Abstract
Salt and fluid absorption is a shared function of many of the body’s epithelia, but its use is highly adapted to the varied physiological roles of epithelia-lined organs. These functions vary from control of hydration of outward-facing epithelial surfaces to conservation and regulation of total body volume. In the most general context, salt and fluid absorption is driven by active Na+ absorption. Cl− is absorbed passively through various available paths in response to the electrical driving force that results from active Na+ absorption. Absorption of salt creates a concentration gradient that causes water to be absorbed passively, provided the epithelium is water permeable. Key differences notwithstanding, the transport elements used for salt and fluid absorption are broadly similar in diverse epithelia, but the regulation of these elements enables salt absorption to be tailored to very different physiological needs. Here we focus on salt absorption by exocrine glands and airway epithelia. In cystic fibrosis, salt and fluid absorption by gland duct epithelia is effectively prevented by the loss of cystic fibrosis transmembrane conductance regulator (CFTR). In airway epithelia, salt and fluid absorption persists, in the absence of CFTR-mediated Cl− secretion. The contrast of these tissue-specific changes in CF tissues is illustrative of how salt and fluid absorption is differentially regulated to accomplish tissue-specific physiological objectives.
In cystic fibrosis, salt and fluid absorption are effectively prevented in gland duct epithelia, but persist in airway epithelia.
ABSORPTION IN GLANDS AND INTESTINES
Exocrine glands are generally involved in two major physiological functions: secretion of macromolecules such as enzymes and electrolyte fluid into the lumen, and postsecretory modification of primary secretions by a process involving absorption of fluid and electrolytes as they pass through the lumen of the ductal system (Fig. 1). For the sake of simplicity, we can take the sweat gland as a representative example of exocrine structure and function. The sweat gland is a morphologically and physiologically simple exocrine model system. The sweat gland has two morphologically distinct regions: a secretory coil that secretes isotonic fluid into its lumen and a reabsorptive duct that reabsorbs most of the secreted Na+, Cl−, and HCO3− from the lumen as the primary sweat passes on to the skin surface to evaporate for cooling (Quinton 1987). Secretion of salt into the lumen of the secretory coil serves the primary function of creating a serosa-to-lumen osmotic gradient to facilitate secretion of water. Because the primary function of the sweat gland is to wet the skin surface for evaporative cooling, losing electrolytes along with water from the body is highly undesirable because it can lead to severe dehydration, electrolyte imbalance, and associated multi-organ disturbances. This physiological threat has been effectively solved in primates as the primary sweat passes through the reabsorptive duct, where most of the secreted salt is returned by active absorption to the extracellular fluid compartment before the sweat deposits onto the skin surface. Most exocrine glands and organs use some variant of this basic principle; that is, first, a primary secretion of an isotonic fluid, followed by a subsequent modification by reabsorption to create a tissue-specific final secretory product so that different exocrine glands exploit this process to create an optimal extracellular aqueous medium to assist in specific physiological functions. For example, although conservation of salt and/or maintenance of electrolyte balance are the primary goals of the absorptive process in the sweat gland and the kidney tubules (Quinton 1990; Bhalla and Hallows 2008), airway epithelial cells may exploit this absorptive process to maintain proper volume and composition of the airway surface liquid (ASL), which is critical for keeping the airways clear of infections and blockage (Widdicombe and Widdicombe 1995; Welsh 1996; Boucher 2001). The physiological significance of this process is emphasized by the fact that abnormal electrolyte absorptive function can lead to severe pathological conditions such as cystic fibrosis (CF), hypertension, pseudohypoaldosteronism (PHA), and Liddle syndrome (Quinton 1990; Bhalla and Hallows 2008).
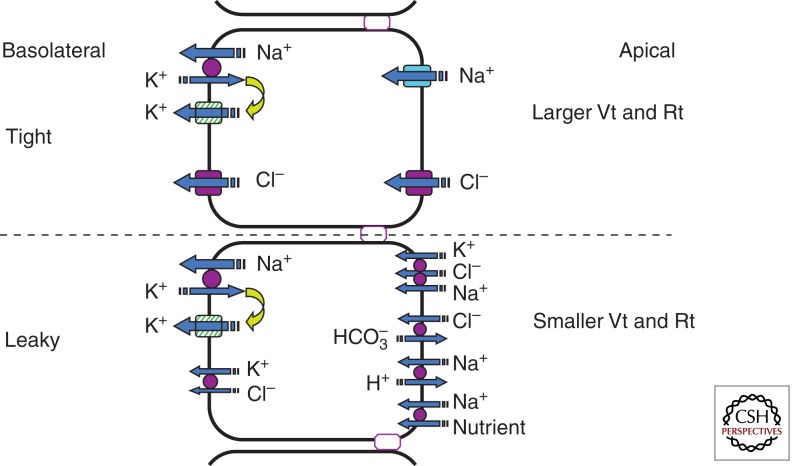
Figure 1.
A simplified model depicting significant transport processes in tight and leaky epithelia. Notice that the tight epithelia predominantly depend on the amiloride-sensitive epithelial Na+ channel and CFTR anion channel in the apical membranes for transporting Na+ and Cl− ions into the cell down the electrochemical gradient. These epithelia are characterized by relatively high electrical resistance (Rt) and transepithelial potentials (Vt). In contrast, the leaky epithelia predominantly depend on carrier-mediated transporters such as NaK2Cl and Na nutrient (e.g., Na+-glucose) cotransporters, Na+/H+, and Cl−/HCO3− exchangers across the apical membranes. Leaky epithelia are also characterized by relatively low values of Rt and Vt. Although most epithelia generate a lumen-negative Vt of varying magnitude, proximal convoluted tubules can generate a lumen-positive Vt (about +3 mV, attributed to paracellular Cl− diffusion potential) in the absence of nutrient transport and decreased luminal NaHHCO3− concentration (Kokko 1973). Although the Na+/K+ pump plays a major role in the extrusion of Na+ from the cell in the salt absorption process, the mechanism of Cl− exit is either mediated by CFTR or by a carrier-mediated transporter such as the KCl cotransporter in the basolateral membrane.
Absorptive Mechanisms Are Tissue Specific
Epithelial cells welded together by tight junctions can accomplish vectorial transport of NaCl and water. Epithelial cells are polarized by an apical membrane (APM) and a basolateral membrane (BLM). Each membrane incorporates specific ion channels and transport proteins such that in an absorptive epithelium, NaCl can enter the cell at the APM and exits the cell across the BLM. Epithelial cells of different exocrine organs use diverse physiological strategies involving coupled ion cotransporters, counter-ion exchangers, and ion channels to absorb salt from the lumen to blood. Significant differences exist in the mechanisms of salt absorption across the apical membranes of different epithelial cells so as to be in compliance with their respective physiological roles in a particular tissue. Because epithelial water permeability varies among tissues, fluid and electrolyte absorption can be either hypo-osmotic (luminal fluid has lower osmolality compared with plasma) (Bijman and Quinton 1984; Quinton 1984) or isotonic (luminal fluid has the same osmolality as plasma). So-called leaky epithelia depend on carrier-mediated Na+-absorptive mechanisms, and they are generally involved in isotonic fluid absorption. In contrast, epithelia using ENaC-dependent active salt transport (e.g., sweat glands, cortical collecting duct, and distal colon) generally tend to be involved in hypo-osmotic absorption (Quinton 1984; Devuyst and Guggino 2002), as described in greater detail in the following discussion.
