Abstract
Although a role for microRNA399 (miR399) in plant responses to phosphate (Pi) starvation has been indicated, the regulatory mechanism underlying miR399 gene expression is not clear. Here, we report that AtMYB2 functions as a direct transcriptional activator for miR399 in Arabidopsis (Arabidopsis thaliana) Pi starvation signaling. Compared with untransformed control plants, transgenic plants constitutively overexpressing AtMYB2 showed increased miR399f expression and tissue Pi contents under high Pi growth and exhibited elevated expression of a subset of Pi starvation-induced genes. Pi starvation-induced root architectural changes were more exaggerated in AtMYB2-overexpressing transgenic plants compared with the wild type. AtMYB2 directly binds to a MYB-binding site in the miR399f promoter in vitro, as well as in vivo, and stimulates miR399f promoter activity in Arabidopsis protoplasts. Transcription of AtMYB2 itself is induced in response to Pi deficiency, and the tissue expression patterns of miR399f and AtMYB2 are similar. Both genes are expressed mainly in vascular tissues of cotyledons and in roots. Our results suggest that AtMYB2 regulates plant responses to Pi starvation by regulating the expression of the miR399 gene.
Phosphorus (P) is an essential component of all organisms, as it is found, among other compounds, in nucleic acids, ATP, and membrane phospholipids. It is an essential nutrient for plants. P can be acquired by plants only as inorganic phosphate (Pi). Therefore, most of the P content of soils is unavailable for plant growth and development (Hinsinger, 2001). To overcome the problem of Pi limitation, plants have developed a variety of adaptive responses that conserve internal P while activating mechanisms that enhance the accessibility and uptake of external P. The accompanying gene expression changes produce changes in root architecture, enhanced Pi uptake activity, secretion of organic acids, and secretion of phosphatases (Raghothama, 1999; Poirier and Bucher, 2002; Yuan and Liu, 2008; Péret et al., 2011). The synchronization of Pi availability with plant growth and development is orchestrated by several phytohormones, including abscisic acid, ethylene, auxin, and cytokinin (Hillwig et al., 2008; Devaiah et al., 2009; Lei et al., 2011).
A few transcription factors have been characterized that appear to regulate subsets of the response to Pi stress, either positively or negatively. PHOSPHATE STARVATION RESPONSE1 (PHR1) is a MYB transcription factor that initiates the up-regulation of Pi starvation-responsive genes in plants and unicellular algae (Rubio et al., 2001). WRKY75, a WRKY transcription factor family member, has been identified as a key regulator of Pi acquisition and root architecture in response to Pi starvation (Devaiah et al., 2007a). MYB62, an R2R3-type MYB transcription factor, connects Pi homeostasis and GA signaling during Pi starvation (Devaiah et al., 2009). ZAT6, a C2H2-type zinc finger transcription factor, regulates Pi homeostasis and exerts some control over root development (Devaiah et al., 2007b). The BHLH32 (for basic helix-loop-helix) transcription factor is a negative regulator of several Pi starvation responses (Chen et al., 2007). PTF1, of rice (Oryza sativa) and maize (Zea mays), encodes a bHLH transcription factor that is involved in Pi signaling (Yi et al., 2005; Li et al., 2011). These transcription factors function in cross talk between Pi starvation signaling and signaling by phytohormones, or photosynthates, to govern physiological responses to Pi limitation (Rouached et al., 2010).
MicroRNAs (miRNAs) are endogenous noncoding RNAs, 21 to 24 nucleotides in length, that contribute to the regulation of gene expression. They have emerged as master regulators in plant development, and they orchestrate adaptive responses to stresses owing to posttranscriptional regulation of gene expression (Bonnet et al., 2006; Mallory and Vaucheret, 2006; Sunkar et al., 2012). Recently, the regulation of phosphate, copper, and sulfate homeostasis in plants was found to involve miRNAs (Jones-Rhoades and Bartel, 2004; Fujii et al., 2005; Chiou et al., 2006; Yamasaki et al., 2007; Liang et al., 2010; Kuo and Chiou, 2011). Pi deprivation induces the expression of several miRNAs in Arabidopsis (Arabidopsis thaliana), including miR156, miR399, miR778, miR827, and miR2111 (Fujii et al., 2005; Hsieh et al., 2009; Pant et al., 2009). Of these, miR2111 up-regulates the expression of At3g27150, which encodes a Kelch repeat-containing F-box protein (Hsieh et al., 2009). miR827 mediates cross talk between Pi and nitrogen limitation signaling, based on the regulation of anthocyanin synthesis. It also down-regulates the expression of At1g02860 that encodes a ubiquitin E3 ligase (Hsieh et al., 2009; Pant et al., 2009). Irrespectively, the precise role of these Pi limitation-induced miRNAs in the regulation of Pi homeostasis remains unknown (Doerner, 2008; Kuo and Chiou, 2011).
In contrast, the mode of action for miR399 during plant responses to Pi starvation is well characterized. Expression of miR399 is strongly induced upon Pi starvation, especially in vascular tissues of the shoot. Mature miR399 is then translocated to roots and binds to the 5′ untranslated region of PHO2 (UBC24, which encodes a ubiquitin-conjugating E2 enzyme) transcripts, leading to the degradation of PHO2 mRNA. The resulting decrease of PHO2 protein level activates the expression of phosphate transporter genes, such as Pht1;8 and Pht1;9, thereby facilitating Pi uptake and transport to the shoot (Fujii et al., 2005; Aung et al., 2006; Bari et al., 2006; Chiou et al., 2006; Pant et al., 2009). Thus, induction of miR399 gene expression by Pi limitation plays an important role as the trigger in the restoration of Pi homeostasis by promoting Pi acquisition in roots and Pi allocation to shoots. Missing are mechanistic details of the regulation of miR399 gene expression in response to Pi starvation. In fact, information on the transcriptional regulation of miRNA genes is generally scarce, although much is known about the genomic organization of miRNA genes, molecular mechanisms of miRNA biogenesis, and miRNA functions in animals and plants (Jones-Rhoades et al., 2006).
We demonstrate here that the positive regulation of miR399 gene expression in response to Pi starvation is mediated at least in part by the transcription factor AtMYB2. AtMYB2, a transcription factor that is known to function in abiotic stress signaling in Arabidopsis (Urao et al., 1996; Abe et al., 1997, 2003; Yoo et al., 2005), directly binds to a MYB-binding site located in the miR399f promoter. This enhances miR399f promoter activity. AtMYB2 is coexpressed with miR399f in vascular tissue, and its transcript level is increased by Pi deprivation like that of miR399f. Constitutive overexpression (OE) of AtMYB2 in Arabidopsis activates the transcription of miR399f and increases a subset of PHOSPHATE STARVATION INDUCED (PSI) gene expression, Pi uptake, and promotes changes in root architecture. These results uncover a missing link between Pi starvation and miR399 transcription that also connects abiotic stress signaling to growth responses and Pi acquisition in the plant.
