neural tube development in mice. Mol Cell Biol. 2004;24:2063–2073. doi: 10.1128/MCB.24.5.2063-2073.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BS, Cavin ST, Booth FW, Olson EN, Marin MC, McDonnell TJ, Butler IJ. Pulmonary hypoplasia in the myogenin null mouse embryo. Am J Respir Cell Mol Biol. 2000;22:304–315. doi: 10.1165/ajrcmb.22.3.3708. [DOI] [PubMed] [Google Scholar]
- Udayakumar T, Shareef MM, Diaz DA, Ahmed MM, Pollack A. The E2F1/Rb and p53/MDM2 pathways in DNA repair and apoptosis: understanding the crosstalk to develop novel strategies for prostate cancer radiotherapy. Semin Radiat Oncol. 2010;20:258–266. doi: 10.1016/j.semradonc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Umehara T, Kida S, Hasegawa S, Fujimoto H, Horikoshi M. Restricted expression of a member of the transcription elongation factor S-II family in testicular germ cells during and after meiosis. J Biochem. 1997;121:598–603. doi: 10.1093/oxfordjournals.jbchem.a021627. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Fariñas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- van Tuyl M, Liu J, Groenman F, Ridsdale R, Han RN, Venkatesh V, Tibboel D, Post M. Iroquois genes influence proximo-distal morphogenesis during rat lung development. Am J Physiol Lung Cell Mol Physiol. 2006;290:L777–L789. doi: 10.1152/ajplung.00293.2005. [DOI] [PubMed] [Google Scholar]
- Vargova J, Vargova K, Skoultchi AI, Stopka T. Nuclear localization of ISWI ATPase Smarca5 (Snf2h) in mouse. Front Biosci (Elite Ed) 2009;1:553–559. doi: 10.2741/e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellanki RN, Zhang L, Guney MA, Rocheleau JV, Gannon M, Volchuk A. OASIS/CREB3L1 induces expression of genes involved in extracellular matrix production but not classical endoplasmic reticulum stress response genes in pancreatic beta-cells. Endocrinology. 2010;151:4146–4157. doi: 10.1210/en.2010-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdeguer F, Le Corre S, Fischer E, Callens C, Garbay S, Doyen A, Igarashi P, Terzi F, Pontoglio M. A mitotic transcriptional switch in polycystic kidney disease. Nat Med. 2010;16:106–110. doi: 10.1038/nm.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Both I, Silvestri C, Erdemir T, Lickert H, Walls JR, Henkelman RM, Rossant J, Harvey RP, Attisano L, Wrana JL. Foxh1 is essential for development of the anterior heart field. Dev Cell. 2004;7:331–345. doi: 10.1016/j.devcel.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Wall NA, Hogan BL. Expression of bone morphogenetic protein-4 (BMP-4), bone morphogenetic protein-7 (BMP-7), fibroblast growth factor-8 (FGF-8) and sonic hedgehog (SHH) during branchial arch development in the chick. Mech Dev. 1995;53:383–392. doi: 10.1016/0925-4773(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004a;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A. 2004b;101:14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, Park HJ, Whitsett JA, Kalinichenko VV. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol. 2010;347:301–314. doi: 10.1016/j.ydbio.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Tamakoshi T, Uezato T, Shu F, Kanzaki-Kato N, Fu Y, Koseki H, Yoshida N, Sugiyama T, Miura N. Forkhead transcription factor Foxf2 (LUN)-deficient mice exhibit abnormal development of secondary palate. Dev Biol. 2003;259:83–94. doi: 10.1016/s0012-1606(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Wang VE, Schmidt T, Chen J, Sharp PA, Tantin D. Embryonic lethality, decreased erythropoiesis, and defective octamer-dependent promoter activation in Oct-1-deficient mice. Mol Cell Biol. 2004;24:1022–1032. doi: 10.1128/MCB.24.3.1022-1032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pasolli HA, Williams T, Fuchs E. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol. 2008;183:37–48. doi: 10.1083/jcb.200804030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Yang H, Livingston DM. Endogenous E2F-1 promotes timely G0 exit of resting mouse embryo fibroblasts. Proc Natl Acad Sci U S A. 1998;95:15583–15586. doi: 10.1073/pnas.95.26.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, Muthusamy N, Man AK, Oshima RG, Leone G, Ostrowski MC. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood. 2009;114:1123–1130. doi: 10.1182/blood-2009-03-211391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development. 1998;125:3615–3623. doi: 10.1242/dev.125.18.3615. [DOI] [PubMed] [Google Scholar]
