Abstract
Lipid asymmetry at the plasma membrane is essential for such processes as cell polarity, cytokinesis and phagocytosis1-3. Here we identify the lipid flippase complex, composed of Lem3, Dnf1 or Dnf24, to play a role in the dynamic recycling of the Cdc42 GTPase, a key regulator of cell polarity5, in yeast. By using quantitative microscopy methods, we show that the flippase complex is required for fast dissociation of Cdc42 from the polar cortex by the guanine nucleotide dissociation inhibitor (GDI). A loss of flippase activity, or pharmacological blockage of the inward flipping of phosphatidylethanolamine (PE), a phospholipid with a neutral head group, disrupts Cdc42 polarity maintained by GDI-mediated recycling. PE flipping may reduce the charge interaction between a Cdc42 C-terminal cationic region with the plasma membrane inner leaflet, enriched for the negatively charged lipid phosphatidylserine (PS). Using a reconstituted system with supported lipid bilayers, we show that the relative composition of PE versus PS directly modulates Cdc42 extraction from the membrane by GDI.
Establishing stable cell polarity is crucial for cellular morphogenesis and differentiation. The Cdc42 small GTPase localizes to the site of polarized growth in yeast and orchestrates structural and signaling events required for budding and mating5,6. Previous work showed that the polarity of Cdc42 distribution is maintained dynamically through two colocalized recycling pathways to counter Cdc42 diffusion7-9. Whereas a slow pathway works through actin-based membrane trafficking, a fast pathway is mediated through Rdi1, the yeast GDI for Rho family GTPases10. GDI is known to extract Rho proteins from the membrane by forming a complex involving both protein-protein contacts and binding of GDI to the prenyl lipid anchor at the COOH terminus of these proteins11,12. When actin polymerization is inhibited, Rdi1-mediated Cdc42 recycling is required for the polarization of Cdc42 at the presumptive bud site9.
In order to gain insights into the molecular mechanisms regulating the location and rate of Rdi1-mediated Cdc42 recycling, we performed a suppressor screen taking advantage of the fact that over-expression of Rdi1 under the Gal1 promoter represses yeast growth, likely due to over-extraction of Cdc42 and other essential Rho family GTPases from membrane compartments13 (also see Fig. 1). We reasoned that deletion of a gene encoding a protein that facilitates Cdc42 extraction by Rdi1 might rescue the Gal-Rdi1 induced growth defect. As there are many possible mechanisms for the suppression of the growth defect, the candidates would be further narrowed down to those encoding proteins colocalizing with Cdc42 at the site of polarization. A centromeric plasmid expressing Rdi1 from the Gal1 promoter was transformed into each strain of the yeast haploid non-essential deletion library14. Growth of each strain over-expressing Rdi1 was quantified and normalized relative to its growth without Rdi1 over-expression. This screen revealed ~ 277 initial candidates rescuing the over-expression growth defect, among which 15 encode plasma membrane-localized proteins (Supplementary Information, Fig. S1b). Among these 15 genes, LEM3 encodes a protein polarized at the site of polarization (Fig. 1a; Supplementary Information, Fig. S1a, S2a). Lem3 is a Cdc50 family protein forming a heterodimeric complex with P-type ATPases Dnf1 or Dnf2, which flips aminophospholipid from the outer to inner plasma membrane leaflet4,15,16. Dnf2 was a weak rescuer from the screen (Supplementary Information, Fig. S1a). Both Dnf1 and Dnf2 also localize to the site of polarization, like Lem3 (Supplementary Information, Fig. S2a-c). This observation and their reported involvement in cell polarity17 led us to perform an in-depth study on the possible role for aminophospholipid flipping in regulating Cdc42 recycling at the polar cortex. To avoid heterogeneity in polarization stages, all the microscopy analyses in this work were performed with unbudded polarized cells with a Cdc42 polar cap at the incipient bud site.
Figure 1. Δlem3 suppresses the growth and Cdc42 localization defects due to RDI1 overexpression.