RESULTS
AtMYB2 Expression, Like mi399f, Is Induced by Phosphate Deficiency
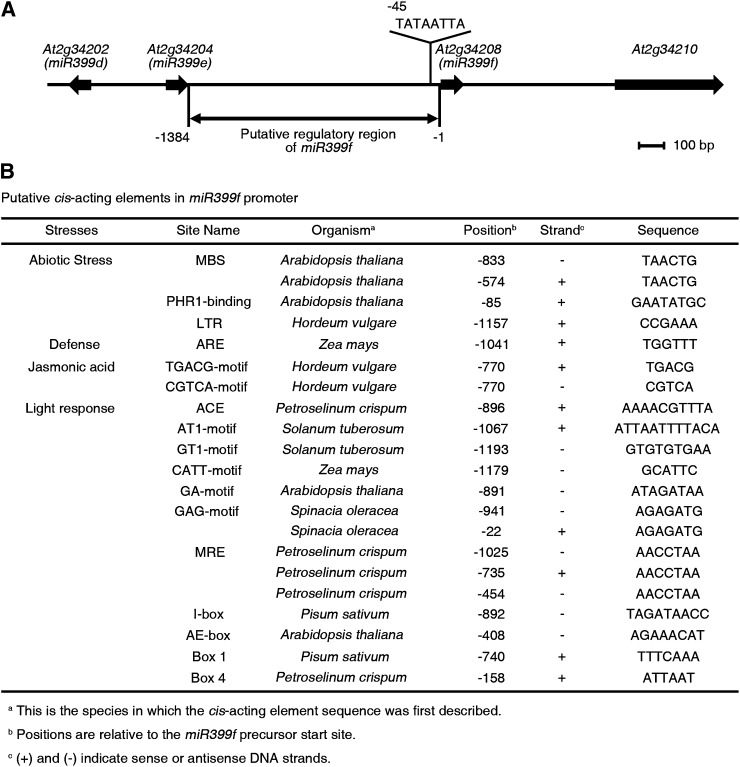
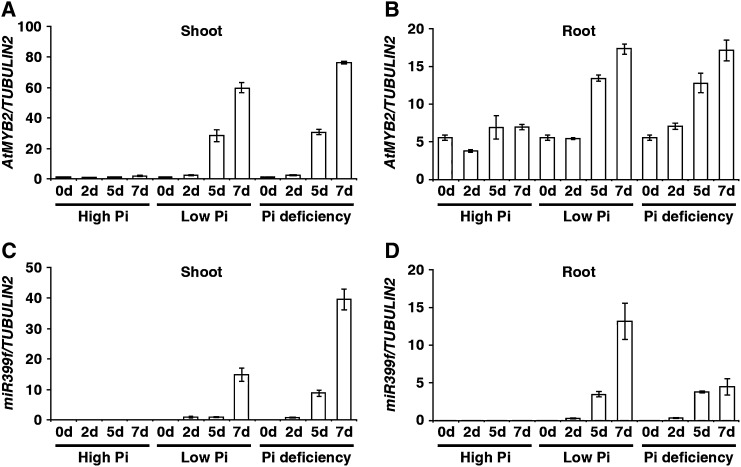
The induction of miR399f gene expression in response to Pi deficit is the earliest known step in the signaling pathway leading from the sensing of Pi deficiency to changes in root architecture and the restoration of Pi homeostasis in Arabidopsis (Fujii et al., 2005; Hsieh et al., 2009). To uncover mechanisms involved in controlling miR399f gene expression, we performed an in silico analysis of its presumptive promoter region using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; Fig. 1). Several cis-acting regulatory elements typically associated with biotic and abiotic stress responses, such as defense, jasmonic acid, and light signaling, were identified in this region. Also found were two canonical binding sites for AtMYB2, a drought-inducible transcription activator of the dehydration-responsive gene RD22 that also participates in abscisic acid and salt stress signaling (Urao et al., 1996; Abe et al., 1997, 2003; Yoo et al., 2005). To test whether AtMYB2 plays a role in miR399f-mediated Pi starvation signaling, we compared the expression of AtMYB2 and the miR399f precursor transcript in wild-type seedlings after transfer from normal growth medium to high-Pi (1.25 mm KH2PO4), low-Pi (0.0125 mm KH2PO4), or Pi-deficient (0 mm KH2PO4) media by quantitative real-time (qRT)-PCR. The temporal expression pattern of miR399f precursor was similar to that of AtMYB2 at all three Pi levels (Fig. 2). Significant increases in the steady-state levels of AtMYB2 and miR399f transcripts were observed in shoots and roots after 5 and 7 d of exposure to low Pi or Pi deficiency. However, no increase in AtMYB2 or miR399f transcript abundance was observed after exposure to high Pi. These results suggested that AtMYB2 may be involved in miR399f-mediated Pi deficiency signaling in Arabidopsis.
Figure 1.
Putative cis-acting regulatory elements in the miR399f promoter. A, Genomic organization of miR399f (At2g34208) flanking regions. The location of the TATA-like sequence (TATAATTA) of the miR399f gene is indicated. B, Putative cis-acting regulatory sequences on the miR399 promoter. An area 1,384 bp upstream of the transcription start site was analyzed using PlantCARE. The selected matrix score for all cis-acting elements was 5 or greater. MBS, MYB2-binding site; LTR, low-temperature response; ARE, anaerobic response element; ACE, ACGT-containing element; MRE, MYB recognition element; AE-box, activating element box.
Figure 2.
Expression of AtMYB2 and the miR399f precursor is induced in response to phosphate deficit. A to D, Wild-type plants were grown on MS medium for 7 d, transferred to high-Pi, low-Pi, or Pi-deficient growth medium, and allowed to grow further for 0, 2, 5, and 7 d. Transcript levels were measured by qRT-PCR in total RNA extracted from shoots and roots at the indicated time points. Transcript levels of AtMYB2 (A and B) and miR399f precursor (C and D), normalized to the transcript level of TUBULIN2, are shown. Bars represent means ± sd of three biological replicates with two technical replicates each.
