Abstract
The ADP receptor P2Y12 belongs to the superfamily of G protein–coupled receptors (GPCRs), and its activation triggers platelet aggregation. Therefore, potent antagonists, such as clopidogrel, are of high clinical relevance in prophylaxis and treatment of thromboembolic events. P2Y12 displays an elevated basal activity in vitro, and as such, inverse agonists may be therapeutically beneficial compared with antagonists. Only a few inverse agonists of P2Y12 have been described. To expand this limited chemical space and improve understanding of structural determinants of inverse agonist-receptor interaction, this study screened a purine compound library for lead structures using wild-type (WT) human P2Y12 and 28 constitutively active mutants. Results showed that ATP and ATP derivatives are agonists at P2Y12. The potency at P2Y12 was 2-(methylthio)-ADP > 2-(methylthio)-ATP > ADP > ATP. Determinants required for agonistic ligand activity were identified. Molecular docking studies revealed a binding pocket for the ATP derivatives that is bordered by transmembrane helices 3, 5, 6, and 7 in human P2Y12, with Y105, E188, R256, Y259, and K280 playing a particularly important role in ligand interaction. N-Methyl-anthraniloyl modification at the 3′-OH of the 2′-deoxyribose leads to ligands (mant-deoxy-ATP [dATP], mant-deoxy-ADP) with inverse agonist activity. Inverse agonist activity of mant-dATP was found at the WT human P2Y12 and half of the constitutive active P2Y12 mutants. This study showed that, in addition to ADP and ATP, other ATP derivatives are not only ligands of P2Y12 but also agonists. Modification of the ribose within ATP can result in inverse activity of ATP-derived ligands.
Introduction
The ADP receptor P2Y12 is a Gi protein–coupled receptor (GPCR) and a key player in platelet aggregation (Hollopeter et al., 2001). Inactivating mutations in P2Y12 are responsible for bleeding disorders in humans and dogs (Hollopeter et al., 2001; Cattaneo et al., 2003, 2005; Shiraga et al., 2005; Remijn et al., 2007; Daly et al., 2009; Fontana et al., 2009; Boudreaux and Martin, 2011). With significant relevance in pathophysiology, P2Y12 is also the major target of the antithrombotic drugs ticlopidine and clopidogrel. The thienopyridine clopidogrel is a prodrug that requires the cytochrome P450 2C19 enzyme for its conversion to an active thiol metabolite. Several mechanisms of antagonistic action have been proposed for the active metabolite of clopidogrel, including interaction with extracellular cysteine residues of P2Y12 (Ding et al., 2003) and receptor dimer disruption (Savi et al., 2006). Although very specific and effective, clopidogrel produces a variable platelet inhibition based on genetic polymorphisms and drug interactions (Munoz-Esparza et al., 2011; Nawarskas and Clark, 2011). This has triggered the search for alternative P2Y12 blockers, such as prasugrel, cangrelor, and ticagrelor. The latter two compounds are ATP analogs and bind reversibly at P2Y12 (Storey, 2011).
P2Y12 displays a high constitutive activity when expressed in vitro (Schulz and Schöneberg, 2003; Chee et al., 2008). Therefore, inverse agonists may be therapeutically beneficial compared with antagonists. Because only a few inverse agonists of P2Y12 have been described (Ding et al., 2006), we therefore screened for compounds that reduce the basal activity of constitutively active P2Y12 mutants.
Functional characterization of P2Y receptors and their mutants in mammalian expression systems is problematic because of the abundance of endogenous nucleotide receptors, nucleosidases, and nucleotide release. In previous experiments, we and others demonstrated that the human P2Y12 is functionally expressed in the yeast system (Schulz and Schoneberg, 2003; Pausch et al., 2004), which lacks such problems. Numerous constitutively activating mutations have been described for GPCRs in natural or recombinant systems, but only a few have been reported for P2Y receptors (Ding et al., 2006). From more than 1000 single point mutations, we identified 28 constitutively active P2Y12 mutants. Screening a purine compound library, we discovered several new agonists and inverse agonists for the wild-type (WT) P2Y12 and constitutively active mutants, respectively.
Materials and Methods
If not stated otherwise, all standard substances were purchased from Sigma-Aldrich (Munich, Germany), Merck (Darmstadt, Germany), and Carl Roth (Karlsruhe, Germany). Cell culture material was obtained from Sarstedt. Salmon sperm DNA, 2-(methylthio)ADP (MeS-ADP) trisodium salt hydrate, lithium acetate (LiAc) dihydrate, lithium chloride (LiCl), polyethylene glycol (PEG) 3350 (catalog no. P-3640), and apyrase from potato (grade III) were obtained from Sigma-Aldrich. MeS-ADP (P2Y12 agonist) was dissolved in water, and aliquots of stock solutions (10 mM) were stored at −20°C. Yeast medium components were purchased from Sigma-Aldrich and from BD Biosciences (Heidelberg, Germany). Restriction enzymes were purchased from New England Biolabs (Frankfurt a. M., Germany), primers were synthesized by Life Technologies (Darmstadt, Germany), and P2Y12 mutant libraries were provided by Sloning BioTechnology (Puchheim, Germany). The adenine nucleotide library was from Jena Bioscience (Jena, Germany). For compound details, see http://www.jenabioscience.com/images/7c63e6fc71/LIB-101.pdf. We additionally included adenine, adenosine, GDP, GTP, GTPγS, IMP, and xanthine (all from Sigma-Aldrich) in our pharmacologic screenings (further referred to as “purine compound library”).
Generation of P2Y12 Mutants.
Mutants were generated by subcloning SlonoMax-SINGLE libraries (synthesized double-stranded DNA fragments containing individual mutants, fragment sizes 100–150 bp) via unique endogenous or silently introduced restriction sites. P2Y12 mutants were introduced into the yeast expression plasmid p416GPD (provided by Dr. Mark Pausch, Wyeth Research, Princeton, NJ) and transformed into Escherichia coli DH5α (Life Technologies). Plasmids from individual clones were isolated (plasmid preparation kit; Promega, Mannheim, Germany), and mutations were identified by DNA sequencing. Because full coverage was not achieved after sequencing of 96 clones, missing mutants (4 mutants per position on average) were generated by polymerase chain reaction–based site-directed mutagenesis using mutant-specific mutagenesis primers.
Expression and Functional Testing of P2Y12 Mutants in Yeast and Mammalian Cells.
The Saccharomyces cerevisiae yeast strain MPY578t5 (provided by Dr. Mark Pausch) was used for yeast expression and functional testing of the P2Y12 mutants. Cells were transformed with plasmid DNA using the LiAc/salmon sperm carrier DNA/PEG method. In brief, an overnight culture grown at 30°C in YPAD (yeast extract, peptone, dextrose medium with adenine) was diluted to an optical density of 0.2 at 600 nm (OD600 nm) in 50 ml YPAD. This culture was incubated at 30°C until the OD600 nm reached 0.7–0.9. Cells were then harvested (2500g for 5 minutes at room temperature) and washed once with 25 ml of water. The pellet was dissolved in 700 µl of LiAc (100 mM) and incubated for 10 minutes at 30°C. A pellet of 50 µl from the yeast cell suspension was then mixed with 90 µl of PEG 3350 (50% w/v), 13.5 µl of LiAc (1 M), 18.75 µl of salmon sperm carrier DNA (2.0 mg/ml), 2.75 µl of sterile water, and plasmid DNA (1 µg) before being incubated for 30 minutes at 30°C and then for 30 minutes at 45°C.
For selection of constitutively active clones, cells were plated on agar plates not containing uracil and histidine (U−/H−). After incubation at 30°C for 4 days, clones were prepared for concentration-response curves. Cells transformed with P2Y12 mutants were precultured for 2 days at 30°C in U−/H− with 10 µM MeS-ADP. To remove MeS-ADP, cells were washed twice with water and grown in U−/H− overnight without MeS-ADP. The yeast cell suspension was then diluted to an OD600 nm of 0.1. From this cell suspension, 100 µl was pipetted into each well of a 96-well plate and to this, 100 µl of a 2× agonist solution or medium was added. Background growth was suppressed by the addition of 20 mM 3-aminotriazole. Mutants were screened for growth and/or constitutive activity at 10 µM MeS-ADP. All positive mutants were further evaluated through MeS-ADP concentration-response (growth) curves.