(a) Images of Wt and the Δlem3 mutant (four replicate spots) grown on solid media with or without Rdi1 over-expression (pGal-RDI1) from the genome-wide screen. (b) FCS experiments were performed in live yeast cells (wild type or Δrdi1) expressing GFP tagged Cdc42, Cdc42R66E, or Cdc42C188S. Example auto-correlation curve of GFP-Cdc42 in Wt cells under its endogenous promoter. FCS data was averaged over 13 cells and fitted with a one-component or two-component diffusion model. Weighted residuals obtained from each fitting are shown in the inset. (c) Autocorrelation curves were fitted to a two-component model to extract percentages of slow and fast diffusing molecules. Error bars represent the estimated standard errors in the parameters determined from fitting an average autocorrelation curves obtained from 12, 10, 13, 22 cells from left to right correspond to each of the Cdc42 mutants (see Methods). (d) Molar concentration (nM) of soluble Cdc42 calculated from the FCS data as described in c (see Methods). (e) The average autocorrelation curve obtained from FCS experiments in live cells (n=12) expressing Rdi1-mCherry was also fitted with one and two-component models in comparison with the Cdc42 curve. Inset shows the distribution of weighted residuals obtained from each fitting. (f) Mobile Cdc42 concentrations (nM) of different pools (as indicated) determined at different times of pGal1-Rdi1 induction in Wt cells. Shown are means and estimated standard errors from the analysis of average autocorrelation curves obtained from 21, 26, 27 cells (from left to right). (g) Representative images of GFP-Cdc42 at the polar cap in unbudded Wt and Δlem3 cells at different times of Rdi1 induction (as indicated above each column). Scale bar: 2 μm. (h) Fluorescence intensity profiles of GFP-Cdc42 along the perimeter of Wt or Δlem3 cells at different time points of Gal-RDI1 induction. Fluorescence traces from 10-12 cells were first peak-aligned and then averaged to yield each of the profiles shown. (i) Same analysis and display as in f for Δlem3 cells (14, 21, 23 cells from left to right).
To test if Δlem3 reduces the ability of Rdi1 to extract Cdc42, we first used fluorescence correlation spectroscopy (FCS)18 to measure the mobile cytosolic concentration of GFP-Cdc42 expressed under the CDC42 promoter7,9 in wild-type (Wt) and Δlem3 cells with or without Rdi1 over-expression. FCS records fluorescence intensity fluctuations using a confocal microscope equipped with a photon counter. The autocorrelation curve of this data can be fitted to extract the molar concentration of the diffusing specie from the amplitude of the curve (G0) and the diffusion time (τD) from the shape of the curve18. Autocorrelation curves of GFP-Cdc42 fitted poorly with a single-component diffusion model but significantly better (p<1 × 10−10) with a two-component diffusion model (Fig. 1b). This suggests the existence of at least two pools of mobile Cdc42 population, 64% displaying fast diffusion (τD = 2.9 ± 0.3 ms) and 36% displaying slow diffusion (τD = 70 ± 8 ms) (Fig. 1c, d). The fast pool was significantly reduced in the Δrdi1 background and for the Cdc42R66E mutant, defective in binding Rdi119 (Fig. 1c, d). The vast majority (> 95%) of Rdi1 exists in a fast diffusing population (τD = 2.2 ± 0.1 ms) (Fig. 1e). The slow pool of Cdc42 was diminished by the cdc42C188S mutation (Fig. 1c, d), which prevents prenylation. This analysis, along with the observed in vivo cross-correlation of Cdc42 and Rdi19, suggests that the fast pool of Cdc42 consists largely of the Cdc42-Rdi1 complex, while the slow pool is dependent on prenylation and likely to be membrane vesicle-associated.
Induction of Rdi1 over-expression resulted in a time-dependent increase in total mobile Cdc42 concentration in the cytosol (Fig. 1f). This increase is accompanied by a similar increase in Cdc42 concentration in the fast but not slow pool, consistent with extraction of Cdc42 by Rdi1 from the plasma membrane into the soluble pool (Fig. 1f). Consistently, depletion of Cdc42 from the plasma membrane, especially from the polar cortex, can be observed (Fig. 1g, h). In Δlem3 cells, the Cdc42 polar cap was more prominent than in Wt cells prior to Gal-Rdi1 induction (Fig. 1g, h), accompanied by a smaller mobile Cdc42 pool (Fig. 1i). Whereas Rdi1 overexpression reduced the concentration of Cdc42 in the polar cap of Wt cells, in Δlem3 cells a nearly Wt pre-extraction level remained (Fig. 1g, h) even after 150 minutes of Rdi1 induction. The cytosol Cdc42 pool also remained constant (Fig. 1i). As the mean fluorescence intensity of GFP-Cdc42 over the entire cell was slightly reduced in Δlem3 compared to that in Wt (by ~15%, Supplementary Information, Fig. S2d), the reduced Cdc42 soluble pool after 150 min Gal induction in Δlem3 (by ~40%, Fig, 1f, i) can be explained as a combined effect of a slightly reduced expression and the reduced extraction from the polar cortex.
We next performed fluorescence recovery after photobleaching (FRAP) experiments to examine the recycling of GFP-Cdc42 at the polar cortex9 and analyzed this data using a previously established mathematical model for Cdc42 recycling that allows extrapolation of the rates for Cdc42 dissociation and association from FRAP data9 (Fig. 2a, b; Supplementary Information, Fig.S2e-g). Model computation found that Δlem3 most significantly reduces m, the rate of Cdc42 dissociation from the polar cap region, but only slightly reduced h, the rate at which Cdc42 is targeted to the polar cap, while n, the internalization rate outside the cap remained similar (Fig. 2c-e). Under such scenario the model also predicts a slightly more pointed polar cap9, which could be observed in Δlem3 cells (Supplementary Information, Fig. S2h, i). The reduced m in Δlem3 was confirmed with inverse FRAP (iFRAP), which directly measures the rate of internalization of GFP-Cdc42 at the polar cap9 (Fig. 2f, g). For comparison, the Cdc42 internalization was not significantly affected by the Δpil1 mutation (iFRAP rate of 0.19 ± 0.028 s−1 compared to 0.21 ± 0.01 s−1 in wild-type), which disrupts the eisosomes and normal ergosterol distribution in the plasma membrane20.