miR399f and AtMYB2 Are Expressed in the Same Plant Tissues
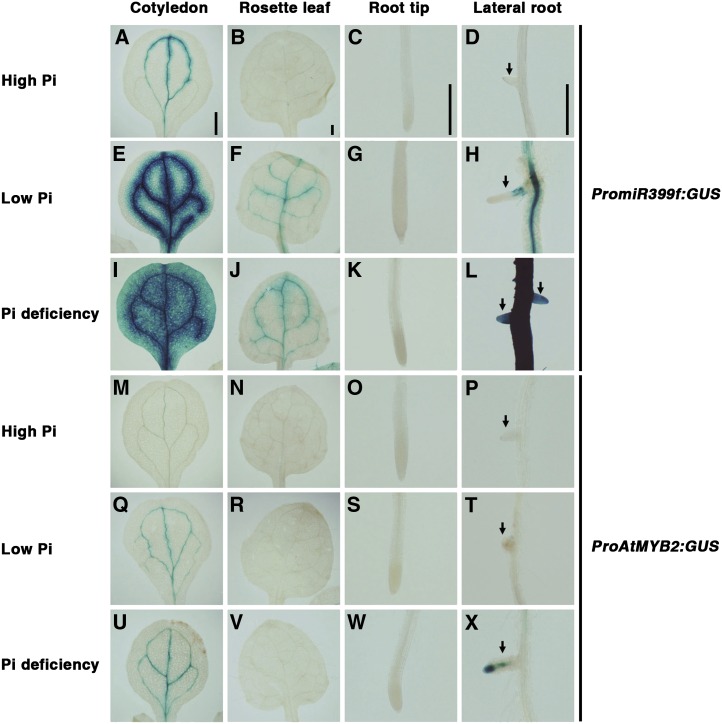
miR399 is expressed mainly in the vascular tissues of cotyledons, leaves, and roots. The expression in these tissues is strongly enhanced by Pi starvation (Aung et al., 2006). In order to investigate whether AtMYB2 is expressed in the same plant organs and tissues, we performed histochemical analysis of GUS expression in tissues of PromiR399f:GUS and ProAtMYB2:GUS transgenic plants grown in high-Pi, low-Pi, and Pi-deficient medium (Fig. 3). As reported earlier (Aung et al., 2006), weak expression of the miR399f promoter was detected by GUS staining in the vascular tissues of cotyledons and leaves but not in roots of seedlings grown in high Pi (Fig. 3, A–D). Strong miR399f promoter activity was observed in vascular tissues of cotyledons, rosette leaves, and primary and lateral roots of seedlings grown under Pi deficit, but no activity was evident in root tips (Fig. 3, E–L). Under high-Pi, low-Pi, and Pi-deficient conditions, GUS activity was weaker in tissues of ProAtMYB2:GUS transgenic seedlings than in the corresponding tissues of PromiR399f:GUS seedlings. Consequently, only weak GUS staining was observed in vascular tissues of cotyledons in ProAtMYB2:GUS seedlings in high-Pi medium, and no GUS stain was observed in rosette leaves or primary and lateral roots (Fig. 3, M–P). Clear induction of AtMYB2 promoter activity was observed in response to Pi limitation in cotyledons (Fig. 3, Q and U) and lateral roots (Fig. 3, T and X). Overall, the GUS reporter expression patterns in tissues of ProAtMYB2:GUS and PromiR399f:GUS seedlings were essentially similar, indicating that AtMYB2 and miR399f are expressed in the same plant tissues, particularly under Pi limitation. These results suggest direct regulation of miR399f expression by AtMYB2.
Figure 3.
Spatial expression patterns of miR399f and AtMYB2. Seeds of PromiR399f:GUS and ProAtMYB2:GUS transgenic lines, which express the GUS reporter from the miR399f and AtMYB2 promoters, respectively, were grown as described in Figure 5. Tissues were stained 7 d after transfer to high-Pi, low-Pi, or Pi-deficient medium. Blue color indicates GUS activity. A to L, Tissues of PromiR399f:GUS transgenic plants. M to X, Tissues of ProAtMYB2:GUS transgenic plants. Arrows indicate lateral roots. Bars = 0.5 mm.
Constitutive OE of AtMYB2 Promotes miR399f Expression and Increases Tissue Pi Content
Next, we generated transgenic CaMV35S:AtMYB2 plants and selected three lines (AtMYB2 OE) that showed constitutive high-, middle-, and low-level OE of AtMYB2 under normal growth conditions (Fig. 4A). RNA gel-blot analysis showed that miR399f mRNA abundance was significantly higher in the AtMYB2 OE transgenic plants compared with wild-type plants grown under identical conditions (Fig. 4C). The level of miR399f accumulation was correlated positively with the level of AtMYB2 expression (Fig. 4, A and C). Thus, OE of AtMYB2 leads to a proportional increase miR399f expression, even in the presence of sufficient Pi.
Figure 4.
OE of AtMYB2 induces miR399f expression and Pi accumulation. Wild type (WT) and three independent lines of CaMV35S:AtMYB2 (AtMYB2 OE) were grown in MS medium. Ten-day-old seedlings were analyzed. A and B, Expression levels of AtMYB2 (A) and UBC24 (B), normalized to the level of TUBULIN2. Transcript levels were analyzed in total RNA extracted from the seedlings by qRT-PCR. Bars represent means ± sd for three biological replicates with two technical replicates each. C, Northern-blot analysis of miR399f expression in total RNA. Ethidium bromide-stained 5S rRNA bands are shown as loading controls. D, Inorganic Pi concentrations were measured in the roots and shoots. Bars represent means ± sd for two biological replicates. Asterisks represent significant differences from the wild type (P ≤ 0.05 from a Student’s t test). F.W., Fresh weight.
Constitutive expression of miR399 leads to degradation of the UBC24 (PHO2) transcript and elevated Pi accumulation in Arabidopsis even under high Pi (Fujii et al., 2005; Chiou et al., 2006). Accordingly, AtMYB2 OE plants grown under high Pi accumulated lower levels of UBC24 transcript than wild-type plants (Fig. 4B). A negative correlation was observed between mRNA levels of UBC24 and AtMYB2 or miR399f (Fig. 4, A–C). The Pi content in shoots of all three AtMYB2 OE lines was significantly higher than that in wild-type plants (Fig. 4D), as predicted from their elevated miR399f expression levels and reduced UBC2 expression levels on the basis of earlier reports (Fujii et al., 2005; Chiou et al., 2006). Elevated Pi accumulation was also observed in roots of the two AtMYB2 OE lines that expressed the highest levels of mi399f transcript. Moreover, it has been reported that elevated Pi accumulation in pho2 mutant and miR399 OE Arabidopsis transgenic plants induced Pi toxicity-mediated chlorosis symptoms on their leaves (Fujii et al., 2005; Aung et al., 2006). To test whether AtMYB2 OE plants also exhibit chlorosis symptoms, we grew wild-type and AtMYB2 OE plants on Pi-sufficient Murashige and Skoog (MS) medium for 3 weeks under constant light conditions. We observed the development of typical chlorosis symptoms on the leaves of AtMYB2 OE plants and also less chlorophyll content in AtMYB2 OE plants compared with wild-type plants, supporting higher Pi content in AtMYB2 OE plants than the wild type (Supplemental Fig. S1, A and B; Supplemental Materials and Methods S1). These results suggest that OE of AtMYB2 affects Pi homeostasis in Arabidopsis by activating miR399f-mediated phosphate starvation signaling.
