Abstract
Background
Adult neurogenesis occurs throughout life in discrete regions of the mammalian brain and is tightly regulated via both extrinsic environmental influences and intrinsic genetic factors. In recent years, several crucial signaling pathways have been identified in regulating self-renewal, proliferation, and differentiation of neural stem cells, as well as migration and functional integration of developing neurons in the adult brain.
Scope of review
Here we review our current understanding of signaling mechanisms, including Wnt, notch, sonic hedgehog, growth and neurotrophic factors, bone morphogenetic proteins, neurotransmitters, transcription factors, and epigenetic modulators, and crosstalk between these signaling pathways in the regulation of adult neurogenesis. We also highlight emerging principles in the vastly growing field of adult neural stem cell biology and neural plasticity.
Major conclusions
Recent methodological advances have enabled the field to identify signaling mechanisms that fine-tune and coordinate neurogenesis in the adult brain, leading to a better characterization of both cell-intrinsic and environmental cues defining the neurogenic niche. Significant questions related to niche cell identity and underlying regulatory mechanisms remain to be fully addressed and will be the focus of future studies.
General significance
A full understanding of the role and function of individual signaling pathways in regulating neural stem cells and generation and integration of newborn neurons in the adult brain may lead to targeted new therapies for neurological diseases in humans.
Introduction
Neural stem cells (NSCs) are characterized by the capacity to continuously self-renew and generate a multitude of neuronal and glial lineages [1,2]. Neurogenesis in the mammalian brain involves multiple, complex processes that include proliferation, fate specification, differentiation, maturation, migration, and functional integration of newborn cells into the existing neuronal circuitry [1]. Following the discovery that neurogenesis persists throughout life in the adult mammalian brain, including in humans [3–5], recent studies have linked variable levels of adult neurogenesis to brain function in the normal and diseased brain. These findings, coupled with the possibility of using NSCs in treatment of neurodegenerative disease and psychiatric disorders, have generated new interest in understanding the molecular mechanisms underlying adult neurogenesis.
Active neurogenesis occurs primarily in two regions of the adult mammalian brain: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) [6,7]. Quiescent or slowly dividing ependymal and subependymal cells expressing GFAP- and Prominin-1/CD133 are thought to be the primary NSCs in the adult SVZ (type B cells) [6,8], although Prominin-1/CD133 is expressed by other non-CNS stem cells as well, such as myogenic and hematopoietic stem cells [9]. These GFAP- and Prominin-1/CD133-expressing cells with stem cell properties reside in the wall of the lateral ventricle and give rise to transit amplifying cells (type C cells) via asymmetric division. Transit amplifying cells, which express the receptor for epidermal growth factor [10,11], give rise to polysialylated neural adhesion molecule (PSA-NCAM)-expressing neuroblasts (type A cells) that migrate into the olfactory bulb via the rostral migratory stream (RMS) and differentiate into GABA- and dopamine-producing granule and periglomerular interneurons [6,12,13]. In the adult hippocampus, the SGZ of the DG contains GFAP-, Nestin-, and Sox2-expressing radial glia-like cells (RGLs) that act as quiescent NSCs. Recent clonal analysis of individual RGLs has revealed both self-renewal and multipotential capacities in this population that can generate additional RGLs, neurons and astroyctes [14]. Asymmetric division of RGLs can give rise to neuronal lineage restricted progenitor daughter cells (type 2 cell) that expresses Nestin and Sox2, but not GFAP [15]. Type 2 cells in turn give rise to neuroblasts expressing doublecortin (DCX) and PSA-NCAM which then differentiate into glutamatergic dentate granule cells [16,17].
Neurogenesis in adults is dynamically regulated by a number of intrinsic as well as extrinsic factors [18]. Endogenous extrinsic factors in the local microenvironment, often referred to as the “neurogenic niche” or “stem cell niche”, include neural precursor cells, surrounding mature cells, cell-to-cell interactions, cilia, secreted factors, and neurotransmitters [6,19]. Microenvironments of the SVZ and SGZ, but not other brain regions, are thought to have specific factors that are permissive for the differentiation and integration of new neurons, as evidenced by a pivotal study showing that adult hippocampal astrocytes promote neuronal differentiation of adult-derived hippocampal progenitor cells in vitro [20].
The importance of the stem cell niche in determining the fate of adult NSCs is highlighted by several different transplantation experiments. SVZ-derived committed neural precursor cells differentiate into glia cells when grafted into ectopic non-neurogenic regions of the brain [21]. Furthermore, SVZ precursor cells generate hippocampal neurons when transplanted into the DG of the hippocampus, whereas SGZ precursors generate olfactory interneurons after transplantation into the RMS [22]. Similarly, neural precursor cells derived from the adult spinal cord differentiate into granule cell neurons after implantation into the hippocampus, but fail to generate neuronal phenotypes and differentiate into glial cells after transplantation back to their original site in the spinal cord [23]. In contrast, Merkle et al. showed that SVZ-derived NSCs maintained their region-specific potential in vivo, and that environmental factors at the host graft site were not sufficient to respecify the grafted cells after heterotopic transplantation [24].
Recently developed tools allowing inducible alteration of gene expression specifically within adult NSCs have provided new insights in the mechanisms regulating neurogenesis in vivo. Viral-mediated gene transfer in vivo utilizes retro- or lentiviruses and allows long-lasting genetic manipulation at the site of virus infusion and transgenic mouse lines enable inducible and cell-specific knock-out, knockdown, or overexpression of a specific gene of interest. With these and other techniques, several soluble and membrane-bound extracellular factors and their intracellular signaling cascades have recently been identified as determinants of the local microenvironment of the SVZ and SGZ, including Wnt, sonic hedgehog, Notch, BMPs, neurotrophins, and neurotransmitters. Furthermore, cell-intrinsic mechanisms including transcription factors and epigenetic regulators of neurogenesis have recently been shown to be crucially involved in modulating neurogenesis in the adult brain. In this review, we summarize the role of both external and cell-intrinsic signals in regulating adult neurogenesis. Furthermore, we discuss key signaling pathways regulating different stages of adult neurogenesis, including neural stem cell proliferation and lineage differentiation, and migration and integration of the developing neuron in the adult brain.
Wnt/beta-catenin pathway
The Wnt signaling pathway is a highly conserved signaling pathway that has been implicated in nervous system development, including neural tube formation, dorsal root ganglia development, and midbrain development [25–27]. Disruption of the physiological Wnt-signaling pathway has been associated with several CNS pathologies, including schizophrenia, mood disorders, autism, and Alzheimer’s Disease [28–31].
Wnt ligands constitute a family of auto- and paracrine secreted glycoproteins that are involved in several diverse developmental cellular processes [27,32,33]. In the absence of Wnt ligand, activated glycogen-synthetase-kinase-3 beta (GSK-3beta), a key modulator of the Wnt pathway, forms a degradation complex, comprising axin, adenomatous polyposis coli, and beta-Transducing repeat-containing protein (beta-TrCP), resulting in phosphorylation and ubiquitination of beta-catenin and subsequent degradation of beta-catenin by the proteosome [34]. Ongoing degradation of beta-catenin in the absence of Wnt maintains a low intracellular beta-catenin level. Since nuclear translocation of beta-catenin and subsequent binding to T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors is required for activation of Wnt-target genes, ongoing sequestration and degradation of beta-catenin in the absence of Wnt ligand prevents transcription of Wnt target genes. However, in the presence of extracellular Wnt ligand, Wnt and its receptor Frizzled form a ternary complex with the co-receptor low-density lipoprotein receptor related protein 5/6 (LRP5/6) [35]. This complex formation leads to phosphorylation and activation of intracellular Dishevelled (Dvl), which in turn inactivates GSK- 3beta by displacing GSK-3beta from the degradation complex. In the absence of ongoing beta-catenin degradation, stabilized beta-catenin enters the nucleus and associates with TCF/LEF transcription factors, resulting in transcription of Wnt-target genes [36].