Isotonic Absorption
Most “leaky” absorptive epithelia (so-called because of their electrically conductive tight junctions) such as proximal tubules, small intestine, and gallbladder are characterized by the presence of small trans-epithelial electrical potentials and relatively high water permeability. These epithelial cells generally use combinations of carrier-mediated transport components, such as Na+/K+/2Cl− and Na+/Cl− cotransporters, as well as Na+/H+ and Cl−/HCO3− exchangers and Na+-coupled nutrient transporters such as Na+-glucose and Na+-amino acid cotransporters, in the apical membrane (Berschneider et al. 1988; Reuss 1985; Reuss et al. 1991; Kunzelmann and Mall 2002). For example, fluid absorption in the intestine and proximal tubules is always coupled to the movement of solutes from the lumen. There are significant regional differences with respect to the relative role of the aforementioned carrier-mediated transport processes even within the gastrointestinal tract and kidney tubules. For example, Na+-dependent glucose and amino acid transporters play a significant role in the process of absorption in the jejunum and ileal regions. In contrast, varying degrees of contribution from Na+/H+ (NHE) and Cl−/HCO3− exchangers to fluid absorption is observed from the duodenum to the distal colon (Berschneider et al. 1987; Quinton 1999). Similarly, along the nephron, Na+-dependent nutrient transporters (e.g., Na+-glucose cotransporters) in the proximal tubule, NHE in the early proximal convoluted tubules, NaCl cotransporters in the distal convoluted tubules, and Na+/K+/2Cl− cotransporters and NHE in the thick ascending limb (Quinton 2007) play tissue-specific roles in absorptive processes. Even though defective functions of these transporters are associated with several disease conditions in various leaky epithelia, most of the abnormalities in carrier-meditated transport processes in cystic fibrosis appear to be secondary to abnormal CFTR (cystic fibrosis transmembrane conductance regulator)–mediated Cl− permeability, as discussed below.
Hypertonic Absorption
In contrast to leaky epithelia, the so-called tight epithelia are generally characterized by the presence of relatively large lumen negative trans-epithelial potentials (<−10 mV, lumen negative) and ion channels involved in electrogenic absorption of NaCl (Boucher et al. 1991; Quinton 1999; Reddy et al. 1999; Kunzelmann and Mall 2002; Reddy and Quinton 2002). These epithelia are mostly, if not exclusively, involved in hypertonic (with reference to plasma) salt absorption and therefore tend to be relatively water impermeable. The human sweat duct, cortical collecting duct, colon, and salivary glandular ducts are examples that share these properties. The absorptive process in tight epithelia is predominantly mediated by ENaC channels and CFTR anion channels in the APM (Reuss et al. 1991; Stutts et al. 1995; Reddy et al. 1999; Kunzelmann and Mall 2002), which involves the following steps: Na+ enters the cells via ENaC channels down a favorable electrochemical gradient, making the cytosolic side of the apical membrane more positive and thereby creating a more favorable electrochemical gradient for negatively charged Cl− ions to enter the cells passively via CFTR Cl− channels. Intracellular Na+ is pumped out of the cell by the Na+/K+ pump in the BLM, which pumps three Na+ ions out of the cell in exchange for two K+ ions. Hence, the Na+/K+ pump exports one net positive charge out of the cell, thereby contributing slightly to the negative electrical potential of the cell. Cl− entering the cell is driven out across the BLM through Cl− channels down a favorable electrical gradient created by a more negative basolateral membrane potential (Welsh 1987; Reddy and Quinton 1991, 1994; Smith et al. 1996; Kunzelmann and Mall 2002). The BLM of epithelial cells expresses a predominant K+ ion selectivity. The basolateral K+ channels not only provide a leakage pathway for extrusion of excess K+ accumulated during pump activity but also play a significant role in maintaining trans-epithelial electrical potential due to significantly larger hyperpolarization of BLM relative to the apical membrane potential, thereby providing a significant driving force for vectorial salt transport by epithelial cells (Reddy and Quinton 1987, 1991; Kunzelmann and Mall 2002). Although the salt absorption creates an osmotic gradient from lumen to serosa (blood side), water movement across the tight epithelia down the osmotic gradient is limited by the epithelial barrier to water.
CFTR in Salt Absorption
CFTR plays a crucial role in diverse physiological functions (regarding absorption and secretion of salt) by numerous epithelial organs (Quinton 1999). CFTR is also unique among ion channels with respect to (1) complex molecular structure (Riordan et al. 1989; Welsh et al. 1992); (2) multiple regulatory controls involving PKC (Berger et al. 1993; Jia et al. 1997), PKA (Cheng et al. 1991; Berger et al. 1993; Dulhanty and Riordan 1994), PKG (Picciotto et al. 1992; French et al. 1995; Vaandrager et al. 1996, 1998), Ca2+ calmodulin-dependent protein kinases (Picciotto et al. 1992), protein tyrosine kinases (Gadsby and Nairn 1999), and different protein phosphatases (Berger et al. 1993; Fischer et al. 1998; Luo et al. 1998; Gadsby and Nairn 1999); and (3) multifunctional behavior involving several transport functions (Schwiebert et al. 1999). Structurally, it is distinguished from most ion channels by the presence of a regulatory domain (R-domain) with multiple consensus phosphorylation sites and two nucleotide-binding domains (NBDs) (Riordan et al. 1989; Welsh et al. 1992). Even though these studies implicated several kinases and phosphatases regulating this channel, we do not know whether all of these regulatory processes are tissue specific or coexist within most epithelial cells. Even if these control mechanisms do exist in vivo, the purpose of such complex regulation remains puzzling. CFTR regulation involves both hydrolytic and nonhydrolytic ATP binding to NBDs (Anderson et al. 1991; Quinton and Reddy 1991; Ko and Pedersen 1995; Reddy and Quinton 1996; Randak et al. 1997; Sheppard and Welsh 1999). Nonhydrolytic ATP binding may be required for coupling trans-epithelial electrolyte transport to cellular energy charge (Quinton and Reddy 1991). However, it is intriguing that, unlike most ion channels that allow passive diffusion of ions (as opposed to energy-dependent active transport), CFTR uses ATP energy during the transport process. Recent studies have indicated that these multiple controls may play a role in determining the anion selectivity of the CFTR channel to HCO3− and Cl− (Reddy and Quinton 2003b). Thus, understanding the regulatory mechanisms is essential not only to better understand how different mutations selectively affect epithelial Cl− and HCO3− conductances in CF (Reddy and Quinton 2003b), but also to develop effective therapeutic strategies to treat diseases like CF.
ENaC in Salt Absorption
The central role that ENaC plays in salt absorption in several diseases including pseudohypoaldosteronism (PHA), Liddle syndrome, and cystic fibrosis (Schild et al. 1995; Rossier 1996; Hummler and Horisberger 1999; Pilewski and Frizzell 1999; Schreiber et al. 1999; Schwiebert et al. 1999; de La Rosa et al. 2000) emphasizes the clinical and physiological significance of this cation channel in epithelial tissues (Quinton 2007). ENaC is expressed in the APM of cells in the salivary gland ducts, sweat gland ducts, cortical collecting tubules of the kidney, the distal colon, and the airways (Duc et al. 1994; Fuller et al. 1995; Stutts et al. 1995; Rossier 1996). ENaC channels in the kidney are directly involved in the maintenance of extracellular volume and blood pressure and indirectly involved in the homeostasis of K+ and H+ ions. The reabsorption of Na+ in the cortical collecting tubule generates a lumen-negative potential that facilitates the secretion of K+ and H+ by the distal nephron. Blocking ENaC with amiloride or inactivation by mutation leads to Na+ wasting, hyperkalemia, and metabolic acidosis. Many regulatory mechanisms such as the renin–angiotensin–aldosterone axis, antidiuretic hormone, and catecholamines modulate the activity of the ENaC channels (de La Rosa et al. 2000). ENaC channels are expressed in the respiratory tract from the nose to the alveoli (de La Rosa et al. 2000). In the lung, ENaC activity is necessary for fluid handling, in particular at birth when the transition from a liquid-filled lung occurs (de La Rosa et al. 2000). The composition of the fluid produced by secretory organs such as salivary glands and sweat glands is also modified by ENaC channels located along the ducts that reabsorb luminal Na+.