The purine compound library was screened for agonists and inverse agonists at the WT P2Y12 and constitutively active mutants. One hundred microliters of the respective yeast cell suspension (OD600 nm, 0.1) was pipetted into each well of a 96-well plate and, to these samples, 100 µl of a 2× ligand solution or medium was added. OD measurements were performed 24 and 48 hours later. Compounds identified as agonists or inverse agonists were further characterized in concentration-response setups. IC50 and EC50 values were calculated using Prism 4 software (GraphPad Software, Inc., La Jolla, CA).
To determine the stability of ATP in the 24-hour yeast assay, we performed a phosphoenolpyruvate (PEP)/pyruvate kinase test. Thus, human WT P2Y12 expressing yeast cells were grown identically as done in the previous assays. One hundred microliters of the respective yeast cell suspension (OD600 nm, 0.1) was pipetted into each well of a 96-well plate, and 100 µl of a 2× ATP solution or medium was added. Further, 2 mM PEP and 2 µl of a pyruvate kinase solution (final 6 U/ml) were added every 5 hours to the experimental solution (the total volume additional contained 100 mM imidazole, 5 mM MgCl2, pH 7.15, to assure proper pyruvate kinase function). In case ADP is formed because of degrading, PEP is used by the pyruvate kinase to produce ATP. Then, the concentration of PEP in the medium was monitored over 24 hours using a coupled optical enzyme test. Thus, 50 µl of yeast medium harvested after 0, 4, and 24 hours (or 1 mM PEP for control purposes) were incubated with 0.7 ml assay buffer (100 mM imidazole, 5 mM MgCl2, pH 7.15), 1.5 µl of lactate dehydrogenase (10 U/ml), 1 µl of pyruvate kinase (10 U/ml), 8 µl of a 100 mM ADP solution, and 8 µl of 2 mM NADH. NADH concentration was determined photometrically at 340 nm.
For expression in mammalian cells, Chinese hamster ovary (CHO)-K1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM supplemented with 10% [v/v] fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in a humidified 5% CO2 incubator. A CHO-K1 cell line stably expressing the chimeric G protein Gαqi4 (Kostenis et al., 2005) was established. Transient transfection experiments of CHO-K1 cells with the respective P2Y12 constructs and inositol-1-phosphate (IP1) accumulation assays were performed as described (Schulz and Schoneberg, 2003). In brief, Gαqi4-stable cells were seeded into 12-well plates (about 0.15 × 106 cells per well), transiently transfected, and about 48 hours after this labeled with 2 μCi/ml [myo-3H]inositol (PerkinElmer Life and Analytical Sciences, Waltham, MA). After a 16 hour-labeling period, cells were washed once with serum-free DMEM containing 10 mM LiCl and then incubated for 60 minutes at 37°C with serum-free DMEM containing 10 mM LiCl with or without a compound. After this time, the assay medium was removed and the reaction was stopped by adding 0.3 ml of 0.1 N NaOH, followed by a 5 minute-incubation at 37°C. The alkaline solution was then neutralized by adding 0.1 ml of 0.2 M formic acid, and the IP1 fraction was isolated by anion exchange chromatography as described (Berridge, 1983) and counted on a liquid scintillation counter.
For cAMP assays, transfected cells were labeled with [3H]adenine (2 μCi/ml; PerkinElmer and Life and Analytical Sciences) for 12 hours and washed once in serum-free DMEM containing 1 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), followed by incubation in the presence of the indicated compounds and forskolin (10 μM) for 1 hour at 37°C. Reactions were terminated by aspiration of the medium and addition of 1 ml of 5% (w/v) trichloroacetic acid. The cAMP content of cell extracts was determined by anion exchange chromatography as described (Salomon et al., 1974).
To measure label-free receptor activation, a dynamic mass redistribution (DMR) assay (Corning Epic Biosensor Measurements; Corning Life Sciences, Lowell, MA) with stably transfected human embryonic kidney (HEK) cells (HEK-FlpIn, P2Y12 in pcDNA5/FRT) was performed as described previously (Schroder et al., 2010; Ritscher et al., 2012). Briefly, cells were seeded into fibronectin-coated Epic 384–well microplates (60,000 cells per well) and exposed to the various compounds. In DMR measurements, polarized light is passed through the bottom of the biosensor microtiter plate, and a shift in wavelength of reflected light indicates intracellular mass redistribution triggered by receptor activation. DMR was recorded as a measure of cellular activity over 60 minutes. Agonist-induced DMR is concentration dependent, and concentration-effect curves were calculated from response peak maxima (approximately 6 minutes after adding the compound) of optical traces.
Generation of a P2Y12 Comparative Model and Ligand Docking.
A comparative model of P2Y12 was constructed using the protein structure prediction software package Rosetta, version 3.2 (Leaver-Fay et al., 2011). The x-ray crystal structure of C-X-C chemokine receptor type 4 (CXCR4) (Protein Data Bank ID: 3ODU) (Gupta et al., 2001) was chosen as a template on the basis of its high similarity to P2Y12 (e-value of 3e−15 with a sequence coverage of 90%) according to a search using National Center for Biotechnology Information BLASTP on sequences from the Protein Data Bank (Supplemental Fig. S1). CXCR4 and P2Y12 also share a conserved disulfide bond between the N-terminal C17 and C270 in extracellular loop 3 (Deflorian and Jacobson, 2011). The backbone coordinates of CXCR4 were retained in the comparative model of P2Y12, whereas the loop coordinates were built in Rosetta using Monte Carlo Metropolis fragment replacement combined with cyclic coordinate descent loop closure. In brief, ϕ-ψ angles of backbone segments from homologous sequence fragments from the Protein Data Bank are introduced into the loop regions. After the fragment substitution, small movements in the ϕ-ψ angles are performed to close breaks in the protein chain. The resulting full sequence models were subjected to eight iterative cycles of side chain repacking and gradient minimization of ϕ, ψ, and χ angles Rosetta Membrane (Yarov-Yarovoy et al., 2006).
Ligand docking into the comparative model of P2Y12 with ADP, ATP, MeS-ADP, MeS-ATP, mant-ADP, mant-ATP, mant-deoxy-ATP [dATP], and mant-deoxy-ADP was performed with Rosetta Ligand (Meiler and Baker, 2006; Davis and Baker, 2009). Each ligand was allowed to sample docking poses in a 5-Å radius centered at the putative binding site for ADP, determined by averaging the coordinates of critical residues for ligand recognition: R256, Y259, and K280 (Hoffmann et al., 2008). Once a binding pose had been determined by the docking procedure, 100 conformations of the ligand created by Molecular Operating Environment (Chemical Computing Group, Toronto, ON, Canada) were tested within the site. Side-chain rotamers around the ligand were optimized simultaneously in a Monte Carlo Metropolis–simulated annealing algorithm. The energy function used during the docking procedure contains terms for van der Waals attractive and repulsive forces, statistical energy derived from the probability of observing a side-chain conformation in the Protein Data Bank, hydrogen bonding, electrostatic interactions between pairs of amino acids, and solvation assessing the effects of both side-chain/side-chain interactions and side-chain/ligand interactions. For each ligand, more than 3000 docked complexes were generated and clustered for structural similarity using bcl::Cluster (Alexander et al., 2011). The lowest energy binding poses from the five largest clusters for each ligand were used for further analysis. The change in free energy with and without ligands bound to P2Y12 was calculated for each residue in the receptor. Residues with the greatest difference in predicted energy are suggested to be important for ligand interaction (Supplemental Fig. S2).
Results
Expression of the Human P2Y12 in Yeast and Determination of Agonist Specificity.
In previous experiments, we and others have already demonstrated that human P2Y12 can be functionally expressed in the yeast system. In this system, P2Y12-expressing yeast grows in 96-well cell plates and regular OD measurements are taken. OD values measure cell growth, which is used as a strong indicator for receptor activity.