Several studies have addressed the role of Wnt signaling in adult neurogenesis. It was recently shown that Wnt3 is strongly expressed in DG hilar cells and in cultured hippocampal astrocytes, and that GSK3beta/beta-catenin-signaling is active in the adult SGZ and dentate granule cell layer [37]. The same study showed that astrocyte-derived Wnt-signaling mediates neuroblast proliferation and neuronal differentiation in adult-derived hippocampal progenitor cells via the beta-catenin pathway. Similarly, injection of lentiviruses expressing dominant-negative Wnt into the adult DG resulted in a marked reduction in neurogenesis when compared to wild-type Wnt, suggesting an important role for Wnt-signaling in adult hippocampal neurogenesis in vivo [37]. In a subsequent study it was shown that NeuroD1, a pro-neurogenic basic helix-loop-helix (bHLH) transcription factor, functions as a downstream mediator of Wnt-induced neurogenesis from adult hippocampal neural progenitors [38]. Interestingly, NeuroD1 is selectively expressed in dividing neural progenitors and in immature granule neurons in the adult DG, but not in Sox2-expressing hippocampal neural progenitors. Furthermore, Kuwabara et al. propose an intriguing link between Wnt-signaling, NeuroD1 expression, and neuronal differentiation: In undifferentiated stem cells the NeuroD1 promoter is silenced by a repressor complex comprising Sox2 and HDAC1. However, in the presence of extracellular Wnt, beta-catenin accumulates in the nucleus forming an activator complex with TCF/LEF, resulting in NeuroD1 transcriptional activation, and subsequent neuronal differentiation [38]. By using NeuroD1 conditional knock-out mice, Gao et al. demonstrated that NeuroD1 is required for neurogenesis in the adult hippocampus in vivo by facilitating survival and maturation of adult-born neurons. Complementary in vitro studies showed that NeuroD1 is sufficient to induce neuronal differentiation and that Wnt-mediated neurogenesis requires NeuroD1 in adult hippocampal neural progenitor cells [39]. Despite this convincing evidence for the role of Wnt/beta-catenin in regulating neurogenesis by promoting neuronal differentiation, a recent study by Mao et al. showed that activation of the Wnt/beta-catenin pathway promotes proliferation rather than differentiation of adult NSCs. By injecting lentivirus expressing Disrupted In Schizophrenia 1 (DISC1) shRNA in the adult hippocampus in vivo, Mao et al. showed that DISC1 promotes neural progenitor cell proliferation in the adult hippocampus by interacting with and inhibiting GSK3beta, resulting in stabilization of beta-catenin and subsequent activation of downstream transcription factors that prevent premature cell cycle exit and neuronal differentiation [40].
In addition, the nuclear orphan receptor Tlx, previously shown to play an essential role in proliferation of adult NSCs [41], directly induces transcription of Wnt7a and promotes proliferation and self-renewal of adult NSCs via the canonical Wnt signaling pathway in neurogenic regions of the adult brain [42]. While these studies have focused mainly on the role of Wnt in neurogenesis in the adult hippocampus, Wnt signaling has also been shown to modulate neurogenesis in the adult SVZ. Overexpression of Wnt3A and Wnt5A promotes proliferation and neuronal differentiation of adult SVZ neural progenitor cells in vitro [43]. In addition, retrovirus-mediated expression of a stabilized beta-catenin was shown to promote proliferation of neural progenitor cells and in the SVZ in vivo, resulting in increased neurogenesis in the olfactory bulb [44].
Notch pathway
Notch signaling impinges on a wide array of cellular processes in the developing nervous system, including cell proliferation, differentiation, and apoptosis [45–47]. Notch receptors are single-pass transmembrane heterodimers that are activated upon forming a binding complex with their membrane-bound ligands on the neighboring cell, Delta and Jagged. Ligand binding results in gamma-secretase mediated cleavage of the transmembrane domain, and subsequent release of the notch intracellular domain (NICD) into the cytosol. NICD then translocates to the nucleus where it forms a complex with the DNA-binding protein RBPj. The NICD-RBPj complex in turn acts as a transcriptional activator and induces the expression of bHLH transcription factors, such as the hairy and enhancer of split (HES) and others [48]. While the role of Notch signaling in neurogenesis has previously been studied mainly during development, there is now growing evidence that Notch has distinct roles in the maintenance and differentiation of NSCs in the adult nervous system. Various studies have shown that components of the Notch pathway are expressed in the SVZ and SGZ of the adult mammalian brain [49–53]. Notch regulates maintenance of adult NSCs by promoting cell cycle exit and decreasing the adult neural progenitor pool [54]. Conditional knock-out of RBPj in the adult SVZ leads to differentiation of all type B-cells into transit-amplifying cells and neurons, resulting in eventual depletion of the neural stem cell pool and subsequent premature cessation of neurogenesis [53]. Similarly, in the adult hippocampus, Notch was found to be required for the expansion and self-renewal of nestin-expressing cells in the adult SGZ in vitro and in vivo [55]. Furthermore, Ehm et al. showed that conditional inactivation of RBPj resulted in an initial increase in hippocampal neurogenesis by inducing premature neuronal differentiation of Sox2-positive progenitors. This in turn resulted in subsequent depletion of the Sox2-positive neural stem cell pool and eventual suppression of adult hippocampal neurogenesis, indicating an important role for Notch signaling in the maintenance of adult NSCs [56]. A related study showed that overexpression of NICD induced proliferation and expansion of the neural stem cell pool in adult hippocampal progenitors in vivo [52]. Notch signaling also appears to be involved in regulating niche cell identity and plasticity. EphB2 acts as a downstream mediator of Notch signaling and prevents differentiation of ependymal cells into niche astrocytes in the adult SVZ [57]. A recent study demonstrated an important interaction between EGF receptor signaling and Notch in maintenance of neural stem and progenitor cells in the adult SVZ. EGF receptor signaling in transit-amplifying (type C cells) non-cell-autonomously inhibited proliferation and self-renewal of type B cells by suppressing Notch signaling in type B cells in the adult SVZ [58]. Interestingly, EGF receptor signaling suppressed Notch signaling by promoting Notch 1 ubiquitination and degradation via induction of Numb, a protein previously shown to mediate Notch receptor degradation [59]. Thus, Notch signaling is required to maintain a reservoir of undifferentiated cells and ensure ongoing neurogenesis during adult life. Furthermore, Notch has been shown to have an important role in dendritic arborization of immature neurons in the adult brain. Conditional knock-out of Notch 1 results in significantly less complex arborization, while overexpression of activated Notch 1 leads to a significant increase in dendritic complexity in newborn, maturing granule cells of the adult DG [52]. Moreover, adult inducible Notch 1 knockout mice have granule cell neurons with smaller dendritic trees in the DG [55]. The role of Notch 1 signaling on dendrite formation seems to be restricted to immature developing neurons, since Notch 1 activation in mature postmitotic neurons was shown to have no effect on dendritic arborization [60].
Sonic Hedgehog pathway
Sonic hedgehog (Shh) is a soluble extracellular signaling protein that was first discovered to have a role in cell differentiation in the neural tube and limb bud [61]. Shh signaling has since been found to be crucial in regulating various processes during development of the nervous system, such as ventral forebrain neuronal differentiation, midbrain dopaminergic differentiation, and cerebellar neuronal precursor proliferation [62–64]. Shh mediates its action via a receptor complex consisting of the transmembrane receptor protein Patched (Ptc), and its G protein-coupled co-receptor Smoothened (Smo), both of which are preferentially located on primary cilia [65]. In the absence of Shh ligand, Ptc represses signal transduction from the co-receptor Smo, thereby inhibiting Shh target gene transcription. However, after Shh ligand binding to Ptc, disinhibition leads to activation of a complex signaling cascade ultimately resulting in transcription of Gli-proteins and other Shh target genes [66,67]. More recently, Shh has been shown to play an important role in neurogenesis in the adult mammalian brain. The Shh receptors Ptc and Smo are expressed in the adult hippocampus and in progenitors derived from this region [68,69]. Furthermore, various components of the Shh signaling cascade are expressed in the early postnatal as well as the adult SVZ [70,71]. Overexpression of Shh in the adult hippocampus via an adeno-associated viral vector delivered to the DG resulted in a significant increase in hippocampal progenitor cell proliferation in vivo [69]. Conversely, cyclopamine, an inhibitor of Shh signaling, reduced granule cell proliferation in the adult DG when directly delivered into the adult hippocampus [69] and the lateral ventricle [72]. Conditional knock-out of signaling via the Shh downstream mediator Smo was shown to result in a significant reduction in proliferation of progenitor cells in the postnatal hippocampus and SVZ [73]. Furthermore, mice lacking Smo in neural precursor cells show defective hippocampal neurogenesis, as indicated by a small DG, and marked reduction in neural stem cell proliferation in the adult dentate gyrus in vivo [74]. A similar phenotype was observed after conditional loss of primary cilia on granule neuron precursors in the adult hippocampus, suggesting that Shh mediated proliferation of adult hippocampal stem cells is dependent on functional primary cilia [74]. Using in vivo genetic fate mapping, Ahn et al. showed that both quiescent NSCs as well as transit-amplifying progenitor cells in the subventricular and SGZ respond to Shh signaling, and contribute to ongoing neurogenesis in the adult forebrain [75]. In addition to playing an important role in promoting self-renewal and proliferation of adult NSCs, Shh was recently found to serve as an important regulator of cellular migration in the adult mammalian brain. Several studies addressed the role of Shh on neuronal migration in the adult SVZ by means of conditional knock-out of Smo as well as adenoviral overexpression of Hip, a negative regulator of the Shh pathway. Interestingly, loss of Shh signaling in the adult SVZ resulted in non cell-autonomous failure of neuroblasts (type A cells) to migrate to the olfactory bulb via the RMS [76,77].