CFTR–ENaC Coordination in the Apical Membrane
Studies on the sweat duct indicated that ENaC and CFTR activation are closely coupled but possibly independent of contemporaneous phosphorylation by PKA, which may suggest molecular interactions between the two channel proteins. The carboxy-terminal motif interacting with PDZ1 or PDZ2 of EBP50 (ERM-binding phosphoprotein 50) or other related proteins may be important for some aspects of CFTR function (Hall et al. 1998; Short et al. 1998), which might include CFTR–ENaC interactions. However, we do not know how closely the two channels function in vivo. Recent studies also indicated that activating G-proteins and A and G-kinases stimulate both CFTR and ENaC simultaneously in the human sweat duct. However, we do not know whether such activation is due to direct action of the agonists on the individual channels or to indirect effects through CFTR interacting with ENaC. Secondary changes in intracellular ion composition seem to control ENaC as well as CFTR channel functions in a complex fashion. Several studies have shown that increasing intracellular Cl− concentration inhibits ENaC activity (Cook et al. 1998; Kunzelmann and Mall 2002; Reddy and Quinton 2003a). It is important to note that activation of CFTR was shown to inhibit ENaC activity in some experimental model systems indicating that changes in intracellular Cl− activity could be one of the mechanisms by which ENaC is inhibited (Dinudom et al. 1993; Reddy and Quinton 2002). Other studies have also indicated that ENaC activity and resultant increases in intracellular Na+ cause feedback inhibition of ENaC (Dinudom et al. 1993). Changing intracellular Na+ could have indirect effects on ENaC activity through changing intracellular pH mediated by basolateral NHE activity (Fig. 2) (Reddy et al. 2008).
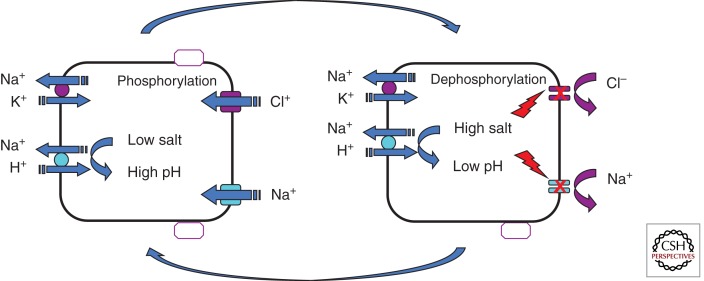
Figure 2.
A simplified model depicting feedback regulation of salt absorption. The model illustrates that as intracellular Na+ concentration rises as a function of ENaC activity, the chemical driving force for proton extrusion by the Na+/H+ exchanger (NHE) in the basolateral membrane decreases and allows cytosolic acidification that would negatively feedback and inhibit both ENaC and CFTR activity and limit salt influx across the apical membrane. The feedback inhibition of salt entry would relax as Na+ is extruded by the Na+/K+ pump in the basolateral membrane. Prevention of salt influx in excess of pump capacity would protect the absorptive cell from disruptive changes in cell volume during trans-epithelial salt absorption.
Cell Specificity of ENaC–CFTR Interaction
Studies on the human airway epithelium found increased Na+ absorption in CF airways, and some studies in heterologous systems (Boucher 1994b; Knowles et al. 1997; Guggino 1999; Pilewski and Frizzell 1999; Schwiebert et al. 1999) detected a negative influence on ENaC activity. No evidence of reciprocal coupling between ENaC exists in other native tissues where it has been studied, such as the sweat duct. It is possible that physiological tissue-specific functions such as absorption versus secretion determine the type of CFTR–ENaC interaction or complicate the interpretation of results. However, in heterologous systems, overexpressed proteins may very well skew or adversely affect normal physiological function. For example, in CFPAC-1 cells, hyperexpressing CFTR actually depressed outward-rectifying Cl− currents and inward-rectifying K+ currents (Mohammad-Panah et al. 1998). Native airway tissues and primary cultures of airway epithelia consist of multiple cell types, some of which may secrete while others absorb NaCl (Welsh 1987; Pilewski and Frizzell 1999). This possibility raises the question of whether the proportional activities of ENaC and CFTR seen in the sweat duct might be present in some cells but overshadowed by functions of others, in more complicated tissues. That is, cAMP-mediated secretion is decreased or absent in CF epithelia (Boucher 1994b; Sato and Sato 1984; Quinton 1999) so that a relatively greater loss of secretory transport in the presence of simultaneous absorptive transport could result in an apparent net increase in Na+ absorption relative to normal tissue (Boucher et al. 1988). In fact, some studies on human airways suggested an increase in salt concentration in CF airway surface fluid (Joris et al. 1993; Zabner et al. 1998; Quinton 1999). These observations seem consistent with recent reports suggesting that increased salt concentration in airway surface fluid may compromise airway defense mechanisms in CF (Smith et al. 1996; Goldman et al. 1997). Furthermore, recent studies involving human airway epithelia (Itani et al. 2011), the CFTR−/−, and CFTRΔF508/ΔF508 (Chen et al. 2010; Ostedgaard et al. 2011) have shown reduced Cl− but not Na+ conductance in CF airways. Furthermore, a recent study on the relationship between airway epithelial Cl− secretion and Na+ absorption balance has led to the conclusion that hCFTR overexpression increases basal secretion but does not regulate Na+ transport in wild-type mice (Grubb et al. 2012). A consensus on this topic is still much needed.
EFFECT OF CF ON THE ABSORPTIVE PROCESSES
Sweat Duct
Absorption of NaCl follows the previously described classic model of salt absorption by epithelia involved in hypertonic absorption. Na+ and Cl− ions enter the cell via ENaC and CFTR channels in the APM. Intracellular Na+ is pumped out by the Na+/K+ pump in the BLM. Cl− diffuses out of the cells through basolateral CFTR Cl− channels. It was shown that the CFTR anion channels are absent in both the BLM and APM of sweat ducts isolated from CF patients. Lack of functional expression of CFTR prevents Cl− exit across the BLM of CF cells (Reddy and Quinton 1992). In addition, absence of CFTR in the APM of CF cells prevents lumen-to-cell Cl− diffusion and causes significant depolarization (makes the membrane more positive) of the apical membrane potential (Reddy and Quinton 1989). APM depolarization abolishes the electrical driving force and prevents passive diffusion of Na+. As a consequence, both Na+ and Cl− ions are retained in the lumen, causing significant loss of electrolytes during sweating, particularly in patients afflicted by overheating (Quinton 1999). Thus, elevated sweat NaCl concentration is the basis of the classic pilocarpine-induced sweat test as a diagnostic feature of CF disease (Quinton 1999).
Salivary Glands
The physiology of salivary glands seems to follow the classic pattern of exocrine function in which the acinar cells of the salivary glands secrete an isotonic fluid, and the ductal system reabsorbs the salt from the primary secretion. The functional properties of salivary gland ducts appear to mimic closely those of the duct cells that are involved in hypertonic absorption of salt from the lumen using ENaC and CFTR channels. Salt absorption by the salivary ducts were significantly inhibited by amiloride and CFTRinh-172 in mouse salivary gland ducts (Catalan et al. 2010). Unlike in the airways, where CFTR seems to inhibit ENaC activity, there is a coordinated activation of CFTR and ENaC in the salivary duct cells so that inhibition of CFTR in the CF mice resulted in the increase in both Na+ and Cl− concentrations (Reddy et al. 1999; Catalan et al. 2010). There are inconsistent and conflicting reports on the effect of CF on salivary gland functions. Abnormal Cl− absorption and HCO3− secretions by the submandibular gland ducts were observed from CF patients (Blomfield et al. 1973; Davies et al. 1989, 1991; McPherson et al. 1992). Several studies have indicated functional and pathological abnormalities in salivary gland functions. It is well known that CFTR and ENaC channels play a predominant role in hypertonic salt absorption in human salivary gland ducts. Early studies indicate elevated NaCl concentration in the parotid gland secretion from CF patients (Prader and Gautier 1955; di Sant’Agnese 1956; Johnson 1956; Barbero and Sibinga 1962). Increased levels of NaCl (and defective β-adrenergic secretions) (Bergler et al. 1994a,b; Catalan et al. 2010) were also observed in the submandibular secretions from mice with the ΔF508 CFTR mutation (Catalan et al. 2010).