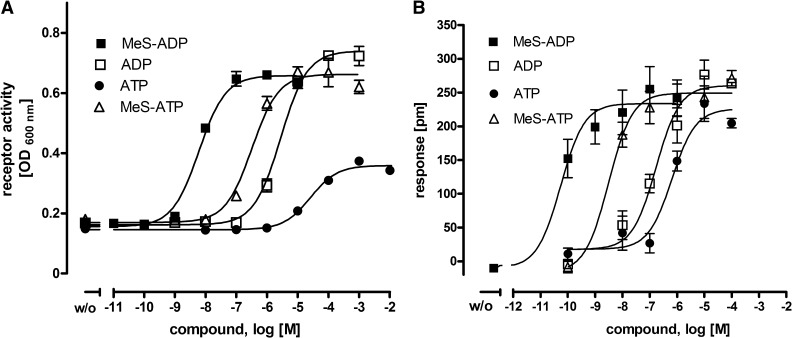
The WT P2Y12 was functionally tested with the compound library for agonists. P2Y12 expressed in yeast showed a similar EC50 value for MeS-ADP (EC50 value, 6 nM; Fig. 1) as when expressed in mammalian cells ranging from low nanomolar to 25–80 nM concentrations (Zhang et al., 2001; Simon et al., 2002; Bodor et al., 2003; Zhong et al., 2004; Ding et al., 2006; Hoffmann et al., 2008). ADP was approximately 500-fold less potent to MeS-ADP, which is consistent with previous findings showing an approximately 30-fold to 1000-fold lower potency in mammalian cells (Zhang et al., 2001; Simon et al., 2002; Bodor et al., 2003). In addition to the highly potent agonist MeS-ADP, we identified additional P2Y12 agonists: ATP and MeS-ATP. ATP was a partial agonist at human P2Y12 when expressed in yeast (Fig. 1A). EC50 values were ranked: MeS-ADP < MeS-ATP < ADP < ATP. We considered the possibility that the registered ATP activity might be due to the fraction of the nucleotides converted to ADP derivatives by nucleotidases or hydrolysis and quantified the possible decay of ATP during the 24-hour assays. Thus, we indirectly quantified ATP degradation in the assay using the PEP/pyruvate kinase system. The pyruvate kinase catalyzes the transfer of a phosphate group from PEP to ADP, yielding one molecule of pyruvate and one molecule of ATP. PEP concentration in the medium is therefore a measure for degraded ATP (see Materials and Methods). We found that PEP concentration in the yeast medium remained almost unchanged during 24-hour yeast growth (∆E0 h = 0.44; ∆E4 h = 0.44; ∆E24 h = 0.48). Only 3.4% of PEP (initial concentration 2 mM) was used by pyruvate kinase for ATP generation. This indicated high stability of ATP (96.6%) in the assay over 24 hours. The functionality of the pyruvate kinase to convert ADP to ATP was verified in control experiments performed in parallel.
Fig. 1.
Nucleotide agonists at the human P2Y12. (A) the human P2Y12 was transformed into yeast cells and incubated with different concentrations of P2Y12 agonists. Receptor activation-dependent growth was measured as OD600 nm after 24 hours. Data are given as mean ± S.D. of three independent experiments, all performed in triplicate. (B) for label-free measurements of receptor activation, a dynamic mass redistribution assay (Epic Biosensor Measurements) with stably transfected HEK cells was performed essentially as described previously (Schroder et al., 2010). The response is shown 6 minutes after compound application. The response of each compound at nontransfected HEK cells was subtracted from the respective response at P2Y12 transfected HEK cells. Data are presented as mean ± S.E.M. of two independent experiments, each carried out in triplicate.
The agonistic properties of the adenine nucleotides were verified in the mammalian cell line COS-7 and CHO cells (data not shown), wherein the human P2Y12 was coexpressed with the chimeric Gαqi4 protein, which redirects receptor activation to the phospholipase C/ inositol phosphate pathway (Kostenis et al., 2005). Because ATP produces a cellular response via endogenous nucleotide receptors in most cell lines, we performed additional measurements of P2Y12 activation on stably transfected mammalian HEK with a dynamic mass redistribution assay (Epic Biosensor Measurements) (Schroder et al., 2010). Responses of endogenous nucleotide receptors were subtracted from the specific response of P2Y12-transfected cells. As shown in Fig. 1B, the concentration-response curves were similar to the data from the yeast expression system except we found that ATP was a full agonist in this mammalian expression system. We also performed Epic measurements in P2Y12-stably transfected astrocytoma cells 1321N1, which should not express nucleotide receptors (Filtz et al., 1994). However, ATP-mediated responses in 1321N1 cells were less than those in HEK cells, having no advantage over transfected HEK cells. In sum, the yeast expression system is free of endogenous nucleotide receptors and, therefore, the most straightforward system to use in analyzing P2Y receptors. P2Y12 expressed in yeast displays pharmacologic properties very similar to those of mammalian expression systems. Our screening revealed additional compounds with agonistic activity at P2Y12: ADPβS, 2′-(OR-3′)-O-(trinitrophenyl) (TNP)-ADP, ATPγS, 2I-ATPγS [2-Iodo-adenosine-5′-(-thio)-triphosphate], and adenosine-5′-(amido)diphosphate (AppNH2) (Table 1). We did not follow the pharmacology of these ADP and ATP derivatives further, but they support the fact that derivatives of ATP, as well as of ADP, also have agonistic activities at the human P2Y12.
TABLE 1.
Mant-dATP is an inverse agonist at different constitutively active mutants
| Position | Mutation | Inverse Activating Substances (Fold over Basal ≤ 0.8) |
|---|---|---|
| F246 | V | mant-dATP |
| F254 | I | mant-dATP, mant-N6-methyl-ATP |
| L | mant-dATP, mant-N6-methyl-ATP | |
| V | mant-dATP | |
| F296 | A | mant-dATP |
| C | mant-dATP, mant-N6-methyl-ATP | |
| M | mant-dATP | |
| F300 | N | mant-dATP |
| L301 | N | mant-dATP |
| M | mant-dATP |
It should also be noted that many nucleotides and nucleosides (e.g., AMP, GTP, cAMP, adenosine) that do not activate P2Y12 in mammalian expression systems (Zhang et al., 2001) did not activate P2Y12 expressed in yeast.
The comprehensive compound library allowed identification of all determinants necessary for agonist function at P2Y12 (Supplemental Table 1). Compounds showing significant agonistic activity are given in Table 1. Substitutions that are not compatible with agonistic activity at the human P2Y12 (at least in two tested compounds) are listed in Table 2. The results for agonistic activity can be roughly summarized for the three major nucleotide components (base, ribose, phosphate groups):
TABLE 2.
Structure of compounds with agonistic properties at the WT human P2Y12
| Backbone | Substances | Side Chain |
||||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | ||
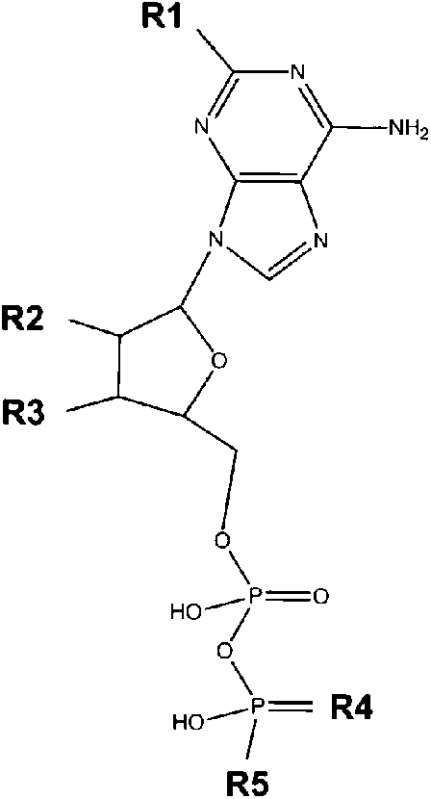 |
ADP | H | OH | OH | O | OH |
| ADPβS | H | OH | OH | S | OH | |
| TNP-ADP | H | C6N3O8H4 | O | OH | ||
| AppNH2 | H | OH | OH | O | NH2 | |
| ATP | H | OH | OH | O | PO4H2 | |
| ATPγS | H | OH | OH | O | PSO3H2 | |
| 2I-ATPγS | I | OH | OH | O | PSO3H2 | |
The purine ring is absolutely required. Some modifications (methylthio, iodo) at the 2-position of adenine are tolerated, but guanine- and inosine-based nucleotides are not agonistic.