Growth factors and neurotrophic factors
Neurotrophic factors are extracellular signaling proteins that play important roles in both the developing and adult central nervous system [78–80]. In mammals, four neurotrophic factors have been identified, namely nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4/5 (NT-4/5) [81,82]. Neurotrophins bind to receptor tyrosine kinases known as Trk receptors and their co-receptor p75NTR. There are three different Trk receptors, namely TrkA, TrkB, and TrC, which have different preferential binding affinities for the different neurotrophins. NGF preferentially binds to TrkA, BDNF and NT-4/5 to TrkB, and NT-3 to TrkC [83,84]. All four neurotrophic factors bind to p75NTR on the cell surface, which serves to augment and facilitate binding of the respective neurotrophin to its specific Trk receptor. Ligand binding induces dimerization of Trk receptors and their autophosphorylation at specific tyrosine residues in the cytoplasmatic domain, leading to recruitment of various downstream effectors and activation of signal transduction cascades [85, 86]. Interestingly, both p75NTR and TrkB are expressed on dividing progenitor cells in the adult subventricular and SGZ [87–91]. BDNF and its role in neurogenesis have been studied more extensively than any of the other neurotrophins. Chronic BDNF infusion directly into the adult DG resulted in increased neurogenesis of granule cells [92]. Similarly, direct intraventricular delivery of BDNF as well as overexpression of BDNF by adenoviral injection of BDNF in the lateral ventricle resulted in a significant increase in newly formed neurons originating from the adult SVZ [93,94]. Conditional loss of TrkB signaling in a Nestin-CreERT2 system resulted in significantly decreased BDNF-induced neurosphere growth in vitro, as well as impaired proliferation and neurogenesis in the adult DG in vivo, suggesting that functional TrkB signaling is required for proliferation of NSCs in the hippocampus [91]. A different study showed that survival, dendritic arborization, and functional integration of newborn neurons in the adult DG is critically dependent on signaling via the TrkB receptor [95]. Moreover, enhancement of hippocampal neurogenesis following environmental enrichment did not occur in BDNF knockout mice [96]. The role of BDNF in neurogenesis in the adult SVZ is less well characterized, however, a recent study suggested that in contrast to its role in adult hippocampal neurogenesis, BDNF signaling does not stimulate neurogenesis in the adult SVZ [97]. Available data on the role of NT-3 on regulating adult neurogenesis are limited, however, conditional knock-out of NT-3 resulted in significant impairment in neuronal differentiation, but not proliferation in the adult hippocampus [98]. Furthermore, NT-3 deficient mice had profound deficits in memory and learning, suggesting that NT-3 mediated neuronal differentiation, but not proliferation, is involved in spatial learning and memory formation in the adult brain [98,99]. Continuous infusion of NGF directly in the lateral ventricle of adult rats had no effect on proliferation of progenitor cells in the DG granule cell layer but resulted in enhanced survival of neurons in the adult hippocampus [99].
Growth factors comprise a large group of extracellular proteins that promote cell growth and maintenance in various biological settings [100–103]. Several growth factors have been shown to be involved in regulating neurogenesis in the adult brain, most importantly Fibroblast growth factor-2 (FGF-2), Insulin-like growth factor-1 (IGF-1), and Vascular endothelial growth factor (VEGF). These growth factors share a common principle of signal transduction, involving binding to a ligand-specific receptor belonging to the tyrosine kinase family. Ligand binding to the receptor results in autophosphorylation and activation of the intracellular domain of the respective receptor and subsequent activation of downstream signaling pathways, including the PI-3 kinase/Akt and the Ras/Raf/MEK/Erk pathway. Several recent studies have implicated FGF-2 as a regulator of neurogenesis in the adult brain. Intraventricular infusion of FGF-2 was accompanied by an increase in number of newly born cells in the adult rat hippocampus [104]. Furthermore, mice with conditional deletion of the FGFR1 gene display significant impairment in neural progenitor cell proliferation and production of new neurons in the adult DG [105]. The role of IGF-1 in regulating adult neurogenesis has been addressed in several studies. Spontaneous euronal differentiation of progenitor cells derived from the adult SVZ is dependent on endogenous IGF-1 signaling in vitro [106]. IGF-1 can directly stimulate proliferation of adult hippocampal progenitor cells in a MAP kinase dependent manner in vitro [107], and IGF-1 increases the rate of neurogenesis in the adult hippocampus in vivo when delivered either via continuous subcutaneous infusion or intraventricular infusion [108,109]. Furthermore, IGF-1 signaling is required for proper migration of neuroblasts from the SVZ to the olfactory bulb via the rostral migratory stream [110]. In addition to promoting adult neurogenesis, IGF-1 was also found to instructively stimulate the differentiation of adult hippocampal progenitor cells into oligodendrocytes in vitro and in vivo by inhibiting BMP signaling [111].
VEGF has emerged as a multifunctional growth factor that is involved in regulation of neurite outgrowth and maturation during development, and can influence complex processes in the adult brain, including learning and memory [112–115]. VEGF signals through two high-affinity receptor tyrosine kinases, Flk-1 and Flt-1 [116,117]. VEGF receptors are expressed on endothelial cells and neural progenitors in the adult hippocampus and SVZ [118–120]. Jin et al. showed that VEGF exerts a direct mitogenic effect on neuronal progenitor cells via an Flk-1 dependent mechanism [121]. The same study demonstrated that direct infusion of VEGF into the lateral ventricle of adult rats increases neurogenesis in the SVZ and the SGZ [121]. A related study subsequently showed that VEGF-Flk1 signaling is required for antidepressant-mediated enhancement of neurogenesis in the adult rat hippocampus [122].
Bone Morphogenetic Proteins
Bone Morphogenetic Proteins (BMPs) comprise a group of multifunctional extracellular signaling molecules of which over 20 members have been identified to date, and constitute the largest subgroup of the transforming growth factor-beta (TGF-beta) superfamily [123]. BMPs are highly expressed in the embryonic and adult nervous system and play pivotal roles in regulating a wide variety of cellular processes, including cell survival, proliferation and fate specification [124, 125]. Activities of BMPs are negatively regulated by Noggin, Chordin and Neurogesin-1, proteins that directly bind and antagonize BMPs extracellularly [126,127]. BMP signaling is transduced via two different types of serine-threonine kinase receptors, namely BMP receptor type I and type II [128,129]. Binding of the BMP ligand results in formation of a tetrameric complex of two BMP type I and two BMP type II receptors, and activates an intracellular signaling cascade that involves phosphorylated Smad proteins [130]. Smad1/5/8 are directly phosphorylated and activated by the BMP type I receptor kinases, and then form a heteromeric complex with a Co-Smad, Smad4. The activated Smad complexes are translocated to the nucleus and, in conjunction with other nuclear cofactors, activate the transcription of various genes [130,131].
In the adult neurogenic niche, BMPs promote glial differentiation and inhibit neuronal fate specification [132,133]. In the adult SVZ, BMP ligands and their receptors are expressed by the neural stem and progenitor cell population, and act as potent inhibitors of neuronal differentiation of Type B and C cells. Furthermore, BMPs were found to be important for promoting survival of neuroblasts migrating along the RMS [132]. Interestingly, the BMP inhibitor Noggin is produced by ependymal cells of the SVZ and antagonizes endogenous BMP signaling and BMP-mediated premature glial differentiation at the expense of neurogenesis, thus promoting the formation of new neurons from SVZ precursors [132]. In the adult SGZ, endogenously produced Noggin had previously been shown to be important for self-renewal and proliferation of adult hippocampal NSCs in vitro and in vivo [134]. Noggin mRNA levels in the dentate gyrus are under the control of the RNA-binding protein FXR2. Loss of function experiments have shown that FXR2 deficiency results in increased expression of Noggin. Increased Noggin levels inhibit endogenous BMP-signaling, which in turn results in increased proliferation of NSCs and thereby increases neurogenesis in the adult hippocampus in vivo [135].
Furthermore, Neurogesin-1, a recently identified astrocyte-derived signaling protein, plays an important role in regulating BMP-mediated cell fate specification in the adult brain. Neurogesin-1 is highly expressed in the adult DG and SVZ, and antagonizes BMP-4 induced astroglial differentiation of adult hippocampal progenitor cells [136]. Blockade of BMP signaling by direct intraventricular Noggin infusion as well as knock-out of Smad4 in adult SGZ neural precursor cells initially increased neurogenesis but subsequently resulted in depletion of precursors and loss of neurogenesis, suggesting that BMP signaling is required for maintenance of neural stem cell properties and neurogenesis [137].
