Abstract
Aldosterone is synthesized in the zona glomerulosa of the adrenal cortex under primary regulation by the renin-angiotensin system. Angiotensin II (A-II) acts through the angiotensin types 1 and 2 receptors (AT1R and AT2R). A-II is metabolized in different tissues by various enzymes to generate two heptapeptides A-III and angiotensin 1-7, which can then be catabolized into smaller peptides. A-II was more potent than A-III in stimulating aldosterone secretion in the adrenocortical cell line HAC15, and A-II, but not A-III, stimulated cortisol secretion. A-II stimulated mRNA expression of steroidogenic acute regulatory protein, 3β-hydroxysteroid dehydrogenase, CYP11B1, and CYP11B2, whereas A-III stimulated 3β-hydroxysteroid dehydrogenase, CYP11B1, and CYP11B2 but decreased the expression of CYP17A1 required for cortisol synthesis. The stimulation of aldosterone secretion by A-II and A-III was blocked by the AT1R receptor blocker, losartan, but not by an AT2R blocker. A-II was rapidly metabolized by the HAC15 cells to mainly to angiotensin 1-7, but not to A-III, and disappeared from the supernatant within 6 h. A-III was metabolized rapidly and disappeared within 1 h. In conclusion, A-II was not converted to A-III in the HAC15 cell and is the more potent stimulator of aldosterone secretion and cortisol of the two. A-III stimulated aldosterone secretion but not cortisol secretion.
Aldosterone is the most important mineralocorticoid for the regulation of extracellular fluid, salt balance, and arterial blood pressure. Excessive aldosterone secretion results in hypertension and cardiovascular and renal damage in patients with primary aldosteronism, the most common form of secondary hypertension (1, 2). Aldosterone is synthesized and secreted by zona glomerulosa cells of the adrenal gland, primarily under the regulation of the renin-angiotensin system. In the human, angiotensin II (A-II) binds to two receptors, the angiotensin type 1 receptor (AT1R) and AT2R, respectively (3). Binding of A-II to AT1R in the zona glomerulosa stimulates aldosterone production via the Ca2+/calmodulin kinase, MAPK, and cAMP cascade (4).
A-II consists of eight amino acids (1–8) and is formed from the decapeptide A-I (1–10) by cleavage of two amino acids, His-Leu (9, 10) by angiotensin-converting enzyme (ACE) (5). Aminopeptidases A and N cleave A-II to form A-III (2–8) and A-III to form A-IV (3–8), respectively (6). Angiotensin 1-7 (Ang1-7) is formed from A-II by ACE2, prolyl endopeptidase, or prolyl carboxypeptidase (7, 8). Expression of the enzymes of angiotensin metabolism varies between tissue and species, and the multiple metabolites of A-II in human adrenocortical cells have not yet been studied.
A-II metabolites bind AT1R or AT2R and newly discovered receptors (9, 10). Radioisotope binding studies showed that A-II and A-III, but not A-IV or Ang1-7, have high affinities for AT1R and that A-II, A-III, A-IV, and Ang1-7 exhibited high to modest affinity for the AT2R (9). Systemic A-III administration increased aldosterone production in the rat, dog, and human (11–16). The effects of these metabolites on the enzymes of steroid production in human adrenocortical cells have not been fully elucidated. In addition, the specific receptor that mediates the effects of A-III has been controversial and may vary between tissues or different species.
In summary, the extent of the regulation of aldosterone production by A-II metabolites in human adrenocortical cells remains uncertain. We investigated how A-II is metabolized and the effect of these metabolites on aldosterone and cortisol synthesis in the HAC15, a human adrenocortical carcinoma cell line used to model human adrenal steroidogenesis.
Materials and Methods
Cell culture and materials
The HAC15 human adrenocortical carcinoma cell line, a subclone of the H295R, a human adrenocortical carcinoma cell (17, 18), was developed and kindly provided by William Rainey (Georgia Health Science University, Augusta, GA). The HAC15 cells were cultured in DMEM:F12 (1:1) supplemented with 10% Cosmic Calf serum (HyClone, Logan, UT) at 37 C under an atmosphere of 5% CO2.
A-II was purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO) and A-III and A-IV from American Peptide Co. Inc. (Sunnyvale, CA). Ang1-7, Losartan potassium (AT1R antagonist), and PD123319 (1-[[4-(Dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid ditrifluoroacetate AT2R antagonist) were from Tocris Bioscience (Ellisville, MO).