Figure 2. Lem3 regulates Rdi1-mediated Cdc42 internalization and polar cap morphology.
(a) Schematic representation of the mathematical model as previously described9. Gray horizontal bar represents the overlapping Rdi1 and actin-based delivery window (χ) approximately 25% of the perimeter (Supplementary information, Fig. S2e-g)9; df, lateral diffusion rate of Cdc42 in the membrane; m and n, internalization rates inside and outside the delivery window, respectively; h: delivery rate inside the window. (b) Example normalized FRAP curves of GFP-Cdc42 from Wt and Δlem3 cells. Solid lines represent the exponential fit of the FRAP data. (c-e) Extracted model parameters from FRAP data. Each point represents a value from a single cell. The small square is the mean; the box range is SEM; whiskers represent standard deviation (SD); line is median. (f) Examples of normalized iFRAP curves from cells expressing GFP-Cdc42. (g) iFRAP rates (1/s) from cells expressing GFP-Cdc42. Box plots are as described in c-e. (h) Example kymographs obtained from bleach corrected movies (300s-385s long) along the perimeter of Δlem3 cells expressing GFP-Cdc42 in the presence of solvent control (DMSO) or 100 μM LatA (3 examples shown). Scale Bar: 2μm
To determine whether Δlem3 specifically slows down Rdi1-mediated Cdc42 internalization, iFRAP was performed in the presence of latrunculin-A (LatA), which disrupts endocytosis21. LatA further slowed down Cdc42 dissociation in Δlem3 to an extent comparable to LatA’s effect on Wt cells (Fig. 2f, g). By contrast, the combined effect of Δrdi1 and Δlem3 on Cdc42 dissociation rate was negligible compared to Δrdi1 alone (Fig. 2g). These results suggest that Lem3 selectively promotes Rdi1-mediated Cdc42 dissociation from the polar cortex. LatA treatment also led to drastic reduction of polarity in Δlem3 cells (Fig. 2h), consistent with a role for Lem3 as an important regulator of Rdi1-mediated Cdc42 recycling in the maintenance of cell polarity.
To test if the flippase complex is involved in fast Cdc42 dissociation from the polar cortex, we performed iFRAP on Δdnf1 and Δdnf2 mutant cells. Cdc42 dissociation was significantly slowed in these mutant cells (Fig. 3a). We further tested if acute inhibition of lipid flipping had the same effect by using the tetracyclic peptide Ro09-0198 (Ro, also called cinnamycin), which specifically binds to PE exposed on the outer plasma membrane leaflet when added to the culture media1,22. Ro peptide at 50 μM, which did not cause significant cell death compare to solvent control (5.6 ± 0.29% compared to 4.3 ± 0.7%), slowed down Cdc42 dissociation from polar cortex in Wt cells but not in Δlem3 cells (Fig. 3b). These results show that PE flipping is required for fast Cdc42 dissociation from the polar cortex.
Figure 3. The Lem3-Dnf1/Dnf2 complex regulates the polar Cdc42 dynamics and phosphatidylserine distribution.
(a) iFRAP rates of GFP-Cdc42 in polarized cells with single or double deletion of DNF1 and DNF2 in box plots as described in Figure 2 legend. (b) iFRAP rates displayed as in a for GFP-Cdc42 in Wt and Δlem3 cells after treatment with 50 μM Ro-peptide for 30 min at room temperature. Solvent control was with the same volume of water-acetonitrile (1:1). (c) Representative confocal images of Wt and Δlem3 cells expressing GFP-Lact-C2, a PS biosensor. White arrows point to polar cap. Scale bar: 2μm. (d) Representative GFP-Lact-C2 fluorescence intensity traces along the cell perimeter. White area represents total fluorescent intensity in the half of the plasma membrane containing the polar cortex; the opposite half is shown in gray. (e) Confocal images of wild-type or Δlem3 cells expressing GFP-2xPH-PLC (a PI(4,5)P2 biosensor). (f) Representative GFP-2xPH-PLC fluorescence intensity traces along the cell perimeter as shown in d. (g) GFP-Lact-C2 fluorescence intensity ratios of the half of the plasma membrane containing the polar cap (white area in d) over the rest. Small Square is the mean; box range shows SEM; whiskers are standard deviation (SD); line is median. (h) GFP-2xPH-PLCδ fluorescence intensity ratio is measured and shown as in g.