Neurotransmitters
Neurotransmitters are small diffusible molecules that serve as the basis of chemical communication between neurons [138–140]. Accumulating evidence also suggests essential roles of neurotransmitters in regulating adult neural progenitor cell proliferation, differentiation, and synaptic integration, as well as activity-dependent adult neurogenesis. Glutamate is an excitatory neurotransmitter that utilizes several different receptor subtypes, namely ionotropic NMDA, AMPA and kainic acid receptors, as well as metabotropic glutamate receptors [141–143]. Electrophysiological as well as immunohistochemical studies have shown expression of various glutamate receptors on neural progenitor cells in the adult subventricular and SGZ [144–146]. Interestingly, in the postnatal SVZ, neuroblasts but not stem cells express various glutamatergic receptors during migration to the olfactory bulb [145,147] and migrating neuroblasts are ensheathed by glutamate-releasing specialized astrocyte-like cells. Single-cell knock-out of the NMDA receptor results in apoptosis of migrating neuroblasts, suggesting that astrocyte derived glutamate mediates survival and proper functional integration of neuroblasts via NMDA receptor signaling [144]. The kainate receptor GLUK5 is tonically activated in migrating SVZ neuroblasts, and that this activation decreases the speed of neuroblast migration [148]. One possible explanation for this seemingly contradictory finding may be a mosaic pattern of expression of different glutamate receptors in neuroblasts that may allow glutamate to exert differential effects on neuroblast production and migration. It has been hypothesized that the observed differences in glutamate receptor expression among neuroblasts may be due their state of differentiation or their ultimate fate in the olfactory bulb [24,148]. Recent studies have addressed the role of glutamatergic signaling in the regulation of proliferation and fate choice in the adult hippocampus. Interestingly, the NMDA receptor subunits NR1 and NR2B are absent from transiently amplifying progenitors, but are found on GFAP-expressing type-1 precursor cells in the adult hippocampus [146]. NMDA receptors on proliferating adult hippocampal progenitors were found to mediate excitation-induced neurogenesis by inhibiting expression of the glial bHLH transcription factors Hes1 and Id2, and by promoting expression of the proneural transcription factor NeuroD [149]. By using a retrovirus-mediated, single-cell gene knockout technique in mice, Tashiro et al. showed that the survival of new neurons is critically regulated by neuronal activity via their own NMDA glutamate receptor within a short period after birth [150]. In addition to the pivotal role of NMDA-receptor mediated glutamate signaling, emerging evidence suggests a role for kainic acid and AMPA receptors in regulating adult hippocampal neurogenesis.
In order to investigate the rate of neurogenesis under pathological conditions such as epilepsy, the kainate receptor agonist kainic acid was used to induce seizures in vivo. Kainic acid-induced seizures result in long-lasting generation of functionally integrated new neurons in the adult rat hippocampus [151]. Additionally, chronic administration of an AMPA receptor potentiator increased progenitor cell proliferation in the adult DG in vivo [152].
GABA is the main inhibitory neurotransmitter in the adult brain. GABA exerts a dual role on immature new granule cells, initially depolarizing, and subsequently hyperpolarizing, depending on the intracellular chloride content determining the transmembrane gradient. The GABA receptor most essential to neurogenesis is the GABAA receptor, which is an ionotropic receptor channel that passes chloride upon GABA binding with the polarity depending on the chloride gradient across the membrane [153–155]. In the postnatal SVZ, GABA released from neuroblasts reduces the rate of proliferation of GFAP-expressing NSCs via tonic GABAA receptor activation, thereby providing a feedback mechanism controlling neural progenitor cell proliferation [156]. Furthermore, GABA was shown to have a direct effect on migrating neuroblasts in the adult SVZ. GABA derived from surrounding astrocyte-like cells decreased the speed of migration of neuroblasts en route to the olfactory bulb via GABAA receptor mediated signaling [157]. Ge et al. showed that newborn granule cells of the DG of the adult hippocampus are tonically activated by ambient GABA [158]. Furthermore, retrovirus-mediated expression of short hairpin RNA against NKCC1, a Na-K-2Cl transporter, lowered the intracellular chloride concentration, resulting in cellular hyperpolarization upon GABA application. As a result, prematurely hyperpolarized immature neurons showed a markedly reduced formation of both GABAergic and glutamatergic synapses, as well as decreased dendritic complexity [158]. In a subsequent study Song et al. showed that in the adult mouse hippocampus GABA derived from local parvalbumin-expressing interneurons promotes quiescence of adult hippocampal radial glia-like neural stem cells in response to neuronal activity and experience [159]. Interestingly, conditional knock-out of the gamma2-subunit of the GABAA receptor resulted in rapid exit from quiescence and enhanced symmetrical self-renewal, suggesting GABA-gamma2 signaling as an important niche mechanism involved in regulating the activation and self-renewal mode of quiescent adult NSCs [159].
Dopamine is a catecholamine neurotransmitter that is implicated in ontogenesis and embryonic germinal zone proliferation during development, and modulates movement, mood and motivation in the adult brain [160–162]. Dopamine receptors are classified as either D1-like (D1 and D5) or D2-like (D2, D3, and D4), according to structural homologies and shared second messenger cascades [163]. In the adult SVZ, D2-like receptors are predominantly expressed on transient amplifying cells (type C cells), which are the target of dopaminergic forebrain afferents. Interestingly, dopaminergic denervation of EGFR-expressing type C cells resulted in a significant reduction in the proliferation rate of SVZ progenitor cells [11,164,165]. A subsequent study showed that the dopaminergic fibers innervating the SVZ originate, at least in part, in the pars compacta of the substantia nigra [166]. Stimulation of D2-like receptors on EGFR-expressing type C cells via chronic Levodopa administration resulted in increased proliferation of neural progenitor cells in the adult SVZ [11]. Furthermore, systemic administration of the D2-like agonist 7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT) significantly increased precursor cell proliferation in the adult SVZ [167]. In the adult hippocampus dopaminergic afferents originating in the ventral tegmental area stimulate proliferation of neural precursors in the SGZ [11].
Transcription Factors
cAMP response element-binding protein (CREB) is a transcription factor that is a central regulator of cellular growth and development, and common final phosphorylation substrate for several kinase-mediated signaling pathways, including the cAMP/Protein kinase A pathway, calcium-calmodulin mediated NMDA receptor signaling, as well as MAP kinase signaling induced by neurotrophins via Trk receptors [168–171]. Upon phosphorylation, CREB dimerizes and binds to cAMP response elements within the promoter regions of target genes [171,172]. More specifically, cAMP mediated phosphorylation of CREB increases neurogenesis by stimulating progenitor cell proliferation and regulates survival of newly born neurons in the adult hippocampus in vivo [173–176]. In addition, cell autonomous knock-out of CREB via retrovirus-mediated overexpression of dominant-negative CREB in newly born cells in the adult mouse hippocampus is accompanied by impaired differentiation and morphological maturation of newborn dentate granule cells [175]. Similarly, cell autonomous CREB signaling is critically involved in regulation of survival, migration and morphological differentiation of neuroblasts in the adult SVZ [177–179]. Interestingly, loss of CREB signaling in immature neurons resulted in significant loss of expression of Pax6, suggesting that the effects of CREB-signaling on survival of migrating immature neurons in the RMS may be mediated by modulation of Pax6 [178].
Pax6, a paired homeobox transcription factor, is crucial for patterning telencephalon during development [180–182]. In the adult SVZ, Pax6 is expressed in immature neurons migrating in the RMS to the olfactory bulb [183–185]. Retroviral overexpression of Pax6 in neurosphere cultures derived from the adult telencephalon was shown to be sufficient to direct almost all neurosphere-derived cells towards a neuronal fate [183]. Moreover, in vivo analysis revealed that Pax6 restricts neural precursor cells in the RMS towards a neuronal fate, and is sufficient to instruct differentiation of neuroblasts to postmitotic dopaminergic periglomerular neurons [184,185].
Ascl1 (Mash1) is a member of the basic helix-loop-helix (bHLH) family of transcription factors involved in regulation of sequential fate commitment of NSCs during embryonic and adult neurogenesis [186]. By using in vivo lineage tracing with inducible Cre recombinase, it was demonstrated that Ascl1 expression in the adult SVZ is restricted to transit amplifying cells destined to differentiate into GABAergic interneurons in the olfactory bulb [187]. In the adult hippocampus, Ascl1 is transiently expressed by type 2 progenitor cells that subsequently develop into glutamatergic granule cell neurons [187]. Interestingly, retroviral-mediated overexpression of Ascl1 in vivo instructed adult hippocampal progenitor cells to generate cells of the oligodendrocytic lineage rather than generate excitatory granule cells, the predominant phenotype generated under physiological condition [188]. This finding is particularly significant since it demonstrates fate plasticity in the adult brain by showing that expression of a single gene can direct fate choice of adult NSCs. Olig2, another bHLH transcription factor, specifies transit-amplifying precursor fate and was found to oppose the role of Pax6 on neurogenesis in the adult SVZ [184].
Distal-less (Dlx) 2 is a homeobox transcription factor that is expressed by transit-amplifying cells and migrating neuroblasts in the adult SVZ [10]. In a study by Brill et al., overexpression of Dlx2 resulted in a significant increase in neuronal differentiation, and increased the migration velocity of neuroblasts migrating to the olfactory bulb. More specifically, Dlx2 promoted differentiation of neuronal precursors into dopaminergic periglomerular neurons in the olfactory bulb in cooperation with Pax6 [189]. This study also demonstrated that Dlx2 is required to maintain the proliferation of SVZ precursors. Inhibition of Dlx2-expression in the adult DG did not affect hippocampal neurogenesis, suggesting region-specificity of neurogenesis regulation by Dlx2 [189]. Subsequent in vitro studies have demonstrated that Dlx2 promotes lineage progression from stem cells to transient amplifying cells by increasing the expression of EGF receptor in SVZ stem cells [190].