Experimental design
HAC15 cells were grown to near confluence in six-, 12-, 24-, or 48-well plates for A-II and A-III metabolites studies, mRNA level detection, SDS-PAGE and immunoblotting analysis, and steroid measurement by ELISA, respectively. Serum deprivation using (Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12) DMEM:F12 supplemented with 0.1% Cosmic Calf serum was performed 24 h before each experiment.
To determine A-II or A-III metabolites, cells were washed three times, A-II or A-III was added in HEPES buffer to media to a final concentration of 100 nm, and media and cells were collected after indicated incubation times. For all other experiments, cells were incubated for 24 h in media with 10 nm A-II, A-III, A-IV, Ang1-7, or no secretagogue. When antagonists were used, they were added to fresh media 30 min before the secretagogue.
RNA extraction and RT-PCR
Total RNA extraction, RT-PCR, and detection of P450, family 11, subfamily B, polypeptide 1 or Aldosterone synthase (CYP11B2), P450, family 11, subfamily B, polypeptide 1 (CYP11B2 or cytochrome P450 11 beta-hydroxylase), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression by the TaqMan Gene expression assay were performed as previously reported (19). mRNA expression of steroidogenic acute regulatory protein (StAR), cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1), 3β-hydroxysteroid dehydrogenase (HSD3B2), cytochrome P450, family 21, subfamily A, polypeptide 2 (CYP21A2), cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17A1), and GAPDH were detected using SYBR Green as previously reported (19). Real-time data were obtained during the extension phase and critical threshold cycle values were calculated at the log phase of each gene amplification curve. Gene expression levels were analyzed as arbitrary units normalized against GAPDH mRNA expression.
SDS-PAGE and immunoblotting analysis
Cells were lysed with radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA) and the lysates added to Laemmli buffer. Equal aliquots of cell lysates were subjected to 12.5% SDS-PAGE and then transferred to polyvinylidene difluoride membranes using a wet technique. Immunoblotting analyses were performed using rabbit antiguinea pig CYP17 antiserum, rabbit antibovine 3βHSD (20), and mouse anti-β-tubulin (Developmental Studies Monoclonal Bank, The University of Iowa, Iowa City, IA) as control. Blots were developed with Super Signal West Pico Chemiluminescent substrate (Pierce, Rockford, IL) and exposed to autoradiography film. Films were scanned and quantified with a Kodak Image Station 440 using the 1D Kodak image analysis software (Kodak, New York, NY). Protein levels were analyzed as arbitrary units normalized against β-tubulin expression.
Steroid and protein assays
Aldosterone and cortisol levels were measured in cell culture supernatants by time-resolved fluorescence (19) using primary antibodies and methods developed in our laboratory as previously described (21, 22). Liquid chromatography-mass spectrometry (LC-MS) of angiotensin metabolites was done as previously described (23, 24).
Statistical analysis
All results were expressed as mean ± sem of at least three separate experiments, in which each sample was assayed in triplicate or quadruplicate. Differences between two groups were analyzed for statistical significance by t test, and multiple groups were analyzed by one-way ANOVA followed by Bonferroni comparisons. The differences were considered to be significant at P < 0.05. Analyses were performed using SPSS for Windows (release 12.0; SPSS, Inc., Chicago, IL).
Results
Effect of A-II, A-III, A-IV, and Ang1-7 on aldosterone and cortisol production
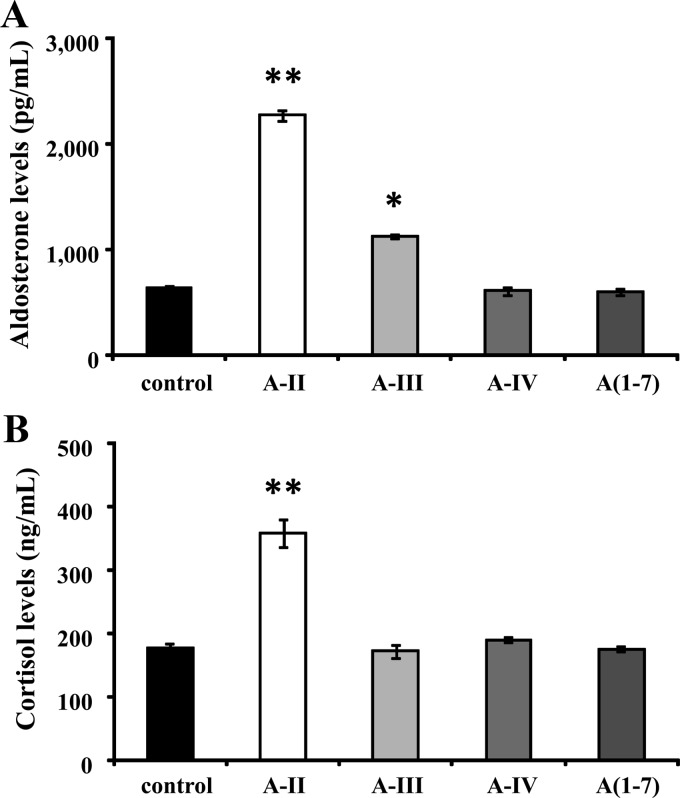
A-II stimulation produced a 3.6- and 2.0-fold increase in aldosterone and cortisol production, respectively (Fig. 1, A and B). A-III significantly stimulated aldosterone production by 1.8 times (Fig. 1A) but had no effect on cortisol production (Fig. 1B). A-IV and Ang1-7, metabolites of A-II and A-III, had no effect on aldosterone or cortisol production (Fig. 1, A and B).