Pi Starvation-Induced Root Architectural Changes Are Exaggerated in AtMYB2 OE Transgenic Plants
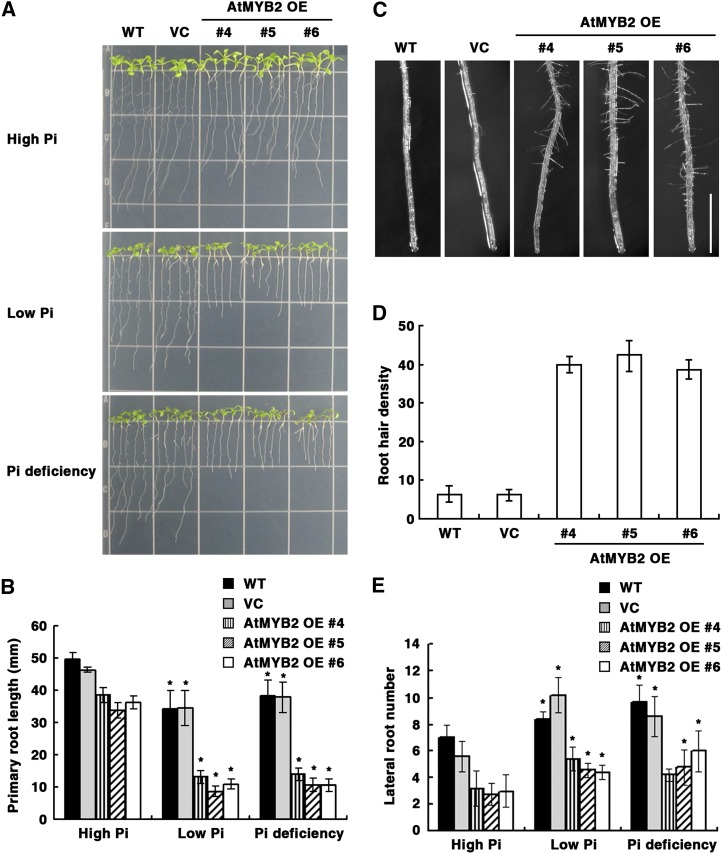
Under Pi limitation, root architecture is altered. Lateral root growth is promoted (increased lateral root number and length), while primary root length is reduced due to reduced cell elongation (Osmont et al., 2007; Desnos, 2008). Based on our results that OE of AtMYB2 activated miR399 accumulation and miR399-mediated Pi starvation signaling, we hypothesized that at least some Pi limitation-induced root architecture changes should be exaggerated in the AtMYB2 OE lines. Accordingly, we investigated root morphology in wild-type plants, transgenic plants expressing empty vector, and AtMYB2 OE transgenic plants 7 d after transfer from normal growth medium to high- and low-Pi media and to Pi-deficient medium (Fig. 5). Compared with plants grown in high-Pi medium, primary root lengths of wild-type and vector control seedlings grown in low-Pi and Pi-deficient media were lower by 20% to 30% (Fig. 5, A and B). However, low-Pi and Pi-deficient conditions resulted in a dramatic reduction (60%–70%) of primary root length of AtMYB2 OE seedlings relative to growth in high Pi. Primary root lengths of AtMYB2 OE seedlings were about 20% less than those of wild-type and vector control seedlings even in high Pi (Fig. 5, A and B). Similarly, AtMYB2 OE plants developed 5-fold more hairs near the tip of the primary root than wild-type plants even under normal Pi conditions (Fig. 5, C and D). The Pi limitation-induced reduction of primary root length and the increase in root hair density were exaggerated in AtMYB2 OE lines, as expected. However, OE of AtMYB2 did not exaggerate the effect of Pi limitation on lateral root development. The lateral root numbers of wild-type, vector control, and AtMYB2 OE plants all increased by 20% to 30% in response to low Pi and Pi deficiency, respectively (Fig. 5E). Thus, OE of AtMYB2 exaggerates some, but not all, Pi limitation-induced root architectural changes.
Figure 5.
AtMYB2 OE enhances Pi deficiency responses in root development and also affects root hair development. A, Seeds of the untransformed wild type (WT), empty vector control (VC) transformants, and three independent lines of AtMYB2 OE transformants were grown on MS agar medium for 5 d and then transferred to nutrient medium containing 1.25 mm (high Pi), 0.0125 mm (low Pi), or 0 mm (Pi deficiency) KH2PO4. Seedlings were photographed 7 d after transfer. B, Quantification of primary root lengths of the seedlings depicted in A. Bars represent means ± se of three replicates with 16 seedlings per replicate. Asterisks represent significant differences from the values of each line under the high-Pi condition (P ≤ 0.05 from a Student’s t test). C, Root hair development at tips of the primary root of seedlings grown in MS medium for 7 d. Bar = 1 mm. D, Quantification of root hair densities at the primary root tip of plants shown in C. Root density is the number of root hairs along 5 mm of each root above the tip. Bars represent means ± se of three replicates with 16 seedlings per replicate. E, Quantification of lateral root numbers per plant of the seedlings depicted in A. Bars represent means ± se of three replicates with 16 seedlings per replicate. Asterisks represent significant differences from the values of each line under the high-Pi condition (P ≤ 0.05 from a Student’s t test).
Taken together, these results suggested that OE of AtMYB2 leads to a constitutive Pi starvation-induced reprogramming of root development, such as suppression of primary root growth and activation of root hair development, under Pi-sufficient conditions. Also, AtMYB2 OE plants become more sensitive to Pi limitation than wild-type plants. These results led us to hypothesize that the inactivation of AtMYB2 should lead to the inhibition of Pi limitation responses of roots, reduced expression of miR399f under Pi limitation, and reduced Pi content under Pi sufficiency or excess.
Inactivation of AtMYB2 Does Not Affect Pi Starvation Responses
To verify the above hypothesis, we obtained an atmyb2 mutant (SALK_045455) that contains a transfer DNA insertion in the third exon of AtMYB2 (Supplemental Fig. S2A). This insertion mutant was designated as atmyb2-3. qRT-PCR analysis of AtMYB2 expression showed that the atmyb2-3 mutant did not produce any detectable AtMYB2 transcript (Supplemental Fig. S2B). In low-Pi and Pi-deficient media, the inhibition of primary root growth and miR399f transcript levels in wild-type and atmyb2-3 plants was comparable (Supplemental Fig. S2, C and D). Furthermore, there was no difference in the Pi contents of roots and shoots of wild-type and atmyb2-3 plants grown in high-Pi medium (Supplemental Fig. S2E). As the inactivation of AtMYB2 did not produce the expected phenotypes, we concluded that there is redundancy of the function that AtMYB2 fulfills in Pi starvation signaling.