Small Intestine
There are conflicting reports on the effect of CF on absorptive process in the intestine. Na+-dependent nutrient transport is the predominant mechanism of absorption in the small intestine. Early reports indicated enhanced Na+-dependent glucose cotransport (Frase et al. 1985). These observations were supported by the subsequent studies showing increased nutrient-dependent short circuit currents (Baxter et al. 1988, 1989, 1990a,b; Hardcastle et al. 1990; Teune et al. 1996). It is well known that secretion of electrolyte fluid is severely compromised throughout the intestine including the jejunum and ileum (Berschneider et al. 1987, 1988; Taylor et al. 1987, 1988). Because the nutrient absorption is driven by the driving force for Na+, absence of a Cl− shunt due to lack of CFTR anion channels in the plasma membranes of CF intestinal epithelium seems to increase the driving force for Na+, causing a significant surge in glucose and other nutrient absorption. To have an increased driving force for Na+ glucose cotransport, it seems necessary that both CFTR and Na+-dependent cotransporters must be present in the plasma membranes of the same cell. In fact, a Cl− conductance resembling CFTR is colocalized with Na+/glucose cotransport in rat and human small intestine, supporting the possibility that abnormalities in glucose absorption in cystic fibrosis may be a secondary effect of defects in Cl− channel function (Baxter et al. 1990a,b; Hardcastle et al. 1990). Russo et al. (2003) have reported that fluid absorption processes are, in fact, reduced in CF jejunum because of a marked depression of passive chloride absorption, but that Na+-glucose cotransport in the CF jejunum is normal. In contrast to human intestine, intestinal glucose absorption seems reduced in transgenic mouse models of CF with the ΔF508 mutation. Therefore, it was suggested that the transgenic mouse models of CF might not accurately reflect all aspects of intestinal dysfunction in the human disease (Hardcastle et al. 2004). It was further reported that the Cftr-null mice lacking one or both copies of the NHE3 (Na+/H+ exchanger) gene exhibited increased fluidity of their intestinal contents, which prevented the formation of obstructions and increased survival (Bradford et al. 2009), indicating that salt absorption in the face of reduced secretions caused by CFTR mutations plays a significant role in the development of intestinal pathology in CF mice. However, increased Na+-dependent nutrient and fluid absorption in the face of reduced CFTR-dependent Cl− secretion in CF intestinal epithelium appears to be a primary cause of dehydrated luminal content leading to the pathogenesis of meconium ileus in CF patients (Berschneider et al. 1988).
Colon
Electrolyte and fluid absorption is the predominant function of the colonic epithelium. The human colon absorbs large quantities of fluid and electrolytes per day (∼1.5 L/d) (Debongnie and Phillips 1978). Absorptive processes in the colon are complex and exhibit significant regional differences. In the ascending colon, electroneutral transport involving Na+/H+ and Cl−/HCO3− exchangers seems to be the predominant mechanism of absorption. In contrast, electrogenic absorption involving ENaC and CFTR channels appears to play a significant role in electrolyte fluid absorption in the descending colon (Clauss et al. 1985; Kunzelmann et al. 2001; Kunzelmann and Mall 2002). Defects in the absorptive processes were observed in both electroneutral as well as electrogenic components of colonic absorptive processes (Kunzelmann and Mall 2001, 2002). Early studies on CF mouse colons show increased electrogenic sodium absorption compared with wild-type tissues. Elevated plasma aldosterone in CF mice was suspected to be responsible for part or all of the increased sodium absorption in CF mouse colons (Cuthbert et al. 1994, 1999). It was also reported that in both CFTR knockout mice and in CF patients, the absence of CFTR-mediated inhibition of ENaC seems responsible for increased amiloride-sensitive short-circuit currents and Na+ absorption in the colonic epithelium compared with non-CF controls (Grubb and Boucher 1997; Briel et al. 1998; Mall et al. 1999; Kunzelmann and Mall 2001; Kunzelmann et al. 2001).
Isotonic Salt Absorption by Airway Epithelia
The essential physiological purpose of human airways is to conduct inspired air to and from the alveolar surfaces where gas exchange occurs. Structurally, the lower airways dichotomously branch from the trachea (∼2 cm diameter) to thousands of terminal airways (0.5–1 mm diameter) (Kilburn 1974). The large aggregate surface of these conducting airways is lined by a continuous epithelium that is protected from desiccation by a thin (∼10 µm) liquid layer called airway surface liquid (ASL). Additionally, ASL supports the innate immune defense mechanism of mucociliary clearance of particles deposited from inhaled air, including infectious agents (Knowles and Boucher 2002). The airway mucosal surface is kept hydrated and mucociliary clearance is maintained by regulation of ASL volume and composition through the fluid transport activities and passive barrier properties of airway epithelia (Boucher 1994a). Airway epithelia are equipped both to absorb Na+ and secrete Cl−, indicative of the importance of regulating airway surface hydration. Electroneutrality requires that Na+ cannot be absorbed without a co-ion, predominantly Cl−, and neither can Cl− be secreted without an co-ion, predominantly Na+. Thus, ASL homeostasis is achieved by balanced isotonic salt absorption and secretion.
Path for Na+ Absorption
Airway epithelia are composed of ciliated and nonciliated cells (Kreda et al. 2005). Ciliated cells not only elaborate the cilia that move ASL cephalad, but are also the cells that accomplish salt transport. Nonciliated cells secrete macromolecules, used in innate defense, including mucins, whose physicochemical properties are dependent on hydration (Kesimer et al. 2010). The path for Na+ absorption through ciliated cells consists of entry at the apical membrane and extrusion from the basolateral membrane. Entry of Na+ from ASL is rate limiting for Na+ absorption and is primarily mediated by the epithelial Na+ channel (ENaC). Other means of Na+ entry have been considered, such as Na+/proton exchange or Na+/glucose cotransport, but have not been found to play a major role in airway epithelia. Na+ extrusion across the basolateral membrane is accomplished by Na+–K+-ATPase (Knowles et al. 1984). This transport element is obviously essential for Na+ absorption by all epithelia, but it does not appear to be the point of regulation in airway epithelia. Thus, Na+ absorption in airways is regulated principally at the ENaC-mediated entry step, as discussed below.
Path for Cl− Absorption
Isotonic absorption of fluid and salt in response to electrochemical driving forces generated by active Na+ transport requires a Cl− conductance. Cl− is predicted to diffuse across the epithelium through available cellular and paracellular routes. The paracellular path of airway epithelia has the properties of free solution (Cotton et al. 1983). The further observation that the ratio of Cl− to mannitol trans-epithelial permeability coefficients exceeds the ratio of their free solution diffusivities suggests that a trans-cellular, in addition to a paracellular, route is available for passive Cl− diffusion (Stutts et al. 1988). Trans-cellular cellular Cl− absorption requires Cl− entry across the apical membrane. Although most work on the elements of Cl− movement across airway epithelia has focused on Cl− secretion, ion channels in the apical cell membrane including CFTR and other Cl− channels, such as ano1, provide an apical Cl− conductance for Cl− to enter or leave the cell, depending on the existing Cl− electrochemical potential (Willumsen et al. 1989; Tarran et al. 2002; Ferrera et al. 2010). Moreover, airway epithelia express SLC family members that exchange intracellular HCO3− for extracellular Cl− (Mount and Romero 2004). Cl− movement from cell to interstitium is thought to require a Cl− basolateral cell membrane conductance, for which electrophysiological data exist, but for which no molecular identity is known (Reddy and Quinton 1992; Uyekubo et al. 1998).
Relationship between Na+ Absorption and Cl− Secretion in Airways
Studies in thin film preparations confirm that both Na+ absorption and Cl− secretion are used to control the depth of liquid on the airway surface (Tarran et al. 2002, 2006). This duality of salt transport mechanisms is extensively documented in studies of freshly excised airway epithelia of many species (Olver et al. 1975), including human (Boucher et al. 1991). Ion flux data from decades of studies show an absorption flux of Na+ that exceeds passive backflux under both short circuit and open circuit conditions (Boucher 1994a,b; Mall 2009), consistent with a classic passive Na+ entry from the luminal solution via ENaC with active extrusion of Na+ by the basolateral Na+/K+ ATPase (Ussing et al. 1974). This net flux is eliminated by amiloride-like ENaC blockers and by ouabain, an inhibitor of the Na+/K+ pump (Olver et al. 1975). The same studies have characterized Cl− secretion by airway epithelia as a secondary active ion transport process (Olver et al. 1975; Boucher et al. 1980). That is, Cl− is not actively transported per se but is accumulated in airway cells above its predicted electrochemical potential by a basolateral cotransporter that use Na+, K+, and Cl− gradients supported by the Na+/K+ pump (Frizzell et al. 1979). Passive Cl− conductance in the apical membrane allows Cl− accumulated above its electrochemical potential to exit into the luminal solution, creating net Cl− secretory transport (cf. Frizzell and Hanrahan 2013). Consistent with the passive nature of this process, Cl− secretion by airway epithelia responds to maneuvers that hyperpolarize the apical membrane potential. Such maneuvers prominently include Na+ channel blockers and clamping trans-epithelial potential difference to zero (so-called short circuit conditions). Even so, under open circuit conditions and in the absence of amiloride, net absorption of both Na+ and Cl− has been documented in native human (Knowles et al. 1984) and canine bronchial epithelium (Boucher et al. 1981).