Fig. 1.
Effect of A-II, A-III, A-IV, and Ang1-7 on aldosterone or cortisol production. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h, then incubated with fresh media with 0.1% serum and no secretagogue or 10 nm A-II, A-III, A-IV, or Ang1-7 for 24 h. *, P < 0.05 vs. control, n = 4; **, P < 0.01 vs. control, n = 4. A(1-7), Ang1-7.
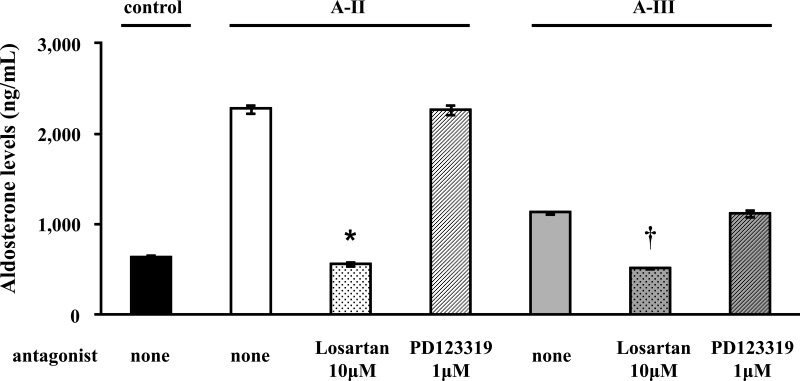
Losartan and PD123319 were used to determine the receptors involved in the regulation of aldosterone by A-II and A-III. Losartan completely inhibited A-II- and A-III-stimulated aldosterone production, whereas inhibition by the AT2R inhibitor PD123319 had no effect (Fig. 2). Similarly, A-II-stimulated cortisol production was abrogated by Losartan but not by PD123319 (data not shown).
Fig. 2.
Effect of AT1R or AT2R blockers on the effect of A-II, A-III, A-IV, and Ang1-7 on aldosterone or cortisol production. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h. Pretreatments with or without Losartan (10 μm) or PD123319 (1 μm) for 30 min were performed for each experiment before adding A-II (10 nm) or A-III (10 nm). *, P < 0.01 vs. A-II stimulation without antagonist, n = 4; †, P < 0.01 vs. A-III stimulation without antagonist, n = 4.
Expression of steroid biosynthetic enzymes after A-II or A-III stimulation
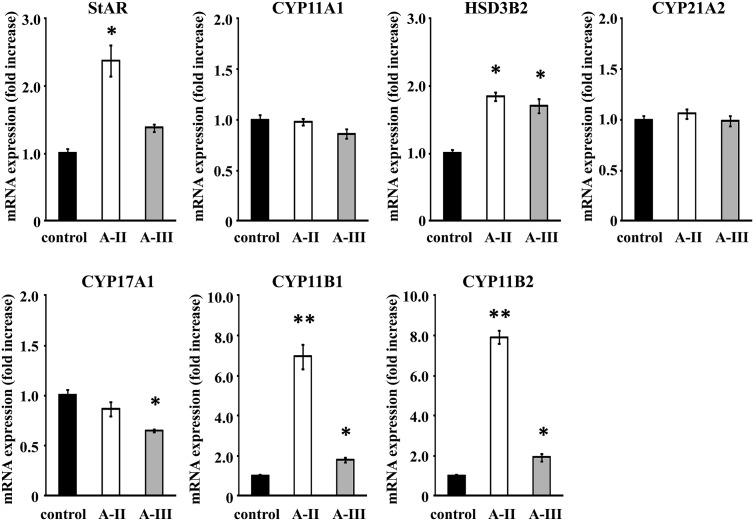
A-II stimulation increased the expression of StAR, HSD3B2, CYP11B1, and CYP11B2 mRNA by 2.4-, 1.9-, 6.9-, and 7.9-fold, respectively, in comparison with control (Fig. 3). A-III stimulation increased mRNA expression of HSD3B2, CYP11B1, and CYP11B2 by 1.7-, 1.8-, and 1.9-fold compared with control, respectively (Fig. 3). Interestingly, CYP17A1 mRNA expression was decreased by A-III, whereas A-II had no effect on it (Fig. 3). CYP11A1 and CYP21A2 were unchanged by A-II or A-III.