OE of AtMYB2 Affects the Expression of PSI Genes
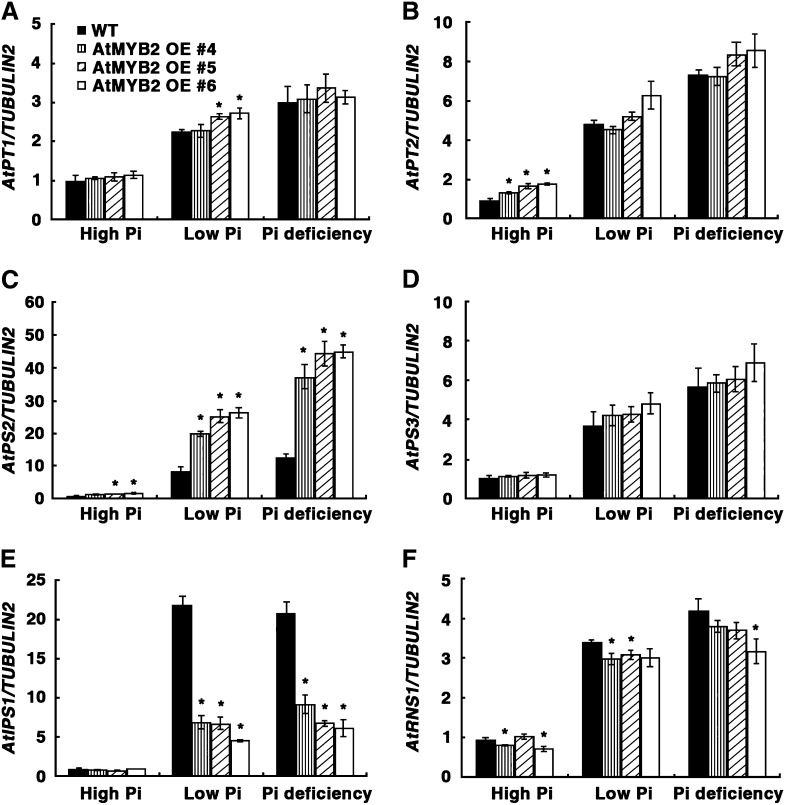
In addition to changes in root architecture, Pi starvation also induces the expression of PSI genes such as the Pi transporters AtPT1 (Pht1;1; Shin et al., 2004) and AtPT2 (Pht1;4; Shin et al., 2004), an acid phosphatase (AtPS2; Baldwin et al., 2001), a glycerol-3-phosphate permease (AtPS3; Ramaiah et al., 2011), an S-like RNase (AtRNS1; Bariola et al., 1999), and a noncoding transcript (AtIPS1; Franco-Zorrilla et al., 2007). To test whether OE of AtMYB2 also affects the expression of PSI genes, the mRNA levels of several PSI genes were analyzed in AtMYB2 OE plants after transfer from normal growth medium to high-Pi, low-Pi, and Pi-deficient medium. qRT-PCR analyses showed that the expression of all PSI genes tested (AtPT1, AtPT2, AtPS2, AtPS3, AtIPS1, and AtRNS1) was highly induced by Pi limitation in wild-type plants (Fig. 6). In AtMYB2 OE plants, abundance of transcripts of the phosphate transporters AtPT1 and AtPT2 was higher than that in wild-type plants under high-Pi and low-Pi limitation conditions but was not evident under Pi deficiency, because the expression level of the wild type was equally high (Fig. 6, A and B). These results provide some explanation of the higher Pi content of AtMYB2 OE plants compared with the wild type under high-Pi growth (Fig. 4D). However, it is possible that other PSI genes, for which we did not analyze the expression patterns in this study, may also play important roles in the enhanced Pi uptake of AtMYB2 OE plants. Expression of the acid phosphatase AtPS2 was comparable in wild type and AtMYB2 OE plants grown under high Pi but was induced to a greater extent in AtMYB2 OE plants compared with the wild type under low Pi and Pi deficiency (Fig. 6C). In contrast, the abundance of AtRNS1 and AtIPS1 mRNA was comparable in wild-type and AtMYB2 OE plants grown under high Pi. Although these transcripts were induced by Pi limitation in the AtMYB2 OE plants, the magnitude of induction was less than that in wild-type plants grown under the same conditions (Fig. 6, E and F). These data indicate that AtMYB2 is involved in the regulation of a subset of PSI gene expression.
Figure 6.
Expression patterns of Pi starvation-induced genes in AtMYB2 OE plants. Seeds of the untransformed wild type (WT) and three independent lines of AtMYB2 OE transformants were grown on MS agar medium for 7 d and then transferred to the high-Pi, low-Pi, or Pi-deficient medium described in Figure 4. Transcript levels of AtPT1 (A), AtPT2 (B), AtPS2 (C), AtPS3 (D), AtIPS1 (E), and AtRNS1 (F) were analyzed by qRT-PCR in total RNA extracted from the seedlings 7 d after transfer. The TUBULIN2 transcript level was used for normalization. Bars represent means ± sd of three biological replicates with two technical replicates each. Asterisks represent significant differences from the wild type (P ≤ 0.05 from a Student’s t test).
AtMYB2 Directly Binds to the miR399f Promoter and Activates miR399f Expression
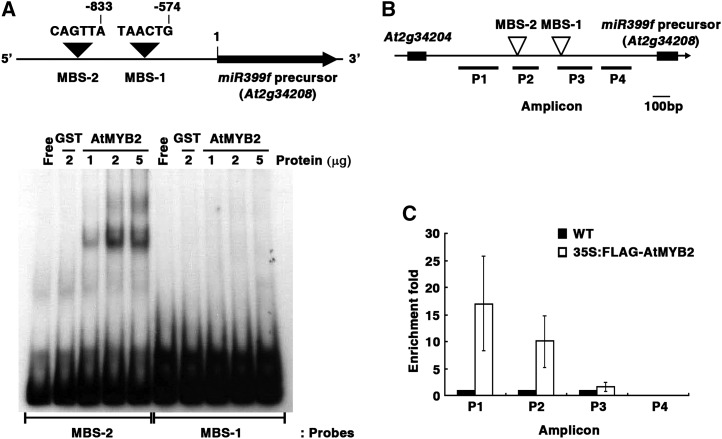
Two putative MYB-binding sites (MBSs; 5′-TAACTG-3′) that have opposite orientations were found by in silico analysis of the putative regulatory region of miR399f (Figs. 1B and 7A). To examine whether the AtMYB2 protein binds to one or both of these MBSs, we performed electrophoretic mobility shift assays (EMSA) with 32P-labeled oligonucleotides corresponding to promoter fragments containing the MBS-1 or MBS-2 motif (140 or 149 bp, respectively) and recombinant glutathione S-transferase (GST)-AtMYB2 or GST proteins. A GST-AtMYB2-specific mobility-retarded band indicating binding to AtMYB2 was observed with the MBS-2 oligonucleotide (Fig. 7A). The intensity of this band was enhanced by increasing the amount of GST-AtMYB2 protein in the binding reaction. No mobility-retarded bands were observed with MBS-1 oligonucleotide, indicating absence of binding.
Figure 7.
AtMYB2 binds to MBS-2 on the miR399f promoter region. A, Top, schematic representation of predicted MYB-binding sites (MBS-1 and MBS-2) in the miR399f promoter. Bottom, EMSA of the binding of recombinant AtMYB2 protein to oligonucleotides spanning the MBS-2 and MBS-1 regions. The autoradiogram shows resolved binding reactions of 32P-labeled DNA probes (MBS-2 and MBS-1) without protein (Free) or with the indicated amounts of AtMYB2-GST (AtMYB2) or GST (negative control). B, Schematic drawing of the miR399f locus and locations of the ChIP assay amplicons (P1–P4). C, ChIP assay for miR399f chromatin regions associated with AtMYB2. The ChIP assay was performed on total protein extracts of MS-grown 3-week-old seedlings of the untransformed wild type (WT) and CaMV35S:FLAG-AtMYB2 transformed Arabidopsis. Fold enrichment is the ratio of CaMV35S:FLAG-AtMYB2 to wild-type signal. Bars represent means ± sd for three technical replicates.
Next, a chromatin immunoprecipitation (ChIP) assay was performed using total protein extracts of wild-type and CaMV35S:FLAG-AtMYB2 transgenic plants. After immunoprecipitation with an antiserum against the FLAG tag, the relative contents of miR399f promoter fragments P1 to P4 (Fig. 7B) in the immunoprecipitates were estimated by qRT-PCR (Fig. 7C). The amplicons P1 and P2, which surrounded and included the MBS-2 region, respectively, were significantly enriched by qRT-PCR. No enrichment of the P3 amplicon that includes MBS-1, or the P4 amplicon, was observed in the CaMV35S:FLAG-AtMYB2 extracts. Together, the results from EMSA and ChIP assays indicate that AtMYB2 directly binds to the MBS-2 region in the miR399f promoter in vitro and in vivo.