Consistent with a previous report23, a defect in PE flipping in Δlem3 correlated with an increase in the negatively charge phospholipid PS at the inner membrane leaflet of the polar cortex, as shown by using the PS biosensor GFP-Lact-C22 (Fig. 3c, d, g; Supplementary Information, Fig. S3a, b). The distribution of PI(4,5)P2, monitored with the biosensor GFP-2xPH-PLCδ24, was not significantly different between Wt and Δlem3 cells (Fig. 3e, f, h). As Cdc42 contains a highly conserved cationic tail immediately adjacent to the prenylation site, the slowed Cdc42 dissociation in Δlem3 may be due to an enhanced charge interaction between Cdc42 and membrane lipids due to PS enrichment. To test this, we increased the Cdc42 polycationic charge by mutating S185 to lysine (KKS185KK to KKK185KK) and performed iFRAP on Wt and Δlem3 cells expressing Cdc42S185K. In Wt cells, the Cdc42S185K dissociation rate from the polar cortex was significantly slower than that of Cdc42, approaching the same slow rate of Cdc42 dissociation in Δlem3 (Fig. 4a). In Δlem3, Cdc42S185K also exhibited a moderate but significant decrease in dissociation rate compared to that of Cdc42 (Fig. 4a). As serine185 might possibly act as an electrostatic switch through phosphorylation25, we also mutated this residue to alanine, but the Cdc42S185A did not exhibit a reduced iFRAP rate compared to Cdc42 in Wt cells and displayed even a slightly increased iFRAP rate compare to Cdc42 in Δlem3 (Fig. 4a). We also mutated the S185 residue to the negatively charged aspartic acid, and the resulting mutant protein (Cdc42S185D), unlike Cdc42S185K, was poorly localized to the polar cap in both Wt and Δlem3 cells (Fig. 4b, c). These observations support the notion that charge interaction between the Cdc42 polycationic tail and the phospholipids at the polar cortex regulates Cdc42 dissociation from the plasma membrane.
Figure 4. The influence of the charge property of a Cdc42 COOH-terminal region on Cdc42 dynamics.
(a) Internalization rates of Cdc42, Cdc42S185K and Cdc42S185A at the polar cortex measured with iFRAP in Wt and Δlem3 cells, box plots as described in Figure 2 legend. (b-c) Representative confocal images of Wt and Δlem3 cells expressing GFP-tagged Cdc42S185K (b) or Cdc42S185D (c). White arrows point to polar cap. Scale bar: 2μm.
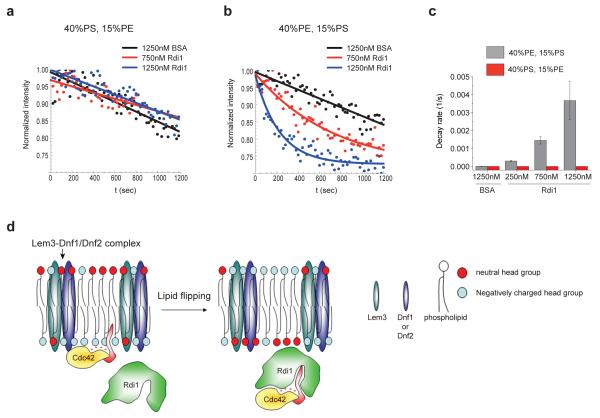
To test if the above regulation is direct, we employed an in vitro system involving supported lipid bilayers (SLB). SLBs were assembled from liposomes with two different lipid compositions, one enriched for PS (referred to as 40% PS, 15% PE, see Methods), and the other for PE (referred to as 40% PE, 15% PS). Prenylated Cdc42 with an N-terminal tetra-cysteine motif (TC-Cdc42) was purified from a yeast plasma membrane fraction and labeled with the biarsenical dye FlAsH26 (Supplementary Information, Fig. S4; see Supplementary Methods). The labeled TC-Cdc42 was added to the SLB and appeared as a fluorescent layer with some bright foci when observed with total internal reflection fluorescence microscopy (TIRF), and its binding to the SLB was dependent on the prenyl group of Cdc42 (Supplementary Information, Fig. S4c-e). In the SLB with 40% PS, 15%PE, the fluorescence intensity of the SLB-bound TC-Cdc42, observed with TIRF, decreased spontaneously and slowly in the presence of the bovine serum albumin (BSA) control, and this decay was not enhanced by added purified, bacterially-expressed Rdi1 (Fig. 5a, c). In the SLB with 40% PE, 15% PS, addition of Rdi1 stimulated the dissociation of Cdc42 from the membrane in a dose-dependent manner (Fig. 5b, c), confirming that PE strongly facilitates Rdi1-mediated Cdc42 dissociation from the membrane.
Figure 5. Lipid composition directly regulates Rdi1-mediated Cdc42 extraction.