Fig. 3.
Effect of A-II and A-III on mRNA expression of the adrenal steroid synthesis StAR, CYP11A1, HSD3B2, CYP21A2, CYP17A1, CYP11B1, and CYP11B2. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h, then incubated with fresh media with 0.1% serum and A-II (10 nm) or A-III (10 nm) for 3 h. After aspiration of media, the cells were collected for RNA extraction and real-time RT-PCR performed. Results were normalized by GAPDH mRNA expression and expressed as fold change vs. control. *, P < 0.05 vs. control, n = 3; **, P < 0.01 vs. control, n = 3.
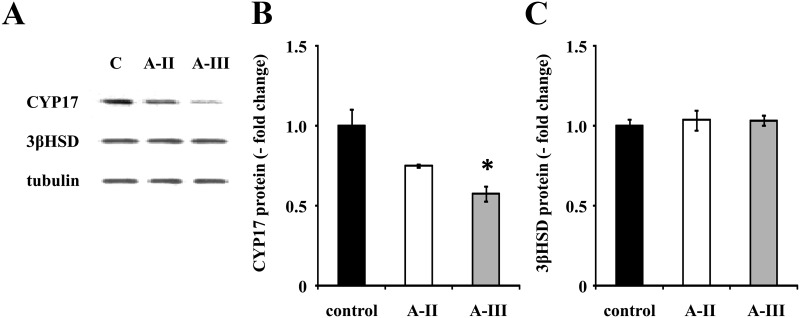
A-III stimulation increased aldosterone but not cortisol production, whereas A-II stimulated the synthesis of both. Both A-II and A-III increased HSD3B2 mRNA but did not change 3β-HSD protein expression, and both increased CYP11B1 and CYP11B2. However, A-III decreased 17-α-hydroxylase protein expression by 42%, whereas A-II had no effect on it (Fig. 4, A and B).
Fig. 4.
Effect of A-II and A-III on protein expression of CYP17A1 and HSD3B2. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h, then incubated with fresh media with 0.1% serum and vehicle, A-II (10 nm), or A-III (10 nm) for 24 h. After aspiration of media, the cells were harvested with protease inhibitor. The cell lysates were subjected to immunoblotting analysis using rabbit antiguinea pig CYP17 antiserum, rabbit antibovine 3b-HSD, or antitubulin antibodies (A). The CYP17 or as in 3b-HSD band intensity of scanned images was adjusted by those of tubulin and expressed as fold change vs. control (graphs B and C). *, P < 0.05 vs. control, n = 3.
Metabolites of A-II and A-III in HAC15 cells
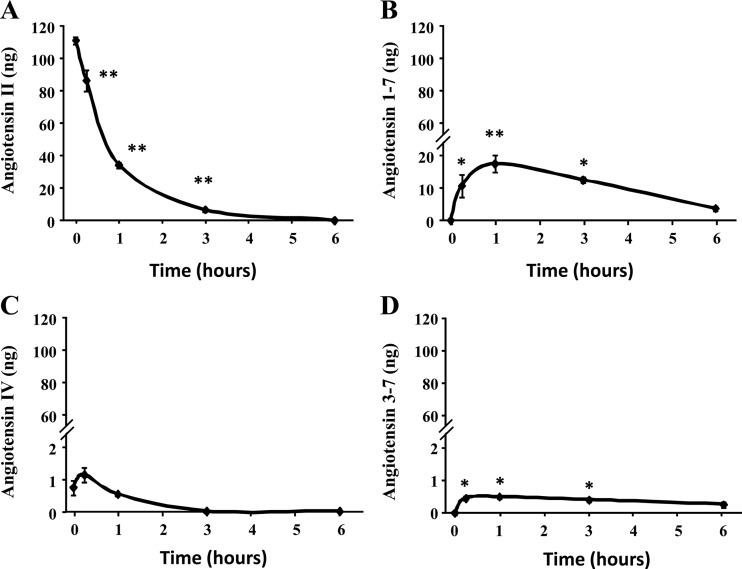
A-II was completely metabolized within 6 h after its addition to HAC15 cells (Fig. 5A). Ang1-7 increased gradually, reaching its maximum within 1 h after the addition of A-II (Fig. 5B), whereas Ang3-7 increased insignificantly (Fig. 5D). HAC15 cells did not covert A-II into A-III, angiotensin A, or Ang2-7. A-IV was detectable at zero times, but we found that A-II batches we used were contaminated with very small amount of A-IV as can be seen at time 0 and the concentration increased at 30 min but was not statistically significant and then decreased to undetectable values (Fig. 5C).