Deoxidation of the ribose is not tolerated. The trinitrophenyl modification (TNP-ADP) is tolerated.
Adenine nucleotides with two or three phosphate residues are agonistic, whereas fewer than 2 phosphate residues or cyclic phosphates are insufficient for agonistic activity. Some substitutions of phosphate moieties, as in ADPβS, ATPγS, and AppNH2, are tolerated.
Adenine nucleotide multimers (P1-(5′-adenosyl) P3-(5′-adenosyl) triphosphate, P1-(5′-adenosyl) P4-(5′-adenosyl) tetraphosphate, P1-(5′-adenosyl) P5-(5′-adenosyl) pentaphosphate, P1-(5′-adenosyl) P6-(5′-adenosyl) hexaphosphate) displayed no agonistic activity.
Structural Model of Agonist Binding.
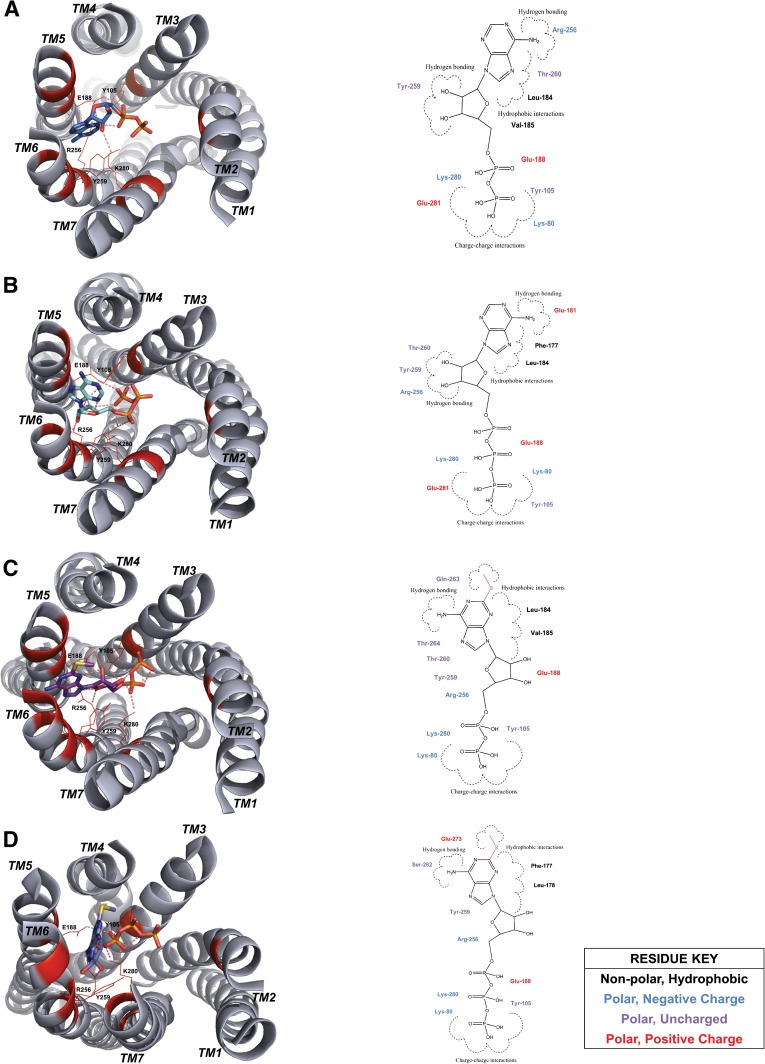
To estimate whether the different agonists may have similar binding properties, we simulated binding by docking the agonists into the comparative model of P2Y12 (Figs. 2 and 3). The model suggested that ADP, ATP, MeS-ADP, MeS-ATP, mant-ADP, mant-ATP, mant-dADP, and mant-dATP bind in the site bordered by transmembrane helices 3, 5, 6 and 7. Ligands were oriented such that the phosphate groups generally pointed toward transmembrane helices 3 and 7, forming hydrogen bonds with Y105 and K280. Adenosine rings frequently interacted with the hydrophobic residues on transmembrane helix 5, namely L184, V185, and F177 in the second extracellular loop. In agreement with previous docking studies, R256 and K280 were found to be critical residues in the ADP binding pocket (Deflorian and Jacobson, 2011; Ignatovica et al., 2011). R256 frequently interacts with the hydroxyl groups and the oxygen from the furanose. K280 is demonstrated to interact with the negatively charged phosphate groups of the ligands. In addition to the R256 and K280, Y105, E188, and Y259 are consistently found to interact with the ligand. Y105 and E188 form hydrogen bonds with the phosphate tail, whereas Y259 seems to stabilize the adenine.
Fig. 2.
P2Y12 docked in complex with agonists ADP, ATP, MeS-ADP, and MeS-ATP. The docked binding poses in the comparative model of P2Y12 for agonists (A) ADP, (B) ATP, (C) MeS-ADP, and (D) MeS-ATP in relation to residues Y105, E188, R256, Y259, and K280. All side chains within the binding site important for ligand interaction according to calculations of free energy change with and without ligands bound to P2Y12 are highlighted in red in the model and also shown in relation to the two-dimensional ligand depiction.
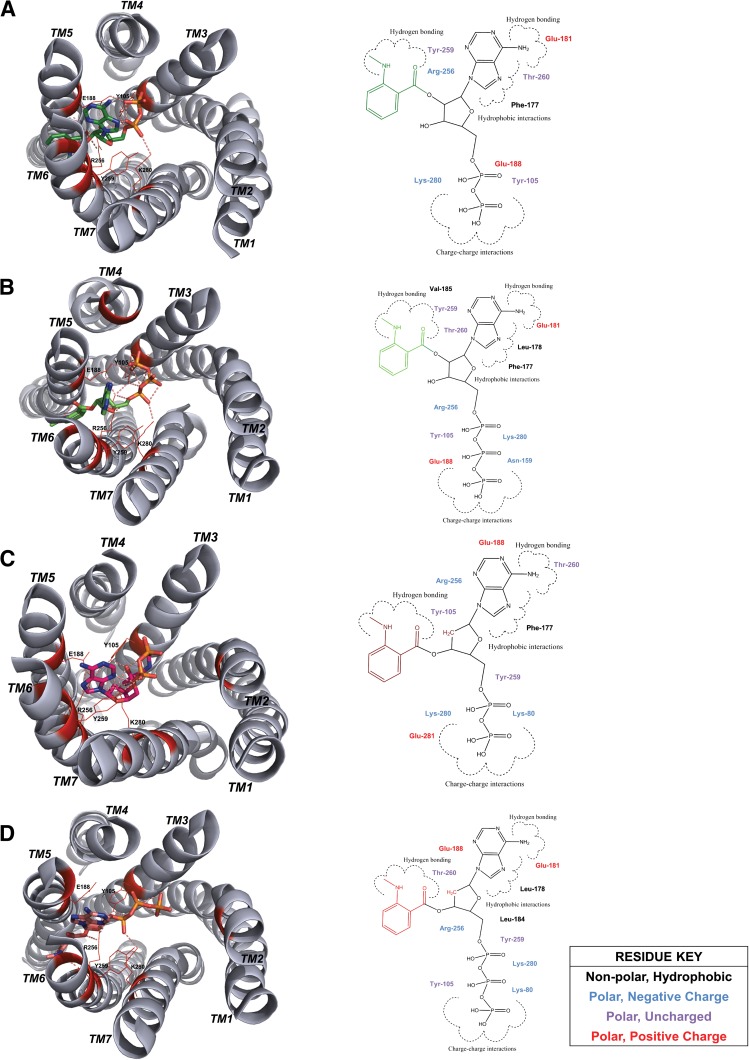
Fig. 3.
