Abstract
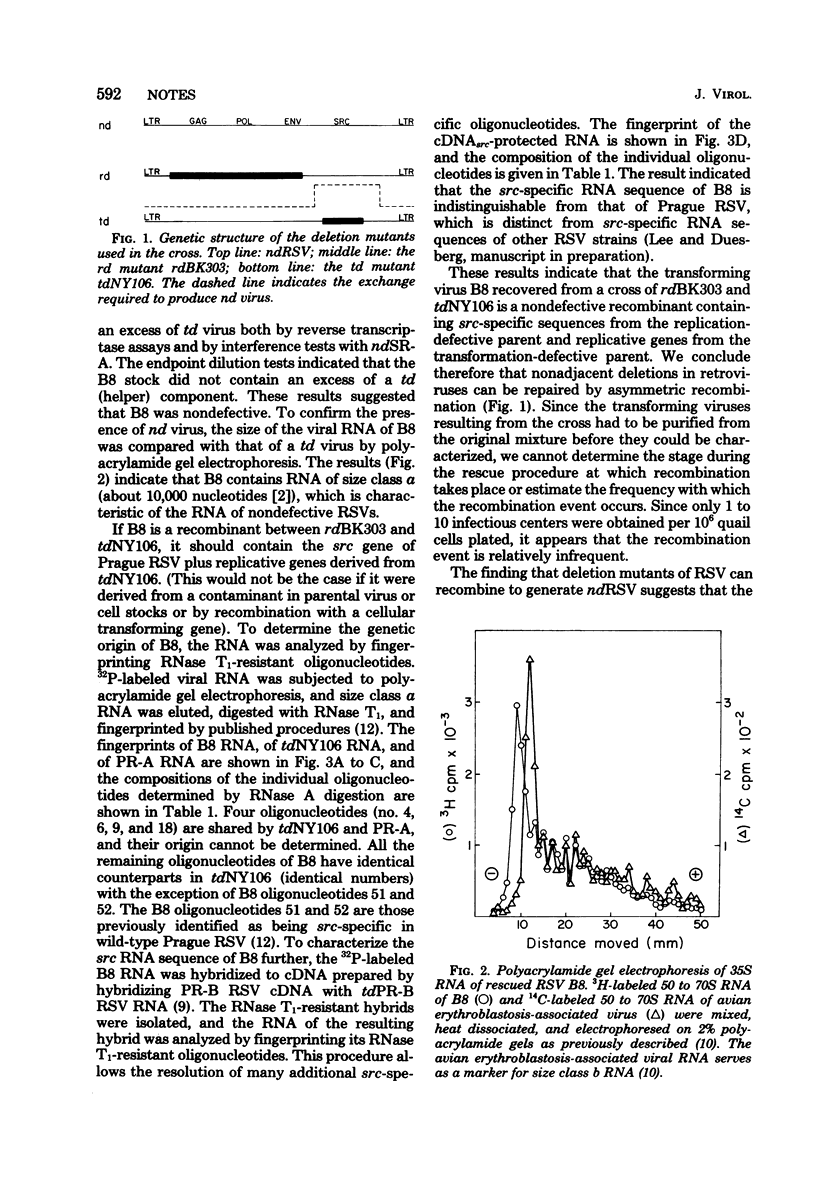
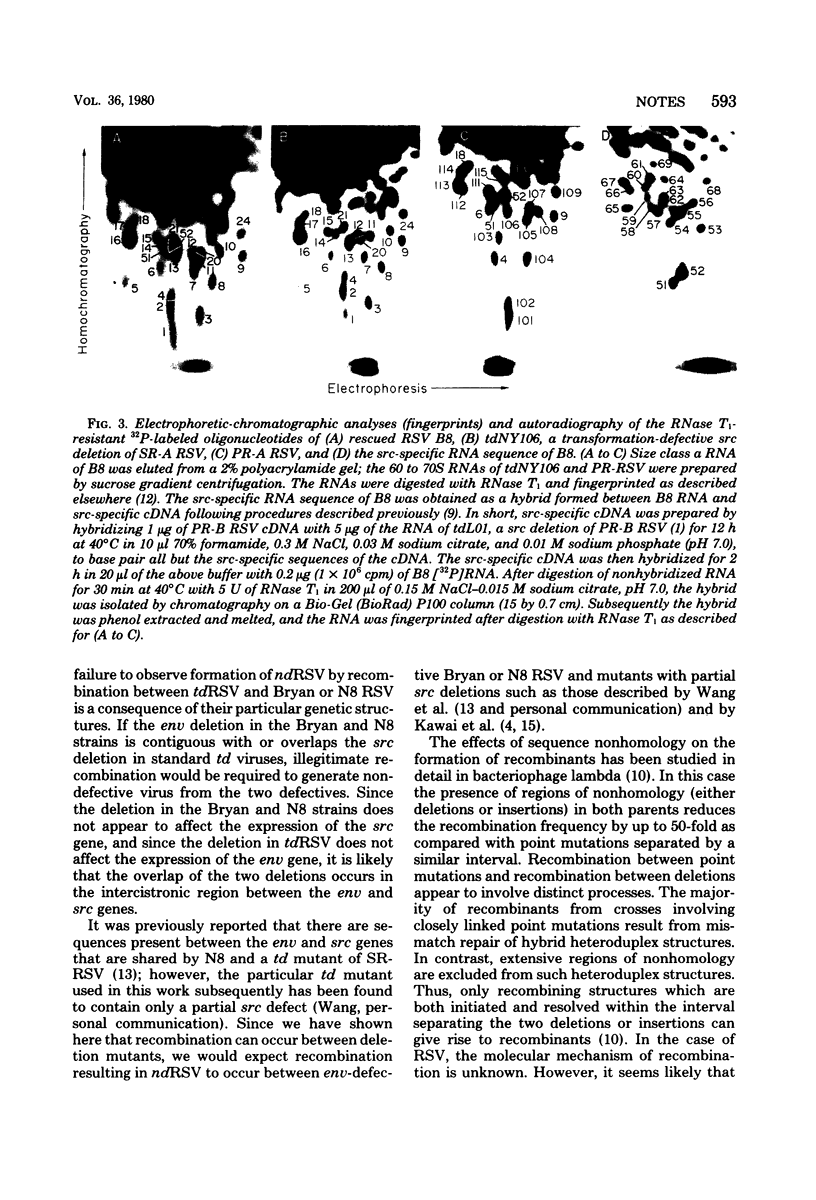
A replication-defective deletion mutant of Prague Rous sarcoma virus (RSV), which lacks functional gag, pol, and env genes, was crossed with a transformation-defective deletion mutant derived from Schmidt-Ruppin RSV. Transformation- and replication-competent viruses were generated in the cross. Characterization of one of these rescued viruses indicated that it was a nondefective recombinant containing the src gene of the replication-defective mutant plus the replicative genes of the transformation-defective virus. These results indicate that, contrary to previous reports, asymmetric recombination between RSV deletion mutants can result in the formation of nondefective RSV.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein A., MacCormick R., Martin G. S. Transformation-defective mutants of avian sarcoma viruses: the genetic relationship between conditional and nonconditional mutants. Virology. 1976 Mar;70(1):206–209. doi: 10.1016/0042-6822(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Recombination between a temperature-sensitive mutant and a deletion mutant of Rous sarcoma virus. J Virol. 1976 Aug;19(2):389–397. doi: 10.1128/jvi.19.2.389-397.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Radke K., Hughes S., Quintrell N., Bishop J. M., Varmus H. E. Mutants of Rous sarcoma virus with extensive deletions of the viral genome. Virology. 1979 Jul 30;96(2):530–546. doi: 10.1016/0042-6822(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren E. J., Fox M. S. Effects of DNA sequence non-homology on formation of bacteriophage lambda recombinants. J Mol Biol. 1979 Jun 5;130(4):357–377. doi: 10.1016/0022-2836(79)90428-5. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Mellon P., Vogt P. K. Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1073–1077. doi: 10.1073/pnas.73.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. A., Beamand J. A. Genetic recombination in Rous sarcoma virus: the genesis of recombinants and lack of evidence for linkage between pol, env and src genes in three factor crosses. J Gen Virol. 1979 May;43(2):349–364. doi: 10.1099/0022-1317-43-2-349. [DOI] [PubMed] [Google Scholar]