Fig. 5.
A-II metabolism by HAC15 cells. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h. After three times washing by HEPES buffer, HEPES buffer with A-II (100 nm) was incubated for indicated time. Buffer was removed, extracted, and analyzed for A-II peptides by LC-MS. The study was done twice. **, P < 0.01; *, P < 0.05.
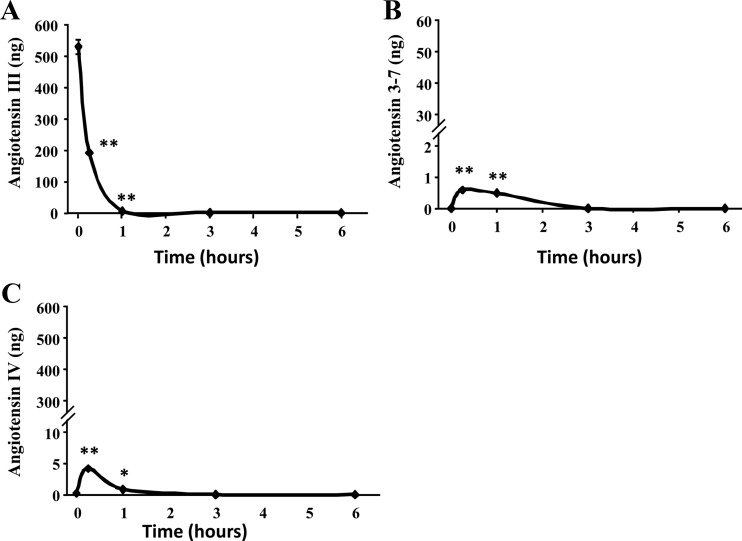
HAC15 cells metabolized exogenous A-III more rapidly than A-II; A-III disappeared within 1 h of its addition to the incubation media (Fig. 6A). Incubation of HAC15 cells with A-III increased A-IV and Ang3-7 slightly (Fig. 6, B and C), whereas Ang2-7 was not detected in the supernatant.
Fig. 6.
A-III metabolism by HAC15 cells. Confluent cells were serum deprived in DMEM:F12 containing 0.1% Cosmic Calf serum for 24 h. After three times washing by HEPES buffer, HEPES buffer with A-III (100 nm) was incubated for indicated time. Buffer was removed, extracted, and analyzed for A-II peptides by LC-MS. The study was done twice. **, P < 0.01; *, P < 0.05.
Effect of repeated A-II or A-III stimulation on aldosterone or cortisol production
Because A-II and A-III completely disappeared from the media in 6 and 1 h, respectively, we added A-II or A-III at 1, 3, 6, or 12 h after the initial addition and measured aldosterone 24 h after the first addition of secretagogue. A slight, but significant, increase in aldosterone production was obtained after additional A-II stimulation at 12 h. Surprisingly, additional A-II stimulation at other times had no effect on aldosterone or cortisol production even though A-II was not detectable in the media after 6 h. Additional A-III had no effect on aldosterone or cortisol production stimulated by treatment at time 0.
Discussion
HAC15 cells in this study did not convert exogenous A-II into A-III, and they metabolized exogenous A-III faster than A-II. Both A-II and A-III acted through the AT1R to increase aldosterone production. However, A-III was a less effective aldosterone secretagogue than A-II. Remarkably, A-II, but not A-III, stimulated cortisol, probably because A-III suppresses CYP17 expression and 17-α-hydroxylase is required for the synthesis of cortisol but not aldosterone.