P2Y12 docked in complex with agonists mant-ADP and mant-ATP and inverse agonists, mant-dADP and mant-dATP. The docked binding poses in the comparative model of P2Y12 are shown for agonists (A) mant-ADP and (B) mant-ATP and the inverse agonists (C) mant-dADP and (D) mant-dATP in relation to residues Y105, E188, R256, Y259, and K280. All side chains within the binding site important for ligand interaction according to calculations of change in free energy with and without ligands bound to P2Y12 are highlighted in red and shown in relation to the two-dimensional ligand depiction.
Identification of Constitutively Active Mutants.
It is still impossible to predict mutations leading to constitutive activity of a given GPCR. Furthermore, at positions where some mutations activate the GPCR, not all mutations will result in constitutive receptor activation (Lalueza-Fox et al., 2007; Bakker et al., 2008 ). Therefore, screening of mutant libraries is required. Mutations induced via error-prone derived mutant libraries cannot provide mutational saturation of every codon, and instead, most alleles will contain more than one mutated codon (Li et al., 2007; Thor et al., 2008). Recent advances in gene synthesis technology (see Materials and Methods) have made it possible to generate comprehensive mutant libraries.
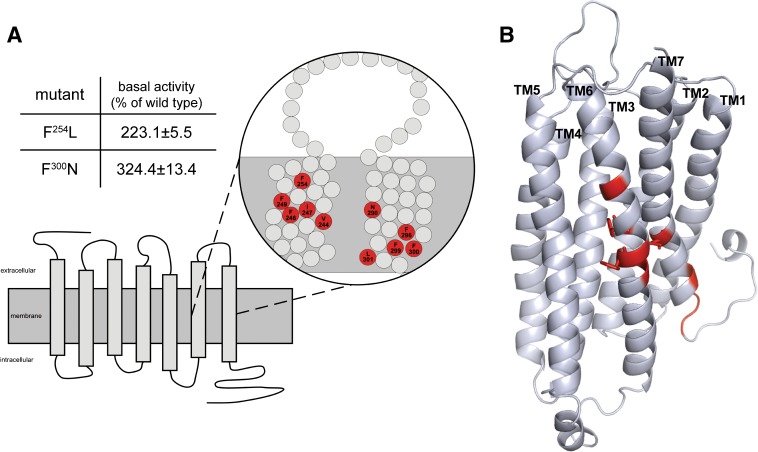
Here, we mutated every single position to all possible amino acids in a receptor region known from other GPCRs to be sensitive for mutational induced constitutive activity. In sum, 1254 P2Y12 mutants were generated covering 66 positions (amino acid positions 236–301) of the receptor and yielding 28 constitutive active mutants at 10 positions (positions are given in Fig. 4). Most mutations were found at positions that faced the lipid, whereas three positions faced the receptor pore (F246, F249, and N290) and three were near the C-terminal receptor tail (F299, F300, and L301). All data are available and organized in a P2Y12 mutant database (http://www.ssfa-7tmr.de/p2y12).
Fig. 4.
Position and basal activity of constitutively active P2Y12 mutants. (A) the position of constitutively active mutations in transmembranes 1 and 7 (TM6 and TM7) are depicted. Basal activities of the individual mutants expressed in yeast are given in the table. Data are presented as mean ± S.D. of three independent experiments, each carried out in triplicate. The basal activity of the WT P2Y12 was OD600 nm: 0.074±0.016. Complete functional data are available and organized in a P2Y12 mutant database (http://www.ssfa-7tmr.de/p2y12). (B) the comparative model of P2Y12 based on the CXCR4 template is depicted. Residues producing constitutively active mutants on TM6 and TM7 are highlighted in red. Residue side chains facing the pore of the receptor (F246, F249, and N290) are shown in sticks.
Identification of Mant-dATP as Inverse Agonist at Constitutively Active P2Y12 Mutants.
Constitutively active mutants were expressed and the purine compound library was tested for inverse agonists. N-methyl-anthraniloyl- (mant-) dATP reduced basal activity of many constitutively active P2Y12 mutants (Table 3). For several mutants, mant-N6-methyl-ATP was also an inverse agonist (see Table 3). There is no obvious structural overlap or difference between the mutants at which the different inverse agonists act or do not act.
TABLE 3.
ADP modifications not compatible with agonistic activity at the WT human P2Y12
| Backbone | Position | Side Chain |
|---|---|---|
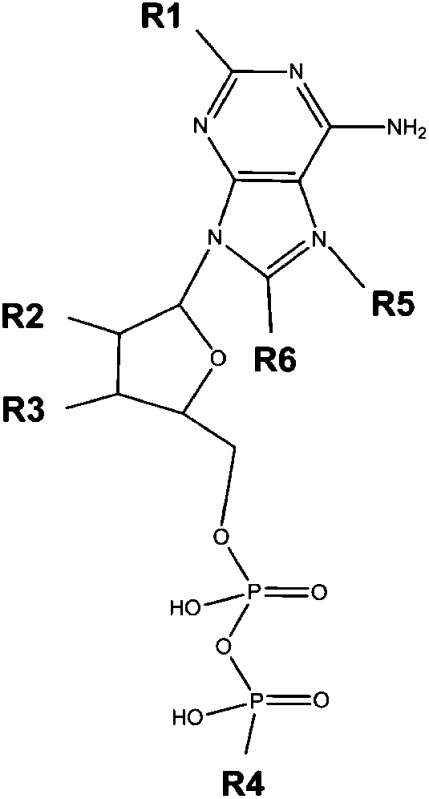 |
R1 | OH |
| R2 | H | |
| R3 | H | |
| CH3 | ||
| I | ||
| Br | ||
| R4 | NH2 | |
| R5 | CH3 | |
| R6 | Br |
The table summarizes the modifications at the ADP backbone that are not compatible with agonistic activity at the WT human P2Y12 at in least two different adenine nucleotides of the compound library (see Materials and Methods).
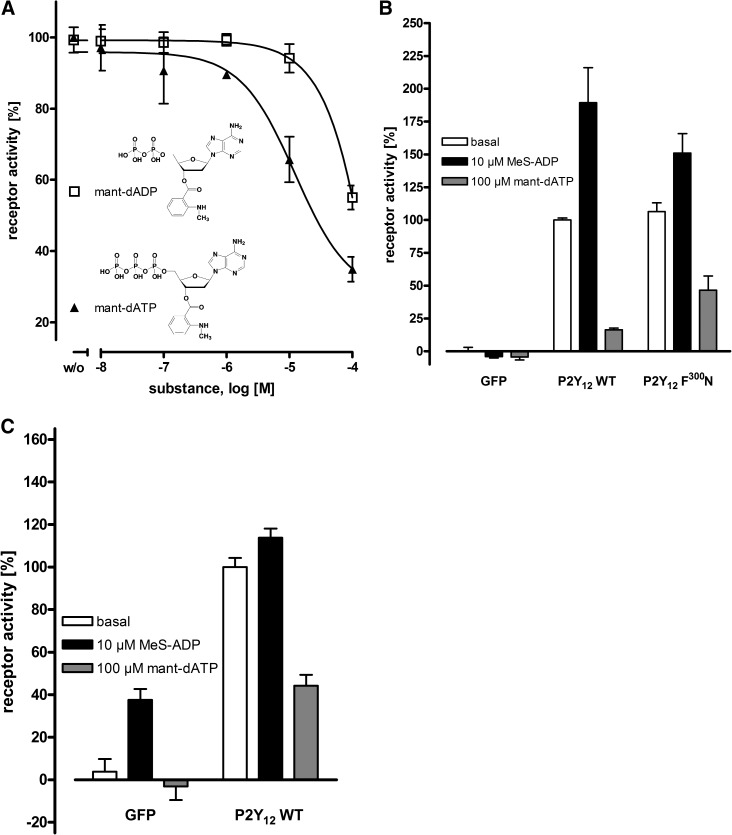
Inverse agonist activity was studied at F254L in more detail. As shown in Fig. 5A, mant-dATP suppressed basal activity in a concentration-dependent manner, with an IC50 value in a micromolar range. Of note, the potency of mant-dADP was lower than that of mant-dATP (see Fig. 5A). Both the deoxy- and the mant- modifications are required because mant-ATP and dATP had no effect on basal activity of P2Y12 mutants. It should be noted that the basal activities of several mutants (V244E, F246C, F246G, F246P, F246S, F246T, I247F, F249Y, N290W, N290Y, F296I, F296L, F296V, F299I, F299V, L301C, L301G, and L301T) were not reduced by mant-dATP or any other compound tested. It is known already that WT P2Y12 displays increased basal activity compared with nontransfected mammalian cells (Schulz and Schoneberg, 2003).