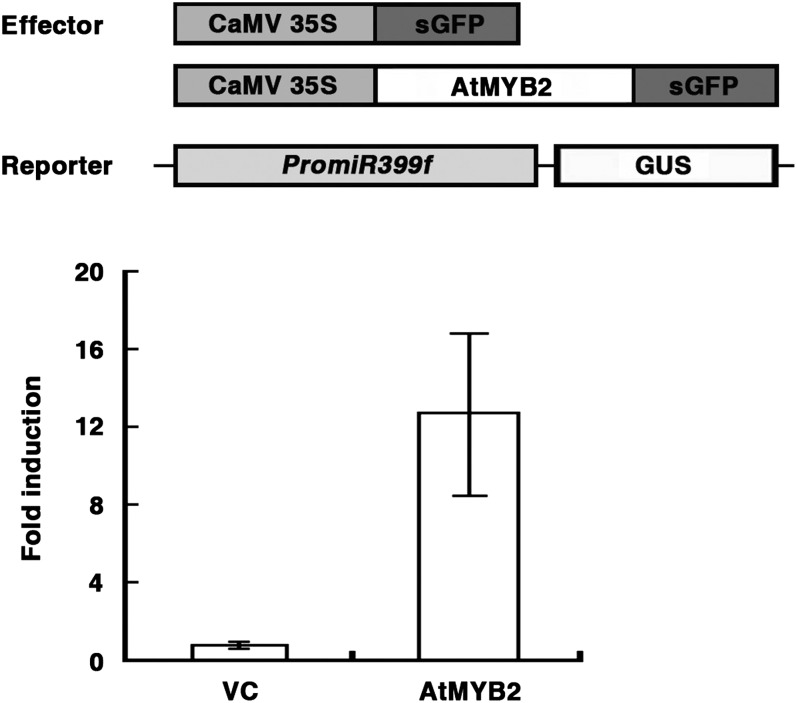
Next, we verified that AtMYB2 was a nucleus-localized protein. As shown in Supplemental Figure S3, the GFP signal in Arabidopsis protoplasts transiently transformed with CaMV35S:AtMYB2-sGFP (for synthetic GFP) was exclusively localized in the nucleus. We then tested whether AtMYB2 can transactivate reporter gene expression from the miR399f promoter. Cotransformation of atmyb2-3 protoplasts with PromiR399f:GUS reporter and CaMV35S:AtMYB2-sGFP or CaMV35S:sGFP (negative control) as effector construct showed that AtMYB2-sGFP greatly increases miR399f promoter activity compared with sGFP alone (Fig. 8). These results indicate that AtMYB2 can function as a transcriptional activator for the miR399f gene in vivo.
Figure 8.
AtMYB2 enhances the miR399f promoter activity. Top, schematic representation of the effector and reporter constructs used in the transient expression assay of miR399f promoter activity. Each effector construct was introduced into atmyb2-3 protoplasts along with the reporter construct and an internal control CaMV35S:LUC construct by polyethylene glycol-mediated transformation. Bottom, GUS reporter activity in each sample was obtained after normalization to LUC activity. Fold induction is the ratio of the GUS activity of CaMV35S:AtMYB2-sGFP transformed protoplasts (AtMYB2) relative to the GUS activity of CaMV35S:sGFP transformed protoplasts (vector control [VC]). Bars represent means ± sd of three technical replicates.
DISCUSSION
Our results show that AtMYB2 binds to the miR399f promoter, leading to the activation of miR399f expression. AtMYB2 and miR399f are expressed in the same tissues, particularly under Pi limitation, and are also induced by Pi limitation, and they activate the same subset of PSI genes. Thus, we infer that AtMYB2 functions as a transcription factor regulating miR399f-mediated signaling in the establishment of Pi homeostasis under Pi limitation. We subsequently were able to support this role for AtMYB2 in the plant response to Pi limitation on the basis of the phenotypes of transgenic AtMYB2 OE lines. As there were no differences with respect to phenotype in the response to Pi starvation between wild-type and null atmyb2-3 plants (Supplemental Fig. S2), we conclude further that AtMYB2 is functionally redundant.
Our discovery of AtMYB2 as a transcription factor activating miR399f expression began with an in silico analysis for cis-acting elements in the 1,384-bp region upstream of the primary transcript of the miR399f gene (pri-miR399f; At2g34208). It has been reported that a TATA box-like sequence is located within 50 nucleotides upstream of the majority of the primary miRNA transcripts of Arabidopsis miRNA genes (Xie et al., 2005a). A TATA box-like sequence, TATAATTA, was mapped at 45 nucleotides upstream of the miR399f precursor (Fig. 1A), indicating that miR399f is a typical RNA polymerase II-transcribed independent transcription unit. In addition to the TATA box-like motif and MYB-binding sites, we found several canonical cis-regulatory elements in the miR399f promoter. A GNATATNC element was located at −84 bp on the miR399f promoter (Fig. 1B). This sequence is known to bind PHR1, a MYB transcription factor that causes up-regulation of Pi-responsive genes (Rubio et al., 2001). The presence of a PHR1-binding motif in the miR399f promoter raises the possibility that the MYB family transcription factor PHR1 compensates at least partly for AtMYB2 function. More experiments are needed in order to both verify the role, if any, of PHR1 in the regulation of miR399f expression and investigate how much the two promoter-binding factors overlap or diverge in the activating capacity.
The miR399 family in Arabidopsis consists of six members, miR399a to miR399f, all of which are induced by Pi starvation and function in Pi homeostasis by regulating the expression of UBC24 (Fujii et al., 2005; Aung et al., 2006; Chiou et al., 2006; Doerner, 2008; Pant et al., 2008; Hsieh et al., 2009; Kuo and Chiou, 2011). We found that the putative promoter regions of miR399a, miR399b, and miR399c also contain GNATATNC elements (data not shown). Soybean (Glycine max) miRNA genes responsible for Pi starvation signaling contain several types of Pi-responsive cis-elements in their promoters, including the PHR1-binding site (Zeng et al., 2010). This suggests that the induction of several miRNA399 family members, including miRNA399f, in response to Pi limitation could be mediated in part by PHR1. Defense-, hormone-, light-, and water stress-responsive cis-regulatory elements were detected in the miR399f promoter (Fig. 1B). This is consistent with a previous report indicating that the cis-acting elements involved in hormone and abiotic stress responses are overrepresented in miRNA promoters when compared with promoters of protein-coding genes (Megraw et al., 2006). It would be interesting to ascertain whether miR399f and other members of this family constitute a hub that is important for coordinating environmental cues with nutrient acquisition to modulate plant growth. It must be noted that AtMYB2, established by us as a transcriptional activator of miR399f, also functions in hormonal and abiotic stress signaling in Arabidopsis (Urao et al., 1996; Abe et al., 1997, 2003; Yoo et al., 2005; Guo and Gan, 2011).
Of the two MBS motifs in the miR399f promoter, only MBS-2, the more distal element in the pri-miR399f sequence, was identified as a functional binding site for the AtMYB2 transcription factor in vitro and in vivo (Fig. 7). This suggests that both the core element sequences and the flanking sequences are important for efficient binding of AtMYB2 on the miR399f promoter. MBS motifs are found in the promoters of many miR399 family members of Arabidopsis and rice (data not shown). From an analysis of 1.5-kb upstream sequences of miR399a, miR399b, miR399c, miR399d, and miR399e, we found that miR399b and miR399c have MBS motifs in their promoter regions (Supplemental Table S2). However, in contrast to miR399f, transcript levels of miR399b and miR399c genes in AtMYB2 OE plants were comparable to those in wild-type plants (Supplemental Fig. S4). These in silico promoter analyses and subsequent gene expression analyses suggest that the transcriptional regulation by AtMYB2 affects specifically the miRNA399f gene among the miR399 family members. The upstream region of other Pi-responsive miRNA genes, including miR156a, miR156e, and miR2111a, also contain MBS motifs (Supplemental Table S2). Additional experimental data will be required to ascertain whether AtMYB2 is also involved in the regulation of miR156a, miR156e, and miR2111a expression in response to Pi starvation or whether this group of miRNAs diversifies into additional functions. Further analyses of promoters of the miRNA genes that regulate low-Pi responses should illuminate other mechanisms and the signaling cross talk that govern their expression.