(a) Example traces of fluorescence intensity decay due to dissociation of FlAsH labeled TC-Cdc42 from the SLB containing 40% PS and 15% PE as measured by TIRF microscopy. Fluorescence intensity values (dots) were normalized to the maximum intensity of each trace and fitted to an exponential decay model (lines). (b) Same experiment and data presentation as in a but from SLB containing higher concentration of PE (40%) relative to PS (15%). (c) Rates of TC-Cdc42 dissociation from the SLB determined from the TIRF movies, for 40% PE, 15% PS (n = 10, 10, 18, 18 SLB areas from left to right) and for 40% PS, 15% PE (n = 10, 10, 15, 10 SLB areas from left to right). Bar graphs show mean and SEM. (d) A schematic model depicting that, by changing the charge property of the inner membrane leaflet, the flippase complex regulates Rdi1-mediated dissociation of Cdc42 from the plasma membrane. On the left, the Cdc42 prenyl group (red) is inserted in the inner leaflet of the plasma membrane at the polar cortex with electrostatic interaction between Cdc42 cationic C-terminal region (+) and the negatively charged phospholipid head groups (blue circles) providing stability for this association. The flippase complex mobilizes phospholipids with neutral head groups such as PE from the outer to inner leaflet, reducing the electrostatic interaction between Cdc42 and the membrane and enhancing the access of Rdi1 to the Cdc42 prenyl group, and thus facilitating the formation of cytosolic Cdc42-Rdi1 complex.
Based on the data presented above we propose a model whereby Lem3-Dnf1/2 flippase complex acts as an electrostatic switch to promote fast Cdc42 dissociation from the plasma membrane by Rdi1 (Fig. 5d). At the steady state, the polar cortex region is enriched for PE in the outer leaflet and PS in the inner leaflet2,17. Such lipid asymmetry facilitates the localization of Cdc42 to the polar cortex27 possibly by enhancing the charge interaction between the polycationic tail of Cdc42 and the negatively charged membrane surface. A transient increase in neutral lipids such as PE as a result of flippase activity destabilizes the charge interaction of Cdc42 with the inner membrane leaflet, increasing the chance of exposure of the prenyl moiety on Cdc42, thus promoting its capture by the cytosolic Rdi1 protein and fast internalization.
A previous study implicated the Lem3 flippase activity in regulating the activity of the GTPase activating proteins (GAP) for Cdc42 and proposed this regulation to be important for the apical to isotropical switch of cell growth at G2/M17. Two lines of considerations suggest that the regulation of Cdc42 dynamics observed in our study by the flippase complex is not a result of GAP regulation. First, the conclusion that PE flipping regulates GAP activity in the previous work was based on the in vitro demonstration that PE and PS stimulated the GAP activity of Rga1 toward Cdc42, contrasting the inhibitory effect of PIP2. However, as shown in this work, the PE flipping defect in Δlem3 is accompanied by an increased enrichment of PS, not PIP2, at the inner membrane leaflet of the polar cortex. As PS and PE are equally effective in stimulating Cdc42 GAP in vitro17, the reciprocal enrichment of PS and PE at the inner and outer membrane leaflet in the mutant, respectively, would predict little net effect on the GAP activity. Second, in the previous study the key in vivo result supporting the authors’ conclusion was that over-expression of the GAP Rga1 rescued the elongated bud morphology in Δlem3, leading to the idea that lipid flipping regulates GAP activity in vivo. However, in budded cells beyond the nascent-bud stage, Rga1 is localized at the bud neck28, while Lem3 at the bud tip16. Thus, it is unlikely that a direct regulation of the GAP by bud tip localized flippase activity underlies the observed genetic interaction. Consistently, Rga1 over-expression does not rescue the defect in Cdc42 dynamics in Δlem3 but shows synthetic growth defect with Δlem3 (Supplementary Information, Fig. S5a, b). Our model, on the other hand, is based on the change in the charge property of the inner membrane leaflet as a result of phospholipid flipping, and the in vitro data supports a direct effect of the lipid composition on Cdc42 dissociation by Rdi1. Thus, our study reveals a mechanism by which dynamic lipid asymmetry regulates a highly conserved signaling process during cellular morphogenesis.