Fig. 5.
Mant-dADP and mant-dATP are inverse agonists at constitutively active P2Y12. (A) yeast cells expressing F254L were incubated with increasing concentrations of the indicated compounds, and yeast growth was measured after 24 hours’ incubation. The ligand-induced decrease of basal activity of F254L is shown relative to the basal activity of the WT P2Y12 (OD600 nm, 0.074; set to 0%) and the basal activity of F254L (OD600 nm, 0.165; set to 100%). Data are given as mean ± S.D. of three independent experiments, all performed in triplicate. (B) to evaluate inverse agonist specificity, CHO-K1 cells, stably expressing the chimeric G-protein Gαqi4, were transfected with plasmids encoding GFP (control), the human ADP receptor or F300N. IP1 formation under basal conditions (white), in the presence of 10 μM MeS-ADP (black bars) and in presence of 100 µM mant-dATP (light gray bars). The basal IP1 for GFP was 321 cpm per well and set to 0%, the basal IP1 for WT P2Y12 was 970 cpm per well and set to 100%. Data are presented as mean ± S.D. (cpm per well) of three independent experiments, each carried out in duplicate. (C) forskolin-induced cAMP levels in CHO-K1 cells stably expressing human ADP receptor were determined under basal conditions (white bars), in the presence of 10 μM MeS-ADP (black bars), and in presence of 100 µM mant-dATP (light gray bars). The decrease of basal activity of WT P2Y12 is shown relative to GFP basal activity (7486 cpm per well; set to 0%) and basal activity of WT P2Y12 (4533 cpm per well; set to 100%). Data are given as mean ± S.D. of three independent experiments, all performed in triplicate.
To verify that mant-dATP mediates its inverse agonistic activity at the constitutive activity of the WT P2Y12 expressed in mammalian cells as well, CHO-K1 cells were co-transfected with chimeric Gαqi4 and IP1 accumulation assays were performed. As shown in Fig. 5B, the WT P2Y12 displayed a high basal activity and MeS-ADP increased IP1 levels only 2-fold. Mant-dATP almost completely blocked basal IP1 formation at the WT P2Y12 and F300N (Fig. 5B). Also in cAMP inhibition assays at CHO-K1 cells, mant-dATP displayed strong inverse agonistic activity on the inhibition of basal cAMP formation at the WT P2Y12 (Fig. 5C).
Some cell lines release receptor function–relevant amounts of nucleotides into the cell culture medium (Parr et al., 1994; Lazarowski et al., 1997). This may account for high basal activity of P2Y12 heterologously expressed in mammalian cell lines. Therefore, we performed similar control experiments with CHO-K1 cells stably transfected with Gαqi4. As shown in Supplemental Fig. S3, Gαqi4-CHO-K1 cells transiently transfected with P2Y12 presented an increased basal IP1 level compared with cells transfected with the control plasmid (GFP). Incubation with apyrase did not reduce this elevated IP1 level. This finding clearly indicates that P2Y12 does induce signal transduction by intrinsic active receptor conformation and not by nucleotides released from the cells into the medium. Proper apyrase function was demonstrated by loss of ADP action on P2Y12.
Mant-dATP Is Most Likely an Orthosteric Ligand at P2Y12.
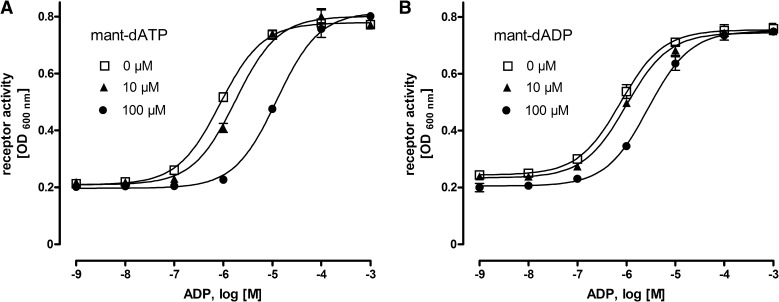
To evaluate whether mant-dATP mediates its inverse agonistic action through an orthosteric or an allosteric binding site, the ADP concentration-response curves at F254L were determined in the presence of different concentrations of mant-dATP. As shown in Fig. 6A, increasing concentrations of mant-dATP shifted the concentration-response curves to higher ADP concentrations. Similar results were obtained for mant-dADP, but with lower potency (Fig. 6B). This competition indicates an orthosteric binding modus for the inverse agonists. Although functional and docking data (see below) support orthosteric binding, we cannot rule out the possibility of an allosteric binding of the inverse agonists given the limited concentration range of mant-dATP investigated herein.
Fig. 6.
Mant-dADP and mant-dATP are most likely orthosteric ligands at P2Y12 F254L. To evaluate the modus of inverse agonist binding, ADP concentration-response curves at F254L-transformed yeast cells were determined in the presence of 0, 10, and 100 µM mant-dATP (A) and mant-dADP (B). Data are given as mean ± S.D. of three independent experiments, all performed in duplicate. Yeast cells expressing different basal active mutants were incubated with a 10 µM purine compound library to identify inverse agonists. In a screen of more than 80 adenine nucleotides and their derivatives, mant-dATP and mant-N6-methyl-ATP showed inverse activity on several constitutively active mutants. All the mutants listed showed activation (more than 2-fold above increased basal activity) upon stimulation with ADP, MeS-ADP, ATP, and mant-ADP. dATP and mant-ATP had no significant effects on the mutants.
Structural Model of Inverse Agonist Binding.
Using our P2Y12 model, we investigated whether mant-dATP can dock into the agonist binding pocket of P2Y12 and whether specific interactions may explain inverse agonistic activity (Fig. 3). As with the other ATP derivatives, mant-dATP sits between (transmembrane helix TMH) 3, 5, 6 and 7 with Y105 and K280, forming hydrogen bonds with the phosphate tail and R256 stabilizing the oxygen connecting the furanose to the mant group. Unlike ATP, the extra bulk of the mant group is further stabilized by interactions with I257, H253, and Q263. However, similar interactions are seen with mant-ATP, which does not exhibit inverse agonism. We conclude that the inverse agonistic activity is probably not the result of a different binding pose. It is possibly caused by smaller-scale modulations in the strengths of specific interactions between ligand and protein. Pin-pointing these changes to reveal the mechanism behind the inverse agonistic activity are beyond the accuracy of the present comparative model but will be the focus of future mutational studies.
Discussion
We used a genetically modified yeast strain (Pausch et al., 2004) to heterologously express and functionally test the human ADP receptor P2Y12. This expression system offers some advantages over mammalian cells lines, specifically in characterizing nucleotide receptors, because it lacks endogenous nucleotide receptors. ADP and MeS-ADP are full agonists in this expression system, with EC50 values of 2.8 µM and 6 nM, respectively (Fig. 1A). Screening a purine compound library, we identified ATP and some derivatives as partial agonists at P2Y12 in addition to ADP and its derivatives (Supplemental Table S1). The agonistic activity of ATP was found not only in the heterologous yeast expression system but also in different mammalian cell lines and signaling assays.