MATERIALS AND METHODS
Plant Materials and Stress Treatments
All Arabidopsis (Arabidopsis thaliana) lines were in the ecotype Columbia-0 background. The atmyb2-3 mutant (SALK_045455) was obtained from the Arabidopsis Biological Resource Center at Ohio State University (http://www.arabidopsis.org/). Transgenic lines were generated by Agrobacterium tumefaciens-mediated transformation using the floral dip method as described (Clough and Bent 1998). Homozygous lines were generated by back-crossing and were used in the experiments. The genotype of the transformants was verified by PCR. Seeds were germinated and grown on MS medium containing 1% Suc and 0.7% (or 1.2%) agar, pH 5.7. For testing the effect of Pi limitation, 5- or 7-d-old seedlings were transferred to growth medium containing 1% Suc, 1/20× micronutrients (Miura et al., 2005), and 1.25 mm KH2PO4 (high Pi; this is equivalent to the Pi content of 1× MS), 0.0125 mm KH2PO4 (low Pi), or 0 mm KH2PO4 (Pi deficiency) for the indicated times (Miura et al., 2005). Plants were grown in a growth chamber at 22°C under a 16-h-light/8-h-dark cycle.
qRT-PCR Analysis
Total RNA was isolated using an RNeasy Kit (Qiagen) according to the manufacturer’s instructions and treated with DNase I (Promega) to remove genomic DNA contamination. Total RNA (2 µg) was used for first-strand complementary DNA (cDNA) synthesis using a cDNA Synthesis Kit (Invitrogen) and subjected to qRT-PCR analysis. The primers used in qRT-PCR analysis are described in Supplemental Table S1. The SsoFast EvaGreen Supermix (Bio-Rad) was used for the PCRs. PCR conditions were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 60 s. The relative expression levels of all the samples were automatically calculated and analyzed three times using CFX Manager software (Bio-Rad).
Northern-Blot Analysis of miRNAs
Northern-blot analysis of miRNAs was performed essentially as described (Xie et al., 2005b). Total RNA was extracted from seedlings using Plant RNA reagent (Invitrogen) following the supplier’s instructions. Briefly, total RNA (20 µg) was resolved on a 15% polyacrylamide gel containing 7 m urea and transferred to an Amersham Hybrid-N+ membrane (GE Healthcare). The probe complementary to miR399f (5′-UGCCAAAGGAGAUUUGCCCGG-3′) was 5′-end labeled with [γ-32P]ATP using Optikinase (Affymetrix/USB). Blots were prehybridized for at least 1 h and hybridized for 24 h in PerfectHyb Plus Hybridization buffer (Sigma) at 37°C. Posthybridization, blots were washed successively at 42°C with 2× SSC and 0.1% SDS for 15 min, 0.5× SSC and 0.1% SDS for 15 min, and 0.1× SSC and 0.1% SDS for 15 min.
Pi Measurement
Total Pi contents were analyzed as described previously (Fujii et al., 2005).
Expression and Purification of Recombinant GST-AtMYB2 Protein
AtMYB2 cDNA was inserted as a BamHI/SalI fragment into the same sites of pGEX-2T (Amersham Biosciences) to create an in-frame GST fusion. The primers used in cDNA cloning are described in Supplemental Table S1. The construct was verified by sequencing. pGEX-2T::AtMYB2 was introduced into Escherichia coli strain BL21 (Merck). For protein expression, cells were induced for 3 h at 30°C with 0.5 mm isopropylthio-β-galactoside. Induced cells were harvested, suspended in 1× GST bind/wash buffer (4.3 mm Na2HPO4, 1.47 mm KH2PO4, 137 mm NaCl, and 2.7 mm KCl, pH 7.3), incubated on ice for 20 min, and lysed by sonication. After centrifugation at 12,000 rpm at 4°C for 30 min, the supernatant was added to 0.5 mL of Glutathione-Agarose 4B (PEPTRON) that had been equilibrated with 1× GST bind/wash buffer. The slurry was mixed gently by shaking at room temperature for 30 min. The resin was then collected and washed two or three times with 10 mL of 1× GST bind/wash buffer. GST-AtMYB2 was eluted in 1 mL of 1× GST elution buffer (50 mm Tris-HCl, pH 8.0, and 10 mm reduced glutathione).
EMSA
To generate the 32P-labeled DNA probes, oligonucleotides spanning the MYB-binding sites on the miR399f promoter, MBS-1 (140 bp) and MBS-2 (149 bp), were annealed and the 5′ overhangs were filled in using the Klenow fragment of DNA polymerase (Takara), dCTP, dGTP, dTTP, and [α-32P]dATP. The DNA-binding reaction was allowed to proceed at 25°C for 20 min in binding buffer (20 mm HEPES, pH 7.9, 0.5 mm dithiothreitol, and 0.1 mm EDTA), 50 mm KCl, 15% glycerol, 1 µg of poly(dI-dC), and various concentrations of purified bacterially expressed AtMYB2 protein. The reaction was started by adding 32P-labeled DNA probe (40,000 cpm) and allowed to proceed at 25°C for 30 min. The reaction mixture was then subjected to electrophoresis on an 8% polyacrylamide gel in 0.5× Tris-borate/EDTA buffer at 80 V for 3 h. The gel was dried, mounted for autoradiography with intensifying screens, and exposed at −70°C.
ChIP Assay
The Gateway system was used to generate a CaMV35S:FLAG-AtMYB2 construct in the pGWB12 vector. This construct expresses FLAG-tagged full-length AtMYB2 protein. The construct was introduced into wild-type Arabidopsis plants through A. tumefaciens-mediated (strain GV3101) transformation. ChIP assays were performed as described by Saleh et al. (2008) using leaf tissue (100 mg) from 3-week-old plants. Monoclonal anti-FLAG M2 (Sigma) was used for immunoprecipitation. The amount of immunoprecipitated DNA was quantified by qRT-PCR. The primers used in the ChIP assay are listed in Supplemental Table S1.
Measurement of Promoter Activity
Transcriptional activity of the miR399f promoter was analyzed in Arabidopsis protoplasts as described by Zhu et al. (2008). The reporter construct was PromiR399f:GUS, and the effector constructs were CaMV35S:AtMYB2-sGFP and CaMV35S:sGFP. Plasmids carrying the reporter and an effector gene construct, along with an internal control plasmid carrying a CaMV35:LUC gene construct, were introduced into protoplasts prepared from leaves of 20-d-old atmyb2-3 plants by polyethylene glycol-mediated transformation as described by Baek et al. (2004). Fluorescence was measured using a SpectraMax GEMINI XPS spectrofluorometer (Molecular Devices) using the SoftMax Pro 5 software. GUS activity was normalized to luciferase activity to eliminate experimental variation between samples.