METHODS
Yeast strains and genome-wide screening for suppressors of Gal-Rdi1
A detailed list of yeast strains used in this study is provided in Supplementary information, Table 1. The yeast haploid non-essential deletion library14 was transformed with a centromeric plasmid containing Rdi1 under a galactose inducible promoter (CEN-pGAL-RDI1:His). Cells were grown for 2 days at 30°C so that all the strains reach maximum growth with approximately similar optical density (OD). At this point strains were diluted (1:100) and spotted in quadruplicates on Sc+2% galactose+2% raffinose (non-transformed control) or Sc-His+2% galactose+2% raffinose agar (transformed experimental strains). A His+ Wt control strain was included on every plate. A Wt strain (RLY2530; Wt-control) on non-selective raffinose plates and Wt strain with Gal-Rdi1 (RLY3811; Wt-experimental) were included in several plates throughout the library to obtain the Wt reference value. The plates were incubated at 23°C for 98 hr prior to saturation. Growth of each spot from the scanned images was quantified as the area and averaged over 4 spots to obtain growth for each strain. Area values were normalized with respect to the His+ Wt control strain on the same plate. A growth-ratio for each deletion strain was then obtained by dividing the normalized growth on the experimental plate by that on the control plate. The growth-ratio for the Wt control obtained by averaging from several plates was 0.26. Based on the distribution shown in Figure S1a, strains with a growth-ratio equal to or above 0.56 were considered as rescuer candidates. Based on gene annotation in the Saccharomyces genome database (SGD), genes encoding proteins involved in galactose-regulated transcription pathway and gene expression were excluded.
Live cell imaging and data analysis
FRAP and iFRAP experiments and data analysis were performed as described9. High signal to noise for the still images were obtained by summing 20 middle confocal slices of short movie of 20 frames. Image analysis and data extraction were performed using imageJ software, statistical analysis and data plotting were performed using OriginLab Pro software.
Fluorescence correlation spectroscopy
FCS experiments were performed as described by Slaughter et al29. In brief, live yeast cells were mounted on coverslips and FCS measurements were taken in the cytosol. GFP-Cdc42 was excited with the 488nm laser line of a Zeiss Confocor 3 through a HFT 488/561 dichroic. HFT565 was used as an emission dichroic and BP 505-540 as an emission filter to collect fluorescence data. Autocorrelation curves generated from the data were fitted to a two component diffusion model30 with a triplet blinking component of 250μs31. Fitting was performed using weighted non-linear least squares32. The autocorrelation curves from many short (4 second) data acquisitions were averaged for analysis. Curves that demonstrated bleaching or non-diffusive dynamics as judged by visual inspection of the binned trajectories and individual correlation traces were eliminated from the analysis. Weights were estimated from the standard error in the mean at each time lag point. Errors in the fitted parameters were estimated by the Monte Carlo method as the standard deviation in fits of 500 simulated correlation curves with Gaussian random errors corresponding to the estimated weights and the observed χ2 parameter32. These errors represent the estimated standard error in the fit parameters. An F-test was performed to compare the one component and two component diffusion model32. The two component model represented a strong improvement in the quality of the fit as judged by the F-test (p<1 × 10−10) and the visual inspection of quality of the fit and residuals (Fig.1b). The FCS data obtained from Rdi1-mCherry was also fitted with one and two-component models. In either case, the autocorrelation curve for Rdi1 is dominated by a rapidly diffusing component (more than 95% of population). An F-test between the models preferred the two-component fit with a p-value of less than 1 × 10−10. However, given the small amplitude of the slow component, we conclude that it represents a relatively insignificant portion of the Rdi1 population.
For measurements of fast and slow pool concentrations in the presence of Gal-Rdi1, it is reasonable to assume that fast and slow diffusion times will remain unchanged. Therefore the diffusion times were fixed to the values obtained for the Wt strain at the 0 time point for Gal induction. Indeed, the curves were reasonably well fit by this model in every case. This allows for unbiased estimation of fast and slow pool concentrations as a function of Gal induction time.
From the fitting we obtained G0, amplitude of correlation function at zero time lag, and the average diffusion time τD required for the molecules to traverse the focal volume. The average number of molecules (N) present in the focal volume was calculated from the following equation 1
| (1) |
where γ, is the shape factor of the focal volume. For one photon excitation, the γ factor is 0.3633. Focal volume was calculated using fluorescein in 0.1 M NaOH with standard method33-36.
Modeling
For each cell, the mathematical model was applied for the analysis of the FRAP movie. Model parameters and assumptions are as described previously9. Considering the overlapping window size between the actin and Rdi1 based recycling mechanisms, we measured the window size from the formin Bni1 distribution in Δlem3 cells (Supplementary Information, Fig. S2e-g) and found it similar to the distribution of Bni1 in Wt cells9.