That MeS-ATP and ATP bind to the human P2Y12 has been shown (Savi et al., 2001), but the ligand properties of ATP at P2Y12 are controversial, ranging from antagonism (Bodor et al., 2004; Springthorpe et al., 2007) to agonism (Barnard and Simon, 2001; Simon et al., 2002). These contrary results are probably due to differences in mammalian expression systems and functional assays used. Introduction of a 2′-methylthio group increased ligand potency at P2Y12 and made ATP a highly potent full agonist (Fig. 1A), consistent with previous findings (Zhang et al., 2001; Simon et al., 2002). Through ligand docking into a structural comparative model of P2Y12, ATP derivatives are found to bind in a similar binding site. Although our structural P2Y12 model is not at the resolution to reveal what fine-structural requirements are essential to turn a nucleotide into an agonist at P2Y12, specific residues critical to ligand interaction can be predicted from the model. Notably, we find that for six of the seven residues indicated to be significant for ligand interaction that are also in the mutant database (H253, I257, Y259, T260, Q263, T264, and K280), mutation of the residues to any other amino acid results in a loss of WT function (see our P2Y12 mutant database: http://www.ssfa-7tmr.de/p2y12). Therefore, there is agreement between the residues predicted to be critical for agonist function through docking studies and experimental results. Our model and the docking studies are consistent with the fact that ATP fits into the same binding pocket as well characterized agonists.
These findings raise a relevant question about whether ATP can serve as a P2Y12 agonist also in vivo. The ATP-to-ADP ratio in human platelet-dense granules is approximately 2 (Weiss et al., 1979; Cattaneo et al., 2000). If one assumes that ATP and ADP secretion from dense granules occurs with the same kinetics, previous data suggest that the surface concentration of ADP after thrombin stimulation will transiently reach 7–10 μM (Beigi et al., 1999). This is sufficient for activation of the platelet P2Y12 by ADP but also by ATP and is consistent with feed-forward autocrine/paracrine activation of platelet responses.
Many WT GPCRs, such as histamine receptors, thyrotropin receptor, and melanocortin receptors, present high basal activity (Seifert and Wenzel-Seifert, 2002). In contrast to antagonists, inverse agonists suppress both agonist-dependent and -independent activity and are therefore developed in priority. For example, many β-blockers and atropine are inverse agonists at β1-adrenoceptors and muscarinic acetylcholine receptors, respectively (Thor et al., 2009; Baker et al., 2011). Therapeutically used P2Y12 ligands are high-affinity antagonists, but inverse activity was described only for the experimental P2Y12 blocker AR-C78511 [(E)-N-[1-[7-hexylamino)-5-(propylthio)-3H-1,2,3-triazolo-[4,5-d]-pyrimidin-3-yl]-1,5,6-trideoxy-β-d-ribo-hept-5-enofuranuronoyl]-l-aspartic acid] (Vasiljev et al., 2003; Ding et al., 2006). AR-C78511 is a 2-alkylthio-substituted ATP analog but, in contrast to mant-dATP, has no modification at the 2′ or 3′ OH residues of the ribose. Mant-dATP most likely binds at the orthosteric ligand-binding site, and inverse agonistic activity mutually depends on the deoxyribose because mant-ATP lacks inverse agonistic activity. At present we cannot explain or predict inverse activity, even with a receptor model in hand, because the pharmacologic properties of a ligand are the result of a tight interplay of the ligand and the receptor molecule. It is, however, of interest that, as for AR-C78511 (Springthorpe et al., 2007), modification of an ATP backbone resulted again in an inverse agonist (mant-dATP). This also supports our findings that P2Y12 naturally recognizes not only ADP but also ATP and that binding of ATP and other ATP derivatives induces conformational changes within P2Y12.
In summary, we clearly show that, in addition to ADP and ATP, some ATP derivatives are not only ligands of P2Y12 but also agonists. Keeping with an ATP/ADP ratio > 1 in vivo and the small differences in concentration-response curves (Fig. 1B), P2Y12 should rather be referred to as an adenine nucleotide receptor without suggesting ADP specificity. Modification of the ribose within ATP can result in inverse activity of ATP-derived ligands.
Supplementary Material
Abbreviations
- AppNH2
adenosine-5′-(amido)diphosphate
- AR-C78511
(E)-N-[1-[7-hexylamino)-5-(propylthio)-3H-1,2,3-triazolo-[4,5-d]-pyrimidin-3-yl]-1,5,6-trideoxy-b-D-ribo-hept-5-enofuranuronoyl]-L-aspartic acid
- 2I-ATPgammaS
2-Iodo-adenosine-5′-(-thio)-triphosphate
- CHO
Chinese hamster ovary
- CXCR4
C-X-C chemokine receptor type 4
- dADP
deoxy-ADP
- dATP
deoxy-ATP
- DMEM
Dulbecco’s modified Eagle’s medium
- DMR
dynamic mass redistribution
- GPCR
G protein–coupled receptor
- HEK
human embryonic kidney
- IP1
inositol-1-phosphate
- LiAc
lithium acetate
- MeS-ADP
2-(methylthio)-ADP
- MeS-ATP
2-(methylthio)-ATP
- OD
optical density
- PEG
polyethylene glycol
- PEP
phosphoenol pyruvate
- TNP-ADP
2′-(OR-3′)-O-(trinitrophenyl)-ADP
- U−/H−
uracil and histidine
- WT
wild type
Authorship Contributions
Participated in research design: Schmidt, Dong, Meiler, Schöneberg.
Conducted experiments: Schmidt, Ritscher, Hermsdorf.
Contributed new reagents or analytic tools: Cöster, Wittkopf.
Performed data analysis: Schmidt, Dong, Ritscher, Hermsdorf, Schöneberg.
Wrote or contributed to the writing of the manuscript: Schmidt, Dong, Meiler, Schöneberg.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft [FOR 748, Scho 624/7-1; Sfb 610] and a student fellowship of the Medical Faculty, University of Leipzig, to D.W. Work in the Meiler laboratory is supported by the National Institutes of Health [Grants R01 GM080403, R01 MH090192, and R01 GM099842] and the National Science Foundation (Career 0742762). E.N.D. is supported through the Paul Calabresi Medical Student Research Fellowship from the PhRMA Foundation.
 This article has supplemental material available at mol.aspetjournals.org.
This article has supplemental material available at mol.aspetjournals.org.