Water Permeability of Airway Epithelia
A striking characteristic of airway epithelia that sets it apart from sweat ductal epithelia is its significant water permeability (Matsui et al. 2000; Verkman et al. 2000). Decreased water permeability is detected in alveoli and airways mice with knocked-down expression of aquaporins, but ASL hydration was unaffected (Verkman 2007). This result was attributed to the relatively small flux of water across airway epithelia, compared with the overall non-aquaporin routes available for water diffusion across airway epithelia (AS 2007). Accordingly, water permeability is not expected to be limiting for fluid absorption by airway epithelia.
Regulation of ENaC
Net salt and fluid absorption, that is, the net of active Na+ absorption (with passive Cl−) and Cl− secretion (with passive Na+), is determined by the driving forces established in response to Na+ conductance of the apical membrane. When Cl− conductance is predominant owing to the activation of CFTR and Na+ conductance is blocked or very small, the apical membrane should hyperpolarize sufficiently to support Cl− secretion. As Na+ conductance increases, the apical cell membrane must depolarize and diminish the driving force on Cl− to passing from cell to lumen (secretion). At some point as Na+ conductance increases, the net driving force on Cl− reverses and Cl− is no longer secreted but moves in the absorptive direction to support net Na+ and Cl− (and fluid) absorption. Because apical Na+ conductance is principally due to ENaC, with a small component of nonselective cation conductance of uncertain molecular origin, regulation of ENaC in airway epithelia is effectively the regulator of fluid absorption.
ENaC Structure and Stoichiometry
ENaC is a heteromeric channel composed of α-, β-, and γ-subunits (Canessa et al. 1994b). Each subunit has two membrane-spanning regions with short amino and carboxyl cytoplasmic tails. The membrane-spanning regions of each subunit are joined by a large extracellular domain (Canessa et al. 1994b). ENaC belongs to a family of channels with similar topology that include acid-sensing channels (ASICs) (Kellenberger and Schild 2002). ASIC1 has been crystallized, revealing a homotrimeric structure (Jasti et al. 2007). This was taken as a strong indication that ENaC has a trimeric structure of α-, β-, and γ-subunits (Stockand et al. 2008), but at this time, ENaC stoichiometry remains a controversial question (Anantharam and Palmer 2007).
ENaC trafficking to and from the apical membrane involves regulated processes that determine the density of ENaC in the apical membrane (Palmer et al. 2012). Liddle’s disease of inherited amiloride-sensitive hypertension is caused by mutations in the carboxy-terminal cytosolic tails of β- and γ-ENaC, which contain highly conserved PY protein interaction motifs that mediate the association of ENaC with the E3 ubiquitin ligase, Nedd4-L (Schild et al. 1996; Staub et al. 2000). Nedd4-L catalyzes ubiquitinylation of ENaC cytosolic amino-terminal lysines, and this posttranslational modification causes ENaC internalization (Rotin 2000). Numerous signaling pathways, including SGK1, PKA, and MAP kinases, adjust the strength of the ENaC–Nedd4-L association through the phosphorylation status of specific residues, giving rise to wide tissue variability in the impact of this regulatory axis (Debonnevilles et al. 2001). For example, the hypertension of patients with Liddle’s disease is attributed to excess ENaC at the apical membrane of renal tubules (Palmer et al. 2012). However, Liddle’s disease patients show no evidence of increased Na+ absorption in respiratory epithelia (Knowles and Boucher 2002), suggesting that other mechanisms exist for controlling Na+ conductance in certain tissues. Nonetheless, Nedd4-L must provide the underlying mechanism of retrieval of ENaC from the surface of airway epithelial cells, because mice with lung-specific Nedd4-L knockdown developed Na+ hyperabsorption and fatal lung disease (Kimura et al. 2011).
ENaC activity at the apical membrane is also subject to regulation by signaling that influences channel open probability (PO). ENaC is stimulated by proteases that cleave sites within the extracellular domains of ENaC (Vuagniaux et al. 2000; Hughey et al. 2004). This action acutely increases PO (Caldwell et al. 2004, 2005). Although still not completely understood, it now appears that cleavage at defined sites within the extracellular domains of α- and γ-ENaC results in conformational changes that allow greater stability of the open state of the channel (Kashlan et al. 2011). It is also evident that numerous proteases can cleave and activate ENaC and that their catalytic activity is opposed by cognate protease inhibitors (Myerburg et al. 2008; Passero et al. 2008; Svenningsen et al. 2009). Thus, the stimulation of ENaC through extracellular proteolysis is likely to be intricately balanced and will depend on the coincidence of proteases and inhibitor expression with ENaC.
ENaC PO is strongly stimulated by binding to membrane phosphoinositides through basic residues in the cytosolic amino termini, especially β- and γ-ENaC (Ma et al. 2002; Yue et al. 2002). Numerous reports have established that exogenous PIP2 or PIP3 is a strong stimulate of ENaC, such that signaling mechanisms that affect the abundance of these phosphoinositides clearly influence ENaC-mediated Na+ absorption (Kunzelmann et al. 2004; Wang et al. 2008).
Regulation of ASL Depth by Embedded Signals in Normal and CF Airways
ENaC-mediated Na+ conductance strongly affects the electrical potential difference across the apical membrane of airway epithelia (Willumsen et al. 1989; Willumsen and Boucher 1991), which determines the balance between isotonic fluid absorption and fluid secretion. Tarran and others, using “thin film” or “air–liquid interface” culture techniques, showed that airway cells cultured under conditions that mimic the ∼10-μm depth of ASL maintained in vivo rely on soluble signals in the small volume on the culture surface to regulate ENaC (Tarran et al. 2001, 2005; Song et al. 2009). In fact, this work identified both protease inhibitors as well as nucleotides as the major inhibitory regulators of ENaC. As ASL depth is reduced, the concentration of these ENaC inhibitory signals increases, slowing NaCl absorption (Myerburg et al. 2006, 2010; Tarran et al. 2006). Moreover, ATP can stimulate Ca2+-dependent Cl− conductance in the apical membrane, and adenosine arising from the catabolism of ATP can stimulate CFTR activity. With ENaC inhibited, driving forces for Cl− secretion can be achieved. In this manner, airway epithelia can temper, or even reverse, the absorption of NaCl and H2O to maintain optimal airway surface hydration. These approaches revealed a striking difference between ASL homeostasis in differentiated cultures derived from normal and CF airways. CF cultures were unable to switch from absorption to secretion, leading to collapse of ASL depth (Tarran et al. 2006). This result was not unpredicted, given the absence of CFTR to mediate Cl− secretion in the CF cultures. However, it was also clear that ENaC remained hyperactive, even as ASL height decreased. A potential basis for ENaC hyperactivity in CF was detected in two laboratories as a much greater extent of ENaC cleavage in differentiated CF cultures compared with normal cultures (Myerburg et al. 2006; Gentzsch et al. 2010). Coexpression of CFTR in Xenopus oocytes with ENaC strongly depressed ENaC proteolysis and amiloride-sensitive currents. Taken together, these results confirm the role of CFTR-mediated Cl− secretion in homeostatic control of ASL volume and further implicate loss of CFTR-mediated inhibition of ENaC proteolysis/stimulation as a potential cause of the dysregulated Na+ absorption that has been reported in CF airways.