Recombinant protein purifications
We constructed a plasmid with a “pGAL1-GST-HA-TC-Cdc42” sequence (referred to as TC-Cdc42). We used a genetically encoded tetracysteine motif (HRWCCPGCCKTF) (TC)37 that binds efficiently to the biarsenical dye FlAsH26 and absorbs at 488nm and emits at 525 nm. The tagged Cdc42 was over-expressed in yeast to maximize the amount of protein at the plasma membrane and purified from a plasma membrane fraction. Cell fractionation to prepare plasma membrane and protein purification was carried out as previous described12,38 with modifications. After homogenization of spheroplasts, unlysed cells were pelleted at 400 x g for 10 min. The pellet (P1) obtained at this step was rewashed with 5mL lysis buffer and the supernatants were pooled (S1). The plasma membrane-containing S1 pool was centrifuged twice at 158,420 x g for 45 min to pellet plasma membrane fractions (P2). P2 was resuspended in solubilization buffer (50mM Tris [pH7.5], 300mM NaCl, 5mM MgCl2, 1% Triton X-100 and 0.8M sorbitol), briefly homogenized 15 times on ice and the resultant solution was incubated under shaking for 45 min at 4°C. The solution was further centrifuged at 9000 x g for 20min and the supernatant (S3) was loaded on glutathione-agarose beads (Sigma, G4510) pre-equilibrated with solubilization buffer. The beads were washed with several column volumes of wash buffer (50mM Tris [pH7.5], 300mM Nacl, 5mM Mgcl2, and 0.1% CHAPS) supplemented with PI, PMSF and DTT, followed by the elution buffer (Tris-HCl [pH8.0] and 0.1% CHAPS]. TC-Cdc42 was eluted with elution buffer containing 10mM reduced glutathione (Sigma-Aldrich, G4251). Recombinant non-prenylated Cdc42 was purified from bacteria using a GST tag. For Rdi1 purification, yeast Rdi1 was subcloned into the pET-28a vector with a C-terminal hexahistidine tag. Protein purification was done essentially as previously described12 with minor modifications.
Liposome Preparation
To prepare liposomes we purchased chloroform solutions of porcine brain PE (catalog # 840022C), porcine brain PS (catalog # 840032C), and porcine brain PC (catalog # 840053C) from Avanti Polar lipids, Inc. Cholesterol was purchased from Nu-Chek prep, and dissolved in chloroform. Lipids were mixed in a clean glass tube and dried under a gentle stream of nitrogen gas and further dried under vacuum for 1hr. The dried lipid mixture was hydrated and resuspended with the TBSM buffer to a final concentration of 2mM39 and bath sonicated for 30 min at 45°C to produce the final liposome solution40.
Cdc42 extraction assay using TIRF microscopy
Supported lipid bilayers were prepared on No. 1.5 glass coverslips. Coverslips were made hydrophilic with a rinse with a concentrated H2SO4 and H2O2 (3:1) mixture and thoroughly washed with ultrapure water and dried with a stream of nitrogen gas. Liposomes with the composition 40% PS, 15% PE, 5% PC and 40% cholesterol, or 40% PE, 15% PS, 5% PC and 40% cholesterol, were added to this coverslip and incubated for 20 min and washed thoroughly with TBSM buffer. Prenylated Cdc42 with the TC FlAsH binding motif was labeled with 166.6μM FlAsH dye (Invitrogen, Carlsbad, CA catalog # T34561) for 1hr at room temperature and added to the lipid bilayer at 63-126 nM final concentration, followed by 10 min incubation. Unbound and loosely bound protein was thoroughly washed from the lipid bilayer with TBSM buffer. TIRF imaging were performed using a Carl Zeiss (Jena, Germany) Axiovert 200 M inverted microscope equipped with the Laser TIRF accessory and a Plan-Apochromat 100X, 1.46 NA objective, and a C9100-13 EM-CCD digital camera from Hamamatsu Photonics (Japan). To quantify Cdc42 extraction, rectangular areas excluding the bright foci of Cdc42 aggregates were sampled from each experiment. The fluorescence intensity (I) decay for each area was fitted for to an exponential decay model, I = A0 + A1exp(-αt), where α is the exponential decay rate using OriginLabPro software.
Statistical analysis
Statistical differences between two sets of data other than FCS data (see above) were analyzed with a two-tailed unpaired Student t-test.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank W. Wiegraebe (Stowers Institute for Medical Research) for advice on imaging and K. Lee (Harvard Medical School) for advice on supported lipid bilayer preparation and N. Pavelka for assistance in genome screening data analysis. This study was done to fulfill, in part, requirements for A.D.’s PhD thesis as a student registered with the Open University. This work was supported by NIH grant RO1-GM057063 to R.L.