Our demonstration that A-III regulates aldosterone production through the AT1R in human adrenocortical cells agrees with the preponderance of information in the literature (3, 25–27). The increase of arterial blood pres-sure in response to A-III in the dog was completely blocked by an AT1R antagonist (12), supporting our results, in which A-III acted via AT1R to stimulate aldosterone secretion (Fig. 2A). Although A-III was reported to stimulate aldosterone secretion through the AT2R in the rat (13), there is significant evidence that aldosterone production is regulated by the AT1R, rather than the AT2R, in the rat and other species (3, 25–27). AT2R mainly exerts inhibitory actions on cellular responses mediated by the AT1R (28, 29).
A-II and A-III administration in the human markedly increased plasma aldosterone, with A-II being approximately 2.4 times more potent than A-III (16). These results are consistent with our in vitro study that A-II was about approximately two times more potent than A-III in stimulating aldosterone production in the HAC15 cells (Fig. 1A). Similarly, A-III was less potent than A-II in causing vessel contractions in rat and rabbit aortas or endothelium-denuded human saphenous veins (30–32) and relaxing precontracted bovine adrenal arteries (24).
It has been suggested that the difference in potency between A-II and A-III is due either to more rapid degradation (32) or to lower affinity of A-III for the AT1R (33). We showed that the concentration of A-III in the human adrenocortical cell culture media decreased faster than that of A-II consistent with a previous report (12). However, our supplementation of the HAC15 culture media with A-III to prevent the decline in its concentration did not enhance its stimulation of aldosterone production. A recent study showed that A-III has a lower affinity for AT1R compared with A-II (9). Our studies did not address the issue of comparative affinity, because we did not do competitive studies with the two agonists. Addition of A-II at several time points after it was completely metabolized at 6 h only increased the 24-h aldosterone production when it was added at 12 h after the initial incubation but had no effect on cortisol production. A-II binding of the AT1R results in its internalization; maximal effect occurs when all available AT1R are fully occupied and internalized (34). Furthermore, adding of A-II from 4 to 12 h suppressed AT1R mRNA levels by more than 80% at 24 h (34). Therefore, the probable explanation for the tachyphylaxis observed for both A-II and A-III was the internalization of all AT1R and perhaps transient suppression of its synthesis.
Aminopeptidase A preferentially cleaves N-terminal acidic amino acids from A-II to form A-III. It is expressed in the brain and contributes to the regulation of blood pressure (6, 35). Central nervous system blockade of aminopeptidase A with a specific inhibitor abrogated the pressor effect of exogenous A-II, suggesting that in the brain, A-II is converted into A-III to increase arterial blood pressure (35). A-III was not formed in HAC15 cells after A-II incubation, suggesting that aminopeptidase A does not exist in human adrenocortical cells. However, this will have to be confirmed in freshly isolated cells of the normal human adrenal gland in case the ability to express this gene was lost during immortalization of these cells. Alternatively, because A-III is rapidly metabolized by HAC15 cells, it is possible that A-II is metabolized to A-III, but A-III does not accumulate due to its rapid metabolism to A-IV and A3-7.
These studies show that the main metabolite for A-II in the HAC15 adrenocortical cell is Ang1-7, most likely due to the conversion by ACE2, prolyl endopeptidase, or prolyl carboxypeptidase. Although ACE2 is reported to have an important role in the metabolism of A-II in kidney, brain, heart, and vessels (36, 37), this is the first report regarding the possible existence of ACE2 in adrenocortical cells. We found the presence of ACE2, glutamyl aminopeptidase, and alanyl aminopeptidase by RT-PCR using specific primers, but they were not regulated by A-II (see Supplemental Material and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Our results are consistent with the previous report that systemic Ang1-7 administration in humans did not change aldosterone concentrations (16). Ang1-7 mainly functions via the G-Protein-coupled receptor Mas 1 (38), and the G-Protein-coupled receptor Mas 1 has not been reported to be expressed in the adrenal. Although binding assays demonstrate that Ang1-7 has low affinity for the AT2R, it does not bind the AT1R (9), probably due to the absence of the C terminus of A-II, which is critical for binding and activation of AT1R. Bovine adrenal zona glomerulosa cells metabolized A-II in a similar fashion as the HAC15 cells with formation of Ang1-7 and A-IV, both of which were inactive releasing aldosterone (39). A-II was not converted to A-III in bovine zona glomerulosa cells (39).