Footnotes
Editors: John R. Riordan, Richard C. Boucher, and Paul M. Quinton
Additional Perspectives on Cystic Fibrosis available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Anantharam A, Palmer LG 2007. Determination of epithelial Na+ channel subunit stoichiometry from single-channel conductances. J Gen Physiol 130: 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ 1991. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell 67: 775–784 [DOI] [PubMed] [Google Scholar]
- Barbero GJ, Sibinga MS 1962. Enlargement of the submaxillary salivary glands in cystic fibrosis. Pediatrics 29: 788–793 [PubMed] [Google Scholar]
- Baxter PS, Dickson JAS, Variend S, Taylor CJ 1988. Intestinal disease in cystic fibrosis. Arch of Dis Child 63: 1496–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Read NW, Hardcastle PT, Wilson AJ, Hardcastle J, Taylor CJ 1989. Abnormal jejunal potential difference in cystic fibrosis. Lancet 464–466 [DOI] [PubMed] [Google Scholar]
- Baxter P, Goldhill J, Hardcastle J, Hardcastle PT, Taylor CJ 1990a. Enhanced intestinal glucose and alanine transport in cystic fibrosis. Gut 31: 817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Goldhill J, Hardcastle J, Hardcastle PT, Taylor CJ 1990b. Enhanced intestinal glucose and alanine transport in cystic fibrosis. Gut 31: 817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger HA, Travis SM, Welsh MJ 1993. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and protein phosphatases. J Biol Chem 268: 2037–2047 [PubMed] [Google Scholar]
- Bergler MK, Dorin JR, Porteous DJ, Quinton PM 1994a. Defective salivary gland function in the CF mouse reveals three distinct phenotypes. Ped Pulmon 10: 211 [Google Scholar]
- Bergler MK, Dorin JR, Porteous DJ, Quinton PM 1994b. Defective salivary gland function in the CF mouse. European Cystic Fibrosis Conference Paris, France, June [Google Scholar]
- Berschneider HM, Azizhan RG, Knowles M, Boucher RC, Powell DW 1987. Intestinal electrolyte in cystic fibrosis. Gastroenterology 92: 1315 [Google Scholar]
- Berschneider HM, Knowles MR, Azizkhan RJ, Boucher RC, Tobey NA, Orlando RC, Powell DW 1988. Altered intestinal chloride transport in cystic fibrosis. FASEB J 2: 2625–2629 [DOI] [PubMed] [Google Scholar]
- Bhalla V, Hallows KR 2008. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854 [DOI] [PubMed] [Google Scholar]
- Bijman J, Quinton PM 1984. Influence of abnormal Cl− impermeability on sweating in cystic fibrosis. Am J Physiol 247: C3–C9 [DOI] [PubMed] [Google Scholar]
- Blomfield J, Warton KL, Brown JM 1973. Flow rate and inorganic components of submandibular saliva in cystic fibrosis. Arch Dis Child 48: 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC 1994a. Human airway ion transport. Part one. Am J Respir Crit Care Med 150: 271–281 [DOI] [PubMed] [Google Scholar]
- Boucher RC 1994b. Human airway ion transport. Part two. Am J Respir Crit Care Med 150: 581–593 [DOI] [PubMed] [Google Scholar]
- Boucher RC 2001. Pathogenesis of cystic fibrosis airways disease. Trans Am Clin Climatol Assoc 112: 99–107 [PMC free article] [PubMed] [Google Scholar]
- Boucher RC Jr, Bromberg PA, Gatzy JT 1980. Airway transepithelial electric potential in vivo: Species and regional differences. J Appl Physiol 48: 169–176 [DOI] [PubMed] [Google Scholar]
- Boucher RC, Stutts MJ, Gatzy JT 1981. Regional differences in bioelectric properties and ion flow in excised canine airways. J Appl Physiol 51: 706–714 [DOI] [PubMed] [Google Scholar]
- Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR 1988. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol (Paris) 405: 77–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC, Chinet T, Willumsen N, Knowles MR, Stutts MJ 1991. Ion transport in normal and CF airway epithelia. Adv Exp Med Biol 290: 105–115 [DOI] [PubMed] [Google Scholar]
- Bradford EM, Sartor MA, Gawenis LR, Clarke LL, Shull GE 2009. Reduced NHE3-mediated Na+ absorption increases survival and decreases the incidence of intestinal obstructions in cystic fibrosis mice. Am J Physiol Gastrointest Liver Physiol 296: G886–G898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briel M, Greger R, Kunzelmann K 1998. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol 508: 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ 2004. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194 [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ 2005. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819 [DOI] [PubMed] [Google Scholar]
- Canessa CM, Merillat AM, Rossier BC 1994a. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol 267: C1682–C1690 [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC 1994b. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467 [DOI] [PubMed] [Google Scholar]
- Catalan MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall AM, Clarke LL, Melvin JE 2010. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588: 713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, et al. 2010. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE 1991. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell 66: 1027–1036 [DOI] [PubMed] [Google Scholar]
- Clauss W, Dürr J, Skadhauge E, Hörnicke H 1985. Effects of aldosterone and dexamethasone on apical membrane properties and Na-transport of rabbit distal colon in vitro. Pflugers Arch 403: 186–192 [DOI] [PubMed] [Google Scholar]
- Cook DI, Dinudom A, Komwatana P, Young JA 1998. Control of Na+ transport in salivary duct epithelial cells by cytosolic Cl− and Na+. Eur J Morphol 36: 67–73 [PubMed] [Google Scholar]
- Cotton CU, Lawson EE, Boucher RC, Gatzy JT 1983. Bioelectric properties and ion transport of airways excised from adult and fetal sheep. J Appl Physiol 55: 1542–1549 [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, MacVinish LJ, Hickman ME, Ratcliff R, Colledge WH, Evans NJ 1994. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflugers Arch 428: 508–515 [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Hickman ME, MacVinish LJ 1999. Formal analysis of electrogenic sodium, potassium, chloride and bicarbonate transport in mouse colon epithelium. Br J Pharmacol 126: 358–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bagg J, Muxworthy S, Goodchild MC, McPherson MA 1989. Electrolyte concentrations in control and cystic fibrosis submandibular saliva. Biochem Soc Trans 18: 447–448 [DOI] [PubMed] [Google Scholar]
- Davies H, Bagg J, Goodchild MC, McPherson MA 1991. Defective regulation of electrolyte and protein secretion in submandibular saliva of cystic fibrosis patients. Acta Paediatr Scand 80: 1094–1095 [DOI] [PubMed] [Google Scholar]
- Debongnie JC, Phillips SF 1978. Capacity of the human colon to absorb fluid. Gastroenterology 74: 698–703 [PubMed] [Google Scholar]
- Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Münster C, Chraïbi A, Pratt JH, Horisberger JD, et al. 2001. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Rosa DA, Canessa CM, Fyfe GK, Zhang P 2000. Structure and regulation of amiloride sensitive sodium channels. Annu Rev Physiol 62: 573–594 [DOI] [PubMed] [Google Scholar]
- Devuyst O, Guggino WB 2002. Chloride channels in the kidney: Lessons learned from knockout animals. Am J Physiol Renal Physiol 283: F1176–F1191 [DOI] [PubMed] [Google Scholar]
- Dinudom A, Young JA, Cook DI 1993. Na+ and Cl− conductances are controlled by cytosolic Cl− concentration in the intralobular duct cells of mouse mandibular glands. J Membr Biol 135: 289–295 [DOI] [PubMed] [Google Scholar]
- di Sant’Agnese PA 1956. Cystic fibrosis of the pancreas. Am J Med 21: 406–422 [DOI] [PubMed] [Google Scholar]
- Duc C, Farman N, Canessa MC, Bonvalet JP, Rossier BC 1994. Cell-specific expression of epithelial sodium channel α, β, and γ subunits in aldosterone-responsive epithelia from the rat: Localization by in situ hybridization and immunocytochemistry. J Cell Biol 127: 1907–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhanty AM, Riordan JR 1994. Phosphorylation by cAMP-dependent protein kinase causes a conformational change in the R domain of the cystic fibrosis transmembrane conductance regulator. Biochemistry 33: 4072–4079 [DOI] [PubMed] [Google Scholar]
- Ferrera L, Caputo A, Galletta LJ 2010. TMEM16A protein: A new identity for Ca2+-dependent Cl− channels. Physiology 25: 357–363 [DOI] [PubMed] [Google Scholar]
- Fischer H, Illek B, Machen TE 1998. Regulation of CFTR by protein phosphatase 2B and protein kinase C. Pflugers Arch 436: 175–181 [DOI] [PubMed] [Google Scholar]
- Frase LL, Strickland AD, Kachel GW, Krejs GJ 1985. Enhanced glucose absorption in the jejunum of patients with cystic fibrosis. Gastroenterology 88: 478–484 [DOI] [PubMed] [Google Scholar]
- French PJ, Bijman J, Edixhoven M, Vaandrager AB, Scholte BJ, Lohmann SM, Nairn AC, De Jonge HR 1995. Isotype-specific activation of cystic fibrosis transmembrane conductance regulator-chloride channels by cGMP-dependent protein kinase II. J Biol Chem 270: 26626–26631 [DOI] [PubMed] [Google Scholar]