Footnotes
AUTHOR CONTRIBUTIONS
A.D. and R.L. designed the experiments; A.D. performed all experiments and prepared the manuscript, with help from B.D.S. and J.R.U.; R.A. and D.B. assisted in the whole genome screening; B.R. assisted in data analysis; R.L. conceived and supervised the project and revised the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Iwamoto K, et al. Local exposure of phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity. Genes Cells. 2004;9:891–903. doi: 10.1111/j.1365-2443.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 2.Yeung T, et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 3.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell. Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomorski T, Menon AK. Lipid flippases and their biological functions. Cell. Mol. Life. Sci. 2006;63:2908–2921. doi: 10.1007/s00018-006-6167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S. Cdc42-the centre of polarity. J. Cell. Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter BD, Smith SE, Li R. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harb perspect biol. 2009;1:a003384. doi: 10.1101/cshperspect.a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell. Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of Cdc42 recycling fine-tune polarized morphogenesis. Dev. Cell. 2009;17:823–835. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda T, et al. Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem. 1994;269:19713–19718. [PubMed] [Google Scholar]
- 11.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JL, Erickson JW, Cerione RA. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J. Biol. Chem. 2009;284:23860–23871. doi: 10.1074/jbc.M109.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richman TJ, et al. Analysis of cell-cycle specific localization of the Rdi1p RhoGDI and the structural determinants required for Cdc42p membrane localization and clustering at sites of polarized growth. Curr. Genet. 2004;45:339–349. doi: 10.1007/s00294-004-0505-9. [DOI] [PubMed] [Google Scholar]
- 14.Winzeler EA, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 15.Pomorski T, et al. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato U, et al. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- 17.Saito K, et al. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev. Cell. 2007;13:743–751. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Slaughter BD, Li R. Toward quantitative “in vivo biochemistry” with fluorescence fluctuation spectroscopy. Mol. Biol. Cell. 2010;21:4306–4311. doi: 10.1091/mbc.E10-05-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson RM, Wilson-Delfosse AL. RhoGDI-binding-defective mutant of Cdc42Hs targets to membranes and activates filopodia formation but does not cycle with the cytosol of mammalian cells. Biochem. J. 2001;359:285–294. doi: 10.1042/0264-6021:3590285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossmann G, et al. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J. Cell. Biol. 2008;183:1075–1088. doi: 10.1083/jcb.200806035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayscough KR, et al. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell. Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki Y, Uenaka T, Aoki J, Umeda M, Inoue K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J. Biochem. 1994;116:291–297. doi: 10.1093/oxfordjournals.jbchem.a124522. [DOI] [PubMed] [Google Scholar]
- 23.Stevens HC, Malone L, Nichols JW. The putative aminophospholipid translocases, DNF1 and DNF2, are not required for 7-nitrobenz-2-oxa-1,3-diazol-4-yl-phosphatidylserine flip across the plasma membrane of Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:35060–35069. doi: 10.1074/jbc.M802379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell. 2002;13:542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forget MA, Desrosiers RR, Gingras D, Beliveau R. Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 2002;361:243–254. doi: 10.1042/0264-6021:3610243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 27.Fairn GD, Hermansson M, Somerharju P, Grinstein S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat cell biol. 2011;13:1424–1430. doi: 10.1038/ncb2351. [DOI] [PubMed] [Google Scholar]
- 28.Caviston JP, Longtine M, Pringle JR, Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell. 2003;14:4051–4066. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slaughter BD, Schwartz JW, Li R. Mapping dynamic protein interactions in MAP kinase signaling using live-cell fluorescence fluctuation spectroscopy and imaging. P. Natl. acad. Sci. USA. 2007;104:20320–20325. doi: 10.1073/pnas.0710336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SA, Heinze KG, Schwille P. Fluorescence correlation spectroscopy in living cells. Nat. Methods. 2007;4:963–973. doi: 10.1038/nmeth1104. [DOI] [PubMed] [Google Scholar]
- 31.Haupts U, Maiti S, Schwille P, Webb WW. Dynamics of fluorescence fluctuations in green fluorescent protein observed by fluorescence correlation spectroscopy. P. Natl. acad. Sci. USA. 1998;95:13573–13578. doi: 10.1073/pnas.95.23.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevington P, Robinson DK. Data Reduction and Error Analysis for the Physical Sciences. 3rd edn McGraw-Hill; New York City: 2003. pp. 194–218. [Google Scholar]
- 33.Thompson NL. Fluorescence Correlation Spectroscopy.Topics in Fluorescence Spectroscopy. Plenum Press; New York: 1991. pp. 337–378. [Google Scholar]
- 34.Coles BA, Compton RG. Photoelectrochemical ESR. Part I. Experimental. J. Electroanal. Chem. and Interfacial Electrochem. 1983;144:87–98. [Google Scholar]
- 35.Daly PJ, Page DJ, Compton RG. Mercury-plated rotating ring-disk electrode. Anal. Chem. 1983;55:1191–1192. [Google Scholar]
- 36.Hess ST, Webb WW. Focal volume optics and experimental artifacts in confocal fluorescence correlation spectroscopy. Biophys. J. 2002;83:2300–2317. doi: 10.1016/S0006-3495(02)73990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin BR, Giepmans BN, Adams SR, Tsien RY. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nature biotechnol. 2005;23:1308–1314. doi: 10.1038/nbt1136. [DOI] [PubMed] [Google Scholar]
- 38.Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Gallop JL, Rambani K, Kirschner MW. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 2010;329:1341–1345. doi: 10.1126/science.1191710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poste G, Papahadjopoulos D, Vail WJ. In Methods in Cell Biology. Academic Press, Inc.; New York: 1976. Lipid Vesicles as Carriers for Introducing Biologically Active Materials into Cells; pp. 34–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.