Tlx is an orphan nuclear receptor that is highly expressed in the developing and the adult brain [191,192]. Several recent studies have addressed the role of Tlx in regulating adult neurogenesis. Tlx is specifically expressed in astrocyte-like GFAP-positive B-type cells in the SVZ, and conditional knock-out of Tlx resulted in a complete loss of ability of NSCs in the adult SVZ to undergo self-renewal [193]. Similarly, in the adult hippocampus, adult NSCs in Tlx-null mice lose their self-renewal capacity and are prone to differentiate into glial cells, suggesting that Tlx is crucial for maintaining NSCs in an undifferentiated, proliferative state. Interestingly, Tlx was found to act as a transcriptional repressor on the GFAP promoter, indicating that Tlx may promote self-renewal of NSCs at least in part by directly inhibiting astroglial differentiation on a transcriptional level [41]. A subsequent study suggested that Tlx regulates NSC proliferation in the adult hippocampus cell-autonomously, and that Tlx-mediated neurogenesis contributes to spatial learning and memory circuits [194]. Recent studies have provided further insight into the mechanism underlying the regulation of neural stem cell proliferation by Tlx. Tlx can activate the Wnt/beta-catenin pathway, which in turn mediates Tlx-induced neural stem cell proliferation and self-renewal in the adult brain [42]. In addition, Tlx was shown to recruit histone deacetylases (HDACs) to the Tlx target genes p21 and pten to repress their expression in adult NSCs, thereby maintaining NSCs in a proliferative state [195]. A feedback regulatory loop involving Tlx and miR-9, an endogenously expressed and highly conserved microRNA, has recently been proposed as an endogenous regulator of neural stem cell proliferation and differentiation [196,197]. MicroRNAs are small RNAs that have been shown to affect various cellular processes during development as well as in the adult via negatively regulating downstream target mRNAs [198,199]. In the proliferative state, Tlx cooperates with HDAC to inhibit transcription of miR-9 in NSCs. During differentiation, Tlx expression decreases, miR-9 becomes predominant, which further inhibits Tlx expression and promotes ongoing neuronal differentiation [196,197].
The Sox family of genes encodes for transcription factors that are crucial during development, and are abundantly expressed in the brain [200–202]. Reduced levels of Sox2 are associated with impaired neural stem cell proliferation and decreased adult neurogenesis [203]. Moreover, Sox2 is required for neuronal maturation, dendrite formation, and differentiation of GABAergic neurons in the adult olfactory bulb [204]. Sox11 expression is temporally restricted and most abundant in neuronally committed precursors and immature neurons of the adult hippocampus and SVZ [205]. Furthermore, overexpression of Sox11 in neurospheres derived from the SVZ resulted in neuronal differentiation of NSCs, suggesting a stage-specific role for Sox2 in the regulation of fate commitment in adult neurogenic regions [205]. In addition, another member of the Sox family, Sox9, was found to act as the downstream mediator of neuronal differentiation induced by the microRNA miR-124. miR-124 is expressed in the adult brain, and expression is increased in developing neuroblasts undergoing differentiation [206,207]. Interestingly, lineage progression of differentiating neuroblasts in the adult SVZ requires downregulation of Sox9 by miR-124 [207].
Emx2 is a homeobox transcription factor that is widely expressed in the developing brain, and important for proper morphogenesis of the CNS [208,209]. In the adult brain, Emx2 is expressed in SVZ precursors as well as in the DG of the hippocampus [210,211]. Emx2 was shown negatively regulate proliferation of SVZ progenitor cells by promoting symmetric division of stem cells generating more differentiated rather than undifferentiated progeny [210]. This was further substantiated by in vitro studies showing that overexpression of Emx2 resulted in decreased proliferation of SVZ-derived NSC clones by promoting asymmetric cell divisions [211].
Tbr2 is a T-box transcription factor widely expressed in neurogenic regions of the developing and the adult brain [212–214]. In the adult DG Tbr2 is expressed in intermediate neuronal progenitors [214]. Conditional knock-out of Tbr2 resulted in impaired neurogenesis in the DG in vivo due to failure of NSCs to differentiate into postmitotic neuroblasts [215.
Epigenetic regulators
Epigenetic mechanisms, including DNA methylation and histone modification, have recently emerged as an important link between external environmental influences and transcriptional control of gene expression in NSCs [216]. Epigenetic modification implies heritable changes in patterns of gene expression that are not encoded in the primary DNA sequence itself, thus resulting in new cellular phenotypes without altering the actual genomic sequence [217]. DNA methylation predominantly occurs at the cytosine residue of CpG dinucleotides to generate 5-methylcytosine on the pyrimidine ring, and the methylation status of DNA plays critical roles in the regulation of gene expression during development [218–220]. Methylation of CpG sites regulates gene expression either by blocking DNA binding of transcription factors or by binding of methyl-CpG binding proteins (MBDs). Binding of MBDs to methyl-CpGs, in turn, results in repression of gene transcription by recruitment of histone deacetylase repressor complexes and subsequent histone deacetylation [218].
Methyl-CpG-binding domain protein 1 (MBD1) is expressed throughout the adult brain with the highest concentration in the adult hippocampus [221]. MBD1 deficient mice develop normally and appear healthy throughout life, but have significantly reduced hippocampal neurogenesis and impaired spatial learning ability [221]. It was subsequently shown that MBD1 facilitates neuronal differentiation by direct binding to the promoter for the NSC mitogen FGF-2. MBD1-induced methylation of the FGF-2 promoter results in downregulation of FGF-2 expression, thus allowing adult hippocampal progenitor cells to undergo neuronal differentiation [222]. Moreover, MBD1 was recently shown to promote neuronal differentiation by decreasing the expression of the microRNA miR-184 [223]. Interestingly, miR-184 promotes proliferation of adult NSCs by downregulation of Numbl, a protein previously shown to be important for cortical brain development [223–225]. Thus, MBD1 promotes neurogenesis in the adult brain via Numbl and requires suppression of miR-184.
Methyl-CpG-binding protein 2 (MeCP2) is another MBD that is predominantly expressed in neurons in the mammalian CNS, and is involved in regulating neurogenesis in the adult [226, 227]. Newborn neurons in the DG of MeCP2-deficient mice exhibit profound deficits in neuronal maturation and spine formation [228]. MeCP2 is important for maintaining neuronal identity and phenotypic features by promoting methylation of the GFAP promoter near the initiation site in neuronal precursors, thus suppressing the expression of GFAP in developing neurons [229]. Moreover, overexpression of MeCP2 was shown to inhibit astroglial and promote neuronal differentiation in embryonic NSCs in vitro and in vivo [230]. While MeCP2 was previously believed to be involved in regulation of neuronal maturation rather than fate choice of progenitors in the adult brain, a recent study demonstrated a role for MeCP2 in regulation of neural stem cell proliferation and differentiation via the microRNA miR-137. MeCP2 in cooperation with Sox2 represses expression of miR-137, thus promoting neuronal differentiation of adult NSCs [231].
Growth arrest and DNA-damage-inducible protein 45 beta (GADD45b), a protein previously implicated in DNA-demethylation, has recently been identified as an important regulator of activity-induced neurogenesis [232,233]. GADD45b is expressed in adult DG and expression is transiently increased in response to activity via electroconvulsion. GADD45b mediates activity-induced adult hippocampal progenitor cell proliferation and dendritic growth of newborn neurons by promoting demethylation and thus induction of target gene promoters critical for adult neurogenesis, including BDNF and FGF [233].
TET1 is a member of the TET protein family, a group of Fe(II)/2-oxoglutarate-dependent dioxygenases that hydroxylate the 5-methyl group of the cytosine ring of the DNA to produce 5-hydroxymethylcytosine [234,235]. TET1 is widely expressed throughout the brain, including the adult hippocampus [236], and it was recently shown that TET1-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine is important for DNA demethylation in mammalian cells [237]. Interestingly, neuronal activity-induced demethylation of the promoters for BDNF and FGF was completely abolished in the adult DG after overexpression of shRNA against TET1 in vivo, suggesting an important role for TET1 in regulating activity-induced neurogenesis in the adult hippocampus [237].
The polycomb family transcriptional repressor Bmi-1 is another important regulator of developmental growth [238]. In the adult brain, Bmi-1 is required for self-renewal of SVZ NSCs by repressing the gene for the cyclin-dependent kinase inhibitor p16Ink4a [239,240]. Furthermore, in vitro overexpression of Bmi-1 significantly increases the number of adult NSCs in the SVZ in cooperation with the forebrain-specific transcription factor Foxg1 and maintains their developmental potential to generate neuronal lineages [241,242].
The histone methyltransferase mixed-lineage leukaemia 1 (Mll1) is expressed in the embryonic as well as the adult SVZ, and critically regulates expression of numerous developmental genes [243,244]. Transgenic mice deficient in Mll1 display intact neural stem cell proliferation, survival, and glial differentiation, but exhibit severely impaired neuronal differentiation in the adult SVZ. Moreover, Mll1-dependent neuronal differentiation of adult SVZ NSCs requires direct interaction and proper transcriptional activation of the Sox2 gene [243].