In conclusion, we showed that A-III was not formed from A-II in the HAC15 cells. A-III stimulated aldosterone, via AT1R, but did not increase cortisol synthesis. The stimulation of aldosterone release by A-III was less than that of A-II in the HAC15 human adrenocortical cells. The smaller effect of A-III is unlikely to be due to more rapid decay of A-III compared with A-II in the incubation, because the stimulatory effect was not restored by addition of agonist at strategic intervals. In fact, the effect on steroidogenensis of both A-II and A-III showed tachyphylaxis, whether this is due to the internalization of the AT1R or to suppression of its synthesis, or both, remains to be demonstrated in the human adrenocortical cells. One limitation of these studies is that the HAC15 is an adrenal carcinoma cell line that might not represent normal aldosterone-producing zona glomerulosa cells. One problem with the use of isolated normal human zona glomerulosa cells is that the CYP11B2 enzyme needed for the synthesis of aldosterone is expressed in a few scattered cells underneath the capsule and in clusters that have been called aldosterone-producing cell clusters, which are mixed under the capsule with a larger component of nonsteroidogenic cells and zona fasciculata cells (40).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL27255, HL105383 (to C.E.G.-S.), and HL-83297 (to W.B.C.), the American Heart Association Midwest Affiliate (P.G.K.), and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award 1018X007080 (to E.P.G.-S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A-II
- Angiotensin II
- ACE
- angiotensin-converting enzyme
- Ang1-7
- angiotensin 1-7
- AT1R
- angiotensin type 1 receptor
- CYP11A1
- cytochrome P450, family 11, subfamily A, polypeptide 1
- CYP17A1
- cytochrome P450, family 17, subfamily A, polypeptide 1
- CYP21A2
- cytochrome P450, family 21, subfamily A, polypeptide 2
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HSD3B2
- 3β-hydroxysteroid dehydrogenase
- LC-MS
- liquid chromatography-mass spectrometry
- StAR
- steroidogenic acute regulatory protein.
References
- 1. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. 2008. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:3266–3281 [DOI] [PubMed] [Google Scholar]
- 2. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. 2005. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45:1243–1248 [DOI] [PubMed] [Google Scholar]
- 3. Kaschina E, Unger T. 2003. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press 12:70–88 [DOI] [PubMed] [Google Scholar]
- 4. Hattangady NG, Olala LO, Bollag WB, Rainey WE. 2012. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350:151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang HY, Erdös EG, Levin Y. 1971. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Ther 177:291–300 [PubMed] [Google Scholar]
- 6. Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. 1996. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci USA 93:11968–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrario CM, Iyer SN. 1998. Angiotensin-(1-7): a bioactive fragment of the renin-angiotensin system. Regul Pept 78:13–18 [DOI] [PubMed] [Google Scholar]
- 8. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. 2000. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243 [DOI] [PubMed] [Google Scholar]
- 9. Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121:297–303 [DOI] [PubMed] [Google Scholar]
- 10. Zhuo JL, Li XC. 2011. New insights and perspectives on intrarenal renin-angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides 32:1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell WB, Brooks SN, Pettinger WA. 1974. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science 184:994–996 [DOI] [PubMed] [Google Scholar]
- 12. Gammelgaard I, Wamberg S, Bie P. 2006. Systemic effects of angiotensin III in conscious dogs during acute double blockade of the renin-angiotensin-aldosterone-system. Acta Physiol (Oxf) 188:129–138 [DOI] [PubMed] [Google Scholar]
- 13. Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. 2011. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology 152:1582–1588 [DOI] [PubMed] [Google Scholar]
- 14. Goodfriend TL, Peach MJ. 1975. Angiotensin III: (DES-aspartic acid-1)-angiotensin II. Evidence and speculation for its role as an important agonist in the renin-angiotensin system. Circ Res 36:38–48 [DOI] [PubMed] [Google Scholar]
- 15. Wamberg C, Plovsing RR, Sandgaard NC, Bie P. 2003. Effects of different angiotensins during acute, double blockade of the renin system in conscious dogs. Am J Physiol Regul Integr Comp Physiol 285:R971–R980 [DOI] [PubMed] [Google Scholar]