- *.Frizzell RA, Hanrahan JW 2013. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a009563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell RA, Field M, Schultz SG 1979. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol 263: F1–F8 [DOI] [PubMed] [Google Scholar]
- Fuller CM, Awayda MS, Arrate MP, Bradford AL, Morris RG, Canessa CM, Rossier BC, Benos DJ 1995. Cloning of a bovine renal epithelial Na+ cannel subunit. Am J Physiol 269: C641–C654 [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nairn AC 1999. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol Rev 79: S77–S107 [DOI] [PubMed] [Google Scholar]
- Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ 2010. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem 285: 32227–32232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zaslof M, Wilson JM 1997. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88: 553–560 [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC 1997. Enhanced colonic Na+ absorption in cystic fibrosis mice versus normal mice. Am J Physiol 272: G393–G400 [DOI] [PubMed] [Google Scholar]
- Grubb BR, O’Neal WK, Ostrowski LE, Kreda SM, Button B, Boucher RC 2012. Transgenic hCFTR expression fails to correct β-ENaC mouse lung disease. Am J Physiol Lung Cell Mol Physiol 302: L238–L247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino WB 1999. Cystic fibrosis and the salt controversy. Cell 96: 607–610 [DOI] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ 1998. A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci 95: 8496–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle J, Taylor CJ, Hardcastle PT, Baxter PS, Goldhill J 1990. Intestinal transport in cystic fibrosis (CF). Acta Univ Carol (Praha) 36: 157–158 [PubMed] [Google Scholar]
- Hardcastle J, Harwood MD, Taylor CJ 2004. Small intestinal glucose absorption in cystic fibrosis: A study in human and transgenic ΔF508 cystic fibrosis mouse tissues. J Pharm Pharmacol 56: 329–338 [DOI] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR 2004. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114 [DOI] [PubMed] [Google Scholar]
- Hummler E, Horisberger JD 1999. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human disease. Am J Physiol 276: G567–G571 [DOI] [PubMed] [Google Scholar]
- Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ 2011. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci 108: 10260–10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E 2007. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Jia Y, Mathews CJ, Hanrahan JW 1997. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem 272: 4978–4984 [DOI] [PubMed] [Google Scholar]
- Johnson W 1956. Salivary electrolytes in fibrocystic disease. Arch Dis Child 31: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris L, Dab I, Quinton PM 1993. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis 148: 1633–1637 [DOI] [PubMed] [Google Scholar]
- Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M 2011. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286: 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L 2002. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767 [DOI] [PubMed] [Google Scholar]
- Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK 2010. Unpacking a gel-forming mucin: A view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol 298: L15–L22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn K 1974. Functional morphology of the distal lung. Int Rev Cytol 37: 153–270 [DOI] [PubMed] [Google Scholar]
- Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D 2011. Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci 108: 3216–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC 2002. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M, Murray G, Shallal J, Askin F, Ranga V, Gatzy J, Boucher R 1984. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol 56: 868–877 [DOI] [PubMed] [Google Scholar]
- Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz MW, Wager GC, Gatzy JT, Boucher RC 1997. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest 100: 2588–2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YH, Pedersen PL 1995. The first nucleotide binding fold of the cystic fibrosis transmembrane conductance regulator can function as an active ATPase. J Biol Chem 270: 22093–22096 [DOI] [PubMed] [Google Scholar]
- Kokko JP 1973. Proximal tubule potential difference dependence on glucose, HCO3− and amino acids. J Clin Invest 52: 1362–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC 2005. Characterization of wild-type and ΔF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell 16: 2154–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M 2001. Pharmacotherapy of the ion transport defect in cystic fibrosis. Clin Exp Pharmacol Physiol 28: 857–867 [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M 2002. Electrolyte transport in the mammalian colon: Mechanisms and implications for disease. Physiol Rev 82: 245–289 [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Schreiber R, Boucherot A 2001. Mechanisms of the inhibition of epithelial Na+ channels by CFTR and purinergic stimulation. Kidney Int 60: 455–461 [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R 2004. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142–143 [DOI] [PubMed] [Google Scholar]
- Luo J, Pato MD, Riordan JR, Hanrahan JW 1998. Differential regulation of single CFTR channels by PP2C, PP2A, and other phosphatases. Am J Physiol 274: C1397–C1410 [DOI] [PubMed] [Google Scholar]
- Ma HP, Saxena S, Warnock DG 2002. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644 [DOI] [PubMed] [Google Scholar]
- Mall MA 2009. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol 94: 171–174 [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K 1999. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol 277: G709–G716 [DOI] [PubMed] [Google Scholar]
- Matsui H, Davis CW, Tarran R, Boucher RC 2000. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest 105: 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson MA, Davies H, Mills CL, Pereira MMC, Goodchild MC, Dormer RL 1992. Role of CFTR in salivary secretion. Elsevier, Amsterdam [Google Scholar]
- Mohammad-Panah R, Demolombe S, Riochet D, Leblais V, Loussouarn G, Pollard H, Baro I, Escande D 1998. Hyperexpression of recombinant CFTR in heterologous cells alters its physiological properties. Am J Physiol 274: C310–C318 [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF 2004. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch 447: 710–721 [DOI] [PubMed] [Google Scholar]
- Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM 2006. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance. J Biol Chem 281: 27942–27949 [DOI] [PubMed] [Google Scholar]
- Myerburg MM, McKenna EE, Luke CJ, Frizzell RA, Kleyman TR, Pilewski JM 2008. Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L932–L941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB 2010. Acute regulation of ENaC in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol 43: 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver RE, Davis B, Marin MG, Nadel JA 1975. Active transport of Na+ and Cl− across the canine tracheal epithelium in vitro. Am Rev Respir Dis 112: 811–815 [DOI] [PubMed] [Google Scholar]
- Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, et al. 2011. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 3: 74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L, Patel A, Frindt G 2012. Regulation and dysregulation of epithelial Na+ channels. Clin Exp Nephrol 16: 35–43 [DOI] [PubMed] [Google Scholar]
- Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR 2008. Plasmin activates epithelial Na+ channels by cleaving the γ subunit. J Biol Chem 283: 36586–36591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Cohn JA, Bertuzzi G, Greengard P, Nairn AC 1992. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 267: 12742–12752 [PubMed] [Google Scholar]
- Pilewski JM, Frizzell RA 1999. Role of CFTR in airway disease. Physiol Rev 79: S215–S255 [DOI] [PubMed] [Google Scholar]
- Prader A, Gautier E 1955. Die Na− and K− Konzentration in gemischten Speichel: II Erhohte Werte bei der Pankreasfibrose. Helv Paediat Acta 10: 56. [PubMed] [Google Scholar]
- Quinton PM 1984. Exocrine glands. In Cystic fibrosis (ed. Taussig LM), pp. 338–375 Thieme-Stratton, New York [Google Scholar]
- Quinton PM 1987. Physiology of sweat secretion. Kidney Int Suppl 21: S102–S108 [PubMed] [Google Scholar]
- Quinton PM 1990. Cystic fibrosis: A disease in electrolyte transport. FASEB J 4: 2709–2717 [DOI] [PubMed] [Google Scholar]
- Quinton PM 1999. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev 79: S3–S22 [DOI] [PubMed] [Google Scholar]
- Quinton PM 2007. Cystic fibrosis: Lessons from the sweat gland. Physiology (Bethesda) 22: 212–225 [DOI] [PubMed] [Google Scholar]
- Quinton PM, Reddy MM 1991. Regulation of absorption in the human sweat duct. Adv Exp Med Biol 290: 159–170 [DOI] [PubMed] [Google Scholar]