- Werling U, Schorle H. Transcription factor gene AP-2 gamma essential for early murine development. Mol Cell Biol. 2002;22:3149–3156. doi: 10.1128/MCB.22.9.3149-3156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth M, Walentin K, Aue A, Schönheit J, Wuebken A, Pode-Shakked N, Vilianovitch L, Erdmann B, Dekel B, Bader M, Barasch J, Rosenbauer F, Luft FC, Schmidt-Ott KM. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development. 2010;137:3835–3845. doi: 10.1242/dev.055483. [DOI] [PubMed] [Google Scholar]
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Matsuzaki Y. Transcriptional regulation of perinatal lung maturation. Pediatr Clin North Am. 2006;53:873–887. viii. doi: 10.1016/j.pcl.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development. 2004;131:4299–4310. doi: 10.1242/dev.01232. [DOI] [PubMed] [Google Scholar]
- Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- Wind M, Reines D. Transcription elongation factor SII. Bioessays. 2000;22:327–336. doi: 10.1002/(SICI)1521-1878(200004)22:4<327::AID-BIES3>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger Q, Huang J, Auman HJ, Lewandoski M, Williams T. Analysis of transcription factor AP-2 expression and function during mouse preimplantation development. Biol Reprod. 2006;75:324–333. doi: 10.1095/biolreprod.106.052407. [DOI] [PubMed] [Google Scholar]
- Xu B, Qu X, Gu S, Doughman YQ, Watanabe M, Dunwoodie SL, Yang YC. Cited2 is required for fetal lung maturation. Dev Biol. 2008;317:95–105. doi: 10.1016/j.ydbio.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, Maki RA, Werb Z, Oshima RG. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Mine N, Mochida K, Sakai Y, Saijoh Y, Meno C, Hamada H. Nodal signaling induces the midline barrier by activating Nodal expression in the lateral plate. Development. 2003;130:1795–1804. doi: 10.1242/dev.00408. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ohneda O, Nagano M, Iemitsu M, Makino Y, Tanaka H, Miyauchi T, Goto K, Ohneda K, Fujii-Kuriyama Y, Poellinger L, Yamamoto M. Abnormal heart development and lung remodeling in mice lacking the hypoxia-inducible factor-related basic helix-loop-helix PAS protein NEPAS. Mol Cell Biol. 2008;28:1285–1297. doi: 10.1128/MCB.01332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Yamauchi J, Kuwata T, Tamura T, Yamashita T, Bae N, Westphal H, Ozato K, Nakatani Y. Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc Natl Acad Sci U S A. 2000;97:11303–11306. doi: 10.1073/pnas.97.21.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, Greenfield A, Niswander LA, Dean CH. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010;19:2251–2267. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Kitagawa H, Fujiki R, Tanabe M, Takezawa S, Takada I, Yamaoka I, Yonezawa M, Kondo T, Furutani Y, Yagi H, Yoshinaga S, Masuda T, Fukuda T, Yamamoto Y, Ebihara K, Li DY, Matsuoka R, Takeuchi JK, Matsumoto T, Kato S. Distinct function of 2 chromatin remodeling complexes that share a common subunit, Williams syndrome transcription factor (WSTF) Proc Natl Acad Sci U S A. 2009;106:9280–9285. doi: 10.1073/pnas.0901184106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci U S A. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Wasylyk C, Ayadi A, Abecassis J, Schalken JA, Rogatsch H, Wernert N, Maira SM, Multon MC, Wasylyk B. The transcription factor Net regulates the angiogenic switch. Genes Dev. 2003;17:2283–2297. doi: 10.1101/gad.272503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chehab R, Tkalcevic J, Naylor MJ, Harris J, Wilson TJ, Tsao S, Tellis I, Zavarsek S, Xu D, Lapinskas EJ, Visvader J, Lindeman GJ, Thomas R, Ormandy CJ, Hertzog PJ, Kola I, Pritchard MA. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 2005;24:635–644. doi: 10.1038/sj.emboj.7600538. [DOI] [PMC free article] [PubMed] [Google Scholar]