Members of the family of fragile X mental retardation proteins, including FMRP, FXR1, and FXR2, regulate translation of mRNAs by direct and selective binding to RNA and association with polyribosomes [245–247]. Loss of functional FMRP in NSCs in the adult DG in vivo resulted in increased astrocyte differentiation at the expense of neuronal differentiation as well as severe defects in hippocampus-dependent learning, suggesting an important regulatory role for FMRP in adult neurogenesis and learning [248,249]. Moreover, FMRP-induced neurogenesis requires intact Wnt signaling as well as presence of the downstream mediator Neurogenin1, a bHLH transcription factor [248]. In the adult olfactory bulb, FMRP is required for cell-autonomous neuronal differentiation by regulating dendritic spine production and morphogenesis [250]. FXR2 regulates hippocampal but not SVZ neurogenesis by facilitating BMP-signaling. FXR2 represses Noggin expression by reducing the stability of Noggin mRNA, thus allowing for proper BMP-mediated NSC proliferation and differentiation [135].
Conclusion
The recent discovery of ongoing neurogenesis in the adult mammalian brain has demonstrated a novel capacity of the mature nervous system to support the integration of de novo populations of neurons. While we are just beginning to understand mechanisms and regulators involved in adult neurogenesis, several common principles have emerged over the last decade. Adult neurogenesis recapitulates many features of embryonic neurogenesis, and several intrinsic and extracellular factors, such as trophic factors and transcription factors, play similar roles in the regulation of embryonic as well as postnatal neurogenesis. Furthermore, neurogenesis in the adult brain is a highly conserved process across all mammalian species sharing the subventricular and subgranular zone as common neurogenic niches. Interestingly, there is a striking similarity in regulation of neurogenesis between the adult SVZ and SGZ, including signaling molecules regulating neural stem cell self-renewal and maintenance of the stem cell pool and the molecular composition of the neurogenic niche. However, these two neurogenic regions also differ in various aspects, such as niche organization, neuronal subtype differentiation and migration. Advances in virally-mediated single-cell genetic manipulation and transgenic animal models allow us to investigate the roles of individual signaling molecules during different stages of neurogenesis, and we are just beginning to understand the complexity of interactions and crosstalk between different signaling pathways, linking extracellular signals via intracellular second messenger pathways to transcription factor activation and epigenetic changes.
Recent evidence elucidating the role of Wnt signaling in adult neurogenesis is a prime example of how application of new technological tools has helped us to characterize and better understand the molecular basis of niche-specific neurogenesis. It had previously been postulated that factors derived from local astrocytes participate in the regulation of proliferation and differentiation in hippocampal neurogenesis [20]. By means of in vivo viral-mediated gene transduction, siRNA-technology, and inducible transgenic mouse lines several subsequent studies not only identified Wnt as the mediator of this interaction, but also identified betacatenin-mediated activation of the NeuroD promoter via interaction with the transcription factor Sox2 as the underlying mechanism, thereby elegantly demonstrating the importance of NeuroD for adult neurogenesis [37–39]. These studies exemplify how methodological advances in recent years have enabled us to link previously observed morphological changes to the underlying molecular mechanisms characterizing the neurogenic niche.
Several other studies have demonstrated interactions and crosstalk between the Wnt-pathway and other signaling molecules important for adult neurogenesis, including Tlx, Disc1, FMRP and hypoxia inducible factor-1 (HIF-1) [40,42,248,251], exemplifying the complexity of molecular interactions implicated in regulating neurogenesis. Signaling networks on various cellular levels define both intrinsic developmental stage-specific changes as well as the identity of the environmental components of the neurogenic niche. However, as more pieces of the puzzle fall into place, more questions arise. The exact molecular and cellular components of the neurogenic niche and their interaction with the putative NSCs are still not fully elucidated. Furthermore, the signaling pathways involved in regulation of self-renewal as well as lineage differentiation of NSCs are just beginning to be understood. In particular, characterization of the role of epigenetic regulation in adult neurogenesis is still in its nascent stage. Interplay between NSCs and other non-neural cell types, such as cells of the immune system, is important for proper maintenance and activation of the endogenous stem cell pool in the adult brain under physiological as well as disease conditions [252]. While we have recently began to appreciate the importance of CNS-resident microglia and other immune cells in fine-tuning proliferation and differentiation of adult NSCs [253,254], future studies are needed to elucidate the exact molecular mechanisms underlying regulatory influences of immune and other non-neural cells on adult neurogenesis and its physiological significance. Lastly, further studies are needed to elucidate causal relationships between neurogenesis and behavior under physiological conditions, and characterize the effect of defective neurogenesis on disease phenotypes. Understanding the functional role of adult neurogenesis as well as its underlying cellular and molecular substrates will enable us to develop targeted new therapies for neurological diseases in humans.
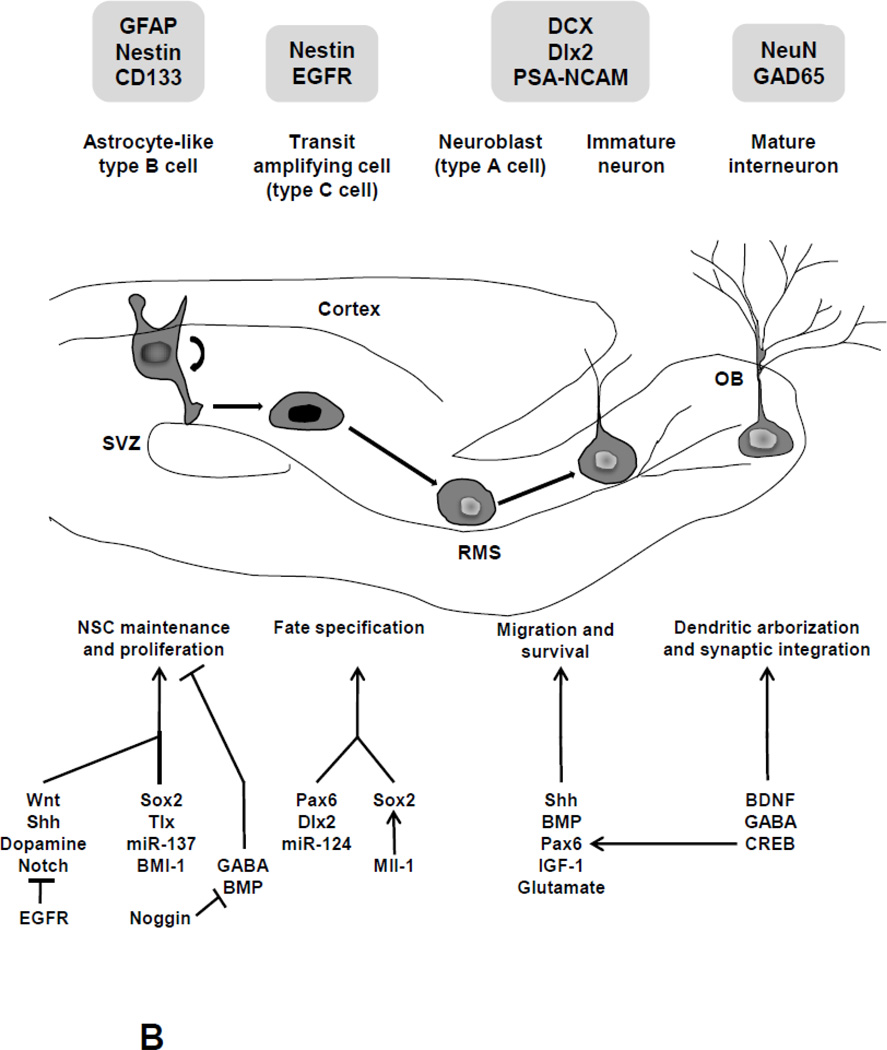
Figure 1. Regulation of Adult Neurogenesis in the Dentate Gyrus of the Hippocampus and the Subventricular Zone of the Lateral Ventricle.
Shown is a schematic diagram illustrating the sequential steps underlying the process of neurogenesis in the adult hippocampus (1A) and the subventricular zone (1B). Figure 1A shows the transition from a radial-like glia cell to neural progenitor cell in the adult SGZ, as well as subsequent stages of neuronal differentiation, maturation and migration. Figure 1B illustrates self-renewal and differentiation of SVZ NSCs and subsequent maturation and migration along the RMS. The expression of cell-specific markers during the various stages of development is shown. The actions of major molecular regulators as well as crosstalk between various molecular players during various stages of adult hippocampal (1A) and subventricular zone (1B) neurogenesis are also presented. GCL, granule cell layer; ML, molecular layer; OB, olfactory bulb; RMS, rostral migratory stream; SGZ, subgranular zone; SVZ, subventricular zone.
Table 1.