- Randak C, Neth P, Auerswald EA, Eckerskorn C, Assfalg-Machleidt I, Machleidt W 1997. A recombinant polypeptide model of the second nucleotide-binding fold of the cystic fibrosis transmembrane conductance regulator functions as an active ATPase, GTPase and adenylate kinase. FEBS Lett 410: 180–186 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1987. Intracellular potentials of microperfused human sweat duct cells. Pflugers Arch 410: 471–475 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1989. Altered electrical potential profile of human reabsorptive sweat duct cells in cystic fibrosis. Am J Physiol 257: C722–C726 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1991. Intracellular potassium activity and the role of potassium in transepithelial salt transport in the human reabsorptive sweat duct. J Membr Biol 119: 199–210 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1992. cAMP activation of CF-affected Cl− conductance in both cell membranes of an absorptive epithelium. J Membr Biol 130: 49–62 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1994. Intracellular Cl activity: Evidence of dual mechanisms of cl absorption in sweat duct. Am J Physiol 267: C1136–C1144 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 1996. Hydrolytic and nonhydrolytic interactions in the ATP regulation of CFTR Cl− conductance. Am J Physiol 271: C35–C42 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 2002. Influence of cytoplasmic Cl− on the effect of CFTR activation on ENaC function. Pediatr Pulmonology 34 (Suppl): 8–359 [Google Scholar]
- Reddy MM, Quinton PM 2003a. Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch 445: 499–503 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM 2003b. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature 423: 756–760 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM 1999. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature 402: 301–304 [DOI] [PubMed] [Google Scholar]
- Reddy MM, Wang XF, Quinton PM 2008. Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol 225: 1–11 [DOI] [PubMed] [Google Scholar]
- Reuss L 1985. Changes in cell volume measured with an electrophysiologic technique. Proc Natl Acad Sci 82: 6014–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L, Segal Y, Altenberg G 1991. Regulation of ion transport across gallbladder epithelium. Annu Rev Physiol 53: 361–373 [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. 1989. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245: 1066–1073 [DOI] [PubMed] [Google Scholar]
- Rossier BC 1996. The renal epithelial sodium channel: New insights in understanding hypertension. Adv Nephrol Necker Hosp 25: 275–286 [PubMed] [Google Scholar]
- Rotin D 2000. Regulation of the epithelial sodium channel (ENaC) by accessory proteins. Curr Opin Nephrol Hypertens 9: 529–534 [DOI] [PubMed] [Google Scholar]
- Russo MA, Hogenauer C, Coates SW Jr, Santa Ana CA, Porter JL, Rosenblatt RL, Emmett M, Fordtran JS 2003. Abnormal passive chloride absorption in cystic fibrosis jejunum functionally opposes the classic chloride secretory defect. J Clin Invest 112: 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato F 1984. Defective β adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest 73: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC 1995. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci 92: 5699–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC 1996. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 15: 2381–2387 [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K 1999. The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci 96: 5310–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB 1999. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev 79: S145–S166 [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ 1999. Structure and function of the CFTR chloride channel. Physiol Rev 79: S23–S45 [DOI] [PubMed] [Google Scholar]
- Short DB, Trotter KW, Reczek D, Kreda SM, Bretscher A, Boucher RC, Stutts MJ, Milgram SL 1998. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J Biol Chem 273: 19797–19801 [DOI] [PubMed] [Google Scholar]
- Smith JJ, Travis SM, Greenberg EM, Welsh MJ 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85: 229–236 [DOI] [PubMed] [Google Scholar]
- Song Y, Namkung W, Nielson DW, Lee JW, Finkbeiner WE, Verkman AS 2009. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: Dependence on Na+ and Cl− channel function. Am J Physiol Lung Cell Mol Physiol 297: L1131–L1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D 2000. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int 57: 809–815 [DOI] [PubMed] [Google Scholar]
- Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU 2008. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 60: 620–628 [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Gatzy JT, Boucher RC 1988. Activation of chloride conductance induced by potassium in tracheal epithelium. Pflugers Arch 411: 252–258 [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC 1995. CFTR as a cAMP-dependent regulator of sodium channels. Science 269: 847–850 [DOI] [PubMed] [Google Scholar]
- Svenningsen P, Uhrenholt TR, Palarasah Y, Skjødt K, Jensen BL, Skøtt O 2009. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol 297: R1733–R1741 [DOI] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC 2001. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol 118: 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Loewen ME, Paradiso AM, Olsen JC, Gray MA, Argent BE, Boucher RC, Gabriel SE 2002. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J Gen Physiol 120: 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Button B, Boucher RC 2005. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol 68: 543–561 [DOI] [PubMed] [Google Scholar]
- Tarran R, Trout L, Donaldson SH, Boucher RC 2006. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis. J Gen Physiol 127: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT 1987. Absence of secretory response in jejunal biopsy samples from children with cystic fibrosis. Lancet 330: 107–108 [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Baxter PS, Hardcastle J, Hardcastle PT 1988. Failure to induce secretion in jejunal biopsies from children with cystic fibrosis. Gut 29: 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, Timmers-Reker AJ, Bouquet J, Bijman J, De Jonge HR, Sinaasappel M 1996. In vivo measurement of chloride and water secretion in the jejunum of cystic fibrosis patients. Pediatr Res 40: 522–527 [DOI] [PubMed] [Google Scholar]
- Ussing HH, Erlij D, Lassen U 1974. Transport pathways in biological membranes. Annu Rev Physiol 36: 17–49 [DOI] [PubMed] [Google Scholar]
- Uyekubo SN, Fischer H, Maminishkis A, Illek B, Miller SS, Widdicombe JH 1998. cAMP-dependent absorption of chloride across airway epithelium. Am J Physiol 275: L1219–L1227 [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Ehlert EM, Jarchau T, Lohmann SM, de Jonge HR 1996. N-terminal myristoylation is required for membrane localization of cGMP-dependent protein kinase type II. J Biol Chem 271: 7025–7029 [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EM, Bot AG, Edixhoven M, Boomaars WE, Lohmann SM, de Jonge HR 1998. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proc Natl Acad Sci 95: 1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS 2007. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol 159: 324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Matthay MA, Song Y 2000. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol 278: L867–L879 [DOI] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E 2000. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol 11: 828–834 [DOI] [PubMed] [Google Scholar]
- Wang J, Knight ZA, Fiedler D, Williams O, Shokat KM, Pearce D 2008. Activity of the p110-α subunit of phosphatidylinositol-3-kinase is required for activation of epithelial sodium transport. Am J Physiol Renal Physiol 295: F843–F850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ 1987. Electrolyte transport by airway epithelia. Physiol Rev 67: 1143–1184 [DOI] [PubMed] [Google Scholar]
- Welsh MJ 1996. Cystic fibrosis. In Molecular biology of membrane transport disorders, 2nd ed. (ed. Schultz SG), Chapter 30, pp. 605–623 Springer, New York [Google Scholar]
- Welsh MJ, Anderson MP, Rich DP, Berger HA, Denning GM, Ostedgaard LS, Sheppard DN, Cheng SH, Gregory RJ, Smith AE 1992. Cystic fibrosis transmembrane conductance regulator: A chloride channel with novel regulation. Neuron 8: 821–829 [DOI] [PubMed] [Google Scholar]
- Widdicombe JH, Widdicombe JG 1995. Regulation of human airway surface liquid. Respir Physiol 99: 3–12 [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC 1991. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am J Physiol 261: C319–C331 [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Davis CW, Boucher RC 1989. Intracellular Cl− activity and cellular Cl− pathways in cultured human airway epithelium. Am J Physiol 256: C1033–C1044 [DOI] [PubMed] [Google Scholar]
- Yue G, Malik B, Yue G, Eaton DC 2002. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969 [DOI] [PubMed] [Google Scholar]
- Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ 1998. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell 2: 397–403 [DOI] [PubMed] [Google Scholar]