- 16. Plovsing RR, Wamberg C, Sandgaard NC, Simonsen JA, Holstein-Rathlou NH, Hoilund-Carlsen PF, Bie P. 2003. Effects of truncated angiotensins in humans after double blockade of the renin system. Am J Physiol Regul Integr Comp Physiol 285:R981–R991 [DOI] [PubMed] [Google Scholar]
- 17. Parmar J, Key RE, Rainey WE. 2008. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab 93:4542–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T, Rainey WE. 2012. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol 351:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. 2012. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology 153:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimoto K, Rigsby CS, Wang T, Mukai K, Gomez-Sanchez CE, Rainey WE, Seki T. 2012. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology 153:1755–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP. 1987. The production of monoclonal antibodies against aldosterone. Steroids 49:581–587 [DOI] [PubMed] [Google Scholar]
- 22. Morra di Cella S, Veglio F, Mulatero P, Christensen V, Aycock K, Zhu Z, Gomez-Sanchez EP, Gomez-Sanchez CE. 2002. A time-resolved fluoroimmunoassay for 18-oxocortisol and 18-hydroxycortisol. Development of a monoclonal antibody to 18-oxocortisol. J Steroid Biochem Mol Biol 82:83–88 [DOI] [PubMed] [Google Scholar]
- 23. Cui L, Nithipatikom K, Campbell WB. 2007. Simultaneous analysis of angiotensin peptides by LC-MS and LC-MS/MS: metabolism by bovine adrenal endothelial cells. Anal Biochem 369:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gauthier KM, Zhang DX, Cui L, Nithipatikom K, Campbell WB. 2008. Angiotensin II relaxations of bovine adrenal cortical arteries: role of angiotensin II metabolites and endothelial nitric oxide. Hypertension 52:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balla T, Baukal AJ, Eng S, Catt KJ. 1991. Angiotensin II receptor subtypes and biological responses in the adrenal cortex and medulla. Mol Pharmacol 40:401–406 [PubMed] [Google Scholar]
- 26. Aguilera G. 1992. Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol Cell Endocrinol 90:53–60 [DOI] [PubMed] [Google Scholar]
- 27. Hajnóczky G, Csordás G, Bagó A, Chiu AT, Spät A. 1992. Angiotensin II exerts its effect on aldosterone production and potassium permeability through receptor subtype AT1 in rat adrenal glomerulosa cells. Biochem Pharmacol 43:1009–1012 [DOI] [PubMed] [Google Scholar]
- 28. Spät A, Hunyady L. 2004. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84:489–539 [DOI] [PubMed] [Google Scholar]
- 29. Hunyady L, Catt KJ. 2006. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20:953–970 [DOI] [PubMed] [Google Scholar]
- 30. Li Q, Zhang L, Pfaffendorf M, van Zwieten PA. 1995. Comparative effects of angiotensin II and its degradation products angiotensin III and angiotensin IV in rat aorta. Br J Pharmacol 116:2963–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Q, Feenstra M, Pfaffendorf M, Eijsman L, van Zwieten PA. 1997. Comparative vasoconstrictor effects of angiotensin II, III, and IV in human isolated saphenous vein. J Cardiovasc Pharmacol 29:451–456 [DOI] [PubMed] [Google Scholar]
- 32. Robertson MJ, Cunoosamy MP, Clark KL. 1992. Effects of peptidase inhibition on angiotensin receptor agonist and antagonist potency in rabbit isolated thoracic aorta. Br J Pharmacol 106:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wright JW, Harding JW. 1995. Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept 59:269–295 [DOI] [PubMed] [Google Scholar]
- 34. Bird IM, Mason JI, Rainey WE. 1994. Regulation of type 1 angiotensin II receptor messenger ribonucleic acid expression in human adrenocortical carcinoma H295 cells. Endocrinology 134:2468–2474 [DOI] [PubMed] [Google Scholar]
- 35. Reaux A, Fournie-Zaluski MC, David C, Zini S, Roques BP, Corvol P, Llorens-Cortes C. 1999. Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc Natl Acad Sci USA 96:13415–13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benter IF, Yousif MH, Cojocel C, Al-Maghrebi M, Diz DI. 2007. Angiotensin-(1-7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292:H666–H672 [DOI] [PubMed] [Google Scholar]
- 37. Grobe JL, Mecca AP, Mao H, Katovich MJ. 2006. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol 290:H2417–H2423 [DOI] [PubMed] [Google Scholar]
- 38. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. 2003. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100:8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanke CJ, Holmes BB, Xu Y, Nithipatikom K, Campbell WB. 2007. Endothelium-derived steroidogenic factor enhances angiotensin II-stimulated aldosterone release by bovine zona glomerulosa cells. Endocrinology 148:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. 2010. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 95:2296–2305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.