Overview of signaling in adult neural stem cells
| Signal | Effect on neurogenesis | Cell type | In vitro/vivo | Reference |
|---|---|---|---|---|
| EXTRINSIC | ||||
| Morphogens | ||||
| Wnt | increases neurogenesis required for neuronal differentiation stimulates NSC proliferation/self-renewal |
SGZ SGZ SGZ/SVZ |
in vivo in vivo in vitro/vivo |
[37] [38] [42, 44] |
| Notch | required for NSC proliferation, maintenance required for dendritic arborization |
SGZ/SVZ SGZ |
in vitro/vivo in vivo |
[52, 53, 55, 56] [52, 55] |
| Shh | required Tor progenitor proliferation required for NSC maintainance required for neuroblast migration |
SGZ/SVZ SVZ SVZ |
in vitro/vivo in vivo in vivo |
[69, 73, 75] [76] [76, 77] |
| BMP | decreases neurogenesis promotes neuroblast survival |
SVZ SVZ |
in vivo in vitro |
[132] [132] |
| Growth Factors | ||||
| BDNF | increases neuragenesis required for dendritic arborization |
SGZ/SVZ SGZ |
in vivo in vivo |
[92, 93, 94] [95] |
| NT-3 | required for neuron al differentiation | SGZ | in vivo | [98] |
| FGF-2 | increases neurogenesis | SGZ | in vivo | [104, 105] |
| IGF-1 | increases neuragenesis required for neuroblast migration |
SGZ SVZ |
in vivo in vivo |
[108, 109] [110] |
| VEGF | increases ne ura genesis | SGZ SVZ | in vitro/vivo | [121] |
| Neurotransmitters | ||||
| Glutamate | required for survival of migrating neuroblasts required for neuronal survival |
SVZ SGZ/SVZ |
in vivo in vivo |
[144] [150] |
| GABA | decreases NSC proliferation required for dendritic arborization required for NSC quiescence |
SVZ SGZ SGZ |
in vivo in vivo in vivo |
[156] [158] [159] |
| Dopamine | required for progenitor proliferation | SGZ/SVZ | in vivo | [11, 164, 165] |
| INTRINSIC | ||||
| Transcription Factors | ||||
| CREB | required for neuron al survival, dendritic arborization | SGZ/SVZ | in vivo | [175, 177] |
| Pax6 | promotes neuronal differentiation | SVZ | in vivo | [184, 185] |
| Ascl1 | overexpression instructs oligodendrocyte fate | SGZ | in vivo | [188] |
| Dlx-2 | increases neuronal differentiation, migration velocity | SVZ | in vitro/vivo | [189, 190] |
| Tlx | required for NSC proliferation/self-renewal | SGZ/SVZ | in vivo/vitro | [41, 42, 193] |
| Sox2 | required for NSC/neural progenior tell proliferation | SGZ/SVZ | in vivo | [203] |
| Tbr2 | required for differentiation of neuronal precursors | SGZ | in vivo | [215] |
| NeuroD | necessary for survival, maturation of neuroblasts | SGZ | in vitro/vivo | [39] |
| Epigenetic Regulators | ||||
| GADD45b | mediates activity-induced NSC5 proliferation | SGZ | in vivo | [233] |
| miR-124 | promotes neuronal differentiation | SVZ | in vitro/vivo | [207] |
| MBD1 | required for neuronal differentiation | SGZ | in vitro/vivo | [221, 222, 223] |
| MeCP2 | required for neuronal maturation, dendrite formation | SGZ | in vivo | [228] |
| miR-137 | required for NSC proliferation, maintainance | SGZ/SVZ | in vitro/vivo | [231] |
| Bmi-1 | required for NSC proliferation, maintainance | SVZ | in vivo | [239, 240] |
| MII-1 | required for neuronal differentiation | SVZ | in vivo | [243] |
| FMRP | required for neuronal differentiation required for activity-dependent dendrite formation |
SGZ SVZ |
in vitro/vivo in vivo |
[248, 249] [250] |
Highlights.
Adult neurogenesis is regulated via both extrinsic environmental influences and intrinsic genetic factors.
We review individual signaling mechanisms and their cross-talk in regulating adult neurogenesis.
We highlight emerging principles in the growing field of adult neural stem cell biology.
Acknowledgement
We thank K. Christian for comments. The research in Dr. Song’s laboratory was supported by NIH (NS047344 and MH087874), IMHRO, and SAFRI. R. Faigle is the recipient of an NIH/National Institute of Neurological Disorders and Stroke R25 training grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roland Faigle, Email: rfaigle1@jhmi.edu.
Hongjun Song, Email: shongju1@jhmi.edu.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 4.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, Zhang Y, Chen R, Song H, Yang Z. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011;21:1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 7.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, de Vellis J, Sun YE. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 10.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 11.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 12.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 13.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J. Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- 18.Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. N. Y. Acad. Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr. Opin. Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 21.Seidenfaden R, Desoeuvre A, Bosio A, Virard I, Cremer H. Glial conversion of SVZderived committed neuronal precursors after ectopic grafting into the adult brain. Mol. Cell. Neurosci. 2006;32:187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampusderived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 23.Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J. Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 25.Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 26.Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 28.Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 30.De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res. Brain Res. Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 31.De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMahon AP. Cell signalling in induction and anterior-posterior patterning of the vertebrate central nervous system. Curr. Opin. Neurobiol. 1993;3:4–7. doi: 10.1016/0959-4388(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 33.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 34.Chen RH, Ding WV, McCormick F. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J. Biol. Chem. 2000;275:17894–17899. doi: 10.1074/jbc.M905336199. [DOI] [PubMed] [Google Scholar]
- 35.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 36.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 37.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 38.Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/betacatenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 42.Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu JM, Kim JH, Song GS, Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- 44.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 45.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 46.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 47.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 48.Bray S, Bernard F. Notch targets and their regulation. Curr. Top. Dev. Biol. 2010;92:253–275. doi: 10.1016/S0070-2153(10)92008-5. [DOI] [PubMed] [Google Scholar]
- 49.Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Deltalike and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech. Dev. 2002;114:153–159. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 50.Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J. Neurosci. Res. 2004;75:330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- 51.Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 52.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell. Stem Cell. 2010;7:730–743. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J. Biol. Chem. 2003;278:23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- 60.Dahlhaus M, Hermans JM, Van Woerden LH, Saiepour MH, Nakazawa K, Mansvelder HD, Heimel JA, Levelt CN. Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J. Neurosci. 2008;28:10794–10802. doi: 10.1523/JNEUROSCI.1348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat. Rev. Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 62.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 63.Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 64.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 65.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 66.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 67.Philipp M, Caron MG. Hedgehog signaling: is Smo a G protein-coupled receptor? Curr. Biol. 2009;19:R125–R127. doi: 10.1016/j.cub.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. J. Neurochem. 1998;70:1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- 69.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 70.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papanikolaou T, Lennington JB, Betz A, Figueiredo C, Salamone JD, Conover JC. In vitro generation of dopaminergic neurons from adult subventricular zone neural progenitor cells. Stem Cells Dev. 2008;17:157–172. doi: 10.1089/scd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 72.Banerjee SB, Rajendran R, Dias BG, Ladiwala U, Tole S, Vaidya VA. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur. J. Neurosci. 2005;22:1570–1580. doi: 10.1111/j.1460-9568.2005.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 74.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 75.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 76.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J. Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells. 2008;26:2311–2320. doi: 10.1634/stemcells.2008-0297. [DOI] [PubMed] [Google Scholar]
- 78.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu. Rev. Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 79.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 80.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat. Rev. Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 81.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 82.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 83.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 85.Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol. Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr. Opin. Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 87.Tonchev AB, Yamashima T, Guo J, Chaldakov GN, Takakura N. Expression of angiogenic and neurotrophic factors in the progenitor cell niche of adult monkey subventricular zone. Neuroscience. 2007;144:1425–1435. doi: 10.1016/j.neuroscience.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 88.Giuliani A, D'Intino G, Paradisi M, Giardino L, Calza L. p75(NTR)-immunoreactivity in the subventricular zone of adult male rats: expression by cycling cells. J. Mol. Histol. 2004;35:749–758. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- 89.Okano HJ, Pfaff DW, Gibbs RB. Expression of EGFR-, p75NGFR-, and PSTAIR (cdc2)-like immunoreactivity by proliferating cells in the adult rat hippocampal formation and forebrain. Dev. Neurosci. 1996;18:199–209. doi: 10.1159/000111408. [DOI] [PubMed] [Google Scholar]
- 90.Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol. Cell. Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 94.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxietylike behavior. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 97.Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J. Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol. Dis. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 101.Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Iwata T, Hevner RF. Fibroblast growth factor signaling in development of the cerebral cortex. Dev. Growth Differ. 2009;51:299–323. doi: 10.1111/j.1440-169X.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 103.Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 104.Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol. Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 106.Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J. Neurosci. Res. 2000;59:332–341. doi: 10.1002/(sici)1097-4547(20000201)59:3<332::aid-jnr6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 107.Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol. Cell. Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 108.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 110.Hurtado-Chong A, Yusta-Boyo MJ, Vergano-Vera E, Bulfone A, de Pablo F, Vicario-Abejon C. IGF-I promotes neuronal migration and positioning in the olfactory bulb and the exit of neuroblasts from the subventricular zone. Eur. J. Neurosci. 2009;30:742–755. doi: 10.1111/j.1460-9568.2009.06870.x. [DOI] [PubMed] [Google Scholar]
- 111.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yasuhara T, Shingo T, Date I. The potential role of vascular endothelial growth factor in the central nervous system. Rev. Neurosci. 2004;15:293–307. doi: 10.1515/revneuro.2004.15.4.293. [DOI] [PubMed] [Google Scholar]
- 113.Wang WY, Dong JH, Liu X, Wang Y, Ying GX, Ni ZM, Zhou CF. Vascular endothelial growth factor and its receptor Flk-1 are expressed in the hippocampus following entorhinal deafferentation. Neuroscience. 2005;134:1167–1178. doi: 10.1016/j.neuroscience.2005.04.064. [DOI] [PubMed] [Google Scholar]