Histochemical Analysis of GUS Activity
Plants expressing the PromiR399f:GUS or ProAtMYB2:GUS transgenes in the wild-type background were used for histological analysis. Seedlings of transgenic plants grown in various levels of Pi were incubated at 30°C for 6 h in the dark in staining buffer (0.5 m Tris, pH 7.0, and 10% Triton X-100) containing 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide. Chlorophyll was removed with an ethanol series consisting of 20%, 35%, 50%, and 70% ethanol washes at room temperature for 30 min each.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Chlorosis phenotype in AtMYB2 OE plants.
Supplemental Figure S2. Responses of atmyb2-3 to Pi starvation.
Supplemental Figure S3. Subcellular localization of AtMYB2.
Supplemental Figure S4. Expression patterns of miR399b and miR399c in AtMYB2 OE plants.
Supplemental Table S1. List of primers used in this study.
Supplemental Table S2. MBS elements in a Pi-responsive miRNA promoter.
Acknowledgments
We thank the Arabidopsis Biological Resource Center for providing mutant seeds.
Glossary
- P
phosphorus
- Pi
phosphate
- miRNA
microRNA
- qRT
quantitative real-time
- MS
Murashige and Skoog
- EMSA
electrophoretic mobility shift assay
- GST
glutathione S-transferase
- ChIP
chromatin immunoprecipitation
- OE
overexpression
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9: 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141: 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Nam J, Koo YD, Kim DH, Lee J, Jeong JC, Kwak SS, Chung WS, Lim CO, Bahk JD, et al. (2004) Bax-induced cell death of Arabidopsis is meditated through reactive oxygen-dependent and -independent processes. Plant Mol Biol 56: 15–27 [DOI] [PubMed] [Google Scholar]
- Baldwin JC, Karthikeyan AS, Raghothama KG. (2001) LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiol 125: 728–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Pant BD, Stitt M, Scheible WR. (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141: 988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola PA, MacIntosh GC, Green PJ. (1999) Regulation of S-like ribonuclease levels in Arabidopsis: antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol 119: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E, Van de Peer Y, Rouzé P. (2006) The small RNA world of plants. New Phytol 171: 451–468 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG. (2007) BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem J 405: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Desnos T. (2008) Root branching responses to phosphate and nitrate. Curr Opin Plant Biol 11: 82–87 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG. (2007a) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143: 1789–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG. (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2: 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG. (2007b) Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol 145: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P. (2008) Phosphate starvation signaling: a threesome controls systemic P(i) homeostasis. Curr Opin Plant Biol 11: 536–540 [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, García JA, Paz-Ares J. (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39: 1033–1037 [DOI] [PubMed] [Google Scholar]
- Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15: 2038–2043 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S. (2011) AtMYB2 regulates whole plant senescence by inhibiting cytokinin-mediated branching at late stages of development in Arabidopsis. Plant Physiol 156: 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillwig MS, Lebrasseur ND, Green PJ, Macintosh GC. (2008) Impact of transcriptional, ABA-dependent, and ABA-independent pathways on wounding regulation of RNS1 expression. Mol Genet Genomics 280: 249–261 [DOI] [PubMed] [Google Scholar]
- Hinsinger P. (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237: 173–195 [Google Scholar]
- Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151: 2120–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP. (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14: 787–799 [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. (2006) MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57: 19–53 [DOI] [PubMed] [Google Scholar]
- Kuo HF, Chiou TJ. (2011) The role of microRNAs in phosphorus deficiency signaling. Plant Physiol 156: 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Zhu C, Liu Y, Karthikeyan AS, Bressan RA, Raghothama KG, Liu D. (2011) Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol 189: 1084–1095 [DOI] [PubMed] [Google Scholar]
- Li Z, Gao Q, Liu Y, He C, Zhang X, Zhang J. (2011) Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth. Planta 233: 1129–1143 [DOI] [PubMed] [Google Scholar]
- Liang G, Yang F, Yu D. (2010) MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J 62: 1046–1057 [DOI] [PubMed] [Google Scholar]
- Mallory AC, Vaucheret H. (2006) Functions of microRNAs and related small RNAs in plants. Nat Genet (Suppl) 38: S31–S36 [DOI] [PubMed] [Google Scholar]
- Megraw M, Baev V, Rusinov V, Jensen ST, Kalantidis K, Hatzigeorgiou AG. (2006) MicroRNA promoter element discovery in Arabidopsis. RNA 12: 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan AS, Raghothama KG, Baek D, Koo YD, Jin JB, Bressan RA, et al. (2005) The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc Natl Acad Sci USA 102: 7760–7765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. (2007) Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58: 93–113 [DOI] [PubMed] [Google Scholar]
- Pant BD, Buhtz A, Kehr J, Scheible WR. (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53: 731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR. (2009) Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol 150: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16: 442–450 [DOI] [PubMed] [Google Scholar]
- Poirier Y, Bucher M. (2002) Phosphate transport and homeostasis in Arabidopsis. The Arabidopsis Book 1: e0024, doi/10.1199/tab.0024 [DOI] [PMC free article] [PubMed]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Ramaiah M, Jain A, Baldwin JC, Karthikeyan AS, Raghothama KG. (2011) Characterization of the phosphate starvation-induced glycerol-3-phosphate permease gene family in Arabidopsis. Plant Physiol 157: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. (2010) Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant 3: 288–299 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J. (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z. (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39: 629–642 [DOI] [PubMed] [Google Scholar]
- Sunkar R, Li YF, Jagadeeswaran G. (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17: 196–203 [DOI] [PubMed] [Google Scholar]
- Urao T, Noji M, Yamaguchi-Shinozaki K, Shinozaki K. (1996) A transcriptional activation domain of ATMYB2, a drought-inducible Arabidopsis Myb-related protein. Plant J 10: 1145–1148 [DOI] [PubMed] [Google Scholar]
- Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC. (2005a) Expression of Arabidopsis MIRNA genes. Plant Physiol 138: 2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Allen E, Wilken A, Carrington JC. (2005b) DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci USA 102: 12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Abdel-Ghany SE, Cohu CM, Kobayashi Y, Shikanai T, Pilon M. (2007) Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem 282: 16369–16378 [DOI] [PubMed] [Google Scholar]
- Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P. (2005) OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol 138: 2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, et al. (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280: 3697–3706 [DOI] [PubMed] [Google Scholar]
- Yuan H, Liu D. (2008) Signaling components involved in plant responses to phosphate starvation. J Integr Plant Biol 50: 849–859 [DOI] [PubMed] [Google Scholar]
- Zeng HQ, Zhu YY, Huang SQ, Yang ZM. (2010) Analysis of phosphorus-deficient responsive miRNAs and cis-elements from soybean (Glycine max L.). J Plant Physiol 167: 1289–1297 [DOI] [PubMed] [Google Scholar]
- Zhu J, Jeong JC, Zhu Y, Sokolchik I, Miyazaki S, Zhu JK, Hasegawa PM, Bohnert HJ, Shi H, Yun DJ, et al. (2008) Involvement of Arabidopsis HOS15 in histone deacetylation and cold tolerance. Proc Natl Acad Sci USA 105: 4945–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]