References
- Alexander N, Woetzel N, and Meiler J (2011) Bcl:Cluster: a method for clustering biological molecules coupled with visualization in the Pymol Molecular Graphics System, in2011 IEEE 1st International Conference on Computational Advances in Bio and Medical Sciences (ICCABS); 2004 February 3–5; Orlando, FL. pp 13-18, Institute of Electrical and Electronics Engineers, Piscataway, NJ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hill SJ, Summers RJ. (2011) Evolution of β-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci 32:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RA, Jongejan A, Sansuk K, Hacksell U, Timmerman H, Brann MR, Weiner DM, Pardo L, Leurs R. (2008) Constitutively active mutants of the histamine H1 receptor suggest a conserved hydrophobic asparagine-cage that constrains the activation of class A G protein-coupled receptors. Mol Pharmacol 73:94–103 [DOI] [PubMed] [Google Scholar]
- Barnard EA, Simon J. (2001) An elusive receptor is finally caught: P2Y(12′), an important drug target in platelets. Trends Pharmacol Sci 22:388–391 [DOI] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 276:C267–C278 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (1983) Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J 212:849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor ET, Waldo GL, Blaesius R, Harden TK. (2004) Delineation of ligand binding and receptor signaling activities of purified P2Y receptors reconstituted with heterotrimeric G proteins. Purinergic Signal 1:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. (2003) Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol 64:1210–1216 [DOI] [PubMed] [Google Scholar]
- Boudreaux MK, Martin M. (2011) P2Y12 receptor gene mutation associated with postoperative hemorrhage in a Greater Swiss Mountain dog. Vet Clin Pathol 40:202–206 [DOI] [PubMed] [Google Scholar]
- Cattaneo M. (2005) The P2 receptors and congenital platelet function defects. Semin Thromb Hemost 31:168–173 [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. (2000) Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol 20:E101–E106 [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Zighetti ML, Lombardi R, Martinez C, Lecchi A, Conley PB, Ware J, Ruggeri ZM. (2003) Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci USA 100:1978–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MJ, Mörl K, Lindner D, Merten N, Zamponi GW, Light PE, Beck-Sickinger AG, Colmers WF. (2008) The third intracellular loop stabilizes the inactive state of the neuropeptide Y1 receptor. J Biol Chem 283:33337–33346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly ME, Dawood BB, Lester WA, et al. (2009) Identification and characterization of a novel P2Y 12 variant in a patient diagnosed with type 1 von Willebrand disease in the European MCMDM-1VWD study. Blood 113:4110–4113 [DOI] [PubMed] [Google Scholar]
- Davis IW, Baker D. (2009) RosettaLigand docking with full ligand and receptor flexibility. J Mol Biol 385:381–392 [DOI] [PubMed] [Google Scholar]
- Deflorian F, Jacobson KA. (2011) Comparison of three GPCR structural templates for modeling of the P2Y12 nucleotide receptor. J Comput Aided Mol Des 25:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. (2003) Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood 101:3908–3914 [DOI] [PubMed] [Google Scholar]
- Ding Z, Kim S, Kunapuli SP. (2006) Identification of a potent inverse agonist at a constitutively active mutant of human P2Y12 receptor. Mol Pharmacol 69:338–345 [DOI] [PubMed] [Google Scholar]
- Filtz TM, Li Q, Boyer JL, Nicholas RA, Harden TK. (1994) Expression of a cloned P2Y purinergic receptor that couples to phospholipase C. Mol Pharmacol 46:8–14 [PubMed] [Google Scholar]
- Fontana G, Ware J, Cattaneo M. (2009) Haploinsufficiency of the platelet P2Y12 gene in a family with congenital bleeding diathesis. Haematologica 94:581–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Pillarisetti K, Thomas RA, Aiyar N. (2001) Pharmacological evidence for complex and multiple site interaction of CXCR4 with SDF-1alpha: implications for development of selective CXCR4 antagonists. Immunol Lett 78:29–34 [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Sixel U, Di Pasquale F, von Kügelgen I. (2008) Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y(12)-receptor. Biochem Pharmacol 76:1201–1213 [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, et al. (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202–207 [DOI] [PubMed] [Google Scholar]
- Ignatovica V, Megnis K, Lapins M, Schioth HB, Klovins J. (2011) Identification and analysis of functionally important amino acids in human purinergic 12 receptor using a Saccharomyces cerevisiae expression system. FEBS J 279:180–191 [DOI] [PubMed] [Google Scholar]
- Kostenis E, Waelbroeck M, Milligan G. (2005) Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci 26:595–602 [DOI] [PubMed] [Google Scholar]
- Lalueza-Fox C, Römpler H, Caramelli D, et al. (2007) A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science 318:1453–1455 [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Homolya L, Boucher RC, Harden TK. (1997) Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272:24348–24354 [DOI] [PubMed] [Google Scholar]
- Leaver-Fay A, Tyka M, Lewis SM, et al. (2011) ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol 487:545–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Scarselli M, Knudsen CD, Kim SK, Jacobson KA, McMillin SM, Wess J. (2007) Rapid identification of functionally critical amino acids in a G protein-coupled receptor. Nat Methods 4:169–174 [DOI] [PubMed] [Google Scholar]
- Meiler J, Baker D. (2006) ROSETTALIGAND: protein-small molecule docking with full side-chain flexibility. Proteins 65:538–548 [DOI] [PubMed] [Google Scholar]
- Muñoz-Esparza C, Jover E, Hernández-Romero D, Saura D, Valdés M, Lip GY, Marín F. (2011) Interactions between clopidogrel and proton pump inhibitors: a review of evidence. Curr Med Chem 18:2386–2400 [DOI] [PubMed] [Google Scholar]
- Nawarskas JJ, Clark SM. (2011) Ticagrelor: a novel reversible oral antiplatelet agent. Cardiol Rev 19:95–100 [DOI] [PubMed] [Google Scholar]
- Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. (1994) Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci USA 91:3275–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausch MH, Lai M, Tseng E, Paulsen J, Bates B, Kwak S. (2004) Functional expression of human and mouse P2Y12 receptors in Saccharomyces cerevisiae. Biochem Biophys Res Commun 324:171–177 [DOI] [PubMed] [Google Scholar]
- Remijn JA, IJsseldijk MJ, Strunk AL, Abbes AP, Engel H, Dikkeschei B, Dompeling EC, de Groot PG, Slingerland RJ. (2007) Novel molecular defect in the platelet ADP receptor P2Y12 of a patient with haemorrhagic diathesis. Clin Chem Lab Med 45:187–189 [DOI] [PubMed] [Google Scholar]
- Ritscher L, Engemaier E, Stäubert C, Liebscher I, Schmidt P, Hermsdorf T, Römpler H, Schulz A, Schöneberg T. (2012) The ligand specificity of the G-protein-coupled receptor GPR34. Biochem J 443:841–850 [DOI] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M. (1974) A highly sensitive adenylate cyclase assay. Anal Biochem 58:541–548 [DOI] [PubMed] [Google Scholar]
- Savi P, Labouret C, Delesque N, Guette F, Lupker J, Herbert JM. (2001) P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun 283:379–383 [DOI] [PubMed] [Google Scholar]
- Savi P, Zachayus JL, Delesque-Touchard N, et al. (2006) The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103:11069–11074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder R, Janssen N, Schmidt J, et al. (2010) Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol 28:943–949 [DOI] [PubMed] [Google Scholar]
- Schulz A, Schöneberg T. (2003) The structural evolution of a P2Y-like G-protein-coupled receptor. J Biol Chem 278:35531–35541 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366:381–416 [DOI] [PubMed] [Google Scholar]
- Shiraga M, Miyata S, Kato H, Kashiwagi H, Honda S, Kurata Y, Tomiyama Y, Kanakura Y. (2005) Impaired platelet function in a patient with P2Y12 deficiency caused by a mutation in the translation initiation codon. J Thromb Haemost 3:2315–2323 [DOI] [PubMed] [Google Scholar]
- Simon J, Filippov AK, Göransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA. (2002) Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem 277:31390–31400 [DOI] [PubMed] [Google Scholar]
- Springthorpe B, Bailey A, Barton P, et al. (2007) From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 17:6013–6018 [DOI] [PubMed] [Google Scholar]
- Storey RF. (2011) Pharmacology and clinical trials of reversibly-binding P2Y12 inhibitors. Thromb Haemost 105 (Suppl 1):S75–S81 [DOI] [PubMed] [Google Scholar]
- Thor D, Le Duc D, Strotmann R, Schöneberg T. (2009) Luciferase activity under direct ligand-dependent control of a muscarinic acetylcholine receptor. BMC Biotechnol 9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor D, Schulz A, Hermsdorf T, Schöneberg T. (2008) Generation of an agonistic binding site for blockers of the M(3) muscarinic acetylcholine receptor. Biochem J 412:103–112 [DOI] [PubMed] [Google Scholar]
- Vasiljev KS, Uri A, Laitinen JT. (2003) 2-Alkylthio-substituted platelet P2Y12 receptor antagonists reveal pharmacological identity between the rat brain Gi-linked ADP receptors and P2Y12. Neuropharmacology 45:145–154 [DOI] [PubMed] [Google Scholar]
- Weiss HJ, Witte LD, Kaplan KL, Lages BA, Chernoff A, Nossel HL, Goodman DS, Baumgartner HR. (1979) Heterogeneity in storage pool deficiency: studies on granule-bound substances in 18 patients including variants deficient in alpha-granules, platelet factor 4, beta-thromboglobulin, and platelet-derived growth factor. Blood 54:1296–1319 [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Schonbrun J, Baker D. (2006) Multipass membrane protein structure prediction using Rosetta. Proteins 62:1010–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, et al. (2001) ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276:8608–8615 [DOI] [PubMed] [Google Scholar]
- Zhong X, Kriz R, Seehra J, Kumar R. (2004) N-linked glycosylation of platelet P2Y12 ADP receptor is essential for signal transduction but not for ligand binding or cell surface expression. FEBS Lett 562:111–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.