- 114.Khaibullina AA, Rosenstein JM, Krum JM. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res. Dev. Brain Res. 2004;148:59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 116.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 117.Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 2003;274:851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- 119.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 120.Maurer MH, Tripps WK, Feldmann RE, Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci. Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 121.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 124.Harvey BK, Hoffer BJ, Wang Y. Stroke and TGF-beta proteins: glial cell line-derived neurotrophic factor and bone morphogenetic protein. Pharmacol. Ther. 2005;105:113–125. doi: 10.1016/j.pharmthera.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 125.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat. Rev. Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 126.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 127.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.ten Dijke P, Miyazono K, Heldin CH. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr. Opin. Cell Biol. 1996;8:139–145. doi: 10.1016/s0955-0674(96)80058-5. [DOI] [PubMed] [Google Scholar]
- 130.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell. Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 131.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev. Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 132.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 133.Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- 134.Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo W, Zhang L, Christopher DM, Teng ZQ, Fausett SR, Liu C, George OL, Klingensmith J, Jin P, Zhao X. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron. 2011;70:924–938. doi: 10.1016/j.neuron.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, Sato K. A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J. Neurosci. 2003;23:11732–11740. doi: 10.1523/JNEUROSCI.23-37-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, Colak D, Gotz M, Farinas I, Gage FH. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell. Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 138.Behar TN, Schaffner AE, Scott CA, O'Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J. Neurosci. 1998;18:6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 140.Platel JC, Boisseau S, Dupuis A, Brocard J, Poupard A, Savasta M, Villaz M, Albrieux M. Na+ channel-mediated Ca2+ entry leads to glutamate secretion in mouse neocortical preplate. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19174–19179. doi: 10.1073/pnas.0504540102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sommer B, Seeburg PH. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol. Sci. 1992;13:291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- 142.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 143.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 144.Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X, Bolteus AJ, Balkin DM, Henschel O, Bordey A. GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes. Glia. 2006;54:394–410. doi: 10.1002/glia.20392. [DOI] [PubMed] [Google Scholar]
- 146.Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 147.Platel JC, Stamboulian S, Nguyen I, Bordey A. Neurotransmitter signaling in postnatal neurogenesis: The first leg. Brain Res. Rev. 2010;63:60–71. doi: 10.1016/j.brainresrev.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Platel JC, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. J. Physiol. 2008;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitationneurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 150.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 151.Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bai F, Bergeron M, Nelson DL. Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44:1013–1021. doi: 10.1016/s0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 153.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 154.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 155.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J. Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012 doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pendleton RG, Rasheed A, Roychowdhury R, Hillman R. A new role for catecholamines: ontogenesis. Trends Pharmacol. Sci. 1998;19:248–251. doi: 10.1016/s0165-6147(98)01218-8. [DOI] [PubMed] [Google Scholar]
- 161.Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J. Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr. Opin. Neurol. 16 Suppl. 2003;2:S3–S9. [PubMed] [Google Scholar]
- 163.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 164.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 165.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp. Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 166.Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC, Hoglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J. Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur. J. Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 168.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 169.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 170.Dworkin S, Mantamadiotis T. Targeting CREB signalling in neurogenesis. Expert Opin. Ther. Targets. 2010;14:869–879. doi: 10.1517/14728222.2010.501332. [DOI] [PubMed] [Google Scholar]
- 171.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 172.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 173.Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J. Neurosci. 2002;22:9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J. Neurosci. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005;25:10105–10118. doi: 10.1523/JNEUROSCI.3512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell. Neurosci. 2011;46:79–88. doi: 10.1016/j.mcn.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 179.Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAPK signalling pathways. Eur. J. Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 180.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 181.Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–1044. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- 182.Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J. Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol. Cell. Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 184.Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat. Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 185.Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 187.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J. Neurosci. 2007;27:12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 2008;11:888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, Ashery-Padan R, Saghatelyan A, Berninger B, Gotz M. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J. Neurosci. 2008;28:6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Suh Y, Obernier K, Holzl-Wenig G, Mandl C, Herrmann A, Worner K, Eckstein V, Ciccolini F. Interaction between DLX2 and EGFR regulates proliferation and neurogenesis of SVZ precursors. Mol. Cell. Neurosci. 2009;42:308–314. doi: 10.1016/j.mcn.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 191.Yu RT, McKeown M, Evans RM, Umesono K. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 1994;370:375–379. doi: 10.1038/370375a0. [DOI] [PubMed] [Google Scholar]
- 192.Monaghan AP, Grau E, Bock D, Schutz G. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development. 1995;121:839–853. doi: 10.1242/dev.121.3.839. [DOI] [PubMed] [Google Scholar]
- 193.Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 195.Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat. Struct. Mol. Biol. 2009;16:365–371. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Denli AM, Cao X, Gage FH. miR-9 and TLX: chasing tails in neural stem cells. Nat. Struct. Mol. Biol. 2009;16:346–347. doi: 10.1038/nsmb0409-346. [DOI] [PubMed] [Google Scholar]
- 198.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–1641. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 199.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 201.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 202.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 203.Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 204.Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, Wanke E, Brunelli S, Favaro R, Ottolenghi S, Nicolis SK. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 205.Haslinger A, Schwarz TJ, Covic M, Chichung Lie D. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur. J. Neurosci. 2009;29:2103–2114. doi: 10.1111/j.1460-9568.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 206.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 207.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mallamaci A, Iannone R, Briata P, Pintonello L, Mercurio S, Boncinelli E, Corte G. EMX2 protein in the developing mouse brain and olfactory area. Mech. Dev. 1998;77:165–172. doi: 10.1016/s0925-4773(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 209.Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Galli R, Fiocco R, De Filippis L, Muzio L, Gritti A, Mercurio S, Broccoli V, Pellegrini M, Mallamaci A, Vescovi AL. Emx2 regulates the proliferation of stem cells of the adult mammalian central nervous system. Development. 2002;129:1633–1644. doi: 10.1242/dev.129.7.1633. [DOI] [PubMed] [Google Scholar]
- 211.Gangemi RM, Daga A, Marubbi D, Rosatto N, Capra MC, Corte G. Emx2 in adult neural precursor cells. Mech. Dev. 2001;109:323–329. doi: 10.1016/s0925-4773(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 212.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Gotz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hodge RD, Nelson BR, Kahoud RJ, Yang R, Mussar KE, Reiner SL, Hevner RF. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J. Neurosci. 2012;32:6275–6287. doi: 10.1523/JNEUROSCI.0532-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat. Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 218.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 219.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 220.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 221.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, Summers RG, Chun J, Lee KF, Gage FH. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li X, Barkho BZ, Luo Y, Smrt RD, Santistevan NJ, Liu C, Kuwabara T, Gage FH, Zhao X. Epigenetic regulation of the stem cell mitogen Fgf-2 by Mbd1 in adult neural stem/progenitor cells. J. Biol. Chem. 2008;283:27644–27652. doi: 10.1074/jbc.M804899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell. Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 225.Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat. Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- 226.Jung BP, Zhang G, Ho W, Francis J, Eubanks JH. Transient forebrain ischemia alters the mRNA expression of methyl DNA-binding factors in the adult rat hippocampus. Neuroscience. 2002;115:515–524. doi: 10.1016/s0306-4522(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 227.Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 228.Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kohyama J, Kojima T, Takatsuka E, Yamashita T, Namiki J, Hsieh J, Gage FH, Namihira M, Okano H, Sawamoto K, Nakashima K. Epigenetic regulation of neural cell differentiation plasticity in the adult mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18012–18017. doi: 10.1073/pnas.0808417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tsujimura K, Abematsu M, Kohyama J, Namihira M, Nakashima K. Neuronal differentiation of neural precursor cells is promoted by the methyl-CpG-binding protein MeCP2. Exp. Neurol. 2009;219:104–111. doi: 10.1016/j.expneurol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 231.Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Barreto G, Schafer A, Marhold J, Stach D, Swaminathan SK, Handa V, Doderlein G, Maltry N, Wu W, Lyko F, Niehrs C. Gadd45a promotes epigenetic gene activation by repairmediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 233.Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 2010;38:e181. doi: 10.1093/nar/gkq684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 239.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fasano CA, Phoenix TN, Kokovay E, Lowry N, Elkabetz Y, Dimos JT, Lemischka IR, Studer L, Temple S. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes Dev. 2009;23:561–574. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell. Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 243.Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 245.Lu R, Wang H, Liang Z, Ku L, O'donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs. Hum. Mol. Genet. 2009;18:3164–3177. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, Li W, Liu C, Jin P, Zhao X. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Guo W, Allan AM, Zong R, Zhang L, Johnson EB, Schaller EG, Murthy AC, Goggin SL, Eisch AJ, Oostra BA, Nelson DL, Jin P, Zhao X. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Scotto-Lomassese S, Nissant A, Mota T, Neant-Fery M, Oostra BA, Greer CA, Lledo PM, Trembleau A, Caille I. Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J. Neurosci. 2011;31:2205–2215. doi: 10.1523/JNEUROSCI.5514-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat. Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 254.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]